Abstract
Anaerobic digestion of microalgal biomass for biogas production may be limited due to the cell wall resulting in an inefficient bioconversion. Enzymatic pretreatments are applied for inducing cell damage/lysis and organic matter solubilisation and this way increasing biogas production. We evaluated enzymatic pretreatments in different conditions for comparing in relation to cell wall rupture, increase of soluble material and increase in biogas production through anaerobic digestion performance in BMP assay. Chlorella sorokiniana cultures were subjected to three different enzymatic pretreatments, each under four different conditions of enzyme/substrate ratio, pH and application time. The results showed increases over 21% in biogas productions for all enzymatic pretreatments. Enzymatic pretreatment was effective at damaging microalgae cell wall, releasing organic compounds and increasing the rate and final methane yield in BMP tests. We observed a synergistic activity between the mixtures enzymes, which would depend on operational conditions used for each pretreatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High biomass productivity, growth conditions that do not depend on fertile land, allied with CO2 fixation are the main advantages of the use of microalgae for bioenergy conversion into biogas through anaerobic digestion [1]. Nonetheless, industrial-scale systems are not yet viable, since there are still limiting steps in the overall process [2]. One of the main bottlenecks is the biodegradation of microalgae cell wall and release of intracellular organic matter during anaerobic digestion [3]. Due to the characteristics of the cell wall structure and composition, extracellular enzymes released by heterotrophic bacteria are not able to degrade the overlayer in totality and, therefore, the available organic matter is limited, as well as the biogas production [4, 5].
This issue may be overcome by applying pretreatment techniques to microalgal biomass for increasing its biodegradability through cell wall disruption and/or partial hydrolysis of resistant cell wall polymers [6,7,8]. In this manner, pretreatments may increase organic matter solubilisation and the rate and extent of anaerobic digestion process [8].
Among pretreatment methods, enzymatic pretreatment perform by means of the selective permeability of microalgae cell walls and, therefore, soluble compound release is more specific compared to mechanical and thermal one [9]. The main advantage of enzymatic techniques relies on its low energy demand, which normally regards only to biomass mixing. Moreover, purified enzymes may be replaced by enzyme production through other microorganisms, by enzyme expression through the microalgae cells to be digested and by the production of hydrolytic enzymes by adding living bacteria or fungi [10,11,12]. Moreover, studies have reported a high release of soluble sugars combined with mild operational conditions, absence of corrosion problems and a lower production of secondary metabolites [13]. The main parameters used for optimising enzymatic pretreatment the dose or concentration between enzyme and substrate, the optimal activity conditions in terms of pH and temperature and the cost associated to the extraction and purification. However, in studies of enzymatic pretreatments for biogas production are not considered all the variables that could affect the enzymatic activity, in addition, not to describe the type of enzymatic action produced in the solubilisation of organic matter. Data needed to describe the biodegradation processes.
Results on enzymatic pretreatment of microalgae carried out up to date are summarised in Table 1. As can be seen, increments in solubilisation and biogas production varied widely, from 36.6 to 243% and from 7.6 to 672%, respectively, compared to non-pretreated microalgae. In fact, control biomass showed a methane yield variation from 203 to 1545 mL CH4/g VS, which indicates that initial biodegradability and pretreatment effectiveness depended on many parameters, such as microalgae species, growing characteristics and pretreatment conditions [19,20,21].
Therefore, the objective of this study was to evaluate the biomethanisation potential of the microalgae Chlorella sorokiniana using enzymatic pretreatment. To this aim, a mix of commercial enzymes was applied for investigating its effect on cell wall disruption, soluble organic matter release, anaerobic digestion rate and final methane yield. For which, we evaluate different variables that affect the enzymatic activity to determine the biodegradation process.
