Abstract
Fungi have received particular attention in regards to alternatives for bioremediation of heavy metal contaminated locales. Enzymes produced by filamentous fungi, such as phosphatases, can precipitate heavy metal ions in contaminated environments, forming metal phosphates (insoluble). Thus, this research aimed to analyze fungi for uranium biomineralization capacity. For this, Gongronella butleri, Penicillium piscarium, Rhodotorula sinensis and Talaromyces amestolkiae were evaluated. Phytate and glycerol 2-phosphate were used as the phosphate sources in the culture media at pH 3.5 and 5.5, with and without uranium ions. After 4 weeks of fungal growth, evaluated fungi were able to produce high concentrations of phosphates in the media. T. amestolkiae was the best phosphate producer, using phytate as an organic source. During fungal growth, there was no change in pH level of the culture medium. After 3 weeks of T. amestolkiae growth in medium supplemented with phytate, there was a reduction between 20 and 30% of uranium concentrations, with high precipitation of uranium and phosphate on the fungal biomass. The fungi analyzed in this research can use the phytic acid present in the medium and produce high concentrations of phosphate; which, in the environment, can assist in the heavy metal biomineralization processes, even in acidic environments. Such metabolic capabilities of fungi can be useful in decontaminating uranium-contaminated environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the United States Environmental Agency, uranium is naturally found in the environment in mineral form and not as a metal, and is part of the soil, rocks, and water. However, radionuclides contaminate the water, soil, and air after industrial processes. Uranium ions present several risks to human health, with the main ones having an effect on the kidneys, and an increased risk of lung and bone cancer (Gadd 2009).
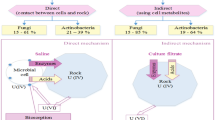
High concentrations of metals in locations with industrial mineral extraction are not the only problem; locales contaminated with heavy metals usually have effluents with acidic water, which is caused by acid mine drainage (AMD). AMD is the natural oxidation of metal sulfides, causing the formation of sulfuric acid and reduced water pH (Mohammed et al. 2017; Kefeni et al. 2017; Sheridan et al. 2018).
The bioremediation of heavy metals from aqueous solutions is often described as a promising technique for wastewater treatment. This technique utilizes the ability of living or dead microorganisms to collect toxic metal ions from wastewater through physical–chemical absorption, or metabolically mediated uptake pathways (El-Naggar et al. 2018).
Biomineralization is a process where, during their growth phase, microorganisms form minerals resulting in the removal of toxic metals from the solution; this process facilitates bio-recovery as well as the reduction of heavy metal toxicity. The main biominerals precipitated by microorganisms are oxides, phosphates, sulfides, and oxalates (Gadd and Pan 2016). Fungi can produce phosphatases and utilize phosphate sources, such as phytate, during the biomineralization process to precipitate heavy metals (Liang et al. 2015).
However, there are other ways for fungi to produce phosphate, such as the accumulation and release of phosphates from cellular components. Despite this, phosphorus sources are limited, and it is necessary to investigate low cost and sustainable alternatives to phosphate rock sources. Some alternatives include recycled animal bones or the production of phosphorus through microorganisms (Khan et al. 2010; Drangert 2012; Elias et al. 2016; Someus and Pugliese 2018).
An excellent alternative to phosphate is myo-inositol 1, 2, 3, 4, 5, 6-hexakisdihydrogenphosphate; mainly myo-inositol hexakisphosphate (InsP6; C6H18O24P6), commonly referred to as phytic acid, or phytate as a salt, a cheaper phosphate donor for metal recovery than glycerol 2-phosphate. It forms a group of organic phosphorus (P) compounds widely found in nature, especially in legumes, cereals, and seed oil crops (Awad et al. 2014). In soil, phytate is the most important form of organic phosphorus, and constitutes up to 60% of the soil organic P which cannot be taken up by the plant. It is dephosphorylated by phytases and phosphatases produced by microorganisms (Paterson-Beedle et al. 2010; Vassilev and de Oliveira Mendes 2017).
