Abstract
Understanding the microbial community structure in soil contaminated with heavy metals (HMs) is a precondition to conduct bioremediation in mine soil. Samples were collected from a typical lead–zinc (Pb–Zn) mine to assess the microbial community structure of the HMs concentrated in the soil. The goal was to analyze the bacterial and fungal community structures and their interactions using the 16S rRNA genes and internal transcribed spacer high-throughput sequencing. Analyses at different sampling sites showed that contamination with HMs significantly reduced the bacterial richness and diversity but increased that of the fungi. The predominant bacteria genera of Acidobacteriales, Gaiellales, Anaerolineaceae, Sulfurifustis, and Gemmatimonadaceae, and predominant fungal genera of Sordariomycetes, Talaromyces, and Mortierella were assumed as HM resistant genera in Pb–Zn mining area. The pH effect on the bacterial and fungal communities was opposite to those of Cd, Pb, and Zn. This study offers comprehensive outlooks for bacterial and fungal community structures upon multiple HM stresses in the soil around a typical Pb–Zn mine area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HMs), such as lead (Pb), cadmium (Cd), and zinc (Zn), are often toxic to organisms; these metals are also persistent in soils (Li et al. 2017). A meta-analysis of HM pollution in farmland and urban soils in China during 2000 to 2019 showed that nearly 33.54% and 44.65% of the sites in farmland and urban soils, respectively, were polluted with Cd (Yuan et al. 2021). Mining activities are one of the primary sources for the contamination of soils with HMs, while Pb–Zn mines are considered to have a serious impact on HM pollution in the soil around them (Du et al. 2019). Zhao et al. (2014) reported that the contents of Pb, Zn, Cd, and copper (Cu) around the Shuikoushan Pb–Zn mining area were 459–3,783, 1,063–5,784, 8.35–79.33, and 53.5–415.8 mg/kg, respectively. Li et al. (2018) also found the contents of Pb, Zn, Cd, Cu, and arsenic (As) around the Xiangxi Tujia and Miao Autonomous District Pb–Zn mine area to be 600 ± 2.11, 2,452 ± 82.2, 22 ± 1.34, 13 ± 0.40, and 2.36 ± 0.08 mg/kg, respectively. Thus, there were generally high concentrations of HMs in the soil around the Pb–Zn mining area. Since microorganisms play a vital role in the remediation of soil contaminated with HMs, it is necessary to evaluate the microbial community structure when it has been subjected to HM stress to provide further reference information for bioremediation.
The soil harbors an enormous diversity of microorganisms, and bacteria and fungi play important roles for ecosystem functioning, such as regulating the decomposition of organic matter and mediating nutrient cycling (Mooshammer et al. 2014). HMs could significantly affect the microbial community and its functions (Liu et al. 2020). Golebiewski et al. (2014) revealed that the microbial diversity and richness decreased in soils contaminated with Zn. Sheik et al. (2012) found that higher concentrations of arsenic (As) increased the microbial community abundance and reduced its diversity. Both single HM and multiple HMs can cause changes in the microbial community. Mierzwa-Hersztek et al. (2018) reported that multiple HMs, such as nickel (Ni), Cd, Pb, Cu, As, Zn, chromium (Cr), and mercury (Hg), have been confirmed to be toxic to soil microorganisms worldwide.
Conversely, the bacterial communities could also resist HM toxicity (Li et al. 2020). Luo et al. (2019) reported that the range of concentration of Cd was approximately 0.3–3 mg/kg in a Pb–Zn mining area in Xiangtan of China where Actinobacteria was found to be tolerant to Cd. Li et al. (2017) reported that Thiobacillus positively correlated with the content of Cd, while Longilinea negatively correlated with the content of Cd in multiple soils contaminated with HMs. In addition, Archaea are resistant to contamination with HMs and could contribute to the adaption to these contaminants. Soil contaminated with different types of HMs caused variations in the microbial richness and community structure (Pan et al. 2020). Li et al. (2020) reported that Acidobacteria, Chloroflexi, and Gemmatimonadetes were more resistant to the higher levels of HMs in the soil contaminated with Cd, Pb, and Zn in mine soil located in Guangxi, China.
Pollution with HMs can affect not only the bacterial community but also the fungal community. Zeng et al. (2020b) investigated the characteristics of a fungal community beside the bacterial community in an arid loess around a large-scale and long-term ore smelting and electroplating area, which caused contamination with Cd, Pb, Ni, Zn, Cu, and Cr in the soil. Ascomycetes was the most abundant fungal phyla with an average abundance of 88.55%, which primarily included Sordariomycetes and Eurotiomycetes. Kerfahi et al. (2020) also found that the phyla Ascomycota (79%) and Basidiomycota (7%) were dominant in metalliferous Gobi Desert soils with high concentrations of Zn, Pb, and Cd. However, Little knowledge is known about both the bacterial and fungal community structure and interaction in a typical Pb–Zn mining soil upon multiple high HM stress.
It is very important to explore the typical HMs and their mobility, as well as their relationship with other soil physicochemical properties and microbial communities in typical Pb–Zn mining areas, which are benefit for Pb–Zn mining soil bioremediation. Therefore, the aims of this study were as follows: (1) to analyze the correlation of HMs concentrations with other soil physicochemical parameters at different sample sites; (2) to analyze the composition and difference of the microbial community composition upon HM stress in the typical Pb–Zn mining area; and (3) to explore the mechanisms of adaptation and interaction of microbial communities under different types of HM stress.
Materials and methods
Soil sampling
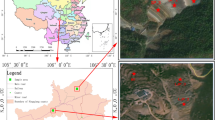
The soil was sampled from a typical Pb–Zn mine in Changning City, Hunan Province, China. Mining and smelting activities were initiated early in 1889 and have developed rapidly since the 1950s (Wei et al. 2009). This area is in a subtropical zone with a warm, wet climate characterized by an annual average temperature of 17.8 °C and an average precipitation of 1,457 mm (Zhao et al. 2014). It has generated a significant quantity of dust, slag, and tailings every year, which contaminates over several km of the surrounding areas. Four soil samples were collected and labeled F1-F4 from the top layer of soils (0–20 cm, approximately 2 kg per soil sample) across a 10 × 10 m area on each sampling site using the plum blossom method. The distance of F1 to F2 was about 0.4 km; F3 to F4 was about 0.1 km, and F1/F2 to F3/F4 was about 3.3 km. The soil samples were taken from the corners and center of an area and mixed thoroughly. The soil samples were then immediately brought back to the laboratory. Approximately 0.5 kg of the soil was frozen at − 20 °C for further DNA extraction, while the rest was dried for soil chemical and physical analyses.
