Abstract
This study used a total of 474 groundwater samples analyzed from 2014 data to evaluate the distribution of groundwater quality in the Water and Sanitation Agency (WASA) jurisdiction of Lahore city, Pakistan. The study further assessed the variations in suitability of groundwater for drinking (emphasis on arsenic and fluoride) and irrigation using spatial correlation technique in GIS. The hydrochemical analysis revealed a predominance of Mg-Ca-HCO3-SO4 and Ca-Mg-HCO3-SO4 type. Distribution analysis indicated relatively higher salinity (TDSmax = 1667 mg/L), total hardness (THmax = 558 mg/L), and alkalinity (HCO3−max = 584 mg/L) in the south-eastern region of the city, while the central part displayed the highest levels of SO4 and NO3. Also, the eastern region (north-south) of Lahore had significantly elevated As concentrations (up to 86 μg/L). The order of exceedance in terms of arsenic was Gunj Bakhsh town (17.4%), Nishter town (16.4%), Iqbal town (9.8%), Aziz Batti and Shalimar town (8.1%), and Ravi town (3%). The groundwater was classified as average saline to highly saline, except few samples in Aziz Batti/Shalimar town that were in non-saline group. Otherwise, the various indices classified the groundwater for irrigation as generally acceptable. With the various irrigation quality indices displaying discernible variations for the entire study area, it was observed from the distribution maps that the groundwater suitability for irrigation is relatively excellent in the areas away from industries and landfill locations. Also, the chloride analysis shows 98.7% of the groundwater samples belong to the very fresh and fresh water class. Thus, continued monitoring and studying the changes in groundwater quality in Lahore is imperative.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most people in developing countries, including Pakistan depend on groundwater for drinking and irrigation. Increased dependence on groundwater, which is presumed to be cleaner than surface water, has resulted in over-reliance and sometimes over-exploitation of the groundwater resource (Mapoma and Xie 2014; Abbas et al. 2015). Groundwater flow, rainfall, climate, geology, human activities, and over-exploitation trigger the mobilization of naturally occurring contaminants and facilitates introduction of anthropogenic pollutant into the subsurface (Rajesh et al. 2015).
Several studies on groundwater quality have been done worldwide (Abou Zakhem and Hafez 2015; Baillieux et al. 2015; Bonton et al. 2010; Ghesquière et al. 2015; Kudoda and Abdalla 2015; Pathak and Limaye 2011) and Pakistan (Abbas et al. 2015; Manzoor et al. 2006; Mullane et al. 2015; Valipour 2014; Kazi et al. 2009; Farooqi et al. 2007; Rafique et al. 2008). The characterization of groundwater quality for various purposes assists in decision-making on either management of water resource or recommending the best measures when planning to use the water.
In Pakistan, studies on groundwater contamination and the subsequent implications on the population are one of the government’s greatest concerns. As such, monitoring and assessment of groundwater quality is paramount and a priority. Lahore city, which is the second largest city in Pakistan, relies on groundwater supply for development (Abbas et al. 2015). Therefore, the elevated concentrations of contaminants in groundwater such as arsenic may affect the health of the population and in turn impede the economic development. The rapid population growth rate of city increases pressure on groundwater supply capacity thereby affecting the natural balance of the aquifers.
Previous studies on groundwater in some parts of Lahore identified serious breaches on quality of groundwater for drinking (Abbas et al. 2015; Bibi et al. 2015; Farooqi et al. 2007). More importantly, these studies identified high arsenic and fluoride levels parts of Lahore city that they studied. However, the studies did not discuss much on the spatial distribution of groundwater quality for drinking and irrigation in the entire city of Lahore. The Water and Sanitation Agency of Lahore reports that the levels of groundwater in the city is decreasing by approximately 1 ft of the water table every year. This is of great concern on both quantity and quality of the groundwater. It is imperative that communities and policy makers get a clear picture of the spatial characteristics of groundwater in Lahore to facilitate in decision making on either management or proper use of the groundwater. Therefore, this study analyzed the spatial distribution of groundwater quality for drinking and irrigation using proper indices. The study takes into consideration the significance of high levels of arsenic in Lahore. Arsenic values in this region closely resemble reports in other countries in southeast Asia such as Bangladesh (Ahmed et al. 2004; Chakraborti et al. 2010; Halim et al. 2010; Rahman et al. 2013), India (Ahamed et al. 2006; Bordoloi et al. 2013; Buragohain et al. 2010), Nepal, Myanmar, Vietnam, Cambodia, Lao People’s Democratic Republic, and China (Rahman et al. 2009).
The results of the study will provide baseline data on groundwater quality in the area and insight into groundwater management and monitoring. Furthermore, the paper will assist in identifying hot spots that need management and policy making through the quality distribution maps produced. The sampling and laboratory analysis were done in the months of August, September, October, and November 2014.
