Abstract
The study of groundwater samples from Patna, which is in the middle Ganga River basin in the state of Bihar, was done to evaluate the hydrogeochemical nature of groundwater and figure out the controlling factors that affect its ionic chemistry. This study’s groundwater samples were taken seasonally from 20 Patna, Bihar locations from 2019 to 2020 and analyzed for their physical and chemical properties using APHA’s standard methods. Multivariate statistical techniques, such as Correlation matrix, Principal Component Analysis and Hierarchical cluster analysis, were carried out on the analytical variables, followed by interpreting the groundwater’s geochemical nature using Durov Plot, Piper plot, ion-scatter plots and Gibb’s diagram. The results obtained were compared with water quality standards prescribed by the Bureau of Indian Standards. The study revealed that groundwater’s alkaline nature and water’s total hardness varied from hard to very hard categories. Total dissolved solids range from 158 to 575 mg/l, where 31.66% of samples have exceeded the desirable TDS limit for drinking water. The abundance of anions was observed in decreasing order (HCO3− > Cl− > SO42−) during all seasons and cations as Ca2+ > Na+ > Mg2+ > K+ in pre-monsoon and post-monsoon, whereas in monsoon season, it was observed as Mg2+ > Na+ > Ca2+ > K+. Ca2+–Mg2+–HCO3− type water was revealed as the significant hydrogeochemical facie, and the reverse ion exchange process was observed to be the governing factor in 77% of the total groundwater samples. Gibbs’s diagram showed rock–water dominance as a major factor in groundwater ionic composition during all seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the most important natural resource of fresh water and is critical for living organisms’ survival (Hui et al. 2020; Sharma and Sharma 2022; Olatunde et al. 2022). Globally about 2.5 billion people depend on it for domestic, agricultural, and industrial purposes (Khan and Jhariya 2017; Gao et al. 2020; Nizam et al. 2022). Several researchers reported that Groundwater quality is deteriorating with the passing decades due to rapid industrialization, increasing population, overexploitation, excessive use of fertilizers and pesticides in intensive agriculture practices, and mixing of untreated municipal and industrial effluents (Liu et al. 2019; Bulut et al. 2020; Kom et al. 2022; Makubura et al. 2022). Besides these, groundwater chemistry is also influenced by the change in lithological characteristics, climatic conditions, general geology, rock–water interaction, quality of recharge water, time of retention of water in the hosted aquifer, and the flow directions of water under the ground (Varol and Davraz 2015; Ahmed and Clark 2016; Eang et al. 2018; Sulaiman et al. 2023; Tiwari et al. 2017). Groundwater in arid and semi-arid regions is more vulnerable to contamination because of changes in the aquifer’s mineral weathering process and uneven resource distribution (Hui et al. 2020). Patna, the state capital and the most populated city of Bihar, is experiencing continuous deterioration in groundwater quality. According to data from the Master plan of Patna (2016), water supply within Patna urban area is from 85 deep tube wells dug in different parts of the city and from deep aquifer pumping by private households. Though, poor and old water supply networks, poor sanitation, improper effluent disposal facilities, and poor sewerage system within Patna pose potential threats to the quality of groundwater reserves. Therefore, knowledge of the study area’s groundwater chemistry can be necessary for determining its use for various purposes. However, extensive groundwater quality assessment becomes difficult as the water quality depends on numerous physicochemical parameters and other controlling factors. Thus, assessing groundwater quality with hydro-geochemical properties over space and time can be a vital tool for solving problems and planning a proper management strategy for groundwater, as it will help us to figure out the mechanism of interaction between groundwater and the environment (Panigrahy et al. 1996; Atwia et al. 1997; Ballukraya and Ravi 1999; Ramappa and Suresh 2000). Different graphical techniques (e.g., Piper, Durov) and bivariate ion scatter plots have been developed to visually understand hydrogeochemical data to show water classification, sources of water solutes, dissimilarities of water composition and quality (Gaikwad et al. 2020). Multivariate statistical analysis and analytical procedures such as principal component analysis (PCA) and hierarchical cluster analysis were also utilized to comprehend and explain the complicated water quality system. Several researchers have agreed with the advantages of using these techniques in concise, large, complex sets of analytical data to relatively simple interpretable numbers (Maliqi et al. 2020; Kadam et al. 2021; Kumar et al. 2023). Singh et al. (2009) reported the fitness of PCA and HCA in water quality assessment, evaluation of the current ecological status of the aquifer system, and identification of the potential source of contamination in the groundwater. Although several researchers have conducted studies on groundwater quality in various districts of the Middle Gangetic Plains, India (Srinivasamoorthy et al. 2011; Prasanna et al. 2011; Kumar et al. 2018; Singh et al. 2015; Shukla and Saxena 2020; Maurya et al. 2020), and in Patna (Rai et al. 2011; Sinha et al. 2011; Sukumaran et al. 2015; Zafar et al. 2022), the literature review revealed that little work had been done on the hydro-geochemical characterization of groundwater of Patna. Hence, the study attempts to identify hydrogeochemical processes in groundwater in Patna to examine the dominant factors responsible for determining the chemical composition of groundwater. The results of this study will contribute to improving the understanding of the factors controlling groundwater quality in Patna, will provide a baseline study in this direction for the study area, and can also help identify suitable water sources for the intended use and proper use management of groundwater resources.