Material and Methods
Microalgae
The microalgal biomass used in this study comprehended the species Chlorella sorokiniana and was obtained from the University of Huelva (Spain). The microalgae was grown using the Sueoka medium (0.72 g KPO4H2/L, 1.44 g K2PO4H/L, 0.061 g MgSO4·7H2O, 0.002 g CaCl2·2H2O, 0.5 g NH4Cl, 0.95 g KNO3 and 5 mL/L of trace minerals, which had 2.28 g of H3BO3, 4.4 g ZnSO4, 1.02 g MnCl2, 1.0 g of FeSO4, 0.32 g of CaCl2·6H2O, 0.32 g of CuSO4·5H2O and 0.22 g of Mo7O24(NH4)6·4H2O). The growth conditions were set at 21 °C (± 2 °C), at pH 7 constantly, 24 h/day artificial light of 24–39 W with an intensity of 127.6 μmol/m2 s and atmospheric aeration of 1.3–1.5 L/min. Biomass concentration was estimated by means of volatile suspended solids (VSS) and optic density (OD) at 600 nm using spectrophotometer (Jenway 6715 UV/VIS). Microalgae were harvested using a centrifuge at 0.31 KW (Hermle Z 400, Labortechnik GmbH) and stored at 4 °C before characterisation and use.
Cellulase Enzymatic Activity
Cellulase activity was measured in terms of “filter paper units” (FPU) per millilitre of enzyme (undiluted) using 2.0 mg of reducing sugar as glucose and 50 mg of filter paper (conversion 4%) in 60 min as the intersection point for calculating cellulase FPU as according to the International Union of Pure and Applied Chemistry (IUPAC). The enzymatic activity for each enzyme mix was evaluated in triplicate, and results were expressed as mean values and standard deviations.
Pretreatment Methods and Biomass Solubilisation
Three commercial enzyme mixtures were evaluated: Cellic ctec2 (cellulase, β-glucosidase, hemicellulase), Ns5003 (cellulase, xylanase, endoglucanase, exoglucanase, β-glucosidase) and Ns22128 (only cellulase). All of them were provided by Novozymes®. Those enzymes were chosen due to their lower commercial cost, acquisition feasibility and the characteristics of microalgae cell wall composition (mainly composed of cellulose, hemicellulose, pectin and glycoprotein). In order to easy interpretation, the enzyme mixtures will be named as follows: mix 1 (Cellic ctec2), mix 2 (Ns5003) and cellulase (Ns22128). Results were expressed as the enzymatic activity of cellulase (common in all three) and volatile solids (VS) solubilisation under different pH and temperature conditions.
The pretreatment conditions were selected considering three different scenarios related to pH and temperature parameters for enzymatic activities’ activation: (i) the optimum conditions for enzymatic activation reported by the manufacturer (Novozymes®), i.e. pH 4.8 and 50 °C; (ii) the condition in which the anaerobic digestion process was performed, i.e. pH 7 and 37 °C; and (iii) the condition corresponding to C sorokiniana growing condition, i.e. pH 7 and 21 °C. The optimal conditions in terms of cellulase activity were chosen for evaluating microalgae biodegradability, i.e. (i) 1% enzyme/substrate, pH 4.8 and 24 h; (ii) 1% enzyme/substrate, pH 7.0 and 24 h; (iii) 2% enzyme/substrate, pH 7.0 and 24 h; and (iv) 1% enzyme/substrate, pH 7.0 and 48 h.
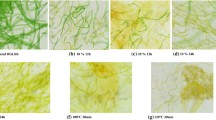
Pretreatment effectiveness was determined by means of microalgae cell wall disruption and/or damage and organic matter solubilisation through Sytox Green staining in unpretreated and pretreated cells [22, 23]. The Sytox Green for Fluorescent labeling (Molecular Probes Inc., Eugene, OR, USA) has a high affinity for the nucleic acid, penetrating only those damaged cell membranes. For the application of Sytox Green probe in microalgal biomass samples were used proportional values. Sytox Green probe was supplied as a 5-mM stock solution in solvent solution. Of this stock solution, 0.5 ml was added to 0.5 ml cell suspension and the mixture was incubated for 5 min at room temperature in the dark. Probe fluorescence and autofluorescence of microalgae were used as dead cell markers (for rupture or damage) and live cells, respectively. The cells were visualised by fluorescence microscopy with double filter band at 473–498 and 548–573 nm for excitation and at 515–535 and 590–620 nm for emission. The fluorescence images are captured by Leica, Germany, camera.
