Abstract
This paper presents a study on the Wular Lake which is the largest fresh water tectonic lake of Kashmir Valley, India. One hundred and ninety-six (196) water samples and hundred (100) sediment samples (n = 296) have been collected to assess the weathering and Anthropogenic impact on water and sediment chemistry of the lake. The results showed a significant seasonal variability in average concentration of major ions being highest in summer and spring and lower in winter and autumn seasons. The study revealed that lake water is alkaline in nature characterised by medium total dissolved solids and electrical conductivity. The concentration of the major ion towards the lake central showed a decreasing trend from the shore line. The order of major cations and anions was Ca2+ > Mg2+ > Na+ > K+ and HCO3 − > SO4 2− > Cl−, respectively. The geochemical processes suggested that the chemical composition lake water is mostly influenced by the lithology of the basin (carbonates, silicates and sulphates) which had played a significant role in modifying the hydrogeochemical facies in the form of Ca–HCO3, Mg–HCO3 and hybrid type. Chemical index of alteration values of Wular Lake sediments reflect moderate weathering of the catchment area. Compared to upper continental crust and the post-Archean Shale, the sediments have higher Si, Ti, Mg and Ca contents and lower Al, Fe, Na, K, P, Zn, Pb, Ni, Cu content. Geoaccumulation index (Igeo) and US Environmental Protection Agency sediment quality standards indicated that there is no pollution effect of heavy metals (Zn, Mn, Pb, Ni and Co).The study also suggested that Wular Lake is characterised by both natural and anthropogenic influences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemistry of river/lake water and sediments plays an important role in elucidating the Earths surface dynamics. The study of the solutes (dissolved load) provides information on chemical erosion processes (Gaillardet et al. 1999; Wu et al. 2005), while as the sediments (suspended/bed load) have been effectively used to evaluate the catchment weathering, paleoclimatic reconstruction (Krishnamurthy et al. 1986; Fontes et al. 1993; Jin et al. 2003; Rose et al. 2004) and for tracing anthropogenic activities (Lerman 1978; Smol 2002). The solutes and sediments of lakes, mostly derived from the weathering and erosion processes in the catchment and anthropogenic interventions, are also influenced and modified by various processes occurring within the lake system. Since many lakes are the final receivers of stream runoff, agricultural and others waste discharges, they become enriched with nutrients, sediments and heavy metals (Skoulikidis et al. 1998; Jeelani and Shah 2006) leading to the deterioration of lake water quality, and thus direct threat to many life forms (Nag and Das 1993; Sujatha et al. 2001).
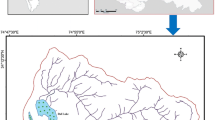
Wular Lake (Fig. 1), the largest freshwater lake of Asia within River Jhelum basin, plays a significant role in the hydrography of the Kashmir valley not only by acting as a huge absorption basin for floodwaters but also for maintaining flows to support agriculture and hydropower generation as well as sports activities. The lake is located 34 km northwest of Srinagar city at an altitude of 1,530 m (amsl) between latitudes 34°17′N and 34°24′N and longitudes 74°31′E and covers an area of 189 km2. The lake is surrounded by Pir Panjal Mountains (locally known as Baba Shakur-Ud-Din Mountains) towards north, northeast and northwest which drain their runoff through various streams, prominent being, Erin and Madhumati apart from River Jhelum (which emerge from Verinag spring and drains whole Kashmir valley). The Lake along with the extensive marshes surrounding is an important habitat for fish, accounting 60 % of the fish production within the state of J&K (Rumysa et al. 2012). The Lake was included in 1986 as a wetland of national importance under the wet lands programme of the Ministry of environment and forests, Govt. of India for intensive convention management purposes. Recognising the importance of the wetland for its biodiversity and socioeconomic values, the lake was designated by India as a wetland of international importance under the Ramsar Convention in 1990. However, a comprehensive study regarding the assessment of hydrochemistry and sediment chemistry of the lake is lacking up to date. The main objectives of the present study are (1) to assess the geochemistry of lake water and sediments, and (2) to examine the natural and anthropogenic influences on water and sediment chemistry of the lake.
