Abstract
Many studies of disinfection by-products (DBPs) in pools have focused on haloacetic acids, trihalomethanes, and chloramines, with less studies investigating the occurrence of other DBPs, such as haloketones, haloacetaldehydes, haloacetonitriles, halonitromethanes, and haloacetamides. Furthermore, while many studies have achieved a broadscreen analysis across several pools, fewer studies have followed the water quality of pools over time, with information regarding the production and fate of DBPs in pools over extended periods (e.g. > 1 year) being limited. This study reports the occurrence of 39 DBPs and several general water quality parameters in two newly built and filled swimming pools over 15 months, where investigations began prior to opening. DBP concentrations measured in this study were generally similar to or higher than those previously reported in chlorinated pools, with concentrations of chloroacetic acid, dichloroacetic acid, trichloroacetic acid, and chloral hydrate (trichloroacetaldehyde) in some samples being higher than previously reported maximum concentrations. Considering both pools, lower concentrations of DBPs were measured in the pool where a steady state non-purgeable organic carbon concentration was achieved, highlighting the importance of the establishment of a steady state balance of mineralisation versus addition of organic carbon to reduce precursors for DBP formation in pools. Pools were found to exhibit significantly higher estimated cytotoxicity than their filling water, which reflects the significantly higher concentrations of DBPs measured in the pools in comparison to the filling water. Chloral hydrate accounted for up to 99% the total estimated cytotoxicity and was found to be correlated to the number of pool entries, suggesting that swimmers may be a potential source of chloral hydrate precursors in pools. The presence and subsequent peak of non-purgeable organic carbon and DBPs prior to, and soon after, opening suggest that the building process and/or new pool infrastructure may have had a significant impact on the chemical water quality, particularly on DBP formation. This study includes the first quantification of bromochloroacetaldehyde, bromodichloroacetaldehyde, bromochloronitromethane, and dichloronitromethane in chlorinated swimming pools, and provides important new knowledge on the long-term trends of DBPs in pools.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While the aim of water disinfection is to kill pathogens and minimise microbial disease risk, it can also lead to the formation of disinfection by-products (DBPs). Many DBPs have been reported to be potentially genotoxic, neurotoxic, and/or cytotoxic, with several DBPs also exhibiting a potentially carcinogenic, teratogenic, and/or mutagenic nature (Richardson et al. 2007). As such, disinfection should be controlled in order to minimise DBP formation while maintaining significant protection against the microbial risk, which is generally much greater than that posed by DBPs. While more is known about DBPs in drinking, waste and recycled waters, where over 700 DBPs have been identified (Plewa and Richardson 2017), less is understood about DBPs in the swimming pool environment. Due to the continual input of organic matter via filling water and bather load (which may include human body excretions, personal care products, and pharmaceuticals) and continual availability of disinfectants (e.g. chlorine), swimming pools are a unique environment compared to other water matrices, particularly in terms of DBPs. Furthermore, over 100 DBPs have been identified exclusively in swimming pools or spas (Daiber et al. 2016; Richardson et al. 2010), highlighting the unique nature of these waters.
As with other water types, many investigations of DBPs in swimming pool waters have focused on the occurrence and/or formation of trihalomethanes (THMs), haloacetic acids (HAAs), and inorganic chloramines (particularly trichloramine), with fewer studies including haloacetonitriles (HANs), particularly dichloroacetonitrile, and chloral hydrate, the hydrated analogue of trichloroacetaldehyde. Very few studies have investigated the occurrence and/or formation of halonitromethanes (HNMs), haloacetamides (HAAms), haloketones (HKs), or other haloacetaldehydes (HALs) in swimming pool waters. Furthermore, very limited studies of DBPs in Australian swimming pools have been reported (Carter et al. 2015; Kelsall and Sim 2001; Teo et al. 2016a, b; Yeh et al. 2014), where higher disinfectant residuals (2 to 4 mg L−1 as Cl2) are employed.
Many studies reported the occurrence of DBPs in pools by carrying out a broadscreen analysis, that is, the analysis of several pools for a suite of DBPs, where different pool types or those employing different treatment methods were investigated (Carter and Joll 2017). While these studies have led to a better understanding of DBPs in pools, they provide minimal information regarding long-term trends of these DBPs and their fate in swimming pools. While some studies have followed DBPs over a matter of days (Gérardin et al. 2015; Judd and Black 2000; Peng et al. 2015; Weng and Blatchley 2011) or months (Golfinopoulos 2000; Kanan and Karanfil 2011; Lahl et al. 1981; Yeh et al. 2014), limited investigations exist for longer periods (e.g. > 1 year) (Kristensen et al. 2010; Simard et al. 2013; Zare Afifi and Blatchley 2015). Of these aforementioned time-based studies, most have limited their focus to the occurrence of THMs and/or chloramines (Gérardin et al. 2015; Golfinopoulos 2000; Judd and Black 2000; Kristensen et al. 2010; Lahl et al. 1981; Peng et al. 2015; Weng and Blatchley 2011; Yeh et al. 2014), with only a few also investigating other DBPs, including HAAs, HNMs, HANs, N-nitrosodimethylamine (NDMA), and/or cyanogen halides (Kanan and Karanfil 2011; Simard et al. 2013; Zare Afifi and Blatchley 2015). With fewer studies encompassing a large number of DBP classes, knowledge of DBP trends over a large time period is limited.
This study expands on the knowledge of DBP occurrence in swimming pools, particularly in Australian conditions, by reporting the occurrence of 39 DBPs (across seven different DBP classes), as well as several general water quality parameters, in two chlorinated swimming pools. This work follows the concentrations of these investigated DBPs over 15 months, providing information to assess any weekly, monthly, or seasonal trends, an area where knowledge is lacking. While Yeh et al. (2014) investigated limited parameters in a pool from a complete water replacement, the current study is the first investigation of the water quality and occurrence of DBPs in newly built and filled swimming pools, where investigations began prior to the opening of the facility. The filling water for the pools was investigated concurrently to assess its impact, if any, on DBP occurrence. Statistical analysis between DBPs, general water quality and/or operational parameters was performed to assess any correlations between these parameters. Furthermore, the chronic cytotoxicity of the pool water samples was estimated based on calculation, in order to provide an idea of the health impact these DBPs may pose, at the concentrations measured.
Methodology
Analytical standards and reagents
All chemicals and reagents used were of analytical grade purity (> 98%) and purchased from a range of suppliers including AccuStandard (CT, USA), CanSyn Chemical Corporation (Ontario, Canada), CDN isotopes (Quebec, Canada), Sigma Aldrich (Sydney, Australia), and Thermo Fisher (Victoria, Australia). Ultrapure water, purified by an ELGA PURELAB Ultra purification system (18.2 MΩ-cm resistivity), was used in all experiments.
Preparation of standard, working, and calibration solutions
DBP standard stock solutions (1 g L−1 of each DBP) in acetone were prepared by DBP class, i.e. one stock solution containing each individual DBP of a given class. Bromochloroacetonitrile (BCAN), trichloronitromethane (TCNM), and 1,1,1-trichloropropanone (1,1,1-TCP) were added to relevant DBP standard stock solutions by dilution of individual purchased solutions (5 g L−1 in acetone). Similarly, haloacetic acid working solutions were prepared by dilution of a purchased stock solution containing all nine HAAs (2 g L−1 in methyl tert-butyl ether (MTBE)). Surrogate standard and internal standard stock solutions were prepared by weighing neat compound(s) into acetone. Working DBP, surrogate standard, and internal standard solutions were prepared by dilution of appropriate stock solution(s) into acetone. Calibration standards were prepared by fortifying ultrapure water samples with the desired DBP standard, surrogate standard, and internal standard working solution(s), as per individual method requirements (“Analytical methods” section).
Analytical methods
Analytical methods employed in this study are summarised in Table SI 1. Free and total chlorine equivalent concentrations, pH, conductivity, dissolved oxygen, and temperature were measured at time of collection using a Pocket Colorimeter (HACH; 5870000) or a portable multimeter (HACH; HQ40D). Non-purgeable organic carbon (NPOC) and total nitrogen (TN) were analysed using high temperature catalytic combustion with non-dispersive infrared detection on a Shimadzu total organic carbon analyser (TOC-L) equipped with a total nitrogen measuring unit (TNM-L). The THMs (trichloromethane (chloroform), bromodichloromethane, dibromochloromethane, and tribromomethane (bromoform)) were analysed by headspace solid-phase microextraction (HS-SPME) gas chromatography-mass spectrometry (GC-MS) using a simplified version of the method of Allard et al. (2012). HKs (chloropropanone, 1,1-dichloropropanone, 1,3-dichloropropanone and 1,1,1-trichloropropanone (CP, 1,1-DCP, 1,3-DCP and 1,1,1-TCP, respectively)) and HALs (dibromoacetaldehyde, bromochlorocetaldehyde, bromodichlorocetaldehyde, dibromochlorocetaldehyde, trichlorocetaldehyde and tribromoacetaldehyde (DBAL, BCAL, BDCAL, DBCAL, TCAL (chloral hydrate; CH), and TBAL, respectively)) were analysed simultaneously by liquid-liquid extraction (LLE) followed by GC-MS by an adaption of Standard Method 551.1 (US EPA 1995). HAAs (chloroacetic acid, bromoacetic acid, dichloroacetic acid, dibromoacetic acid, bromochloroacetic acid, bromodichloroacetic acid, dibromochloroacetic acid, trichloroacetic acid, and tribromoacetic acid (CAA, BAA, DCAA, DBAA, BCAA, BDCAA, DBCAA, TCAA, and TBAA, respectively)) were analysed as their methyl esters by LLE derivatisation GC-MS as per Standard Method 552.2 (US EPA 2003). HANs (chloroacetonitrile, bromoacetonitrile, dichloroacetonitrile, dibromoacetonitrile, bromochloroacetonitrile, and trichloroactetonitrile (CAN, BAN, DCAN, DBAN, BCAN, and TCAN, respectively)), HNMs (dichloronitromethane, dibromonitromethane, bromochloronitromethane, bromodichloronitromethane, dibromochloronitromethane, trichloronitromethane, and tribromonitromethane (DCNM, DBNM, BCNM, BDCNM, DBCNM, TCNM (chloropicrin), and TBNM (bromopicrin), respectively)), and HAAms (dichloroacetamide, dibromoacetamide, and trichloroacetamide (DCAAm, DBAAm, and TCAAm, respectively)) were analysed simultaneously by LLE GC-MS, using a simplified version of the method of Carter et al. (2019). All analyses were performed in duplicate with average results presented. For GC-MS analysis, selective ion monitoring (SIM) was used for quantification with results normalised by the use of internal and/or surrogate standards where appropriate.
