Abstract
The aquaculture industry is an efficient edible protein producer and grows faster than any other food sector. Therefore, it requires enormous amounts of fish feed. Fish feed directly affects the quality of produced fish, potential health benefits, and cost. Fish meal (FM), fis oil (FO), and plant-based supplements, predominantly used in fish feed, face challenges of low availability, low nutritional value, and high cost. The cost associated with aquaculture feed represents 40–75% of aquaculture production cost and one of the key market drivers for the thriving aquaculture industry. Microalgae are a primary producer in aquatic food chains. Microalgae are expanding continuously in renewable energy, pharmaceutical pigment, wastewater treatment, food, and feed industries. Major components of microalgal biomass are proteins with essential amino acids, lipids with polyunsaturated fatty acids (PUFA), carbohydrates, pigments, and other bioactive compounds. Thus, microalgae can be used as an essential, viable, and alternative feed ingredient in aquaculture feed. In recent times, live algae culture, whole algae, and lipid-extracted algae (LEA) have been tested in fish feed for growth, physiological activity, and nutritional value. The present review discusses the potential application of microalgae in aquaculture feed, its mode of application, nutritional value, and possible replacement of conventional feed ingredients, and disadvantages of plant-based feed. The review also focuses on integrated processes such as algae cultivation in aquaculture wastewater, aquaponics systems, challenges, and future prospects of using microalgae in the aquafeed industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture is one of the fastest-growing food sectors globally. Aquaculture provides ~50% of the fish for human consumption, and the trend is on the continuous rise over recent years (Prem and Tewari 2020). In the last five decades, worldwide fish consumption per capita has increased significantly from 9kg in 1961 to 20.2kg in 2015 (Cordeiro 2019). By 2050, aquaculture production needs to be doubled from the current production capacity to cater to the global per capita fish consumption rates without further burden on wild fisheries (Waite et al. 2014).
Despite the fast growth rate of the aquaculture industry, globally, meeting the demand for quality fish is still a challenge. Many health authorities recognize fish as good primary dietary sources of omega-three fatty acids and proteins, which can potentially improve human health, including reducing the risk of heart disease and cancer and helping to uplift brain growth and function (Ruxton et al. 2007; Tacon et al. 2020). As a result, consumption of animal and fish proteins has increased due to heightened health awareness, resulting in higher demand for quality fish (Yaakob et al. 2014).
Fish provide essential fatty acids for humans such as ALA (α-linolenic acid, C18:3ω3), DHA (docosahexaenoic acid, C22:6ω3), and EPA (eicosapentaenoic acid, C20:5ω3) (Lagarde 2008; Spolaore et al. 2006). These polyunsaturated fatty acids are mainly supplied through the diet; ALA comes from vegetable oils and nuts, while DHA and EPA from fish and seafood (Kalogeropoulos et al. 2010). Some dietary ALA may be converted to EPA and DHA in the human diet, although this is limited (Lagarde 2008). Essential PUFAs (polyunsaturated fatty acid) must be provided in the diet at recommended daily levels of 140–667mg/day (Lemahieu et al. 2013); much higher levels, up to 4g/day, may be required for those suffering from CVD (cardiovascular diseases) and other pathologies.
Thus, it is imperative to ensure that farmed fish feeds have an appropriate balance of ingredients to accumulate essential fatty acids in fish. Unlike industrialized nations, people in some highly populated, emergent, and small island developing states obtain most proteins and essential fatty acids through fish consumption. Therefore, many factors influence the production of high-quality farmed fish, including feed quality, size and shape, and water quality (Miranda et al. 2020; Gupta 2020).
The balanced nutritional feed has emerged as a critical limiting factor to increase production quality and efficiency. As a result, the aquafeed market has a global annual value of around $60b (Tibbetts et al. 2015b).
Fish need feed with all required ingredients for energy, movement, normal function, defense against diseases, maintenance, and metabolic growth. Globally, aquaculture production depends (>70%) on formulated feed, and 50–70% of total production cost is associated with feed (Gong et al. 2019; Llagostera et al. 2019). Formulated feeds are made of different constituents such as proteins, oils, carbohydrates, essential amino acids, vitamins, minerals, and pigments. These constituents are derived from various sources. Fish meal (FM), chicken feather meal, and soybean meal are commonly used for protein, and maize and wheat bran are employed as a carbohydrate source. The inclusion percentage of each ingredient (protein, oil, carbohydrate, mineral, vitamin, etc.) varies according to fish species and growth stages (i.e., larvae, fry, juvenile, adult, and spawning). The protein requirement is higher during early development (e.g., larvae or juvenile) due to higher metabolic growth when compared against grower and finisher stages. Among all feed constituents, ingredients that provide protein are a major contributor to the total feed cost. The quality of fish feed ensures not only reasonable growth rates but also has physiological benefits to fish. Therefore, it is imperative to ensure that those feed ingredients are relevant (Kiron 2012). Most conventional protein sources provide basic nutrition but lack essential amino acids, long-chain polyunsaturated fatty acids (LC-PUFA), and pigments. Furthermore, the addition of these expensive essential ingredients in feed significantly increases the cost of fish feed (Hass et al. 2016). FM is one of the best protein sources for fish feed due to its high digestible protein content (60–72%), essential fatty acids, and balanced essential amino acid profile (Cho and Kim 2011). Fish oil (FO) contains a good proportion of ALA, EPA, and DHA. FM is also a good source of natural vitamins (e.g., biotin, B12 and choline) and trace elements (selenium and iodine). Hence, FM and FO are considered essential ingredients in diets for different fish species (carnivorous, omnivorous). An alternative good-quality feed ingredient availability will be significantly constrained for the future development of the aquaculture industry (Palmegiano et al. 2005).
To reduce the overall cost of fish feed, low levels of FM are supplemented in feed formulations. The inadequate inclusion of FM in feed is not adequately compensated for by plant-based proteins and oil sources. Thus, final products may lack critical nutritional components such as PUFAs and essential amino acids (Turchini et al. 2009). It has also been estimated that 25% of the world’s captured fish are utilized for FM and FO production (De Silva et al. 2010). Approximately 70% of the FM supplies globally are utilized by the aquaculture industry (Subhash et al. 2020). The increased use of FM in fish feed adversely affects the sustainability of the marine environment. Therefore, it is a significant challenge for the aquaculture industry to find replacements for FM that can provide proteins, oil, pigments, etc. There is a need to find sustainable alternative feed sources for aquaculture that can provide all essential requirements, including proteins, oil, carbohydrates, pigments, vitamins, and minerals (Tibbetts et al. 2015c; Tibbetts et al. 2017). Many alternative plants (soya bean meal) and animal-based ingredients (feather meal, poultry meal) have been explored and deemed to have their advantages and disadvantages.
Microalgal biomass contains important biochemical constituents such as lipid, protein, carbohydrate, and various pigments (Apandi et al. 2018). They are a major source of food for fishes in their natural habitat, making them suitable ingredients for aquaculture feed production. Algae provide nearly all essential nutrients such as PUFA, amino acids, vitamins, and minerals (Benemann 1992; Becker 1994; Knuckey et al. 2005; Carneiro et al. 2020). Due to their balanced nutritional content, microalgae are rapidly gaining importance as a feed/feed supplement, potentially replacing FM and other conventional constituents in aquaculture and animal feed. Algae such as Spirulina, Chlorella sp., Scenedesmus sp., Dunaliella sp., and Nannochloropsis sp. are most commonly used in aquaculture feed due to their excellent nutritional value and suitability (Khatoon et al. 2010). Microalgae have been used as feed additives at a large scale for fish and prawn larvae, crustaceans, and molluscan (Belay et al. 1996; Borowitzka 1997).
Dry whole algae and lipids and pigment-extracted biomass could also be used to supplement aquaculture feed. For example, globally, 30% of algal biomass produced is used in animal feed (Becker 2007). This is due to the many advantages of algae, such as fast growth rate, year-round growth, and because they do not require fertile land, they can grow in wastewater and sequester 1.83 kg of CO2 for the production of 1 kg of algal biomass (Chisti 2007; Bhola et al. 2016; Chauton et al. 2015; Gupta et al. 2016; Rawat et al. 2011; Shriwastav et al. 2014). In addition, microalgal yields can be improved for PUFA, protein, pigment content, etc. by altering cultivation conditions (viz. light intensity, nutrient, and temperature).
In this review, the prospects of microalgae in aquaculture feed are summarized. The advantages and disadvantages of conventional feed ingredients, using the nutritional composition of microalgae, the mode of application of microalgae in aquaculture feed, and the possible substitution of conventional ingredients by microalgae are critically evaluated. The review also discusses the potential use of lipid-extracted algae (LEA) in aquaculture feed. Challenges of using microalgae in aquaculture feed are discussed. An integrated biorefinery approach, including microalgae cultivation using aquaculture wastewater and aquaponics systems, is emphasized to make aquaculture and microalgal cultivation processes economically viable.
Conventional aquaculture feed formulations and their limitations
Conventional fish feed formulations make use of resources that are traditional and commonly available in local markets. These ingredients are mixed in a balanced ratio to provide the required nutrition to the fish. Many components of conventional feeds are non-competitive in terms of human use, less expensive by-products, or waste products from many process industries. These constituents include feedstuffs from the plant (soya bean whole, soya bean oil extracted, wheat middling, groundnut cake, palm kernel cake, rice brans, maize, sorghum, etc.), brewery (brewery dried yeast, brewery dried grains), and animal by-product sources (chicken feather meal, fish head, poultry by-product, etc.) (Table 1).
Soybean and FM are the essential constituents in aquaculture feed, used as a source of proteins and oil. Approximately 70% of the global FM supplies are utilized by the aquaculture industry (Subhash et al. 2020). Cheap plant-based protein sources are often used in conventional feeds. Plant-based proteins (soybean, sunflowers meal, rapeseed meal, corn gluten, wheat gluten, peameal, rice products, etc.) are widely used as a supplement to FM protein or as a protein source in fish feed. The plant-based proteins lack essential amino acids such as methionine, lysine, tryptophan, and threonine (Li et al. 2009). Methionine is involved in the initiation of peptide synthesis required for protein synthesis. Thus, it is crucial to provide methionine in soybean-based fish feed to get optimum growth, health, and nutritional quality. Plant-based protein sources are also associated with compromised fish health. Soybean meal is not a natural fish food and, in aquafeed, has been reported to induce intestinal inflammation and reduce the survival rate of fish (Bravo-Tello et al. 2017). Poultry processing produces many by-products like feathers, blood, head, leg, and skin. These discards are processed to make a protein source for fish feed.
In the early stages of growth, fish require more significant amounts of protein, which decline over the growth period. Thus, protein requirements generally depend on fish and growth conditions, with average protein requirements for marine shrimp being 18–20%, catfish 28–32%, tilapia 32–38%, and hybrid, striped bass 38–42% (Craig and Helfrich 2009). Typically, lesser protein is required for herbivorous and omnivorous fish than for carnivores.
Fish themselves do not synthesize PUFA; it comes from the algae, which are primary producers in the aquatic food chain. A fish diet deficient in omega-3 fatty acids negatively affects fertility, fertilization, and hatching rates. The inclusion of critical ingredients quantitatively and qualitatively in the feed has great importance in maintaining the nutritional value of fish in aquaculture (Patil et al. 2005). FO is used as a source of PUFA essential for fish nutrition. Conventional feed constituents like soybean cannot meet omega-3 fatty acids and pigment requirements in fish feed. Soybean has high-fat content and trypsin inhibitors, hemagglutinin, and antivitamins, while brewery dried yeast has limited methionine and cysteine (Nguyen 2008). Soybean meal contains high crude protein and a more suitable amino acid profile among plant-based protein sources but contains antinutritional factors (ANF) (Huang et al. 2017). Brewery dried grain has higher crude fiber content, limiting its use in fish feed as a protein source (Abowei and Ekubo 2011).
Carbohydrate is the cheapest energy source in fish diets for which starch and glucose are most commonly used. Non-essential carbohydrate sources are used to reduce overall feed cost, which gives the shape and serves binding purposes during feed manufacturing. For floating feeds, dietary starches are very important during the extrusion process (Craig and Helfrich 2009). Fish utilizes glucose and glycogen as an energy source during muscle exercise and, ≈30-fold glucose utilization increase during peak activity (Hemere et al. 2002). In fish feed, carbohydrates have an essential role in digestibility. Rice bran, wheat bran, and maize are commonly used to provide carbohydrates due to their low cost.
