Abstract
High-altitude lakes (HALs) can be used as a supplement or alternative source of water in areas where there is a water shortage. When these lakes are efficiently managed, they can supply more water resources to fulfil the increasing demand. Water quality assessment aids in the identification of adequate and safe drinking water sources. It minimizes threats to the public’s health by making sure that lake water extraction fulfills safety and health regulations. Water quality and hydrogeochemical study was conducted on six HALs of the Tawang district of Arunachal Pradesh during the year 2022. The water quality index (WQI) values varied from excellent to poor (33.87 to 101.95). Lake 6 stands out with its exceptional water quality as it had the minimum average WQI value of 52.98. In contrast, Lake 5 had the lowest water quality among the studied lakes with the maximum average WQI value of 95.31. However, the water might not be safe to drink due to the elevated levels of fluoride in these lakes. It is crucial to address and minimize the high fluoride levels to ensure the safety and acceptability of the water for consumption. The Piper diagram showed that Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42−, respectively, were the primary cations and anions present in these lakes. The Gibbs diagram also demonstrated the effect of rock weathering and precipitation dominance on the water chemistry in the research area. These results provide insightful information about the water quality of HALs, which is essential information for concerned government departments and agencies to manage water issues more efficiently. Based on current research, the HALs in this region have a lot of potential to meet the growing demand for drinking water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lakes play a significant role on our planet by supporting a variety of crucial processes (Hairston and Fussmann 2014). Lakes are considered open system water sources because they interact with their surroundings by absorbing energy and matter (Guo et al. 2020). It helps in regulating the temperature, regional hydrological cycle, and aquatic ecosystems (Wetzel 2001). High-altitude lakes (HALs), which are perennial, are mainly fed by meltwaters from glaciers, snow, and seasonal monsoon (Xu et al. 2009). As natural storage systems, they are also essential for the hydrology and ecology of rivers (Upadhyay et al. 2016). These alpine mountain ecosystems are of the utmost importance because over 1.3 billion people in several Asian countries rely on them for their freshwater needs (Rasul 2014; Mukherji et al. 2015). HALs are currently facing a variety of issues including sediment build-up, microbiological contamination, dangerous heavy metals, and eutrophication (Ravikumar et al. 2013; Meena et al. 2017). These concerns jeopardize the water quality and overall health of these lakes, seeking urgent attention and conservation measures.
Research on HALs is crucial because it sheds light on how the climate is changing globally (Thies et al. 2007). The quality of water in alpine lakes is significantly threatened by atmospheric deposition and climate change, even though direct human activity has not affected it much (Jimenez et al. 2018; Burpee and Saros 2020). Extensive research has been conducted over the years to assess the state of Himalayan lakes and shed light on their water quality (Ramanathan 2007; Zhu et al. 2010; Khadka and Ramanathan 2013; Kanakiya et al. 2014; Sheikh et al. 2014). It is reported that human caused and natural factors have affected the deterioration in the quality of the water in these lakes. Elevated temperatures in these lakes have resulted in higher mineral weathering, glacier melting, and improved hydrological connectivity, all of which have resulted in higher ion and nutrient concentrations (Gudasz et al. 2010; Zindros et al. 2020; Kumar et al. 2022). High-altitude regions often have minimal human induced pollution sources like industrial activity and urbanization owing to their isolation and challenging climatic conditions (Akhtar et al.2021). It is more likely that the primary causes of contamination are linked to geological phenomena and naturally occurring processes that release contaminants into the environment (Gaur et al. 2022). These may include geological weathering, volcanic activity, mineral deposits, and naturally occurring elements or compounds that might leach into surrounding water bodies, consequently influencing the water quality of HALs (Smedley and Kinniburgh 2002; Bhateria and Jain 2016). According to Finger et al. (2013) and Hundey et al. (2016), atmospheric deposition is changing the naturally oligotrophic character of alpine aquatic ecosystems, which are characterized by shallow catchment soils, thin vegetation cover, and insoluble bedrock. Apart from temperature variations, air deposition is a noteworthy catalyst for altering the ecosystem in alpine lakes (Burpee and Saros 2020). Yang et al. (2007), Deka et al. (2016a), and Machate et al. (2023) reported that long-range air pollutants transferred from the lowlands contaminated the mountainous lakes. As a result, monitoring these natural and geogenic events is critical for assessing and managing potential contamination hazards in such high-altitude environment. Hydrogeochemical research has become increasingly significant all over the world to assess how geological formations affect the quality of natural water bodies (Kumar et al. 2023). These investigations elucidate the interactions between water and rock, which are crucial to the existence of particular elements in the water. The minerals are primarily produced through rock weathering and the disintegration of minerals that occur in geological strata (Feng et al. 2020). Such studies have clarified the mechanisms of rock water interaction and their impact on water quality by evaluating the mineral composition and determining the various kinds and amounts of elements present (Smedley and Kinniburgh 2002; Elango and Kannan 2007; Gaus 2010; Nordstrom 2011). Many water quality parameter concentrations in the lakes are directly determined by the region’s rocks that surround it. Erosion and weathering processes of carbonate and silicate minerals such as calcite, dolomite, pyrite, carbonic acid, and gypsum play a major part in the disintegration of these types of minerals under various environmental circumstances (Li and Zhang 2008; Vishwakarma et al. 2018). Tyagi et al. (2013), Wu et al. (2018), and Uddin et al. (2021) reported that the water quality index (WQI) can be used to reflect more simply the overall quality of surface water. This method is useful because physical, chemical, and biological factors influence the quality of surface water. Instead of assessing each component individually, the WQI integrates many parameters into a single numerical number, offering a comprehensive assessment of water quality. Dealing with complex and vast data matrices when computing the WQI can be a challenging undertaking (Tripathi and Singal 2019; Akhtar et al. 2021). Multiple multivariate analysis techniques, including principal component analysis (PCA) and factor analysis, were used to overcome this difficulty and ensure a more objective study (Floyd and Widaman 1995; Güler et al. 2002). These techniques aid in interpreting the results and simplifying data comprehension.
Arunachal Pradesh is the largest state in the northeastern region of India with an area of 83,743 km2 or 2.54% of the country’s geographical area (ISFR 2021). It is located between 26°28′ N and 29°30′ N latitude and 91°30′ E to 97°30′ E longitude, stretching from the snow-capped mountains of the Eastern Himalayas in the north to the plains of the Brahmaputra valley in the south. The state is adorned with a vast number of high-altitude lakes that vary in size, depth, and ecological qualities. A total of 1672 HALs have been identified throughout the state, covering an area of 11,864 hectares (Panigrahy et al. 2012). However, limited studies have been conducted on HALs located in Arunachal Pradesh due to the difficult terrain. Water quality study has been focused on lower level lakes, leaving alpine lakes severely under monitored and under researched. Based on existing published literature, only a few studies on high-altitude lakes in Arunachal Pradesh were accessible. Deka et al. (2015) discussed the seasonal variation and trace element levels transferred via atmospheric activities on two lakes. Deka et al. (2016a) reported on Penga Teng Tso Lake that alterations in the lake’s water chemistry may be associated with long distance transported pollutants. A recent study on the Tawang district was also done to determine whether surface water is suitable for drinking and agricultural uses (Gaur et al. 2022). The finding suggested that geological processes possibly affected the water quality of the studied location. HALs are frequently used as drinking water sources for local communities or as reservoirs for downstream consumers. Maintaining water quality benefits aquatic life, which is critical for biodiversity conservation in these fragile ecosystems. Since these lakes are the only water source in the area, there have been more military installations built next to them. In addition, there may also be an indirect effect of the growing tendency of tourism on these HALs. Water quality monitoring detects changes or emerging issues such as pollution, nutrient imbalance, or changes in ion concentrations early, allowing for rapid response. Therefore, to protect and preserve such fragile ecosystems, regular surveillance and the setting up of efficient mitigating measures are necessary. Considering the above viewpoints, the main goals of the current study are to assess the water quality and monitor the hydrogeochemical processes taking place in the selected HALs in the Tawang region of Arunachal Pradesh. Given the region’s difficult topography and restricted accessibility, conducting regular and systematic lake monitoring can be logistically challenging.
