Abstract
Fishmeal is an indispensable ingredient for most aquatic animals. However, the finite supply and escalating price of fishmeal seriously limit its use in aquaculture. Thus the development of new, sustainable protein ingredients has been a research focus. Microalgae are potential fishmeal alternatives owing to their high protein content and balanced amino acid profile. Studies suggest that suitable replacement of fishmeal with microalgae is beneficial for fish growth performance, but excessive replacement would induce poor growth and feed utilization. Therefore, this paper aims to review research on the maximum substitutional level of fishmeal by microalgae and propose the main issues and possible solutions for fishmeal replacement by microalgae. The maximum replacement level is affected by microalgal species, fish feeding habits, quality of fishmeal and microalgal meals, and supplemental levels of fishmeal in the control group. Microalgae could generally replace 100%, 95%, 95%, 64.1%, 25.6%, and 18.6% fishmeal protein in diets of carp, shrimp, catfish, tilapia, marine fish, and salmon and trout, respectively. The main issues with fishmeal replacement using microalgae include low production and high production cost, poor digestibility, and anti-nutritional factors. Possible solutions to these problems are recommended in this paper. Overall, microalgae are promising fishmeal alternatives in aquaculture.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture is the fastest-growing food-producing sector in the world and the primary source of aquatic products for humans (Houston et al. 2020; Subasinghe et al. 2009). The rapid development of aquaculture depends heavily on the supply of aquafeed. It is estimated that the total compound aquafeed usage in aquaculture was 51.23 million tonnes in 2017, and is expected to rise to 73.15 million tonnes by 2025 (Tacon 2020). In fish farming, aquafeed generally represents approximately 50% to 70% of total aquaculture production costs (Foster et al. 1995; Vassiliou et al. 2015). Protein is the most expensive component in aquafeed (Craig and Helfrich 2009). Among various protein ingredients in aquafeed, fishmeal is regarded as the ideal protein source for most aquatic animals owing to its balanced amino acid profile and palatability (Jannathulla et al. 2019). However, the limited stock, unstainable growth, and unstable supply escalate the price of fishmeal (Abbott et al. 2021). Therefore, great efforts have been made to look for fishmeal alternatives (Glencross et al. 2007; Tacon et al. 2011; Teves and Ragaza 2016).
Microalgae, a diverse collection of O2-evolving, photosynthetic organisms, are characterized by wide distribution, rapid growth and reproduction, and strong tolerance to extreme environments, etc. (Mata et al. 2010; Richmond and Hu 2013). Some microalgae are rich in protein (e.g., Spirulina) and could be effective solutions to the problem of fishmeal alternatives in aquaculture (Ahmad et al. 2022; Nagappan et al. 2021). Although many reviews have been done on applications of microalgae in aquaculture (Ahmad et al. 2020; Alagawany et al. 2021; Hemaiswarya et al. 2011; Ragaza et al. 2020; Tham et al. 2023), little attention was given to fishmeal replacement. Accordingly, the following discussion focuses on microalgal meals as fishmeal alternatives and summarizes advances as a basis for future improvements.
Current status of fishmeal production
Fishmeal is a brown powder obtained after cooking, pressing, drying, and milling fresh raw fish and byproducts derived from fish processing (Shepherd and Jackson 2013). Raw fishes used to manufacture fishmeal are mainly composed of small marine pelagic species (e.g., anchovy, mackerel, herring, and sardine) (Péron et al. 2010). Production of these raw fishes is easily affected by climate conditions (mainly El Niño Events). It is estimated that an El Niño year can decrease 4–5 million tonnes of raw fish and 1 million tonnes of fishmeal in Peru and northern Chile (Hardy 2010). Therefore, global fishmeal production fluctuates according to changes in the catches of these raw fish species and averages 5 million tonnes annually (Fig. 1). From 2001 to 2010, the average yearly fishmeal production was above 5.5 million tonnes, while from 2011 to 2020 it was around 5 million tonnes (European Commission 2021). According to FAO, global fishmeal production is expected to reach approximately 6 million tonnes in 2030 owing to an increased amount of the production being obtained from fish waste and byproducts of the processing industry (FAO 2020). Since fishmeal stocks are limited, and face growing sustainability concerns, the fishmeal price has been increasing dramatically for the last 20 years. According to FAO (2020), the fishmeal price is projected to reach 1800 USD/ton by 2030 (Fig. 1).
Aquaculture is the primary consumer of fishmeal (Boyd et al. 2022; Hua et al. 2019). No dramatic changes were made in the global fishmeal use by sector throughout 2009–2019 (Fig. 2). In 2009 and 2010, 63% and 73% of world fishmeal were consumed by aquaculture (Shepherd and Jackson 2013). In 2017–2019, the share increased up to 78% (European Commission 2021). In 2019, around 25% of the fishmeal going into aquaculture was used to feed crustaceans, 15% to feed salmon and trout, 17% to feed marine fish, and 21% to feed freshwater species. The rest was divided between tilapias, cyprinids, and eels (Fig. 2) (European Commission 2021). With the rapid growth of aquaculture, fishmeal could hardly meet the demands.
Current status of microalgal production
Microalgal production comprises several major stages: microalgae cultivation, dewatering, and drying (Fig. 3) (Halim et al. 2012). Microalgae cultivation is conducted in either open systems or closed systems (Chisti 2007). The commonly used open systems include circular ponds and raceway ponds; the closed systems contain tubular photobioreactors, flat plate photobioreactors, and fermenters (Perez-Garcia et al. 2011; Posten 2009). Both systems have advantages and disadvantages (Dębowski et al. 2020). The open systems have a lower production cost and operating cost but also have some disadvantages, including water evaporation, difficulties in managing culture conditions, poor microalgae growth, and susceptibility to microbial contamination (Goswami et al. 2021). On the other hand, the closed systems have higher cell densities and are easier to control culture conditions and microbial contamination, but have higher production and operating costs (Pulz 2001). Flocculation is regarded as the most advantageous dewater technology owing to its low energy requirement (Wijffels and Barbosa 2010). Spraying drying, drum drying, and freeze drying are common drying methods for obtaining microalgae biomass in microalgal production (Grima et al. 2013). Microalgae biomass applied in aquaculture could be classified into two categories: whole microalgae and lipid-extracted microalgae which are the protein-rich byproducts of diesel production from microalgae (Fig. 3) (Maisashvili et al. 2015; Sarker et al. 2018).
Presently, large-scale production of microalgae is dominated by several genera (e.g., Spirulina sp., Chlorella sp., Dunaliella sp., Aphanizomenon sp., Haematococcus sp., Crypthecodinium sp., and Schizochytrium sp.) (Rizwan et al. 2018). It is estimated that the annual production of microalgae ranged from 19,000 to 20,000 tonnes (dry weight) between 2016 and 2018, and was projected to increase up to 27,500 tonnes (dry weight) in 2024 (Benemann et al. 2018; Transparency Market Research 2016). Among the main microalgal species, Spirulina and Chlorella account for 65.78% and 26.32%, respectively (Fig. 4).
Microalgae as fishmeal alternatives
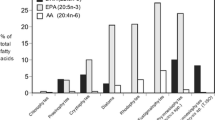
The high protein content and balanced amino acid profiles are considered the main reasons for microalgae as protein sources in aquafeed (Kovač et al. 2013). Microalgae can synthesize all amino acids, thus containing all the essential amino acids in significant amounts (Ibañez and Cifuentes 2013). The amino acid composition of the microalgae is similar, irrespective of algal class (Fig. 5), which suggests that protein quality also is similar (Brown et al. 1997). Besides, they also demonstrate the varying potential of digestibility, with digestibility coefficients ranging from 59.4% to 95.1% (Becker 2004b). It was suggested that the average protein quality of most microalgae could be superior to conventional plant feedstuffs (Becker 2007). Spirulina and Chlorella are rich in protein and possess great potential in fishmeal replacement in aquaculture (Alagawany et al. 2021). Besides Spirulina and Chlorella, there are some protein-rich microalgae with great potential to replace fishmeal, such as Scenedesmus, Dunaliella, and Synechococcus (Table 1).
Partial replacement of fishmeal with microalgae significantly increases feed intake and fish growth through bioactive compounds (e.g., pigments, vitamins, minerals, and fatty acids) in microalgae (Alagawany et al. 2021; Chen et al. 2021). However, accumulating studies suggest that excessive replacement would induce poor growth and feed utilization (Binh Van et al. 2020; Olvera‐Novoa et al. 1998). Several aspects could account for the phenomenon. First, the high inclusion of Spirulina induces mineral deficiency, resulting in decreased biomass productivity (Olvera‐Novoa et al. 1998). Minerals are essential for maintaining the normal life processes of aquatic animals (Lall 2022), and ash content in fishmeal is higher than that in Spirulina meal (Kim et al. 2013; Olvera‐Novoa et al. 1998). Olvera‐Novoa et al. (1998) found that the addition of phosphorus (P) in the 100% replacement group significantly increased fish growth and feed efficiency over groups without P addition in Mozambique tilapia (Oreochromis mossambicus), similar results were also found in gibel carp (Carassis auratus gibelio) (Cao et al. 2018a). Second, the high replacement of fishmeal by microalgal meals decreased feed palatability, leading to reductions in feed intake and fish growth (Walker and Berlinsky 2011). Fishmeal replacement with S. maxima meal increased feed hardness, decreasing the feed palatability and feed intake in Mozambique tilapia (Olvera‐Novoa et al. 1998). Walker and Berlinsky (2011) utilized Nannochloropsis sp. and Isochrysis sp. to replace fishmeal in Atlantic cod (Gadus morhua) and also found a palatability problem in groups with excessive fishmeal replacement by microalga. Third, anti-nutritional factors (ANFs) in microalgae may inhibit fish growth when microalgae meals replace high levels of fishmeal (Ahmad et al. 2022). ANFs are known to inhibit fish growth and some ANFs have been reported in microalgae (National Research Council 2011; Silva et al. 2020; Jacob-Lopes et al. 2019). Therefore, in the present review, we concentrate on the maximum replacement level of fishmeal by microalgae meals without affecting fish growth performance.