Conventional feedstuffs often lack antioxidant factors, so it becomes necessary to obtain these antioxidants from other sources. The most common antioxidants used are BHA (butylated hydroxyanisole), BHT (butylated hydroxytoluene), or ethoxyquin (FAO 1980). The inclusion level of each antioxidant varies according to fish species and their growing stage. The by-products of crustacea provide carotenoid pigment that enhances fish color (Abowei and Ekubo 2011).
Mineral and vitamins are required in trace amounts but are essential for growth and disease resistance. Both water-soluble (vitamins like B and C) and fat-soluble vitamins (vitamins A, D, E, and K) are essential for fish growth and are required in the correct proportions. Extruder-based processing of feed components increases the probability of destroying vitamins due to the process conditions. Therefore, it is important to add more than enough vitamins in fish feed formulation to fulfill the minimum requirement.
Natural and synthetic pigments impact the color of fish flesh and skin. The most frequent colors used are red and yellow. Synthetic astaxanthin and naturally occurring colors are usually obtained from microalgae, dried shrimp meal, extract from marigold, etc. that are used in aquaculture feed. To keep all feed ingredients together, binding agents are critical in feed formulations.
Binding agents provide strength to the pellet and enhance stability in water. Gelatin agents derived from seaweed products and carbohydrates (agar, carrageenan, starch, cellulose, pectin, and other polysaccharides) are the most widely used binding agents in fish feed formulations (Abowei and Ekubo 2011). The cost of fish production has increased sharply as the cost of the aquaculture feed ingredients is subjected to the hike in their market price. The commodity price index (CPI) increased more than 50% to a hike in the ingredients’ price (FAO, 2009). Correspondingly, the price of almost all types of oils used in fishmeal has increased. The cumulative increase resulted in the rise in the aquaculture feed price.
Antinutritional factors in plant-based feed ingredient
Plant-based aquafeed is widely used globally to meet the high demand in the aquaculture industry. However, the application of plant-based protein sources has shown lower dry matter digestibility than FM, leading to increased waste production (Kokou and Fountoulaki 2018). The main reason for lower digestibility is ANF, such as indigestible non-starch polysaccharides and fibers (Francis et al. 2001; Krogdhal et al. 2010). Antinutrients are substances that interfere with food utilization directly or via products generated through metabolic processes and adversely affect the physiology of the animal (Subhash et al. 2020).
Protease inhibitors (trypsin, chymotrypsin elastases, etc.) are ANF types, mainly found in legumes. The effectiveness of protease inhibitors depends on the source of the inhibitor and the target enzyme. Commercially available soybean products, which are used in a fish feed, contain trypsin. Dabrowski and Kozak 1979) used commercial soybean meal as FM for grass carp; results showed that growth performance decreased with an increase in soybean content. High concentrations (>4–5gm/kg of feed) of protease inhibitors in diets significantly affect protein digestibility resulting in high nitrogenous waste production (Kokou and Fountoulaki 2018). Fish species differ in their ability to tolerate trypsin inhibitors. Trypsin concentration lower than 4–5gm/kg did not significantly affect the feed efficiency of either salmonids or gilthead sea bream (Kokou and Fountoulaki 2018). To reduce ANF concentration, various techniques such as moist heat treatment, and chemical and organic solvents have been employed. However, these techniques are expensive and time-consuming. Moist heat treatment is widely accepted to reduce trypsin inhibitors. However, this treatment might result in loss of nutritional quality of feed.
Phytates or phytic acid (phytate in its salt form) are responsible for adverse effects on growth performances, thyroid function, feed conversion ratio, and feed intake (von Danwitz and Schulz 2020). Phytates are commonly found in plant seeds and chelate with di and trivalent minerals ions, including Ca+2, Mg+2, Cu+3, and Fe+3, which reduces mineral availability for consumers. Non-ruminant animals cannot break down phytates, and their presence in feed reduces the availability of phosphorus. In addition, phytates form digestible phytic acid-proteins complexes that reduce the availability of dietary proteins (Kokou and Fountoulaki 2018; Subhash et al. 2020). The presence of phytic acids in the diet of commonly reared fish, including trout, tilapia, carp, and salmon, inhibits their performance. The role of phytates in inducing adverse effects has been explored in feeding studies of aquatic animals where synthetic phytates were supplemented in the fish diet of rainbow trout (Spinelli et al. 1983) and channel catfish (Satoh et al. 1989). Denstadli et al. (2006) found a decrease in phosphorus retention by approximately 50% when 2% of phytic acid was added to Atlantic salmon diets. The negative effect depends on phytate concertation and fish species. Milling of feedstuffs, fermentation, and heat treatment (autoclaving) are widely accepted techniques to reduce the effects of phytates. Other common ANFs are lectins, allergen, saponins, polyphenols, alkaloids, and glucosinolates found in soybean meal, soybean protein concentrate, wheat, sunflower lupins, and rapeseed meal. Processes to reduce ANF levels include heat treatment, microbial fermentation, and aqueous extraction (Kokou and Fountoulaki 2018). However, these processes increase the cost of feed production. The challenges mentioned above act as limiting factors for a higher level of supplementation of plant-based protein ingredients in fish feed.
Microalgae as feed for aquaculture
Microalgae are one of the oldest organisms inhabiting the earth. Over the last 50 years, microalgae have been intensively investigated to produce oils, proteins, carbohydrates, pigments, and other value-added products for various purposes. For thousands of years, humans have consumed microalgae as a food source. In China, people eat Nostoc and other algae such as Arthrospira (Spirulina) and Aphanizomenon (Milledge 2011). Chad and Mexico have both used Spirulina as food since ancient times (Chisti 2006). During the Second World War, German scientists cultivated algae for the first time at a demonstration scale in open systems to produce proteins (Soeder 1986). Microalgae are a diverse group of organisms with varying characteristics. Some species contain biogenic toxins (purines) and non-biogenic toxins (heavy metals). Certain species rapidly accumulate high concentrations of heavy metals from their surroundings; others generate pathological metabolites that cause neurodegenerative disorders (Lum et al. 2013). Thus, not all algae can be used for consumption or feed applications.
Nevertheless, some algal species are commonly used in aquaculture systems due to their ease in cultivation, balanced nutritional profile, etc. For example, in Japan, dried Spirulina was used for koi carp and juvenile shrimp (Benemann 1992). Live microalgae also play crucial roles in culturing numerous zooplanktons, which are suitable feed for the larval stages of marine fish and crustaceans.
Microalgal biomass contains proteins, fats, amino acids, coloring the flesh and enhancing metabolic activities, and carbohydrates, making it a potential source for FM alternative (Hemaiswarya et al. 2011). Microalgae, which provide pigments, antioxidants, and other bioactive compounds, are primary natural feed sources for zooplankton in aquatic systems, having primary nutritional value (Tibaldi et al. 2015). Brown et al. (1997) found that microalgae, on average, contain 10–20% lipids, 5–15% carbohydrates, and 30–40% proteins in the late log phase of growth. However, there is a variation in the biochemical composition of microalgae biomass depending on the microalgae species and strategies applied for cultivation conditions and cultivation strategies (Table 2). Apart from favorable biochemical composition, several other factors, including shape size, palatability, digestibility, and cell wall composition, make microalgae a good feed ingredient for fish. Therefore, microalgae have tremendous potential as an aquaculture feed; fish consume it as live cultures or in the form of dried microalgae supplemented to conventional feeds (Hemaiswarya et al. 2011; Radhakrishnan et al. 2016). Using microalgae for aquaculture feed has several commercial as well as environmental advantages. They can improve palatability and feed nutritional quality and contribute to reduced feed costs, thereby improving the economic viability of the sector. Other vital benefits of microalgae as fish feed include providing proteins with balanced essential amino acids, omega-three fatty acids, and pigments. The composition of fatty acid and antioxidant content differs between microalgal strains according to the taxonomic group.
Many studies have reported that microalgae-supplemented feed enhances fish morphometric characteristics (size, girth, weight) and nutritional value (Rossi and Davis 2012; Abdulrahman 2014a; Babuskin et al. 2014; Chen et al. 2015). Therefore, it is crucial to determine the various criteria such as level of inclusion, ease in cultivation, lack of toxicity, balanced nutritional value, and proper cell shape and size before the use of algae in aquaculture feed. The inclusion level of microalgae in fish feed depends strongly on species and fish. Microalgae can be used in aquaculture feed in different forms, and the mode of application depends on the fish, type of aquaculture, and product. Fish with high nutritional requirements can be fed with live microalgae or whole microalgae biomass, whilst fish with lower nutritional requirements can be fed with lipid-extracted algal biomass.
Beal et al. (2018) in their case study reported an integrated system for the production of microalgae oil and proteins consisting of both photobioreactors and raceway ponds to replace FM and FO in aquaculture feed. They reported 2750 tonnes/year of protein and 2330 tonnes/year of algal oil can be produced by adopting this system for 11 ha plant at the cost of $29.3 M. They also reported that setting up of such 100 plants can replace 10% world fishmeal production. Selection of right microalgae strains and cultivation in raceway pond could be more feasible and economical to replace FM in aquaculture feed.
Nutritional composition of microalgae
Some groups of microalgae (mostly green microalgae) have similar nutritional composition to higher plants. Apart from the primary metabolites (lipid, protein, and carbohydrates), they also contain vitamins, pigments, antioxidants, and trace metals. The composition of these nutrients in microalgae varies considerably according to the strains and cultivation conditions, viz. temperature, light, pH, and nutrient levels in growth medium (Ho et al. 2012; Pancha et al. 2015). Nitrogen limitation in microalgae has shown high lipid and carbohydrate accumulation while protein content was compromised (Chen et al. 2013; Pancha et al. 2015; Singh et al. 2015).
Microalgal biomass rich in proteins in terms of quantity compete favorably with conventional protein sources (eggs, soybean, etc.) and have balanced essential amino acid profiles (Becker 2007; Sarker et al. 2018; Carneiro et al. 2020). Spirulina sp. contains 50–70% protein (depending on the strain), while Dunaliella sp. can produce 50–100% higher proteins per unit area than conventional plants and animals (Ejike et al. 2017). There are ten essential amino acids, which animals and fish cannot synthesize. Thus, these essential amino acids must be supplied through diets. Microalgal amino acid compositions have shown the presence of all essential amino acids. Brown et al. (1997) studied 40 species of microalgae and reported that all algal species contain a similar profile of amino acids. The composition of essential amino acids in whole microalgae and LEA in different microalgae are shown in Table 3. Microalgal protein has great potential to be used as a feedstock in aquaculture industries. Future studies must determine suitable microalgae species that are easily cultivable at a commercial scale and produce a high protein yield with balanced essential amino acids.
The carbohydrate in heterotrophic microalgae is mainly composed of starch in the chloroplast and cellulose on inner cell walls. The algal cell does not contain lignin in combination with cellulose (Ho et al. 2012). The most common sugars found in polysaccharides of many algal species are glucose and 28–86% of the total carbohydrate. The most commonly used microalgal spp. in aquafeeds as a carbohydrate source are Isochrysis galbana, P. lutheri, C. vulgaris, Pseudoisochrysis paradoxa, and Pyramimonas virginica (Roy and Pal 2014). Chlorella vulgaris accumulates 50% (DCW) carbohydrate under autotrophic conditions (Wang et al. 2014). Zhao et al. (2013) reported that Chlamydomonas could contain around 60% of carbohydrates, of which 44% was starch, which can easily be hydrolyzed and converted into glucose. Thus, the application of microalgal carbohydrate as an aquaculture feedstock ingredient has excellent potential. Still, more research is required to find suitable microalgal species that have desired qualities such as high carbohydrate and high growth rate while growing on low-cost media for large-scale cultivation. Heterotrophically cultivated algae meal incorporated into aquafeed can improve feed consumption and growth performances (Kupchinsky et al. 2015).