Water samples
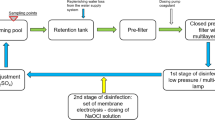
Two newly built and filled public swimming pools at one facility in Perth, Western Australia, were investigated from prior to opening (November 2015) until March 2017, with permission granted by the Department of Health (Western Australia). In order to ensure confidentiality, pools have been de-identified and coded as Pool A and Pool B. Both pools were filled and regularly topped-up, with disinfected distributed drinking water from one source. Pool A was a 20-m (4 lane) outdoor/covered leisure pool (300 kL), disinfected by chorine gas and equipped with ultraviolet (UV) treatment, with a target operational temperature of 30 °C. Pool B was a 50-m (10 lane) outdoor lap pool (1.86 ML), disinfected by chlorine gas and employing cyanuric acid (20 to 50 mg L−1) as a chlorine stabiliser, with a target operational temperature of 27 °C. Both pools were filtered (~ 1 μm) via individual diatomaceous earth filters and the pools were operated/treated independently from one another. The target pH of both pools was 7.2 to 7.8, with free chlorine equivalent residual concentration targets of 2.5 to 3 and 3 to 3.5 mg L−1 for Pools A and B, respectively. Additional information regarding the operation of the pools is presented in Tables SI 2 and SI 3.
Samples were collected at the centre of the longest side of each pool, from approximately 20 to 30 cm below the water’s surface and 50 cm from the pools edge. Samples were collected directly into amber bottles with no headspace and the oxidant residual quenched (110% of the measured total chlorine equivalent molar concentration as per Table SI 1), before storage at 4 °C until analysis (24 to 72 h). Where possible, water samples were collected at the same time of the day (5 to 6 am) on each sampling occasion. Initially, samples were collected twice daily (morning and night), then when sampling frequency decreased, they were collected daily, weekly, biweekly, and finally monthly for the duration, totalling thirty-three sampling events. The pools’ filling water, disinfected distributed drinking water, was collected regularly for analysis.
Cytotoxicity evaluation
The chronic cytotoxicity for most investigated DBPs was evaluated based upon C50 values available in the literature (Wagner and Plewa 2017). HKs were excluded from cytotoxicity evaluation as C50 values do not currently exist for these compounds. In these calculations, the concentration (M) of each DBP measured was divided by its C50 value (M), resulting in a dimensionless number. Finally, these results were multiplied by 106 to make the numbers more readable.
Statistical evaluation
Spearman’s rank correlation coefficient was used to evaluate any correlations that may exist amongst the DBPs and other water quality parameters of the investigated swimming pools. Statistical analysis was performed using SPSS Statistics version 24 software (IBM, Armonk, New York).
Results and discussion
General water quality parameters
A summary of several general water quality and operational parameters, free and total chorine equivalent residual concentrations, NPOC and TN concentrations, pH, temperature, dissolved oxygen, and conductivity measured in each pool is presented in Table SI 4. Free chlorine equivalent concentrations were, on average, 3.4 and 3.3 mg L−1 in Pools A and B, respectively, although concentrations up to 6.6 mg L−1 were measured. Minimum free chlorine equivalent concentrations that met local regulations (greater than 3 and 2 mg L−1 for stabilised and non-stabilised pools, respectively (Standards Australia 2002)) were measured in only 88% and 54% of samples taken from Pools A and B, respectively. Total chlorine equivalent concentrations, measured between 1.8 and 7.1 mg L−1 across both pools, were outside the local guideline (must not exceed 30% of the measured free chlorine (Standards Australia 2002)) on three occasions (< 11% of the time), in which all cases were limited to Pool A. It should be noted, however, that due to technical issues with the chlorine dosing unit, unusually high free and total chlorine equivalent concentrations (15 to 26 and 15 to 29 mg L−1, respectively) were measured in Pool A on three successive days soon after opening (days 1.5, 2, and 3). These concentrations were excluded in the subsequent statistical analysis, as well as in the determination of minimum, maximum, and average values for free and total chlorine equivalent concentrations. pH levels in Pools A (6.6 to 7.7) and B (6.8 to 7.8) met local regulations (7.2 to 7.8 (Standards Australia 2002)) in only 73 and 76% of the samples, respectively. NPOC concentrations ranged between 2.8 and 30 mg L−1 in Pool A and between 1.7 and 21 mg L−1 in Pool B, while TN concentrations between 0.1 and 16 and 4.5 and 21 mg L−1 (for Pools A and B, respectively) were measured. It should be noted that the use of isocyanuric acid in Pool B contributed to the concentrations of NPOC and TN measured in Pool B.
Occurrence of disinfection by-products in the swimming pool waters
Of the 39 investigated DBPs, only 13 were not detected in any samples of either Pool A or B. Despite being measured in previous pool water studies at concentrations up to 53 μg L−1 (Carter et al. 2015; Daiber et al. 2016; Hang et al. 2016; Kanan 2010; Manasfi et al. 2016; Tardif et al. 2015, 2016; Yeh et al. 2014), BAA, DBCAA, TBAA, BAN, DBAL, TBAL, DBAAm, DCAAm, TCAAm, and TBNM were all below their respective limits of detection (0.2 to 1.1 μg L−1) in all pool samples investigated in the current study. DBCNM, DBNM, and BDCNM were below their respective limits of detection (0.7 μg L−1) in all pool samples in the current study, which, apart from Daiber et al. (2016) who identified their presence in some of the brominated and chlorinated pools and spas of their study, is the only known investigation of these HNMs in pools. The majority of these DBPs are brominated and their absence in the investigated pools may be attributed to the lower availability of bromine (via bromide oxidation) compared to that of chlorine, and hence lower formation of brominated DBPs. In addition, HNMs, particularly brominated tri-HNMs, have been shown to be unstable in chlorinated waters (Liew et al. 2012), which may explain the absence of DBCNM and BDCNM to date in swimming pool waters and although reported at concentrations up to 1.2 μg L−1 by Yeh et al. (2014), the absence of TBNM in all pools investigated by Manasfi et al. (2016). In the presence of free chlorine, HAAms are rapidly degraded, presumably due to their conversion to HAAs (Chu et al. 2010), the most likely reason for their absence in the current study.
A detailed summary of the concentrations of DBPs measured in Pools A and B is provided in Table 1. Furthermore, Table 2 summarises the contribution of each DBP class to the total concentration of DBPs measured, on any given day in each pool, where concentrations were compared on a molar basis.
Haloacetic acids were generally the predominant class of DBP measured in both pools, where total HAA concentrations (also referred to as HAA9; the sum of CAA, BAA, DCAA, DBAA, BCAA, BDCAA, DBCAA, TCAA, and TBAA concentrations) represented between 34 and 99% and between 58 and 97% of the total measured DBP molar concentrations in any sample, of Pools A and B, respectively. DCAA and TCAA were detected in all pool samples at concentrations significantly higher than any other HAA measured in this study, up to 26 and 11 mg L−1, respectively. These concentrations are generally higher than those previously reported for chlorinated swimming pools (as summarised by Carter and Joll (2017)), and were up to 11× and 4× higher than the maximum previously reported concentrations for DCAA and TCAA, respectively (Yeh et al. 2014). TCAA and DCAA are known degradation products of CH in waters containing chlorine (Barrott 2004). As the pools in this study were found to contain significant CH concentrations (discussed in detail below), we theorise that chlorine degradation of CH may be a significant formation pathway for the high DCAA and TCAA concentrations measured in this study.