Study area
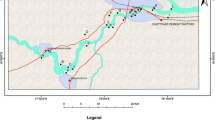
The study was conducted in Patna, India’s capital and largest city, Bihar. Patna is situated in the middle Ganga River basin on the northern edge of the south bank of River Ganga, 47 m above mean sea level between latitude 25°13' N–25°45' N and longitude 84°43' E–86°44' E, covering an area of 3172 Km2 (Fig. 1). The study area has a monotonous flat topography. It is laid down by the alluvial sediments of River Ganga and northerly flowing streams of the Precambrian period, deposited unconformably on the Archaean basement (Sandhu et al. 2011; USGS 2022). The majority of land in Patna is under residential usage (47.5%) (Patna Master Plan 2031 2016). Agriculture (17.66%), public and semi-public (10.18%), transportation (5.90%), and commercial use (4.46%) are some of the other contributors (Zafar et al. 2022). According to the Census of India (2011), the population of Patna is 2,049,156, of which 1,087,285 are males and 961,871 are females. Groundwater aquifer of the Middle Gangetic Plain is characterized as unconfined or semi-confined sandy sediments that move through the joints, fissures, and other associated rocks of the Pre-Cambrian age like granites gneisses, mica-schist and quartzite (CGWB 2013; Kumar et al. 2019a). The climate is subtropical and is characterized by hot summers with an average temperature of 32.4 °C and relatively cold winters with an average temperature of 17.2 °C. The average annual rainfall is 1076 mm, with substantial rain falling in July and August. Relative humidity can reach 100% during summer (Sulaiman et al. 2021). River Ganga drains the city in the north, Sone in the west, and the central part of the city is drained by Punpun, Phalgu and their tributaries (CGWB 2013).
Materials and methods
Water sample collection and chemical analysis
The study adopted a systematic random sampling method to collect groundwater samples at a regular interval of ~ 3 km from one site to another. Groundwater samples were collected seasonally in pre-monsoon with the onset of the summer season (March–April), near the end of the monsoon (August–September) and in the post-monsoon month of October–November. The samples were collected from 250 to 300 ft. deep boreholes located at 20 different sites in the study area during 2019–2020. Water samples were collected in one-litre acid-washed, well-rinsed high-density polyethylene (HDPE) bottles and filtered through 0.45 µm Millipore membrane filter paper. The samples were transported to the laboratory in an ice box. The methods given by APHA (2012) were employed in the chemical analysis of samples, and the result obtained was compared with water quality standards prescribed by BIS and WHO. A Portable field kit was used to measure pH, Electrical conductivity (EC) and Total alkalinity (TA) at the sampling sites. The EDTA titrimetric method measured Ca2+ and Mg2+ ions in the groundwater samples, while the Argentometric and Turbidimetric methods measured Cl− and SO42− ions concentration. Na+ and K+ ions were estimated using Flame Photometer. Each parameter has been analyzed in duplicates, with fresh sample blanks and standards prepared during sample analysis as the quality control of analytical data. Interpretation of the geochemical nature of the groundwater has been carried out using the Piper plot (1944), Durov plot (1948), Gibbs diagram (1970) and Scatter plot diagrams. Piper and Durov plots were constructed using Grapher (version 14.0) software, while an ion Scatter plot was prepared using SPSS (version 22.0) software. Further, the analytical data has been subjected to descriptive statistical analysis, including correlation matrix, principal component analysis, and cluster analysis using XLSTAT (version 14.0) and SPSS (version 22.0).
Result and discussion
Hydro-geochemistry of groundwater
Groundwater samples were analyzed, and the value of physicochemical parameters obtained seasonally, including statistical measures such as minimum, maximum, mean and standard deviation, are presented in Table 1. pH of the groundwater samples varied from 7.1 to 8.1 with an average of 7.3 ± 0.24, indicating a slightly alkaline to alkaline nature of water samples. Electrical conductivity was measured from 300 to 1700 µS/cm, with an average value of 727.5 ± 296.8 µS/cm. The majority of water samples were found to be more than the BIS (2012) prescribed desirable limit (750 µS/cm) for drinking water’s electrical conductivity (Table 1).
The higher value of EC in the groundwater might be due to the increased dissolved solids and ions in water (Al Dahaan et al. 2016; Gebresilasie et al. 2021; Zafar et al. 2022). The concentration of TDS ranged from 236 to 1195 mg/l, with an average value of 462.7 ± 231.8 mg/l, indicating the presence of a higher concentration of dissolved salt (Gulgundi and Shetty 2018), and this is also in agreement with higher value of EC. A slight increase in the value of EC and TDS was observed during the end of monsoon and post-monsoon, indicating the dissolution of salts with rainwater infiltrations with local anthropogenic influence (Vidana et al. 2012; Kupwade and Langade 2013; Wagh et al. 2019). The total hardness of groundwater varied from hard to very hard, indicating a high concentration of calcium and magnesium salts (Kumar et al. 2015). The abundance of cations Ca2+, Na+, Mg2+ and K+ was found in decreasing order (Fig. 2), and mean concentration ranged from 39.67 to 88.95 mg/l, 58.24–83.45 mg/l, 18.45–34.72 mg/l and 5.11–14.92 mg/l for Ca2+, Na+, Mg2+ and K+ respectively.
The results suggest that Ca2+ + Mg2+ contributes 63.56% of the total cationic budget, followed by Na+ and K+ with 33.76% and 2.67%, respectively. Ca2+ was dominant among cations because of the dominance of calcium-rich minerals in the earth’s crust and its higher solubility (Ali and Ali 2018). An unusual increase in the concentration of Na+ and K+ was observed during the post-monsoon season at site S20 (Fig. 2) that even exceeded the maximum permissible limit set by BIS (2012), which might be due to the seepage of sewage and domestic wastes into the groundwater. The concentration of anions HCO3−, Cl− and SO42− ranged between 228 and 456 mg/l, 7–169 mg/l and 1–113.8 mg/l respectively. In addition, HCO3− contributes 71.16% of the total anionic budget, followed by Cl− and SO42− with 20.17% and 8.65%, respectively. The concentration of Cl− ion in all water samples of the study area was within the desirable limits prescribed by BIS (2012). However, three samples (S3, S4 and S20) showed relatively higher values throughout the year, possibly due to the seepage of domestic and municipal effluents in the groundwater (Srinivasamoorthy et al. 2014; Kumar et al. 50,51,c). The SO42− ion was within their desirable limit (200 mg/l) for drinking water set by BIS (2012).