The increment in soluble organic matter content after pretreatment was calculated as described in Eq. 1.
where VSSs represents the soluble volatile suspended solids after pretreatment and VSSo represents soluble volatile suspended solids without pretreatment.
Biochemical Methane Potential Tests
Biochemical methane potential (BMP) tests were carried out for comparing the anaerobic digestion rate and extent of C. sorokiniana before and after enzymatic pretreatment conditions, according to standardised procedure [24]. The anaerobic inoculum used in the BMP tests was obtained from a lab-scale anaerobic reactor that treats sewage sludge. BMP tests were performed in 100-mL flasks under mesophilic conditions (37 °C) at pH 7. In all cases, the substrate to inoculum ratio was maintained at 0.5 g VSsubstrate/g VSinoculum. Bubbles were made in the bottles using a mix of gases (80% N and 20% CO2) in order to ensure anaerobic conditions and were then sealed. Control bottles for enzymatic activity, microalgal biomass, and inoculum were performed for each BMP assay in order to correct for inoculum methane yields. Methane production from the inoculum was determined in blank assays with medium and no microalgal biomass, which is subtracted from the methane production obtained with microalgal biomass assays. BMP assay ended once the methane production had stopped.
For CH4 production was quantified by displacement of a strongly alkaline solution of sodium hydroxide (NaOH) at a concentration of 25 g/L. CO2 contained in biogas is absorbed by the alkaline medium; CH4 produced was calculated by measuring the volume or weight of the displaced liquid.
All tests were performed in triplicated. Results were reported as mean values and standard deviations of the methane yield, which was calculated by the methane produced, divided by the volatile solids content fed to each bottle.
For evaluating the kinetics effect of enzymatic pretreatment conditions, the tests were modelled using a modified version of the Gompertz model [25] expressed by the Eq. 2.
where B represents the methane accumulated in a time t (mL CH4), P represents the maximum potential of methane production (mL CH4/g VS), Rm represents the maximum rate of methane production (mL CH4/g VS·d), λ represents the duration of the lag phase (days) and t represent the digestion time (days).
Statistics
Statistical analyses were carried out for verifying differences in microalgae anaerobic biodegradability before and after pretreatment conditions. To this aim, the statistical software Statistica 13 (StatSoft Inc., Tulsa, USA, 2016) was used. For all cases in which the differences were noticed, the Bonferroni post-hoc test was implemented [26].
Analytical Methods
The biomass was quantified by means of total (TS) and volatile solids (VS) and optical density (OD). TS and VS were determined as indicated in the Standard Methods [16]. OD was measured at 600 nm using a spectrophotometer (Jenway 6715 UV/VIS). pH was analysed through a pHmeter (HI/111 Hanna Instrumen).
Results and Discussion
Effectiveness of Enzymatic Pretreatment
Enzymatic pretreatment was firstly evaluated by means of cellulase enzyme activity (Fig. 1). Cellulase activity was used as parameter since all enzymatic mixtures had cellulase as a component. This enzyme is a glucosyl hydrolase and is responsible for the degradation of cellulose, one of the main constituents of microalgae cell wall [17]. The best conditions were then tested in BMP tests. For this, cell wall integrity using Sytox staining for life/dead cells and organic matter solubilisation were assessed for analysing the pretreatment effectiveness. The results obtained are summarised in Table 2.