Sampling and analysis
Water samples (n = 196) were collected in high density polyethylene bottles across Wular Lake. Initial characterisation of water samples was done at site by measuring their temperature, pH, and electrical conductivity with the help of digital water analysis kit. Before analysis, the water samples were filtered through <0.45 μm nucleopore filter paper. For major ion analysis the samples were brought to the Geochemistry Laboratory, Department of Earth Sciences, University of Kashmir. Samples were analyzed as per the standard methods (APHA 1999). HCO3 − was determined by HCl titration. Total hardness as CaCO3 and Ca2+ was analyzed titrimetrically, using EDTA titration. Magnesium (Mg++) was calculated from the total hardness and Ca++. Cl− was determined by standard AgNO3 titration. Flame emission photometry has been used for the determination of Na+ and K+. Sulphate, Silica and Nitrate were determined by spectrophotometric method. Waters were characterised by their specific charge balance as indicated by the normalised inorganic charge balance [NICB = (Σ+ Σ−)/Σ+]. The observed charge balance between cations (TZ+) and anions (TZ−) is further proof of procession of these data (Fig. 2).
Freshly deposited bed sediments samples (n = 100) were also collected from Wular Lake, with the help of a scoop from shallow sites (up to 1 m) and by means of an Ekman–Birge dredge from deeper sites (1–6 m). Samples were packed in polythene bags prior to analysis, dried and properly mixed and an aliquot of each sample was powdered in a tima to 200 mesh. Geochemical analysis of the sediments was carried out by decomposition of each sample with HNO3, HCl, H2SO4 and HF mixture (Shapiro and Brannock 1962). Atomic absorption spectrophotometer was used for the determination of Si, Al, Ca, Mg, Fe, P, Ti, Mn, Zn, Cu, Ni, Pb, Co. Na and K were analyzed by flame emission photometer and the results were expressed in μg/g.
Results and discussion
Water chemistry
The summary of the results of physico-chemical analyses of the Wular Lake water samples collected in spring, summer, autumn and winter season is presented in Table 1. The lake waters are alkaline with pH ranging between 7.1 and 9.8. The total dissolved solids (TDS) and electrical conductivity (EC) of the lake water samples range from 77–418 mg/l to 120–653 μS/cm, respectively. Lowest values are observed for the samples collected in the lake central and outlet and the highest TDS levels were measured near the shore line.
Calcium and magnesium, the most abundant cations, contribute 84 to 94 % to the major cation budget, with average concentrations ranging from 14.4–26.7 mg/l to 6.9–13.5 mg/l, respectively. Calcium contributes 51–66 % to the major cation budget. Average sodium concentration of the water samples ranges between 1.7 and 5.1 mg/l. Potassium is the least abundant cation with average concentration ranging from 0.06 to 0.35 mg/l. Bicarbonate, the most abundant major anion, contributes 69–84 % to the major anion budget with average concentration ranging from 50 to 98 mg/l. Sulphate, the second dominant major anion, comprises 12–25 % of the measured anions, with average concentrations ranging from 11.2 to 28.6 mg/l. Average chloride concentration ranges from 2.2 to 6.0 mg/l. The concentration of nitrate varied from 1.1 to 3.9 mg/l. Higher concentration of major ions is observed for the samples collected near shore line and from the major streams/rivers (Jhelum, Erin and Madhumati) entering the lake (Fig. 3). The concentration of the major ion towards the lake central showed a decreasing trend from the shore line. The order of major cations and anions was Ca2+ > Mg2+ > Na+ > K+ and HCO3 − > SO4 2− > Cl−, respectively.
The average concentration of major ions showed a significant seasonal variability. The higher and lower average concentration of calcium is observed in summer and spring seasons, respectively. Magnesium concentration was found to be higher in summer and lower in autumn season. However, some shore line samples collected away from the inlet streams showed higher calcium and magnesium content in winter season. The concentration of sodium and sulphate was found to be higher in spring and lower in winter season. The average concentration of bicarbonate is higher in summer and lower in winter and/or autumn seasons. The average concentration of chloride is higher in spring season and lower in winter season.