Maximum replacement level of fishmeal with Spirulina meal
Spirulina is a multicellular, filamentous, photoautotrophic cyanobacteria that naturally lives in tropical and subtropical water bodies with high pH and alkalinity (Vonshak 1997). S. maxima and S. platensis are the two most studied Spirulina for fishmeal replacement in aquaculture (Table 2). As a fishmeal alternative, Spirulina has many advantages. Firstly, it contains high protein (60%-70% dry weight); secondly, Spirulina cells do not have cellulose walls, which makes their protein highly digestible (Altmann and Rosenau 2022; Ragaza et al. 2020). Spirulina has already been tested as a substitute for fishmeal protein source for many aquatic animals, including sturgeon, trout, carp, catfish, tilapia, prawn, and shrimp.
Research on Spirulina as a fishmeal alternative in sturgeons was mainly conducted by Palmegiano et al. (2005, 2008). In 2005, Palmegiano et al. evaluated Spirulina sp. (60% crude protein) as fishmeal alternatives in Siberian sturgeon (Acipenser baeri) by setting three replacement levels (63.0%, 75.9%, and 88.9%) and found that sturgeon in the 88.9% replacement group exhibited higher final body weight (FBW) and protein efficiency ratio (PER), and lower feed conversion ratio (FCR) compared to the control (P < 0.05) (Palmegiano et al. 2005). In another trial with white sturgeon (Acipenser transmontanus), Palmegiano et al. (2008) reported that Spirulina sp. could replace 63% fishmeal (34% absolute content) in white sturgeon without adverse effects on specific growth rate (SGR), FCR, PER, and survival rate.
As for trout, Teimouri et al. (2013) investigated the effect of replacing fishmeal with Spirulina meal (62.0% crude protein) using four substitutional levels (6.0%, 11.9%, 17.9%, and 23.8%) on growth performance of rainbow trout (Oncorhynchus mykiss). After ten weeks of feeding, they observed that Spirulina sp. could replace 23.8% fishmeal (10% absolute content) without affecting FBW, weight gain rate (WGR), SGR, and FCR (Teimouri et al. 2013).
Several studies have been conducted on the effects of dietary Spirulina as a fishmeal alternative on the growth performance of carps. According to the study of Abdulrahman and Ameen (2014), common carp (Cyprinus carpio) were fed diets containing different substitutional levels (10.3%, 20.7%, 30.6%, and 41.2%) of fishmeal (about 68% crude protein) by Spirulina sp. (34% crude protein), and fish weight gain in the 41.2% replacement group (15.0 g) nearly doubled compared to the control (8.4 g). In addition, Nandeesha et al. (1998) evaluated S. platensis (54.5% crude protein) as a fishmeal substitute in common carp and concluded that S. platensis could replace 100% fishmeal without detrimental impacts on FBW, SGR, FCR, and PER. Similarly, Cao et al. conducted two studies to evaluate fishmeal replacement with S. platensis in gibel carp and found that S. platensis could replace 100% fishmeal without adverse effects on feed intake (FI), FBW, SGR, feed efficiency (FE), and PER (Cao et al. 2018a, 2018b). These results were in line with findings reported for two Indian major carps catla (Catla catla) and rohu (Labeo rohita) (Nandeesha et al. 2001).
Similar to carps, four kinds of catfish species were reported in fishmeal replacement by Spirulina meal. Akter et al. (2023) investigated fishmeal replacement with S. platensis by setting four substitutional levels (10%, 15%, 20%, and 25%) in pabda catfish (Ompok pabda) and found that S. platensis could replace 25% fishmeal (7.82% absolute content) and simultaneously increase FBW, WGR, and SGR. Raji et al. (2020) evaluated the effect of 100% fishmeal replacement with S. platensis and found that 100% fishmeal replacement significantly increased FBW, FI, SGR, and PER, and decreased FCR (P < 0.05) in African catfish (Clarias gariepinus). Similar results were also reported in Mekong giant catfish (Pangasianodon gigas) (Tongsiri et al. 2010). However, in yellow catfish (Pelteobagrus fulvidraco), S. platensis can only replace 80% fishmeal and 100% fishmeal replacement significantly decreased FBW, feeding rate (FR), SGR, and FE (Liu et al. 2019). Differences in catfish species may partially account for the phenomenon.
Similar to carps and catfish, four kinds of tilapia species were used to investigate Spirulina meal as fishmeal alternatives. In Mozambique tilapia, Olvera‐Novoa et al. (1998) substituted fishmeal with S. maxima (66.86% crude protein) and found that microalgae could replace 40% fishmeal. In red tilapia fingerlings (Oreochromis sp.), Rincón et al. (2012) evaluated three substitutional levels (10%, 20%, and 30%) of fishmeal with S. maxima and found no significant difference in WGR, PER, and FE among all groups. In hybrid red tilapia (O. niloticus x O. mossambicus), S. platensis replaced 100% fishmeal, increased FBW, WGR, PER, and survival rate, and decreased FCR (P < 0.05) (El-Sheekh et al. 2014). In Nile tilapia (O. niloticus), Velasquez et al. (2016) evaluated four substitutional levels (30%, 47%, 75%, and 100%) of fishmeal (65.65% crude protein) by S. platensis (56.7% crude protein) and found that up to 75% fishmeal could be replaced by microalgae without adversely influencing FBW, SGR, and survival rate.
Different from results in carps and catfish, Spirulina replaces less fishmeal in mullet (Mugil liza), silver seabream (Rhabdosargus sarba), and barramundi (Lates calcarifer). In an eighty-day-feeding trial with juvenile mullet, the effect of S. platensis (61.61% crude protein) in replacing fishmeal on fish growth performance was evaluated with four substitutional levels (30.7%. 50.0%, 69.2%, and 100%) (Rosas et al. 2019). Fish in the 69.2% replacement group had similar FBW, WGR, SGR, FCR, and PER compared to the control (P > 0.05), while fish in the 100% replacement group showed a lower SGR, PER, and survival rate, and a higher FCR (P < 0.05) (Rosas et al. 2019). El-Sayed (1994) evaluated Spirulina sp. as protein source for silver seabream with four substitutional levels (25%, 50%, 75%, and 100%) and found that Spirulina sp. (61.88% crude protein) could only replace up to 50% fishmeal without negative effects on FBW, WGR, SGR, FC, and PER. In agreement with these results, raw S. platensis (64.1% crude protein) can only replace 20% fishmeal (14% absolute content) in barramundi. However, the substitutional level of fishmeal could be increased up to 40% when S. platensis was treated using cellulose and serine endo-peptidase, suggesting that these enzymes can improve the quality of Spirulina meal.
In studies of fishmeal replacement with Spirulina meal in Pacific whiteleg shrimp (Penaeus vannamei), Pakravan et al. (2017) and Macias-Sancho et al. (2014) incorporated the same amount of fishmeal in the control group (40%), used the similar quality of microalgal meals (68.0% vs. 66.9%), but drew different conclusions. The former found that microalgae could replace 100% fishmeal without adverse effects on FBW, SGR, FCR, and survival rate. However, the latter observed that 100% replacement induced lower FBW, WGR, SGR, and a higher FCR compared to the control (Macias-Sancho et al. 2014). Differences in fishmeal quality could be the main reason for the phenomenon. Pakravan et al (2017) used fishmeal with 51% crude protein, while Macias-Sancho et al. utilized fishmeal with 68.6% crude protein. Inclusion of the same content of microalgae substituted higher levels of inferior fishmeal than superior fishmeal.
Two prawn species were used for fishmeal replacement with Spirulina meal. Sivakumar et al. (2018) evaluated the growth performance of giant tiger prawn (Penaeus monodon) fed diets containing different substitutional levels (14.3%, 28.6%, 42.8%, and 57.1%) of fishmeal by S. platensis. After a sixty-day-feeding trial, they found that 28.6% and 42.8% fishmeal replacement significantly increased FI, SGR, and FE, while 57.1% fishmeal replacement significantly decreased PER without adverse effects on FI, FCR, and SGR (Sivakumar et al. 2018). Radhakrishnan et al. (2016) investigated the impact of fishmeal replacement with S. platensis (62.1% crude protein) on the growth performance of giant river prawn (Macrobrachium rosenbergii) and found that microalgae could replace 100% fishmeal without adverse effects on WGR and SGR.
In addition, Spirulina meal was also used to replace fishmeal in hybrid striped bass (Morone crhysops × M. saxatilis), parrot fish (Oplegnathus fasciatus), and red drum (Sciaenops ocellatus). The results showed that microalgae could replace 50% fishmeal in hybrid striped bass (Perez-Velazquez et al. 2019), 37.7% fishmeal in parrot fish (Kim et al. 2013), and 50% fishmeal in red drum without detrimental effects on WGR, SGR, FE, and PER (Table 2).
Maximum replacement level of fishmeal with Chlorella meal
Chlorella is a genus of unicellular green microalgae and is rich in protein (42%-58%), minerals, vitamins, and carotenoids (Oh et al. 2022; Safi et al. 2014a). Compared with Spirulina, Chlorella has a rigid cellulose-rich cell wall, which makes it difficult for the digestive enzymes of aquatic animals to access the cellular components (Kotrbáček et al. 2015; Yamada and Sakaguchi 1982) and thus greatly limits its application in fishmeal replacement in aquaculture. Many effective methods have been employed to disrupt the cell wall of Chlorella to increase the digestibility of protein (Becker 2007; Safi et al. 2014b). Similar to Spirulina meal, work on Chlorella meal as fishmeal alternatives was carried out in many aquatic animals, including freshwater fish (e.g., carps and tilapias), marine fish (e.g., red drum), and crustaceans (Table 3).