Hydro-geochemical process
The hydrogeochemical processes that influence groundwater’s physicochemical characteristics depend on different relationships between the dissolved ions and processes of weathering of rock present in the earth’s crust (Wang and Shao 2013). Ca2+ + Mg2+ was plotted against HCO3− + SO42− to identify the parental rock responsible for the ion exchange process in the groundwater (Maurya et al. 2019; Kumar et al. 50,51,c). Figure 3a shows that the majority of water, mainly in PRM and MON, was close to the equiline, depicting silicate and carbonate weathering as a significant geochemical process in groundwater (Kumar et al. 2006; Tiwari and Singh 2014; Su et al. 2021) except for those samples, that were well below the equiline, and are indicative of silicate weathering (Bouderbala 2017; Kaur et al. 2019). Apart from that, a significant portion of water during POM falls above the equiline, suggesting reverse ion exchange involvement in contributing Ca2+ and Mg2+ to the cationic budget (Okiongbo and Douglas 2015; Rao et al. 2015; Subba Rao et al. 2019). Na+ + K+ ions plotted against Cl− + SO42− (Fig. 3b) have revealed that a significant portion of the groundwater of all seasons (~ 79%) plots near and below 1:1 line suggesting mineral dissolution as the process that dominates major-ion chemistry (Zakaria et al. 2021). Few groundwater samples that found their place near or above the 1:1 indicates the dominance of Cl− and SO42− and this might be due to the use of fertilizers in agricultural activities (Rajmohan and Elango 2006), use of road salt and from livestock wastes (Kelly et al. 2012). The plot of Na+ + K+ versus total cations (TZ+) (Fig. 3c) in all seasons fall below the 1:1 equiline, with an average value ranging from 0.33 ± 0.08 to 0.40 ± 0.007 in different seasons (Table 2), suggests silicate weathering has a significant role that contributes Na+ and K+ ions to the water system along with anthropogenic inputs in soil (Stallard and Edmond 1983; Kanagaraj and Elango 2019; Ansari and Umar 2019). Similarly, the ion Scatter plots of Ca2+ + Mg2+ against TZ+ (Fig. 3d) revealed the distribution of samples below but close to the theoretical line (1:1). The average value ranged from 0.60 ± 0.07 to 0.67 ± 0.08 in different seasons (Table 2), suggests weathering of silicate and calc–silicate minerals, and reverse ion exchange processes are responsible for ionic characteristics of the groundwater in study area contributing Ca2+ and Mg2+ ions to the groundwater (Brindha et al. 2020; Pradhan et al. 2022).
An Ion Scatter plot for Na+/Cl− against EC was used to examine the influence of evaporation on the groundwater quality. It identifies the source of salinity in the groundwater. A value of Na+/Cl− molar ratio equal to 1 suggests halite dissolution is responsible for the release of Na+ ion to the groundwater, whereas a ratio > 1 indicates Na+ ion is added to the groundwater from the silicate weathering process (Meyback 1987). The ratio of Na+/Cl− in the groundwater of the study area varied from 0.51 to 16.95, with a significant portion of groundwater samples, irrespective of seasons, having a Na+/Cl− ratio above 1 (89%), which revealed an excess of Na+ is introduced to the groundwater system either from silicate weathering or by anthropogenic influences (Li et al. 2018). However, 11% of groundwater samples have a Na+/Cl− molar ratio value < 1, indicative of halite dissolution (Fig. 3e). The source of Ca2+ and Mg2+ in the groundwater environment was determined using an ion Scatter plot of the Ca2+/Mg2+ ratio (Mayo and Loucks 1995). A value of Ca2+/Mg2+ = 1 is indicative of the dissolution of dolomite, whereas a ratio > 1 indicates the dissolution of calcite rock (Mayo and Loucks 1995), and a ratio > 2 signifies silicate mineral dissolution in groundwater (Katz et al. 1997). Figure 3f showed that Ca2+ and Mg2+ in the groundwater of the study area largely contributed due to dolomite dissolution. A more significant proportion of MON’s sample (~ 70%) lies close to the one ratio line, suggesting reverse ion exchange with Na+ increased Mg2+ ions. A similar finding was also observed by (Srinivasamoorthy et al. 2014). Samples that lay well below the 1 ratio line revealed that Ca2+ has precipitated as CaCO3, resulting in a decline in Ca2+ value during MON and few samples of POM compared to PRM. While most of the water in the PRM season (~ 90%) shows a Ca2+/Mg2+ ratio > 2, indicating that silicate dissolution contributes to the Ca2+ and Mg2+ in the groundwater (Katz et al. 1998). However, few water samples in all three seasons showed a ratio between 1 and 2, indicating the source of ions is from the dissolution of calcite rock. The C-ratio value around 0.50 marks the presence of carbonate termination reaction along with sulfide corrosion, and a value closer to 1, indicates carbonation reactions and atmospheric input of CO2-deriving protons. The C-ratio (HCO3– /HCO3− + SO42+) observed during the study period was 0.90 ± 0.07 to 0.94 ± 0.06, showing carbonation reactions along with the atmospheric inputs.
Hydro-geochemical facies
Hydrochemical facies depend on solution kinetics, rock–water interactions, the geology of that area, sources of contamination and flow pattern of the aquifers (Kumar et al. 2023; Zaidi et al. 2019; Liu et al. 2020). It enables us to describe the water quality that differs in chemical composition (Srinivasamoorthy et al. 2014). The concentrations of major ionic species of groundwater for the three seasons were plotted in the Piper diagram in order to identify, classify and compare the water type of the study area (Fig. 4). The diamond-shaped graph in the Piper plot is divided into four different fields based on of hydrochemical facies and nine subfields that identify the combination of distinct cation and anions. The plot revealed that Ca2+–Mg2+–HCO3− type water had dominated the study area’s groundwater samples, indicating temporary hardness contributed due to sufficient freshwater recharge (Handa 1979; Herojeet et al. 2016). Cationic triangular fields suggest that most groundwater is observed as no dominant cation type in all seasons, with 17% in PRM and 9% in POM, as Ca2+ types. Also, 15% of monsoon and 10% of post-monsoon water was Mg2+ type, and about 5% of monsoon and post-monsoon groundwater was Na+–K+ type. The triangular plot for anions has revealed bicarbonate-type water, suggesting silicate weathering dominance in the study area. Hence, two different types of main hydrochemical facies were observed for groundwater samples, such as Ca–HCO3 and Mg–HCO3 dominant in pre-monsoon and post-monsoon and Mg–HCO3 was observed to be dominant during monsoon season in the study area.