Study area
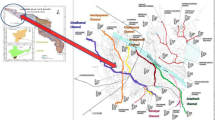
Lahore city lies between 31° 15′–31° 45′ N and 74° 01′–74° 39′ E in the Punjab province, Southeast Pakistan (Fig. 1). The city experiences five seasons of characteristic semi-arid and subtropical continental climate. Foggy winters (15 November–15 February), pleasant spring (16 February–15 April), summer (15 April–15 June), rainy monsoon (16 July–16 September), and dry but pleasant autumn (16 September–14 November) are distinguishing characteristics of the climate in Lahore. The mean annual maximum temperature as high as 41 °C has been recorded for the period May to June, while a mean annual minimum of 4 °C for January (the coldest month) is on record (Abbas et al. 2015). The average annual rainfall for Lahore is 510 mm (Gabriel and Khan 2010).
Lahore is in the Punjab alluvial plain, which displays similar characteristics to the arsenic laden aquifers of West Bengal and Bangladesh (Bibi et al. 2015). The city covers a total land area of 1232 km2, which is underlain by unconsolidated alluvial deposits with intermittent clay lenses. The thickness of sedimentary aquifers is about 400 m. The well logs show that the lenses of less permeable materials are neither thick nor continuous so the aquifer is treated as a single (homogenous) unconfined layer. Various aquifer formations exist in the region. Most of these formations consist of sand, silt, and clay. Gravels of mudstone or siltstone and calcareous concretion locally known as canker exist dispersed in places. The lithological analysis from one well log agrees with other studies showing a sequential depth conformation consisting of clay, fine sand, medium sand, coarse + canker, and medium to coarse + canker (Fig. 2). Important hydrological features in the area are Ravi River in the Northwest and Bambanwala Ravi Bedian Depalpur (BRBD) canal in the East (Fig. 1). Isotopic and chemical analysis data shows that Lahore aquifer has major contributions from River Ravi, and flow path is from the river to the center of the city. The aquifer has mixed recharge from River Ravi and direct rainfall infiltration (Ahmad et al. 2002). However, there is poor management of wastewater in the city where disposal is directly into River Ravi that would result in groundwater contamination. Besides, an unconfined and poorly managed landfill is located in the northeastern part of the city that could add to pollution of the groundwater through infiltration. The abstraction of groundwater of 44.6 MGD (million gallon per day) in 1960 has increased to 784 MGD in 2010 translating to a decline of 1.27 m per year.
The current population of Lahore city is 9.24 million. Water and Sanitation Agency (WASA) of Lahore is responsible for monitoring the quality of drinking water for 6.8 million people while the remaining population is served by private urban schemes. The scope of this research was WASA jurisdiction area, which consists of seven towns (Aziz Batti, Shalimar town, Gunj Bakhsh, Gulberg, Iqbal, Nishter, and Ravi town).
Materials and methods
Sampling
The study used 474 samples from groundwater sources of depth ranging from 110 to 245 m in the study area (Fig. 1). Groundwater was purged for 10 to 15 min before sampling. Samples for major cations and anions analysis were collected in 250 and 100 ml glass bottles, respectively. Immediately after sampling, a drop of standard nitric acid (HNO3) into the sample meant for cation analysis adjusted the pH (< 2). This procedure prevents trace elements from bacterial conversion of nitrite to nitrate. Also, a separate HCl acidified sample was preserved.
Hydrochemical analysis
All the field, titration, and analytical methods followed the prescribed APHA techniques (Rice et al. 2012). Calibrated portable meters (JENCO USA) determined the pH, turbidity, electric conductivity (EC), and total dissolved solids (TDS) in groundwater samples on-site.
The EDTA Titrimetric Method determined TH (mg CaCO3 /L) and calcium (mg/L). Subtracting calcium hardness from TH determined magnesium hardness, which in turn computed the concentration of magnesium (mg/L). Similarly, APHA titration determined total alkalinity (mg/L), while subsequent titration provided results for carbonate concentrations (CO32−) (mg/L) in the samples. Gravimetric method determined Sulfate (SO42−) with drying of residue. Also, argentometric method determined concentration of chloride (Cl−). This method is suitable for use in clear water.