Mechanisms controlling the major element chemistry
Weathering inputs
Use of Gibbs diagram (Gibbs 1970, Fig. 4) indicates that the major mechanism controlling the water chemistry of Wular Lake is the chemical weathering of rocks [TDS: 77–418 mg/l and weight ratio of Na/(Na + Ca): 0.014–0.225]. The primary source of major ions in the river and lake waters is atmospheric deposition, dissolution of evaporates (halite, gypsum), weathering of carbonates and silicates by sulphuric acid or carbonic acid and anthropogenic activities. Ca, Mg and HCO3 are derived from weathering of carbonates rocks by carbonic acid if the ratio of HCO3/(Ca + Mg) is close to 1 (Stallard and Edmond 1983; Chakrapani 2002) as the carbonate rocks weather congruently. HCO3/(Ca + Mg) ratio of 70–80 % of the lake water samples is close to 1 indicating the carbonate weathering by carbonic acid as the dominant source of Ca, Mg and HCO3. However, If sulphuric acid is the weathering agent then Ca/SO4 ratio in the waters should be 1:1 for calcite and 1:2 for dolomite (Stallard and Edmond 1983). In spring season, about 40 % of the lake water samples show a possibility of carbonate weathering by sulphuric acid as the Ca/SO4 is close to 1. Na/Cl ratio in some water samples is more than 1 indicating the silicate weathering.
Using piper diagram (Fig. 5), three main water types were identified: Mg–-HCO3, Ca–HCO3 and Hybrid type. Most of the water samples (84 %) were Mg–HCO3 type followed by Ca–HCO3 type (9 %) and hybrid type (7 %). The plotting of the water samples near the left corner of the diamond in the Piper diagram shows that the lake water is rich in Ca2+, Mg2+ and HCO3 − and possesses temporary hardness. This pattern also shows that the lake water is dominated by alkaline earth (Ca2+, Mg2+) and weak acids such as HCO3 −. The presence of hybrid water type indicates the role of multiple lithology including carbonates and silicates as the source of major ions.
To study the relative importance of different weathering regimes and dominance of major ions, binary plots are plotted. In Ca2+ + Mg2+ versus Na+ + K+ plot (Fig. 6a), the data points fall below 1:1 equiline indicating carbonate lithology as the dominant source of major ions. In the plots of Ca2+ + Mg2+ versus HCO3 − (Fig. 6b) and Ca2+ + Mg2+ versus HCO3 − + SO4 2− (Fig. 6c), most of the points fall below 1:1 equiline suggesting some contribution from silicates or/and sulphates too. In the Langelier–Ludwig plot (Fig. 7), most of the samples confirm to the chemistry of meteoric waters, i.e. Ca2+ − Mg2+ − HCO3 − type. This also confirms the carbonate lithology as the dominant source of major ions. However, some samples in spring and summer seasons showed some increase in SO4 + Cl indicating their origin from other sources.
Anthropogenic inputs
The Wular Lake reflects anthropogenic inputs in the form of sewage, tilling of slopes, eutrophication and unplanned application of agrochemicals from surrounding areas, especially from Saderkoot, Papchan, Garoora, Dachigam, Nasoo, Baba Gund, Kunis, Zurmanz, Ashtung, Haritar, Sumbal, Nadihal, Mangnipora and Watlab besides siltation load from the river Jhelum and its various tributaries mainly Erin and Madhumati streams have led to increased quantum of various chemicals, which have considerably changed the hydrogeochemistry of the lake. In addition to this, the encroachment due to the high population density (particularly north eastern side) within the immediate vicinity of the catchment, the impact of land use and human influence on water chemistry seems to be more important because NO3 −, K+, P and Cl− which are index of anthropogenic activities commonly derived from agricultural fertilizers, animal waste, and municipal sewage (Grosbois et al. 2000; Jeong 2001; Han and Liu 2004; Liu et al. 2006) are sufficient to accelerate the processes of eutrophication, which has become a widely recognised problem of water quality deterioration in the Wular Lake. The influence of human and agricultural activities in Wular Lake has been estimated (Table 2) by comparing results of the present study of the waste water samples with arranged limit as given by Chetelat et al. (2008). The study suggested that the concentration of Mg and Cl ions was influenced by both human settlements and agricultural inputs while as K, Cl and P, NO3 by agricultural inputs. The higher concentration of these elements was observed near the periphery of the lake (Nasoo, Nadihal, Dachigam, Baba Gund and Saderkoot) which significantly marks the role of the anthropogenic input by sewage run-off and excessive use of agrochemicals in the surrounding areas of the catchment. The higher concentration of heavy metals Cu, Pb and Zn in basin sediments points to the source input of domestic wastes and sewage runoff as the lake lies in the vicinity of maximum habitation and there is no industrial or mining activity prevalent in the area.