Materials and methods
Study area
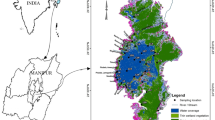
Tawang district of Arunachal Pradesh lies between 27° 52′ N and 27° 28′ N latitudes and 91° 32′ E and 92° 23′ E longitudes with altitude varying from 1800 to 4500 m above mean sea level (amsl) (Fig. 1). The geological age of the region is Archaean and Palaeozoic (Mishra 2007). The lesser Himalayan range is made up of several rocks of varying geological ages that are separated by thrust faults (Singh et al. 2014). The Se La group, a collection of crystalline formations, is the dominant group along this region of the strata. According to Bhattacharjee and Nandy (2008), these formations include tourmaline bearing leucogranite, streaky gneiss, kyanite sillimanite bearing garnet biotite schist, psammitic gneiss, and amphibolite. The Tawang section’s topography consists of extraordinarily steep hills and glacial era features. According to Srivastava et al. (2011), faults and thrusts are to blame for the dramatic changes in the geomorphology of this area. The district’s rugged geography contributes to its peculiar climate due to its location in the Himalayas. The existence of high peaks and valleys influences local weather patterns, such as temperature fluctuations and precipitation levels. The monthly mean maximum and lowest temperatures are 20.88 °C and − 0.07 °C, respectively (Nimasow et al. 2016). During the monsoon season (June to September), the district receives substantial rainfall due to the impact of the southwest monsoon. The range of average annual rainfall varied from 1300 to 4500 mm as per the Indian Meteorological Department (IMD) from 1980 to 2022. During the pre-monsoon sampling period, a total of 373.83 mm was recorded as per IMD, while January and February are typically the driest months in the year. The Monpa tribes of Tibeto-Mongoloid stock, who worship Buddhism, are the majority of the district’s population. Lakes are referred to as “Tso” in the local dialect. Six lakes were chosen for this study based on geography, accessibility, logistical assistance, ecological diversity, tourism, and traditional beliefs (Fig. 1; Table 1). The Village Forest Management Committee, a part of the Department of Environment and Forest, Tawang, provided all the information about these lakes that are mentioned here. The ArcGIS software was used to construct maps associated with this study.
Sampling
A total of 60 samples were collected, i.e., ten samples each from six different lakes based on a random sampling method during the year 2022. For better representation, five sampling points were considered from each lake periphery to ensure that the data was represented clearly and unambiguously. This decision was based on the observation of in situ parameters like pH, electrical conductivity (EC), salinity, total dissolved solids (TDS), and oxidation reduction potential (ORP). These parameters exhibited minimal or negligible variation, due to the small size of the lakes. The present assessment aims to account for potential geographical variations and acquire a thorough understanding of the water quality characteristics of these lakes. Polypropylene sampling bottles (500 mL and 125 mL) were thoroughly washed with deionized water and dried before visiting the field site. A maximum depth of 20 cm below the water’s surface was maintained when collecting water samples from the lakes’ periphery. The bottles were cleaned three times with lake water before collecting the actual water sample, and correct labelling was completed following APHA (2005) standards. The water samples collected in 125 mL bottles were double filtered using 0.45 µm (PVDF) and thereafter with 0.22 µm (QVWP) pore sized filter papers (Kumar et al. 2019b). They were then stored at 4°C for further analysis of major cations and anions. Figure 2 shows the pinpoint sampling locations using a GPS (Model: Oregon 750 Make: Garmin, USA) having an accuracy of 3–5 m.
Physicochemical analysis
Parameters including temperature, EC, pH, salinity, and TDS were measured using a multi parameter probe (Model: PCSTestr35 Eutech). ORP (Model: ORPTestr10 Eutech) and DO (Model: HI9146 Hanna) were measured using a field test probe. Alkalinity and total hardness were analyzed in the field using titration methods. Following APHA (2005), the instrument ion chromatography (Model: EcoIC Metrohm) was used to measure the calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), fluoride (F−), nitrate (NO3−), phosphate (PO43−), and sulphate (SO42−). The sodium (Na+) and potassium (K+) were analyzed using a flame photometer (Model: 128 Systronics). Carbonate (CO32−) and bicarbonate (HCO3−) were computed using the empirical formula by APHA (2005).
Evaluation of water quality index (WQI)
The WQI is a detailed mathematical evaluation that offers an overall summary of the parameters that influence water quality (Saini et al. 2008). The criteria used to select the parameters were based on the concentration of different physiochemical parameters. Based on their harmful impact on human health, each water quality characteristic has on the total quality of the water; weights (Wi) (1–5) are assigned to each characteristic to calculate WQI (Ravikumar et al. 2013). The relative weightage (Wr) for individual metrics in the calculation of the WQI can be determined using the following equation:
where i is the number of variables needed to calculate WQI. The rating scale (Qi) for each parameter of water quality is calculated based on BIS (2012) standards using the following equation:
where Ci denotes parameter concentration and Si denotes BIS (2012) drinking water quality guidelines for all parameters. The sub-indices (SIi) and overall WQI are then computed using the following equations:
Based on total WQI values, the water quality was evaluated and grouped as excellent (WQI < 50), good (between 50 and 100), poor (between 100 and 200), very poor (between 200 and 300), and unfit or unsuitable (WQI > 300) (Judran and Kumar 2020). This classification method provides an in-depth assessment of total drinking water quality, facilitating simple interaction and recognizing areas that require attention or change based on the designated groups.
Hydrogeochemical evaluation
The Piper tri-linear diagram (Piper 1944) and Durov’s diagram (Durov 1948) are two extensively used hydro-chemical diagrams that offer important insights into the geochemical and hydrochemical mechanisms taking place in water. Grapher 13 was used to generate both Piper and Durov diagrams. Chadha (1999) introduced a modified hydrochemical diagram that gives a thorough knowledge of water quality variables. Understanding the chemistry of the water, where it comes from any potential problems with salinity, mineralization or contamination are all made easier with the use of this knowledge. The chemical composition of lake water samples was examined, following the above mentioned diagrams or plots. The cationic and anionic ratios in water samples are graphically represented using Gibbs diagrams as a function of total dissolved solid content (Gibbs 1970). In geochemistry, these diagrams are frequently used to evaluate and explain the main geochemical processes taking place in each water system. Some of these processes include rain, weathering of rocks, and evaporation dominance (Drever 1997; Ramanathan 2007). Charts for Chadha and Gibbs were created using Microsoft Excel. Changes within the concentration of multiple water quality parameters, notably those involving the ions Ca2+, Mg2+, Na+, and K+, can result from the ion exchange process.
The amount (meq/L) of various cations and anions was calculated using scatter plots (Kumar et al. 2020) to identify the primary sources of important ions in the research area. The Chloro-alkaline indices (CAI) 1 and 2 were first established by Schoeller (1965) as measures of the ion exchange processes between water bodies and their surroundings. In the indices above, all ions (meq/L) were determined using Eqs. (5) and (6) (Kumar et al. 2009). The positive readings in these indicators reflect the direct base exchange, whereas negative readings suggest the Chloro-alkaline disequilibrium (Kant et al. 2018; Barik and Pattanayak 2019; Dey et al. 2023).
Data analysis
To find patterns and relationships between variables, multivariate statistics techniques were used including principal component analysis (PCA) and Pearson’s correlation coefficient. The Shapiro–Wilk test was conducted to check the normal distribution of data. It was checked that the variables were normally distributed. In the present study, the correlation analysis method of Pearson was implemented to examine the correlations and find the interdependencies between various characteristics of water samples. Correlation coefficients (r) suggest a strong positive correlation when they are greater than 0.80 and a positive correlation when they are between 0.50 and 0.79. PCA was used to provide insight into changes in hydrogeochemical relationships among different water parameters (Prusty et al. 2018; Alam et al. 2020). To ensure the validity of the interpretation acquired through PCA, Kaiser–Meyer–Olkin (KMO) and Bartlett’s tests were performed before PCA. A score near 1.00 show that the data are suitable for PCA, while a value below 0.50 shows that the data may not be suitable for PCA (Gaur et al. 2022; Krishan et al. 2023). The KMO test decides whether the data is suitable for PCA. Student t-test was also performed to evaluate the effect of TDS on cations and anions. Data were analyzed using the Microsoft Excel and IBM SPSS (Version 22).
Results
Samples collected from six separate lakes in the Tawang district were subjected to an analysis of a total of 18 physicochemical parameters. Descriptive statistical analysis was performed on the collected data, which included calculating absolute and relative deviations as well as determining the percentage of samples that were beyond the BIS-permitted limits depicted in Table 2. The temperature of water samples varies from 2.50 to 10.00 °C, with an average of 6.14 ± 0.37 °C during the pre-monsoon season. The water samples had a pH between 6.03 and 8.00, with an average of 6.98 ± 0.11. Lake 4 had the highest pH value (8.00), showing an alkaline nature, whereas Lake 1 had the lowest pH value (6.03), indicating a slightly acidic character. TDS in water refers to the presence of all dissolved substances, including salts, organic materials, and minerals. The average value of the TDS content was 20.38 ± 5.19 mg/L, with a range of 4.20 and 147.00 mg/L. Lake 3 has the highest TDS value of 147.00 mg/L of the six lakes examined, yet it is still below BIS (2012) permissible limits. The EC values ranged from 6.30 to 206.00 µS/cm, with an average of 27.11 ± 7.28 µS/cm. The highest EC value was observed in Lake 3, out of all the lakes investigated. The average salinity was found to be 0.01 p.s.u., with Lake 3 having the highest salinity of 0.09 p.s.u. was the saltiest lake.