As can be seen, the highest enzymatic activity was shown for the conditions of pH 4.8 and 50 °C for all enzymatic mixtures. This was expected, since it represents the optimal condition for the enzymes according to the manufacturer. On the other hand, the lowest activity was shown for the conditions of pH 7.0 and 21 °C, which corresponds to the conditions in which microalgae was cultivated (Fig. 1). Regarding the results, the conditions chosen for the anaerobic biodegradability assay were as follows: (i) 1% enzyme/substrate, pH 4.8 and 24 h; (ii) 1% enzyme/substrate, pH 7.0 and 24 h; (iii) 2% enzyme/substrate, pH 7.0 and 24 h; and (iv) 1% enzyme/substrate, pH 7.0 and 48 h.
Moreover, all pretreatment conditions tested showed rupture and/or damage in its cell wall structure according to the Sytox staining analysis. This indicates that enzymatic pretreatment was effective at impacting the outer membrane, which may ease the release of readily available biodegradable compounds. In fact, organic matter solubilisation was significantly increased after all pretreatment conditions, reaching values from 23 to 50% (Table 2). The increments found are in accordance with those previously reported in the literature, which varied from 36.6 to 243% (Table 1). The high range of values was mostly due to the different microalgae species studied and the operational conditions for applying the pretreatments.
In general, the highest solubilisation values were reached for the enzyme Mix 2 (cellulase, xylanase, endoglucanase, exoglucanase, β-glucosidase), followed by the enzyme Mix 1 (cellulase, β-glucosidase y hemicellulase) and, finally, for cellulase. The enzyme mixtures effectiveness depends of a chain degradation and, therefore, of the presence of endoglucanase, which hydrolyse the 1,4-β-glucosides bonds in an average way to the microfiber of cellulose; exoglucanase, which also performs by hydrolysing the 1,4 β-glucosides bonds releasing cellobiose; and β-glucosidases, which catalases cellobiose and releases β-d-glucose [27]. The cooperative integration of those three enzymes results in the synergetic degradation of cellulose, i.e. the enzymes combined are more effective if compared to the sum of their effectiveness alone [14]. This synergistic activity between different cellulases depends on some factors such as the characteristics of the substrate to be degraded, concentration and enzymatic process, affinity of the cellulases with the substrate and the ratio enzymes [15].
Moreover, the enzyme xylanase is responsible for xylan hydrolysis, the major component of hemicellulose [18] and a synergistic activity has been described between different types of xylanases as well [15, 28]. This synergistic effect would result in higher percentages of solubilisation, as observed for 1% pH 4.8–24 h condition, which is described as optimal for the enzymatic activation of cellulose and for 2% pH 7–24 h condition, in which the enzyme–substrate ratio increases for the enzyme mixes 1 and 2, which contain both types of enzymes (cellulases and hemicellulases). Conditions, in which the highest solubilisation was achieved with only cellulases, are likely to operate under these conditions other such factors. For example, under these conditions, a synergistic effect would not be achieved and/or conditions to increased cellulase activity.
Increase in Anaerobic Biodegradability in BMP Tests
Accumulated methane yield before and after enzymatic pretreatments in BMP tests are shown in Fig. 2. As can be seen, for all cases, enzymatic pretreatment increased the final methane yield when compared to non-pretreated biomass. Differences obtained were significantly higher according to the ANOVA test applied.
Accumulated methane yield in BMP tests of C. sorokiniana before (C) and after enzymatic pretreatment with mix 1, mix 2 and cellulase (E1–E12) at the four different conditions: a 1% enzyme/substrate, pH 4.8 and 24 h; b 1% enzyme/substrate, pH 7.0 and 24 h; c 2% enzyme/substrate, pH 7.0 and 24 h; and d 1% enzyme/substrate, pH 7.0 and 48 h
The methane yield increase ranged from 22 to 86%, which were among those previously reported in the literature (Table 1). In fact, enzymatic pretreatment with cellulase, protease, β-glucanase and xylanase of microalgae Acutodesmus obliquus increased methane yield in 14% [29], while pretreatment of Arthrospira maxima using esterase and protease showed an increase up to 672% in the final methane yield attained in BMP tests [30].