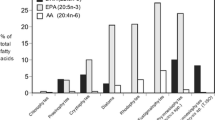
Microalgal lipids serve as the source of essential fatty acids, including omega-3 fatty acids EPA, ALA, and DHA (Table 4) (Guedes and Malcata, 2012; Ratledge 2010). The lipid content in microalgae varies from strain to strain and according to the mode of cultivation and stage of harvesting. The composition of fatty acids also depends on the microalgal strain and conditions provided for cultivation. Due to stress conditions, microalgae can accumulate up to 70% of lipid (DCW) (Stephenson et al. 2011). The lipid yield in Chlorella vulgaris ranges from 12 to 26% and Bortryococcus braunii from 14 to 75% (D’Alessandro and Filho 2016). Many strategies enhance lipid accumulation, such as stress-induced lipid accumulation (different concentrations of nutrients, e.g., N, P, K, EDTA, and Fe), high salinity, temperature, light intensity, and different carbon sources (Singh et al. 2015). Some marine microalgae have significantly higher DHA content than freshwater species. Heterotrophically cultivated algae contain higher DHA contents, e.g., Amphidium caryerea (17%) Schizochytrium mangrove (33–39%), and Thrautocytrium (16.1%). Crypthecodinium cohnii produced 30–50% of DHA of total constituents (Yaakob et al. 2014). It is essential to focus on the inclusion of ALA, DHA, and EPA in aquaculture feed as these are essential ingredients for proper growth and development. The percentage of PUFA in different microalgae is shown in Table 4. Nannochloropsis sp. is predominantly used in aquaculture as a supplement due to the high content of EPA. Chaeotoceros mulleri and Isochrysis galbana produce EPA at 3.5% and 4.8%, respectively (DCW) (Yaakob et al. 2014). ALA is also an essential PUFA; C. vulgaris (7.5%), Micractinium reisseri (6.2%), N. bacillris (9.5%), and Tetracystis sp. (9.5%) are rich in ALA (Tibbetts et al. 2015a). The application of microalgal lipids in aquaculture requires further in-depth studies on the physiological role of the nutrients and mechanisms of stress on the microalgae to enhance lipid accumulation and identify suitable microalgae high in PUFA content.
Pigments are one of the most important constituents found in microalgae. They help in algal photosynthetic metabolism and pigmentation. In addition, pigments have biological functions such as antioxidants, anti-inflammatory agents, and anti-obesity, and are neuroprotective (D’Alessandro and Filho 2016). Carotenoids are the most abundant pigment, widely distributed, and extensively used to protect microalgae cells from various stress conditions, including reactive oxygen and high light intensity. The carotenoids are divided into two groups based on the presence of oxygen (lutein and astaxanthin) or the absence of oxygen molecules (β-carotene). Thus, the carotenoids produced by microalgae are astaxanthin, lutein, α-carotene, β-carotene, and zeaxanthin (Novoveská et al. 2019).
Potential substitution of various conventional constituents by microalgae in aquaculture feed
Fishmeal
FM is obtained after different pressing, drying, and milling of small bony fish species. FM is a good source of highly digestible proteins and contains all essential amino acids needed for fish growth. FM contains the requisite fatty acids as well as vitamins and minerals. The inclusion of FM in aquafeed enhances digestibility and feed utilization, which increases the growth rate of fish. However, the inclusion of FM increases overall production costs, which eventually results in high fish prices in the world market. The policies of governments across the globe for capture fishing have been revised to consider environmental concerns causing gaps in demand and supply of FM. This makes FM even more limited and costly for the aquafeed industry. Over the last 20 years, FM production has remained constant, which has stifled progress in aquaculture due to limited availability. FM prices have jumped from 608 US$/tonne in May 2003 (Origin oil 2013) to approximately 1700–2000 US$/tonne in May 2013 (FAO 2014). The use of high-cost FM in aquafeed hampers its commercial feasibility. Microalgae have similar nutritional characteristics to FM due to their balanced nutritional composition and do not contain inhibitors (Becker 2007). Radhakrishnan et al. (2014) used Arthrospira platensis and Chlorella vulgaris to replace FM in post-larvae of freshwater prawn (Macrobrachium rosenbergii) diets to assess the effect on vitamin (vit. C and E), enzymatic antioxidant, catalase, and lipid peroxidation activity. After 90 days, results showed that diet replacement of 50% in all three microalgal strains showed better performance. The activity of digestive enzymes, e.g., protease and amylase activity, was significantly improved in the prawn fed with a diet containing 50% of A. platensis followed by 25% and 75% diets. Their study concluded that 50% included feed fed showed the optimal performance level compared to control. In an investigation by Abdulrahman (2014a), five different formulations of Spirulina meal were used in common carp (Cyprinus carpio) diets to replace FM; they found that replacement of 10% FM resulted in fish with higher body weight and inclusion of 5% and 15% had a higher survival rate. These results, however, showed that microalgae could not entirely replace FM. Basri et al. (2015) used green water meals to make five (0%, 10%, 20%, 30%, and 40%) different isonitrogenous and is olipidic diets to replace FM in juvenile Pacific white shrimp (Litopenaeus vannamei) diets. Results showed that the growth of white shrimp decreases with increasing green water meal inclusion. Replacement of 20–40% FM protein by green water meal resulted in more unsatisfactory performance than control diet-fed animals. The diatom Phaeodactylum tricornutum can replace up to 6% of fishmeal without any adverse effects on digestibility feed utilization efficiency, and growth performance of Atlantic cod, Salmo salar (Sørensen et al. 2016). High microalgal inclusion increases fiber content and decreases feed digestibility. Badwy et al. (2008) partially replaced FM with Chlorella spp and Scenedesmus sp. in Nile tilapia fingerling diets. The partial replacement did not affect fish morphometric characteristics, feed conversion, or body composition. Their results showed that 50% FM substitution with algae could be achieved in fish diets without any adverse effect. Higher algae inclusion adversely affected growth performance due to the high content of carbohydrates and ash that leads to a negative effect on digestibility. However, further investigations are needed with various microalgal strains for achieving a possible higher percentage replacement of FM with microalgae in fish feeds.
Soybean meal
Soybean meal predominantly uses plant-based protein (47–50%) sources used in fish feed preparation (Sharawy et al. 2016; Huang et al. 2017). The production process of soybean meal is similar to FM. Soybean or any other plant-based proteins cannot be used alone as protein sources due to factors such as palatability and digestibility. The use of soybean meal in fish has advantages such as easy availability, environmentally friendly, and low cost. The disadvantages of using soybean meal are low nutrient availability, unbalanced amino acid profile, and lack of EPA and DHA (Carneiro et al. 2020). The primary amino acid content (e.g., methionine and lysine) is very low in soybean meals. The application of microalgae over soybean has many advantages. Microalgae meal contains pigments, long-chain fatty acids, and a balanced amino acid profile.
Ding et al. (2015) investigated the use of fermented soybean meal to replace FM partially (0%, 25%, 50%, 75%, and 100%) or entirely in juvenile prawns (Macrobrachium nipponense) diets. In their experiments, the replacement of 25% FM by fermented soybean meal significantly increased the prawns’ weight and specific growth rate by week 8. They did not find any significant differences among other inclusion levels. Increasing the inclusion level decreased the total hemocyte count and hemolymph phagocytic activity. However, the replacement of 100% FM by fermented soybean meal showed the highest total antioxidant activity competence and malondialdehyde level in the hepatopancreas. Sharawy et al. (2016) used four-inclusion levels of solid-state fermented soybean meal with Saccharomyces cerevisiae to replace FM in prawns (Fenneropenaeus indicus). Results revealed that 50% FM could be replaced using solid-state fermented soybean meal with S. cerevisiae. High replacement levels of FM by soybean significantly decreased morphometric characteristics. Silva-Carrillo et al. 2012) used four different (0%, 20%, 40%, and 60%) soybean meal inclusions to replace FM in juvenile spotted rose snapper feed. After 12 weeks of treatment, growth performances, SPR, and PER were unaffected by the 20% FM replacement. Fish growth performance was reduced at higher levels (60%) of inclusion. Moreover, no significant changes were found in mortality in fish fed with different diets. Studies showed that higher inclusion levels of soybean in aquafeed negatively affect the fish growth performance, and it is not a suitable alternative to the FM.
Poultry by-products and feather meal
The application of poultry by-products and feather meal in fish feed has been practiced for decades. These are good sources of proteins, containing 69% crude proteins. Hydrolyzed feather meal enhances feed digestibility in fish. Positive results were found in catfish diets when feather meal at the level of 15% was supplemented. The limiting amino acids, lysine, and methionine are present in poultry by-products (Rossi and Davis 2012). Poultry meal, however, is unable to provide pigments in diets and lacks essential PUFAs. Microalgae meal has a balanced essential amino acid profile and contains essential PUFAs. Apart from proteins and oil, microalgae are rich in pigment, vitamins, and minerals, which provide color to fish flesh and act as an antioxidant agent. Thus, microalgae could represent better feed ingredients than poultry by-products and feather meal. However, in-depth investigations are necessary to ascertain the replacement of poultry by-products and feather meal with microalgae.
Pigments
Pigments play crucial roles in aquatic organisms. They especially provide the flesh colorants in the salmonid, sea urchin roe, red sea bream, snapper, and koi/ornamental. Pigments are an essential constituent of feed. Good-quality aquaculture products should fulfill many requirements to attract consumers. The color of the aquaculture mentioned above produces is essential as a quality parameter in the industry. Fish flesh color illustrates freshness as well as a healthy appearance. Carotenoids are widely used in salmon and ornamental fish feed to give the vibrant and attractive pink-red color of flesh. Yellow corn, corn gluten meal, and alfalfa are generally supplemented as carotenoid sources in aquaculture feed. It has been reported that supplementation of pigments in rainbow trout produced a quality product (Sommer et al. 1991). Pigments also have biological functions such as growth improvement, reproduction, and immune system enhancement. Different non-microalgal pigments have been used in conventional feed preparations, but their performance and effectiveness are inconsistent with natural pigments obtained from microalgae (Spolaore et al. 2006; Gouveia et al. 1996; Hu et al. 2018). Apart from coloring agents, specific carotenoids have therapeutic and nutritional functions, which act as provitamin -A (Kalogeropoulos et al. 2010). Most importantly, pigments act as an anti-inflammatory agent and control reactive oxygen species (Hu et al. 2018).
The production cost and market value of natural pigments are very high. For example, the price of natural phycobilin pigments in the world market varied from 3 US$ to 25 US$/mg. According to Brennan and Owende 2010), the production cost of phycobilin from Spirulina sp. is 16.61 US$/mg. The production cost of microalgae is 5 to 10 US$/ kg. Their astaxanthin value is US$ 2500/kg to US$ 3000/kg, and by 2020, it is estimated to reach 670 metric tons with a price of US$ US$ 1.1 billion (D’Alessandro and Filho 2016; Hu et al. 2018). D. salina is a natural producer of β-carotene with market price of US$ 300–US$ 500/kg and by 2019, it is estimated to reach US$532 million (Tang et al. 2020).
The high price of pigments increases the price of fish feed formulation. Microalgae naturally produce these pigments and thus, if used in fish feed formulation, could serve as the inexpensive source of these pigments. Microalgae such as Haematococus pluvialis–supplemented feed is gaining popularity in the market as a pigment source (Waldenstedt et al. 2003; Spolaore et al. 2006; Hu et al. 2018). The pigment synthesis in microalgae varies depending on the species and cultivation conditions.