Soil physicochemical analysis
The air-dried soil samples were filtered through a 2 mm sieve, and 10 g was added to a 200 mL beaker with 25 mL of distilled water. The mixture was first stirred at 3000 rpm for 2 min and incubated stationary for 30 min. The pH of the supernatant was finally measured by a pH meter (FiveEasy 20; Mettler-Toledo, Changzhou, China). The soil water content (moisture) was determined by oven drying (DF205; Hebei Haowei Electrical Equipment Technology Co., Ltd., Cangzhou, China) at 105 °C for 12 h, while the total organic carbon (TOC) was determined by comparing the change in mass after high temperature firing (550 ℃, 8 h) (Liang et al. 2017). The water extract of organic carbon (WEOC) was measured as follows: 1:10 soil was added to distilled water (w/v), shaken at 150 rpm in the air at a constant temperature shock chamber (HZQ-C; Harbin Donglian Electronic Technology Development Co., Ltd., Harbin, China) for 3 h, separated by centrifugation at 821 g for 20 min, and filtered by 0.45 µm nylon membrane filters to remove the suspended particulate matter (MD nylon 66; Changde BKMAM Biotechnology Co., Ltd., Changde, China). The TOC in the extracted solution was determined as WEOC using a TOC analyzer (TOC-V CPH; Shimadzu, Tokyo, Japan).
The total HMs in the soil were extracted by a graphite furnace digestion apparatus assisted acid (HNO3: HCl: HF = 6:3:2 [v/v/v]) digestion method (Li et al. 2020; Liang et al. 2017). The concentrations of Cd, Pb, Cu, Zn, As, Cr, and Ni were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS 7900; Agilent Technologies, Santa Clara, CA, USA) using an SPS4 autosampler and Masshunter software.
The mobility of HMs was evaluated using the method of monitoring calcium chloride (CaCl2)-extractable metal concentrations. The CaCl2 was extracted in a 50 mL centrifuge tube. A volume of 30 mL of 0.01 mol CaCl2 was added to a centrifuge tube that contained 3 g soil. After mixing and shaking at 60 rpm on a thermostatic shaker at 25 °C for 24 h, the samples were centrifuged (821 g, 20 min), and the supernatants were filtered through a 0.45 μm syringe filter. The concentrations of HMs in the filtrate were determined by ICP-MS.
DNA extraction, PCR amplification, and Illumina MiSeq sequencing
The genomic DNA was extracted from each soil sample in triplicate using an E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions. A NanoDrop 2000 UV–Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the concentration of DNA and its quality. Primers of 515FmodF/806RmodR were used to amplify the V4 hypervariable regions of both the bacterial and archaeal 16S rRNA gene (Zeng et al. 2019). Primers of 1737F/2043R were used to amplify the internal transcribed spacers (ITS) of the fungi (Zhang et al. 2016). The PCR products were purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor™-ST (Promega, Madison, WI, USA) according to the manufacturer’s instructions. High-throughput sequencing was conducted by Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) using an Illumina MiSeq platform (Illumina, San Diego, CA, USA).
Bioinformatics analysis
Quality control was conducted using the Trimmomatic software as previously reported (Xiao et al. 2019). The qualified data were then clustered and classified into operational taxonomic units (OTUs) at a 97% sequence similarity threshold using USEARCH v. 7.0. The alpha index values of the ACE, Chao, Shannon, and Simpson indices were calculated using Mothur v. 1.30.1. The taxonomy was determined with the Ribosomal Database Project (RDP) Classifier v. 2.6 with a confidence threshold of 70% (Zeng et al. 2020a). The composition and abundance of the microorganisms were determined using R software v. 3.3.1 at the phylum and genus levels. Non-metric multidimensional scaling (NMDS) was conducted to evaluate the difference among samples at the OTUs level based on the Bray_curtis distance. A Kruskal–Wallis H test was performed to assess the significant differences in the abundance of taxa. Pearson correlation coefficients were calculated by R to assess the effect of environmental factors on the microbial community. Molecular ecological networks (MENs) were constructed using Network software to determine the coexistence of taxa among the samples (Liu et al. 2019). BugBase (https://bugbase.cs.umn.edu/index.html) was used to predict the tolerance of the bacterial communities to HM stress at genus level (Deng et al. 2022). FUNGuild (http://www.funguild.org/) was utilized to predict the fungal functional profiles (Zhang et al. 2019). Finally, an Illumina HiSeq 2500 platform (Guangzhou Magigene Biotechnology Co., LTD., China) was used for sequencing and microbial community analysis.
Statistical analysis
All the soil samples were tested in triplicate. Origin 2018 (OriginLab, Northampton, MA, USA) was used to statistically analyze the degree of contamination with HMs at different sample sites (F1, F2, F3, and F4). The correlation analysis of physicochemical properties of the sample sites and a one-way analysis of variance (ANOVA) of the soil properties, content of HMs, and the alpha indices of microorganisms in the different sample sites (p < 0.05) were calculated using SPSS 20.0 (IBM, Inc., Armonk, NY, USA). Typically, p < 0.05 was considered to be a significant difference. The sequences were submitted to the Sequence Read Archive (SRA) database in the National Center for Biotechnology Information (NCBI), and the accession numbers were SRR14278453-14278464 and SRR14279054-14279065.