A flame photometer determined Na+ and K+ concentrations. To minimize interference, standard addition technique was chosen to determine these two elements from the standard calibration graphs. The concentration of F− was examined by the SPADNS method using a spectrophotometer (NOVA) at wavelength 570 nm. The inductively coupled plasma-optical emission spectroscopy (ICP) method (ICP-OES, Perkin Elmer) detected arsenic concentration in the samples. Temperature was kept constant to keep analytical accuracy when analyzing samples and standards. The colorimetric method established nitrite (NO2) by using spectrophotometer (NOVA) at 543 nm following a standard procedure that ensures minimal interference from metal ions. Sample analysis happened on the day of sampling to prevent bacterial conversion of nitrite to nitrate. Finally, automated hydrazine reduction method determined nitrate (NO3) levels. The analytical error (ion balance) of the dataset was less than 10%, which is desirable in groundwater quality analysis.
Data analysis
A summary of data (minimum, maximum, and mean) were determined for both observed and computed water quality parameters. All statistics (ANOVA and least squared deviation) were done using SYSTAT. Tables and figures present the data summary obtained from specific hydrological software. To discern the variations in suitability of groundwater for both drinking and irrigation among towns within Lahore, various indices were computed after converting the values to meq/L, as follows:
The geographical information system was used to analyze the spatial distribution of groundwater quality parameters as well as the computed quality indices for the study area. Kriging is the interpolation method that was used to generate the spatial maps. This method relies on the assumption that distance or direction between points reflects a spatial correlation that can be used to explain the variations in the output surfaces. Kriging enables one to optimize the method through the evaluation of the errors associated with the models. Kriging has been widely used in several studies to understand the distribution of groundwater quality (Salcedo-Sánchez et al. 2016; Bohling 2005).
Results and discussion
General hydrochemistry
Firstly, this section shows and discusses data from the field and laboratory analyses arranged according to arsenic levels to facilitate general description of groundwater physico-chemical characteristics (Table 1). Table 2 shows the variation of groundwater physico-chemistry among towns within Lahore. From Table 1, the major physico-chemical parameters varied across the groups, although the variations were not significant (p > 0.05). The piper plot (Fig. 3) illustrates the general groundwater characteristics in the study area. The supplementary material lists the various water facies for individual sampling points. From the piper plot (Fig. 3), it is observed that the groundwater types are predominantly of Ca-Mg-HCO3-SO4 and Mg-Ca-HCO3-SO4. The groundwater containing arsenic higher than 50 μg/L is mainly of Mg-Ca-HCO3-SO4 type (see “supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”). The water types for each town are sorted according to frequency of occurrence for individual sampling points listed in “supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)” as shown in Table 2. The diversity in hydrochemical facies reflects the heterogeneous geological conditions of the aquifers in Lahore (Farooqi et al. 2007) consequently explaining the variations in hydrochemistry within and among the towns studied. The differences in local geology are important to consider when prospecting new tube-wells for drinking water in Lahore.
The concentration of cations and anions varied in different areas as shown in Tables 1 and 2. In most cases, divalent cations (Ca2+ and Mg2+) dominate over univalent cations (Na+ and K+) (Tables 1 and 2). In terms of anions, the HCO3− dominates the group (Tables 1 and 2). It is possible that CO2 (dissolving as HCO3−) from organic matter decomposition is one good supplement to observed alkalinity although organic matter in aquifer sediments was not measured in this study. Besides decomposition product from overburden materials, atmospheric CO2 can find its way into the groundwater by diffusion. Above all, it is stated earlier in this paper that the aquifer is rich in sedimentary rocks whose dissolution could account for the elevated HCO3 levels. Therefore, carbon dioxide dissolution and carbonate weathering can increase HCO3− concentration in groundwater, although alkaline conditions favor precipitation of carbonate minerals (e.g., calcite) over dissolution (Mapoma et al. 2014; Farooqi et al. 2007).
With respect to Na+, Fig. 4 shows excess of Cl− over Na+(stoichiometric ratio ≠ 1), which implies minimal external sources of Na+ and aids in dismissing halite dissolution as a significant source of Na+. Whenever the plot of Na vs Cl falls on the 1:1 line, it signifies halite dissolution, which is not the case in this study. The other probable source of Na+ could be a result of cation exchange. The other sources of Na+ could be cation exchange and industrial activities. Similarly, wastewater pollution and human activities such as agriculture and industries contribute to elevated chlorides in the system.
Fig 5a, b depicts detailed hydrochemical evolution scenarios. Despite differences in correlation with TDS, both cations and anions display a discernible increase with increase in salinity. The linear relationship between TDS and major groundwater quality descriptors is significant with higher regression coefficients for Na+ and HCO3−(p < 0.05). Although the observed correlation between Na+ and TDS is strong, the salinity is more dependent on Ca2+ and Mg2+, while HCO3− and SO42− dominate over Cl− (Fig. 5a, b). This observation affirms earlier findings in Lahore (Abbas et al. 2015) that noted the prevalence of Mg-HCO3 or Ca-HCO3 type in almost all samples studied. With these observations, cation exchange between divalents and monovalent is plausible, although minimal as shown earlier. This necessitates further experimental studies to confirm the mechanisms.