In freshwater fish, studies on Chlorella meal for fishmeal replacement were concentrated on zebrafish, (Danio rerio), crucian carp (Carassius auratus), largemouth bass (Micropterus salmoides), African catfish, and Nile tilapia. In zebrafish (Carneiro et al. 2020) and crucian carp (Shi et al. 2017), fishmeal can be 100% substituted by Chlorella meal without affecting FBW, WGR, SGR, and PER. In largemouth bass (Micropterus salmoides), Chlorella meal could replace 75% fishmeal (30% absolute content) without influencing fish growth and feed utilization (Xi et al. 2022); when the replacing level reached 100% (40% absolute content), FBW, WGR, SGR, FI, and FE were all significantly decreased (Xi et al. 2022).
African catfish with different body weight was used to evaluate the effect of Chlorella sp. (58% crude protein) as fishmeal alternatives. In the small fish (7.8 g), C. vulgaris (58% crude protein) significantly increased FBW, SGR, FI, and PER, and decreased FCR in fish fed 75% replacement level diets (Raji et al. 2019). In the big fish (58.1 g), only the 100% fishmeal replacement level was assessed and fish in the 100% replacement group by C. vulgaris (58% crude protein) had higher FBW, SGR, and PER, and lower FCR compared to the control (P < 0.05) (Raji et al. 2020).
Badwy et al. (2008) investigated the effect of Chlorella sp. (46.78% crude protein) as fishmeal alternatives with four replacement levels (10%, 25%, 50%, and 75%) in Nile tilapia and found that Chlorella sp. could only replace 50% fishmeal (11.11% absolute content). In a later study, juvenile Nile tilapia were fed diets in which fishmeal was replaced by Chlorella sp. (47.2% crude protein) using three substitutional levels (34.3%, 67.2%, and 100%) (Lupatsch and Blake 2013) and Chlorella sp. could only replace 34.3% fishmeal (22% absolute content) without adverse influence on growth performance. It is strange that the former replaced less absolute fishmeal but got a higher relative replacement level. The difference in replacement level could be mainly due to the fishmeal supplemental level in the control group. The former added 22.23% fishmeal, while the latter supplemented 64% fishmeal. Therefore, a higher supplemental level of fishmeal in the control group will decrease the relative substitution level of fishmeal by microalgae.
Work on Chlorella meal for substitution of fishmeal in marine fish was mainly done in red drum, olive flounder (Paralichthys olivaceus), and gilthead seabream (Sparus aurata). In red drum, four substitutional levels (5%, 10%, 20%, and 25%) of fishmeal by lipid-extracted Chlorella sp. (21.2% crude protein) were assessed and only 5% and 10% fishmeal (2.8% absolute content) groups did not affect WGR, FE, and PER (Patterson and Gatlin 2013). However, the study on olive flounder (Paralichthys olivaceus) showed 10% fishmeal replacement (6% absolute content) by defatted C. vulgaris (57% crude protein) increased FBW, WGR, and SGR compared to the control (P < 0.05) (Rahimnejad et al. 2017). Moreover, 30% fishmeal (16.1% absolute content) could be replaced in gilthead seabream without affecting fish growth performance (Karapanagiotidis et al. 2022).
Research on Chlorella meal as fishmeal alternatives in crustaceans was primarily conducted in Pacific whiteleg shrimp and giant river prawn. Pacific whiteleg shrimp were fed a diet in which fishmeal was replaced by C. vulgaris (51.5% crude protein) for eight weeks and showed no adverse effects on FBW, SGR, FCR, and survival rate in the 100% fishmeal replacement group (Pakravan et al. 2018). Similarly, Radhakrishnan et al. (2015) assessed the effect of dietary replacement of fishmeal with C. vulgaris (55.7% crude protein) on the growth performance of giant river prawn and observed that C. vulgaris could replace 100% fishmeal without influencing shrimp growth, survival rate, and feed utilization.
Maximum replacement level of fishmeal with Scenedesmus meal
Scenedesmus is a common freshwater green algal genera whose cells usually form flattened coenobia, arranged in linear or alternating series (Shubert and Gärtner 2015; Sucunthowong et al. 2023). Scenedesmus is easily cultured in the laboratory owing to its strong ability to adapt to harsh environmental conditions, simple nutritional requirements, and rapid growth rates (Lürling 2003; Trainor et al. 1976). At present, Scenedesmus has been used in many biotechnological applications due to its high nutritional content and bioactivities (Ishaq et al. 2016), including fishmeal replacement (Table 4). Scenedesmus possesses high protein content (50%-56%) (Becker 2007), contains all essential amino acids and a good amount of lipid and essential minerals (Geldenhuys et al. 1988), and its amino acid pattern could compare favorably with that of other food proteins (e.g., egg and soybean) (Becker 2004a). Compared to Spirulina and Chlorella meal, fewer aquatic animals were used to investigate the effect of Scenedesmus meal as fishmeal substitutes, including salmon and trout, tilapia, gilthead seabream, and spotted wolffish (Anarhichas minor) (Table 4).
S. almeriensis (46.7% crude protein) from an integrated system waste-nutrient was assessed in rainbow trout and induced significantly lower FBW, SGR, FI, PER, and FE in the 5% fishmeal replacement group (Tomas-Almenar et al. 2018). Similarly, Scenedesmus sp. (45.7% crude protein) induced lower WGR, SGR, and PER in Atlantic salmon (Salmo sala) compared to the control in the 75% fishmeal replacement group, but did not affect fish growth performance in the 50% fishmeal replacement group (Gong et al. 2019).
Badwy et al. (2008) made use of Scenedesmus sp. (51.17% crude protein) to replace fishmeal in Nile tilapia and found that replacing 50% fishmeal increased FBW, WGR, and SGR, while replacing 75% fishmeal significantly decreased FBW, WGR, SGR, and FI, but had no significant effects on FCR and PER.
Gilthead seabream (Sparus aurata) were fed diets containing different substitutional levels (15.7%, 23.4%, 31.2%, and 46.9%) of fishmeal by S. almeriensis (43.2% crude protein) and showed no significant difference in FBW, SGR, FCR, and PER among all treatments (Vizcaíno et al. 2014). Nevertheless, S. obliquus (45.7% crude protein) could replace only 15% fishmeal without negative effects on FBW, WGR, and SGR in spotted wolffish juveniles (Knutsen et al. 2019).
Maximum replacement level of fishmeal with other microalgal meals
In addition to these common microalgae, some other microalgae could be also utilized to replace fishmeal (Table 5). These microalgae are mainly marine algae and are usually abundant in lipids and essential fatty acids, such as Nannochloropsis spp, Phaeodactylum tricornutum, Tetraselmis, and Grammatophor in EPA, Isochrysis, Schizochytrium in DHA, and Nanofrustulum, Navicula, and Desmodesmus for biodiesel production. As fishmeal alternatives, these microalgae were used in the form of either lipid-extracted algae or whole algae. Compared with Spirulina, Chlorella, and Scenedesmus, these microalgae are inferior in fishmeal replacement and are mainly utilized as fishmeal alternatives in Atlantic salmon, Atlantic cod, Nile tilapia, common carp, European sea bass (Dicentrarchus labrax), turbot (Scophthalmus maximus), red drum, and Pacific whiteleg shrimp (Table 5).
Kiron et al. evaluated three kinds of microalgae as fishmeal alternatives and found that Nanofrustlum sp. (11.9% crude protein) (Kiron et al. 2012), Tetraselmis sp. (27.9% crude protein) (Kiron et al. 2012), and defatted Desmodesmus sp. (about 63% crude protein) (Kiron et al. 2016) could replace 5%, 5%, and 26.1% fishmeal without affecting FBW, SGR, FCR, PER, and survival rate in Atlantic salmon. Similarly, Sørensen et al. found that P. tricornutum (49% crude protein) (Sørensen et al. 2016) and defatted Nannochloropsis oceania (43% crude protein) (Sørensen et al. 2017) could replace 11% and 14.5% fishmeal in Atlantic salmon, respectively. However, in Atlantic cod, a microalgal mix (Nannochloropsis sp. and Isochrysis sp.) (42.1% crude protein) as protein sources decreased FBW, FI, and FE in the two substitutional levels (14.1% and 26.5%). The phenomena can be explained by differences in species of microalgae and fish and protein content in microalgae.
Nile tilapia and common carp can tolerate high levels of dietary microalgae. In Nile tilapia, Nannochloropsis oculata could replace 100% fishmeal (7% absolute content) and simultaneously increase FBW, WGR, and SGR compared to the control (Sarker et al. 2020). Two substitutional levels (25% and 39.9%) of fishmeal by Nanofrustlum sp. (11.9% crude protein) and Tetraselmis sp. (27.9% crude protein) were evaluated in common carp and the results showed that both microalgae could replace 39.9% fishmeal (6.4% absolute content) without affecting WGR, SGR, FI, FCR, PER, and survival rate (Kiron et al. 2012).
European sea bass were fed diets containing three substitutional levels (15%, 30%, and 45%) of fishmeal by Tetraselmis suecica (48.7% crude protein) and Tisochrysis lutea (46.3% crude protein) (Cardinaletti et al. 2018). The microalgae mix (T. lutea:T. suecica = 2:1) could replace 45% fishmeal without (12.5% absolute content) affecting FBW, SGR, FCR, and PER (Cardinaletti et al. 2018). Moreover, lipid-extracted Nannochloropsis sp. (45.2% crude protein) could replace 31.3% fishmeal (9.4% absolute content) without influencing FBW and FCR in European sea bass (Valente et al. 2019), in agreement with results reported by Qiao et al. who found that Nannochloropsis sp. (50.72% crude protein) could replace up to 15.5% fishmeal (7.7% absolute content) in juvenile turbot (Qiao et al. 2019).