While some studies (Dehghani et al. 2018; Wang 2011) have reported higher CAA concentrations (up to 377 μg L−1) in some of the chlorinated pools in their respective studies, CAA concentrations measured in Pool B (3.3 to 180 μg L−1) were similar to, or only slightly higher than, those reported in most other studies, < 0.5 to 120 μg L−1 (Berg et al. 2000; Cardador and Gallego 2011; Carter et al. 2015; Hang et al. 2016; Sa et al. 2012; Tardif et al. 2016; Yeh et al. 2014). In Pool A, however, CAA concentrations were generally higher than the previously reported concentrations, with CAA being measured at concentrations of 93 to 6092 μg L−1 in Pool A. As observed with DCAA and TCAA, maximum concentrations of CAA measured in Pool A were significantly higher (approximately 6× greater) than any previous study, where a maximum concentration of 300 μg L−1 has been reported (Wang 2011). A significantly higher CAA concentration (1000 μg L−1) has been reported by Loos and Barceló (2001); however, this study was not included for comparison as no information regarding pool type or treatment method was provided.
BCAA and BDCAA were also detected in some of the Pool A and B samples, where concentrations of up to 187 and 43 μg L−1 were measured, respectively. BCAA concentrations in this study were generally higher than most previously reported concentrations (e.g. Daiber et al. 2016; Tardif et al. 2016; Wang 2011), although up to 1353 μg L−1 has been reported (Hang et al. 2016). Only two studies have reported higher concentrations of BDCAA, up to 912 and 110 μg L−1 (Kanan 2010; Loos and Barceló 2001), with concentrations measured in this study (4.2 to 43 μg L−1) found to be similar to, or only slightly higher than, the other previously reported studies (e.g. Kanan 2010; Yeh et al. 2014). Compared to other HAAs, significantly lower concentrations (0.9 to 8.3 μg L−1) of DBAA were measured in some pool samples in this study. Although higher DBAA concentrations have been reported, up to 88 μg L−1 (Dehghani et al. 2018; Wang 2011), concentrations measured in this study were generally similar to those reported for other chlorinated pools, 1 to 28 μg L−1 (e.g. Carter et al. 2015; Daiber et al. 2016; Hang et al. 2016). The high NPOC content of the pools may help explain the occurrence of HAAs, as HAA formation from the chlorination of filling water organic matter and body fluid analogue has been demonstrated (Kanan and Karanfil 2011).
Based on molar concentrations, HALs represented 1 to 62% and 0.5 to 20% of the total measured DBP concentrations in Pools A and B, respectively (Table 2). CH was the only HAL detected in all samples of each pool, where concentrations up to 3202 and 151 μg L−1 were measured in Pools A and B, respectively (Table 1). While CH concentrations measured in Pool B were generally similar or lower to those previously reported, 17 to 301 μg L−1 (Carter et al. 2015; Daiber et al. 2016; Lee et al. 2010; Manasfi et al. 2016; Serrano et al. 2011; Yeh et al. 2014; Zhang et al. 2015), concentrations measured in Pool A (2434 to 3202 μg L−1) are the highest ever reported in chlorinated swimming pools, where a previous maximum concentration of 400 μg L−1 was reported in our previous study (Carter et al. 2015). CH is known to form from the chlorination of both humic and fulvic substances, as well as amino acids, as summarised by Barrott (2004). The high NPOC concentrations (up to 30 mg L−1) measured in the pools may, in part, explain the elevated CH concentrations measured over this study, as amino acids are a likely contributor to the organic carbon (OC) content in pools. Although not in all samples, only one other HAL, BDCAL, was detected in both pools, where concentrations of 1.6 to 31 μg L−1 were measured. BCAL and DBCAL were detected in some samples of Pool A (up to 3.2 and 1.7 μg L−1, respectively), only slightly higher than the DBCAL concentrations (0.3 μg L−1) in our previous study of swimming pool water (Carter et al. 2015). This is the first known quantification of BDCAL and BCAL in swimming pool waters. As mentioned for CH, the occurrence of other HALs in the current study may also be attributed to the high NPOC concentrations, suggesting the potential presence of HAL precursors. Furthermore, as discussed for CH (Barrott 2004), the degradation of these HALs to their corresponding HAAs is consistent with the significant concentrations of most of the corresponding HAAs measured in this study.
THMs represented between 0.1 and 18% and between 1.6 and 18% of the total molar DBP concentrations measured in Pools A and B, respectively (Table 1). Trichloromethane and bromodichloromethane were the only THMs detected in all samples of both pools. Trichloromethane was measured at concentrations of up to 4400 and 92 μg L−1 in Pools A and B, while bromodichloromethane was found at concentrations up to 8.2 and 7.7 μg L−1, respectively. Although some studies have reported higher concentrations of bromodichloromethane in chlorinated pools (e.g. up to 318 μg L−1, Hang et al. (2016)), concentrations measured in this study were generally similar to those observed in most other studies of chlorinated pools (Carter and Joll 2017 and references therein). Similarly, the majority of the trichloromethane concentrations measured in this study (0.3 to 194 μg L−1) were generally similar to those reported previously (e.g. Hang et al. 2016; Yeh et al. 2014), although elevated concentrations (1.7 to 4.4 mg L−1) were observed in Pool A at the beginning of this study. The trichloromethane concentrations measured at the beginning of this study were significantly higher than those reported in any other study, where a previous maximum of 980 μg L−1 was reported in an indoor chlorinated pool (Lahl et al. 1981). Trichloromethane has been shown to be a degradation product of CH and TCAA (Barrott 2004; Zhang and Minear 2002), and, as previously discussed, these DBPs were observed at elevated concentrations, potentially accounting for the elevated trichloromethane concentrations also observed. Although not detected in all samples, dibromochloromethane and tribromomethane were measured in both Pools A and B, up to 1.5 and 0.9 μg L−1, respectively, concentrations which were generally similar to those previously reported in chlorinated pools (as summarised by Carter and Joll 2017).
Only three of the five HNMs investigated were detected in the pools, although not in all samples. BCNM, DCNM, and TCNM were found at concentrations up to 5.3, 5.9, and 6.2 μg L−1 in Pool A and up to 9.2, 2.9, and 4.4 μg L−1 in Pool B, respectively. Concentrations of TCNM measured throughout this study were generally similar to concentrations previously reported in other chlorinated pools, e.g. up to 5 μg L−1 (Carter and Joll 2017; Tardif et al. 2015). Only one other study has identified BCNM in pools, at concentrations between 0.8 and 11 μg L−1 (Kanan 2010), which generally reflect those observed in the pools in this study. Furthermore, we report here the first known quantification of DCNM in swimming pools, where concentrations of between 0.2 and 5.9 μg L−1 were measured.
Although not in all samples, all HKs investigated (1,1-DCP, 1,3-DCP, CP and 1,1,1-TCP) were detected in Pool A, while only 1,1-DCP and 1,1,1-TCP were detected in Pool B. 1,1,1-TCP was the predominant HK in both pools, routinely measured at concentrations of 1.1 to 45 μg L−1, which are generally similar to, or only slightly higher than, most previous studies (Carter et al. 2015; Daiber et al. 2016; Font-Ribera et al. 2016; Hang et al. 2016; Manasfi et al. 2016; Spiliotopoulou et al. 2015; Tardif et al. 2015, 2016; Yeh et al. 2014; Zhang et al. 2015). It should be noted, however, that 1,1,1-TCP concentrations in several samples of Pool A soon after opening were significantly higher (91 to 140 μg L−1) than the routine measurements. Higher concentrations (up to180 μg L−1) have also been reported in one other study (Hang et al. 2016). These previous studies of 1,1,1-TCP also reported the occurrence of 1,1-DCP, where concentrations were similar to those observed in the current study (0.2 to 3.4 μg L−1). Our previous study is the only other known quantification of both CP and 1,3-DCP in pool water (1.9 and 0.8 μg L−1, respectively) (Carter et al. 2015). While only measured in Pool A of the current study, concentrations were generally similar for CP (0.7 to 2.5 μg L−1), but were generally higher for 1,3-DCP (0.4 to 27 μg L−1), in comparison to our previous study (Carter et al. 2015).
BCAN, DBAN, and TCAN were detected (up to 12 μg L−1) in some samples from each pool, at concentrations generally comparable to those previously reported (Carter and Joll 2017 and references therein). These previous studies also reported the occurrence of DCAN in chlorinated pools, at concentrations generally similar to those in the current study (2 to 263 and 0.5 to 148 μg L−1 in Pools A and B, respectively). Furthermore, several samples in the current study were found to contain DCAN at concentrations higher than the previously reported maximum, 206 μg L−1 (Hang et al. 2016). CAN was also observed (0.4 to 9.1 μg L−1) in the current study, which for some samples was higher than any previously reported concentration (3 μg L−1) (Carter et al. 2015; Kanan 2010). In swimming pools, HAN formation has been reported to occur from human-derived compounds high in nitrogen, such as urea or hair proteins (Kim et al. 2002). Hypochlorite has also been shown to oxidise HANs, resulting in the formation of HAAms and HAAs (Glezer et al. 1999; Yu and Reckhow 2015), which is consistent with the low HAN and high HAA concentrations observed in the current study.
Contribution of filling water
The filling water, i.e. distributed disinfected drinking water, used to fill and regularly top up the swimming pools, was also investigated. Detection frequency and concentrations of the investigated DBPs measured in the filling water are presented in Table 1, with details of several general water quality parameters also presented in Table SI 4.