In addition, the Durov diagram was used to extract more information on the hydro-geochemical processes (mixing, ion exchange, reverse ion exchange dissolution) that influence groundwater quality (Aly et al. 2015; Singh et al. 2020). In a Durov diagram, the cations (Na+, K+, Ca2+, Mg2+) and anions (Cl−, HCO3−, SO42−) values are plotted on two separate ternary diagrams, projected onto a square grid placed at the base of each triangle. The cation triangle suggests that most groundwater is not the dominant cation-type water in all seasons. Based on the classification of Lloyd and Heathcote (1985), 80% (PRM), 76%(MON) and 74% (POM) of the groundwater samples were found in the field 6 indicating the reverse ion exchange process dominated by HCO3− and Mg2+. The Remaining water, about 20% in PRM, 24% in MON and 26% in POM, was observed in field 5, indicating that the groundwater exhibits simple dissolution or mixing type with no dominant anion or cation (Fig. 5). This indicates simple dissolution or mixing influence, suggesting recent freshwater recharge that has controlled the geochemical processes in groundwater (Thakur et al. 2018). The HCO3− ion surplus in the study area may be due to silicate weathering by carbonic acid in the groundwater (Kumar et al. 2006; Singh et al. 2020). Thus, the Durov diagram revealed weathering of rocks and reverse-ion exchange as two dominant hydrogeochemical processes involved in controlling the groundwater chemistry in the study area.
Gibb’s diagram was employed to identify hydrogeochemical processes in determining water quality based on the value of TDS and cation/anion ratio to predict the dominant hydrogeochemical factor responsible for groundwater quality. Gibbs ratio was calculated using an equation developed by Gibbs (1970):
In this study, the diagram revealed that rock–water interaction involving rock-weathering, dissolution–precipitation and ion-exchange process is the primary source of dissolved ions during all seasons. Furthermore, Gibb’s ratio significantly determines water quality (Fig. 6). Very few samples fall in an evaporation–crystallization zone, possibly due to higher TDS resulting from increased chemical weathering and anthropogenic activities (Subba Rao et al. 2019).
Statistical analysis
Correlation matrix
In this study, Pearson’s correlation coefficient (r) was employed to identify the relationship between various physicochemical parameters of groundwater, with values ranging between − 1 and + 1. Correlation coefficient (r) > 0.7 suggest a strong correlation, moderate correlation (0.5 < r < 0.7), weak correlation (0.0 < r < 0.5), and no correlation found when r ~ 0 (Mudgal et al. 2009) (Table 3).
In our study, TH was strongly correlated with Mg2+ (0.830) and Ca2+ (0.792) cations, as well as the anions Cl− (0.903) and SO42− (0.717) and suggests the dominant factor responsible for TH are Mg2+ and Cl−. A high positive correlation of TH with Cl− (r = 0.903) suggests the significant contribution of chloride to non-carbonate or permanent hardness (Chemistry for environmental engineering 1994). TDS was strongly correlated with Ca2+ (0.889) and Na+ (0.732) cations, as well as anions Cl− (0.888) and SO42− (0.770), and it appears that Ca2+ and Cl− are important ions for predicting TDS. Sojobi (2016) and Viswanath et al. (2015) have reported similar results in their study. EC is strongly correlated to TDS (0.965), TH (0.914), Ca2+ (0.848), Na+ (0.776), Cl− (0.898) and SO42− (0.824), and Ca2+, Cl− and SO42− has a significant contribution in EC. The major anion strongly associated with Ca2+ is Cl− (r = 0.775) and SO42− (0.848), whereas Mg2+ are moderately associated with Cl− (r = 0.705), which suggests a similar source origin or geochemical behaviour. A strong correlation between Ca2+ and Cl− ion can be attributed to the dissolution of carbonate rocks and reverse cation exchange of Na+ ions for Ca2+ (Biglari et al. 2016; Mohapatra et al. 2011; Ghodbane et al. 2022). A negative correlation of pH with all parameters explains the minimal contribution of alkaline media in the dissolution process of host rock and soil minerals (Helena et al. 2000). Hierarchical Cluster analysis was further used to analyze the correlation matrix with the help of SPSS software (SPSS version 22.0).
Hierarchical cluster analysis of water parameters exhibited two distinct clusters, as shown in Fig. 7, cluster 1 (C1) comprises two sub-clusters. The First group (Sub-cluster 1) showed a robust correlation between EC, TDS, TH and Cl− while the second group (Sub-cluster 2) exhibited a close association between SO42− and Ca2+, which are ultimately correlated with Mg2+. Sub-cluster I and sub-cluster 2 also showed a strong correlation. Cluster 2 marked a strong correlation between Na+ and K+, moderately correlated with TA. The outcomes of HCA confirm the results from the correlation analysis.
Principal component and factor analysis
In the present study, PCA has been carried out on a dataset of groundwater quality parameters using varimax rotation in XLSTAT to understand the problem arising from different measurement scales of the original variable, which is avoided by the diagonalization of the correlation matrix. Results of PCA have been represented in the form of a Scree plot showing principal factors and their eigenvalue together with cumulative variability (Fig. 8a), a score plot showing positive and negative factor loading in groundwater (Fig. 8b) and a loading matrix for different physicochemical parameters for groundwater along with eigenvalue, variability and cumulative percentage summarized in Table 4. PCA results showed that two PCs having eigenvalues > 1 explain 77.44% of the total variance; therefore, these two PCs, namely PC factors1 and PC factors 2 have been taken for consideration (Fig. 8a) since eigenvalues ≥ 1.0, are considered significant (Kim and Mueller 1978). Factor analysis of the study revealed that PC1 describes 63.31% of the total variance and has an eigenvalue of 7.07; therefore, it is considered a primary factor (Table 4). According to Liu et al. (2003), loading signifies “strong” (loading values > 0.75) marked as bold in Table 4, “moderate” (0.5 < loading values < 0.75) and “weak” (0.3 < loading values < 0.5). Therefore, physicochemical parameters having a loading value greater than 0.6 were considered for the interpretation (Singh et al. 2011; Kumar et al. 50,51,c).