Patterson and Gatlin (2013) assessed three microalgae as fishmeal alternatives in juvenile red drum and found that fish fed diets in which 5% fishmeal replacement by lipid-extracted Nannochloropsis salina and lipid-extracted Navicula sp. had a lower WGR compared to the control, while whole Navicula sp. could replace 10% fishmeal without negative effects on WGR, FE, and survival rate. The quality of microalgae could partly explain the difference in fishmeal replacement by whole Navicula sp. and lipid-extracted Navicula sp. Whole Navicula sp. contained 19.4% crude protein and 18.8% crude lipid, while the lipid-extracted Navicula sp. possessed 13.3% crude protein and 4.9% crude lipid (Patterson and Gatlin 2013). Microalgae with higher protein levels generally substitute more fishmeal than those with lower protein content.
Five kinds of microalgae were used to replace fishmeal in Pacific whiteleg shrimp. Nanofrustlum sp. (11.9% crude protein) and Tetraselmis sp. (27.9% crude protein) could replace 40% fishmeal without influencing WGR, SGR, FI, FCR, PER, and survival rate (Kiron et al. 2012). Defatted H. pluvialis (40.3% crude protein) could replace 50% fishmeal without adverse effects on FBW, WGR, SGR, and survival rate, and significantly increased PER and FE compared to the control (Ju et al. 2012). Schizochytrium sp. (9.09% crude protein) and Grammatophora sp. (7.12% crude protein) could replace 9.1% fishmeal without adverse effects on FBW, WGR, SGR, and survival rate (Pacheco-Vega et al. 2018).
Summary of fishmeal replacement by microalgal meals
The maximum substitution level of fishmeal by microalgae was affected by several factors.
The first is the microalgal species. In general, maximum replacement levels of fishmeal were the highest in Spirulina, and the lowest in other microalgae (Tables 2, 3, 4, and 5). Protein content and protein digestibility in microalgae could partially explain the phenomenon. Spirulina meal generally has a much higher protein content, and its protein is more digestible because its peptidoglycan cell walls are much softer and more digestible for most fish than cellulose-based cell walls (e.g., Chlorella) (Teuling et al. 2017).
The second is the fish species and feeding habits. Carnivorous fish (such as salmon and trout) generally has lower maximum substitution level than omnivorous fish (e.g., tilapia, carps, crustacean, and catfish). Kiron et al. (2012) evaluated two marine microalgae (Nanofrustulum and Tetraselmis) as fishmeal alternatives in Atlantic salmon and common carp and found that two marine microalgae could replace over 40% fishmeal (6.21–6.39% absolute content) in common carp, but just 5% fishmeal (1.4% absolute content) in Atlantic salmon. Feeding habits or striking morphological and physiological differences in their digestive tracts could partially account for the phenomena (Kamalam et al. 2017). Carbohydrates are important components of microalgal biomass composition (Markou et al. 2012). The ability to use carbohydrates in fish are determined by their natural feeding habits (Kamalam et al. 2017). Atlantic salmon are carnivorous and cannot tolerate high amounts of carbohydrates (Krogdahl et al. 2003; Torstensen et al. 2008), whereas common carp are omnivorous and can digest substantial amounts of carbohydrates from plants (Kiron et al. 2012; Stone 2003).
Other factors include the difference in fishmeal quality, the supplemental level of fishmeal in the control group, and microalgal meal quality, which have been discussed in the previous sections.
Based on the results in studies with upper limits of the relative substitutional level of fishmeal (Supplemental Table), we made a summary of fishmeal replacement by microalgae. Microalgae could generally replace 100% (30.8% absolute content), 95% (28.3% absolute content), 95% (32% absolute content), 64.1% (20% absolute content), 25.6% (11.1% absolute content), and 18.6% (4.5% absolute content) fishmeal protein in carp, shrimp, catfish, tilapia, marine fish, and salmon and trout, respectively (Fig. 6). Nowadays marine fish and carnivorous fish (e.g., salmon and trout) are major consumers of fishmeal (Oliva-Teles et al. 2015), thus more attention should be paid to these species in future studies. Although a positive linear relationship exists between the relative substitutional level and the absolute substitutional level of fishmeal (Fig. 6), the absolute substitution level seemed to be a more reliable indicator than the relative substitution level due to huge differences in fishmeal supplemental level in the control group.
Main problems and possible solutions of microalgal meals in fishmeal replacement
Low production and high production cost
At present, the global autotrophic microalgae biomass production is about 20,000 tonnes (dry weight) (Benemann et al. 2018), while the annual production of fishmeal used in aquaculture is estimated to be 3,900,000 tonnes (European Commission 2021). Therefore, the huge gaps in production at present cannot be filled. Moreover, microalgal production costs are much higher than fishmeal. Presently, the production cost of Spirulina and Chlorella meal ranges from about 10 USD/kg to 30 USD/kg (Benemann et al. 2018). However, the maximum price of fishmeal achieved in 2013 was just 1.74 USD/kg. Thus the price difference makes it impossible for fishmeal replacement by microalgal meals at present.
Heterotrophic culture seems an effective solution to these problems. Heterotrophic culture occurs in a closed fermentation process that uses organic carbon sources for microalgal growth in the absence of light. Compared to the most commonly used autotrophic cultivation of microalgae, heterotrophic cultivation is much cheaper, simpler in construct facilities, easier to maintain on a large scale, and higher in cell densities (up to 100 g/L in heterotrophic vs. 0.5–2 g/L in autotrophic) (Barclay et al. 2013; Chen 1996; Perez-Garcia et al. 2011). Presently, some microalgae have been achieving commercial success via fermentation, such as Chlorella, Crypthecodinium, and Schizochytrium (Barclay et al. 2013). Although the protein content of heterotrophic cells (10.3–25.8%) was much lower than that of autotrophic cells (up to 52.6%), an over-compensation strategy (Xie et al. 2017) and a novel two-stage heterotrophic cultivation for starch-to-protein method (Xiao et al. 2022) have been used to increase the protein content of heterotrophic Chlorella which could be comparable in protein content to that cultured under autotrophic conditions. Furthermore, efforts in the improvement of the algal medium, culture facility, and harvesting methods are necessary to increase global microalgal production. Improvements in harvesting methods could reduce the production cost of microalgal meals to some extent since microalgal harvesting accounts for 30% of the total production cost for microalgae (Yang et al. 2021).
Poor digestibility
Poor digestibility of microalgae was mainly associated with their high content of carbohydrates which are mainly starch (cell content) and non-starch polysaccharides (NSP) (cell wall) (de Farias Silva and Bertucco 2016; Maia et al. 2020; Velazquez-Lucio et al. 2018).
The ability to utilize starch in fish varies in species and is affected by many aspects, such as fish feeding habits (mainly variations in the anatomical structure and function of the gastrointestinal tract), dietary starch levels, starch sources, processing conditions of starch, and rearing conditions (Krogdahl et al. 2005). Generally, carnivorous fish have a lower ability to utilize starch than herbivorous and omnivorous fish (Krogdahl et al. 2005; Polakof et al. 2012). Yamamoto et al. (2001) compared the starch digestibility in common carp and rainbow trout and found that the starch digestibility in common carp and rainbow trout was 90% and 78%, respectively.
NSPs are predominantly structural components of cell walls, comprising cellulose, β-glucans, hemicellulose, pectins, and gums (National Research Council 2011; Sinha et al. 2011). NSPs in microalgal cell wall includes cellulose, hemicellulose, and pectins (Domozych et al. 2012, 2007; Scholz et al. 2014). NSPs are considered to be unavailable as energy sources for the majority of fish due to the absence of adequate gut microbiota for their digestion and the lack of specific NSP-degrading digestive enzymes (Maas et al. 2020; Polakof et al. 2012; Sinha et al. 2011). The presence of NSPs in aquafeed has induced adverse effects on feed utilization and fish growth (Sinha et al. 2011). Sarker et al. (2018) evaluated the substitution of fishmeal with lipid-extracted N. oculata in diets of Nile tilapia and attributed the growth retardation of fish in the high fishmeal substitution group to high levels of cellulose (3.7%) and hemicellulose level (43.3 ug/mg) in microalgae.
Several solutions can be adopted to address the digestibility problem in microalgae. Firstly, physical treatment (e.g., bead milling and freezing) (Velazquez-Lucio et al. 2018) or enzymatic digestion (addition of NSP-degrading enzymes) (Córdova et al. 2019) could be used to alleviate the adverse effects of NSPs. Compared to the control (without disruption), disruption of the cell wall in Nannochloropsis gaditana by bead milling improved protein digestibility, weight gain, and feed utilization by 16.3%, 13%, and 11%, respectively, in African catfish (Agboola et al. 2019). Treatment with cellulose, β-glucosidase, and hemicellulose was proved effective in damaging the cell wall and releasing cellular organic compounds of C. sorokiniana (Córdova et al. 2019). Secondly, some starch in microalgae can be separated before the use of microalgal biomass as aquafeed ingredients. The aqueous two-phase system (ATPS) is considered a potential scalable method to isolate starch from other cellular components in microalgae after cell wall disruption (Di Caprio et al. 2022; Suarez Ruiz et al. 2020).