The presence of phytic acid in plant matter suggests that plant waste products containing InsP6 may be a cheaper and useful source of phosphate for bioremediation. Particularly, wastes from biodiesel production, fermentation processes, or agricultural industry have been suggested as good candidates for phytic acid degradation by fungal phytases (Paterson-Beedle et al. 2010). Despite several studies on bioremediation, the authors recognize that most of the studies are not economically viable. New research needs to be conducted to determine new, more viable, and cheaper bioremediation alternatives for the treatment of residual water contaminated with heavy metals (Gadd 2009; Fomina and Gadd 2014).
The current research study focuses on the fungi Gongronella butleri, Penicillium piscarium, Rhodotorula sinensis, and Talaromyces amestolkiae strains, previously isolated from a contaminated uranium mine in Brazil (Coelho et al. 2020a); and their capacity/ability for phosphate release. For this purpose, two phosphate sources were used: phytic acid and glycerol 2-phosphate phosphate. Subsequently, the best phosphate producer was selected for uranium biomineralization experiments. The results of this research will contribute to the existing knowledge in the biotechnology field, and, in the future, these microorganisms could possibly be utilized for the bioremediation of contaminated sites. This study was conducted on uranium, however, the potential for application in sites contaminated with other heavy metals is significant.
Materials and methods
Fungal strains
Fungal strains used in the present research were previously isolated from a uranium mine located in the state of Minas Gerais, southeastern Brazil (Coelho et al. 2020a, b). The fungi evaluated were: G. butleri, P. piscarium, and T. amestolkiae isolated from soil, and R. sinensis was isolated from water. These fungal strains were maintained through lyophilization.
Phosphate production
Initial experiments investigated fungal growth and stimulation of phosphatase activity; to identify which fungus released more phosphate into the solution, and the best organic phosphate donor. Two organic phosphate sources were used: phytic acid and glycerol 2-phosphate.
The lyophilized fungi were cultured in Petri dishes using potato dextrose agar (PDA) for 7 days at 25 °C. Then, 1 cm² of hyphae was removed using a sterilized blade, and added to a 100 mL Erlenmeyer flask containing one of two different culture media.
First media: yeast nitrogen base (YNB) without amino acids and phosphate + glucose + phytic acid/phytate (Insp6). Second media: YNB without amino acids and phosphate + glucose + glycerol 2-phosphate.
The pH level of each solution was adjusted to pH 5 using KOH and HCl. The media was sterilized by autoclaving at 121 °C at 15 psi, for 20 min. Autoclave-sensitive chemicals (InsP6) and glycerol 2-phosphate solutions were sterilized through syringe filtration [0.2 μm, polyethersulfone (PES) membrane] and then mixed with the sterilized media under aseptic conditions, with the following concentrations: 3 g L−1 (Olstorpe et al. 2009). Sampling was conducted at 1, 2, 3, and 4 weeks. After sampling, the released phosphate concentration was analyzed as described under the phosphate determination section.
Biomineralization experiments
Three treatments were evaluated for biomineralization capacity: (a) T. amestolkiae cultured in YNB broth, without uranium and phosphate (YNB); (b) T. amestolkiae cultured in YNB broth + phytate (3 g L−1) (YNBp); and finally, (c) T. amestolkiae cultured in YNB broth + phytate (3 g L−1) + uranium (50 mg L−1) (YNBpU). All experiments were performed in triplicate at pH 3.5 and 5.5.
The flasks were incubated in an orbital shaker at 25 ºC and 150 rpm for 21 days (3 weeks). On days 1, 7, 15, and 21, analyses were performed to determine phosphate and uranium concentration, and pH of the solutions.
Phosphate determination
Colorimetric phosphate determination was performed according to the Peterson method, as modified by Qvirist et al. (2015). This procedure involves mixing 0.5 mL of filtered samples with 0.9 mL of 50 g L−1 sodium dodecyl sulfate (SDS) solution, 1 mL of 12.5 g L−1 ammonium heptamolybdate tetrahydrate (AHT) in 73 g L−1 hydrochloric acid solution, and 0.1 mL of 1 g L−1 ascorbic acid (AscA). This mixture reacts with phosphate in solution to form a blue color; the intensity of which, over a particular range, is linearly correlated with phosphate concentration.