PC factor1 showed strong positive loading of EC, TDS, Total Hardness, Ca2+, Na+, Cl− and SO42−, moderate positive loading of Total Alkalinity and K+. The value of EC, TDS and TH in the first factor is high, which might be due to the presence of high loading of Ca2+ and Na+ (cations), Cl− and SO42− (anions) in groundwater, which was the dominant factor responsible for EC, TDS and TH. Reghunath et al. (2002) also found that the EC and TDS in the groundwater water of Karnataka were mainly due to Na+ and Cl−, though bicarbonate also plays a substantial role in determining EC and TDS. High EC in the water might be due to increased dissolved solids in groundwater (Ahipathi and Puttaiah 2006). PC factor 2, explaining 13.14% of the total variance, has an eigenvalue of 1.44 and shows strong positive loading of K+, which might be due to the impact of anthropogenic activities from agricultural processes (Kaur et al. 2019) and moderate negative loading of Mg2+. The negative loading of Mg2+ and the weak loading of HCO3− suggests the removal of magnesium in the form of magnesium bicarbonate precipitate from groundwater. The Bartlett’s sphericity test revealed that the observed χ2 = 271.18 is greater than the critical value χ2 = 73.31 (‘degree of freedom’ 55, p values < 0.0001, significance level 0.05) and shows the PCA significantly reduced the dimensionality of the original data (Kumar et al. 2018b, 2019b; Helena et al. 2000).
Conclusion
The final section of the study presents preliminary but essential information on the hydrogeochemical characteristics of the groundwater in Patna, which is located in the state of Bihar. The research investigated the regional and temporal differences in the chemical makeup of the groundwater and found that the changing of the seasons had minimal effect on the ionic chemistry of the aquifer. The findings provided substantial evidence that multivariate statistical tools, such as correlation matrices, principal component analyses, and hierarchical cluster analyses, effectively locate the origin of the contamination and forecast geochemical behaviours that are analogous to those found there. Further, it summarizes the principal role of natural and geogenic processes in contributing to the ionic budget of the study area and also identifies deterioration in water quality at some locations as a result of some anthropogenic disturbances. The ionic composition of the groundwater is mainly controlled by the rock weathering process, where mineral dissolution from silicate rocks has dominated the studied region. The result has disclosed the role of cal–silicate and carbonate rock weathering, along with the reverse ion exchange of Ca2+ with Na+, to the high concentration of calcium and bicarbonate ions in the groundwater of Patna. It concludes that the Ca–Mg–HCO3 water type was the dominant hydrogeochemical facies in the groundwater. This study is the most recent and pioneer work in this direction and can be helpful for the authorities concerned to strengthen a sustainable management plan for the study area. However, the study has certain limitations in not estimating the concentration of NO3−, F− and bacterial load in the groundwater samples. Finally, the study emphasizes regular monitoring of groundwater quality and recommends potential health risk assessment of non-carcinogenic toxicity and trace metal contamination in Patna’s groundwater, which can further benefit a comprehensive understanding.
Data Availability
The datasets used or analyzed during the current study are available upon reasonable request from the corresponding author.
References
Ahipathi MV, Puttaiah ET (2006) Ecological characteristics of Vrishabhavathi River in Bangalore (India). Environ Geol 49:1217–1222
Ahmed A, Clark I (2016) Groundwater flow and geochemical evolution in the Central Flinders ranges South Australia. Sci Total Environ 572:837–851
Al Dahaan S, Al-Ansari N, Knutsson S (2016) Influence of groundwater hypothetical salts on electrical conductivity total dissolved solids. Engineering 8(11):823–830
Ali SA, Ali U (2018) Hydrochemical characteristics and spatial analysis of groundwater quality in parts of Bundelkhand Massif India. Appl Water Sci 8:39. https://doi.org/10.1007/s13201-018-0678-x
Aly AA, Al-Omran AM, Alharby MM (2015) The water quality index and hydrochemical characterization of groundwater resources in Hafar Albatin, Saudi Arabia. Arab J Geosci 8:4177–4190
Ansari JA, Umar R (2019) Evaluation of hydrogeochemical characteristics and groundwater quality in the quaternary aquifers of Unnao District, Uttar Pradesh, India. HydroResearch 1:36–47
APHA (2012) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Atwia MG, Hassan AA, Ibrahim A (1997) Hydrogeology, log analysis and hydrochemistry of unconsolidated aquifers south of El-Sadat city, Egypt. J Hydrol 5:27–38
Ballukraya PN, Ravi R (1999) Characterization of groundwater in the unconfined aquifers of Chennai City, India; Part I: hydrogeochemistry. J Geol Soc India 54:1–11
Biglari H, Saeidi M, Alipour V, Rahdar S, Sohrabi Y, Khaksefidi R, Ahamadabadi M (2016) Review on hydrochemical and health effects of it in Sistan and Baluchistan groundwaters Iran. Int J Pharm Technol 8(3):17900–17920
Bouderbala A (2017) Assessment of water quality index for the groundwater in the upper Cheliff plain, Algeria. J Geol Soc India 90:347–356. https://doi.org/10.1007/s12594-017-07237
Brindha K, Paul R, Walter J, Tan ML, Singh MK (2020) Trace metals contamination in groundwater and implications on human health: comprehensive assessment using hydrogeochemical and geostatistical methods. Environ Geochem Health 42(11):3819–3839
Bulut OF, Duru B, Çakmak Ö, Günhan Ö, Dilek FB, Yetis U (2020) Determination of groundwater threshold values: a methodological approach. J Clean Prod 253:120001