Anti-nutritional factors (ANFs)
ANFs refer to endogenous substances present in food and feedstuffs that adversely affect health and nutrition when ingested by humans and animals (Gemede and Ratta 2014). The detrimental effects induced by prolonged ingestion of ANFs include disturbance of digestive processes and growth, decreased feed intake and feed utilization, pancreatic hypertrophy, hypoglycemia, liver dysfunctions, and suppression of immunity (National Research Council 2011). ANFs in plants include tannins, phytate, oxalate, saponins, lectins, alkaloids, protease inhibitors, and cyanogenic glycosides (Francis et al. 2001; Gemede and Ratta 2014). Some ANFs have been reported in microalgae (Table 6), such as lectin in Scenedesmus acutus (Silva et al. 2020) and Chlorella (Jacob-Lopes et al. 2019). Lectin could disrupt the small intestinal metabolism and induce morphological damage to the villi (Francis et al. 2001). Diets containing tannins, alkaloids, or protease inhibitors have been proven to reduce feed intake and fish growth (de la Higuera et al. 1988; Mukhopadhyay and Ray 1999; Shiau et al. 1987). Adverse effects induced by these ANFs are similar to those when microalgae replace high fishmeal in aquatic animals. However, the direct relationship between these is unknown. Whether these adverse effects are induced by ANFs in microalgae needs further investigation.
Heat treatment (such as autoclaving) or treatment with chemical reagents (such as alkali) are suggested to reduce the content of tannins, alkaloids, lectin, and protease inhibitors (Francis et al. 2001; Samtiya et al. 2020). However, more care should be taken to minimize the loss of nutrients of microalgae in the treatment. Moreover, breeding for microalgae with low content of these ANFs could be an ideal solution (Sims et al. 2019).
Conclusion
In summary, microalgal meals could partially or completely replace fishmeal, with levels of substitution ranging from 0 to 100%. The maximum replacement level is affected by microalgal species, fish feeding habits, quality of fishmeal and microalgal meals, and supplemental levels of fishmeal in the control group. Generally, microalgae could replace 100%, 95%, 95%, 64.1%, 25.6%, and 18.6% fishmeal protein in diets of carp, shrimp, catfish, tilapia, marine fish, and salmon and trout, respectively.
The main problems and possible solutions concerning the application of microalgal meals in fishmeal replacement were proposed. Firstly, heterotrophic cultures and improvement of the algal medium, culture facility, and harvesting methods could increase production and decrease the production cost of microalgae. Secondly, physical treatment, enzymatic digestion, and starch separation could improve the poor digestibility of microalgae. Thirdly, heat treatment (such as autoclaving), treatment with chemical reagents, and breeding could decrease the ANFs in microalgae.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abbott JK, Willard D, Xu J (2021) Feeding the dragon: The evolution of China’s fishery imports. Mar Policy 133:104733. https://doi.org/10.1016/j.marpol.2021.104733
Abdulrahman NM, Ameen HJH (2014) Replacement of fishmeal with microalgae Spirulina on common carp weight gain, meat and sensitive composition and survival. Pak J Nutr 13:93–98
Agboola JO, Teuling E, Wierenga PA, Gruppen H, Schrama JW (2019) Cell wall disruption: An effective strategy to improve the nutritive quality of microalgae in African catfish (Clarias gariepinus). Aquacult Nutr 25:783–797. https://doi.org/10.1111/anu.12896
Ahmad MT, Shariff M, Yusoff FMd, Goh YM, Banerjee S (2020) Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquacult 12:328–346. https://doi.org/10.1111/raq.12320
Ahmad A, Hassan SW, Banat F (2022) An overview of microalgae biomass as a sustainable aquaculture feed ingredient: food security and circular economy. Bioengineered 13:9521–9547. https://doi.org/10.1080/21655979.2022.2061148
Akter T, Hossain A, Rabiul Islam M, Hossain MA, Das M, Rahman MM, Aye AT, Abdel-Tawwab M (2023) Effects of spirulina (Arthrospira platensis) as a fishmeal replacer in practical diets on growth performance, proximate composition, and amino acids profile of pabda catfish (Ompok pabda). J Appl Aquacult 35:69–82. https://doi.org/10.1080/10454438.2021.1936740
Alagawany M, Taha AE, Noreldin A, El-Tarabily KA, Abd El-Hack ME (2021) Nutritional applications of species of Spirulina and Chlorella in farmed fish: A review. Aquaculture 542:736841. https://doi.org/10.1016/j.aquaculture.2021.736841
Altmann BA, Rosenau S (2022) Spirulina as animal feed: opportunities and challenges. Foods 11:965. https://doi.org/10.3390/foods11070965
Badwy TM, Ibrahim EM, Zeinhom MM (2008) Partial replacement of fishmeal with dried microalgae Chlorella spp. and Scenedesmus spp.) in Nile tilapia (Oreochromis niloticus) diets. In: Elghobashy H, Fitzsimmons K, Diab AS (eds) From the pharaohs to the future: proceedings of the 8th international symposium on tilapia in aquaculture. Egypt Ministry of Agriculture, Cairo, pp 801–810
Barclay W, Apt K, Dong XD (2013) Commercial production of microalgae via fermentation. In: Richmond A, Hu Q (eds) Handbook of microalgal culture, 2nd edn. John Wiley & Sons, UK, pp 134–145
Becker W (2004a) Microalgae in human and animal nutrition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 312–351
Becker W (2004b) Microalgae for aquaculture. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 352–364
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Benemann JR, Woertz I, Lundquist T (2018) Autotrophic microalgae biomass production: from niche markets to commodities. Ind Biotechnol 14:3–10. https://doi.org/10.1089/ind.2018.29118.jrb
Boyd CE, McNevin AA, Davis RP (2022) The contribution of fisheries and aquaculture to the global protein supply. Food Secur 14:805–827. https://doi.org/10.1007/s12571-021-01246-9
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331. https://doi.org/10.1016/S0044-8486(96)01501-3
Cao S-P, Zou T, Zhang P-Y, Han D, Jin J-Y, Liu H-K, Yang Y-X, Zhu X-M, Xie S-Q (2018a) Effects of dietary fishmeal replacement with Spirulina platensis on the growth, feed utilization, digestion and physiological parameters in juvenile gibel carp (Carassis auratus gibelio var. CAS III). Aquac Res 49:1320–1328. https://doi.org/10.1111/are.13590
Cao S, Zhang P, Zou T, Fei S, Han D, Jin J, Liu H, Yang Y, Zhu X, Xie S (2018b) Replacement of fishmeal by spirulina Arthrospira platensis affects growth, immune related-gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge against Aeromonas hydrophila infection. Fish Shellfish Immun 79:265–273. https://doi.org/10.1016/j.fsi.2018.05.022
Cardinaletti G, Messina M, Bruno M, Tulli F, Poli BM, Giorgi G, Chini-Zittelli G, Tredici M, Tibaldi E (2018) Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 485:173–182. https://doi.org/10.1016/j.aquaculture.2017.11.049
Carneiro WF, Castro TFD, Orlando TM, Meurer F, Paula DAdJ, Virote BdCR, Vianna ARdCB, Murgas LDS (2020) Replacing fish meal by Chlorella sp. meal: Effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 528:735612. https://doi.org/10.1016/j.aquaculture.2020.735612
Chen F (1996) High cell density culture of microalgae in heterotrophic growth. Trends Biotechnol 14:421–426. https://doi.org/10.1016/0167-7799(96)10060-3
Chen W, Luo L, Han D, Long F, Chi Q, Hu Q (2021) Effect of dietary supplementation with Chlorella sorokiniana meal on the growth performance, antioxidant status, and immune response of rainbow trout (Oncorhynchus mykiss). J Appl Phycol 33:3113–3122. https://doi.org/10.1007/s10811-021-02541-w
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Córdova O, Passos F, Chamy R (2019) Enzymatic pretreatment of microalgae: cell wall disruption, biomass solubilisation and methane yield increase. Appl Biochem Biotechnol 189:787–797. https://doi.org/10.1007/s12010-019-03044-8
Craig S, Helfrich LA (2009) Understanding fish nutrition, feeds and feeding. Virginia Cooperative Extension (Publication 420-256). http://hdl.handle.net/10919/48950. Accessed 7 Apr 2014
de Farias Silva CE, Bertucco A (2016) Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem 51:1833–1842. https://doi.org/10.1016/j.procbio.2016.02.016
de la Higuera M, Garcia-Gallego M, Cardenete G, Suarez MD, Moyano FJ (1988) Evaluation of Lupin seed meal as an alternative protein source in feeding of rainbow trout (Salmo gairdneri). Aquaculture 71:37–50. https://doi.org/10.1016/0044-8486(88)90271-2
Dębowski M, Zieliński M, Kazimierowicz J, Kujawska N, Talbierz S (2020) Microalgae cultivation technologies as an opportunity for bioenergetic system development—advantages and limitations. Sustainability 12:9980. https://doi.org/10.3390/su12239980
Di Caprio F, Chelucci R, Francolini I, Altimari P, Pagnanelli F (2022) Extraction of microalgal starch and pigments by using different cell disruption methods and aqueous two-phase system. J Chem Technol Biotechnol 97:67–78. https://doi.org/10.1002/jctb.6910
Domozych DS, Serfis A, Kiemle SN, Gretz MR (2007) The structure and biochemistry of charophycean cell walls: I. Pectins of Penium margaritaceum. Protoplasma 230:99–115. https://doi.org/10.1007/s00709-006-0197-8