Although ANOVA did not identify the significant differences among the four groups in terms of HCO3− concentrations, LSD analysis showed that the average HCO3 value for As > 50 μg/L group was significantly higher (p < 0.05) (Table 1). Traces of NO2− in all but three samples (99.37%) (“supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”) is a sign of bacterial activities as it is the end product of aerobic decomposition of organic nitrogenous matter (Abbas et al. 2015). This calls for further studies in the area.
Spatial variations in drinking water quality
This section discusses the groundwater quality variations among towns within Lahore city. The values were compared with the WHO (2011) standards and identifies towns with disparities. Overall, pH and turbidity values were within the acceptable range for human consumption. When using a threshold limit of less than 1000 mg/L TDS in drinking water, 44.7% of the total samples exceeded this value (166 ≤ TDS ≤ 1668 mg/L). Groundwater with higher salinity is unsuitable for drinking, and palatability depends on the element contributing most to the overall salinity. In some samples, higher values exceeding the WHO threshold values for alkalinity (Table 2) is apparent (see “supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”). High alkalinity observed in 50.0% of total samples (“supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”) is unacceptable particularly for children (Abbas et al. 2015). In Lahore, groundwater is distributed to consumers by the Integrated Water Supply System, and such high alkalinity may adversely impact on the circulation system by enhancing scale formation through carbonate precipitation and impinge on water flow regime. However, the Langelier saturation index (LSI), computed in Aquachem 2011 (− 35.6 < LSI < − 33.9), recognized the water as undersaturated with respect to calcium carbonate. The index range (LSI < 0) dismissed any evidence of scale forming potential of groundwater in the studied samples. Furthermore, there were no traces of carbonates (CO32−) detected in all the samples. Chloride and SO42− values were both below the WHO limit value of 250 mg/L, respectively, except some samples in GBT (Table 2 and “supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”).
Table 2 shows groundwater quality according to different parts of the study area. The spatial distribution map for TDS, alkalinity, TH, SO42−, NO3−, and As is presented in Figs. 6 and 7. The distribution characteristics of TDS and HCO3− are similar, and higher concentrations are found mainly in the central, south-eastern, and north-eastern regions of the study area (Fig. 6a, b). Nishter town recorded the highest salinity (and highest exceedance) value, while the lowest occurred in Ravi town. Similarly, the highest mean value for alkalinity is eminent in Nishter town, while Ravi town recorded the lowest mean alkalinity value (Table 2). TH has a sporadic (high to low) distribution throughout the area (Fig. 6c). High SO42− and NO3 concentrations are mainly in the central part (Fig. 7a, b), while). The Ravi town had the highest mean NO2− value observed. The highest mean value for NO3 was determined for Aziz Batti and Shalimar town. There was a weak inverse relationship between NO3 and NO2− (r = − 0.02, p = 0.61) which suggests little significance of NO2− oxidation to NO3−. As stated earlier, this needs further confirmation in the next studies. It is possible that NO3− is controlled by dissolution process rather than evaporation as the correlation between nitrate and chloride was also poor (r = 0.07, p = 0.15) (Abou Zakhem and Hafez 2015). However, infiltrating water from River Ravi that is laden with wastewater effluents contributes NO3 into the aquifer.
Spatial distribution maps of TDS (a), HCO3− (b), and TH (c) in the study area showing areas of high concentrations against those displaying low concentrations in Lahore city. Names of towns are included based on Fig. 1
Table 1 shows that As co-varied with HCO3− and alkaline earth metals (Na+ and K+) in terms of mean values across the groups. The correlation analysis confirmed this observation (r = 0.8, 0.7, and 0.5, respectively, p < 0.05). From the summary of results (Table 1), As in majority of the samples exceeds the WHO recommended value (As = 10 μg/L). Six out of 474 samples (representing 1.27% of total samples) showed values of arsenic above 50 μg/L (“supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”); only 15% of the samples were within permissible range (< 10 μg/L). This is further illustrated in the distribution map (Fig. 7c). The map shows that the eastern region (north-south) displayed relatively higher As distribution. From the supplementary material, sample (NI-41) of Nishter town (industrial area) has the highest value of As (86 μg/L). On average, Gunj Bakhsh town exceeded the limit by the most (17.4%) followed by Nishter town area (16.4%). The lowest exceedance (3%) was for Ravi town. The HCO3− dominated groundwater facies observed in all towns in Lahore is conducive for As mobilization. Similarly, the pH observed for all samples in this study is ideal for arsenic mobilization. However, the average arsenic value (Table 1) observed is relatively lower than most of the cases reported in arsenic laden regions (Bangladesh, China, India, and West Bengal) (Smedley and Kinniburgh 2002). However, the situation seriously threatens the health of the population that solely depends on groundwater for drinking in the city.