The DO content was determined to be in the range of 4.50 to 9.70 mg/L, having an average of 7.24 ± 0.28 mg/L and a spatial variability of 20.90%. The tested water samples also showed positive oxidation reduction potential (ORP) values, with an average ORP value of 181.83 ± 5.69 millivolts (mV) and a range from 116.00 to 222.00 mV. An oxidizing environment in the water is present when the ORP value is positive. The strong correlation (Pearson correlation, r = 0.97) between DO and ORP further supported this finding. Lake 1 showed DO content ranging from 6.50 to 7.05 mg/L, Lake 2 from 8.60 to 9.30 mg/L, Lake 3 from 5.00 to 5.80 mg/L, Lake 4 from 4.50 to 5.80 mg/L, Lake 5 from 8.90 to 9.70 mg/L, and Lake 6 from 6.90 to 7.50 mg/L, respectively. Values for bicarbonate (HCO3−) varied between 10.33 and 216.98 mg/L. Bicarbonate levels were calculated to be 35.14 ± 7.17 mg/L on average. Total hardness varied from 8.50 to 135.00 mg/L, with an average hardness of 23.48 ± 4.68 mg/L. According to Briggs and Ficke (1977) classification of water hardness, the maximum percentage of water samples (93.33%) belonged to the soft water category. The remaining samples were divided into two categories, with 3.33% being classified as moderately hard water and the other 3.33% being classified as hard water. The measured water samples had magnesium (Mg2+) contents between 0.36 and 17.01 mg/L and calcium (Ca2+) between 2.00 and 26.03 mg/L. Potassium (K+) content was averaged at 0.88 mg/L, while sodium (Na+) was at 1.20 mg/L on average. The highest concentration of Na+ was observed in Lake 5 (2.68 mg/L) and the lowest in Lake 6 (0.37 mg/L). Lake 3 had the maximum concentration of K+ (1.51 mg/L), and Lake 5 had the minimum of 0.30 mg/L. The chloride (Cl−) was determined to be 14.91 ± 1.14 mg/L on average, ranging from 7.10 and 28.40 mg/L. Average values for NO3− and SO42− were 0.22 ± 0.04 mg/L and 0.77 ± 0.05 mg/L, while the highest concentration was recorded from Lake 5. Phosphate (PO43−) concentration ranges from below the detection limit (BDL) and 0.04 mg/L. Fluoride (F−) levels varied from 1.50 to 7.11 mg/L having an average of 5.78 ± 0.25 mg/L. The samples (100%) were found to be beyond the BIS acceptable level of 1 mg/L. The water sample from Lake 5 had the highest fluoride levels of all the samples, measuring 7.11 mg/L. Ca2+ and Mg2+ were observed as the two most dominant cations whereas HCO3− and Cl− as anions, in the current study.
Correlation analysis
Based on Pearson correlation analysis (Table 3), EC has a strong correlation between salinity (r = 0.99) and TDS (r = 0.99). The strong correlation among EC, TDS, and salinity may be influenced by the concentration of dissolved salts and ions in the water. Furthermore, bicarbonate (HCO3−) (r = 0.98) had the highest anion concentration while Ca2+ (r = 0.97) and Mg2+ (r = 0.91) for cation concentration in the study, produced a strong correlation with EC. This correlation reveals that both cations and anions affect the water’s overall conductivity. Total hardness (r = 0.97) is related to Ca2+ and Mg2+ ions that are present in water sources as a result of dissolved minerals and also have a strong correlation with EC. ORP and DO were found to be strongly correlated (r = 0.97). Their similarity to redox processes that take place in water is the basis for this relationship. Dissolved oxygen levels show the oxygenation of the water, while ORP indicates the overall redox state and the potential for oxidation reduction reactions. The strong correlation between the larger contribution of fluoride (F−) and the WQI suggests that it has an impact on the evaluation of water quality (r = 0.94). Finally, a positive correlation between sodium and potassium was found (r = 0.77), showing that one parameter can affect the other. This correlation can be explained by common sources that both supply sodium and potassium to the water samples, such as natural geologic processes and anthropogenic inputs. Overall, the correlation analysis sheds light on how the various parameters are related to one another, aiding in the understanding of their interdependencies and the variables affecting water quality.
WQI-based evaluation of water quality
Using the WQI (Table 4), the suitability of water for drinking purposes was assessed in the selected HALs. The calculated WQI values ranged from 33.87 and 101.95 with an average of 83.92. Approximately 6.67% of the samples had excellent water quality, 83.33% had good water quality, and 10.00% had poor water quality as shown in Fig. 3. Lake 6 stands out with its exceptional water quality as it had the lowest average WQI value of 52.98. On the other hand, Lake 5 had the lowest water quality among the studied lakes with the highest average WQI values of 95.31. The average WQI values of the other lakes were 84.68 (Lake 1), 85.39 (Lake 4), 90.45 (Lake 3), and 94.72 (Lake 2), respectively. The WQI was significantly affected by the concentration of dissolved ions including F−, HCO3−, and Ca2+ as well as other factors like pH, EC, and TH. The excessive amount of fluoride in these lakes poses a serious hazard to public health if consumed, even though the water samples were categorized as having excellent to poor water quality based on the WQI.
Hydrogeochemical facies
Piper diagram
The hydrogeochemical facies of water samples are analyzed and interpreted using the Hill-Piper tri-linear diagram. The diamond-shaped area in the center of Fig. 4 depicts the chemical composition of the water as a function of its predominant water type, while two triangles at the bottom show the relative proportions of primary cations (Ca2+, Mg2+, Na+, K+) and major anions (HCO3−, Cl−, SO42−). The plot reveals that the Ca2+-HCO3− type (86.67%) characterizes the majority of the geochemical facies in the water samples, showing temporary hardness. The remaining samples (13.33%) were shown as mixed type (neither cation nor anion dominant). The cationic triangle showed that, correspondingly, 56.67% of the samples were plotted in the Ca2+ type and 43.33% of the samples had no dominating type. While 97.00% of the samples in the anionic triangle were displayed as the HCO3− type, the remaining 3.00% were split between the Cl− type and no dominating type, respectively. The HCO3− type is dominant in the anionic triangle, which is a sign that the research area has seen weathering of silicate minerals.
Durov diagram
The expanded Durov diagram, in comparison to the Piper diagram, offers insights into potential geochemical processes (such as mixing, ion exchange, and reverse ion exchange dissolution) affecting water genesis. Its expanded tail part describes pH and TDS values, respectively (Singh et al. 2020; Sulaiman et al. 2023). The cation triangle shows that water samples are dominated by larger concentrations of Ca2+ and Mg2+ ions than alkali metal ions (Fig. 5). The water samples were primarily composed of the bicarbonate (HCO3−), carbonate (CO32−), and chloride (Cl−) ions in terms of the anion triangle. Almost 93.00% of the samples were found to fit into field 5, which shows a simple dissolving or mixing type without a prominent anion or cation, according to Lloyd and Heathcote (1985) classification. The Durov diagram further reveals that the lake water consists of calcium bicarbonate (Ca (HCO3)2) and magnesium bicarbonate (Mg (HCO3)2), with TDS values reaching 147.00 mg/L and pH values between 6.03 and 8.00 (Fig. 5).
Chadha diagram
Chadha diagram displays the properties of water samples as well as their overall ionic concentrations and geochemical categories. The prevalent rock type and dissolution pattern are reflected in the water type, which is an important indicator. The distribution of the sample data points over the two fields (5 and 6) on the diagram (Fig. 6) shows that the Ca2+-Mg2+-HCO3− and Ca2+-Mg2+-Cl− type of water predominate, which suggests that the studied lakes have both temporary and permanent hardness. The data points also coincide with the vicinity of field 1, showing a larger amount of Ca2+ and Mg2+ than Na+ and K+.
Gibbs diagram
Gibbs diagram remains a useful tool for figuring out the composition, origin, and distribution of dissolved elements in the water samples. The background of the underlying aquifer and the water quality are also important pieces of information presented. Gibbs (1970) first proposed the notion and identified the key factors affecting the composition of dissolved salts in surface or groundwater. According to Gibbs, the chemistry of water bodies is governed by three processes, viz., precipitation dominance, weathering dominance, and evaporation dominance. Thirty samples from the current investigation have been shown on Gibbs diagrams (Fig. 7 a and b). Based on the Gibbs classification, it was found that a significant portion of the samples belong to precipitation (Fig. 7a) and rock weathering (Fig. 7b) categories. This shows that these mechanisms are crucial in determining how the geochemical properties of lake water are shaped. It also suggests that these activities have a significant impact on the geochemical characteristics of lake water.