For reaction vessels, 24 well multiwell trays (Corning 25820) were used. The samples and reaction mixtures were added, a lid placed over the plates, and then the plates placed on an orbital shaker at a moderate agitation speed at room temperature for 30–60 min.
The absorbance of the reaction mixture was read at 700 nm (A700) using an Epoch Biotek multiplate reader and recorded with the Gen 5 1.11 software. For this equipment, the linear detection range was determined to be between 0 and 30 mg L−1.
Samples were compared to a standard curve of known values (0–30 mg L−1). Standards were run before each set of analyses, although day-to-day variation in the measured absorbance values was low.
The background phosphate concentration for each media tested was defined as the mean recorded value of phosphate across all samples taken before inoculation. The phosphate released was defined as the measured phosphate concentration recorded at each temporal sampling point after the background value had been subtracted.
Uranium measurement
For uranium measurement, aliquots of the uranium solution (1 mL) were filtered using a sterile syringe filter (0.2 μm) and add to 9 mL of 2% citric acid solution. The uranium concentration was determined using inductively coupled plasma atomic emission spectroscopy (ICP OES), at the Institute of Chemistry and Environment/IPEN. All tests were performed in triplicate.
Scanning electron microscopy and X-ray fluorescence
To identify precipitated uranium on the fungal cell surface after biomineralization, scanning electron microscopy (SEM + EDS) and X-ray fluorescence analysis were performed at the Institute of Physics, University of São Paulo, Brazil, with a JEOL® model 6460LV. Energy dispersive X-ray fluorescence (ED-XRF) using a portable Amptek® setup composed of a mini X-ray tube [silver (Ag) target] and a Si drift X-ray semiconductor detector (25 mm2 × 500 μm 0.5 mil−1) with a thin beryllium end window of 3.8 cm and energy resolution of 125 eV FWHM at 5.9 keV (55Fe). The XRF measurements were carried out with 30 kV voltage and 50 µA current; and an excitation/detection time of 300 with a fixed distance of ~ 3(1) mm. For the U measurements, a filter of W (25 μm) and Al (250 μm) foil was used in the X-ray tube exit.
Results
Phosphate production by fungi
After 4 weeks, all evaluated fungi were able to degrade phytate and glycerol 2-phosphate; and release phosphate into the solution with glucose. The phosphate production in phytate supplemented media was: 632.55, 492.36, 275.85, and 1531.30 mg L−1 for G. butleri, P. piscarium, R. sinensis, T. amestolkiae, respectively; and, in media supplemented with glycerol 2-phosphate: 403.08, 210.11, 19.86, and 60.78 mg L−1, respectively (Fig. 1).
Biomineralization experiments
After determining the best phosphate producing fungus (T. amestolkiae) and the best organic phosphate donor (phytate), biomineralization tests were performed to verify the decrease of uranium in media during fungal growth in the presence of phytate (without glucose). The results with standard deviation are given in Table 1.
The biomineralization test results showed increases in phosphate production over the weeks. At pH 3.5, a level close to that found in the mine, we observed phosphate production of 605.00 mg L−1 (using YNBp) and 319.60 mg L−1 (using YNBpU) after 3 weeks. At pH 5.5, phosphate production was 584.40 mg L−1 (using YNBp) and 404.98 mg L−1 (using YNBpU) (Fig. 2).
In both experiments (pH 3.5 and 5.5), the pH of the solution did not change during the duration (weeks) of fungal growth.
Phosphate and uranium precipitation on T. amestolkiae cell surface
During T. amestolkiae growth in YNBpU culture medium, there was a reduction in uranium ion concentration and increased precipitation onto the fungal biomass. After 3 weeks at pH 3.5, there was a 26% reduction in uranium concentration (20.8 to 15.2 ± 0.2 mg L−1), and at pH 5.5 the reduction was 27% after 3 weeks of fungal growth (4 to 2.9 ± 0.2 mg L−1). In both cases, the reduction of uranium in the solution ranged from 18 to 27% (Fig. 3). During the experiment it was also observed that there was high uranium precipitation when initially mixed with the culture media, therefore the initial uranium concentration was reduced to 20 and 4 mg L−1, respectively.