Results and discussion
Correlation analysis of the HMs and other soil physicochemical parameters
The relative concentration was obtained by dividing the measured concentration by the background value (local standard of Hunan province DB43-T1125-2016). The relative concentrations of Cd, Pb, Zn, Cu, and As in F1 and F2 were higher than those in F3 and F4 by 4.89–6.90-, 22.09–45.12-, 9.73–10.99-, 5.39–10.15-, and 7.70–11.18-fold (Fig. 1). In addition, the bioavailable HM concentrations of F1 and F2 were also higher than those of F3 and F4. Previous studies showed there were generally high levels of the other HMs, such as Cd, As, and Cu, associated in the Pb–Zn mines (Huang et al. 2017; Lee et al. 2014; Li et al. 2018; Mouni et al. 2017). The pH of the mine soils ranged from 4.9 to 6.2, while the TOC and WEOC ranged from 4.90% to 10.68% and 66.85 to 153.22 mg/kg, respectively. Compared with F3 and F4, the F1 and F2 soils contained higher levels of WEOC and TOC. However, the pH and moisture content were lower (Table 1).
The content of total and available Cd, Pb, Zn, Cu, and As significantly positively correlated with each other (p < 0.01) (Table 2). Typically, the concentration of HMs that originated from the same or a similar source tended to have a significant correlation (Antonio et al. 2008). The total HMs also significantly positively correlated with the WEOC and TOC (p < 0.01), but there was a significantly negative correlation with the pH and moisture (p < 0.01). Previous studies have also shown that the soil pH was one of the most important factors that impacted the Cd fractions, which negatively correlated with the concentration of available Cd by enhancing the competition for negative ion surfaces between Cd2+ and H+ in the soil (Muhammad et al. 2017). Li et al. (2020) also found that the soil pH negatively correlated with the available concentrations of Cd, Pb, and Zn. The pH value significantly negatively correlated with the available HMs and total HMs in this study, which could indicate that the pH enhanced the competition between Cd, Pb, Zn, Cu, As, and H+ on the surface of negative ions in the soil. A correlation analysis also showed that the TOC significantly negatively correlated with the pH and positively correlated with the available HMs, which was also similar to the findings of previous studies (Liu et al. 2020). This result suggested that the concentrations of HMs and other soil physicochemical parameters are correlated.
Microbial community diversity
Herein, a comprehensive microbial community analysis on the diversity, composition, differences and interactions were conducted. At least 34,973 and 51,907 valid reads of bacteria and fungi, respectively, were obtained through quality filtering from 12 soil samples (F1 to F4 with three replicates). After the sequence data had been normalized, they were clustered into OTUs with 97% sequence identity. The number of OTUs of the bacteria and fungi ranged from 1,983 to 3,037 and 418 to 787, respectively (Tables S1 and S2).
Both the ACE and Chao indices were used to evaluate the microbial richness with a positive correlation relationship. The microbial diversity positively correlated with the Shannon index but negatively correlated with the Simpson index (Zeng et al. 2019). Typically, both bacterial richness and diversity were higher than those of the fungi (Tables S1 and S2). F1 and F2 had lower bacterial richness and diversity than those of F3 and F4. Conversely, F1 and F2 had higher fungal richness and diversity than those of F3 and F4. Thus, it can be inferred that the contamination with HMs in the Pb–Zn mine soil affected the bacterial richness and diversity but promoted the fungal richness and diversity. An ANOVA showed that there were no significant differences in the bacterial richness and diversity between F1 and F2, as well as the fungal richness and diversity. However, as shown in Tables S1 and S2, there were differences in both the bacterial and fungal richness and diversity between F3 and F4, as well as between F1 and F2 (high level of HMs) and F3 and F4 (low level of HMs). These results suggest that the soil physicochemical parameters, particularly the content of HMs, have some effect on the diversity and richness of the bacteria and fungi.
A correlation analysis was conducted to evaluate the relationship of bacterial/fungal richness and diversity with soil physicochemical properties in more detail (Table 3). The total HMs (T_Cd, T_Pb, T_Zn, T_Cu, and T_As) negatively correlated with bacterial richness and diversity, but they had no significant correlation with fungal richness or diversity. This indicated that the bacterial richness and diversity were affected under high HM stress (Gao et al. 2022). However, there was no apparent influence of the HMs on the fungal richness and diversity. The available HMs (A_Cd, A_Pb, A_Zn, A_Cu, and A_As) had a similar effect on both the bacterial and fungal richness and diversity, but they had an opposite effect on the pH. The toxicity of HMs may have changed the microbial diversity by causing the death of sensitive microorganisms and increasing the abundance of tolerant microorganisms (Qian et al. 2023). Previous studies also demonstrated that the bacterial diversity and richness were affected by the HMs. Li et al. (2020) reported that the bacterial community richness and diversity were significantly lower in soil polluted with high levels of HMs than that polluted with low levels of HMs. Beattie et al. (2018) reported that the bacteria significant negatively correlated with Pb, Cd, Zn, and Mg in the soils decades after mining had ceased. Previous studies also showed that the fungal diversity was not significantly correlated with the concentrations of HMs in the soil. Zeng et al. (2020b) reported that there were no significant differences (p > 0.05) in the fungal community richness and diversity among the soils contaminated with Zn, Pb, Cd, Cu, and Cr. Kerfahi et al. (2020) also reported that the contents of Pb, Zn, and Cu had no significant effect on fungal diversity, which in the soil with high samples of metal was no lower than that with normal samples of metal. Thus, under the stress of HMs in the Pb–Zn mine soil, the bacterial richness and diversity were significantly affected, while the fungal richness and diversity were more stable.
Microbial community difference
The Venn diagram of the four samples of soil contaminated with the HMs based on the bacterial genus is shown in Fig. S1, and it shows the similarity and overlap among the microbial communities (Zeng et al. 2020a). The number of bacterial and fungal genera shared among all the samples was 392 and 105, respectively. The number of core bacterial genera was more than that of fungi by 2.4- to 3.5-fold. The numbers of bacterial genera of F1 to F4 were 654, 569, 808, and 652, respectively, while the numbers of fungal genera of F1 to F4 were 268, 240, 231, and 271, respectively. The F3 sample had the most bacterial genera (808) but the least fungal genera (231). The shared bacterial common genera (392) were more than half of the average genera of the four samples, which indicated that the shared bacterial genera occupied a large proportion of the microbial communities. These results were consisted with previous studies that shared OTUs occupied majority in the HMs contaminated industry wastes (Li et al. 2017), and much more bacterial core OTUs than fungal ones presented in HMs contaminated soils (Lin et al. 2019).