Considering average molar concentrations, the different classes of DBPs in order of highest to lowest concentrations measured in the filling water were THMs > HAAs > HANs > HNMs > HAAms > HKs/HALs. Generally, the brominated DBPs were detected at significantly higher concentrations in the filling water compared to the pool waters which is likely due to (i) the transformation of brominated DBPs to mixed bromo-chloro-DBPs due to the constant availability of free chlorine in the pool waters and (ii) the faster degradation of brominated DBPs (compared to chlorinated DBPs) by UV (Hansen et al. 2013b). The presence of bromide/bromine in the filling water, measured at ~ 0.2 mg L−1 bromide after quenching the oxidant residual, may lead to the formation of brominated DBPs in the pool waters (Hunter and Jiang 2010). If the filling water contains residual disinfectant, bromide will be present in its oxidised form, bromine, which can react with organic matter in pools. Alternatively, if no residual disinfectant is present in the filling water, the bromide can be oxidised to bromine via chlorine in pools (Hunter and Jiang 2010). For these reasons, the filling water is likely the major source of brominated DBPs in swimming pool waters. As mentioned, neither Pool A nor pool B contained detectable concentrations of DBAAm, DBCAA, TBAA, or BAN, despite their detection in the filling water. Thirteen DBPs (BCAL, BDCAL, DBAL, CH, CAA, CAN, TCAN, BCNM, DCNM, and the four HKs) were detected in at least one of the swimming pools but were not detected in the filling water, and as such, the filling water can be eliminated as a source of these DBPs in the swimming pool waters. The remaining thirteen DBPs were detected in both the filling water and at least one of the swimming pools. Of these, the chlorinated DBPs were generally at much higher concentrations in the pools compared to the filling water and hence the filling water is not considered a major source of these chlorinated DBPs in the pool waters. NPOC concentrations measured prior to opening (~ 16 and 9 mg L−1 for Pools A and B, respectively) were significantly higher than those observed in the filling water (~ 2 mg L−1). While the filling water contributed a small portion of the NPOC measured prior to opening, it is clear that filling water was not the major source of NPOC in these pools. Similarly, the total DBP concentrations measured prior to opening (104 and 2.9 μM for Pools A and B, respectively) were significantly higher (382 and 11 times higher) than those measured in the filing water (0.27 μM). As pools contained DBPs at much higher concentrations than their filling water, it can be concluded that the filling water was generally an insignificant source of DBPs in the investigated pools.
Comparison of the swimming pools
Table 1 summarises the concentrations of DBPs measured in Pools A and B, while Table 2 summarises the contribution of each DBP class to the total DBP concentrations measured (based on molar concentrations), for both Pool A and pool B. Considering average molar concentrations, the different classes of DBPs in order of highest to lowest concentrations were found to be HAAs > HALs > THMs > HANs > HKs > HNMs > HAAms for Pool A and HAAs > HANs > THMs > HALs > HKs > HNMs > HAAms for Pool B. HAAs were also found to be the dominant species by Lee et al. (2010) in their investigation of 30 chlorinated pools (representing 73%), followed by THMs (14%), CH (10%), and HANs (3%), in terms of total average molar concentrations. Although some differences can be observed in the order of dominant DBP classes between Pool A and pool B, these are likely due to a range of factors including bather load, water recirculation, and DBP volatilisation which naturally differ between the pools, also noted by Lee et al. (2010).
On average, total molar DBP concentrations were approximately 23× higher in Pool A compared to Pool B. Excluding the first 2 days where concentrations measured in Pool A were up to 38 and 190 times higher than Pool B, for HKs and THMs, respectively, concentrations of these DBPs were generally similar between the pools. Concentrations of HAAs were roughly one order of magnitude higher in Pool A compared to those observed in Pool B, possibly due to the higher NPOC concentrations measured in Pool A, as HAA formation from organic matter from filling waters and body fluid analogue has been reported (Kanan and Karanfil 2011). HAN concentrations measured in Pool A were approximately double those measured in Pool B, while HALs were approximately 20 times higher in Pool A compared to Pool B. Although similar concentrations were observed in some cases, HNMs measured in Pool A were generally higher (up to 8×) than those measured in Pool B. As discussed below, the higher concentrations of nitrogenous DBPs (N-DBPs) observed in Pool A compared to Pool B are potentially due to higher release of anthropogenic compounds (e.g. sweat) which are nitrogen rich. As HALs have been shown to convert to HAAs (Barrott 2004), HAA precursors may also act as precursors to HALs, which therefore may help explain the higher concentrations of HALs in Pool A compared to Pool B.
While not explicitly investigated in this study, the main anion contributing to conductivity is presumably chloride, which remains after some reactions of chlorine with organic matter. Chloride has been shown to promote the formation of some DBPs, attributed to its effect on oxidant speciation, where a shift from HOCl to the more reactive Cl2 was observed to increase with increasing chloride concentration (E et al. 2016). Consistent with observations of this study, the higher levels of DBPs measured in Pool A compared to those in Pool B may be a reflection of the higher conductivity measured in Pool A compared to Pool B, 1.4 to 4.5 and 0.5 to 2.7 mS cm−1, respectively.
The generally higher concentrations of DBPs observed in Pool A may also result from a range of operational factors including swimming pool size, bather load, swimmer activities, and treatment. Due to its smaller size (Pool A is approximately six times smaller than Pool B when comparing total water volumes), and assuming the number of swimmers and hence bather load inputs are comparable between both pools, a higher concentration of bather-derived inputs would be observed in Pool A, which may lead to higher DBP formation in comparison to ool B. Similarly, the higher operating temperature of Pool A (30 °C) compared to Pool B (27 °C) may have increased the formation of some DBPs, as observed in previous studies (Kanan 2010; Simard et al. 2013). The higher temperature in Pool A may also have slightly increased the release rate of bather load derived precursors (Keuten et al. 2012). The use of UV treatment in Pool A may also be a contributing factor for the higher concentrations observed for some DBPs in pool A, as UV treatment in swimming pools has been shown to increase the formation of DCAN, 1,1,1-TCP, 1,1-DCP, CH, THMs, and TCNM (Cimetiere and De Laat 2014; Hansen et al. 2013b; Spiliotopoulou et al. 2015).
Potential health effects and estimation of cytotoxicity
Although no swimming pool specific guidelines exist for these DBPs, DCAA, TCAA, and CH concentrations were greater than their respective Australian Drinking Water Guideline (ADWG) values (100 μg L−1; (ADWG 2011)) in all samples taken from Pool A, and in 61, 97, and 16% of samples taken from Pool B, respectively. DCAA and TCAA were also measured at concentrations greater than their respective World Health Organisation (WHO) drinking water guidelines (50 and 200 μg L−1 for DCAA and TCAA, respectively; (WHO 2011)) in 100 and 90% of samples taken from Pool A and in 90 and 97% of samples taken from Pool B. Although not detected in all samples, CAA was greater than both the ADWG and WHO values (150 and 20 μg L−1, respectively; (ADWG 2011; WHO 2011)) in 97 and 100% of samples from Pool A, as well as in 20 and 40% of samples from Pool B. Although DCAN is currently not regulated in Australian drinking water, concentrations measured in 48 and 34% of samples taken from Pool A and Pool B, respectively, were higher than the WHO guideline value (20 μg L−1; (WHO 2011)). Total THMs (sum of trichloromethane, bromodichloromethane, dibromochloromethane, and tribromomethane) were the only other DBPs to exceed their ADWG value (250 μg L−1; (ADWG 2011), although this only occurred in Pool A within the first 2 days from opening, where concentrations of 1.7 to 4.4 mg L−1 were measured. However, considering the WHO value (80 μg L−1; (WHO 2011)), total THM concentrations exceeded this drinking water guideline in 48 and 6% of samples taken from Pool A and Pool B, respectively. Germany has imposed a swimming pool specific guideline for total THMs, being 20 μg L−1 as trichloromethane equivalents (Deutsches Institut für Normung e. V. (German Institute for Standardization) 2012). Concentrations of total THMs measured in all samples of Pool A and in 94% of samples from Pool B exceeded this German swimming pool guideline.
As few swimming pool specific guidelines exist and guidelines for drinking waters are unlikely to represent the true risk associated with swimming pools as more than one uptake mechanism is viable in pools, the chronic cytotoxicity of the swimming pool waters was estimated (via calculation, “Cytotoxicity evaluation” section) to indicate the potential health effect of these DBPs at the concentrations measured in this study. Table 2 summarises the contribution of each DBP class to the overall theoretically calculated chronic cytotoxicity.
Excluding the sample prior to opening for Pool B, for all pool samples, the estimated cytotoxicity was significantly higher than that estimated for the filling water, being between 108 to over 46000 and 20 to over 2300 times higher for Pools A and B, respectively. Furthermore, Pool A demonstrated a consistently higher (between 7 and 113 times) level of estimated cytotoxicity than Pool B, when considering total estimated cytotoxicity values.
Compared to all investigated DBP classes, HALs were found to contribute the most to the estimated cytotoxicity in pools, representing up to 98 and > 99% of the total calculated cytotoxicity in some samples of Pools A and B, respectively. This cytotoxicity was found to be predominantly due to CH, which represented over 93% of the estimated cytotoxicity associated with HALs. Considering HALs only represented 0.5 to 62% of the total measured DBP molar concentration, HALs, more specifically CH, were found to pose the highest health risk (in terms of cytotoxicity) in the investigated pools. Furthermore, as CH was below its detection limit (1.0 μg L−1) in all filling water samples, the increase in CH concentration in the pools is likely to account for the significant increase in the estimated cytotoxicity in both pools.