SEM analysis determined that the biomass of T. amestolkiae in YNB medium (control) was smooth, non-aggregated aspect and without the presence of precipitated compounds; while the live fungal biomass grown in YNBp medium presented a smooth and more aggregated aspect without the presence of precipitates. However, in the YNBpU medium, uranium precipitates can be observed on the biomass surface (Fig. 4).
Energy-dispersive spectroscopy (EDS) determined the elements that are part of the fungal biomass composition.
The EDS results for live fungal biomass grown in YNB (control) show the composition of fungal biomass without (P) and (U). However, after 3 weeks of T. amestolkiae growth in YNBp media, was observed a phosphate (P) peak in the biomass composition. In YNBpU media, we noticed uranium (U) and phosphate (P) peaks on the biomass composition (Figs. 5 and 6, and 7, respectively). The quantitative results show that the mass of phosphate and uranium present in the fungal biomass is 2.72 and 2.08%, respectively (Figs. 6, 7).
The ED-XRF analysis confirmed that a high concentration of uranium precipitated onto the fungal biomass after 21 days of growth in media containing uranium and phosphate; the analysis also indicated that uranium (α, Lβ, Lγ) uptake occurred (Fig. 8). The standard spectrum showed three peaks for uranium alpha, beta, and gamma. After 21 days no uranium peaks were observed in the control. However, comparing the standard with the biomass after growth in media supplemented with uranium and phytate, three similar uranium peaks were identified; which confirmed the precipitation of the metal onto fungal biomass.
The IAEA quality assurance reference material RGU- 238 (uranium standard) was measured using the same specifications and conditions and was used to confirm the U L-lines.
Discussion
In the current study, the evaluated fungi had a higher production of phosphate in media supplemented with phytic acid than glycerol 2-phosphate. However, in both media, all the fungi produced phosphate in different concentrations.
In media supplemented with phytic acid, all the fungi tested were able to produce phosphate, but T. amestolkiae displayed the best capacity to assimilate phytate and enzymatically produce phosphate.
Gongronella butleri showed the best capacity to produce phosphate in media supplemented with glycerol 2-phosphate. However, after 4 weeks, the amount of phosphate had decreased during fungal growth. According to Lima et al. (2003), during the cell growth cycle of G. butleri, phosphate use occurs due to the synthesis of cellular compounds.
The phosphate present in microorganism biosorption processes is essential to the biomineralization of heavy metals, such as uranium. The phosphate can combine with the uranium ions in the medium and form different uranium-phosphate mineral complexes (Zheng et al. 2018). Studies have shown that the addition of phosphate during the biosorption processes can increase the removal capacity of the heavy metals (Khijniak et al. 2005; Gadd 2009; Shelobolina et al. 2009). Phytases are the primary enzymes responsible for the hydrolysis of phytic acid (Awad et al. 2014).
Fungi have been reported to have a vital ability to solubilize phosphates, due to their metabolic capacities. Research in the field of microbial solubilization of phosphates focus on filamentous fungi (Vassilev and de Oliveira Mendes 2017). Gongronella species are often described as a good producer of enzymes, G. butleri isolated from soil samples in Colombia have demonstrated that they are excellent phosphate producers utilizing iron phosphate sources (Vera et al. 2011; Cavalheiro et al. 2017).
In the present study, T. amestolkiae exhibited a better ability to assimilate phytate, and to produce phosphate during growth in the media supplemented with phytate. Talaromyces, Penicillium, and Rhodotorula species have all been described as optimal phytase producers, thus demonstrating optimum potential for the hydrolysis of organic phosphate sources (Ocampo et al. 2012; Khan et al. 2014; Kaur 2017).