Bureau of Indian Standards (2012) Specification for drinking water. 1S: 10500. Bureau of Indian Standards, New Delhi
Census of India (2011) Primary census abstract for slum, Office of the Registrar General and Census Commissioner of India
CGWB Ground Water Information Booklet (2013) Central Ground water Board Ministry of Water Resources (Govt. of India), Mid-Eastern Region, Patna
Chemistry for Environment and Engineering (1994) fourth edition international editions
Durov SA (1948) Natural waters and graphic representation of their composition. DoklAkadNauk SSSR 59(3):87–90
Eang KE, Igarashi T, Kondo M, Nakatani T, Tabelin CB, Fujinaga R (2018) Groundwater monitoring of an open-pit limestone quarry: water–rock interaction and mixing estimation within the rock layers by geochemical and statistical analyses. Int J Min Sci Technol 28:849–857
Gaikwad S, Gaikwad S, Meshram D et al (2020) Geochemical mobility of ions in groundwater from the tropical western coast of Maharashtra, India: implication to groundwater quality. Environ Dev Sustain 22:2591–2624. https://doi.org/10.1007/s10668-019-00312-9
Gao Y, Qian H, Ren W, Wang H, Liu F, Yang F (2020) Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J Clean Prod 260:121006
Gebresilasie KG, Berhe GG, Tesfay AH, Gebre SE (2021) Assessment of some physicochemical parameters and heavy metals in hand-dug well water samples of Kafta Humera Woreda, Tigray, Ethiopia. Int J Anal Chem 2021:1–9
Ghodbane M, Benaabidate L, Boudoukha A, Gaagai A, Adjissi O, Chaib W, Aouissi HA (2022) Analysis of groundwater quality in the lower Soummam Valley, North-East of Algeria. J Water Land Dev, 1–12
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Gulgundi MS, Shetty A (2018) Groundwater quality assessment of urban Bengaluru using multivariate statistical techniques. Appl Water Sci 8:43. https://doi.org/10.1007/s13201-018-0684-z
Handa BK (1979) Groundwater pollution in India proceedings of national symposium on hydrology. IAHS, Publication University of Roorkee, India, pp 34–49
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34(3):807–816
Herojeet R, Rishi MS, Lata R et al (2016) Application of environmetrics statistical models and water quality index for groundwater quality characterization of alluvial aquifer of Nalagarh Valley, Himachal Pradesh, India. Sustain Water Resour Manag 2:39–53
Hui T, Jizhong Du, Ma S, Zhuang K, Yan G (2020) Application of water quality index and multivariate statistical analysis in the hydrogeochemical assessment of shallow groundwater in Hailun, northeast China. Hum Ecol Risk Assess: Int J 27:651–667
Kadam A, Wagh V, Patil S, Umrikar B, Sankhua R (2021) Seasonal assessment of groundwater contamination, health risk and chemometric investigation for a hard rock terrain of western India. Environ Earth Sci 80(5):1–22
Kanagaraj G, Elango L (2019) Chromium and fluoride contamination in groundwater around leather tanning industries in southern India: implications from stable isotopic ratio δ53Cr/δ52Cr, geochemical and geostatistical modelling. Chemosphere 220:943–953
Katz BG, Tyler BC, Thomas DB, Davis JH (1997) Use of chemical and isotopictracers to characterize the interactions between groundwater and surface water in mantled karst. Groundwater 35(6):1014–1028
Katz BG, Coplen TB, Bullen TD, Davis JH (1998) Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled Karst. Groundwater 35:1014–1028
Kaur L, Rishi MS, Sharma S et al (2019) Hydrogeochemical characterization of groundwater in alluvial plains of River Yamuna in Northern India: an insight of controlling processes. J King Saud Univ Sci. https://doi.org/10.1016/J.JKSUS.2019.01.005
Kelly WR, Panno SV, Hackley K (2012) The sources, distribution, and trends of chloride in the water of Illinois
Khan R, Jhariya DC (2017) Groundwater quality assessment for drinking purpose in Raipur City, Chhattisgarh using water quality index and geographic information system. J Geol Soc India 90:69–76
Kim JO, Mueller CW (1978) Factor analysis: statistical methods and practical issues. Sage, Beverly Hills
Kom KP, Gurugnanam B, Bairavi S (2022) Non-carcinogenic health risk assessment of nitrate and fluoride contamination in the groundwater of Noyyal basin, India. Geod Geodyn
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical process inthe groundwater environment of Delhi India. Envir Geol. https://doi.org/10.1007/s00254-006-0275-4
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343
Kumar R, Kumar R, Singh S, Singh A, Bhardwaj A, Kumari A, Randhawa SS, Saha A (2018) Dynamics of suspended sediment load with respect to summer discharge and temperatures in Shaune Garang glacierized catchment, Western Himalaya. Acta Geophys 66:1109–1120
Kumar R, Kumar R, Singh S, Singh A, Bhardwaj A, Chaudhary H (2019a) Hydro-geochemical characteristics of glacial meltwater from Naradu Glacier catchment Western Himalaya. Environ Earth Sci 78:683. https://doi.org/10.1007/s12665-019-8687-0
Kumar D, Singh A, Jha RK, Sahoo BB, Sahoo SK, Jha V (2019b) Source characterization and human health risk assessment of nitrate in groundwater of middle Gangetic Plain, India. Arab J Geosci 12(11):1–12
Kumar R, Kumar R, Bhardwaj A, Singh A, Singh S, Kumari A, Sinha RK (2022a) Multivariate statistical analysis and geospatial approach for evaluating hydro-geochemical characteristics of meltwater from Shaune Garang Glacier, Himachal Pradesh, India. Acta Geophys 71(1):323–339
Kumar R, Kumar R, Singh A, Arif M, Kumar P, Kumari A (2022b) Chemometric approach to evaluate the chemical behavior of rainwater at high altitude in Shaune Garang catchment, Western Himalaya. Sci Rep 12(1):1–14
Kumar R, Qureshi M, Vishwakarma DK, Al-Ansari N, Kuriqi A, Elbeltagi A, Saraswat A (2022c) A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud Chem Environ Eng 6:100219