Major ion sources
Figure 8 a and b showed the concentrations of alkaline earth metals and alkali metals plotted against total cations, demonstrating larger contributions from total cations with data points drawn below the equiline (1:1 ratio). The scatter plot of Ca2+ + Mg2+ versus Na+ + K+ is displayed over the divider (equiline) in Fig. 8c, indicating a higher concentration of Ca2+ and Mg2+, implying that carbonate rock weathering, rather than silicate weathering, is the major mechanism in the examined lakes. The scatter plot in Fig. 8d (Ca2+ + Mg2+ versus HCO3−) shows a trend where most of the data points (93.33% of samples) are below the equiline, indicating increased bicarbonate alkalinity concentration. It also implies that bicarbonate alkalinity is influenced not only by alkaline earth metals but also by alkali metals and silicate weathering. While the remaining (6.67% of samples) are on the equiline, this indicates that alkaline earth metals predominate over bicarbonate due to carbonate rock weathering. Figure 8 e compares Ca2+ + Mg2+ versus SO42− + HCO3− in the scatter plot that has been used as a tool to comprehend the significance of the ion exchange process. The presence of silicate weathering in conjunction with the process of ion exchange was shown by the locations of the data points close to or below the equiline, where the concentration of Ca2+ + Mg2+ decreased while the concentration of SO42− + HCO3− increased. The points along the equiline, however, may be the consequence of gypsum, calcite, dolomite, and anhydrite dissolution in addition to carbonate weathering (Mora et al. 2017). In the scatter plot of Fig. 8f that compares Ca2+/SO42− and Mg2+/SO42−, the sample points are primarily located over the equiline. This data confirms that calcite minerals are a supply of SO42− in these lakes, highlighting their significant impact on total water chemistry. The points found close to the dividing line show a similar contribution from calcite, dolomite and gypsum, while those located below the equiline reflect a stronger influence of the dissolution of dolomite and gypsum. However, the current analysis has decided that the mineral calcite is the principal contributor to SO42− in the studied lakes. TDS has a significant effect (p < 0.05) on Ca2+, Mg2+, and HCO3−. Chloro-alkaline indices (CAI) determined yielded positive values, showing the existence of direct base exchange or Chloro-alkaline equilibrium. These activities, which altered the chemistry of the lake water, specifically involved the exchange of alkali metals with alkaline earth metals, where surrounding rocks served as the main sources of dissolved solids in water. While a few samples showed negative values, indicating the occurrence of reverse ion reaction or Chloro-alkaline disequilibrium.
Analysis of the principal component (PCA)
Using varimax rotation in XLSTAT, PCA was conducted on 18 water quality parameters. The KMO value in the current study was discovered to be higher than 0.50 (KMO = 0.64), showing that PCA is appropriate for the dataset. Four principal components were found to have eigenvalues greater than 1.00, suggesting their relevance in interpreting the variation in the set of data, based on Scree plot analysis (Kim and Mueller 1978). The loading values in Table 5 are the significance of the correlation between the physicochemical parameters and the main components (PCs), following Liu et al. (2003). The table’s bolding designates “strong” loading levels, which are those that are greater than 0.75. Weak loading is defined as values between 0.30 and 0.50, while moderate loading is defined as values between 0.50 and 0.75. Four PCs were identified using PCA accounting for 86.34% of the variability in the hydrogeochemical variables. PC-1, 2, 3, and 4 are responsible for 49.32%, 67.84%, 78.91%, and 86.34% of the variations within the variables, respectively.
PC-1 contains significant positive loadings of EC, TDS, salinity, total hardness, HCO3−, Ca2+, and Mg2+, which account for approximately 49.30% of the variation in the variables. However, some variables in PC-1, including ORP, DO, NO3−, and SO42− have negative loading values. The presence of a significant amount of Ca2+, Mg2+, and HCO3− ions may be the cause of the strong positive correlation between EC, TDS, salinity, and TH. This suggests that processes like weathering of rocks, exchange of ions, and mineral dissolution have considerable consequences on the concentration of major ions present in the lake water systems. The findings provide light on the mechanisms controlling the composition of important ions in lakes by demonstrating a complex interaction between water chemistry and the geological characteristics of the surrounding environment. The redox reactions in the lake water, which occur when reducing substances or activities deplete oxygen and generate negative correlations with other parameters, may be the reason for the negative loading values for DO and ORP. Sulphate and nitrate concentrations might also decrease because of processes like assimilation by aquatic organisms or microorganisms, which could change how they relate to other variables in the PCA analysis. Similarly, PC-2 shows 18.52% of variations in the variables and positive loading with DO, F−, and WQI, as well as negative loading with TH, Ca2+, Mg2+, PO43−, and NO3−. The absence of anthropogenic influence is indicated by the negative loading of NO3− in PCs 1 and 2. Positive SO42− loading in PC-3, which accounts for 11.07% of the overall variance, may be caused by the impact of rock minerals that contain pyrite. The positive fluoride (F−) loading reflects the abundance of fluorides, fluorosilicates, and fluorocarbonates mineral sources within the catchment area of these lakes. These minerals might aid in the release of fluoride ions into the water, which would result in a favorable relationship between PC-2 and fluoride ions. The WQI positive loading indicates that the factors it considers when analyzing water quality have a predictable pattern of change. This demonstrates that issues with water quality that affect the WQI are likely affecting the overall variability in PC-2. Furthermore, significant variations across the variables are anticipated due to geochemical restrictions and dilution by rainfall.
Discussion
The pH level of a water body is a fundamental property that shows whether the water is acidic or basic and has a substantial impact on water quality. pH alterations may also affect other physicochemical properties of water. Average pH values measured from each lake were 6.13 (Lake 1), 7.63 (Lake 2), 7.33 (Lake 3), 7.00 (Lake 4), 7.34 (Lake 5), and 6.43 (Lake 6). The pH levels of the water samples taken were determined to meet the BIS-approved limit of 6.50 to 8.50. The observed variation in pH levels in the studied lakes is similar to earlier findings reported by various authors from the Eastern Himalayan regions (Deka et al. 2015, 2016b; Gaur et al. 2022).
Temperature changes affect EC as well since they change the ionic composition and salt solubility. The dissolution of lithophilic ions might be attributed to excessive amounts of parameters such as EC, TDS, and salinity in the studied lakes. These ions are usually formed because of the weathering and erosion of rocks in the surrounding geological formations (Ritter et al. 2002), which is supported by the high significance between EC and TDS (r = 0.99), EC, and salinity (r = 0.98) as well as TDS and salinity (r = 0.98). There are several explanations for the elevated levels of salinity, TDS, and EC in comparison to other parameters. These include (1) the existence of standing water, (2) extended contact with sediment, (3) a larger interface between distinct phases, and (4) interaction between water and rocks (Sharma et al. 2012). These circumstances cause dissolved solids to accumulate and concentrate in the water, which increases the concentration of EC, TDS, and salinity. Higher ion concentration in Lake 3 may be from human settlement (military camps) in some areas of the catchment regions of the lake, which results in higher levels of EC, total hardness, and other related parameters.
The concentration of major dissolved ions found in Lake 1 water samples ranged from 10.33 to 20.00 mg/L of HCO3−, 7.10 to 10.65 mg/L of Cl−, 5.47 to 6.91 mg/L of F−, 0.01 to 0.02 mg/L of PO43−, 0.01 to 0.17 mg/L of NO3−, 0.29 to 0.76 mg/L of SO42−, 2.00 to 3.40 mg/L of Ca2+, 1.26 to 1.94 mg/L of Mg2+, 0.39 to 1.09 mg/L of Na+, and 0.57 to 0.98 mg/L of K+. Major ion concentrations in Lake 2 ranged from 2.90 to 4.79 mg/L of Ca2+, 1.52 to 1.92 mg/L of Mg2+, 0.97 to 1.24 mg/L of Na+, 0.74 to 1.17 mg/L of K+, 19.96 to 26.67 mg/L of HCO3−, 7.10 to 14.20 mg/L of Cl−, 6.11 to 7.09 mg/L of F−, BDL to 0.01 mg/L of PO43−, 0.33 to 0.67 mg/L of NO3−, and 1.17 to 1.29 mg/L of SO42−. The major ion concentrations in Lake 3 varied from 10.61 to 26.03 mg/L of Ca2+, 3.16 to 17.01 mg/L of Mg2+, 2.10 to 2.28 mg/L of Na+, 1.13 to 1.51 mg/L of K+, 53.33 to 216.98 mg/L of HCO3−, 14.20 to 28.40 mg/L of Cl−, 5.91 to 6.67 mg/L of F−, 0.02 to 0.04 mg/L of PO43−, 0.31 to 0.43 mg/L of NO3−, and 1.03 to 1.30 mg/L of SO42−. The cation concentrations in Lake 4 yielded from 2.00 to 4.00 mg/L of Ca2+, 2.43 to 3.04 mg/L of Mg2+, 0.47 to 0.71 mg/L of Na+, and 0.36 to 0.52 mg/L of K+, and anions concentration ranged from 20.00 to 26.66 mg/L of HCO3−, 10.65 to 17.75 mg/L of Cl−, 5.66 to 6.87 mg/L of F−, 0.01 to 0.02 mg/L of PO43−, 0.27 to 0.44 mg/L of NO3−, and 0.68 to 1.20 mg/L of SO42−. Lake 5 major ion concentrations were measured in the range from 2.60 to 4.00 mg/L (Ca2+), 0.36 to 1.58 mg/L (Mg2+), 2.34 to 2.68 mg/L (Na+), 0.30 to 0.75 mg/L (K+), 19.99 to 53.33 mg/L (HCO3−), 17.75 to 28.40 mg/L (Cl−), 6.14 to 7.11 mg/L (F−), 0.03 to 0.04 mg/L (PO43−), 0.41 to 0.78 mg/L (NO3−), and 1.09 to 1.39 mg/L (SO42−). Lake 6 water chemistry analysis resulted in ion concentrations varied from 2.60 to 3.40 mg/L of Ca2+, 0.49 to 0.73 mg/L of Mg2+, 0.37 to 0.66 mg/L of Na+, 0.32 to 0.41 mg/L of K+, BDL to 0.02 mg/L of PO43−, 0.07 to 0.25 mg/L of NO3−, 0.29 to 0.37 mg/L of SO42−, 1.50 to 3.10 mg/L of F−, 13.33 to 20.00 mg/L of HCO3−, and 10.65 to 21.30 mg/L of Cl−. Ca2+ > Mg2+ > Na+ > K+ and HCO3− > Cl− > SO42− > NO3− > PO43− are the major cations and anions determined within the lakes, respectively. As a result, all the lakes tested had Ca2+ and Mg2+ as dominating cations, with 51.54% and 27.15% contributions, respectively. Major dissolved ion metrics are found within the BIS acceptable levels except for fluoride. Out of the six studied lakes, 1, 3, and 5 are famous tourist spots; therefore, anthropogenic activities might be one of the biggest reasons for altering the water chemistry of these lakes.