For uranium biomineralization, T. amestolkiae showed high potential in the production of phytases and degradation of phytic acid. Exponential growth was exhibited in the production of free phosphate. These results are important for the bioremediation of heavy metals in the environment, where the ions released during the degradation process of phytic acid can help in the treatment of water contaminated with uranium.
Talaromyces species present an essential ability for uranium bioremediation, and recent studies demonstrate that these fungi have a high capacity for remediation of other heavy metals from aqueous solutions (Bengtsson et al. 1995; Cárdenas González et al. 2019; Das et al. 2019; Wang et al. 2019).
Regarding the medium supplemented with phytate and uranium, a reduction in the production of phosphate was observed. This may have happened due to the toxicity of the uranium, which may reduce the growth index of the fungus; another factor may also have been that phosphate precipitated with the uranium ions in solution or the fungal biomass. This hypothesis was confirmed through SEM, EDS, and XRF analysis. The micrographs showed the formation of uranium “granules” on the surface of the live fungal biomass.
In ED-XRF, X-rays emitted by atoms are specific to each element, generating peaks that cannot be seen with the naked eye. Uranium can emit alpha, beta, and gamma radiation, which are dangerous types of radiation (Coelho et al. 2020b). Thus, the identification of these elements in the analyses and the evident peaks indicate the precipitation of uranium in T. amestolkiae.
Biomineralization is generally defined as a process where living organisms form minerals, and this can result in the removal of metals from the solution; providing a means of detoxification and bio-recovery of contaminated environments (Gadd and Pan 2016). In biomineralization, the phosphatase activity of microorganisms cleaves organic phosphate to release inorganic phosphate; which precipitates with U(VI) as extracellular hydrogen minerals, such as uranyl phosphate [HUO2PO4] (Newsome et al. 2014). The formation of these compounds was demonstrated in research carried out with fungi and heavy metals such as Cr and Pb (Qian et al. 2017).
Fungi have a high metabolic capacity, which often makes them very resistant to the toxicity of metal ions, being described as excellent microorganisms to be used in biomineralization processes. It is believed that the mechanisms for biomineralization of uranium phosphate by fungi occur when the available phosphate binders appear after the action of fungal acid phosphatases on organic substrates containing P (Liang et al. 2015; Cumberland et al. 2016).
In the current study, we identified that T. amestolkiae can assist in the decontamination processes of environments contaminated with uranium; even if the contaminated site has an acidic pH. The current study shows that, media supplemented with phytate and other nutrients allows the microorganisms to produce and increase the formation of minerals, such as uranium phosphate; and consequently, to improve the bioremediation efficiency.
Conclusions
The fungi G. butleri, P. piscarium, T. amestolkiae, R. sinensis, isolated from a uranium mine in Brazil, were shown to be excellent phosphate producers. However, the best phosphate donor was phytate. Talaromyces amestolkiae, growing in culture medium supplemented with phytate, was the best phosphate producer. During fungal growth, pH changes in the medium were not observed. After 3 weeks of T. amestolkiae growth in medium supplemented with phytate, there was a reduction of between 20 and 30% in the uranium concentration, with high precipitation of uranium and phosphate in the fungal biomass. These results demonstrate that fungi possess the ability to use metabolism for phytate degradation and phosphate production. In the environment, this fungus can control the dispersion of heavy metal ions, even in highly contaminated places and acidic pH levels, helping to support the restoration and decontamination of affected environments.