While HAAs represented up to 99% of the total measured DBP concentrations (molar), they were found to only represent a maximum of 24% of the total estimated cytotoxicity in some pool samples. Similarly, while N-DBPs (such as HANs or HNMs) were generally detected at lower concentrations, accounting for up to 23% of total molar concentrations, they were found to represent almost half the estimated cytotoxicity (up to 47% in some samples). Furthermore, while it was observed that THMs accounted for a similar portion of the total measured DBP concentrations (up to 18% in some samples) compared to HANs (up to 23% in some samples), THMs only accounted for less than 0.4% of the estimated cytotoxicity. These observations highlight that DBPs measured at higher concentrations, e.g. HAAs, may not be as significant as those detected in lower concentrations (e.g. HANs), when considering health effects of DBPs in swimming pools.
Trends over time and correlations
General water quality parameters NPOC and TN can be easily used to assess overall trends in swimming pool water quality. Fig. 1 presents the concentrations of NPOC and TN for Pools A and B, both newly built and filled, measured over the duration of this study. NPOC concentrations measured prior to opening were much higher in the pools than in the filling water, being 16, 7, and < 1 mg L−1 for Pool A, pool B, and the filling water, respectively. For Pool A, NPOC concentrations generally increased soon after opening (summer 2015), after which they gradually decreased until approximately day 250 (winter 2016). A gradual increase was then observed for the duration of the study, until summer 2017. These trends in measured NPOC concentrations suggest NPOC follows a seasonal trend in Pool A, that is highest during times of peak periods (summer) and lowest at times of minimal bather loads (winter). While an initial increase (and subsequent maximum) in NPOC concentration was also observed for Pool B (containing cyanuric acid which contributes to the NPOC concentration), after this, NPOC concentrations were found to be relatively constant in Pool B. In a previous 6-month study of a newly re-filled and chlorinated swimming pool, total organic carbon (TOC) was also reported to be fairly uniform (3.1 to 3.9 mg L−1) (Yeh et al. 2014), which the authors attributed to the TOC reaching steady state due to mineralisation of TOC, presumably due to chlorine oxidation, offsetting continual TOC input. Similarly, in a model pool operated under conditions reflective of those of full-scale swimming pools, concentrations of TOC were reported to reach steady state after 200 to 500 h, again attributed to the mineralisation of organic carbon (OC) (Judd and Bullock 2003). Consistent with these previous two studies, the relatively constant NPOC concentrations measured after around 50 days in Pool B are indicative of the NPOC reaching a steady state due to mineralisation (i.e. oxidation) processes balancing the addition of fresh OC. Judd and Bullock (2003) highlighted the importance of the establishment of a steady state balance of mineralisation versus addition of OC in pools to reduce precursors for DBP formation. A steady state for NPOC was not observed in Pool A, possibly due to (a) the higher NPOC concentrations, such that a longer time was required to achieve steady state levels and (b) seasonal trends in NPOC input. Unfortunately, the shorter duration of the Yeh et al. (2014) study (6 months, over summer period) limits observation of any seasonal trends in NPOC concentration that may have been present, as observed in Pool A. The generally lower concentrations of DBPs in Pool B than in Pool A are consistent with the likely establishment of mineralisation balancing addition of OC in Pool B, which was not observed in Pool A.
Concentrations of (a) non-purgeable organic carbon (NPOC) and (b) total nitrogen (TN) measured in Pool A (left) and Pool B (right). Y-axis represents concentration (presented in mg L−1). X-axis represents time (presented in days), where t = 0 represents initial samples collected prior to the opening of the pools
Interestingly, the initial peak in NPOC concentration (16 and 7 mg L−1 in Pools A and B, respectively) in both pools of the current study was not observed in the study by Yeh et al. (2014), where the initial TOC concentration was 3.5 mg L−1. As similar TOC concentrations were measured in the filling water and the pool investigated by Yeh et al. (2014), and with both swimmers and filling water excluded as a major source of NPOC in the current study, these observations suggest that newly built and filled pools may, at least initially, differ significantly in water quality compared to those simply re-filled, presumably as a result of the pool building process and/or new pool infrastructure.
TN may be used in swimming pools as an indication of bather load-derived chemical input, as many bather load compounds are high in nitrogen content (Keuten et al. 2012). In relation to initial concentrations measured prior to opening, TN concentrations gradually increased, with maximum concentrations being observed at day 114 (16 and 21 mg L−1 for Pool A and Pool B (containing cyanuric acid), respectively) after which a gradual decrease (until day 200) was observed. This increase is likely due to the input of swimmers, where perhaps the decrease may be attributed to the TN reaching steady state levels, as observed for NPOC. Fairly constant TN concentrations were measured for the remainder of the study for both pools. Yeh et al. (2014) reported a similar trend for TN, where a somewhat linear increase was observed in the newly re-filled pool of their study, where input of swimmers was reported to be the major source of TN. Unlike the current study, however, no subsequent decrease or constant concentrations of TN were reported, which, as with NPOC, is likely a consequence of the shorter duration of their study.
One key finding of this study is the occurrence of many DBPs at high concentration soon after the opening of the pool facility. Most DBPs showed a significant increase in concentration during these initial days (up to day 52, Fig. 2); however, significant concentrations were also measured prior to opening; hence, bather load is not the only contributor to the observed DBP formation. Using Pool B as an example, in comparison to its concentration measured in the filling water (< 4 μg L−1 (LOD)), higher levels of TCAA were measured both prior to and soon after opening: 345 and 4347 μg L−1 at t = 0 and t = 52 days, respectively. The significantly higher NPOC concentrations measured prior to the opening of the facility (16 and 7 mg L−1 for Pool A and Pool B, respectively), i.e. no contribution from swimmers, compared to that measured in the filling water (< 1 mg L−1) suggest relatively large amounts of potential DBP precursors were present even prior to the opening of the pools. The high levels of NPOC (and subsequent DBPs) observed prior to opening (and their subsequent increase soon after) appear to have been generated from the pool building process and/or new pool infrastructure.
Concentration of (a) haloketones (HKs), (b) trihalomethanes (THMs), (c)haloacetic acids (HAAs), (d) haloacetaldehydes (HALs), (e) haloacetonitriles (HANs), and (f) halonitromethanes (HNMs) measured in Pool A (left) and Pool B (right). Data is presented as total concentration by DBP class. Y-axis represents concentration (μM) and, where required (Figures (a) and (b) for Pool A), a secondary y-axis with a scale change has been included with its data represented by a triangle. X-axis represents time (presented in days), where t = 0 represents initial samples collected prior to the opening of the pools
Spearman’s rank coefficients (summarised in Tables SI 5 and 6) were used to assess any correlation(s) that exists between (i) the 39 measured DBPs (on a molar basis) and (ii) the measured general water quality parameters, for each of the investigated pools. Parameters that resulted in a rank coefficient between 0.01 and 0.05 (0.01 < p < 0.05) were said to exhibit a moderate significant correlation, while parameters resulting in a rank coefficient of 0.01 or less (p < 0.01) were said to have a significantly strong correlation.
While some correlations differed between Pool A and Pool B, correlations between several parameters and/or DBPs were observed for both pools. Residual free chlorine equivalent concentrations were also found to be negatively weakly correlated with HANs in Pool A (r = − 0.44), which is consistent with the findings of Yu and Reckhow (2015), where the instability of HANs in waters containing residual hypochlorite was demonstrated. This negatively weak correlation differs to other studies where no significant correlation (Lee et al. 2010), and a significant positive correlation (Yang et al. 2018), between HANs and free residual chlorine was reported. No other DBP class was found to correlate with either free or total chlorine equivalent residual concentrations in either pool, which is consistent with Lee et al. (2010) who, in addition to HANs, reported no correlation was observed between chlorine residual concentrations and the concentrations of THMs, HAAs, or CH in their studied pools. While Zhang et al. (2015) reported no correlation between chlorine residual concentrations and CH in their study of 14 swimming pools, a correlation was reported for chlorine residual concentrations and both HAAs and THMs (r = 0.31 to 0.40), with THMs and TCNM also previously reported to be positively correlated to free chlorine residual concentrations (Yang et al. 2018). Zhang et al. (2015) attributed these unusual correlations to the level of chlorine concentrations measured, suggesting that correlations between chlorine concentrations and DBPs are dependent on the residual concentration employed. Consistent with correlations being suggested to be more evident for pools where lower residuals are employed (< 2.2 mg L−1; (Yang et al. 2018; Zhang et al. 2015)), correlations between chlorine residual concentration and levels of DBPs were not observed for the pools of the current study as relatively high chlorine residuals were employed (target free chlorine residuals of 2.5 to 3.5 mg L−1). Both free and total chlorine equivalent concentrations were however found to weakly correlate with conductivity in Pool A (r = 0.42 to 0.46), suggesting that residual chloride, remaining after some reactions of chlorine with organic matter, is likely responsible for much of the conductivity measured. Conductivity (presumably mostly chloride) was also found to be moderately to strongly correlated with TN and HKs for both pools (r = 0.53 to 0.97) and weakly correlated with THMs in Pool B (r = 0.46). While HKs and TN were not target parameters of their study, E et al. (2016) also reported a significant relationship between chloride and several DBPs (e.g. trichloromethane and DCAN; r = 0.62 to 0.98), in both bench scale studies and swimming pool waters. As chlorine is continually added into the pools, the chloride concentration increases and more DBPs are formed, likely resulting in the apparent correlation between conductivity and some DBP concentrations.