Modes of application
Live microalgae as aquaculture feed
During the early development stages, mollusks and shrimp larvae consume microalgae, which provide basic nutritional needs (Muller-Feuga 2000). In addition, microalgae are a feed source of zooplankton and stabilize the conditions of culture medium by improving water quality through oxygen production, pH stabilization, providing important biochemical components, and increasing the esthetic appeal of finfish bred in captivity (Muller-Feuga 2000; Sirakov et al. 2015). Therefore, it is essential to choose the right microalgae for the fish species fed. The application of live microalgae as feed has many advantages. All nutrients and bioactive compounds are preserved in live algae, which can be directly made available for fish. Live microalgae can provide protein, essential amino acids, oil with PUFAs, carbohydrates, pigment, minerals, and vitamins. In the green water technique, one or more types of microalgae are added to larval rearing tanks of marine fish hatchery systems. Due to several factors, larval production by the green water technique is often superior (Faul and Holt 2005; Palmer et al. 2007). Larvae directly ingest the microalgae as their first food, which may provide all essential nutrients (Brown 2002). Tetraselmis, Nannochloropsis, Isochrysis, Thalassiosira, and Chaetoceros are the most frequently used microalgae in larval feeds (Priyadarshani and Rath 2012). Microalgae stimulate enzyme and polysaccharide synthesis and stimulate the non-specific immune system (Abdulrahman 2014b). The application of commercially available microalgae concentrates as an alternative for live microalgae in sandfish hatcheries, supporting the growth and is simple to apply in rearing protocols for this species. Duy et al. (2017) used two live algae (Isochrysis aff. Galbana, Chaetoceros muelleri) and six concentrated microalgae products obtained from Instant algae, Mariculture Inc. early juveniles of sandfish, Holothuria scabra. Their results revealed that live C. muelleri improved juvenile growth performance compared to three microalgae concentrates fed either alone or in combination.
One of the major obstacles in using algae supplements in aquaculture is the storage and maintenance of live cultures on a commercial scale. It is challenging to maintain stock culture strains having high biomass and nutrient yields. Therefore, various techniques are being practiced to maintain such cultures. The use of preserved algal feedstock instead of using live algal cultures could improve hatchery efficiencies. Being affordable and inexpensive, freezing and freeze-drying represent the most common techniques for preserving live algal cultures. These techniques have been proven effective in maintaining the viability of algal cultures for comparatively more extended periods (Ben-Amotz and Gilboa 1980; Buitrago 1992). Previous studies have reported the effectiveness of these techniques to preserve freshwater live microalgae (Takano et al. 1973; Morris 1976a, 1976b); however, viability loss is expected due to the formation of intracellular ice crystals resulting in irreversible cell injuries (McLellan, 1989). The decline of viability over time has been observed for live culture preserved by freezing and freeze-drying, even when the protective agents such MnSO4 and glycerol are used, preventing intracellular biochemical fractions due to the formation of hydrogen bonds (Cordero and Voltolina 1997). The H-bond prevents the development of an ice front and the diffusion of water molecules at freezing temperatures. Ben-Amotz and Gilboa. (1980) reported that lipoprotein solubilization and protein denaturation due to salt saturation and formation of ice crystals make this relatively ineffective for the marine algal species. Cordero and Voltolina (1997) reported that preservatives, i.e., protective agents, are also ineffective in controlling the loss in the lipid content if the algal cells are vacuum dried after freezing. Similarly, a significant decrease in the carbohydrate and protein content of Chaetoceros and Phaeodactylum sp. was also observed over time, even if the cells are preserved at −20°C without preservatives. Despite the many benefits of live microalgae in aquaculture, challenges such as high operating cost, maintenance, and concentrating and storage difficulties are evident (Guedes and Malcata 2012).
Whole microalgae as a supplement in aquaculture feed
Microalgae biomass without extracting any component is known as whole algae and their value is much higher than the lipid-extracted algae (LEA). Whole algae biomass exhibits significant potential to improve the nutritional content in aquafeed due to the presence of balanced amino acids, protein, lipid, fatty acids, carbohydrates, minerals, and vitamins. Application of whole microalgae in aquafeed as a protein source has been used in which FM is partially replaced. Brown et al. (1997) comprehensively studied 40 microalga sp. of the same class and found all algal has similar amino acid composition and rich in essential amino acids. Studies have shown that Scenedesmus sp., Spirulina spp., Isochyris spp., and Chlorella spp. have potential as a protein source for different fishes (carnivorous, omnivorous, and freshwater prawns at early stages) (Apandi et al. 2018).
The inclusion of different microalgae species offers required nutrition and enhanced fish growth compared to feed containing single algae species (Spolaore et al. 2006; Hemaiswarya et al. 2011). Studies have shown that slight whole algae inclusion increased the growth performance of the fish, protein retention, and critical fatty acid contents, and provided good protein with essential amino acids (Abdulrahman 2014a; Radhakrishnan et al. 2014). Substitution of FM, FO, and pigments with microalgae thereby can be used as an alternative ingredient to reduce the price of fish feed. Brune (2011) suggested that algae have the potential to improve aquaculture production 3–4-fold and simultaneously reduce the production costs by US$0.05–US$0.1/pound. Nakagawa and Gomez-Diaz 1995) reported that the whole biomass of Spirulina supplemented feed for giant freshwater shrimp and Penaeus japonicas showed better growth performances, lower mortality rates, and improved feed utilization. Kousoulaki et al. (2016) found that supplementation of 5% whole biomass of Schizochtrum sp. obtained through heterotrophic cultivation conditions in the extruded meal of salmon effectively substituted FO without any adverse effect on growth performance. Thus, inclusions of Schizochtrum sp. biomass in salmon diets have the potential to enhance their preservative ability of nutritional value. Vizcaíno et al. (2014) conducted a study to evaluate S. almeriensis supplementation (0%, 12%, 20%, 25%, and 39%) in the diets of sea bream (Sparus aurata). After 45 days, they observed that S. almeriensis–incorporated feed has no adverse effect on nutrient utilization efficiency or growth performances. Results also showed that fish feed supplemented with 12% of S. almeriensis–included diets improved trypsin levels.
In a study by Walker and Berlinsky (2011), three experimental diets were used to replace 0%, 15%, and 30% of FM proteins in diets of juvenile Atlantic cod (Gadus morhua), which showed that 30% of algae inclusion caused palatability problems resulting in reduced feed intake and fish growth. They observed that 15% of algal biomass supplementation as FM protein replacement improved the feed intake, while for 30% replacement, the fish were approaching starvation. In another study by García-Ortega et al. (2016), FM and FO were replaced by a mixture of the algal meal (Schizochytrium limacinum) and soybean protein in the diet for giant grouper Epinephelus lanceolatus. Their experimental study included three formulated diets to replace 20%, 40%, and 80% FM. After 12 weeks of the experiment, the result showed a low level of FM (80%, FM20) significantly reduced weight, specific growth rate, feed intake rate, lipid retention, and methionine accumulation in giant grouper. The results showed that algae can be used as the primary lipid source and can replace at least 40% of the marine protein in diets for giant grouper. Sprague et al. (2015) used two inclusion levels (5.5% and 11%) Schizochytritum sp. (rich in DHA) for replacement of FO in Atlantic salmon (Salmo salar) post-smolts and compared to fish fed a FO diet of southern or northern hemisphere origin. Results showed that replacing FO with Schizochytritum sp. significantly reduces dietary and flesh fillet persistent organic pollutant levels compared to FO-based diet. Similarly, Aranda-Burgos et al. (2014) used four mono- and multi-algal inclusions to see the effect of microalgal diets on growth performances, survival, and fatty acid quality during larval growth in grooved carpet shell (Ruditapes decussatus). The results revealed that the feeding regime improved larval growth performances, survival, and fatty acid composition. A low level of microalgae meal inclusion in fish feed increased the fish growth rate and showed positive morphometric effects. Li et al. (2009) studied the effect of inclusion level of dried algae Schizochytrium sp., on growth performances, sensory quality, and fatty acid composition, of channel catfish (Ictalurus punctatus). They formulated five isonitrogenous (28%) and isocaloric (2.78kcal/g) feeds with plant diets containing 0%, 0.5%, 1%, 1.5%, and 2% dried algae. After 9 weeks of study, they found that as inclusion level algae increased (2%) and improved the levels of total n-3 LC-PUFA and DHA in channel catfish. Fish obtained higher body weight consuming diets containing 1% and 1.5% dried algae than fish fed diets with 0% and 0.5% algae. Dallaire et al. (2007) incorporated three levels (12.5%, 25%, and 50%) of microalgae in rainbow trout (Oncorhynchus mykiss) fry feed to evaluate its growth rate and nutritional value. They found that higher inclusion levels of microalgae feeds significantly affected rainbow trout growth rates negatively. Results suggested that a maximum of 12.5% microalgae could be incorporated to avoid negative effects on rainbow trout fry. Younis et al. (2018) carried out a 12-week feeding experiment to evaluate the effect of Gracilaria arcuata supplementation on growth performance, body composition, and feed utilization of Nile tilapia. Fish were fed with conventional feed (without algae supplementation) as control and three (20%, 40%, and 60%) different inclusion levels as replacement of FM. Results showed that supplementation of less than 20% Gracilaria arcuata could be feasible in the Nile tilapia diet. High inclusion levels increase the fiber contained in the feed, which affects the digestibility and metabolic processes (Ju et al. 2012; Hussein et al. 2013).
Most of the studies concluded that algae could not replace 100% FM or use higher inclusion levels in fish feed. In Table 5, microalgae in aquaculture feed and their effect on fish growth performances and feed utilization have been summarized.
Lipid extracted algae as a supplement in aquaculture feed
Microalgae are considered suitable candidates for biofuel production (Guldhe et al. 2016). Lipid extraction results in massive amounts of residual biomass, which is commonly termed lipid-extracted algae (Ansari et al. 2017b). This residual biomass still contains proteins, carbohydrates, and many other valuable components (Ansari et al. 2020; Ju et al. 2012). The proteins left in residual biomass can partially replace conventional protein sources like FM and soybean meal in fish diets (Sørensen et al. 2017). Ju et al. (2012) conducted a study in which the LEA of H. pluvialis was used as a source of protein. Their study included four experimental diets (3%, 6%, 9%, and 12%) to substitute FM at level of 12.5%, 25%, 37.5%, and 50% in Pacific shrimp diets. After 56 days of the experiment, they found that diets containing 12.5% substitution of FM improved growth performances compared to the control—similarly, Patterson and Gatlin. (2013) included three LEA obtained from N. salina Chlorella sp. and Naviculla sp., as a substitute of protein source in diets of red drum. In the first trial, 5% and 10% crude protein were replaced with LEA of Naviculla sp. Their results revealed that the replacement of 10% LEA dietary proteins negatively affects energy retention and protein value. In the second trial, four substitution levels (5%, 10%, 20%, and 25%) were selected with LEA of Chlorella sp. to replace crude protein in the reference diet. The results indicated that 20% and 25% replacement of crude protein with LEA of Chlorella sp. decreased growth performances, but no changes were observed in body composition. Their last trial reduced the inclusion levels (5%, 7.5%, 10%, and 15%) of LEA obtained from N. salina as crude protein in reference diets. Their results indicated that a 10% substitution level is the optimal level for LEA of N. salina as a crude protein source in diets of juvenile red drum. Gong et al. (2018) used LEA of Nannochloropsis sp. and Desmodesmus sp. to feed Atlantic salmon post-smolts in seawater to observe the apparent digestibility coefficient (ADC). Two sets of experiments were done, the first one using cold-pelleted while the second one using extruded pellets to examine the ADC, protein, ash, and energy. Nannochloropsis LEA-incorporated cold-pelleted feed resulted in higher ADC and protein than Desmodesmus sp. Nannochloropsis sp. LEA in extruded feed improved the ADC and energy as compared to Desmodesmus sp. The results showed that the LEA of Nannochloropsis sp. was better than Desmodesmus sp. The extrusion process improved the digestibility. Bryant et al. (2012) employed hedonic pricing methods to calculate the values of constituent nutrients (total digestible nutrient, protein, etc.) of N. oculata LEA-based feed for aquaculture and found lesser value than soybean meal and menhaden FM. Thus, LEA has great potential to replace crude proteins in aquaculture diets. LEA appears to possess greater potential than terrestrial plant–based feed ingredients as a replacement protein in aquafeed (Table 6). LEA inclusion in fish feed highly depends on algal species and types of fish. Therefore, it is vital to know the supplementation level of LEA in the diet before feed formulation and preparation. The application of LEA in aquaculture will also make the algal biodiesel production process more economical and sustainable by opening a new gateway to generate revenue from residual biomass.