Sediment chemistry
In addition to water chemistry, the lake sediments have also been analyzed and the summarised data are presented in Table 3, which reveal that Si and Al were the most abundant elements which together constitute nearly 80 % of the total elemental composition, with average concentrations ranging from 100490–100853 μg/g to 20265–23169 μg/g, respectively. Lowest average elemental composition was recorded for p 269–388 μg/g and followed by Na 692–796 μg/g. Zn was abundant heavy metal accounting nearly 60 % of total heavy metal composition; with average concentrations ranging from 37 to 47 μg/g. Higher concentration of elements and heavy metal was observed for the samples collected near shore line. However, the concentrations showed a decreasing trend in the Lake central (Fig. 8). The order of elements and heavy metals was Si > Al > Ca > Mg > Fe > K > Ti > Na > P and Zn > Pb > Ni > Cu, respectively. The higher concentration of elements in the Wular Lake sediments near Bunyar (Fig. 9) might be due to weathering of the catchment. The elemental concentration showed sudden drop in Mongpor mix1 and inside lake which is attributed to dilution effect and consumption by aquatic life; as these areas are occupied by dense growth of macrophytes. The abrupt increase of the ions in Outlet may be attributed to accumulation of load near outlet (as Wular Lake is Ox-bow type lake). The average concentration of major ions showed a significant seasonal variability. The highest and lower average concentrations of Si and Na were observed in summer and spring seasons, respectively. The highest average concentrations of Al were observed in summer season; however, some stream samples and some shoreline samples showed lowest average concentrations in summer season. The average concentrations of K and Fe were to be higher in autumn and lower in spring season. The average concentrations of Ca and Mg were observed higher in winter and lowest in autumn season; however, some stream samples showed lowest average concentrations in summer season. The average concentration of P was observed highest in summer and lowest in autumn season. Highest average concentration of Pb and Ni was observed lowest in summer and highest in winter season; however at some sampling stations highest average concentrations were observed in spring season. The highest average concentrations of Zn were observed in summer and lowest in spring seasons; however, at some sampling locations (stream samples) the highest concentrations were recorded in winter season.
Concentration of SiO2 was highest in summer and lowest during winter reflecting its origin from weathering of silicate rocks. Mg and Ca concentrations were recorded higher during winter and lowest during summer, such trends are mainly attributed to dilution effects and to some extent may be due to the intake of these elements by plants, as Ca2+ is an important plant nutrient used in various metabolic activities. Concentrations of phosphorus were recorded generally higher during summer, such trends are attributed to anthropogenic activities, like use of fertilizers and tourist pressure. Concentrations of Ni were recorded elevated mainly during spring and modest during summer (Fig. 10) which is attributed to the dilution and concentration effects. To asses the source and extent of weathering in the study area, data of the Lake sediments has been compared with average upper continental crust (UCC), post-Archean Shale (PAS) and North American Shale Composite (NASC) in the form of normalised multi-element patterns. The positive anomalies of the SiO2, TiO2, MnO, MgO and CaO indicate moderate weathering at the source and significant contribution from carbonate lithology. The negative anomaly for elemental oxides like Al2O3, Fe2O3, Na2O, K2O, P2O5 and heavy metals Zn, Pb, Ni, and Cu may be ascribed lower than the limits (Fig. 9), which could be attributed to the dilution effects of CaCO3 as envisaged from the positive Ca anomaly (Das and Dhiman 2003). Nesbitt and Young (1982) defined a chemical index of alteration (CIA) to quantify the degree of weathering (in molecular proportions) using the following equation:
where CaO* represents Ca in the silicate fraction only. CIA values for average shale ranges from 70 to 75, which reflects the composition of muscovites, illites and smectite. Intensely weathered rock yields mineral compositions trending towards kaolinite or gibbsite and a corresponding CIA that approaches 100. CIA value of 45–55 indicates no weathering, whereas CIA of 100 indicates extreme weathering. In Wular Lake bed sediments CIA values ranged from 74 to 77 during spring, 66 to 78 during summer, 77 to 81 during autumn and 73 to 75 during winter reflecting moderate chemical weathering in the provenance.