For assessing anaerobic digestion rate, experimental data was modelled using a modified version of the Gompertz model. The results achieved are summarised in Table 3. As can be seen, lowest lag phase was shown for the condition using 2% enzyme/substrate. This indicates that higher enzyme concentration affects the initial biomass hydrolysis. Moreover, commercial enzyme mixtures use stabilisers that contain simple sugars, which could be quickly consumed by bacteria. Thus, by increasing the concentration of enzymes, the bacteria would have higher concentration of sugars available to be consumed immediately. Regarding the values of maximum potential of methane production (P) and maximum rate of methane production (Rm), all cases tested showed significantly higher values for pretreated conditions compared to non-pretreated ones.
In this study, no clear correlation was observed when comparing biomass solubilisation and methane yield increase, although both showed increments when compared to control biomass. This may be due to the release of metabolites that are not easily degradable or that are toxic to anaerobic microorganisms. In fact, the longest lag phase for pretreated microalgae in BMP tests would support this hypothesis (Table 3). In this manner, for each enzymatic mix used as pretreatment, there were different effects on the solubilisation of the microalgal biomass through the permeability achieved by the organic matter by each enzyme and/or by the synergy between enzymatic activities.
Finally, enzymatic pretreated was effective at disrupting and/or damaging microalgae cell wall, releasing organic compounds and increasing the rate and final methane yield in BMP tests (Fig. 3). Moreover, this technology had no thermal or electricity energy input, which is an advantage in respect to physical pretreatment methods. This suggests that the process energy balance is generally more favourable, i.e. energy output from methane yield increase is higher than the low energy required for the pretreatment step. Nonetheless, in this case, enzyme cost should be considered. It is known that the high cost of the enzymatic industrial production may be a drawback for its commercialisation and use. In any case, during the last 6 years, manufacturers have reduced enzyme costs by focusing efforts on improving its efficiency, identifying new and more active enzymes, creating mixtures to optimise the degradation of specific substrates and minimising production costs [31]. In addition, the enzyme cost may also be overcome by enzyme production through other microorganisms, by enzyme expression through the microalgae cells to be digested and by the production of hydrolytic enzymes by adding living bacteria or fungi.
Conclusions
The aim of this study was to assess enzymatic pretreatment using two enzyme mixtures and cellulase only for disrupting microalgae cell wall, releasing soluble organic compounds and increasing anaerobic digestion rate and extent in BMP tests. Results showed that for all conditions, tested enzymes were able to improve microalgae anaerobic biodegradability, reaching an increase from 22 to 86% higher than non-pretreated biomass. Moreover, modelled data indicated that pretreatment also increased significantly biogas production rate. The best conditions among the applied ones were showed for the highest concentration of enzyme (i.e. 2% enzyme/substrate) and for the optimal conditions for the enzyme activity (i.e. pH 4.8).
References
Bohutskyi, P., & Bouwer, E. (2013). Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In W. J. Lee (Ed.), Adv. Biofuels Bioprod (pp. 873–975). New York, NY: Springer New York. https://doi.org/10.1007/978-1-4614-3348-4_36.
Zamalloa, C., Vulsteke, E., Albrecht, J., & Verstraete, W. (2011). The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresource Technology, 102(2), 1149–1158. https://doi.org/10.1016/j.biortech.2010.09.017.
Ward, A. J., Lewis, D. M., & Green, F. B. (2014). Anaerobic digestion of algae biomass: a review. Algal Research, 5, 204–214. https://doi.org/10.1016/j.algal.2014.02.001.
González-Fernández, C., Sialve, B., Bernet, N., & Steyer, J. P. (2012). Impact of microalgae characteristics on their conversion to biofuel. Part I: focus on cultivation and biofuel production. Biofuels, Bioprod. Biorefining., 6, 246–256. https://doi.org/10.1002/bbb.
González-Fernández, C., Sialve, B., Bernet, N., & Steyer, J. P. (2012). Impact of microalgae characteristics on their conversion to biofuel. Part II: focus on biomethane production. Biofuels, Bioprod. Biorefining, 6, 205–218. https://doi.org/10.1002/bbb.