The dissolution of F− containing minerals including fluorite, fluorapatite, hornblende, mica, and clay minerals constitute the sources of F− in natural water, as reported by Boyle and Chagnon (1995), Rao (2003), and Chae et al. (2007). A study conducted in Ulungur Lake in Xinjiang, China, revealed that biotite and hornblende are important F-containing minerals in the region, with biotite accounting for over 75% of the fluorine (Tong et al. 2023). Bhattacharjee and Nandy (2008) confirmed the occurrence of garnet biotite schist in the Tawang area. Therefore, the excessive fluoride content in the current study region may be due to biotite as well as other fluoride-containing minerals found in rocks and sediments. Other researchers have also reported a high concentration of fluoride in the current study location (Dutta and Gupta 2022; Gaur et al. 2022). The water quality index for the current investigation was observed in the range of 33.87 to 101.95, indicating “excellent to poor” and suitable for drinking (Fig. 3). However, the water might not be safe to drink due to the elevated levels of fluoride in the lakes. The possible health risks associated with pollutants, such as fluoride, are not specifically considered by the WQI although the fact that it provides a complete review of water quality. It is crucial to address and minimize the high fluoride levels to ensure the safety and acceptability of the water for consumption. Earlier investigations from several lakes revealed that they were polluted as a result of the increased impact of anthropogenic activity (Abbas et al. 2015; Gaury et al. 2018). Fluoride (F−) consumption causes fluorosis in about 66 million individuals in India, which can lead to several health problems such as joint and neck stiffness, enamel erosion in children’s teeth, spinal and limb abnormalities and crippling diseases (Ayoob and Gupta 2006). Aquatic life may also be harmed by high fluoride concentrations (Jha et al. 2011). The high concentration of F− can build up in fish bone, tissues and invertebrate exoskeletons, potentially posing harm to aquatic life in lakes (Gonzalo and Camargo 2012). It can also restrict invertebrate growth, disrupt egg hatching, impede fish growth, and cause breathing issues (Camargo 2003). As a result, the increasing fluoride concentration raises concerns about the safety of humans when consuming water for drinking or domestic reasons.
The Piper plot shows that the majority of the water points fall in field 1, indicating Ca2+-HCO3− type water (Fig. 4). Based on Kumar et al. (2019a), this dominance is explained by the greater levels of the HCO3− and Ca2+ characteristics that are present year round. A few points showed up in fields 3 and 4, demonstrating mixed water types of Ca2+-Mg2+-Cl− and Ca2+-Na2+-HCO3−. The cationic triangular plot shows that Ca2+ is the most abundant cation in the water samples. One of the causes of this water composition, as reported by Islam et al. (2017), is the occurrence of calcium and dissolved carbonate in the form of HCO3− entering the lake system as freshwater recharge. Several studies have observed similar types of water with alkaline earth metal dominance over alkali metals (Kumar et al. 2006; Singh et al. 2016; Gaury et al. 2018). Furthermore, it was revealed throughout the investigation that weak acid (HCO3−) dominated over strong acid (SO42−), which is consistent with findings from Chakrapani (2002) and Gaury et al. (2018) from Rewalsar Lake and other Himalayan lakes such as Nainital, Bhimtal, etc. The dominance of bicarbonate-type water in the anion plot indicates silicate weathering dominates in the studied area (Sulaiman et al. 2023). In Durov’s plot (Fig. 5), a considerable number of samples that fall in Field 5 demonstrate that processes like mineral dissolution or the blending of different water sources predominate in the lake water’s chemistry. Data points plotted in field 6 (Fig. 5) indicate a form of water that is uncommon and suggests mixing or unusual dissolving influences (Ravikumar et al. 2015). The outcome of the Durov plot is in line with previous literature from Thakur et al. (2018), Gaur et al. (2022), and Sulaiman et al. (2023), which observed the importance of recent freshwater recharge in controlling the lake water geochemistry characteristics. According to several studies (Kumar et al. 2006; Vishwakarma et al. 2018; Singh et al. 2020), the weathering of silicate minerals is the cause of the surplus HCO3− ions present in the study area. Chadha diagram (Fig. 6) depicts the existence of two main types of water, i.e., Ca2+-Mg2+-HCO3− and Ca2+-Mg2+-Cl−, which were plotted in fields 5 and 6. Temporary hardness may predominate in the studied lakes as maximum water samples exhibit soft water (TH < 60) characteristics. The current finding is similar to the previous study reported by Gaur et al. (2022) in the Tawang district of Arunachal Pradesh. Also, the sample points close to field 1 indicate that alkaline earth metals dominate over alkali metals; this finding was supported by earlier research (Kumar et al. 2009, 2020).
As per Gibbs’ diagram (Fig. 7), precipitation and rock dominance are the key drivers affecting the ion composition of these studied lakes. The greater solute concentrations seen in the high-altitude lakes may be ascribed to water filtering through rocky lithology and ongoing interaction between water and rocks. As a result, it may be inferred that the rocks in the lake’s surroundings have a significant impact on its water quality. Previous research in Eastern and Western Himalayan regions has provided supporting evidence for the role of precipitation (Deka et al. 2015, 2016b; Gaur et al. 2022) and rock weathering (Bhat et al. 2014; Gaury et al. 2018; Kumar et al. 2019a) in controlling the ionic composition of water bodies. Similar studies were also observed from Mansarovar Basin Lake, Tibet by Yao et al. (2015) and Alam et al. (2020) in the Chandel district of Manipur.
Using scatter plots, it has been observed that the studied lakes have a higher contribution of alkaline earth metals than alkali metals due to carbonate weathering (Fig. 8a), alkali metals versus total cations (Fig. 8b) and alkaline earth metals versus alkali metals (Fig. 8c). Similar trend was observed in different Himalayan lakes by various authors (Mora et al. 2017; Gaury et al. 2018; Kumar et al. 2019a). However, Gupta et al. (2012) and Gaur et al. (2022) have reported that there is weathering of silicate minerals from their respective study areas. Alkaline earth metals and bicarbonate are contrasted in a scatter plot in Fig. 8d, with bicarbonate having a larger concentration. This finding shows that alkaline earth metals, as well as the presence of alkali metals and silicate weathering, influence bicarbonate concentration (Prasad and Ramanathan 2005; Bhat et al. 2014; Gaury et al. 2018; Kumar et al. 2020). Furthermore, reverse cation exchange may contribute to the high concentrations of bicarbonate in the water system (Ebrahimi et al. 2015). These outcomes indicate that a combination of silicate weathering and reverse cation exchange mechanisms is likely to be involved in regulating the excess bicarbonate concentration found in the study. The plot of Ca2+ + Mg2+ versus SO42− + HCO3− (Fig. 8e) shows the values underneath and near the equiline. The values underneath the equiline are characterized by greater SO42− + HCO3− concentrations, which are caused by the weathering of silicate minerals combined with the ion exchange mechanism. The above results were found to be consistent with earlier studies conducted in the Penna-Chitravathi river basin and Godavari basin watershed by Reddy and Kumar (2010) and Marghade et al. (2021). Mora et al. (2017) and Gaury et al. (2018) noted in their study that the points along the equiline may be the result of gypsum, calcite, dolomite, and anhydrite dissolution in addition to carbonate weathering. It has been reported that the weathering of silicate minerals may be the dominant source of significant ions if the point is below the equiline (Lakshmanan et al. 2003; Elango and Kannan 2007). The fact that more than 90.00% of the locations in Fig. 8f are above the equiline indicates that calcite minerals are more influential in the studied lakes. On the other hand, a single point below the equiline indicates that dolomite and gypsum contribute significantly. Points that fall around the equiline, however, indicate that calcite, dolomite, and gypsum all contributed equally. Similar results were observed in previous studies from various Himalayan locales by Kumar et al. (2019a) and Kumar et al. (2020).
Based on the coefficient of determination (R2), there is a substantial relationship between Ca2+, Mg2+, and HCO3− (R2 = 0.93, 0.82, and 0.96, respectively) with TDS. These results lend credence to the idea that TDS in the lake water in the study area is significantly influenced by the dissolution of carbonate minerals (Abbas et al. 2015, 2018). The CAI values were found to be positive, indicating a direct base exchange process, with the surrounding rocks which serve as the most important contributors of dissolved solids in the water. In the groundwater of Anekal Taluk, Bangalore, similar results were reported by Ravikumar et al. (2011).