In Al2O3–(CaO* + Na2O)–K2O plot (Fig. 10a), most of points fall towards Al2O3 apex and are away from PAAS attesting to moderate weathering conditions. By plotting the samples on K2O–Fe2O3–Al2O3 triangular diagram (Fig. 10b), most of the points fall in the field of shales, eventually at the junction of overlapping residual clays which may be formed due to leaching of illite. This also indicates moderate chemical weathering in the source area.
Many efforts have been undertaken to develop criteria for the assessment of environmental impacts on sediments, but there are no universally accepted guidelines for sediment quality (Chapman 1986; Calmano et al. 1996; Jeelani and Shah 2006). The degree of pollution of a given area can be estimated by comparison of the obtained heavy metal contents with those considered to represent the pre-civilisation level (Irabien and Velasco 1999). Many authors prefer to express the metal contamination with respect to average shale to quantify the degree of pollution (Förstner and Muller 1973; Singh and Husnain 1998). Concentration above these reference values is presumed to be anthropogenically affected. In this study, average shale composition proposed by Turekian and Wedepohl (1961) was used because metal contents in fossil argillaceous sediments are a global standard in general use (Müller 1973). Average heavy metals (Mn, Zn, Pb, Ni and Co) concentrations were higher in the study area compared to the average shale values (Table 3), thus demonstrating the role of anthropogenic activities in the study area.
The geo-accumulation index (Igeo) for quantification of metal accumulation in sediments (Müller 1973) is calculated as Igeo = log2 Cn/1.5Bn; Where Cn is the measured concentration of the heavy metal (n) of sediment and Bn is the concentration in average shale (Turekian and Wedepohl 1961) of element n. The calculated Igeo values (Table 4) suggest that all studied heavy metals (Zn, Mn, Pb, Ni and Co) fall to zero class with no pollution effects.
The US Environmental Protection Agency (USEPA 1997) has established sediment quality guidelines in the form of level-of-concern concentrations for several trace metals which include the threshold effect level (TEL) and the probable effect level (PEL). As per EPA, toxic effects usually or frequently occur at concentrations above PEL. The concentrations near or greater than the respective threshold effective levels (TEL) in sediment samples indicate greater risk to aquatic organisms (Jeelani and Shah 2006). The sediment quality guidelines have been developed for individual elements and not for the complex combinations that are seen in the natural environment (Ingersoll et al. 1996). Actual toxicity to aquatic organisms is not usually observed if only one metal exceeds the sediment quality guideline (Christensen and Juracek 2001). Zn, Pb and Ni concentrations in the lakebed sediments were well below TEL and PEL (Table 3) may be attributed to the minimal industrial and mining activities in the catchment and nearer the lake. Metal mining, industrial and municipal are the most significant sources of Cu, Pb and Zn in sediments (Moore and Ramamoorthy 1984; Johansson and Iverfeldt 1994; Thuy et al. 2000). The Wular Lake lies in the vicinity of maximum habitation and there is no industrial or mining activity prevalent in the area. Therefore, point source input of domestic wastes and urban runoff are major contributors of elevated concentration of these heavy metals in the basin sediments.