Kumar R, Kumari A, Kumar R, Sulaiman MA, Zafar MM, Singh A, Prabhakar R, Pippal PS (2023) Assessing the geochemical processes controlling groundwater quality and their possible effect on human health in Patna, Bihar. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-26203-1
Kupwade RV, Langade AD (2013) Pre and post monsoon monitoring of ground water quality in region near Kupwad MIDC, Sangli, Maharashtra. Int J Chem Tech Res 5(5):291–294
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ 37(2):222–237
Liu CW, Lin KH, & Kuo YM (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Tot Environ 313(1–3): 77–89
Liu D, Qi X, Li M, Zhu W, Zhang L, Faiz MA, Khan MI, Li T, Cui S (2019) A resilience evaluation method for a combined regional agricultural water and soil resource system based on Weighted Mahalanobis distance and a Gray-TOPSIS model. J Clean Prod 229:667–679
Liu J, Wang H, Jin D, Xu F, Zhao C (2020) Hydrochemical characteristics and evolution processes of karst groundwater in Carboniferous Taiyuan formation in the Pingdingshan coalfield. Environ Earth Sci 79(6):1–14
Lloyd JW, Heathcote JAA (1985) Natural inorganic hydrochemistry in relation to ground water 109(3)
Makubura R, Meddage DPP, Azamathulla HM, Pandey M, Rathnayake U (2022) A simplified mathematical formulation for water quality index (WQI): a case study in the Kelani River basin Sri Lanka. Fluids 7(5):147
Maliqi E, Jusufi K, Singh SK (2020) Assessment and spatial mapping of groundwater quality parameters using metal pollution indices, graphical methods and geoinformatics. Anal Chem Lett 10(2):152–180
Maurya P, Kumari R, Mukherjee S (2019) Hydrochemistry in integration with stable isotopes (δ18O and δD) to assess seawater intrusion in coastal aquifers of Kachchh district, Gujarat India. J Geochem Explor 196:42–56
Maurya J, Pradhan SN, Seema Ghosh AK (2020) Evaluation of ground water quality and health risk assessment due to nitrate and fluoride in the Middle Indo-Gangetic plains of India. Hum Ecol Risk Assess Int J 27(5):1349–1365
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the central Wasatchrange Utah. J Hydrol 172:31–59
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Mohapatra PK, Vijay R, Pujari PR, Sundaray SK, Mohanty BP (2011) Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city, India: a multivariate statistical approach. Water Sci Technol 64(4):809–817
Mudgal KD, Kumari M, Sharma DK, dev Mudgal K, Kumari M (2009) Hydrochemical analysis of drinking water quality of Alwar district, Rajasthan. Nat Sci 7(2):30–39
Nizam S, Virk HS, Sen IS (2022) High levels of fluoride in groundwater from Northern parts of Indo-Gangetic plains reveals detrimental fluorosis health risks. Environ Adv 8:100200
Okiongbo KS, Douglas RK (2015) Evaluation of major factors influencing the geochemistry of groundwater using graphical and multivariate statistical methods in Yengoa city, Southern Nigeria. Appl Water Sci 5:27–37
Olatunde K, Patton SK, Cameron L, Stankus T, Milaham PJ (2022) Factors affecting the quality of drinking water in the United States of America: a ten-year systematic review. Am J Water Resour 10(1):24–34
Panigrahy PIC, Sahu SD, Sahu BK, Sathyanarayana D (1996) Studies on the distribution of calcium and magnesium in Visakhapatnam harbour waters, Bay of Bengal. In: International symposium on applied geochemistry. Osmania University, Hyderabad, pp 353–340
Patna Master Plan 2031 (2016) Town and Country Planning Organisation, Urban Development & Housing Department, Patna, Bihar. http://udhd.bihar.gov.in/PMP2031/
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
Pradhan RM, Behera AK, Kumar S, Kumar P, Biswal TK (2022) Recharge and geochemical evolution of groundwater in fractured basement aquifers (NW India): insights from environmental isotopes (δ18O, δ2H, and 3H) and hydrogeochemical studies. Water 14(3):315
Prasanna MV, Chidambaram S, Shahul HA, Srinivasamoorthy K (2011) Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin, Tamil Nadu, India. J Earth Syst Sci 120:85–98
Rai AK, Paul B, Mudra L, Kishor N (2011) Studies of selected water quality parameters of river Ganges at Patna, Bihar. J Adv Lab Res Biol 2(4):136–140
Rajmohan N, Elango L (2006) Hydrogeochemistry and its relation to groundwater level fluctuation in the Palar and Cheyyar River basins, southern India. Hydrol Process: Int J 20:2415–2427
Ramappa R, Suresh TS (2000) Quality of groundwater in relation to agricultural practices in Lokapavani river basin, Karnataka, India. In: Proceedings of international seminar on applied hydrogeochemistry. Annamalai University, pp 136–142
Rao S, Mahesh J, Surinaidu L, Dhakate R (2015) Hydrochemical assessment ofgroundwater in alluvial aquifer region, Jalandhar District, Punjab India. Environ Earth Sci 73(12):8145–8153
Reghunath R, Murthy TRS, Raghavan BR (2002) The utility of multivariate statistical techniques in hydro-geochemical studies: an example from Karnataka India. Water Res 36(2002):2437–2442
Sandhu C, Grischek T, Schoenheinz D, Prasad T, Thakur AK (2011) Evaluation of bank filtration for drinking water supply in Patna by the Ganga River, India. Riverbank filtration for water security in desert countries. Springer, Dordrecht, pp 203–222
Sharma P, Sharma A (2022) Heavy metals in ground water affect the human health global challenge. Computational intelligence and applications for pandemics and healthcare. IGI Global, Hershey, pp 139–158
Sheikh MA, Azad C, Mukherjee S, Rina K (2017) An assessment of groundwater salinization in Haryana state in India using hydrochemical tools in association with GIS. Environ Earth Sci 76(13):1–13
Shukla S, Saxena A (2020) Water quality index assessment of groundwater in the Central Ganga Plain with reference to Raebareli district, Uttar Pradesh, India. Curr Sci 119(8):1308–1315