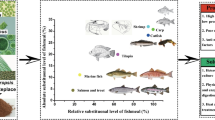
Microalgae- and aquaculture-based integrated biorefinery approach
The biorefinery approach, where microalgal cultivation and aquaculture are integrated for mutual benefit, could be economical and sustainable (Shaalan et al. 2018) (Fig. 1). Aquaculture wastewater contains nitrate, nitrite, ammonia, phosphate, and organic carbon, which are required for microalgae cultivation. Thus, this aquaculture wastewater can potentially be used for microalga cultivation and wastewater treatment. The biomass can be potentially used as a feed ingredient in different aquafeeds. Microalgae cultivation in wastewater has unique advantages on ecological restoration by removing nutrients and produced biomass without additional cost. After harvesting the biomass, the treated water can be further used for fish rearing or other purposes subject to its suitability. This novel approach of cultivation can reduce aquafeed production costs and make the overall process environmentally friendly. Guldhe et al. (2017) heterotrophically cultivated Chlorella sorokiniana in aquaculture wastewater obtained from the tilapia rearing tank. They found removal efficiency of 75.56%, 84.51%, 73.35%, and 71.88% for ammonia, nitrate, phosphate, and COD (chemical oxygen demand). The productivity of carbohydrates, lipids, and proteins was 172.91, 150.19, and 141.57mg/L/day. The productivity of lipid, protein, and carbohydrate indicated the quality of microalgae grown in aquaculture wastewater alongside remediation. Ansari et al. (2017a) reported a similar pattern of lipid, carbohydrate, and protein productivities in microalgae propagated in tilapia wastewater when compared to the productivities of algae grown in BG11 medium. Microalgae cultivation in tilapia wastewater showed nutrients and COD removal efficiencies in the range of 86.45–98.21%, 75.76–80.85%, 98.52–100%, and 42–69%, for ammonia, nitrate, phosphate, and COD, respectively (Table 7). Tejido-Nuñez et al. (2020) used the wastewater of the recirculating aquaculture system (RAS) as a nutrient medium for the co-cultivation of two species of microalgae (C. vulgaris and Tetradesmus obliquus).
They found that co-cultivation was more efficient in nutrient removal than monoculture, and average removal efficiencies of 98.73±0.06% and 99.46±0.04%, respectively, for nitrate and phosphate, were observed. Similarly, Guo et al. (2013) employed aquaculture wastewater as a growth medium for cultivating microalgae Platymonas subcordiformis. They found that P. subcordiformis efficiently removed nutrients with a range of 87–95% and 98–99%, respectively, for nitrogen and phosphorus.
Different types of microalgae were cultivated in aquaculture wastewater for nutrient removal efficiency and biomass production (Nasir et al. 2019; Peng et al. 2020; Tejido-Nuñez et al. 2020; Gupta and Yadav 2017). However, the integration processes such as aquaculture wastewater for microalgae cultivation and high-quality biomass production for feed applications are not explored. Simultaneous inclusion of microalgae biomass in aqua feed as proteins, pigments, oil, and carbohydrates sources further needs to be studied. It is vitally important to identify suitable, indigenous, and robust microalgae species (high in PUFA, proteins, and balanced essential amino acids) that adapt to cultivation in aquaculture wastewater.
Aquaculture-aquaponics-based integrated approach
Aquaponics technology is a combination of aquaculture and hydroponic plants. This is an emerging technology well adopted by several aquaculture industries in many countries. Aquaponics systems are one of the most intriguing strategies for improving aquaculture, where fish and plants are grown simultaneously in a symbiotic environment (Paudel 2020). It provides many benefits by improving water quality, reducing water demand, and creating better environmental conditions for fish growth (Diver 2006). Aquaponics is applicable for both fresh and marine aquatics species, ultimately enhancing net revenue from both fish and plant production (Estim et al. 2019).
Several studies employed different strategies specific to their particular design and operating conditions to improve the performance of aquaponics systems (Lam et al. 2015; Paudel 2020). For example, Endut et al. (2010) found improvement in nitrogen uptake and plant growth (water spinach) when hydraulic loading rates were varied. In addition, Ahn et al. (2010) reported that reduced ammonium, nitrate, and nitrite concentration might reduce the potential of nitrogen oxide emission.
Enduta et al. (2011) studied the efficiency of aquaponics recirculation systems in removing nutrients (N and P)) from aquaculture wastewater using water spinach and mustard green. In their study, the removal efficiency of 78.32–85.48%, 82.93–92.22%, 79.17–87.871%, and 75.36–84.94%, for total ammonia nitrogen, nitrite, nitrate, and orthophosphate, respectively, for water spinach was observed. At the same time, the nutrient removal efficiencies were 69–75.85% for total ammonia nitrogen, 72.32–79.34% for nitrite, 66.67–80.65% for nitrate, and 75.36–84.94% for orthophosphate, respectively by mustard green. The higher nutrient removal efficiency of water spinach is attributed to the more bacterial attachment to a greater surface area of root structure than mustard green. In the aquaponics system, Estim et al. (2019) conducted a trial using Nile tilapia, green beans, and Chinese cabbage over 70 days. They found improvement in plant growth without having any adverse effect on fish growth.
In hydroponic beds, appropriate plant and plant density selection are critical design parameters to improve fish’s nutrient recovery and growth performance in aquaponics systems. Aquaponics systems have the potential to add further value to the aquaculture industry. Further expansion in aquaponics at a pilot scale is vital due to water scarcity globally, especially in arid areas. Application of live algae in aquaponic systems might add more value to improve nutrient removal efficiency and plant growth performances. Studies are needed to establish a design for algae, aquaponic systems, and aquaculture integrated systems.
Challenges
There are some techno-economic barriers, which need to be addressed while using microalgae as an aquaculture feed. The high production costs of algal biomass, contamination of algal cultivation systems, and changes in the biochemical composition still pose problems successful application of microalgae for aquaculture operations (Ansari et al. 2020; Tredici et al. 2016). Similarly, LEA as a protein source for aquaculture also faces suitability issues due to organic solvent usage during extraction (Yun et al. 2014; Ansari et al. 2017b). The high production cost of microalgal biomass due to typical cultivation requirements and energy-intensive harvesting/dewatering could hamper the economics. The microalgal biomass quality is greatly affected by environmental factors, and it varies in different geographical regions. There are variations in the digestibility of certain microalgal strains among fish, which need to be further investigated. As per estimates, 5 million kg/year of algal biomass is produced annually, and the use of algal biomass in aquaculture was approximately 1000 tonnes in 1999 (Spolaore et al. 2006; Gagneux-Moreaux et al. 2007). Out of total global algal biomass used in aquaculture, only 16% is used for fish, 21% for shrimps, whereas the significant proportion, i.e., approximately 62% is used for mollusks (Spolaore et al. 2006; Gagneux-Moreaux et al. 2007).
The major challenge being faced by large-scale algae cultivation is the cost of chemical nutrients required for growth. Various studies have demonstrated the economical production of algal biomass using wastewater, leachates, or some industrial effluents (Zhao et al. 2014; Gupta et al. 2016; Wang et al. 2016). The use of such biomass, produced from wastewater or industrial effluent, could reduce the overall cost of aquaculture feed production. However, most algal species are phyco-scavengers for various organic and inorganic nutrients and toxicants. The accumulation of toxicants in the algal biomass would render it incompatible for aquaculture feed applications. Therefore, the use of wastewater-grown microalgae for aquaculture is questionable due to the ultimate risk to human health.
Moreover, wastewater-grown biomass is also susceptible to pathogenic contamination. Therefore, even though the techniques are available for the sterilization of biomass, the effectiveness of sterilization for microbial contaminants without compromising the nutritional qualities of the feedstock is of concern. Furthermore, if such biomass is used in aquaculture, the translocation of such accumulated toxicants/pathogens to the aquaculture species and products is a significant concern. Therefore, the consumption of such aquaculture products may pose a risk to the consumer. Therefore, thorough risk assessment studies are required to apply wastewater-grown microalgal biomass in aquaculture feed application.
Recommendations and conclusions
The application of microalgae in aquaculture feed has tremendous potential for improving the nutritional quality of the product. The microalgae have also shown the potential to replace FM and other conventional ingredients from the fish feed, reducing overall cost and environmental concerns. However, the high production cost of microalgae and varying nutrient composition are the major challenges that need to be addressed. More studies still need to investigate the potential of different microalgal species to be used in aquaculture feed, formulation of microalgae-based feed, reducing the microalgal cultivation cost, and ensuring the high nutritional quality of microalgae use of lipid extracted microalgae for aquaculture feed. An integrated cultivation approach where microalgae are used as aquaculture feed and aquaculture wastewater is employed as a medium for biomass production could be a sustainable and economical approach. A “cradle to grave” lifecycle assessment (LCA) is essential for assessing the economic sustainability of the algae-based aquaculture feed formulations and their impact on fish. The assessment of environmental sustainability is also equally crucial for the evaluation of its ecological impacts. The application of algae in aquafeed could improve the quality of produced fish and lead to a sustainable aquaculture industry due to its environmental benefits.
Data availability
Data and materials will be available by reaching to corresponding author Prof. Faizal Bux (faizalb@dut.ac.za).