Conclusions
The geochemical chemical study of the Wular Lake sediments reflects moderate weathering of the catchment area. The hydrogeochemical results indicated that water of the lake is generally alkaline in nature with moderate TDS and EC. Three water types were identified viz, Ca–HCO3, Mg–HCO3 and Hybrid type. The concentration of the major ion towards the lake central showed a decreasing trend from the shore line which may be due to the hydrological conditions in the watershed as well as biological productivity in the lake. The order of elements and heavy metals was Si > Al > Ca > Mg > Fe > K > Ti > Na > P and Zn > Pb > Ni > Cu, respectively. Compared to upper continental crust (UCC) and the post-Archean Shale (PAS), the sediments have higher Si, Ti, Mg and Ca contents and lower Al, Fe, Na, K, P, Zn, Pb, Ni, Cu content. Geoaccumulation index (Igeo) and US Environmental Protection Agency sediment quality standards indicated that there is no pollution effect of heavy metals (Zn, Mn, Pb, Ni and Co). The study suggested that Wular Lake is characterised by both natural and anthropogenic influences.
References
APHA, AWWA, WPCF (1999) Standard methods for the examination of water and waste water, 14th edn. American Public Health Agency, Washington, DC
Calmano W, Ahlf W, Förstner U (1996) Sediment quality assessment: chemical and biological approaches. In: Calmano W, Fo¨rstner U (eds) Sediments and toxic substances. Springer Berlin, Heidelberg, New York, pp 1–35
Chakrapani GJ (2002) Water and Sediment geochemistry of major Kumaun Himalayan Lakes, India. Environ Geol 43:99–107
Chapman PM (1986) Sediment quality criteria from the sediment quality triad. Environ Toxicol Chem 5:957–964
Chetelat B, Liu CQ, Zahao ZQ, Wang QL, Li SL, Li J, Wang BL (2008) Geochemistry of the dissolved load of the Chagjiang Basin Rivers: anthropogenic impacts and chemical weathering. Geochim Cosmochim Acta 72:4254–4277
Christensen VG, Juracek KE (2001) Variability of metals in reservoir sediment from two adjacent basins in the central Great Plains. Environ Geol 40:470–481
Das BK, Dhiman SC (2003) Water and sediment chemistry of Higher Himalayan lakes in the Spiti Valley: control on weathering, provenance and tectonic setting of the basin. Environ Geol 44:717–730
Fontes JC, Mélières F, Gibert E, Qing L, Gasse F (1993) Stable isotope and radiocarbon balances of two Tibetan lakes (Sumxi Co, Longmu Co) from 13000 B.P. Quat Sci Rev 12:875–887
Förstner U, Muller G (1973) Heavy metals accumulations in river sediments: a response to environmental pollution. Geoforum 14:53–71
Gaillardet J, Dupre B, Allegre CJ (1999) Geochemistry of larger river suspended sediments. Silicate weathering or recycling tracer? Geochem Cosmochim Acta 63:4037–4051
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170:1088–1090
Grosbois C, Négrel P, Fouillac C, Grimaud D (2000) Dissolved load of the Loire River: chemical and isotopic characterization. Chem Geol 170:179–201
Han G, Liu CQ (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21
Ingersoll CG, Haverland PS, Brunson EL, Canfield TJ, Dwyer FJ, Henke CE, Kemble NE, Mount DR, Fox RG (1996) Calculation and evaluation of sediment effect concentrations for the amphipod Hyalella azteca and the midge Chironomus riparius. Gt Lakes Res 22(3):602–623
Irabien MJ, Velasco F (1999) Heavy metals in Oka river sediments (Urdaibai National Biosphere Reserve northern Spain): lithogenic and anthropogenic effects. Environ Geol 37(1–2):54–63
Jeelani G, Shah AQ (2006) Geochemical characteristics of water and sediment from the Dal Lake, Kashmir Himalaya: constraints on weathering and anthropogenic activity. Environ Geol 50:12–30
Jeong CH (2001) Effects of land use and urbanization on hydrochemistry on contamination of groundwater Tarjon area Kore. J Hydrol 253:194–210