Domozych D, Ciancia M, Fangel J, Mikkelsen M, Ulvskov P, Willats W (2012) The cell walls of green algae: a journey through evolution and diversity. Front Plant Sci 3:82. https://doi.org/10.3389/fpls.2012.00082
El-fayoumy EA, Shanab S, Shalaby EA (2020) Metabolomics and biological activities of Chlorella vulgaris grown under modified growth medium (BG11) composition. CMU J Nat Sci 19:91–123. https://doi.org/10.12982/CMUJNS.2020.0007
El-Sayed A-FM (1994) Evaluation of soybean meal, spirulina meal and chicken offal meal as protein sources for silver seabream (Rhabdosargus sarba) fingerlings. Aquaculture 127:169–176. https://doi.org/10.1016/0044-8486(94)90423-5
El-Sheekh M, E-Shourbagy I, Shalaby S, Hosny S (2014) Effect of feeding Arthrospira platensis (Spirulina) on growth and carcass composition of hybrid red tilapia (Oreochromis niloticus x Oreochromis mossambicus). Turkish J Fish Aquat Sci 14:471–478. https://doi.org/10.4194/1303-2712-v14_2_18
European Commission (2021) Fishmeal and fish oil production and trade flows in the EU. Publication Office of the European Union, Luxembourg. https://op.europa.eu/en/publication-detail/-/publication/984cee38-475a-11ec-91ac-01aa75ed71a1/language-en
FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action. Food and Agriculture Organization of the United Nations, Rome, Italy. https://www.fao.org/3/ca9229en/ca9229en.pdf
Foster M, Petrell R, Ito MR, Ward R (1995) Detection and counting of uneaten food pellets in a sea cage using image analysis. Aquacult Eng 14:251–269. https://doi.org/10.1016/0144-8609(94)00006-M
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227. https://doi.org/10.1016/s0044-8486(01)00526-9
Geldenhuys DJ, Walmsley RD, Toerien DF (1988) Quality of algal material produced on a fertilizer-tap water medium in outdoor plastic-enclosed systems. Aquaculture 68:157–164. https://doi.org/10.1016/0044-8486(88)90238-4
Gemede HF, Ratta N (2014) Antinutritional factors in plant foods: potential health benefits and adverse effects. Int J Nutr Food Sci 3:284–289. https://doi.org/10.11648/j.ijnfs.20140304.18
Glencross BD, Booth M, Allan GL (2007) A feed is only as good as its ingredients - a review of ingredient evaluation strategies for aquaculture feeds. Aquacult Nutr 13:17–34. https://doi.org/10.1111/j.1365-2095.2007.00450.x
Gong Y, Bandara T, Huntley M, Johnson ZI, Dias J, Dahle D, Sørensen M, Kiron V (2019) Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 501:455–464. https://doi.org/10.1016/j.aquaculture.2018.11.049
Goswami RK, Mehariya S, Obulisamy PK, Verma P (2021) Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour Technol 320:124301. https://doi.org/10.1016/j.biortech.2020.124301
Grima EM, Acién Fernández FG, Robles Medina A (2013) Downstream processing of cell mass and products. In: Richmond A, Hu Q (eds) Handbook of microalgal culture, 2nd edn. John Wiley & Sons, UK, pp 267–309
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: A review. Biotechnol Adv 30:709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Hardy RW (2010) Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res 41:770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Hemaiswarya S, Raja R, Kumar RR, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746. https://doi.org/10.1007/s11274-010-0632-z
Hetta M, Mahmoud R, El-Senousy W, Ibrahim MK, El-Taweel G, Ali G (2014) Antiviral and antimicrobial activities of Spirulina platensis. World J Pharm Pharm Sci 3:31–39
Houston RD, Bean TP, Macqueen DJ, Gundappa MK, Jin YH, Jenkins TL, Selly SLC, Martin SAM, Stevens JR, Santos EM, Davie A, Robledo D (2020) Harnessing genomics to fast-track genetic improvement in aquaculture. Nat Rev Genet 21:389–409. https://doi.org/10.1038/s41576-020-0227-y
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1:316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Ibañez E, Cifuentes A (2013) Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric 93:703–709. https://doi.org/10.1002/jsfa.6023
Ishaq AG, Matias-Peralta HM, Basri H (2016) Bioactive compounds from green microalga - Scenedesmus and its potential applications: a brief review. Pertanika J Trop Agric Sci 39:1–15
Jacob-Lopes E, Maroneze MM, Deprá MC, Sartori RB, Dias RR, Zepka LQ (2019) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci 25:1–7. https://doi.org/10.1016/j.cofs.2018.12.003
Jannathulla R, Rajaram V, Kalanjiam R, Ambasankar K, Muralidhar M, Dayal JS (2019) Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac Res 50:3493–3506. https://doi.org/10.1111/are.14324
Ju ZY, Deng D-F, Dominy W (2012) A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 354–355:50–55. https://doi.org/10.1016/j.aquaculture.2012.04.028
Kamalam BS, Medale F, Panserat S (2017) Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27. https://doi.org/10.1016/j.aquaculture.2016.02.007
Karapanagiotidis IT, Metsoviti MN, Gkalogianni EZ, Psofakis P, Asimaki A, Katsoulas N, Papapolymerou G, Zarkadas I (2022) The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 561:738709. https://doi.org/10.1016/j.aquaculture.2022.738709
Kavisri M, Marykutty A, Gopal P, Manickam E, Meivelu M (2021) Phytochemistry, bioactive potential and chemical characterization of metabolites from marine microalgae (Spirulina platensis) biomass. Biomass Convers Bior 13:1147–1154. https://doi.org/10.1007/s13399-021-01689-2
Kim S-S, Rahimnejad S, Kim K-W, Lee K-J (2013) Partial replacement of fish meal with Spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus). Turkish J Fish Aquat Sci 13:197–204. https://doi.org/10.4194/1303-2712-v13_2_01
Kiron V, Phromkunthong W, Huntley M, Archibald I, De Scheemaker G (2012) Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquacult Nutr 18:521–531. https://doi.org/10.1111/j.1365-2095.2011.00923.x
Kiron V, Sørensen M, Huntley M, Vasanth GK, Gong Y, Dahle D, Palihawadana AM (2016) Defatted Biomass of the Microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front Mari Sci 3:67. https://doi.org/10.3389/fmars.2016.00067
Knutsen HR, Ottesen OH, Palihawadana AM, Sandaa W, Sorensen M, Hagen O (2019) Muscle growth and changes in chemical composition of spotted wolffish juveniles (Anarhichas minor) fed diets with and without microalgae (Scenedesmus obliquus). Aquacult Rep 13:100175. https://doi.org/10.1016/j.aqrep.2018.11.001
Kotrbáček V, Doubek J, Doucha J (2015) The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol 27:2173–2180. https://doi.org/10.1007/s10811-014-0516-y
Kovač DJ, Simeunović JB, Babić OB, Mišan AČ, Milovanović IL (2013) Algae in food and feed. Food Feed Res 40:21–31
Krogdahl Å, Bakke-McKellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquacult Nutr 9:361–371. https://doi.org/10.1046/j.1365-2095.2003.00264.x
Krogdahl Å, Hemre GI, Mommsen TP (2005) Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquacult Nutr 11:103–122. https://doi.org/10.1111/j.1365-2095.2004.00327.x
Lall SP (2022) The minerals. In: Hardy RW, Kaushik SJ (eds) Fish Nutrition, 4th edn. Academic Press, London, pp 469–554
Liu C, Liu H, Xu W, Han D, Xie S, Jin J, Yang Y, Zhu X (2019) Effects of dietary Arthrospira platensis supplementation on the growth, pigmentation, and antioxidation in yellow catfish (Pelteobagrus fulvidraco). Aquaculture 510:267–275. https://doi.org/10.1016/j.aquaculture.2019.05.067
Lupatsch I, Blake C (2013) Algae alternative: Chlorella studied as protein source in tilapia feeds. Glob Aquacult Advocate 16:78–79
Lürling M (2003) Phenotypic plasticity in the green algae Desmodesmus and Scenedesmus with special reference to the induction of defensive morphology. Ann Limnol-Int J Lim 39:85–101. https://doi.org/10.1051/limn/2003014
Maas RM, Verdegem MCJ, Wiegertjes GF, Schrama JW (2020) Carbohydrate utilisation by tilapia: a meta-analytical approach. Rev Aquacult 12:1851–1866. https://doi.org/10.1111/raq.12413
Macias-Sancho J, Poersch LH, Bauer W, Romano LA, Wasielesky W, Tesser MB (2014) Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: Effects on growth and immunological parameters. Aquaculture 426–427:120–125. https://doi.org/10.1016/j.aquaculture.2014.01.028
Maia JLd, Cardoso JS, Mastrantonio DJdS, Bierhals CK, Moreira JB, Costa JAV, Morais MGd (2020) Microalgae starch: A promising raw material for the bioethanol production. Int J Biol Macromol 165:2739–2749. https://doi.org/10.1016/j.ijbiomac.2020.10.159
Maisashvili A, Bryant H, Richardson J, Anderson D, Wickersham T, Drewery M (2015) The values of whole algae and lipid extracted algae meal for aquaculture. Algal Res 9:133–142. https://doi.org/10.1016/j.algal.2015.03.006
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biot 96:631–645. https://doi.org/10.1007/s00253-012-4398-0
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: A review. Renew Sust Energ Rev 14:217–232. https://doi.org/10.1016/j.rser.2009.07.020
Mukhopadhyay N, Ray AK (1999) Utilisation of copra meal in the formulation of compound diets for rohu, Labeo rohita, fingerlings. J Appl Ichthyol 15:127–131. https://doi.org/10.1046/j.1439-0426.1999.00132.x