Angelidaki, I., & Batstone, D. J. (2010). Anaerobic digestion. In Solid Waste Technology and Management, 1(2), 583–600.
Mendez, L., Mahdy, A., Timmers, R. A., Ballesteros, M., & González-Fernández, C. (2013). Enhancing methane production of Chlorella vulgaris via thermochemical pretreatments. Bioresource Technology, 149, 136–141. https://doi.org/10.1016/j.biortech.2013.08.136.
Passos, F., Uggetti, E., Carrère, H., & Ferrer, I. (2014). Pretreatment of microalgae to improve biogas production: a review. Bioresource Technology, 172, 403–412. https://doi.org/10.1016/j.biortech.2014.08.114.
Córdova, O., Santis, J., Ruiz-Fillipi, G., Zuñiga, M. E., Fermoso, F. G., & Chamy, R. (2018). Microalgae digestive pretreatment for increasing biogas production. Renewable and Sustainable Energy Reviews, 82, 2806–2813. https://doi.org/10.1016/j.rser.2017.10.005.
Yin, L. J., Jiang, S. T., Pon, S. H., & Lin, H. H. (2010). Hydrolysis of chlorella by Cellulomonas sp. YJ5 cellulases and its biofunctional properties. Journal of Food Science, 75(9), H317–H323. https://doi.org/10.1111/j.1750-3841.2010.01867.x.
Chen, C. Y., Der Bai, M., & Chang, J. S. (2013). Improving microalgal oil collecting efficiency by pretreating the microalgal cell wall with destructive bacteria. Biochemical Engineering Journal, 81, 170–176. https://doi.org/10.1016/j.bej.2013.10.014.
Muñoz, C., Hidalgo, C., Zapata, M., Jeison, D., Riquelme, C., & Rivas, M. (2014). Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Applied and Environmental Microbiology, 80(14), 4199–4206. https://doi.org/10.1128/AEM.00827-14.
Yun, Y. M., Kim, D. H., Oh, Y. K., Shin, H. S., & Jung, K. W. (2014). Application of a novel enzymatic pretreatment using crude hydrolytic extracellular enzyme solution to microalgal biomass for dark fermentative hydrogen production. Bioresource Technology, 159, 365–372. https://doi.org/10.1016/j.biortech.2014.02.129.
Zhang, Y. H. P., & Lynd, L. R. (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnology and Bioengineering, 88(7), 797–824. https://doi.org/10.1002/bit.20282.
Yang, B., Dai, Z., Ding, S.-Y., & Wyman, C. E. (2014). Enzymatic hydrolysis of cellulosic biomass. Biofuels., 2(4), 421–449. https://doi.org/10.4155/bfs.11.116.
APHA-AWWA-WPCF, Standard methods for the examination of water and wastewater, (20th Ed.)Washingt. (1999).
Percival Zhang, Y. H., Himmel, M. E., & Mielenz, J. R. (2006). Outlook for cellulase improvement: screening and selection strategies. Biotechnology Advances, 24(5), 452–481. https://doi.org/10.1016/j.biotechadv.2006.03.003.
Thomas, L., Joseph, A., & Gottumukkala, L. D. (2014). Xylanase and cellulase systems of Clostridium sp.: an insight on molecular approaches for strain improvement. Bioresource Technology, 158, 343–350. https://doi.org/10.1016/j.biortech.2014.01.140.
Passos, F., Hom-Diaz, A., Blanquez, P., Vicent, T., & Ferrer, I. (2016). Improving biogas production from microalgae by enzymatic pretreatment. Bioresource Technology, 199, 347–351. https://doi.org/10.1016/j.biortech.2015.08.084.