NMDS results showed F1 and F2 clearly differed in their composition of both bacteria and fungi, although they were both extremely polluted with HMs (Fig. S2). While the bacterial compositions of F3 and F4 samples were similar, their fungal compositions differed. Min et al. (2017) revealed that there was significantly less structural diversity in the microbial communities in chromite ore processing residue with a high content of total Cr (> 300 mg/kg) than that in the low group (< 90 mg/ kg), which indicated that the group of microorganisms in the highly polluted soil was more scattered with greater heterogeneity. Zeng et al. (2020b) also reported dissimilarity between the bacterial and fungal communities among the soil sampled from multiple types of HM contamination in an arid loess region. Thus, contamination with HMs could be the primary cause of heterogeneity in the bacterial and fungal community structures.
Microbial community composition
The taxonomic composition in each sample was evaluated at the levels of phylum (Fig. S3). Chloroflexi was the predominant bacterial phylum in all the samples with relative abundances of 33.5%-45.4%. Among the bacterial phyla with relative abundances > 1%, Actinobacteriota (9.8%-17.3%), Proteobacteria (9.1%-15.6%), Acidobacteriota (6.6%-15.6%), and Planctomycetota (2.0%-7.4%) were the other predominant bacterial phyla in all the samples (Fig. S3a). Kruskal–Wallis H test showed no significant differences (P > 0.05) on the abundances of the bacterial phyla Chloroflexi, Actinobacteriota, Proteobacteria, Patescibacteria, and Firmicutes among F1 to F4 samples (Fig. S4a). Previous studies also showed that Chloroflexi, Actinobacteriota, Proteobacteria, and Acidobacteriota were highly abundant in a mine area polluted with Cd, Pb, and Zn (Li et al. 2020) or in rare earth mining soil after an in situ leaching mining operation (Liu et al. 2021). Thus, the above dominant phyla were the results of the adaptation of bacterial community to HM stress.
Additionally, Gemmatimonadota (1.4%-2.9%), Myxococcota (1.5%-2.2%), Verrucomicrobiota (1.0%-1.5%), Patescibacteria (0.9%-1.8%), and Firmicutes (1.0%-1.2%) were unique dominating at F1 and F2. Cyanobacteria (3.4%-7.9%), Verrucomicrobiota (1.7%-3.2%), Myxococcota (0.9%-1.8%), Patescibacteria (1.0%-1.6%), Bacteroidota (1.4%-2.0%), and Armatimonadota (1.5%-2.2%) were unique dominating at F3 and F4. Notably, the archaea Crenarchaeota was also present in F1 (4.1%) and F2 (2.1%), wherein with low proportions of 0.2% and 0.3% in F3 and F4 samples, respectively. Crenarchaeota was also found to be enriched in HMs soils in previous report (Luo et al. 2021). Kruskal–Wallis H test showed Acidobacteriota, Cyanobacteria, Verrucomicrobiota, Crenarchaeota, Myxococcota, Gemmatimonadota, Bacteroidota, Armatimonadota, and Methylomirabilota differed significantly (P < 0.05) in the F1 to F4 samples, which indicated that these bacteria contributed to the microbial community differences in the F1 to F4 soils (Fig. S4a). These varieties were probably due to the multiple causes of HMs concentrations, mobility, and soil physicochemical properties, et al.
The fungal community was much simpler than that of the bacteria at the phylum level. The top three dominant fungal phyla were Ascomycota (65.0%-89.8%), Basidiomycota (3.6%-15.2%), and Mortierellomycota (1.6%-4.3%) in the four soils sampled (Fig. S3b). Kruskal–Wallis H test showed the fungal phyla Ascomycota, Glomeromycota, Rozeccomycota, Zoopagomycota, Aphelidiomycota, Monoblepharomycota, and Kichxellomycota differed significantly (P < 0.05) in the F1 to F4 samples (Fig. S4b). Ascomycota was previously found to be the primary dominant fungus (79.0% to 88.5%) in multiple HMs contaminated soils (Zn, Pb, Cd, Cu, and Cr) (Kerfahi et al. 2020; Zeng et al. 2020b). Lin et al. (2019) also reported Ascomycetes, Zygomycetes, and Basidiomycetes dominated at four different levels of soil contaminated with HMs (Cd, Pb, Ni, As, Cr, Cu, Zn, and Hg) with abundances variation. Thus, the presence of dominant phyla of Ascomycota, Basidiomycota, and Mortierellomycota were the results of the adaptation of fungal community to HM stress.
The taxonomic composition of bacteria (Fig. 2a) and fungi (Fig. 2b) in each sample was evaluated at the level of genus. The four samples of F1 to F4 had similar bacterial community composition with Acidobacteriales (0.65%-9.60%), Acidothermus (0.18%-4.8%), Gemmataceae (0.40%-2.45%), Sulfurifustis (< 1% to 4.6%), and Gaiellales (1.13%-2.28%) (Fig. 2a). Acidothermus is a member of the Actinobacteriota phylum, whose content increased with that of the metals in grassland soil contaminated with Cu, Zn, Cd, and Pb in a previous study (Vetrovsky and Baldrian 2015). Gaiella is also a member of the Actinobacteriota phylum, while both Sulfurifustis and Thiobacillus are members of Proteobacteria. The three bacteria genera described above were found to be predominant in seven tailings sites from Sb, Pb–Zn, and Sn mining and smelting industries in Hechi, Guangxi Province, China (Liu et al. 2019). Thus, the presence of predominant bacteria genera of Acidothermus, Sulfurifustis, Gaiella, and Thiobacillus were survived well upon HM stress in Pb–Zn mining area.