Abbreviations
- ADC:
-
Apparent digestibility coefficient
- ALA:
-
α-Linolenic acid
- ANF:
-
Antinutritional factor
- COD:
-
Chemical oxygen demand
- CSM:
-
Cottonseed meal
- CVD:
-
Cardiovascular diseases
- DCW:
-
Dry cell weight
- DHA:
-
Docosahexaenoic acid
- DO:
-
Dissolve oxygen
- EPA:
-
Eicosapentaenoic acid
- FAO:
-
Food and Agricultural Organization
- FM:
-
Fish meal
- FO:
-
Fish oil
- LEA:
-
Lipid extracted algae
- NREL:
-
National Renewable Energy Laboratory
- PUFA:
-
Polyunsaturated fatty acid
- RAS:
-
Recirculating aquaculture system
- SBM:
-
Soybean meal
- USD:
-
United States dollar
References
Abdulrahman NM (2014a) Effect of replacing fishmeal with Spirulina spp. on carcass chemical composition of common carp (Cyprinus carpio L.). Iraqi J Vet Sc 28:67–70
Abdulrahman NM (2014b) Evaluation of Spirulina spp. as food supplement and its effect on growth performance of common carp fingerlings. Int J Fish Aquat Stud 2(2):89–92
Abomohra AE, Almutairi AW (2020) A close loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhancing biofuel recovery. Bioresour Technol 317:124027
Abowei JFN, Ekubo AT (2011) A review of conventional and unconventional feeds in fish nutrition. Brit J Pharmacol Toxicol 2(4):179–191
Ahn JH, Kim S, Park H, Rahm B, Pagilla K, Chandran K (2010) N2O emissions from activated sludge processes 2008-2009: results of a National Monitoring Survey in the United States. Environ Sci Technol 44(12):4505–4511
Ansari FA, Shriwastav A, Gupta SK, Rawat I, Guldhe A, Bux F (2015) Lipid extracted algae as a source for protein and reduced sugar : a step closer to the biorefinery. Bioresour Technol 179:559–564
Ansari FA, Singh P, Guldhe A, Bux F (2017a) Microalgal cultivation using aquaculture wastewater: integrated biomass generation and nutrient remediation. Algal Res 21:169–177
Ansari FA, Gupta SK, Shriwastav A, Guldhe A, Rawat I, Bux F (2017b) Evaluation of various solvent systems for lipid extraction from wet microalgal biomass and its effects on primary metabolites of lipid-extracted biomass. Environ Sci Pollut Res 24(18):15299–15307
Ansari FA, Ravindran B, Gupta SK, Nasr M, Rawat I, Bux F (2019) Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J Environ Manag 240:293–302
Ansari FA, Nasr M, Guldhe A, Gupta SK, Rawat I, Bux F (2020) Techno-economic feasibility of algal aquaculture via fish and biodiesel production pathways: a commercial-scale application. Sci Total Environ 704:135259
Ansari FA, Ratha SK, Renuka N, Ramanna L, Gupta SK, Rawat I, Bux F (2021) Effect of microplastics on growth and biochemical composition of microalga Acutodesmus obliquus. Algal Res 56:102296
Apandi NM, Mohamed RMSR, Al-Gheethi A, Mohd AH (2018) Microalgal biomass production through phycoremediation of fresh market wastewater and potential of fresh market wastewater and potential applications as aquaculture feed. Environ Sci Pollut Res 26:3226–3242
Aranda-Burgos JA, da Costa F, Nóvoa S, Ojea J, Martínez-Patiño D (2014) Effects of microalgal diet on growth, survival, biochemical and fatty acid composition of Ruditapes decussatus larvae. Aquaculture 420-421:38–48
Arney B, Liu W, Forster IP, McKinley RS, Pearce CM (2015) Feasibility of dietary substitution of live microalgae with spray-dried Schizochytrium sp. or Spirulina in the hatchery culture of juveniles of the Pacific geoduck clam (Panopea generosa). Aquaculture 444:117–133
Babuskin S, Krishnan KR, Saravana PA (2014) Functional foods enriched with marine microalga Nannochloropsis oculata as a source of w -3 fatty acids. Food Technol Biotechnol 9862:292–299
Badwy TM, Ibrahim EM, Zeinhom MM (2008) Partial replacement of fish meal with dried microalga (Chlorella spp. and Scenedesmus spp.) in Nile tilapia (Oreochromis niloticus) diets. In 8th International Symposium on Tilapia in Aquaculture 801-811
Basri NA, Shaleh SRM, Matanjun P, Noor NM, Shapawi R (2015) The potential of microalgae meal as an ingredient in the diets of early juvenile Pacific white shrimp, Litopenaeus vannamei. J Appl Phycol 27:857–863
Beal CM, Gerber LN, Thongrod S, Phromkunthong W, Kiron V, Granados J, Archibald I, Greene CH, Huntley ME (2018) Marine microalgae commercial production improves sustainability of global fisheries and aquaculture. Sci Rep 8(1):1–8
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25(2):207–210
Belay A, Kato T, Ota Y (1996) Spirulina (Arthrospira); potential application as an animal feed supplement. J Appl Phycol 8:303–311
Ben-Amotz A, Gilboa A (1980) Cryopreservation of marine unicellular algae. A survey of algae with regard to size, culture age. Photosynthetic activity and chlorophyll-to-cell ratio. Mar Ecol Prog Ser 2:157–161
Benemann JR (1992) Microalgae aquaculture feeds. J Appl Phycol 4:233–245
Bhola KM, Swalaha FM, Nasr M, Kumari S, Bux F (2016) Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. Eur J Phycol 51(4):401–412
Bohutskyi P, Ketter B, Chow S, Adams KJ, Betenbaugh MJ, Allnutt FT, Bouwer EJ (2015) Anaerobic digestion of lipid-extracted Auxenochlorella protothecoides biomass for methane generation and nutrient recovery. Bioresour Technol 183:229-39
Borowitzka MA (1997) Microalgae for aquaculture; opportunities and constraints. J Appl Phycol 9:393–401
Bravo-Tello K, Ehrenfeld N, Solis CJ, Ulloa PE, Hedrera M, Pizarro-Guajardo M (2017) Effect of microalgae on intestinal inflammation triggered by soybean meal and bacterial infection in zebrafish. PLoS One 12(11):e0187696
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Brown MR (2002) Nutritional value and use of microalgae in aquaculture. CSIRO Marine Res 3:291–292
Brown MR, Jeffrey SW, Volkman JK, Dunstan G (1997) Nutritional properties of microaglae for mariculture. Aquaculture 151:315–331
Brune DE (2011) Aquaculture and biofuels: three decades of microalgae lessons, Southeast Bioenergy Conference,
Bryant HL, Gogichaishvili I, Anderson D, Richardson JW, Sawyer J, Wickersham T, Drewery ML (2012) The value of post-extracted algae residue. Algal Res 1:185–193
Buitrago EB (1992) Concentracibn y presetvacibn de microalgas coma reserva de aliment de organismos marinos cultivados. Prog Iberoam Cienc Tee Subprog II – Acuicultura Cyted-D 1:47–53
Carneiro WF, TFD C, Orlando TM, Meurer F, DAdeJ P, BdoC V, ARdaCB V, LDS M (2020) Replacing fish meal by Chlorella sp. meal: effects on zebrafish growth, reproductive performances, biochemical parameters and digestive enzymes. Aquaculture 528:735612
Carrero A, Vicente G, Rodríguez R, del Peso GL, Santos C (2015) Synthesis of fatty acids methyl esters (FAMEs) from Nannochloropsis gaditana microalga using heterogeneous acid catalysts. Biochem Eng J 97:119-24
Chauton MS, Reitan KI, Norsker NH, Tveterås R, Kleivdal HT (2015) A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture 436:95–103
Chen C-Y, Zhao X-Q, Yen H-W, Ho S-H, Cheng C-L, Lee D-J, Bai F-W, Chang J-S (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10
Chen SM, Tseng KY, Huang CH (2015) Fatty acid composition, sarcoplasmic reticular lipid oxidation, and immunity of hard clam (Meretrix lusoria) fed different dietary microalgae. Fish Shellfish Immunol 45(1):141–145
Chisti Y (2006) Microalgae as sustainable cell factories. Environ Eng Manag J 5(3):261–274
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Cho JH, Kim IH (2011) Fish meal-nutritive value. J Ani Physiol Ani Nutri (Berl) 95(6):685–692
Cordeiro CM (2019) A corpus-based approach to understanding market access in fisheries and aquaculture international business research: a systematic literature review. Aquac Fisheries 4(6):219–230
Cordero B, Voltolina D (1997) Viability of mass algal cultures preserved by freezing and freeze-drying. Aquac Eng 16:205–211
Craig S, Helfrich LA (2009). Understanding fish nutrition, feeds and feeding. Virginia Polytechnic Institute and State University. Publication 420-256.
D’Alessandro EB, Filho NRA (2016) Concepts and studies on lipid and pigments of microalgae: a review. Renew Sust Energ Rev 58:832–841
Dabrowski K, Kozak B (1979) The use of fishmeal and soybean meal as a protein source in the diet of grass carp fry. Aquaculture 18:107–114
Dallaire V, Lessard P, Vandenberg G, de la Noue J (2007) Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour Technol 98(7):1433–1439
Daneshvar E, Antikainen L, Koutra E, Kornaros M, Bhatnagar A (2018) Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: treatment of wastewater and lipid extraction. Bioresour Technol 255:104–110
Dang VT, Li Y, Speck P, Benkendorff K (2011) Effects of micro and macroalgal diet supplementations on growth and immunity of greenlip abalone, Haliotis laevigata. Aquaculture 320(1-2):91–98
Daroch M, Shao C, Liu Y, Geng S, Cheng JJ (2013) Induction of lipids and resultant FAME profiles of microalgae from coastal waters of Pearl River Delta. Bioresour Technol 146:192–199
De Silva SS, Francis DS, Tacon AGT (2010) Fish oils in aquaculture Fish oil replace. Altern Lipid sources Aquact Feed:1–20
Delaporte M (2003) Effect of a mono-specific algal diet on immune functions in two bivalve species - Crassostrea gigas and Ruditapes philippinarum. J Exp Biol 206(17):3053–3064
Denstadli V, Skrede A, Krogdahl Å, Sahlstrøm S, Storebakken T (2006) Feed intake, growth, feed conversion, digestibility, enzyme activities and intestinal structure in Atlantic salmon (Salmo salar L.) fed graded levels of phytic acid. Aquaculture 256(1-4):365–376
Ding Z, Zhang Y, Ye J, Du Z, Kong Y (2015) An evaluation of replacing fish meal with fermented soybean meal in the diet of Macrobrachium nipponense: growth, nonspecific immunity, and resistance to Aeromonas hydrophila. Fish Shellfish Immunol 44(1):295–301
Diver S (2006) Aquaponics-Integration of hydroponics with aquaculture. National Sustainable Agricultural Information Service.
Duy NDQ, Francis DS, Southgate PC (2017) The nutritional value of live and concentrated micro-algae for early juveniles of sandfish, Holothuria scabra. Aquaculture 473:97–104
Ejike CECC, Collins SA, Balasuriya N, Swanson AK, Mason B, Udenigwe CC (2017) Porspects ofmicroalgae proteins in producing peptide- based functional foods for promoting cardiovascular health. Trends Food Sci Technol 59:30–36
Endut A, Jusoh A, Ali N, Nik WBW, Hassan A (2010) A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour Technol 101(5):1511–1517
Enduta A, Jusoh A, Ali N, Nik WBW (2011) Nutrient removal from aquaculture wastewater by vegetable production in aquaponics recirculation system. Desalin Water Treat 32(1-3):422–430
Estim A, Saufie S, Mustafa S (2019) Water quality remediation using aquaponics sub-systems as biological and mechanical filters in aquaculture. J Water Process Eng 30:100566
FAO (1980) The state of world fisheries and aquaculture. Fisheries and Aquaculture Department, Rome
FAO (2014) The state of world fisheries and aquaculture. Fisheries and Aquaculture Department, Rome
Faul CN, Holt GJ (2005) Advances in rearing cobia Rachycentron canadum larvae in recirculating aquaculture systems: live prey enrichment and greenwater culture. Aquaculture 249:231–243
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227
Gagneux-Moreaux S, Moreau C, Gonzalez JL, Cosson RP (2007) Diatom artificial medium (DAM): a new artificial medium for the diatom Haslea ostrearia and other marine microalgae. J Appl Phycol 19:549–556
Gamboa-Delgado J, Morales-Navarro YI, Nieto-López MG, Villarreal-Cavazos DA, Cruz-Suárez LE (2019) Assimilation of dietary nitrogen supplied by fish meal and microalgal biomass from Spirulina (Arthrospira platensis) and Nannochloropsis oculata in shrimp Litopenaeus vannamei fed compound diets. J Appl Phycol 31(4):2379–2389
García-Ortega A, Kissinger KR, Trushenski JT (2016) Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus. Aquaculture 452:1–8
Gladyshev MI, Makhutova ON, Kravchuk ES, Anishchenko OV, Sushchik NN (2016) Stable isotope fractionation of fatty acids of Daphnia fed laboratory cultures of microalgae. Limnologica – Ecol Manag Inland Waters 56:23–29
Gong Y, Guterres HADS, Huntley M, Sorensen M, Kiron V (2018) Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquact Nut 24:56–64
Gong Y, Bandara T, Huntley M, Johnson ZI, Dias J, Dahle D, Sørensen M, Kiron V (2019) Microalgae Scenedesmus sp. as a potential ingredient in low fishmeal diets for Atlantic salmon (Salmo salar L.). Aquaculture 501:455–464
Goo BG, Baek G, Choi DJ, Park YI, Synytsya A, Bleha R, Seong DH, Lee CG, Park JK (2013) Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour Technol 129:343–350
Gouveia L, Gomes E, Empis J (1996) Potential use of a microalga (Chlorella vulgaris) in the pigmentation of rainbow trout (Oncorhynchus mykiss) muscle. Eur Food Res Technol 202(1):75–79
Guedes AC, Malcata FX (2012) Nutritional value and uses of microalgae in aquaculture. Aquaculture 390.
Guldhe A, Misra R, Singh P, Rawat I, Bux F (2016) An innovation electrochemical process to alleviate the challenges for harvesting of small size microalgae by using non-sacrificial carbon electrodes. Algal Res 19:292–298
Guldhe A, Ansari FA, Singh P, Bux F (2017) Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol Eng 99:47–53
Guo Z, Liu Y, Guo H, Yan S, Mu J (2013) Microalgae cultivation using an aquaculture wastewater as growth medium for biomass and biofuel production. J Environ Sci 25:S85–S88
Gupta PK (2020) Fate, transport, and bioremediation of biodiesel and blended biodiesel in subsurface environment: a review. J Environ Eng 146(1):03119001
Gupta PK, Yadav BK (2017) Bioremediation of non-aqueous phase liquids (NAPLS) polluted soil and water resources. Environmental Pollutants and their Bioremediation Approaches. Taylor and Francis Group LLC Boca Raton, Florida (FL) United States of America (U.S.A) 241-256.