Fluoride ranged from below detection limit (< D.L) to 1.4 mg/L comparably lower than the 1.5 mg/L prescribed by WHO guidelines (WHO 2011). The results echo those of earlier studies (Farooqi et al. 2007; Abbas et al. 2015). In the right amounts, fluoride is an essential element for human health; therefore, there is need for constant monitoring of fluoride levels in potable groundwater. Fluoride was found highest in Gunj Bakhsh town while the lowest mean value is for Iqbal and Nishter towns.
The industries occupying the northeast and southeast and a solid waste dumpsite located in the northeast parts of Lahore (Fig. 1) may have an impact on groundwater quality through leaching processes (Aiman et al. 2016). Leachates from landfill and seepage from wastewater drains are increasing pollutants in ground water of Lahore. As mentioned earlier (and illustrated in Figs. 6a, b and 7a, respectively), the eastern region of Lahore has the highest distribution of TDS, HCO3−, and SO42−, which may be attributed to industrial activities, poor solid waste, and wastewater management.
Irrigation water quality distribution
Salinity values
As mentioned earlier, salinity refers to total amount of dissolved inorganic solids in natural water. Thus, an increase in TDS and overall chemical load of the water is a predictor of salinization. The excessive dissolved ions, especially Na+, HCO3−, and CO32− in irrigation water affect the soil fertility and crop yield. It is established already in this paper that there are higher salinity values in the city. The WHO recommends that irrigation water should not exceed a TDS value of 450 mg/L with values greater than 2000 mg/L classified as unsuitable. The maximum value observed in this study is 1667 mg/L, which is not bad (Zouahri et al. 2015). The maximum EC value in all the cities is 2010 μS/cm falling in the category of highly saline water. The EC plot shows some highly saline groundwater samples in the city (Fig. 8). As earlier shown (Table 2), Nishter town had the highest percent of groundwater samples exceeding the WHO limit value for drinking in terms of EC. This is no surprise that the same town surpasses the other towns in number of samples falling within the highly saline water (Fig. 8). Increased EC results in decreased water intake by the plants and therefore reduction in productivity. Groundwater samples in GBT, Iqbal, and Nishter fall either in average salinity (C2) or highly saline (C3). Only a few samples in Aziz Batti/Shalimar and Ravi towns had groundwater falling within the non-saline group (C1). However, there were no samples with salinity values in the class of very highly saline (C4) and extremely saline water (C5). In places were irrigation is practiced in the city of Lahore, it is better to be aware of which groundwater abstraction point to use. If no option is available in terms of abstraction points, implementing mitigation measures to obviate the effect of high salinity is a necessary.
Sodium adsorption ratio and magnesium hazard
In terms of sodium adsorption ratio (SAR), the values were found to show that the groundwater is suitable for irrigation SAR (< 9) (Fig. 8). The distribution of SAR values is clearly shown in Fig. 9a. The eastern margin of the city is characterized by relatively higher values of SAR. This is where Nishiter town is located with the highest mean SAR. It is further observed that certain samples (27.2%) in all studied towns exceeded the MH limit value of 50% on individual basis (see “supplementary material (http://doi.pangaea.de/10.1594/PANGAEA.847769)”). Furthermore, ABST showed the highest average and a wider range of MH values while RAT had the lowest mean MH value. Most of the groundwater for RAT fall within the acceptable region for irrigation in terms MH (Fig. 9b) unlike other towns. A high MH value indicates higher Mg2+ compared to Ca2+ ions, which may affect the soil quality by converting it to alkaline conditions and thereby decreasing crop yield. In this case, the eastern region is more prone to development of alkaline soil conditions unlike the northwestern margin (Fig. 9b). Therefore, it is better to identify properly the areas within the towns that have high risk of magnesium for irrigation.
Percent sodium (%Na) and exchangeable sodium ratio (ESR)
Most groundwater samples (%) indicate a %Na within the excellent water class for irrigation, i.e., %Na < 20. Except for one sample in NIT falling in the permissible region (40–60%), the remaining samples were on average classified as excellent for irrigation in terms of %Na (Fig. 9c). In the supplementary material, it shows that the highest mean %Na is observed for NIT while the lowest is for RAT. However, it should be noted that the southern part of the city is relatively poor in terms of %Na than the northern part. Despite this distribution, the results rule out issues of poor permeability of soils due to excess Na in relation to Ca and Mg and hence the water being safer for irrigation (Zouahri et al. 2015; Rajesh et al. 2015). Coupled to %Na, the ESR or Kelly Index (KI) (Fig. 9d) supports the conclusion that groundwater in all towns in Lahore is suitable for irrigation. This relies on the fact that waters with a KI > 1 are unsuitable for irrigation while those of KI less than 1 are suitable. In this study, the average KI in all samples from the five towns was less than 1 with the northern region better than the southern part (industrial area).