Composition and relative average abundance of the bacterial (a) and fungal (b) communities at the genus level, and the variation of 15 predominant bacterial (c) and fungal (d) genera. F1 to F4 represent four sampled soils. Genera with relative abundance below 2% were grouped as others. *: 0.01 < P < 0.05
The predominant fungal taxa in all the samples were Sordariomycetes (6.6%-19.0%), Talaromyces (1.0%-13.8%), and Mortierella (1.6%-4.1%) at the genus level (Fig. 2b). Both Sordariomycetes and Mortierella were found to be the core fungal genera in the soil located in Hechi City that was contaminated with mining pollutants (Choi et al. 2020). Sordariomycetes could serve as an endophyte or decomposer and is involved in nutrient cycling, while Mortierella had a vast potential for the yield of lipids and high-value fatty acids (Choi et al. 2020). Talaromyces was found to be resistant to Pb, Cu, Zn, and As (Khanthawong et al. 2018; Nam et al. 2019; Wang et al. 2017). Thus, the predominant fungal genera of Sordariomycetes, Talaromyces, and Mortierella were survived well upon HM stress.
In addition, the bacterial genera of Anaerolineaceae (0.20% to 12.95%), Bathyarchaeia (< 1% to 3.37%), and Thiobacillus (< 1% to 2.57%) were unique present at F1 and F2. Chloroplast (2.17%-4.10%), and Ktedonobacteraceae (0.14%-7.69%) were unique present at F3 and F4 (Fig. 2a). For fungi, the taxa of Agaricomycetes (2.9%-9.3%), Pyrenochaetopsis (4.9%-5.4%), unclassified Ascomycota (1.3%-2.2%), Fusarium (2.6%-3.1%), and Penicillium (1.2%-3.6%) were predominant in the F1 and F2 samples. These fungal genera were also found to be predominant in areas polluted with HMs (Chen et al. 2020; Choi et al. 2020; Zeng et al. 2020b), which indicated that they could be tolerant to extreme contamination with HMs in the present study. Arnium (5.6–13.6%), Curvularia (1.8%-11.5%), Coniosporium (1.5%-5.5%), Tubeufiaceae (2.2%-4.7%), Nigrospora (1.4%-1.5%), and Acidomelania (1.3%-3.9%) predominated in the F3 and F4 samples. Arnium, Curvularia, and Nigrospora were previously found to be prevalent or resistant to Zn, Cu, and Pb contamination (Chen et al. 2020; Kerfahi et al. 2020; Wong et al. 2018). Kruskal–Wallis H test showed most of the abundant taxa differed significantly at the genus level of both bacteria and fungi in the F1 to F4 samples (Fig. 2c-d). Thus, the abundances of most bacteria and fungi varied in the four different soil sites.
Correlation analysis of environmental factors and the microbial community
Previous studies showed that contamination of the soil with HMs and the pH was the most important factors that affected the microbial communities in soils (Wang et al. 2019). Herein, the pH and the primary HMs of Cd, Pb, and Zn were selected as the environmental factors to evaluate their impacting on microbial community at the phylum level (Fig. S5). This study showed that the pH and HMs (Cd, Pb, and Zn) had opposite effects on the bacterial and fungal phyla. The pH had a significant positive impact on bacteria in the phylum Armatimonadota (P < 0.05 or 0.01), but it significant negatively affected Crenarchaeota, Gemmatimonadota, and Myxococcota (P < 0.05, 0.01 or 0.001). This suggests that the pH primarily regulates the abundance of Armatimonadota, Crenarchaeota, Gemmatimonadota, and Myxococcota in this sample site. Similarly, Cd, Pb, and Zn significantly positively correlated with the phyla Firmicutes, Acidobacteriota, Crenarchaeota, and Gemmatimonadota but significantly negatively correlated with Verrucomicrobiota, Armatimonadota, Bacteroidota, and Cyanobacteria (Fig. S5a).
The fungal phyla Aphelidiomycota, Basidiomycota, Monoblepharomycota, Mucoromycota, Chytridiomycota, and Rozellomycota significantly negatively correlated with the pH but positively correlated with Cd, Pb, and Zn (Fig. S5b). In addition, the fungal phyla Ascomycota and Calcarisporiellomycota negatively correlated with Cd, Pb, and Zn. The fungal communities have been shown to be sensitive to changes in environmental factors, such as pH or HMs (Pan et al. 2020). The present results showed that the pH and HMs primarily regulated the fungi Aphelidiomycota, Basidiomycota, Monoblepharomycota, and Rozellomycota at the phylum level.
The environmental impacting on bacterial and fungal taxa at the genus level was shown in Fig. 3. There were also opposite effects of the pH and HMs (Cd, Pb, and Zn) on the bacterial and fungal community at the genus level. The bacterial genus Anaerolineaceae positively correlated with Cd, Pb, and Zn but significantly negatively correlated with the pH. Acidobacteriales significantly positively correlated with Cd, and Pb but no significantly correlation with Zn and pH. Anaerolineaceae, and Acidobacteriales were previously found to be positively correlation with Pb (Qi et al. 2022; Zarate et al. 2021), which indicated these two bacterial genera were HM resistant. Ktedonobacteraceae significantly negatively correlated with Cd, Pb, and Zn but positively correlated with the pH. Chloroplast significantly negatively correlated with Cd, Pb, and Zn but no significantly correlation with the pH (Fig. 3a).
Heatmap of the Pearson’s correlation coefficient between environmental factors and the bacterial (a) and fungi (b) taxa at the genus level. F1 to F4, four sampled soils. Only the top 15 phyla are shown in this figure. Color key for the correlation values is shown on the right panel inset. Red, positive correlations. Green, negative correlations. *0.01 < P < 0.05. **0.001 < P < 0.01. ***P < 0.001
The fungal genera Pyrenochaetopsis, Agaricomycetes, Sordariaceae, and Pseudophialophora positively correlated with Cd, Pb, and Zn but significantly negatively correlated with the pH. Coniosporium positively correlated with the pH but negatively correlated with Cd, Pb, and Zn. Talaromyces significantly negatively correlated with the pH but no significantly correlation with Cd, Pb, and Zn. Additionally, the fungal genera Melanconiella only positively correlated with Pb (Fig. 3b). Pyrenochaetopsis was previously found to be positively correlation with Pb/Zn (Chen et al. 2020). Talaromyces had not previously been found in typical Pb–Zn mining areas, but it had desirable effects on Pb (Sharma et al. 2020), As (Nam et al. 2019) and uranium (U) (Coelho et al. 2022), such as absorbing and transforming them. Our findings confirmed that the HMs concentration in Pb–Zn mining soil stimulated the abundance of typical HM resistant bacteria and fungi.