Gupta SK, Ansari FA, Shriwastav A, Sahoo NK, Rawat I, Bux F (2016) Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J Clean Prod 115:255–264
Hass S, Bauer JL, Adakli A, Meyer S, Lippemeier S, Schwarz S, Schulz C (2016) Marine microalgae Pavlova viridis and Nannochloropsis so. As n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax). J Appl Phycol 28:1011–1021
Hawrot-Paw M, Koniuszy A, Gałczyńska M, Zając G, Szyszlak-Bargłowicz J (2020) Production of microalgal biomass using aquaculture wastewater as growth medium. Water 12(1):106
He Y, Lin G, Rao X, Chen L, Jian H, Wang M, Guo Z, Chen B (2018) Microalga Isochrysis galbana in feed for Trachinotus ovatus: effect on growth performance and fatty acid composition of fish fillet and liver. Aquac Int 26(5):1261–1280
Hemaiswarya S, Raja R, Kumar RR, Ganesan V, Anbazhagan C (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27(8):1737–1746
Hemere G-I, Mommsen TP, Akrogdahl Å (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8(3):175–194
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36(1):54–67
Huang F, Wang L, Zhang C, Song K (2017) Replacemnet of fishmeal with soybean meal and mineral supplemnets in diets of Litopeanaeus vannamei reared in low-salinity water. Aquaculture 473:172–180
Hussein EE, Dabrowski K, El-Saidy DMSD, Lee B (2013) Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquac Res 44(6):937–949
Jiang X, Han Q, Gao X, Gao G (2016) Conditions optimising on the yield of biomass, total lipid, and valuable fatty acids in two strains of Skeletonema menzelii. Food Chem 194:723–732
Jiang M, Zhao HH, Zai SW, Shepherd B, Wen H, Deng DF (2019) A defatted microalgae meal (Haematococcus pluvialis) as a partial protein source to replace fishmeal for feeding juvenile yellow perch Perca flavescens. J Appl Phycol 31(2):1197–1205
Ju ZY, Deng D-F, Dominy W (2012) A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 354-355:50–55
Kalogeropoulos N, Chiou A, Gavala E, Christea M, Andrikopoulos NK (2010) Nutritional evaluation and bioactive microconstituents (carotenoids, tocopherols, sterols and squalene) of raw and roasted chicken fed on DHA-rich microalgae. Food Res Int 43(8):2006–2013
Kassim MA, Kirtania K, De La Cruz D, Cura N, Srivatsa SC, Bhattacharya S (2014) Thermogravimetric analysis and kinetic characterization of lipid-extracted Tetraselmis suecica and Chlorella sp. Algal Res 6:39–45
Kent M, Welladsen HM, Mangott A, Li Y (2015) Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS One 10(2):e0118985
Khatoon N, Sengupta P, Homechaudhuri S, Pal R (2010) Evaluation of algae based feed in goldfish (Carassius auratus) nutrition. Proc Zool Soc 63:109–114
Khatoon H, Banerjee S, Syahiran MS, Noordin NBM, Bolong AMA, Endut A (2016) Re-use of aquaculture wastewater in cultivating microalgae as live feed for aquaculture organisms. Desalina Water Treat 57(60):29295–29302
Kim SS, Ly HV, Kim J, Lee EY, Woo HC (2015) Pyrolysis of microalgae residual biomass derived from Dunaliella tertiolecta after lipid extraction and carbohydrate saccharification. Chem Eng J 263:194-199
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol 173:111–133
Knuckey RM, Semmens GL, Mayer RJ, Rimmer MA (2005) Development of an optimal micro algal diet for the culture of the calanoid copepod Acartia sinjiensis: effect of algal species and feed concentration on copepod development. Aquaculture 249:339–351
Knutsen HR, Johnsen IH, Keizer S, Sørensen M, Roques JAC, Hedén I, Sundell K, Hagen Ø (2019a) Fish welfare, fast muscle cellularity, fatty acid and body-composition of juvenile spotted wolffish (Anarhichas minr) fed a combination of plant proteins and microalgae (Nannochloropsis oceanica). Aquaculture 506:212–223
Knutsen HR, Ottesen OH, Palihawadana AM, Sandaa W, Sørensen M, Hagen Ø (2019b) Muscle growth and changes in chemical composition of spotted wolffish juveniles (Anarhichas minor) fed diets with and without microalgae (Scenedesmus obliquus). Aquacult Rep 13:100175
Kokou F, Fountoulaki E (2018) Aquaculture waste production associated with antinutrient presence in common fish feed plant ingredients. Aquaculture 495:295–310
Kousoulaki K, Mørkøre T, Nengas I, Berge RK, Sweetman J (2016) Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.). Aquaculture 451:47–57
Krogdhal A, Penn M, Thorsen J, Refstie S, Bakke AM (2010) Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac Res 41:333–344
Kuo CM, Jian JF, Lin TH, Chang YB, Wan XH, Lai JT, Chang JS, Lin CS (2016) Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour Technol 221:241–250
Kupchinsky ZA, Coyle SD, Bright LA, Tidwell JH (2015) Evaluation of heterotrphiv algae meal as a diet ingredient for Channel catfisg, Ictaluurus punctatus. J World Aquaclt Soc 46(4):445–452
Lagarde M (2008) Docosahexaenoic acid: Nutrient and precursor of bioactive lipids. Eur J Lipid Sci Technol 110(8):673–678
Lam SS, Ma NL, Jusoh A, Ambak MA (2015) Biological nutrient removal by recirculating aquaponic system: optimization of the dimension ration between the hydroponic and rearing tank components. Int Biodeterior Biodegrad 102:107e115
Lemahieu C, Bruneel C, Termote-Verhalle R, Muylaert K, Buyse J, Foubert I (2013) Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem 141(4):4051–4059
Li MH, Robinson EH, Tucker CS, Manning BB, Khoo L (2009) Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 292:232–236
Llagostera PF, Kallas Z, Reig L, de Gea DA (2019) The use of insect meal as a sustainable feeding alternative in aquaculture: current situation, Spanish consumers’ perceptions and willingness to pay. J Clean Prod 229:10–21
Lober M, Zeng C (2009) Effect of microalgae concentration on larval survival, development and growth of an Australian strain of giant freshwater prawn Macrobrachium resenbergii. Aquaculture 289:95–100
Lum KK, Kim J, Lei XG (2013) Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J Ani Sci Biotechnol 4:53
Macias-Sancho J, Poersch LH, Bauer W, Romano LA, Wasielesky W, Tesser MB (2014) Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: effects on growth and immunological parameters. Aquaculture 426-427:120–125
Malibari R, Sayegh F, Elazzazy AM, Baeshen MN, Dourou M, Aggelis G (2018) Reuse of shrimp farm wastewater as growth medium for marine microalgae isolated from Red Sea- Jeddah. J Clean Prod 198:160–169
Matos AP, Feller R, Moecke EH, Sant'Anna ES (2015) Biomass, lipid productivities and fatty acids composition of marine Nannochloropsis gaditana cultured in desalination concentrate. Bioresour Technol 197:48–55
McLellan MR (1989) Cryopreservation of diatoms. Diatom Res 4:301-318
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol 10:31–41
Miranda LE, Coppola G, Boxrucker J (2020) Reservoir fish habitats: a perspective on coping with climate change. Rev Fish Sci Aquac 4:478–498
Misurcova L, Bunka F, Vavra Ambrozova J, Machu L, Samek D, Kracmar S (2014) Amino acid composition of algal products and its contribution to RDI. Food Chem 151:120–125
Morris GJ (1976a) The cryopreservation of Chlorella.1. Interactions of rate of cooling, protective addition and warming rate. Arch Microbiol 107:57–62
Morris GJ (1976b) The cryopreservation of Chlorella. 2. Effect of growth temperature on freezing tolerance. Arch Microbiol 107:309–312
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12:527–534
Nakagawa H, Gomez-Diaz G (1995) Usefulness of Spirulina sp. meal as feed additive for giant freshwater prawn, Macrobrachium rosenbergii. Suisan Zoshoku 43:521–526
Nasir NM, Yunos FHM, Jusoh HHW, Mohammad A, Lam SS, Jusoh A (2019) Subtopic: advances in water and wastewater treatment harvesting of Chlorella sp. microalgae using Aspergillus niger as bio-flocculant for aquaculture wastewater treatment. J Environ Manag 249:109373
Neumann P, Torres A, Fermoso FG, Borja R, Jeison D (2015) Anaerobic co-digestion of lipid-spent microalgae with waste activated sludge and glycerol in batch mode. Int Biodeterior Biodegrad 100:85–88
Nguyen NT (2008) The utilization of soybean products in Tilapia Feed. 8th International Symposium on Tilapia in Aquaculture 53-65.
Novoveská L, Ross ME, Stanley MS, Pradellles R, Wasiolek V, Sassi JF (2019) Microalgal carotenoids: a review of production, current markets, regulations, and future direction. Mar Drugs 17:1–21
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13(3):1594–1618
Origin oil (2013) Use of algae as aquafeed to improve production in aquaculture operations. Los Angeles. CA 90016.
Palmegiano GB, Agradi FG, Gai F, Gasco L, Rigamont E, Sicuro B, Zoccarato I (2005) Spirulina as a nutrient source in diets for growing sturgeon (Acipenser baeri). Aquac Res 36(2):188–195
Palmer PJ, Burke MJ, Palmer CJ, Burke JB (2007) Development in controlled green-water larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture 272:1–21
Pancha I, Chokshi K, Ghosh T, Paliwal C, Maurya R, Mishra S (2015) Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 193:315–323
Parimi NS, Singh M, Kastner JR, Das KC, Forsberg LS, Azadi P (2015) Optimization of protein extraction from Spirulina platensis to generate a potential co-product and a biofuel feedstock with reduced nitrogen content. Front Energy Res 3:1–9
Patil V, Reitan KI, Knutsen G, Mortensen LM, Källqvist T, Olsen E, Vogt G, Gislerød HR (2005) Microalgae as source of polyunsaturated fatty acids for aquaculture. Curr Top Plant Biol 6:57–65
Patterson D, Gatlin DM (2013) Evaluation of whole and lipid-extracted algae meals in the diets of juvenile red drum (Sciaenops ocellatus). Aquaculture 416-417:92–98
Paudel SR (2020) Nitrogen transformation in engineered aquaponics with water celery (Ocenathe javanica) and koi carp (Cyprinus carpio): Effects of plant to fish biomass ratio. Aquaculture 520:734971
Peng YY, Gao F, Yang HL, Li C, Lu MM, Yang ZY (2020) Simultaneous removal of nutrient and sulfonamides from marine aquaculture wastewater by concentrated and attached cultivation of Chlorella vulgaris in an algal biofilm membrane photobioreactor (BF-MPBR). Sci Total Environ 725:138524
Prajapati SK, Malik A, Vijay VK (2014) Comparative evaluation of biomass production and bioenergy generation potential of Chlorella spp. through anaerobic digestion. Appl Energy 114:790–797
Prem R, Tewari VK (2020) Development of human-powered fish feeding machine for freshwater aquaculture farms of developing countries. Aquac Eng 88:102028
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae – a review. J Algal Biomass Uti 3:89–100
Qiao H, Cong C, Sun C, Li B, Wang J, Zhang L (2016) Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 452:311–317
Radhakrishnan S, Bhavan PS, Seenivasan C, Shanthi R, Muralisankar T (2014) Replacement of fishmeal with Spirulina platensis, Chlorella vulgaris and Azolla pinnata on non-enzymatic and enzymatic antioxidant activities of Macrobrachium rosenbergii. J Basic Appl Zool 67(2):25–33
Radhakrishnan S, Belal IEH, Seenivasan C, Muralisankar T, Bhavan PS (2016) Impact of fishmeal replacement with Arthrospira platensis on growth performance, body composition and digestive enzyme activities of the freshwater prawn, Macrobrachium rosenbergii. Aquacult Rep 3:35–44
Ratledge C (2010) “Single cell oils for the 21st century,” in: single cell oils. Microbial and algal oils, Cohen, Z. and Ratledge, C. (ed.), AOCS Press, Urbana, 3.