NPOC concentrations were found to weakly correlate with HANs in both pools (r = 0.45 to 0.46) and moderately with HALs in Pool A (r = 0.51), although no other correlations of NPOC with any other DBP class were observed. While Lee et al. (2010) also reported TOC to be correlated to CH (the monohydrate of trichloroacetaldehyde; r = 0.68), HANs and TOC were not found to correlate in their study of chlorinated pools. No correlation between HANs and TOC, nor THMs or TCNM and TOC, were reported by Yang et al. (2018) in their recent study of 35 outdoor chlorinated pools. Zhang et al. (2015) reported no correlation to exist between TOC and CH in their study of 14 chlorinated pools, which is consistent with observations with Pool B but opposite to those for Pool A in the current study. Zhang et al. (2015) did, however, report no correlation between TOC and either THMs or HAAs, consistent with the current study. Furthermore, consistent with this study is the correlation between HANs and UV254 (an indicator of TOC) observed by Hang et al. (2016), although unlike this study, a correlation between THMs was also reported. THMs have also been reported to be correlated with either dissolved organic carbon (DOC) or TOC (Chu and Nieuwenhuijsen 2002; Peng et al. 2015), although Peng et al. (2015) report a time delay of 2 days.
TN concentrations were found to be moderately correlated to HAAs in both pools (r = 0.49 to 0.64), which is likely why a weak to moderate correlation between TN and total DBPs was also observed (r = 0.38 to 0.66) in these pools, as HAAs accounted for a significant portion of the total DBP molar concentrations (34 to 99%) across both pools. While no correlation between TN and THMs was observed by Yang et al. (2018) in their study of 35 outdoor chlorinated pools, and although only observed for Pool B, TN concentrations were found to strongly correlate with HKs, HALs, and THMs in the current study. These correlations observed in the current study suggest that nitrogenous compounds may act as precursors to these DBPs, although due to conflicting previously published results, further investigations under more controlled conditions (e.g. bench scale) are required to confirm these correlations. A weak correlation between TN concentrations and number of pool entries (r = 0.45) was also only observed in Pool B, which is likely due to the release of nitrogen-rich compounds from swimmers (Keuten et al. 2012, 2014).
A number of pool entries were found to weakly to moderately correlate with HKs, HALs, and NPOC in Pool A (r = 0.40 to 0.61), and weakly to moderately correlate with THMs and TN in Pool B (r = 0.55 and 0.45, respectively); however, no significant correlation between number of pool entries and either HAAs, HNMs, or HANs was observed in either pool. While a correlation (r = 0.50) between TOC and number of swimmers was reported by Chu and Nieuwenhuijsen (2002), no significant correlation was observed between number of swimmers and DOC concentrations in other studies (Hang et al. 2016; Peng et al. 2015), with the differing observations also seen between the two pools of this study. Similarly, while both HAAs and THMs have been correlated (r = 0.70 to 0.72) to number of swimmers (Chowdhury et al. 2016; Chu and Nieuwenhuijsen 2002), negative or no significant correlations have also been reported (Chowdhury et al. 2016; Hang et al. 2016; Peng et al. 2015). Consistent with the current study, Hang et al. (2016) reported no significant correlation between number of swimmers with HNMs and HANs; however, no significant correlation with number of swimmers and both HAAs, HKs, and HALs was also reported. These reports are neither in agreement nor disagreement with the current study, as these correlations were observed in one of the pools, while being absent in the other. The differing correlations reported suggest that the number of swimmers may not be a reliable indication of DBP levels in pools, although swimmers habits (e.g. pre-swim showering or urinating while swimming) and their activity (e.g. water agitation and splashing) have been demonstrated to have a significant impact on DBPs in pools, as discussed in more detail elsewhere (e.g. Carter and Joll 2017).
A weak negative correlation between pH and number of pool entries was observed in Pool A (r = − 0.43), which can potentially be explained by the likely release of bodily fluids (e.g. sweat and urine), which are generally acidic, pH 4.5 to 7 (Rose et al. 2015). This release is presumably higher for Pool A (compared to Pool B) as Pool A is designed for use by children and babies. HANs were found to be weakly and negatively correlated with pH in Pool A (r = − 0.42), which is consistent with several other studies (Lee et al. 2010; Yang et al. 2018) who also reported a negative correlation between pH and HANs, which is presumably due to a higher pH supressing their formation (Hansen et al. 2013a). Although not observed in the current study and an opposite observation was reported by Kanan (2010), a negative correlation between TCNM and pH was reported by Yang et al. (2018), which the authors attribute to the more complicated precursors that exist in real swimming pools. No other significant correlations between pH and other DBP classes were observed in this study. This is consistent with other studies (Chu and Nieuwenhuijsen 2002; Lee et al. 2010), where no significant correlation between pH and THMs, HAAs, CH, or TOC were also reported, with Yang et al. (2018) also reporting no correlation to exist between THMs and pH in their study of chlorinated pools.
Water temperature was weak to moderately correlated with HANs for both pools (r = 0.38 to 0.52) and weakly with both HKs and HALs in Pool B (r = 0.41 to 0.43). These correlations suggest higher temperatures lead to an increase in the formation of these DBPs, as reported for THMs and HAAs (Kanan and Karanfil 2011). The absence of any correlation between THMs and water temperature, particularly for Pool A, is potentially, in part, due to the operating temperatures of the pools (24 to 32 °C). While the higher operating temperatures likely increased the formation rate of THMs (as reported by Kanan and Karanfil (2011)), they likely also increased their volatilisation, resulting in an overall decrease in THM concentrations in the pools. The loss of volatile DBPs is supported further as a weak negative correlation between total molar DBP concentrations and temperature in Pool A was also observed (r = − 0.36). Furthermore, the relatively high operating temperature of Pool A (30 °C) is likely to promote the release of sweat from swimmers, which in addition to supporting the negative correlation observed with pH and number of pool entries, is consistent with the weak correlation between temperature and TN concentration observed in Pool A (r = 0.46). While not observed in this study, THMs were found to correlate with temperature (r = 0.50) in a study of chlorinated pools (Chu and Nieuwenhuijsen 2002), although as summarised by Carter and Joll (2017), it has been suggested that THM correlations in general are highly dependent on both swimmer activity and water agitation, both of which affect THM volatilisation (hence THM water concentrations) and inherently any correlation with other parameters. Chu and Nieuwenhuijsen (2002) did, however, report a correlation between temperature and TOC (r = 0.40), for which a moderate correlation was also observed for Pool B in the current study (r = 0.53).
As expected, due to their high dominance in each pool, HAAs were found to be strongly significantly correlated (r = 0.94 to 0.98) to the total DBP concentrations for both pools, which is consistent with other studies (Hang et al. 2016; Lee et al. 2010). Furthermore, the dominance of both HALs and HANs in Pool B (each up to 23% of the total molar DBP concentration) is likely the reason they were observed to weakly to moderately correlated to the total DBP concentrations measured in Pool B (r = 0.43 to 0.59). While some results differ for each pool in the current study, Lee et al. (2010) also reported correlations between both THMs and CH to total DBP concentrations (r = 0.51 to 0.58), although HANs were not found to be correlated.
Between the investigated DBP classes, only HANs and HALs were found to be correlated to one another for both pools, where a significantly strong correlation (r = 0.68 to 0.71) was observed. These results are similar to those of Lee et al. (2010), where a significant correlation (r = 0.67) between HANs and CH was reported in their study of 30 chlorinated pools. This correlation is potentially a result of the formation of nitriles as transformation products of aldehydes, via reactions involving monochloramine and two intermediate species, N-chloramino alcohols and N-chloraldimines, as we have previously demonstrated for valine in model compound studies (How et al. 2017). The only other correlation observed in Pool A was that between THMs and HNMs (r = 0.49), likely a consequence of their similar chemical structures and hence similar precursors, with the lower HNM concentrations measured in Pool B a possible reason for the absence of this correlation in Pool B. Yang et al. (2018) also reported a correlation (r = 0.76) between THMs and TCNM in their study of 35 chlorinated pools; however, unlike the current study, correlations between THMs and HANs, as well as between HANs and TCNM, were also reported. Although only observed for Pool B, a moderate to strong correlation was observed between HKs and HANs, HALs and THMs (r = 0.62, 0.76, and 0.58, respectively). Hang et al. (2016) also reported a correlation between HKs and THMs in their study of 13 chlorinated pools, although no correlation between HKs and HANs was observed.
The observed DBP correlations in the current study suggest a potential relationship between HANs, HKs, HALs, and THMs. Methyl ketones (i.e. the HKs investigated in this study) can be converted to THMs via the haloform reaction, supporting the observed correlation between HKs and THMs. Although not a direct decomposition product (it is suggested to be a result of a secondary reaction when organic matter is present), CH (i.e. a HAL) has been observed as a result of the decomposition of 1,1,1-TCP (i.e. a HK) (Nikolaou et al. 2001). While this study was limited to the relationship between CH and 1,1,1-TCP, it is reasonable to suggest that a similar relationship may exist between other HALs and their corresponding HKs, supporting the observed correlation between HKs and HALs in the current study. How et al. (2018) provide a multi-pathway reaction scheme for the formation of several DBPs from the chlorination of amino acids, summarising and linking the findings of several earlier studies (How et al. 2017; Kimura et al. 2015; Ueno et al. 1996; Yu and Reckhow 2015). Here, aldehydes have been shown to be transformed to their corresponding nitriles via several reaction steps involving monochloramine. Although demonstrated for isobutyraldehyde (a chlorination by-product of valine) by detection of several of its corresponding transformation products (e.g.1-(chloroamino)-2-methylpropan-1-ol, N-chloroisobutyraldimine, and isobutyronitrile) (How et al. 2017), this pathway may be applicable to other compounds, e.g. HALs such as CH. The potential conversion of HKs to their corresponding HANs via the pathways proposed by Nikolaou et al. (2001) and How et al. (2017) supports the correlation observed between HKs and HANs in the current study.