Permeability index (PI)
Long-term use of water for irrigation affects soil permeability influenced by Na+, Ca2+, Mg2+, and HCO3− contents of the soil. The PI predicts the possible impact of water quality on soil permeability. The PI is classified into first class (PI > 75%), second class (25 < PI < 75%), and third/unsuitable (PI < 25%). In this study, suitability for irrigation varies among (and within) the towns based on PI values observed (Fig. 9e). However, most samples for all towns belonged to the second class and some samples in the first class. These two classes define groundwater as good for irrigation (Fig. 9e). In terms of distribution, it is noted that the mean PI values for IQT and NIT belong to the first class while those of ABST, GBT, and RAT falling in the range of second class. The PI distribution observed for the southern part of the city explains the poor quality of groundwater in the area relative to the northern part in that good permeability is prone to increased infiltration of polluting chemicals. None of the groundwater samples from these towns had their PI less than 25% (unsuitable).
Risk related to bicarbonate
Residual sodium bicarbonate (RSBC) value of less than 5 meq/L classifies water samples as satisfactory for irrigation. In this study, most samples had RSBC values less than 5 meq/L except few samples (8.2%) (Fig. 9f). Overall, the RSBC values among towns indicate mean values below the RSBC prescribed upper limit of 5 meq/L (Gupta and Gupta 1987). Despite having some values higher than 5 meq/L, the groundwater remains within suitable range with maximum value falling below 10 meq/L. The areas in the center and eastern margin where industries are located as well as the northeastern margin of the city (where a landfill is found) have RSBC comparably higher. These areas coupled with poor wastewater management result in increased contamination of the groundwater through infiltrating water.
Risk related to chloride
Whenever the chloride ion concentration in irrigation water is higher than 4 meq/L, toxicity problems to plants may occur (Zouahri et al. 2015). The Cl− toxicity affects sensitive crops such as maize. In this study, majority of the groundwater samples had Cl− concentration less than 4 meq/L and a few between 4 and 10 mg/L (Table 2). Since none of the groundwater samples from the five towns exceeded 10 meq/L, the groundwater remains within the category of good water for irrigation. But, precautionary measures are necessary for the samples with Cl between 4 and 10 meq/L before using the water for irrigation. It is also noted that majority of samples (52.3%) fall within the very fresh water group (0.14 > Cl− > 0.85), 46.4% classified as fresh water (0.85 < Cl− < 4.23 meq/L), and the remaining few (1.3%) falling within the fresh brackish (4.23 < Cl− < 8.46) category (Zouahri et al. 2015; Stuyfzand 1989). The second group of samples (fresh brackish) requires careful consideration when planning to use for irrigation in Lahore.
Conclusions
From the 474 groundwater samples in Lahore city (WASA jurisdiction), the study highlights the bicarbonate (HCO3) type of groundwater dominated by Ca2+ and Mg2+, in that order. The eastern area (north-south) of the city was relatively elevated in terms of cations and anions. This area is dominated by industrial activities and a waste dumpsite in the northeastern part. The study shows that geogenic processes inherently influence the variation in groundwater quality. However, the elevated Cl and NO3 indicate influence of industrial, poor wastewater, and landfill management on the quality of the groundwater. The activities explain the spatial distribution of the groundwater suitability for drinking and irrigation. Despite this, the groundwater in the city remains suitable for irrigation except where 1.3% of the water remains classified as fresh-brackish water. Proper management of solid waste, wastewater, and regulation of industrial activities could lessen the burden on groundwater quality violations.
References
Abbas, Z., Su, C., Tahira, F., Mapoma, H. W. T., & Aziz, S. Z. (2015). Quality and hydrochemistry of groundwater used for drinking in Lahore, Pakistan: analysis of source and distributed groundwater. Environmental Earth Sciences, 74(5), 4281–4294. https://doi.org/10.1007/s12665-015-4432-5.
Abou Zakhem, B., & Hafez, R. (2015). Hydrochemical, isotopic and statistical characteristics of groundwater nitrate pollution in Damascus Oasis (Syria). Environmental Earth Sciences, 74(4), 2781–2797. https://doi.org/10.1007/s12665-015-4258-1.
Ahamed, S., Kumar Sengupta, M., Mukherjee, A., Amir Hossain, M., Das, B., Nayak, B., Pal, A., Chandra Mukherjee, S., Pati, S., Nath Dutta, R., Chatterjee, G., Mukherjee, A., Srivastava, R., & Chakraborti, D. (2006). Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: a severe danger. Sci Total Environ, 370(2–3), 310–322. https://doi.org/10.1016/j.scitotenv.2006.06.015.