Jin ZD, Wang S, Shen J, Wang Y (2003) Carbonate versus silicate Sr isotope in lake sediments and its response to the little ice age. Chin Sci Bull 48:95–100
Johansson K, Iverfeldt A (1994) The relation between mercury content in the soil and the transport of mercury from small catchments in Sweden. In: Watras CJ, Huckabee JW (eds) Mercury pollution, integration and synthesis. Lewis, Boca Raton, pp 323–328
Krishnamurthy RV, Bhattacharya SK, Kusumgar S (1986) Palaeoclimatic changes deduced from 13C/12C and C/N ratios of Karewa Lake sediments, India. Nature 323:150–152
Lerman A (1978) Lake: chemistry, geology, physics. Springer, Berlin
Liu CQ, Li SL, Lang YC, Xiao HY (2006) Using δ15N and δ18O values to identify nitrate sources in karst ground water, Guiyang, Southwest China. Environ Sci Technol 40:6928–6933
Moore JW, Ramamoorthy S (1984) Heavy metals in natural waters: applied monitoring and impact assessment. Springer, New York, Berlin, Heidelberg, p 268
Müller G (1973) Schwermetalle in den sediment des Rheins-Vernderungen seit 1971. Umschau 79(24):778–783
Nag JK, Das AK (1993) Trace metal levels in drinking water scenario of Hooghly district. Ind Environ Prot 13:20–22
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motion inferred from element chemistry of lutites. Nature 299:715–717
Rose NL, Boyle JF, Du Y, Yi C, Dai X, Appleby PG, Bennion H, Cai S, Yu L (2004) Sedimentary evidence for changes in the pollution status of Taihu in the Jiangsu region of eastern China. J Paleolimnol 32:41–51
Rumysa K, Sharique AA, Tariq Z, Farooq M, Bilal A, Pinky K (2012) Physico chemical status of Wular Lake in Kashmir. J Chem Biol Phys Sci November 2012 –January 201 3(1):631–636
Shapiro L, Brannock WW (1962) Rapid analyses of silicate, carbonate and phosphate rocks. USGS Bull 1144A:A1–A32
Singh AK, Husnain SI (1998) Major ion chemistry and weathering control in a high altitude basin: alaknanda River, Garhwal Himalaya, India. Hydrol Sci 43:825–843
Skoulikidis NT, Bertahas I, Koussouris T (1998) The environmental state of freshwater resources in Greece (rivers and lakes). Env Geol 36(1–2):1–17
Smol J (2002) Pollution of lakes and rivers: a paleoenvironmental perspective. Arnold, London
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon, the influence of geology and weathering environment on the dissolved load. Geophys Res 88:9671–9688
Sujatha SD, Sathyanarayanan S, Satish PN, Nagaraju D (2001) A sewage and sludge treated lake and its impact on the environment Mysore, India. Environ Geol 40:1209–1213
Thuy HTT, Tobschall HJ, An PV (2000) Trace element distributions in aquatic sediments of Danang-Hoian area, Vietnam. Environ Geol 39(7):733–740
Turekian SR, Wedepohl KH (1961) Distribution of the elements in some major units of the Earths crust. Geol Soc Am Bull 72:175–192
USEPA (1997) The incidence and severity of sediment contamination in surface waters of the United States. National sediment quality survey US Environmental Agency Report 1:823-R-97–006
Wu L, Huh Y, Qin J, Du G, Van Der Lee S (2005) Chemical weathering in the upper Huang He (Yellow River) draining the eastern Qinghai-Tibet Plateau. Geochim Cosmochim Acta 69(22):5279–5294
Acknowledgments
The authors are highly thankful to HOD, Department of Earth Sciences, University of Kashmir and HOD, Department of Environmental Sciences University of Pune, for providing laboratory and other facilities. The authors also express gratitude to the anonymous reviewers whose comments and suggestions improved the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheikh, J.A., Jeelani, G., Gavali, R.S. et al. Weathering and anthropogenic influences on the water and sediment chemistry of Wular Lake, Kashmir Himalaya. Environ Earth Sci 71, 2837–2846 (2014). https://doi.org/10.1007/s12665-013-2661-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2661-z