Conclusion
Sixty water samples were collected from six separate lakes in the Tawang district of Arunachal Pradesh and for better representation, 30 samples were selected for an analysis of a total of 18 physicochemical parameters to check their suitability for drinking and other domestic purposes. As per BIS permissible criteria, all physicochemical parameters were under the acceptable range except F−. WQI value from the present work was found to be in the range between 33.87 and 101.95, indicating ‘excellent to poor’ categories for drinking purposes. Lake 6 had excellent water quality and Lake 5 had the poorest of all the studied lakes. However, the water might not be safe to drink due to the elevated levels of fluoride in the lakes. It is crucial to address and minimize the high fluoride levels to ensure the safety and acceptability of the water for human consumption. The major hydrogeochemical facie noticed in the water samples, as indicated by the Piper plot, is Ca2+-HCO3− type. As per Gibbs’ diagram, precipitation and rock dominance are the key drivers affecting the ion composition of these selected lakes. Chadha’s diagram revealed that simple dissolution and mixing, driven by recent freshwater inflows, are the main sources influencing the chemistry of lake water. Using scatter plots, it has been observed that the studied lakes have a higher contribution of alkaline earth metals than alkali metals due to carbonate weathering. CAI indices yielded positive results indicating the existence of direct base exchange. These results on water quality may help policy makers to create effective regulations and management strategies to protect these pristine ecosystems. Further research on water quality fosters a deeper understanding of high altitude ecosystems, aiding in scientific advancements and better management practices. Therefore, it is crucial to monitor the water quality in these valuable natural assets, especially in light of the global climate change, growing population, and an increase in tourists visiting these HALs of Tawang district, Arunachal Pradesh, India.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
Abbas Z, Su C, Tahira F, Mapoma HWT, Aziz SZ (2015) Quality and hydrochemistry of groundwater used for drinking in Lahore, Pakistan: analysis of source and distributed groundwater. Environ Earth Sci 74(5):4281–4294. https://doi.org/10.1007/s12665-015-4432-5
Abbas Z, Mapoma HWT, Su C, Aziz SZ, Ma Y, Abbas N (2018) Spatial analysis of groundwater suitability for drinking and irrigation in Lahore Pakistan. Environ Monit Assess 190(7):391. https://doi.org/10.1007/s10661-018-6775-3
Akhtar N, Ishak MIS, Bhawani SA, Umar K (2021) Various natural and anthropogenic factors responsible for water quality degradation: a review. Water 13(19):2660. https://doi.org/10.3390/w13192660
Alam W, Singh KS, Gyanendra Y, Laishram RJ, Nesa N (2020) Hydrogeochemical assessment of groundwater quality for few habitations of Chandel district, Manipur (India). Appl Water Sci 10:123. https://doi.org/10.1007/s13201-020-01208-0
APHA (2005) Standards methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36(6):433–487. https://doi.org/10.1080/10643380600678112
Barik R, Pattanayak SK (2019) Assessment of groundwater quality for irrigation of green spaces in the Rourkela city of Odisha, India. Groundw Sustain Dev 8:428–438. https://doi.org/10.1016/j.gsd.2019.01.005
Bhat NA, Jeelani G, Bhat MY (2014) Hydrogeochemical assessment of groundwater in karst environments, Bringi watershed, Kashmir Himalayas, India. Curr Sci 106(7):1000–1007
Bhateria R, Jain D (2016) Water quality assessment of lake water: a review. Sustain Water Resour Manag 2(2):161–173. https://doi.org/10.1007/s40899-015-0014-7
Bhattacharjee S, Nandy S (2008) Geology of the Western Arunachal Himalaya in parts of Tawang and West Kameng districts Arunachal Pradesh. J Geol Soc India 73(4):589–590. https://doi.org/10.1007/s12594-009-0043-7
BIS (2012) Indian standard drinking water-specification IS 10500:2012. 2nd Revision. Bureau of Indian Standards, New Delhi, India. https://bis.gov.in/wp-content/uploads/2020/10/PM-IS-10500.pdf. Accessed 12 Dec 2023
Boyle DR, Chagnon M (1995) An incidence of skeletal fluorosis associated with groundwaters of the maritime carboniferous basin, Gaspé region, Quebec Canada. Environ Geochem Health 17(1):5–12. https://doi.org/10.1007/BF00188625
Briggs JC, Ficke JF (1977) Quality of rivers of United States, 1975 water year-based on the National Stream Quality Accounting Network (NASQAN). Open File Report 78–200. US Geological Survey, USA. https://doi.org/10.3133/ofr78200
Burpee BT, Saros JE (2020) Cross-ecosystem nutrient subsidies in arctic and alpine lakes: implications of global change for remote lakes. Environ Sci Process Impacts 22:1166–1189. https://doi.org/10.1039/C9EM00528E
Camargo JA (2003) Fluoride toxicity to aquatic organisms: a review. Chemosphere 50(3):251–264. https://doi.org/10.1016/S0045-6535(02)00498-8
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7(5):431–439. https://doi.org/10.1007/s100400050216
Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, Kim K, Koh YK (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385(1–3):272–283. https://doi.org/10.1016/j.scitotenv.2007.06.038
Chakrapani GJ (2002) Water and sediment geochemistry of major Kumaun Himalayan lakes India. Environ Geol 43(1–2):99–107. https://doi.org/10.1007/s00254-002-0613-0
Deka JP, Tayeng G, Singh S, Hoque RR, Prakash A, Kumar M (2015) Source and seasonal variation in the major ion chemistry of two eastern Himalayan high altitude lakes India. Arab J Geosci 8(12):10597–10610. https://doi.org/10.1007/s12517-015-1964-7
Deka JP, Singh S, Jha PK, Singh UK, Kumar M (2016a) Imprints of long-range-transported pollution on high-altitude Eastern Himalayan lake water chemistry. Environ Earth Sci 75(4):285. https://doi.org/10.1007/s12665-015-4813-9
Deka JP, Yamanaka T, Singh S, Choudhry R, Tyang G, Kumar M (2016b) Tracing the imprints of climate change through hydrogeochemical studies on the Eastern Himalayan high altitude lakes. J Clim Chang 2(1):69–78. https://doi.org/10.3233/JCC-160008
Dey S, Raju NJ, Gossel W, Mall RK (2023) Hydrogeochemical characterization and geochemical modeling for the evaluation of groundwater quality and health risk assessment in the Varuna river basin India. Environ Geochem Health 45(7):4679–4702. https://doi.org/10.1007/s10653-023-01521-y
Drever JI (1997) The geochemistry of natural water: Surface and groundwater environments. Prentice Hall, New Jersey, USA
Durov SA (1948) Natural waters and graphical representation of their composition. Dokl Akad Nauk SSSR 59:87–90
Dutta A, Gupta RK (2022) Geochemistry and utilization of water from thermal springs of Tawang and West Kameng districts Arunachal Pradesh. J Geol Soc India 98(2):237–244. https://doi.org/10.1007/s12594-022-1964-7
Ebrahimi M, Nematollahi MJ, Moradian A, Adineh S, Esmaeili R (2015) Surface water quality assessment in Gilan Province Iran. J Bio Env Sci 6(5):269–280
Elango L, Kannan R (2007) Rock-water interaction and its control on chemical composition of groundwater. In: Sarkar D, Datta R, Hannigan R (eds) Concepts and Applications in Environmental Geochemistry. Dev Environ Sci 5:229–243. https://doi.org/10.1016/S1474-8177(07)05011-5
Feng F, Jia Y, Yang Y, Huan H, Lian X, Xu X, Xia F, Han F, Jiang Y (2020) Hydrogeochemical and statistical analysis of high fluoride groundwater in northern China. Environ Sci Pollut Res 27(28):34840–34861. https://doi.org/10.1007/s11356-020-09784-z
Finger D, Wueest A, Bossard P (2013) Effects of oligotrophication on primary production in peri-alpine lakes. Water Resour Res 49(8):4700–4710. https://doi.org/10.1002/wrcr.20355
Floyd FJ, Widaman KF (1995) Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess 7(3):286–299. https://doi.org/10.1037/1040-3590.7.3.286
Gaur N, Sarkar A, Dutta D, Gogoi BJ, Dubey R, Dwivedi SK (2022) Evaluation of water quality index and geochemical characteristics of surface water from Tawang India. Sci Rep 12:11698. https://doi.org/10.1038/s41598-022-14760-3
Gaury PK, Meena NK, Mahajan AK (2018) Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh India. Environ Monit Assess 190(2):84. https://doi.org/10.1007/s10661-017-6451-z
Gaus I (2010) Role and impact of CO2–rock interactions during CO2 storage in sedimentary rocks. Int J Greenh Gas Control 4(1):73–89. https://doi.org/10.1016/j.ijggc.2009.09.015
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170(3962):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Gonzalo C, Camargo JA (2012) Fluoride bioaccumulation in the signal crayfish Pacifastacus leniusculus (Dana) as suitable bioindicator of fluoride pollution in freshwater ecosystems. Ecol Indic 20:244–251. https://doi.org/10.1016/j.ecolind.2011.12.019