No further significant correlations were observed between any of the investigated DBP classes in either of the pools investigated, which is consistent with most observations of Lee et al. (2010), who reported no significant correlations existed between HANs and either HAAs or THMs, or between CH and HAAs. In contrast to the current study, Lee et al. (2010) reported correlations to exist between THMs and both HAAs and CH (r = 0.49 and 0.42, respectively), as did Zhang et al. (2015), r = 0.35 to 0.55, who also reported a correlation to exist between HAAs and CH (r = 0.42). As discussed, the absence of correlations between THMs and other DBP classes may be due to the volatilisation of THMs, as suggested to occur for other volatile DBPs (e.g. Schmalz et al. 2011; Zwiener and Schmalz 2015), likely to be more pronounced in pool A due to the higher operating temperature and/or the higher splashing potential (leisure pool).
Conclusions
This study is the first investigation of the water quality and occurrence of DBPs in newly built and filled swimming pools, where investigations occurred for 500 days and began prior to the opening of the facility. A range of DBPs (THMs, HAAs, HANs, HNMs, HKs, and HALs) was detected throughout the duration of the study, where many of the DBPs were generally measured at higher concentrations than previously reported for chlorinated swimming pools. The maximum concentrations of CAA, DCAA, TCAA, and CH were significantly greater than any previously reported concentrations. HAAs were the dominant class (based on molar concentrations) for both pools, followed by HALs, THMs, HANs, HKs, and HNMs for Pool A, and by HANs, THMs, HALs, HKs, and HNMs for Pool B. HAAms were not detected in either pool. This study is the first known quantification of four DBPs (BCAL, BDCAL, BCNM, and DCNM) in swimming pools.
Considering total molar concentrations, on average, Pool A contained 23× higher levels of DBPs compared to Pool B, with both pools found to contain significantly higher total molar concentrations than their filling water. In most cases, similar concentrations of THMs and HKs were found in both pools, although HANs, HNMs, HAAs, and HALs were generally higher (on average 2, 8, 10, and 20 times higher, respectively) in Pool A compared to Pool B. These differences are likely due to the NPOC concentration measured prior to opening, the potential organic input from bather load, as well as operational parameters such as water temperature and chlorine residual, all of which were higher in Pool A compared to Pool B. The lower concentrations of DBPs in Pool B, where a steady state NPOC concentration was achieved, highlight the importance of the establishment of this steady state balance of mineralisation versus addition of OC to reduce precursors for DBP formation.
Filling waters were found to be the major source of brominated DBPs in the pools, but were an insignificant source of other DBPs, NPOC, and TN, while swimmers were found to be the major source of TN in the pools. Significant concentrations of NPOC were measured prior to opening. Furthermore, compared to the filling water, a significant concentration of DBPs was measured in both pools prior to opening, suggesting that DBP precursors (encompassed in NPOC concentrations) existed prior to the opening of the facility. Almost all DBPs and NPOC significantly increased soon after opening, where maximum concentrations were generally observed at approximately 50 days after opening. The pool building process and/or new pool infrastructure appears to have had a major impact on the chemical water quality of the pools, particularly with regard to the significant concentrations of NPOC and DBPs prior to, and after, opening of the facility.
Pool A exhibited higher estimated cytotoxicity compared to Pool B and, in almost all cases, pool water samples exhibited higher cytotoxicity than their filling water. HALs were found to contribute the most to the total estimated cytotoxicity, predominantly due to CH. With correlations between number of pool entries and HALs also observed, findings suggest that swimmers may be a potential source of HAL precursors and in turn may have significant impact on the cytotoxicity of pool waters. While HAAs were found to contribute significantly to the total molar DBP concentrations, they only accounted for up to 24% of the total estimated cytotoxicity. Furthermore, other DBP classes (e.g. N-DBPs), while measured at lower concentrations, were found to account for almost half the total estimated cytotoxicity. These observations highlight that the predominant DBPs (e.g. HAAs or THMs) are not necessarily the significant DBPs in terms of potential health effects from swimming pools.
References
ADWG (2011) Australian Drinking Water Guidelines 6: Version 3.1
Allard S, Charrois JWA, Joll CA, Heitz A (2012) Simultaneous analysis of 10 trihalomethanes at nanogram per liter levels in later using solid-phase microextraction and gas chromatography mass-spectrometry. J Chromatogr A 1238:15–21
Barrott L (2004) Chloral hydrate: formation and removal by drinking water treatment. J Water Supply Res Technol AQUA 53:381–390
Berg M, Müller SR, Mühlemann J, Wiedmer A, Schwarzenbach RP (2000) Concentrations and mass fluxes of chloroacetic acids and trifluoroacetic acid in rain and natural waters in Switzerland. Environ Sci Technol 34:2675–2683
Cardador MJ, Gallego M (2011) Haloacetic acids in swimming pools: swimmer and worker exposure. Environ Sci Technol 45:5783–5790
Carter RAA, Joll CA (2017) Occurrence and formation of disinfection by-products in the swimming pool environment: a critical review. J Environ Sci 58:19–50
Carter RAA, Linge KL, Heitz A, Liew DS, Allard S, Joll CA (2015) Disinfection by-products: not just an issue for drinking water, but also potentially for swimming pool waters. Water 42:82–87
Carter RAA, Liew DS, West N, Heitz A, Joll CA (2019) Simultaneous analysis of haloacetonitriles, haloacetamides and halonitromethanes in chlorinated waters by gas chromatography-mass spectrometry. Chemosphere 220:314–323
Chowdhury S, Mazumder AJ, Husain T (2016) Predicting bromide incorporation in a chlorinated indoor swimming pool. Environ Sci Pollut Res 23:12174–12184
Chu H, Nieuwenhuijsen MJ (2002) Distribution and determinants of trihalomethane concentrations in indoor swimming pools. Occup Environ Med 59:243–247
Chu W h, Gao N y, Deng Y (2010) Formation of haloacetamides during chlorination of dissolved organic nitrogen aspartic acid. J Hazard Mater 173:82–86
Cimetiere N, De Laat J (2014) Effects of UV-dechloramination of swimming pool water on the formation of disinfection by-products: a lab-scale study. Microchem J 112:34–41
Daiber EJ, DeMarini DM, Ravuri SA, Liberatore HK, Cuthbertson AA, Thompson-Klemish A, Byer JD, Schmid JE, Afifi MZ, Blatchley ER, Richardson SD (2016) Progressive increase in disinfection byproducts and mutagenicity from source to tap to swimming pool and spa water: impact of human inputs. Environ Sci Technol 50:6652–6662
Dehghani MH, Farhang M, Zarei A (2018) Data on the level of haloacetic acids in indoor swimming pools of Iran: a case study of Tehran. Data Brief 19:326–330
Deutsches Institut für Normung e. V. (German Institute for Standardization) (2012) Treatment of water of swimming pools and baths - Part 1: General requirements: DIN 19643-1 (English translation of DIN 19643-1:2012-11)
E Y, Bai H, Lian L, Li J, Blatchley ER (2016) Effect of chloride on the formation of volatile disinfection byproducts in chlorinated swimming pools. Water Res 105:413–420
Font-Ribera L, Kogevinas M, Schmalz C, Zwiener C, Marco E, Grimalt JO, Liu J, Zhang X, Mitch W, Critelli R, Naccarati A, Heederik D, Spithoven J, Arjona L, de Bont J, Gracia-Lavedan E, Villanueva CM (2016) Environmental and personal determinants of the uptake of disinfection by-products during swimming. Environ Res 149:206–215
Gérardin F, Cloteaux A, Midoux N (2015) Modeling of variations in nitrogen trichloride concentration over time in swimming pool water. Process Saf Environ Prot 94:452–462
Glezer V, Harris B, Tal N, Iosefzon B, Lev O (1999) Hydrolysis of haloacetonitriles: linear free energy relationship, kinetics and products. Water Res 33:1938–1948
Golfinopoulos S (2000) Volatile halogenated organics in swimming pools. Toxicol Environ Chem 76:219–228
Hang C, Zhang B, Gong T, Xian Q (2016) Occurrence and health risk assessment of halogenated disinfection byproducts in indoor swimming pool water. Sci Total Environ 543:425–431
Hansen KMS, Albrechtsen HJ, Andersen HR (2013a) Optimal pH in chlorinated swimming pools - balancing formation of by-products. J Water Health 11:465–472
Hansen KMS, Zortea R, Piketty A, Vega SR, Andersen HR (2013b) Photolytic removal of DBPs by medium pressure UV in swimming pool water. Sci Total Environ 443:850–856
How ZT, Linge KL, Busetti F, Joll CA (2017) Chlorination of amino acids: reaction pathways and reaction rates. Environ Sci Technol 51:4870–4876