Microbial community interactions
The MEN is a powerful tool to reveal potential patterns of microbial interaction (Wang et al. 2021a). MEN images and its topological properties are shown in Fig. S6 based on the bacterial and fungal phyla. The degree reflects the connect links with the samples, while the weighted degree reflects the importance of nodes (Shi et al. 2020). The total nodes of the bacterial and fungal phyla were 59 and 16, respectively, among which 33 and 9 nodes connected with the F1 to F4 samples with a degree of 4. Thus, the bacterial network contained more complex interactions than the fungal network. This showed that the 10 most weighted degree bacterial phyla were Chloroflexi, Actinobacteriota, Proteobacteria, Acidobacteriota, Planctomycetota, Cyanobacteria, Verrucomicrobiota, unclassified Bacteria, Crenarchaeota, and Myxococcota with a weighted degree of 53,022 to 2,087 (Table S3). They were also the core phyla of the bacterial community in the F1 to F4 soils. In contrast, the most 8 weighted degree fungal phyla were Ascomycota, unclassified Fungi, Basidiomycota, Mortierellomycota, Glomeromycota, Rozellomycota, Chytridiomycota, and Mucoromycota with a weighted degree of 154,044 to 183 (Table S3).
Moreover, there were many more links present at the genus level of both the bacteria and fungi (Fig. 4). There were 980 and 441 total nodes of the bacterial and fungal genera, respectively. The soil microorganisms in environments that contain HMs have been hypothesized to develop resistant taxa and microbial interaction strategies for survival (Li et al. 2017; Zeng et al. 2020b). Greater network complexity will stabilize the communities with mixed interaction types and improve the community’s resistance to HMs stress (Wang et al. 2021a). According to the results described above, more tolerant bacteria than fungi have adapted to HMs mining soil environment.
Prediction of the microbial functions
Based on 16S rRNA gene sequencing, a potential HM tolerate phenotype was predicted by the BugBase tool (Fig. 5a). The bacteria with HM tolerate were mainly composed of Acidobacteriales (1.85%-28.53%) and Gaiellales (1.31%-2.53%) at F1-F4. Anaerolineaceae (2.32%-6.84%), Sulfurifustis (0.19%-6.28%), and Gemmatimonadaceae (1.07%-4.08%) were dominant at F1 and F2. Chloroplast (4.21%-7.44%) and Terracidiphilus (1.00%-3.18%) were dominant at F3 and F4. Deng et al. (2022) found that the relative abundance of stress-tolerant phenotypes showed a significant positive relationship with total-Cd and available-Cd. The authors also found Anaerolineaceae positively correlated with exchangeable-Zn, organic matter bound-Cu, and Fe–Mn oxide-bound-Pb/Zn (Sha et al. 2023). Therefore, Acidobacteriales, Gaiellales, Anaerolineaceae, Sulfurifustis, and Gemmatimonadaceae might play great roles in the tolerance of HMs toxicity.
Moreover, the community composition of the fungal functional guilds was evaluated by the FUNGuild database (Fig. 5b). The undefined saprotroph (12.91%-35.16%) and plant pathogen (1.40%-14.93%) existed at F1-F4, while the dung saprotroph-plant saprotroph (5.27%-15.30%) and endophyte-lichen parasite-undefined saprotroph (4.90%-5.45%) existed only at F1/F2. Animal pathogen-plant pathogen-undefined saprotroph (2.18%-6.43%) and dung saprotroph-undefined saprotroph (5.58%-13.60%) were mainly present at F3/F4. Wang et al. (2021b) reported that Sordariomycetes, Talaromyces, and Mortierella were placed in the saprotroph/symbiotroph guild. These results suggested that the HMs might affect the saprotrophs and pathogens fungal genera of Sordariomycetes, Talaromyces, Mortierella (Zhang et al. 2019). The authors also found Sordariomycetes and Talaromyces positively correlated with carbonate-bound Cd/Zn and residual form Zn/As, while Mortierella positively correlated with carbonate-bound Pb/As and Fe–Mn oxide-bound Cu/As in previous report (Sha et al. 2023). Thus, both of bacterial and fungal communities have developed strategies to survive upon HM stress, which conduced to further bioremediation of Pb–Zn mining soil.
Conclusions
The results showed that the soils in a typical Pb–Zn area were acidic with a pH from 4.9 to 6.2. Higher levels of contamination with HMs caused a significant reduction in bacterial richness and diversity, but they increased the fungal richness and diversity. The predominant bacteria genera of Acidobacteriales, Gaiellales, Anaerolineaceae, Sulfurifustis, and Gemmatimonadaceae and predominant fungal genera of Sordariomycetes, Talaromyces, and Mortierella were assumed as HM resistant taxa in Pb–Zn mining area. And HMs concentration in Pb–Zn mining soil stimulated the abundance of typical HM resistant bacteria and fungi. Microbial interactions results showed that the bacterial network played a greater role on the resistance of the microbial community to HM stress than the fungal network. Microbial function prediction also confirmed the HM-tolerant bacteria and fungi survival in toxic environment. These results provide comprehensive outlooks for the bacterial and fungal community structure upon multiple HMs coexist, and it also offers useful reference data for the bioremediation of the HM soil around the typical Pb–Zn mine area for future research.
Data availability
All data generated or used during the study appear in the submitted article.