Gudasz C, Bastviken D, Steger K, Premke K, Sobek S, Tranvik LJ (2010) Temperature-controlled organic carbon mineralization in lake sediments. Nature 466(7305):478–481. https://doi.org/10.1038/nature09186
Güler C, Thyne GD, McCray JE, Turner KA (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10(4):455–474. https://doi.org/10.1007/s10040-002-0196-6
Guo C, Chen Y, Xia W, Qu X, Yuan H, Xie S, Lin LS (2020) Eutrophication and heavy metal pollution patterns in the water suppling lakes of China’s south-to-north water diversion project. Sci Total Environ 711:134543. https://doi.org/10.1016/j.scitotenv.2019.134543
Gupta S, Nayek S, Saha RN (2012) Major ion chemistry and metal distribution in coal mine pit lake contaminated with industrial effluents: constraints of weathering and anthropogenic inputs. Environ Earth Sci 67(7):2053–2061. https://doi.org/10.1007/s12665-012-1644-9
Hairston Jr NG, Fussmann FG (2014) Lake ecosystems. In: eLS. John Wiley & Sons, Ltd, Chichester. https://doi.org/10.1002/9780470015902.a0003191.pub2
Hundey EJ, Russell SD, Longstaffe FJ, Moser KA (2016) Agriculture causes nitrate fertilization of remote alpine lakes. Nat Commun 7:10571. https://doi.org/10.1038/ncomms10571
ISFR (2021) India State Forest Report 2021. Forest Survey of India. Ministry of Environment, Forest and Climate Change, Government of India, Dehradun, India. https://fsi.nic.in/forest-report. Accessed 3 June 2023
Islam SMDU, Majumder RK, Uddin MJ, Khalil MI, Alam MF (2017) Hydrochemical characteristics and quality assessment of groundwater in Patuakhali district, Southern coastal region of Bangladesh. Expo Health 9(1):43–60. https://doi.org/10.1007/s12403-016-0221-y
Jha SK, Mishra VK, Sharma DK, Damodaran T (2011) Fluoride in the environment and its metabolism in humans. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, vol 211. Springer, New York, pp 121–142. https://doi.org/10.1007/978-1-4419-8011-3_4
Jimenez L, Ruhland KM, Jeziorski A, Smol JP, Perez-Martinez C (2018) Climate change and Saharan dust drive recent cladoceran and primary production changes in remote alpine lakes of Sierra Nevada Spain. Glob Chang Biol 24(1):e139–e158. https://doi.org/10.1111/gcb.13878
Judran NH, Kumar A (2020) Evaluation of water quality of Al-Gharraf river using the water quality index (WQI). Model Earth Syst Environ 6(3):1581–1588. https://doi.org/10.1007/s40808-020-00775-0
Kanakiya RS, Singh SK, Sharma JN (2014) Determining the water quality index of an urban water body Dal Lake, Kashmir, India. IOSR J Environ Sci Toxic Food Tech 8(12):64–71
Kant N, Singh PK, Kumar B (2018) Hydrogeochemical characterization and groundwater quality of Jamshedpur urban agglomeration in Precambrian Terrain Eastern India. J Geol Soc India 92(1):67–75. https://doi.org/10.1007/s12594-018-0954-2
Khadka UR, Ramanathan AL (2013) Major ion composition and seasonal variation in the lesser Himalayan lake: case of Begnas Lake of the Pokhara valley Nepal. Arab J Geosci 6(11):4191–4206. https://doi.org/10.1007/s12517-012-0677-4
Kim J, Mueller CW (1978) Factor analysis: statistical methods and practical issues. Sage Publications, CA, USA
Krishan G, Bhagwat A, Sejwal P, Yadav BK, Kansal ML, Bradley A, Singh S, Kumar M, Sharma LM, Muste M (2023) Assessment of groundwater salinity using principal component analysis (PCA): a case study from Mewat (Nuh), Haryana India. Environ Monit Assess 195(1):37. https://doi.org/10.1007/s10661-022-10555-1
Kumar V, Rai SP, Singh O (2006) Water quantity and quality of Mansar Lake located in Himalayan foothills India. Lake Reservoir Manag 22(3):191–198. https://doi.org/10.1080/07438140609353896
Kumar M, Kumari K, Singh UK, Ramanathan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57(4):873–884. https://doi.org/10.1007/s00254-008-1367-0
Kumar P, Meena NK, Mahajan AK (2019a) Major ion chemistry, catchment weathering and water quality of Renuka Lake, north-west Himalaya India. Environ Earth Sci 78(10):319. https://doi.org/10.1007/s12665-019-8315-z
Kumar P, Meena NK, Diwate P, Mahajan AK, Bhushan R (2019b) The heavy metal contamination history during ca 1839–2003 AD from Renuka Lake of lesser Himalaya, Himachal Pradesh India. Environ Earth Sci 78(17):549. https://doi.org/10.1007/s12665-019-8519-2
Kumar P, Mahajan AK, Kumar A (2020) Groundwater geochemical facie: implications of rock-water interaction at the Chamba city (HP), northwest Himalaya India. Environ Sci Pollut Res 27(9):9012–9026. https://doi.org/10.1007/s11356-019-07078-7
Kumar R, Manzoor S, Vishwakarma DK, Al-Ansari N, Kushwaha NL, Elbeltagi A, Sushanth K, Prasad V, Kuriqi A (2022) Assessment of climate change impact on snowmelt runoff in Himalayan region. Sustainability 14(3):1150. https://doi.org/10.3390/su14031150
Kumar R, Pippal PS, Kumar R, Kumar P, Singh A, Sharma P (2023) The global scenario of hydrogeochemical research on glacier meltwater: a bibliometric and visualization analysis. Environ Sci Pollut Res 30(30):74612–74627. https://doi.org/10.1007/s11356-023-27642-6
Lakshmanan E, Kannan R, Kumar MS (2003) Major ion chemistry and identification of hydrogeochemical processes of ground water in a part of Kancheepuram district, Tamil Nadu India. Environ Geosci 10(4):157–166. https://doi.org/10.1306/eg.0820303011
Li S, Zhang Q (2008) Geochemistry of the upper Han river basin, China, 1: spatial distribution of major ion compositions and their controlling factors. Appl Geochem 23(12):3535–3544. https://doi.org/10.1016/j.chemgeo.2009.02.021
Liu CW, Lin KH, Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Tot Environ 313(1–3):77–89. https://doi.org/10.1016/S0048-9697(02)00683-6
Lloyd JW, Heathcote J (1985) Natural inorganic hydrochemistry in relation to groundwater. Oxford University Press, New York, USA
Machate O, Schmeller DS, Schulze T, Brack W (2023) Mountain lakes as freshwater resources at risk from chemical pollution. Environ Sci Eur 35(3). https://doi.org/10.1186/s12302-022-00710-3
Marghade D, Malpe DB, Duraisamy K, Patil PD, Li P (2021) Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra Central India. Environ Sci Pollut Res 28(15):18471–18494. https://doi.org/10.1007/s11356-020-10032-7
Meena NK, Prakasam M, Bhushan R, Sarkar S, Diwate P, Banerji U (2017) Last-five-decade heavy metal pollution records from the Rewalsar Lake, Himachal Pradesh India. Environ Earth Sci 76(1):39. https://doi.org/10.1007/s12665-016-6303-0
Mishra DK (2007) Evidence of neotectonic activity along active faults in Arunachal Himalay NE India. Himalayan Geol 28(2):75–78
Mora A, Mahlknecht J, Rosales-Lagarde L, Hernández-Antonio A (2017) Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León Mexico. Environ Monit Assess 189(8):394. https://doi.org/10.1007/s10661-017-6096-y
Mukherji A, Molden D, Nepal S, Rasul G, Wagnon P (2015) Himalayan waters at the crossroads: issues and challenges. Int J Water Resour Dev 31(2):151–160. https://doi.org/10.1080/07900627.2015.1040871
Nimasow G, Nimasow OD, Rawat JS, Tsering G, Litin T (2016) Remote sensing and GIS-based suitability modeling of medicinal plant (Taxus baccata Linn.) in Tawang district, Arunachal Pradesh. India. Curr Sci 110(2):219–227
Nordstrom DK (2011) Hydrogeochemical processes governing the origin, transport and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl Geochem 26(11):1777–1791. https://doi.org/10.1016/j.apgeochem.2011.06.002
Panigrahy S, Murthy TVR, Patel JG, Singh TS (2012) Wetlands of India: inventory and assessment at 1:50,000 scale using geospatial techniques. Curr Sci 102(6):852–856
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans Am Geophys Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Prasad MBK, Ramanathan AL (2005) Solute sources and processes in the Achankovil river basin, Western Ghats Southern India. Hydrol Sci J 50(2):341–354. https://doi.org/10.1623/hysj.50.2.341.61798
Prusty P, Farooq SH, Zimik HV, Barik SS (2018) Assessment of the factors controlling groundwater quality in a coastal aquifer adjacent to the Bay of Bengal India. Environ Earth Sci 77(22):762. https://doi.org/10.1007/s12665-018-7943-z
Ramanathan AL (2007) Seasonal variation in the major ion chemistry of Pandoh Lake, Mandi district, Himachal Pradesh India. Appl Geochem 22(8):1736–1747. https://doi.org/10.1016/j.apgeochem.2007.03.045
Rao NS (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh India. Hydrol Sci J 48(5):835–847. https://doi.org/10.1623/hysj.48.5.835.51449
Rasul G (2014) Food, water, and energy security in South Asia: a nexus perspective from the Hindu Kush Himalayan region. Environ Sci Policy 39:35–48. https://doi.org/10.1016/j.envsci.2014.01.010