Ahmad, M., Rafiq, M., Akram, W., Tasneem, M., Ahmad, N., Iqbal, N., & Sajjad, M. I. (2002). Assessment of aquifer system in the city of Lahore, Pakistan using isotopic techniques. Major Urban Areas, 33, 109–133.
Ahmed, K. M., Bhattacharya, P., Hasan, M. A., Akhter, S. H., Alam, S. M. M., Bhuyian, M. A. H., Imam, M. B., Khan, A. A., & Sracek, O. (2004). Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Applied Geochemistry, 19(2), 181–200. https://doi.org/10.1016/j.apgeochem.2003.09.006.
Aiman, U., Mahmood, A., Waheed, S., & Malik, R. N. (2016). Enrichment, geo-accumulation and risk surveillance of toxic metals for different environmental compartments from Mehmood Booti dumping site, Lahore city, Pakistan. Chemosphere, 144, 2229–2237. https://doi.org/10.1016/j.chemosphere.2015.10.077.
Baillieux, A., Moeck, C., Perrochet, P., & Hunkeler, D. (2015). Assessing groundwater quality trends in pumping wells using spatially varying transfer functions. Hydrogeology Journal, 23(7), 1449–1463. https://doi.org/10.1007/s10040-015-1279-5.
Bibi, M., Hashmi, M. Z., & Malik, R. N. (2015). Human exposure to arsenic in groundwater from Lahore district, Pakistan. Environmental Toxicology and Pharmacology, 39(1), 42–52. https://doi.org/10.1016/j.etap.2014.10.020.
Bohling, G. (2005). Introduction to geostatistics and variogram analysis. Kansas geological survey, 1, 1–20.
Bonton, A., Rouleau, A., Bouchard, C., & Rodriguez, M. J. (2010). Assessment of groundwater quality and its variations in the capture zone of a pumping well in an agricultural area. Agricultural Water Management, 97(6), 824–834. https://doi.org/10.1016/j.agwat.2010.01.009.
Bordoloi, S., Nath, S. K., Gogoi, S., & Dutta, R. K. (2013). Arsenic and iron removal from groundwater by oxidation-coagulation at optimized pH: laboratory and field studies. Journal of Hazardous Materials, 260, 618–626. https://doi.org/10.1016/j.jhazmat.2013.06.017.
Buragohain, M., Bhuyan, B., & Sarma, H. P. (2010). Seasonal variations of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam, India. [journal article]. Environmental Monitoring and Assessment, 170(1), 345–351. https://doi.org/10.1007/s10661-009-1237-6.
Chakraborti, D., Rahman, M. M., Das, B., Murrill, M., Dey, S., Chandra Mukherjee, S., Dhar, R. K., Biswas, B. K., Chowdhury, U. K., Roy, S., Sorif, S., Selim, M., Rahman, M., & Quamruzzaman, Q. (2010). Status of groundwater arsenic contamination in Bangladesh: a 14-year study report. Water Research, 44(19), 5789–5802. https://doi.org/10.1016/j.watres.2010.06.051.
Farooqi, A., Masuda, H., & Firdous, N. (2007). Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environmental Pollution, 145(3), 839–849. https://doi.org/10.1016/j.envpol.2006.05.007.
Gabriel, H., & Khan, S. (2010). Climate responsive urban groundwater management options in a stressed aquifer system. IAHS-AISH Publication, 338, 166–168.
Ghesquière, O., Walter, J., Chesnaux, R., & Rouleau, A. (2015). Scenarios of groundwater chemical evolution in a region of the Canadian Shield based on multivariate statistical analysis. Journal of Hydrology: Regional Studies, 4, 246–266. https://doi.org/10.1016/j.ejrh.2015.06.004.
Gupta, S. K., & Gupta, I. C. (1987). Management of saline soils and waters. New Delhi: Oxford & IBH Publishing Company.
Halim, M. A., Majumder, R. K., Nessa, S. A., Hiroshiro, Y., Sasaki, K., Saha, B. B., Saepuloh, A., & Jinno, K. (2010). Evaluation of processes controlling the geochemical constituents in deep groundwater in Bangladesh: spatial variability on arsenic and boron enrichment. Journal of Hazardous Materials, 180(1–3), 50–62. https://doi.org/10.1016/j.jhazmat.2010.01.008.
Kazi, T. G., Arain, M. B., Jamali, M. K., Jalbani, N., Afridi, H. I., Sarfraz, R. A., Baig, J. A., & Shah, A. Q. (2009). Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicology and Environmental Safety, 72(2), 301–309. https://doi.org/10.1016/j.ecoenv.2008.02.024.