References
Antonio RJ, Nikos N, Manuel GJ, Luis G, Manuel LA (2008) Multiscale analysis of heavy metal contents in Spanish agricultural topsoils. Chemosphere 70(6):1085–1096
Beattie RE, Henke W, Campa MF, Hazen TC, McAliley LR, Campbell JH (2018) Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol Biochem 126:57–63
Chen ZM, Wang Q, Ma JW, Zou P, Yu QG, Jiang LN (2020) Fungal community composition change and heavy metal accumulation in response to the long-term application of anaerobically digested slurry in a paddy soil. Ecotoxicol Environ Saf 196:110453
Choi D, Bang J, Kim T, Oh Y, Hwang Y, Hong J (2020) In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J Hazard Mater 400:123308
Coelho E, Reis TA, Cotrim M, Mullan TK, Renshaw J, Rizzutto M, Correa B (2022) Talaromyces amestolkiae uses organic phosphate sources for the treatment of uranium-contaminated water. Biometals 35(2):335–348
Deng Y, Fu S, Sarkodie EK, Zhang S, Jiang L, Liang Y, Yin H, Bai L, Liu X, Liu H, Jiang H (2022) Ecological responses of bacterial assembly and functions to steep Cd gradient in a typical Cd-contaminated farmland ecosystem. Ecotoxicol Environ Saf 229:113067
Du Y, Chen L, Ding P, Liu LL, He QC, Chen BZ, Duan YY (2019) Different exposure profile of heavy metal and health risk between residents near a Pb-Zn mine and a Mn mine in Huayuan county, South China. Chemosphere 216:352–364
Gao T, Wang X, Liu Y, Wang H, Zuo M, He Y, Li H, Li G, Li C, Lis X, Li X, Yang Y (2022) Characteristics and diversity of microbial communities in lead-zinc tailings under heavy metal stress in north-west China. Lett Appl Microbiol 74(2):277–287
Golebiewski M, Deja-Sikora E, Cichosz M, Tretyn A, Wrobel B (2014) 16S rDNA pyrosequencing analysis of bacterial community in heavy metals polluted soils. Microb Ecol 67(3):635–647
Huang SH, Yuan CY, Li Q, Yang Y, Tang CJ, Ouyang K, Wang B (2017) Distribution and risk assessment of heavy metals in aoils from a typical Pb-Zn mining area. Pol J Environ Stud 26(3):1105–1112
Kerfahi D, Ogwu MC, Ariunzaya D, Balt A, Davaasuren D, Enkhmandal O, Purevsuren T, Batbaatar A, Tibbett M, Undrakhbold S, Boldgiv B, Adams JM (2020) Metal-tolerant fungal communities are delineated by high zinc, lead, and copper concentrations in metalliferous gobi desert soils. Microb Ecol 79(2):420–431
Khanthawong S, Pratanaphon R, Wongsawan K, Vanittanakom N (2018) Expression of Cu, Zn superoxide dismutase recombinant protein from Talaromyces marneffei in Pichia pastoris and its resistance to oxidative stress. Med Mycol 56:S120–S120
Lee SH, Ji W, Lee WS, Koo N, Koh IH, Kim MS, Park JS (2014) Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J Environ Manag 139:15–21
Li XQ, Meng DL, Li J, Yin HQ, Liu HW, Liu XD, Cheng C, Xiao YH, Liu ZH, Yan ML (2017) Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ Pollut 231(Pt 1):908–917
Li ZY, Yang SX, Peng XZ, Li FM, Liu J, Shu HY, Lian ZH, Liao B, Shu WS, Li JT (2018) Field comparison of the effectiveness of agricultural and nonagricultural organic wastes for aided phytostabilization of a Pb-Zn mine tailings pond in Hunan Province, China. Int J Phytoremediation 20(12):1264–1273
Li CZ, Wang XF, Huang H, Wang L, Wei F, Zhang CL, Rong Q (2020) Effect of multiple heavy metals pollution to bacterial diversity and community structure in farmland soils. Hum Ecol Risk Assess 27(3):724–741
Liang J, Yang ZX, Tang L, Zeng GM, Yu M, Li XD, Wu HP, Qian YY, Li XM, Luo Y (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288
Lin YB, Ye YM, Hu YM, Shi HK (2019) The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol Environ Saf 180:557–564
Liu JL, Yao J, Wang F, Min N, Gu JH, Li ZF, Sunahara G, Duran R, Solevic-Knudsen T, Hudson-Edwards KA, Alakangas L (2019) Bacterial diversity in typical abandoned multi-contaminated nonferrous metal(loid) tailings during natural attenuation. Environ Pollut 247:98–107
Liu HK, Wang C, Xie YL, Luo Y, Sheng MP, Xu F, Xu H (2020) Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J Hazard Mater 392:122478
Liu JJ, Liu W, Zhang YB, Chen CJ, Wu WX, Zhang TC (2021) Microbial communities in rare earth mining soil after in-situ leaching mining. Sci Total Environ 755(Pt 1):142521
Luo LY, Xie LL, Jin DC, Mi BB, Wang DH, Li XF, Dai XZ, Zou XX, Zhang Z, Ma YQ, Liu F (2019) Bacterial community response to cadmium contamination of agricultural paddy soil. Appl Soil Ecol 139:100–106
Luo JP, Guo XY, Tao Q, Li JX, Liu YK, Du YL, Liu YY, Liang YC, Li TQ (2021) Succession of the composition and co-occurrence networks of rhizosphere microbiota is linked to Cd/Zn hyperaccumulation. Soil Biol Biochem 153:108120
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014) Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Mierzwa-Hersztek M, Gondek K, Klimkowicz-Pawlas A, Baran A, Bajda T (2018) Sewage sludge biochars management—Ecotoxicity, mobility of heavy metals, and soil microbial biomass. Environ Toxicol Chem 37(4):1197–1207
Min XB, Wang YY, Chai LY, Yang ZH, Liao Q (2017) High-resolution analyses reveal structural diversity patterns of microbial communities in Chromite Ore Processing Residue (COPR) contaminated soils. Chemosphere 183:266–276
Mouni L, Belkhiri L, Bouzaza A, Bollinger J-C (2017) Interactions between Cd, Cu, Pb, and Zn and four different mine soils. Arab J Geosci 10(4):77–85
Muhammad S, Camille D, Sana K, Khan NN, C APM (2017) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Nam IH, Murugesan K, Ryu J, Kim JH (2019) Arsenic (As) removal using talaromyces sp. KM-31 isolated from As-contaminated mine soil. Minerals. 9(10):568
Pan XM, Zhang SR, Zhong QM, Gong GS, Wang GY, Guo X, Xu XX (2020) Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci Total Environ 715:136904