Nagappan S, Das P, AbdulQuadir M, Thaher M, Khan S, Mahata C, Al-Jabri H, Vatland AK, Kumar G (2021) Potential of microalgae as a sustainable feed ingredient for aquaculture. J Biotechnol 341:1–20. https://doi.org/10.1016/j.jbiotec.2021.09.003
Nandeesha MC, Gangadhar B, Varghese TJ, Keshavanath P (1998) Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp, Cyprinus carpio L. Aquac Res 29:305–312. https://doi.org/10.1046/j.1365-2109.1998.00163.x
Nandeesha MC, Gangadhara B, Manissery JK, Venkataraman LV (2001) Growth performance of two Indian major carps, catla (Catla catla) and rohu (Labeo rohita) fed diets containing different levels of Spirulina platensis. Bioresource Technol 80:117–120. https://doi.org/10.1016/S0960-8524(01)00085-2
National Research Council (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington DC
Oh Y-K, Kim S, Ilhamsyah DPA, Lee S-G, Kim JR (2022) Cell disruption and lipid extraction from Chlorella species for biorefinery applications: Recent advances. Bioresource Technol 366:128183. https://doi.org/10.1016/j.biortech.2022.128183
Oliva-Teles A, Enes P, Peres H (2015) Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In: Davis DA (ed) Feed and feeding practices in aquaculture. Woodhead Publishing, Oxford, pp 203–233
Olvera-Novoa M, Dominguez-Cen L, Olivera-Castillo L, Martínez-Palacios CA (1998) Effect of the use of the microalga Spirulina maxima as fish meal replacement in diets for tilapia, Oreochromis mossambicus (Peters), fry. Aquac Res 29:709–715. https://doi.org/10.1046/j.1365-2109.1998.29100709.x
Pacheco-Vega JM, Gamboa-Delgado J, Alvarado-Ibarra AG, Nieto-López MG, Tapia-Salazar M, Cruz-Suárez LE (2018) Nutritional contribution of fish meal and microalgal biomass produced from two endemic microalgae to the growth of shrimp Penaeus vannamei. Lat Am J Aquat Res 46:53–62. https://doi.org/10.3856/vol46-issue1-fulltext-7
Pakravan S, Akbarzadeh A, Sajjadi MM, Hajimoradloo A, Noori F (2017) Partial and total replacement of fish meal by marine microalga Spirulina platensis in the diet of Pacific white shrimp Litopenaeus vannamei: Growth, digestive enzyme activities, fatty acid composition and responses to ammonia and hypoxia stress. Aquac Res 48:5576–5586. https://doi.org/10.1111/are.13379
Pakravan S, Akbarzadeh A, Sajjadi MM, Hajimoradloo A, Noori F (2018) Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile Pacific white shrimp Litopenaeus vannamei. Aquacult Nutr 24:594–604. https://doi.org/10.1111/anu.12594
Palmegiano GB, Agradi E, Forneris G, Gai F, Gasco L, Rigamonti E, Sicuro B, Zoccarato I (2005) Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquac Res 36:188–195. https://doi.org/10.1111/j.1365-2109.2005.01209.x
Palmegiano GB, Gai F, Daprà F, Gasco L, Pazzaglia M, Peiretti PG (2008) Effects of Spirulina and plant oil on the growth and lipid traits of white sturgeon (Acipenser transmontanus) fingerlings. Aquac Res 39:587–595. https://doi.org/10.1111/j.1365-2109.2008.01914.x
Patterson D, Gatlin DM (2013) Evaluation of whole and lipid-extracted algae meals in the diets of juvenile red drum (Sciaenops ocellatus). Aquaculture 416:92–98. https://doi.org/10.1016/j.aquaculture.2013.08.033
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res 45:11–36. https://doi.org/10.1016/j.watres.2010.08.037
Perez-Velazquez M, Gatlin DM, González-Félix ML, García-Ortega A (2018) Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture 487:41–50. https://doi.org/10.1016/j.aquaculture.2018.01.001
Perez-Velazquez M, Gatlin DM, González-Félix ML, García-Ortega A, de Cruz CR, Juárez-Gómez ML, Chen K (2019) Effect of fishmeal and fish oil replacement by algal meals on biological performance and fatty acid profile of hybrid striped bass (Morone crhysops ♀ × M. saxatilis ♂). Aquaculture 507:83–90. https://doi.org/10.1016/j.aquaculture.2019.04.011
Péron G, François Mittaine J, Le Gallic B (2010) Where do fishmeal and fish oil products come from? An analysis of the conversion ratios in the global fishmeal industry. Mar Policy 34:815–820. https://doi.org/10.1016/j.marpol.2010.01.027
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol 182:1015–1045. https://doi.org/10.1007/s00360-012-0658-7
Posten C (2009) Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9:165–177. https://doi.org/10.1002/elsc.200900003
Prabakaran G, Moovendhan M, Arumugam A, Matharasi A, Dineshkumar R, Sampathkumar P (2018) Quantitative analysis of phytochemical profile in marine microalgae Chlorella vulgaris. Int J Pharm Biol Sci 8:562–565
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biot 57:287–293. https://doi.org/10.1007/s002530100702
Qiao H, Hu D, Ma J, Wang X, Wu H, Wang J (2019) Feeding effects of the microalga Nannochloropsis sp. on juvenile turbot (Scophthalmus maximus L.). Algal Res 41:101540. https://doi.org/10.1016/j.algal.2019.101540
Radhakrishnan S, Saravana Bhavan P, Seenivasan C, Muralisankar T (2015) Effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbergii postlarvae. Int J Fish Aquac 7:62–70. https://doi.org/10.5897/IJFA15.0471
Radhakrishnan S, Belal IEH, Seenivasan C, Muralisankar T, Bhavan PS (2016) Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquacult Rep 3:35–44. https://doi.org/10.1016/j.aqrep.2015.11.005
Ragaza JA, Hossain MS, Meiler KA, Velasquez SF, Kumar V (2020) A review on Spirulina: alternative media for cultivation and nutritive value as an aquafeed. Rev Aquacult 12:2371–2395. https://doi.org/10.1111/raq.12439
Rahimnejad S, Lee S-M, Park H-G, Choi J (2017) Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters, and antioxidant enzyme activity in olive flounder, Paralichthys olivaceus. J World Aquacult Soc 48:103–112. https://doi.org/10.1111/jwas.12320
Raji AA, Junaid OQ, Milow P, Taufek NM, Fada AM, Kolawole AA, Alias Z, Razak SA (2019) Partial replacement of fishmeal with Spirulina platensis and Chlorella vulgaris and its effect on growth and body composition of African catfish Clarias gariepinus (Burchell 1822). Indian J Fish 66:100–111. https://doi.org/10.21077/ijf.2019.66.4.87193-13
Raji AA, Jimoh WA, Abu Bakar NH, Taufek NHM, Muin H, Alias Z, Milow P, Razak SA (2020) Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. J Appl Phycol 32:1763–1770. https://doi.org/10.1007/s10811-020-02070-y
Richmond A, Hu Q (2013) Handbook of microalgal culture: applied phycology and biotechnology. John Wiley & Sons, UK
Rincón DD, Velásquez HA, Dávila MJ, Semprun AM, Morales ED, Hernández JL (2012) Substitution levels of fish meal by Arthrospira (=Spirulina) maxima meal in experimental diets for red tilapia fingerlings (Oreochromis sp.). Rev Colomb Cienc Pec 25:430–437
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew Sust Energ Rev 92:394–404. https://doi.org/10.1016/j.rser.2018.04.034
Rosas VT, Bessonart M, Romano LA, Tesser MB (2019) Fishmeal substitution for Arthrospira platensis in juvenile mullet (Mugil liza) and its effects on growth and non-specific immune parameters. Rev Colomb Cienc Pec 32:3–13. https://doi.org/10.17533/udea.rccp.v32n1a01
Roy SS, Pal R (2015) Microalgae in aquaculture: a review with special references to nutritional value and fish dietetics. Proc Zool Soc 68:1–8. https://doi.org/10.1007/s12595-013-0089-9
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014a) Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew Sust Energ Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Safi C, Ursu AV, Laroche C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014b) Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res 3:61–65. https://doi.org/10.1016/j.algal.2013.12.004
Samtiya M, Aluko RE, Dhewa T (2020) Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Process Nutr 2:6. https://doi.org/10.1186/s43014-020-0020-5
Sarker PK, Kapuscinski AR, Bae AY, Donaldson E, Sitek AJ, Fitzgerald DS, Edelson OF (2018) Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS One 13:e0201315. https://doi.org/10.1371/journal.pone.0201315
Sarker PK, Kapuscinski AR, McKuin B, Fitzgerald DS, Nash HM, Greenwood C (2020) Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Sci Rep 10:19328. https://doi.org/10.1038/s41598-020-75289-x
Sauvant D, Perez J-M, Tran G, Ponter AA (2004) Tables of composition and nutritional value of feed materials: pigs, poultry, cattle, sheep, goats, rabbits, horses and fish. Wageningen Academic, France
Scholz MJ, Weiss TL, Jinkerson RE, Jing J, Roth R, Goodenough U, Posewitz MC, Gerken HG (2014) Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell 13:1450–1464. https://doi.org/10.1128/EC.00183-14
Sheih IC, Fang TJ, Wu T-K (2009) Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem 115:279–284. https://doi.org/10.1016/j.foodchem.2008.12.019
Shepherd C, Jackson A (2013) Global fishmeal and fish-oil supply: inputs, outputs and marketsa. J Fish Biol 83:1046–1066. https://doi.org/10.1111/jfb.12224
Shi X, Luo Z, Chen F, Wei C-C, Wu K, Zhu X-M, Liu X (2017) Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquac Res 48:3244–3256. https://doi.org/10.1111/are.13154
Shiau SY, Chuang JL, Sun CL (1987) Inclusion of soybean meal in tilapia (Oreochromis aureus×O.niloticus) diets at two protein levels. Aquaculture 65:251–261. https://doi.org/10.1016/0044-8486(87)90238-9