References
Awad GEA, Helal MMI, Danial EN, Esawy MA (2014) Optimization of phytase production by Penicillium purpurogenum GE1 under solid state fermentation by using Box–Behnken design. Saudi J Biol Sci 21:81. https://doi.org/10.1016/J.SJBS.2013.06.004
Bengtsson L, Johansson B, Hackett TJ, McHale L, McHale AP (1995) Studies on the biosorption of uranium by Talaromyces emersonii CBS 814.70 biomass. Appl Microbiol Biotechnol 42:807–811. https://doi.org/10.1007/BF00171965
Cárdenas González JF, Rodríguez Pérez AS, Vargas Morales JM, Martínez Juárez VM, Rodríguez IA, Cuello CM, Fonseca GG, Escalera Chávez ME, Muñoz Morales A (2019) Bioremoval of cobalt(II) from aqueous solution by three different and resistant fungal biomasses. Bioinorg Chem Appl 2019:8757149. https://doi.org/10.1155/2019/8757149
Cavalheiro GF, Sanguine IS, da Silva Santos FR, da Costa AC, Fernandes M, da Paz MF, Fonseca GG, Leite RSR (2017) Catalytic properties of amylolytic enzymes produced by Gongronella butleri using agroindustrial residues on solid-state fermentation. BioMed Res Int 2017:1–8. https://doi.org/10.1155/2017/7507523
Coelho E, Reis TA, Cotrim M, Mullan TK, Corrêa B (2020a) Resistant fungi isolated from contaminated uranium mine in Brazil shows a high capacity to uptake uranium from water. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126068
Coelho E, Reis TA, Cotrim M, Rizzutto M, Corrêa B (2020b) Bioremediation of water contaminated with uranium using Penicillium piscarium. Biotechnol Prog. https://doi.org/10.1002/btpr.3032
Cumberland SA, Douglas G, Grice K, Moreau JW (2016) Uranium mobility in organic matter-rich sediments: a review of geological and geochemical processes. Earth Sci Rev 159:160–185. https://doi.org/10.1016/J.EARSCIREV.2016.05.010
Das D, Chakraborty A, Santra SC (2019) Assessment of lead tolerance in gamma exposed Aspergillus niger van Tieghem & Penicillium cyclopium Westling. Int J Radiat Biol 95:771–780. https://doi.org/10.1080/09553002.2019.1569769
de Lima MAB, Nascimento AE, de Souza W, Fukushima K, de Campos-Takaki GM (2003) Effects of phosphorus on polyphosphate accumulation by Cunninghamella elegans. Braz J Microbiol 34:363–372. https://doi.org/10.1590/S1517-83822003000400016
Drangert J-O (2012) Phosphorus—a limited resource that could be made limitless. Procedia Eng 46:228–233. https://doi.org/10.1016/J.PROENG.2012.09.469
Elias F, Woyessa D, Muleta D (2016) Phosphate solubilization potential of rhizosphere fungi isolated from plants in Jimma Zone, southwest Ethiopia. Int J Microbiol 2016:1–11. https://doi.org/10.1155/2016/5472601
El-Naggar NEA, Hamouda RA, Mousa IE, Abdel-Hamid MS, Rabei NH (2018) Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb2+ removal from aqueous solutions. Sci Rep. https://doi.org/10.1038/s41598-018-31660-7
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14. https://doi.org/10.1016/j.biortech.2013.12.102
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Gadd GM, Pan X (2016) Biomineralization, bioremediation and biorecovery of toxic metals and radionuclides. Geomicrobiol J 33:175–178
Kaur R (2017) Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nusant Biosci. https://doi.org/10.13057/nusbiosci/n090112
Kefeni KK, Msagati TAM, Mamba BB (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Clean Prod 151:475–493
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi—current perspective. Arch Agron Soil Sci 56:73–98. https://doi.org/10.1080/03650340902806469
Khan S, Zaid MA, Ahmad E, Musarrat J (2014) Phosphate solubilizing microorganism: principles and applications of microphos technology. Springer, Berlin
Khijniak TV, Slobodkin AI, Coker V, Renshaw JC, Livens FR, Bonch-Osmolovskaya EA, Birkeland N-K, Medvedeva-Lyalikova NN, Lloyd JR (2005) Reduction of uranium(VI) phosphate during growth of the thermophilic bacterium Thermoterrabacterium ferrireducens. Appl Environ Microbiol 71:6423–6426. https://doi.org/10.1128/AEM.71.10.6423-6426.2005