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2011) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Bangalore urban district, Karnataka India. Environ Monit Assess 179(1–4):93–112. https://doi.org/10.1007/s10661-010-1721-z
Ravikumar P, Mehmood MA, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey tank and Mallathahalli Lake, Bangalore urban district, Karnataka India. Appl Water Sci 3(1):247–261. https://doi.org/10.1007/s13201-013-0077-2
Ravikumar P, Somashekar RK, Prakash KL (2015) A comparative study on usage of Durov and Piper diagrams to interpret hydrochemical processes in groundwater from SRLIS river basin, Karnataka, India. Elixir Earth Sci 80:31073–31077
Reddy AGS, Kumar KN (2010) Identification of the hydrogeochemical processes in groundwater using major ion chemistry: a case study of Penna-Chitravathi river basins in Southern India. Environ Monit Assess 170(1–4):365–382. https://doi.org/10.1007/s10661-009-1239-4
Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, Linton B (2002) Sources, pathways, and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health Part A 65(1):1–142. https://doi.org/10.1080/152873902753338572
Saini RK, Swain S, Patra A, Khanday GJ, Gupta H, Purushothaman P, Chakrapani GJ (2008) Water chemistry of three Himalayan Lakes: Dal (Jammu & Kashmir), Khajjiar (Himachal Pradesh) and Nainital (Uttarakhand). Himalayan Geol 29(1):63–72
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Schoeller H (ed) Methods and Techniques of Groundwater Investigation and Development. UNESCO, Paris, France, pp 54–83
Sharma A, Singh AK, Kumar K (2012) Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River basin, western India. Environ Earth Sci 65(4):1231–1250. https://doi.org/10.1007/s12665-011-1371-7
Sheikh JA, Jeelani G, Gavali RS, Shah RA (2014) Weathering and anthropogenic influences on the water and sediment chemistry of Wular Lake Kashmir Himalaya. Environ Earth Sci 71(6):2837–2846. https://doi.org/10.1007/s12665-013-2661-z
Singh CD, Kohli A, Kumar P (2014) Comparison of results of BIS and GSI guidelines on macrolevel landslide hazard zonation - a case study along highway from Bhalukpong to Bomdila, West Kameng district Arunachal Pradesh. J Geol Soc India 83(6):688–696. https://doi.org/10.1007/s12594-014-0101-7
Singh VB, Ramanathan AL, Mandal A (2016) Hydrogeochemistry of high-altitude lake: a case study of the Chandra Tal, Western Himalaya India. Arab J Geosci 9(4):308. https://doi.org/10.1007/s12517-016-2358-1
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) Multivariate analysis and geochemical signatures of groundwater in the agricultural dominated taluks of Jalandhar district Punjab India. J Geochem Explor 208:106395. https://doi.org/10.1016/j.gexplo.2019.106395
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568. https://doi.org/10.1016/S0883-2927(02)00018-5
Srivastava HB, Srivastava V, Srivastava RK, Singh CK (2011) Structural analyses of the crystalline rocks between Dirang and Tawang, West Kameng district Arunachal Himalaya. J Geol Soc India 78(1):45–56. https://doi.org/10.1007/s12594-011-0066-8
Sulaiman MA, Zafar MM, Prabhakar R, Kumar R, Sinha RK, Kumari A (2023) A multivariate statistical approach to evaluate the hydro-geochemistry of groundwater quality in the middle Ganga river basin. Acta Geophys, Patna, India. https://doi.org/10.1007/s11600-023-01071-y
Thakur N, Rishi M, Sharma DA, Keesari T (2018) Quality of water resources in Kullu Valley in Himachal Himalayas, India: perspective and prognosis. Appl Water Sci 8:20. https://doi.org/10.1007/s13201-018-0668-z
Thies H, Nickus U, Mair V, Tessadri R, Tait D, Thaler B, Psenner R (2007) Unexpected response of high alpine lake waters to climate warming. Environ Sci Technol 41(21):7424–7429. https://doi.org/10.1021/es0708060
Tong L, Liu X, Liu Y, Zhou K, Zhang S, Jia Q, Lu W, Huang Y, Ni G (2023) Accumulation of high concentration fluoride in the Ulungur Lake water through weathering of fluoride containing rocks in Xinjiang China. Environ Pollut 323:121300. https://doi.org/10.1016/j.envpol.2023.121300
Tripathi M, Singal SK (2019) Use of principal component analysis for parameter selection for development of a novel water quality index: a case study of river Ganga India. Ecol Indic 96(1):430–436. https://doi.org/10.1016/j.ecolind.2018.09.025
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Res 1(3):34–38. https://doi.org/10.12691/ajwr-1-3-3
Uddin MG, Nash S, Olbert AI (2021) A review of water quality index models and their use for assessing surface water quality. Ecol Indic 122:107218. https://doi.org/10.1016/j.ecolind.2020.107218
Upadhyay J, Chakraborty R, Medhi K (2016) Ecological and socio-cultural assessment of the high altitude wetland: A case study of the Bhagajang Wetland Complex in Western Arunachal Pradesh, India. Int Conf Ecosyst Serv Wetl-Ardrabhum 2016:57–62
Vishwakarma CA, Sen R, Singh N, Singh P, Rena V, Rina K, Mukherjee S (2018) Geochemical characterization and controlling factors of chemical composition of spring water in a part of Eastern Himalaya. J Geol Soc India 92(6):753–763. https://doi.org/10.1007/s12594-018-1098-0
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, San Diego, USA
Wu Z, Wang X, Chen Y, Cai Y, Deng J (2018) Assessing river water quality using water quality index in Lake Taihu basin, China. Sci Total Environ 612:914–922. https://doi.org/10.1016/j.scitotenv.2017.08.293
Xu J, Grumbine E, Shrestha A, Eriksson M, Yang X, Wang Y, Wilkes A (2009) The melting Himalayas: cascading effects of climate change on water, biodiversity, and livelihoods. Conserv Biol 23(3):520–530. https://doi.org/10.1111/j.1523-1739.2009.01237.x
Yang R, Yao T, Xu B, Jiang G, Xin X (2007) Accumulation features of organochlorine pesticides and heavy metals in fish from high mountain lakes and Lhasa river in the Tibetan Plateau. Environ Int 33(2):151–156. https://doi.org/10.1016/j.envint.2006.08.008
Yao Z, Wang R, Liu Z, Wu S, Jiang L (2015) Spatial-temporal patterns of major ion chemistry and its controlling factors in the Manasarovar basin Tibet. J Geogr Sci 25(6):687–700. https://doi.org/10.1007/s11442-015-1196-5
Zhu L, Ju J, Wang Y, Xie M, Wang J, Peng P, Zhen X, Lin X (2010) Composition, spatial distribution, and environmental significance of water ions in Pumayum Co catchment, southern Tibet. J Geog Sci 20(1):109–120. https://doi.org/10.1007/s11442-010-0109-x
Zindros A, Radoglou K, Milios E, Kitikidou K (2020) Tree line shift in the Olympus mountain (Greece) and climate change. Forests 11(9):985. https://doi.org/10.3390/f11090985
Acknowledgements
We are thankful to the Director of NERIST and Head of the Department of Forestry for providing the laboratory facilities and Department of Environment, Forest and Climate Change, Government of Arunachal Pradesh for permission and field support. The authors would like to acknowledge Dr. Wazir Alam, Assistant Professor, Department of Forestry and Environmental Science, Manipur University and research scholars for providing lab facilities and assistance with the analysis of the samples. The authors are also thankful to the local field guides for their help and support during the field study.
Funding
This research was funded by the Department of Science and Technology (DST/CCP/HICAB/SN-AP/160/2018(C) and (DST/CCP/HICAB/SN-AP/160/2018(G)), New Delhi, Government of India.
Author information
Authors and Affiliations
Contributions
Aribam Rocky Sharma: field work and data collection, conceptualization, methodology, analysis, interpretation, writing of original draft; Reetashree Bordoloi: field work and data collection, conceptualization, review and editing; Ashish Paul: conceptualization, interpretation, review and editing; Yumnam Gyanendra: conceptualization, methodology, analysis and review; Om Prakash Tripathi: conceptualization, interpretation, review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. This manuscript is the author’s original work, which has not been previously published elsewhere. The manuscript is not currently being considered for publication elsewhere. The manuscript reflects the author’s research and analysis truthfully and completely.
Consent to participate
No participants involved in the present research work.
Consent for publication
All the authors have participated and agreed to publish on the submission of this manuscript to your esteemed journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A.R., Bordoloi, R., Paul, A. et al. Water quality and geochemical facie of high-altitude lakes in Tawang, Eastern Himalaya, India. Environ Sci Pollut Res 31, 24492–24511 (2024). https://doi.org/10.1007/s11356-024-32712-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32712-4