Mahdy, A., Ballesteros, M., & González-Fernández, C. (2016). Enzymatic pretreatment of Chlorella vulgaris for biogas production: influence of urban wastewater as a sole nutrient source on macromolecular profile and biocatalyst efficiency. Bioresource Technology, 199, 319–325. https://doi.org/10.1016/j.biortech.2015.08.080.
He, S., Fan, X., Katukuri, N. R., Yuan, X., Wang, F., & Guo, R. B. (2016). Enhanced methane production from microalgal biomass by anaerobic bio-pretreatment. Bioresource Technology, 204, 145–151. https://doi.org/10.1016/j.biortech.2015.12.073.
Córdova, O., Passos, F., & Chamy, R. (2018). Physical pretreatment methods for improving microalgae anaerobic biodegradability. Applied Biochemistry and Biotechnology, 185(1), 114–126. https://doi.org/10.1007/s12010-017-2646-6.
Sato, M., Murata, Y., Mizusawa, M., Iwahashi, H., & Oka, S. (2004). A simple and rapid dual-fluorescence viability assay for microalgae. Microbiology and Culture Collections, 20, 53–59 http://www.jscc-home.jp/journal/No20_2/No20_2_53.pdf.
Angelidaki, I., Alves, M., Bolzonella, D., Borzacconi, L., Campos, J. L., Guwy, A. J., Kalyuzhnyi, S., Jenicek, P., & van Lier, J. B. (2009). Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Science and Technology, 59(5), 927–934. https://doi.org/10.2166/wst.2009.040.
Donoso-Bravo, A., Pérez-Elvira, S. I., & Fdz-Polanco, F. (2010). Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chemical Engineering Journal, 160(2), 607–614. https://doi.org/10.1016/j.cej.2010.03.082.
Doncaster, C. P., & Davey, A. J. H. (2007). Analysis of variance and covariance: how to choose and construct models for the life sciences. https://doi.org/10.1017/CBO9780511611377.
Kumar, R., Singh, S., & Singh, O. V. (2008). Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. Journal of Industrial Microbiology & Biotechnology, 35(5), 377–391. https://doi.org/10.1007/s10295-008-0327-8.
Moraïs, S., Barak, Y., Caspi, J., & Hadar, Y. (2010). Cellulase-xylanase synergy in designer cellulosomes for enhanced degradation of a complex cellulosic substrate. MBio., 1, 3–10. https://doi.org/10.1128/mBio.00285-10.Editor.
Gruber-Brunhumer, M. R., Jerney, J., Zohar, E., Nussbaumer, M., Hieger, C., Bochmann, G., Schagerl, M., Obbard, J. P., Fuchs, W., & Drosg, B. (2015). Acutodesmus obliquus as a benchmark strain for evaluating methane production from microalgae: influence of different storage and pretreatment methods on biogas yield. Algal Research, 12, 230–238. https://doi.org/10.1016/j.algal.2015.08.022.
Ometto, F., Quiroga, G., Psenicka, P., Whitton, R., Jefferson, B., & Villa, R. (2014). Impacts of microalgae pre-treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Research, 65, 350–361. https://doi.org/10.1016/j.watres.2014.07.040.
Merino, S. T., & Cherry, J. (2007). Progress and challenges in enzyme development for biomass utilization. Advances in Biochemical Engineering/Biotechnology, 108, 95–120. https://doi.org/10.1007/10_2007_066.
Funding
The authors want to thank Pontificia Universidad Católica de Valparaiso for the financial support. Olivia Córdova appreciates her scholarship funded by the CONICYT, Beca Nacional Doctorado, 21121012.
Author information
Authors and Affiliations
Contributions
OC conceived the study, designed, and performed the experiments, evaluated the data and drafted the manuscript. FP evaluated the data and drafted the manuscript. RC supervised the work and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Córdova, O., Passos, F. & Chamy, R. Enzymatic Pretreatment of Microalgae: Cell Wall Disruption, Biomass Solubilisation and Methane Yield Increase. Appl Biochem Biotechnol 189, 787–797 (2019). https://doi.org/10.1007/s12010-019-03044-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03044-8