Liang X, Hillier S, Pendlowski H, Gray N, Ceci A, Gadd GM (2015) Uranium phosphate biomineralization by fungi. Environ Microbiol 17:2064–2075. https://doi.org/10.1111/1462-2920.12771
Mohammed NH, Atta M, Wan Yaacub WZ (2017) Remediation of heavy metals by using industrial waste by products in acid mine drainage. Am J Eng Appl Sci 10:1001–1012. https://doi.org/10.3844/ajeassp.2017.1001.1012
Newsome L, Morris K, Lloyd JR (2014) The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol 363:164–184. https://doi.org/10.1016/J.CHEMGEO.2013.10.034
Ocampo M, Patiño LF, Marín M, Salazar M, Gutiérrez PA (2012) Isolation and characterization of potential phytase-producing fungi from environmental samples of Antioquia (Colombia)/aislamiento y caracterizacin de hongos productores de fitasa a partir de muestras ambientales de Antioquia (Colombia). Rev Fac Nal Agr Medellín 65(1):6291–6303
Olstorpe M, Schnürer J, Passoth V (2009) Screening of yeast strains for phytase activity. FEMS Yeast Res 9:478–488. https://doi.org/10.1111/j.1567-1364.2009.00493.x
Paterson-Beedle M, Readman JE, Hriljac JA, Macaskie LE (2010) Biorecovery of uranium from aqueous solutions at the expense of phytic acid. Hydrometallurgy 104:524–528. https://doi.org/10.1016/J.HYDROMET.2010.01.019
Qian X, Fang C, Huang M, Achal V (2017) Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. J Clean Prod 164:198–208. https://doi.org/10.1016/J.JCLEPRO.2017.06.195
Qvirist L, Carlsson N-G, Andlid T (2015) Assessing phytase activity—methods, definitions and pitfalls. J Biol Methods 2:16. https://doi.org/10.14440/jbm.2015.58
Shelobolina ES, Konishi H, Xu H, Roden EE (2009) U(VI) sequestration in hydroxyapatite produced by microbial glycerol 3-phosphate metabolism. Appl Environ Microbiol 75:5773–5778. https://doi.org/10.1128/AEM.00628-09
Sheridan C, Akcil A, Kappelmeyer U, Moodley I (2018) A review on the use of constructed wetlands for the treatment of acid mine drainage. In: Constructed wetlands for industrial wastewater treatment. Wiley, Chichester, pp 249–262
Someus E, Pugliese M (2018) Concentrated phosphorus recovery from food grade animal bones. Sustainability 10:1–17. https://doi.org/10.3390/su10072349
Vassilev N, de Oliveira Mendes G (2017) Solid-state fermentation and plant-beneficial microorganisms. Curr Dev Biotechnol Bioeng. https://doi.org/10.1016/b978-0-444-63990-5.00019-0
Vera DF, Perez H, Valencia H (2011) Aislamiento de hongos solubilizadores de fosfatos de la rizosfera de araza (Eugenia stipitata, Myrtaceae). Acta Biol Colomb 7:33–40. https://doi.org/10.1016/j.riam.2009.03.005
Wang N, Qiu Y, Xiao T, Wang J, Chen Y, Xu X, Kang Z, Fan L, Yu H (2019) Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: high performance, selectivity and application. J Hazard Mater 373:39–49. https://doi.org/10.1016/j.jhazmat.2019.03.056
Zheng XY, Shen YH, Wang XY, Wang TS (2018) Effect of pH on uranium(VI) biosorption and biomineralization by Saccharomyces cerevisiae. Chemosphere 203:109–116. https://doi.org/10.1016/J.CHEMOSPHERE.2018.03.165
Acknowledgements
The author thanks Fundação de Amparo à Pesquisa do Estado de São Paulo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for funding and research support (FAPESP: 2015/06757-1).
Author information
Authors and Affiliations
Contributions
EC, JR, MR, and BC devised the research. EC, TAR, and MC conducted the experiments. EC, TM, JR, and BC conducted the modeling. EC, TAR, and BC wrote the manuscript. All authors discussed the data, results and commented on the manuscript. BC and JR supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing financial interests and non-financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coelho, E., Reis, T.A., Cotrim, M. et al. Talaromyces amestolkiae uses organic phosphate sources for the treatment of uranium-contaminated water. Biometals 35, 335–348 (2022). https://doi.org/10.1007/s10534-022-00374-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00374-9