Qi Q, Hu C, Lin J, Wang X, Tang C, Dai Z, Xu J (2022) Contamination with multiple heavy metals decreases microbial diversity and favors generalists as the keystones in microbial occurrence networks. Environ Pollut 306:119406
Qian L, Lin H, Li B, Dong Y (2023) Physicochemical characteristics and microbial communities of rhizosphere in complex amendment-assisted soilless revegetation of gold mine tailings. Chemosphere 320:138052
Sha HC, Li J, Wang LQ, Nong HD, Wang GH, Zeng TT (2023) Preparation of phosphorus-modified biochar for the immobilization of heavy metals in typical lead-zinc contaminated mining soil: Performance, mechanism and microbial community. Environ Res 218:114769
Sharma R, Talukdar D, Bhardwaj S, Jaglan S, Kumar R, Kumar R, Akhtar MS, Beniwal V, Umar A (2020) Bioremediation potential of novel fungal species isolated from wastewater for the removal of lead from liquid medium. Environ Technol Innov 18:100757
Sheik CS, Mitchell TW, Rizvi FZ, Rehman Y, Faisal M, Hasnain S, McInerney MJ, Krumholz LR (2012) Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS One 7(6):e40059
Shi QH, Liu YT, Shi AQ, Cai ZD, Nian H, Hartmann M, Lian TX (2020) Rhizosphere soil fungal communities of aluminum-tolerant and -sensitive soybean genotypes respond differently to aluminum stress in an acid soil. Front Microbiol 11:1177
Vetrovsky T, Baldrian P (2015) An in-depth analysis of actinobacterial communities shows their high diversity in grassland soils along a gradient of mixed heavy metal contamination. Biol Fertil Soils 51(7):827–837
Wang NN, Xu XJ, Li HY, Wang QY, Yuan LZ, Yu HW (2017) High performance and prospective application of xanthate-modified thiourea chitosan sponge-combined Pseudomonas putida and Talaromyces amestolkiae biomass for Pb(II) removal from wastewater. Biores Technol 233:58–66
Wang X, Gao P, Li DP, Liu J, Yang N, Gu WZ, He XH, Tang WZ (2019) Risk assessment for and microbial community changes in farmland soil contaminated with heavy metals and metalloids. Ecotoxicol Environ Saf 185:109685
Wang H, Zeng YF, Guo CL, Zheng XK, Ding C, Lu GN, Dang Z (2021a) Soil rehabilitation shaped different patterns of bacterial and archaeal community in AMD-irrigated paddy soil. Chemosphere 263:128259
Wang Y, Liu Y, Li J, Bai S, Tian T (2021) Fungal community composition and diversity in the rhizosphere soils of Argentina (syn. Potentilla) anserina, on the Qinghai Plateau. Fungal Ecol 54:101107
Wei CY, Wang C, Yang LS (2009) Characterizing spatial distribution and sources of heavy metals in the soils from mining-smelting activities in Shuikoushan, Hunan Province, China. J Environ Sci 21(9):1230–1236
Wong C, Tan LT, Mujahid A, Lihan S, Wee JLS, Ting LF, Muller M (2018) Biosorption of copper by endophytic fungi isolated from Nepenthes ampullaria. Lett Appl Microbiol 67(4):384–391
Xiao SQ, Zhang Q, Chen XM, Dong FQ, Chen H, Liu MX, Ali I (2019) Speciation distribution of heavy metals in uranium mining impacted soils and impact on bacterial community revealed by high-throughput sequencing. Front Microbiol 10:1867
Yuan XH, Xue ND, Han ZG (2021) A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J Environ Sci (china) 101(03):217–226
Zarate A, Dorador C, Valdes J, Molina V, Icaza G, Pacheco AS, Castillo A (2021) Benthic microbial diversity trends in response to heavy metals in an oxygen-deficient eutrophic bay of the Humboldt current system offshore the Atacama Desert. Environ Pollut 286:117281
Zeng TT, Rene ER, Hu Q, Lens PNL (2019) Continuous biological removal of selenate in the presence of cadmium and zinc in UASB reactors at psychrophilic and mesophilic conditions. Biochem Eng J 141:102–111
Zeng TT, Mo GH, Hu Q, Wang GH, Liao W, Xie SB (2020a) Microbial characteristic and bacterial community assessment of sediment sludge upon uranium exposure. Environ Pollut 261:114176
Zeng XY, Li SW, Leng Y, Kang XH (2020b) Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci Total Environ 723:138081
Zhang LL, Zhang HQ, Wang ZH, Chen GJ, Shan WL (2016) Dynamic changes of the dominant functioning microbial community in the compost of a 90–m3 aerobic solid state fermentor revealed by integrated meta-omics. Biores Technol 203:1–10
Zhang W, Sun R, Xu L, Liang J, Wu T, Zhou J (2019) Effects of micro-/nano-hydroxyapatite and phytoremediation on fungal community structure in copper contaminated soil. Ecotoxicol Environ Saf 174:100–109
Zhao XL, Liu JF, Xia XL, Chu JM, Wei Y, Shi SQ, Chang EM, Yin WL, Jiang ZP (2014) The evaluation of heavy metal accumulation and application of a comprehensive bio-concentration index for woody species on contaminated sites in Hunan, China. Environ Sci Pollut Res 21(7):5076–5085
Funding
This research was supported by National Natural Science Foundation of China (52170164), the Science and Technology Innovation Program of Hunan Province of China (2022RC1184) and the Funds for Innovative Province Construction of Hunan Province of China (2019RS3012). The authors thank Yuanyuan Deng for providing help on ICPMS detection.
Author information
Authors and Affiliations
Contributions
Taotao Zeng: Methodology, Formal analysis, Data curation, Writing – original draft, Funding acquisition. Haichao Sha: Investigation, Data curation, Software, Writing – original draft. Qingqing Xie: Validation, Writing –review & editing. Yue Lu: Conceptualization, Writing – review & editing, Funding acquisition, Supervision. Haidu Nong and Liangqin Wang: Investigation, Data curation. Lin Tang: Conceptualization, Writing–review & editing, Funding acquisition, Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, T., Sha, H., Xie, Q. et al. Comprehensive assessment of the microbial community structure in a typical lead–zinc mine soil. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33377-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33377-9