Rawat I, Kumar RR, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88(10):3411–3424
Ren H, Tuo J, Addy MM, Zhang R, Lu Q, Anderson E, Chen P, Ruan R (2017) Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour Technol 245:1130–1138
Rossi W, Davis DA (2012) Replacement of fishmeal with poultry by-product meal in the diet of Florida pompano Trachinotus carolinus L. Aquaculture 338-341:160–166
Roy SS, Pal R (2014) Microalgae in aquaculture: a review with special references to nutritional value and fish dietetics. Proc Zool Soc 68(1):1–8
Ruxton CHS, Reed SC, Simpson MJA, Millington KJ (2007) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 20:275–285
Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160:393–400
Sahu A, Pancha I, Jain D, Paliwal C, Ghosh T, Patidar S, Bhattacharya S, Mishra S (2013) Fatty acids as biomarkers of microalgae. Phytochemistry 89:53–58
Sarker PK, Kapuscinski AR, Bae AY, Donaldson E, Sitek AJ, Fitzgerald DS, Edelson OF (2018) Towards sustainable aquafeeds: evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS One 13(7):e0201315
Satoh S, Poe WE, Wilson RP (1989) Effect of supplemented phytates and/or tricalcium phosphate on weight gain, feed efficiency and zinc content in vertebrae of channel catfish. Aquaculture 80:155–161
Schwenzfeier A, Wierenga PA, Gruppen H (2011) Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour Technol 102(19):9121–9127
Sepúlveda C, Acién FG, Gómez C, Jiménez-Ruíz N, Riquelme C, Molina-Grima E (2015) Utilization of centrate for the production of the marine microalgae Nannochloropsis gaditana. Algal Res 9:107–116
Shaalan M, El-Mahdy M, Saleh M, El-Matbouli M (2018) Aquaculture in Egypt: insights on the current trends and future perspectives for sustainable development. Rev Fish Sci Aquac 26(1):99–110
Sharawy Z, Goda AMAS, Hassaan MS (2016) Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, Postlarvae. Anim Feed Sci Technol 212:90–99
Shriwastav A, Gupta SK, Ansari FA, Rawat I, Bux F (2014) Adaptability of growth and nutrient uptake potential of Chlorella sorokiniana with variable nutrient loading. Bioresour Technol 174:60–66
Silva-Carrillo Y, Hernández C, Hardy RW, González-Rodríguez B, Castillo-Vargasmachuca S (2012) The effect of substituting fish meal with soybean meal on growth, feed efficiency, body composition and blood chemistry in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquaculture 364-365:180–185
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2015) Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem Eng J 94:22–29
Sirakov I, Velichkova K, Stoyanova S, Staykov Y (2015) The importance of microalgae for aquaculture industry. Review. Int J Fish Aquat Stud 2:81–84
Soeder CJ (1986) An historical outline of applied algology. CRC Press, Boca Raton, FL
Solana M, Rizza CS, Bertucco A (2014) Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J Supercrit Fluids 92:311–318
Sommer TR, Potts WT, Morrisy NM (1991) Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 94(1):79–88
Sørensen M, Berge GM, Reitan KI, Ruyter B (2016) Microalga Phaeodactylum tricornutum in feed for the Atlantic salmon (Salmo salar) - effect on nutrient digestibility, growth and utilization of feed. Aquaculture 460:116–123
Sørensen M, Gong Y, Bjarnason F, Vasanth GK, Dahle D, Huntley M, Kiron V (2017) Nannochloropsis oceania-derived defatted meals as an alternative to fishmeal in Atlantic salmon feeds. PLoS One 12(7):e0179907
Southgate PC, Braley RD, Militz TA (2017) Ingestion and digestion of micro-algae concentrates by veliger larvae of the giant clam, Tridacna noae. Aquaculture 473:443–448
Spinelli J, Houle CR, Wekell JC (1983) The effect of phytates on the growth of rainbow trout (Salmo gairdneri) fed purified diets containing varying quantities of calcium and magnesium. Aquaculture 30(1-4):71-83
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96
Sprague M, Walton J, Campbell PJ, Strachan F, Dick JR, Bell JG (2015) Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar) post-smolts. Food Chem 185:413–421
Stephenson PG, Moore CM, Terry MJ, Zubkov MV, Bibby TS (2011) Improving photosynthesis for algal biofuels: toward a green revolution. Trends Biotechnol 29:615–623
Subhash GV, Chugh N, Iyer S, Waghmare A, Musale AS, Nandru R, Dixit RB, Gaikwad MS, Menon D, Throat R, Kumar GRK, Nagle V, Sagaram US, Dasgupta S (2020) Application of invitro protein solubility for selection of microalgae biomass as protein ingredient in animal and aquafeed. J Appl Phycol 32:3955–3970
Suh S-S, Kim SJ, Hwang J, Park M, Lee T-K, Kil E-J, Lee S (2015) Fatty acid methyl ester profiles and nutritive values of 20 marine microalgae in Korea. Asian Pac J Trop Med 8(3):191–196
Tacon AGJ, Lemos D, Metian M (2020) Fish for health: improved nutritional quality of cultured fish for human consumption. Rev Fish Sci Aquac 28(4):449–458
Takano M, Sado JI, Ogawa T, Terui G (1973) Freezing and freeze-drying of Spin F a platensis. Cryobiology 10:440–444
Tang DYY, Khoo KS, Chew KW, Tao Y, Ho SH, Show PL (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997
Tejido-Nuñez Y, Aymerich E, Sancho L, Refardt D (2020) Co-cultivation of microalgae in aquaculture water: interactions, growth and nutrient removal efficiency at laboratory-and pilot-scale. Algal Res 49:101940
Tibaldi E, Zittelli GC, Parisi G, Bruno M, Giorgi G, Tulli F, Venturini S, Tredici MR, Poli BM (2015) Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp. (T. ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 440:60–80
Tibbetts SM, Bjornsson WJ, McGinn PJ (2015a) Biochemical composition and amino acid profiles of Nannochloropsis granulata algal biomass before and after supercritical fluid CO2 extraction at two processing temperatures. Anim Feed Sci Technol 204:62–71
Tibbetts SM, Whitney CG, MacPherson MJ, Bhatti S, Banskota A, Stefanova R, McGinn PJ (2015b) Biochemical characterization of microalgal biomass from freshwater species isolated in Alberta, Canada for animal feed applications. Algal Res 11:435–447
Tibbetts SM, Melanson RJ, Park KC, Banskota AH, Stefanova R, Mcginn PJ (2015c) Nutritional evaluation of whole and lipid-extracted biomass of the microalga Scenedesmus sp. AMDD isolated in Saskatchewan, Canada for animal feeds : proximate, amino acid, fatty acid, carotenoid and elemental composition. Curr Biotechnol 4:1–17
Tibbetts SM, Yasumaru F, Lemos D (2017) In Vitro prediction of digestible protein content of marine microalgae (Nannochloropsis granulata) meals for Pacific white shrimp (Litopenaeus vannamei) and rainbow trout (Oncorhynchus mykiss). Algal Res 21:76–80
Tibbetts SM, Patelakis SJ, Whitney-Lalonde CG, Garrison LL, Wall CL, MacQuarrie SP (2020) Nutrient composition and protein quality of microalgae meals produced from the marine prymnesiophyte Pavlova sp. 459 mass-cultivated in enclosed photobioreactors for potential use in salmonid aquafeeds. J Appl Phycol 32:299–318
Tossavainen M, Lahti K, Edelmann M, Eskola R, Lampi AM, Piironen V, Korvonen P, Ojala A, Romantschuk M (2019) Integrated utilization of microalgae cultured in aquaculture wastewater: wastewater treatment and production of valuable fatty acids and tocopherols. J Appl Phycol 31:1753–1763
Tredici MR, Rodolfi L, Biondi N, Bassi N, Sampietro G (2016) Techno-economic analysis ofmicroalgal biomass production on 1-ha green wall panel (GWP ®) plant. Algal Res 19:253–263
Tsai HP, Chuang LT, Chen CN (2016) Production of long chain omega-3 fatty acids and carotenoids in tropical areas by a new heat-tolerant microalga Tetraselmis sp. DS3. Food Chem 19:82–90
Turchini GM, Torstensen BE, Ng W-K (2009) Fish oil replacement in finfish nutrition. Rev Aquac 1(1):10–57
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178-87
Viegas C, Gouveia L, Gonçalves (2021) Aquaculture wastewater treatment trough microalgal.Biomass potential appplications on animal feed, agricultural, and energy. J Environ Manag 286:112187
Vizcaíno J, López G, Sáez MI, Jiménez JA, Barros A, Hidalgo L, Camacho-Rodríguez J, Martínez TF, Cerón-García MC, Alarcón FJ (2014) Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 431:34–43
von Danwitz A, Schulz C (2020) Effects of dietary rapeseed glucosinolates, sinapic acid and phytic acid on feed intake, growth performance and fish health in turbot (Psetta maxima L.). Aquaculture 516:734624
Waite R, Beveridge M, Brummett R, Castine S, Chaiyawannakarn N, Kaushik S, Mungkung R, Nawapakpilai S, Phillips M (2014) Improving productivity and environmental performance of aquaculture. World Fish.
Waldenstedt L, Inborr J, Hansson I, Elwinger K (2003) Effects of astaxanthin-rich algal meal (Haematococcus pluvalis) on growth performance, caecal campylobacter and clostridial counts and tissue astaxanthin concentration of broiler chickens. Anim Feed Sci Technol 108:119–132
Walker A, Berlinsky DL (2011) Effects of partial replacement of fish meal protein by microalgae on growth, feed intake, and body composition of atlantic cod. N Am J Aquac 73:76–83
Wang Y, Guo WQ, Lo YC, Chang JS, Ren NQ (2014) Characterization and kinetics of bio-butanol production with Clostridium acetobutylicum ATCC824 using mixed sugar medium simulating microalgae-based carbohydrates. Biochem Eng J 91:220–230
Wang Y, Ho S, Cheng C, Guo W, Nagarajan D, Ren N (2016) Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour Technol 222:485–497
Wuang SC, Khin MC, Chua PQ, Luo YD (2016) Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res 15:59–64
Yaakob Z, Ali E, Zainal A, Mohamad M, Takriff M (2014) An overview: biomolecules from microalgae for animal feed and aquaculture. J Biol Res 21:6Yun Y-M, Cho, S-K, Jung K-W, Kim M-S, Shin HS, Kim DH (2014) Inhibitory effect of chloroform on fermentative hydrogen and methane production from lipid etracted microalgae. Int J Hydrog Energy 39:19256–19261
Yadav G, Meena DK, Sahoo AK, Das BK, Sen R (2020) Effective valorization of microalgal biomass for the production of nutritional fish-feed supplements. J Clean Prod 243:118697
Younis ES, Al-Quffail AS, Al-Asgah NA, Abdel-Warith AW, Al-Hafedh YS (2018) Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J Biol Sci 25(2):198–203
Yun YM, Cho SK, Jung KW, Kim MS, Shin HS, Kim DH (2014) Inhibitory effect of chloroform on fermentative hydrogen and methane production from lipid-extracted microalgae. Int J hydrogen energy 39:19256-19261
Zhao G, Chen X, Wang L, Zhou S, Feng H, Chen WN, Lau R (2013) Ultrasound assisted extraction of carbohydrates from microalgae as feedstock for yeast fermentation. Bioresour Technol 128:337–344
Zhao X, Zhou Y, Huang S, Qiu D, Schideman L, Chai X, Zhao Y (2014) Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour Technol 156:322–328
Zhou W, Wang Z, Xu J, Ma L (2018) Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J Biosci Bioeng 126(5):644–648
Funding
The authors are grateful to the Durban University of Technology (DUT), Technology Innovation Agency (TIA), Department of Science and Innovation (DSI), Economic Development, Tourism and Environmental Affairs (EDTEA, KZN), and National Research Foundation (NRF) for providing financial assistance. In addition, one of the authors, Dr. Abhishek Guldhe, is thankful to the Department of Biotechnology, Govt. of India, for the award of Ramalingaswami Fellowship.
Author information
Authors and Affiliations
Contributions
F.A Ansari thought of the original concept and drafted the manuscript. A Guldhe finalized the manuscript contents and edited the manuscript. S.K Gupta and I Rawat edited the manuscript. F. Bux critically edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansari, F.A., Guldhe, A., Gupta, S.K. et al. Improving the feasibility of aquaculture feed by using microalgae. Environ Sci Pollut Res 28, 43234–43257 (2021). https://doi.org/10.1007/s11356-021-14989-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14989-x