Singh S, Singh C, Kumar K, Gupta R, Mukherjee S (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. J Hydrol Hydromech 57(1):45–54
Singh CK, Kumari R, Singh RP, Shashtri S, Kamal V, Mukherjee S (2011) Geochemical modeling of high fluoride concentration in groundwater of Pokhran area of Rajasthan, India. Bull Environ Contam Toxicol 86:152–158
Singh SK, Srivastava PK, Pandey AC (2013a) Fluoride contamination mapping of groundwater in northern India integrated with geochemical indicators and GIS. Water Sci Technol 8:4687–4702
Singh A, Sharma CS, Jeyaseelan AT, Chowdary VM (2015) Spatio–temporal analysis of groundwater resources in Jalandhar district of Punjab state India. Sustain Water Res Manag 1(3):293–304
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) (2020) Multivariate analysis and geochemical signatures of groundwater in the agricultural dominated taluks of Jalandhar district, Punjab India. J Geochem Explor 208:106395
Sinha MR, Avnisha D, Amita P, Mausumi G, Tagore RN (2011) Physicochemical examination and quality assessment of groundwater (Hand-Pump) around Patna main town, Bihar state, India. J Chem Pharm Res 3(3):701–705
Sojobi AO (2016) Evaluation of groundwater quality in a rural community in North Central of Nigeria. Environ Monitor Assess 188:1–17
Srinivasamoorthy K, Nanthakumar C, Vasanthavigar M, Vijayaraghavan K, Rajivgandhi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2011) Groundwater quality assessment from a hard rock terrain, Salem District of Tamil Nadu, India. Arab J Geosci 4:91–102. https://doi.org/10.1007/s12517-009-0076-7
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu India. J King Saud Univ Sci 26:37–52
Stallard RF, Edmond JM (1983) Geochemistry of Amazon, the influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Su H, Kang W, Kang N, Liu J, Li Z (2021) Hydrogeochemistry and health hazards of fluoride-enriched groundwater in the Tarim Basin. China Environ Res 200:111476
Subba Rao N, Srihari C, Deepthi Spandana B, Sravanthi M, Kamalesh T, Abraham Jayadeep V (2019) Comprehensive understanding of groundwater quality and hydrogeochemistry for the sustainable development of suburban area of Visakhapatnam, Andhra Pradesh, India. Hum Ecol Risk Assess Int J 25(1–2):52–80
Sukumaran D, Saha R, Saxena RC (2015) Ground water quality index of Patna, the capital city of Bihar India. Am J Water Resour 3(1):17–21
Sulaiman MA, Zafar MM, Afshan N, Kumari A (2021) Impact of lockdown on air pollutants during COVID-19 at Patna, India. Asian J Atmos Environ 15(4):62–77
Sulaiman MA, Zafar MM, Divya, Prabhakar R, Kumari A (2023) Evaluation of ionic composition and hydrogeochemical processes of the groundwater in Muzaffarpur, district, Bihar, India. Ind J Environ Protect 43(3):263–269
Thakur N, Rishi M, Sharma DA, Keesari T (2018) Quality of water resources in Kullu Valley in Himachal Himalayas, India: perspective and prognosis. Appl Water Sci 8(1):20
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83(3):329–343. https://doi.org/10.1007/s12594-014-0045-y
Tiwari AK, Singh AK, Singh AK, Singh MP (2017) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh district Uttar Pradesh, India. Appl Water Sci 7:1609–1623
USGS (2022) Maps showing geology, oil and gas fields and geologic provinces of South Asia. https://pubs.usgs.gov/of/1997/ofr-97-470/OF97-470C/ofr97470C.pdf
Varol S, Davraz A (2015) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 73:1725–1744
Vidana SM, Banoeng-Yakubo B, Sakyi PA (2012) Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. J Earth Syst Sci 121:491–507
Viswanath NC, Kumar PD, Ammad KK (2015) Statistical analysis of quality of water in various water shed for Kozhikode City, Kerala, India. Aqua Proc 4:1078–1085
Wagh VM, Mukate SV, Panaskar DB, Muley AA, Sahu UL (2019) Study of groundwater hydrochemistry and drinking suitability through Water Quality Index (WQI) modelling in Kadava river basin India. SN Appl Sci 1(10):1–16
Wang YQ, Shao MA (2013) Spatial variability of soil physical properties in a region of the loess plateau of PR China subject to wind and water erosion. Land Degrad Dev 24(3):296–304
Zafar MM, Sulaiman MA, Prabhakar R, Kumari A (2022) Evaluation of the suitability of groundwater for irrigational purposes using irrigation water quality indices and geographical information systems (GIS) at Patna (Bihar), India. Int J Energy Water Resour 1–14. https://doi.org/10.1007/s42108-022-00193-1
Zaidi FK, Salman A, Hag-Elsafi S, Alfaifi HJ (2019) Assessment of hydrological processes operating in a multi-layered sedimentary aquifer system in Saudi Arabia using integrated chemical and statistical approach. Environ Monit Assess 191(7):1–18
Zakaria N, Anornu G, Adomako D, Owusu-Nimo F, Gibrilla A (2021) Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw Sustain Dev 12:100489
Acknowledgements
The authors are thankful to all members of the Environmental Biology Laboratory at Patna University, Patna.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
MAS, MMZ, RP contributed to Data curation, Formal analysis and investigation, MAS, MMZ contributed to Writing original draft, MAS, MMZ, RK contributed to Software, AK, RKS contributed to Resources, AK, MAS, RK contributed to Writing review and editing, AK, RKS contributed to Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Edited by Dr. Michael Nones (CO-EDITOR-IN-CHIEF).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sulaiman, M.A., Zafar, M.M., Prabhakar, R. et al. A multivariate statistical approach to evaluate the hydro-geochemistry of groundwater quality in the middle Ganga river basin, Patna, India. Acta Geophys. 72, 1913–1926 (2024). https://doi.org/10.1007/s11600-023-01071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11600-023-01071-y