How ZT, Linge KL, Busetti F, Joll CA (2018) Formation of odorous and hazardous by-products from the chlorination of amino acids. Water Res 146:10–18
Hunter GL, Jiang H (2010) Bromine, bromine chloride, BCDMH, and iodine. In: White’s handbook of chlorination and alternative disinfectants. John Wiley & Sons, Inc., pp 848–892
Judd SJ, Black SH (2000) Disinfection by-product formation in swimming pool waters: a simple mass balance. Water Res 34:1611–1619
Judd SJ, Bullock G (2003) The fate of chlorine and organic materials in swimming pools. Chemosphere 51:869–879
Kanan A (2010) Occurrence and formation of disinfection by-products in indoor swimming pools water (Doctral Thesis)
Kanan A, Karanfil T (2011) Formation of disinfection by-products in indoor swimming pool water: the contribution from filling water natural organic matter and swimmer body fluids. Water Res 45:926–932
Kelsall H, Sim M (2001) Skin irritation in users of brominated pools. Int J Environ Health Res 11:29–40
Keuten MGA, Schets FM, Schijven JF, Verberk JQJC, van Dijk JC (2012) Definition and quantification of initial anthropogenic pollutant release in swimming pools. Water Res 46:3682–3692
Keuten MGA, Peters MCFM, Daanen HAM, De Kreuk MK, Rietveld LC, van Dijk JC (2014) Quantification of continual anthropogenic pollutants released in swimming pools. Water Res 53:259–270
Kim H, Shim J, Lee S (2002) Formation of disinfection by-products in chlorinated swimming pool water. Chemosphere 46:123–130
Kimura SY, Vu TN, Komaki Y, Plewa MJ, Mariñas BJ (2015) Acetonitrile and N-chloroacetamide formation from the reaction of acetaldehyde and monochloramine. Environ Sci Technol 49:9954–9963
Kristensen GH, Klausen MM, Hansen VA, Lauritsen FR (2010) On-line monitoring of the dynamics of trihalomethane concentrations in a warm public swimming pool using an unsupervised membrane inlet mass spectrometry system with off-site real-time surveillance. Rapid Commun Mass Spectrom 24:30–34
Lahl U, Bätjer K, Düszeln JV, Gabel B, Stachel B, Thiemann W (1981) Distribution and balance of volatile halogenated hydrocarbons in the water and air of covered swimming pools using chlorine for water disinfection. Water Res 15:803–814
Lee J, Jun MJ, Lee MH, Lee MH, Eom SW, Zoh KD (2010) Production of various disinfection byproducts in indoor swimming pool waters treated with different disinfection methods. Int J Hyg Environ Health 213:465–474
Liew D, Linge KL, Joll CA, Heitz A, Charrois JWA (2012) Determination of halonitromethanes and haloacetamides: an evaluation of sample preservation and analyte stability in drinking water. J Chromatogr A 1241:117–122
Loos R, Barceló D (2001) Determination of haloacetic acids in aqueous environments by solid-phase extraction followed by ion-pair liquid chromatography–electrospray ionization mass spectrometric detection. J Chromatogr A 938:45–55
Manasfi T, De Méo M, Coulomb B, Di Giorgio C, Boudenne JL (2016) Identification of disinfection by-products in freshwater and seawater swimming pools and evaluation of genotoxicity. Environ Int 88:94–102
Nikolaou AD, Lekkas TD, Kostopoulou MN, Golfinopoulos SK (2001) Investigation of the behaviour of haloketones in water samples. Chemosphere 44:907–912
Peng D, Saravia F, Abbt-Braun G, Horn H (2015) Occurrence and simulation of trihalomethanes in swimming pool water: a simple prediction method based on DOC and mass balance. Water Res 88:634–642
Plewa MJ, Richardson SD (2017) Disinfection by-products in drinking water, recycled water and wastewater: formation, detection, toxicity and health effects: Preface. J Environ Sci 58:1
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636(636):178–242
Richardson SD, DeMarini DM, Kogevinas M, Fernandez P, Marco E, Lourencetti C, Balleste C, Heederik D, Meliefste K, McKague AB, Marcos R, Font-Ribera L, Grimalt JO, Villanueva CM (2010) What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118:1523–1530
Rose C, Parker A, Jefferson B, Cartmell E (2015) The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol 45:1827–1879
Sa CSA, Boaventura RAR, Pereira IMB (2012) Analysis of haloacetic acids in water and air (aerosols) from indoor swimming pools using HS-SPME/GC/ECD. J Environ Sci Heal A 47:176–183
Schmalz C, Frimmel FH, Zwiener C (2011) Trichloramine in swimming pools - formation and mass transfer. Water Res 45:2681–2690
Serrano M, Silva M, Gallego M (2011) Micro liquid–liquid extraction combined with large-volume injection gas chromatography–mass spectrometry for the determination of haloacetaldehydes in treated water. J Chromatogr A 1218:8295–8302
Simard S, Tardif R, Rodriguez MJ (2013) Variability of chlorination by-product occurrence in water of indoor and outdoor swimming pools. Water Res 47:1763–1772
Spiliotopoulou A, Hansen KMS, Andersen HR (2015) Secondary formation of disinfection by-products by UV treatment of swimming pool water. Sci Total Environ 520:96–105
Standards Australia (2002) HB 241-2002: water management for public swimming pools and spas, 2nd edn. Standards Australia International
Tardif R, Catto C, Haddad S, Rodriguez M (2015) Studies and research projects assessment of worker exposure to disinfection byproducts at indoor swimming pools in Québec, Quebec
Tardif R, Rodriguez M, Catto C, Charest-Tardif G, Simard S (2016) Concentrations of disinfection by-products in swimming pool following modifications of the water treatment process: an exploratory study. J Environ Sci (China) 58:163–172
Teo TLL, Coleman HM, Khan SJ (2016a) Presence and select determinants of organophosphate flame retardants in public swimming pools. Sci Total Environ 569–570:469–475
Teo TLL, Coleman HM, Khan SJ (2016b) Occurrence and daily variability of pharmaceuticals and personal care products in swimming pools. Environ Sci Pollut Res 23:6972–6981
Ueno H, Moto T, Sayato Y, Nakamuro K (1996) Disinfection by-products in the chlorination of organic nitrogen compounds: by-products from kynurenine. Chemosphere 33:1425–1433
US EPA (1995) Method 551.1: determination of chlorination disinfection byproducts, chlorinated solvents , and halogenated pesticides/ herbicides in drinking water by liquid-liquid extraction and gas chromatography with electron-capture detection
US EPA (2003) Method 552.3: Determination of haloacetic acids and dalapon in drinking water by liquid-liquid microextraction, derivatization, and gas chromatography with electron capture device
Wagner ED, Plewa MJ (2017) CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: an updated review. J Environ Sci 58:64–76
Wang Z (2011) Body fluid analogues and personal care products as potential DBP precursors (Masters Thesis). University of Toronto (Canada), Ann Arbor
Weng SC, Blatchley ER (2011) Disinfection by-product dynamics in a chlorinated, indoor swimming pool under conditions of heavy use: national swimming competition. Water Res 45:5241–5248
WHO (2011) Guidelines for drinking water quality, 4
Yang F, Yang Z, Li H, Jia F, Yang Y (2018) Occurrence and factors affecting the formation of trihalomethanes, haloacetonitriles and halonitromethanes in outdoor swimming pools treated with trichloroisocyanuric acid. Environ Sci Water Res Technol 4:218–225
Yeh RYL, Farré MJ, Stalter D, Tang JYM, Molendijk J, Escher BI (2014) Bioanalytical and chemical evaluation of disinfection by-products in swimming pool water. Water Res 59:172–184
Yu Y, Reckhow DA (2015) Kinetic analysis of haloacetonitrile stability in drinking waters. Environ Sci Technol:150814123802000
Zare Afifi M, Blatchley ER (2015) Seasonal dynamics of water and air chemistry in an indoor chlorinated swimming pool. Water Res 68C:771–783
Zhang X, Minear RA (2002) Decomposition of trihaloacetic acids and formation of the corresponding trihalomethanes in drinking water. Water Res 36:3665–3673
Zhang X, Yang H, Wang X, Zhao Y, Wang X, Xie Y (2015) Concentration levels of disinfection by-products in 14 swimming pools of China. Front Environ Sci Eng 9:995–1003
Zwiener C, Schmalz C (2015) Ion mobility spectrometry to monitor trichloramine in indoor pool air. In: Karanfil T, Mitch B, Westerhoff P, Xie Y (eds) Recent advances in disinfection by-products. American Chemical Society, pp 431–446
Acknowledgements
This work was supported by Water Research Australia, ChemCentre, Curtin University and the Australian Government (through a Research Training Program Stipend Scholarship to R. Carter). The authors would like to thank the Department of Health (Western Australia) and the local pool operators for their assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 180 kb)
Rights and permissions
About this article
Cite this article
Carter, R.A.A., Allard, S., Croué, JP. et al. 500 days of swimmers: the chemical water quality of swimming pool waters from the beginning. Environ Sci Pollut Res 26, 29110–29126 (2019). https://doi.org/10.1007/s11356-019-05861-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05861-0