Kudoda, A. M., & Abdalla, O. A. E. (2015). Hydrochemical characterization of the main aquifers in Khartoum, the capital city of Sudan. Environmental Earth Sciences, 74(6), 4771–4786. https://doi.org/10.1007/s12665-015-4464-x.
Manzoor, S., Shah, M. H., Shaheen, N., Khalique, A., & Jaffar, M. (2006). Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. Journal of Hazardous Materials, 137(1), 31–37. https://doi.org/10.1016/j.jhazmat.2006.01.077.
Mapoma, H. W. T., & Xie, X. (2014). Basement and alluvial aquifers of Malawi: an overview of groundwater quality and policies. African Journal of Environmental Science and Technology, 8(3), 190–202. https://doi.org/10.5897/ajest2013.1639.
Mapoma, H. W. T., Xie, X., & Zhang, L. (2014). Redox control on trace element geochemistry and provenance of groundwater in fractured basement of Blantyre, Malawi. Journal of African Earth Sciences, 100, 335–345. https://doi.org/10.1016/j.jafrearsci.2014.07.010.
Mullane, J. M., Flury, M., Iqbal, H., Freeze, P. M., Hinman, C., Cogger, C. G., & Shi, Z. (2015). Intermittent rainstorms cause pulses of nitrogen, phosphorus, and copper in leachate from compost in bioretention systems. Sci Total Environ, 537, 294–303. https://doi.org/10.1016/j.scitotenv.2015.07.157.
Pathak, H., & Limaye, S. N. (2011). Study of seasonal variation in groundwater quality of Sagar City (India) by principal component analysis. E-Journal of Chemistry, 8(4), 2000–2009. https://doi.org/10.1155/2011/765749.
Rafique, T., Naseem, S., Bhanger, M. I., & Usmani, T. H. (2008). Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environmental Geology, 56(2), 317–326. https://doi.org/10.1007/s00254-007-1167-y.
Rahman, M. M., Naidu, R., & Bhattacharya, P. (2009). Arsenic contamination in groundwater in the Southeast Asia region. Environmental Geochemistry and Health, 31(1), 9–21. https://doi.org/10.1007/s10653-008-9233-2.
Rahman, M. M., Asaduzzaman, M., & Naidu, R. (2013). Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. Journal of Hazardous Materials, 262, 1056–1063. https://doi.org/10.1016/j.jhazmat.2012.06.045.
Rajesh, R., Brindha, K., & Elango, L. (2015). Groundwater quality and its hydrochemical characteristics in a shallow weathered rock aquifer of southern India. Water Quality, Exposure and Health, 7(4), 515–524. https://doi.org/10.1007/s12403-015-0166-6.
Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (Eds.). (2012). Standard methods for the examination of water and wastewater (22nd ed.). Washington, DC: American Public Health Association, American Water Works Association, Water Environment Federation.
Salcedo-Sánchez, E. R., Hoyos, S. E. G., Alberich, M. V. E., & Morales, M. M. (2016). Application of water quality index to evaluate groundwater quality (temporal and spatial variation) of an intensively exploited aquifer (Puebla valley, Mexico). Environmental Monitoring and Assessment, 188(10), 573.
Smedley, P., & Kinniburgh, D. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568. https://doi.org/10.1016/S0883-2927(02)00018-5.
Stuyfzand, P. J. (1989). Nonpoint source of trace element in potable groundwater in Netherland. In Proceedings of the 18th TWSA Water Workings, KIWA, 1989. Nieuwegein: Testing and Research Institute.
Valipour, M. (2014). Drainage, waterlogging, and salinity. Archives of Agronomy and Soil Science, 60(12), 1625–1640. https://doi.org/10.1080/03650340.2014.905676.
WHO. (2011). Guidelines for drinking-water quality (4th ed.). Geneva, Switzerland: World Health Organization.
Zouahri, A., Dakak, H., Douaik, A., El Khadir, M., & Moussadek, R. (2015). Evaluation of groundwater suitability for irrigation in the Skhirat region, northwest of Morocco. Environmental Monitoring and Assessment, 187(1), 4184. https://doi.org/10.1007/s10661-014-4184-9.
Funding
The authors acknowledge the material support from Water and Sanitation Agency Lahore, Pakistan. The research work was financially supported by National Natural Science Foundation of China (No. 41521001, No. 40802058, and No. 41502230).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abbas, Z., Mapoma, H.W.T., Su, C. et al. Spatial analysis of groundwater suitability for drinking and irrigation in Lahore, Pakistan. Environ Monit Assess 190, 391 (2018). https://doi.org/10.1007/s10661-018-6775-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6775-3