Shubert E, Gärtner G (2015) Nonmotile coccoid and colonial green algae. In: Wehr JD, Sheath RG, Kociolek JP (eds) Freshwater algae of North America, 3rd edn. Academic Press, Boston, pp 315–373
Silva AJ, Cavalcanti VLR, Porto ALF, Gama WA, Brandão-Costa RMP, Bezerra RP (2020) The green microalgae Tetradesmus obliquus (Scenedesmus acutus) as lectin source in the recognition of ABO blood type: purification and characterization. J Appl Phycol 32:103–110. https://doi.org/10.1007/s10811-019-01923-5
Sims S, Ajayi O, Roubach R (2019) Microalgae in aquatic animal feeds. FAO Aquacult Newslett 60:50–50
Sinha AK, Kumar V, Makkar HPS, De Boeck G, Becker K (2011) Non-starch polysaccharides and their role in fish nutrition – A review. Food Chem 127:1409–1426. https://doi.org/10.1016/j.foodchem.2011.02.042
Sivakumar N, Sundararaman M, Selvakumar G (2018) Evaluation of growth performance of Penaeus monodon (Fabricius) fed diet with partial replacement of fishmeal by Spirulina platensis (Sp) meal. Indian J Anim Res 52:1721–1726. https://doi.org/10.18805/ijar.B-3438
Sørensen M, Berge GM, Reitan KI, Ruyter B (2016) Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar) —Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 460:116–123. https://doi.org/10.1016/j.aquaculture.2016.04.010
Sørensen M, Gong Y, Bjarnason F, Vasanth GK, Dahle D, Huntley M, Kiron V (2017) Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS One 12:e0179907. https://doi.org/10.1371/journal.pone.0179907
Stone DAJ (2003) Dietary carbohydrate utilization by fish. Rev Fish Sci 11:337–369. https://doi.org/10.1080/10641260390260884
Suarez Ruiz CA, Baca SZ, van den Broek LAM, van den Berg C, Wijffels RH, Eppink MHM (2020) Selective fractionation of free glucose and starch from microalgae using aqueous two-phase systems. Algal Res 46:101801. https://doi.org/10.1016/j.algal.2020.101801
Subasinghe R, Soto D, Jia J (2009) Global aquaculture and its role in sustainable development. Rev Aquacult 1:2–9. https://doi.org/10.1111/j.1753-5131.2008.01002.x
Sucunthowong K, Lee JH, Powtongsook S, Nootong K (2023) Simultaneous utilization of CO2 and nitrate wastes from compact recirculating aquaculture system for improving algal biomass (Scenedesmus armatus) production. Algal Res 74:103224. https://doi.org/10.1016/j.algal.2023.103224
Sultana R, Khatoon H, Rahman MR, Haque ME, Nayma Z, Mukta FA (2022) Potentiality of Nannochloropsis sp. as partial dietary replacement of fishmeal on growth, proximate composition, pigment and breeding performance in guppy (Poecilia reticulata). Bioresour Technol Rep 18:101112. https://doi.org/10.1016/j.biteb.2022.101112
Tacon AGJ (2020) Trends in global aquaculture and aquafeed production: 2000–2017. Rev Fish Sci Aquac 28:43–56. https://doi.org/10.1080/23308249.2019.1649634
Tacon AG, Metian MR, Tacon MAG, Hasan MR, Metian M (2011) Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects. Food and Agriculture Organization of the United Nations (FAO), Rome
Teimouri M, Amirkolaie AK, Yeganeh S (2013) The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquaculture 396–399:14–19. https://doi.org/10.1016/j.aquaculture.2013.02.009
Teuling E, Schrama JW, Gruppen H, Wierenga PA (2017) Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 479:490–500. https://doi.org/10.1016/j.aquaculture.2017.06.025
Teves JFC, Ragaza JA (2016) The quest for indigenous aquafeed ingredients: a review. Rev Aquacult 6:1–18. https://doi.org/10.1111/raq.12089
Tham PE, Lim HR, Khoo KS, Chew KW, Yap YJ, Munawaroh HSH, Ma Z, Rajendran S, Gnanasekaran L, Show PL (2023) Insights of microalgae-based aquaculture feed: A review on circular bioeconomy and perspectives. Algal Res 74:103186. https://doi.org/10.1016/j.algal.2023.103186
Tomas-Almenar C, Larran AM, de Mercado E, Sanz-Calvo MA, Hernandez D, Riano B, Garcia-Gonzalez MC (2018) Scenedesmus almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss). Aquaculture 497:422–430. https://doi.org/10.1016/j.aquaculture.2018.08.011
Tongsiri S, Mang-Amphan K, Peerapornpisal Y (2010) Effect of replacing fishmeal with Spirulina on growth, carcass composition and pigment of the Mekong giant catfish. Asian J Agric Sci 2:106–110
Torstensen BE, Espe M, Sanden M, Stubhaug I, Waagbø R, Hemre GI, Fontanillas R, Nordgarden U, Hevrøy EM, Olsvik P, Berntssen MHG (2008) Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285:193–200. https://doi.org/10.1016/j.aquaculture.2008.08.025
Trainor FR, Cain JR, Shubert LE (1976) Morphology and nutrition of the colonial green alga Scenedesmus: 80 years later. Bot Rev 42:5–25. https://doi.org/10.1007/BF02860860
Transparency Market Research (2016) Algae market (by application, by cultivation technology, and geography) – Global industry analysis, size, share, growth, trends, and forecast 2016–2024. Transparency Market Research. https://www.transparencymarketresearch.com/algae-market.html. Accessed 5 Dec 2017
Valente LMP, Custódio M, Batista S, Fernandes H, Kiron V (2019) Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol Biochem 45:1067–1081. https://doi.org/10.1007/s10695-019-00621-w
Van Binh V, Siddik MAB, Fotedar R, Chaklader MR, Abu Hanif M, Foysal MJ, Huy Quang N (2020) Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture 529:73541. https://doi.org/10.1016/j.aquaculture.2020.735741
Vassiliou V, Charalambides M, Menicou M, Chartosia N, Tzen E, Evagelos B, Papadopoulos P, Loucaides A (2015) Aquaculture feed management system powered by renewable energy sources: investment justification. Aquacult Econ Manag 19:423–443. https://doi.org/10.1080/13657305.2015.1082115
Velasquez SF, Chan MA, Abisado RG, Traifalgar RFM, Tayamen MM, Maliwat GCF, Ragaza JA (2016) Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile Nile tilapia (Oreochromis niloticus). J Appl Phycol 28:1023–1030. https://doi.org/10.1007/s10811-015-0661-y
Velazquez-Lucio J, Rodríguez-Jasso RM, Colla LM, Sáenz-Galindo A, Cervantes-Cisneros DE, Aguilar CN, Fernandes BD, Ruiz HA (2018) Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J 5:780–791. https://doi.org/10.18331/brj2018.5.1.5
Vizcaíno AJ, López G, Sáez MI, Jiménez JA, Barros A, Hidalgo L, Camacho-Rodríguez J, Martínez TF, Cerón-García MC, Alarcón FJ (2014) Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 431:34–43. https://doi.org/10.1016/j.aquaculture.2014.05.010
Vonshak A (1997) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology. CRC Press, London
Walker AB, Berlinsky DL (2011) Effects of partial replacement of fish meal protein by microalgae on growth, feed Intake, and body composition of Atlantic cod. N Am J Aquacult 73:76–83. https://doi.org/10.1080/15222055.2010.549030
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799. https://doi.org/10.1126/science.1189003
Wu L-c, J-aA Ho, Shieh M-C, Lu I-W (2005) Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J Agr Food Chem 53:4207–4212. https://doi.org/10.1021/jf0479517
Xi L, Lu Q, Liu Y, Su J, Chen W, Gong Y, Han D, Yang Y, Zhang Z, Jin J, Liu H, Zhu X, Xie S (2022) Effects of fish meal replacement with Chlorella meal on growth performance, pigmentation, and liver health of largemouth bass (Micropterus salmoides). Anim Nutr 10:26–40. https://doi.org/10.1016/j.aninu.2022.03.003
Xiao X, Zhou Y, Liang Z, Lin R, Zheng M, Chen B, He Y (2022) A novel two-stage heterotrophic cultivation for starch-to-protein switch to efficiently enhance protein content of Chlorella sp. MBFJNU-17. Bioresour Technol 344:126187. https://doi.org/10.1016/j.biortech.2021.126187
Xie T, Xia Y, Zeng Y, Li X, Zhang Y (2017) Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour Technol 233:247–255. https://doi.org/10.1016/j.biortech.2017.02.099
Yamada T, Sakaguchi K (1982) Comparative studies onChlorella cell walls: Induction of protoplast formation. Arch Microbiol 132:10–13. https://doi.org/10.1007/BF00690809
Yamamoto T, Shima T, Furuita H, Suzuki N, Shiraishi M (2001) Nutrient digestibility values of a test diet determined by manual feeding and self-feeding in rainbow trout and common carp. Fish Sci 67:355–357. https://doi.org/10.1046/j.1444-2906.2001.00251.x
Yang L, Li H, Lu Q, Zhou W (2021) Emerging trends of culturing microalgae for fish-rearing environment protection. J Chem Technol Biot 96:31–37. https://doi.org/10.1002/jctb.6563
Acknowledgements
Great thanks were given to Qiang Hu for his instructive suggestions for the review.
Funding
This work was supported by the Doctoral Scientific Research Foundation of Henan University of Science and Technology (13480087; 13480088), Henan Provincial Science and Technology Research Project (222102320144), and the National Natural Science Foundation of China (NSFC, no. 32202952).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception. Data collection was performed by Weijun Chen, Shenping Cao, Xiaochan Gao, and Ping Sun. The first draft of the manuscript was written by Shiyang Gao. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, S., Chen, W., Cao, S. et al. Microalgae as fishmeal alternatives in aquaculture: current status, existing problems, and possible solutions. Environ Sci Pollut Res 31, 16113–16130 (2024). https://doi.org/10.1007/s11356-024-32143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32143-1