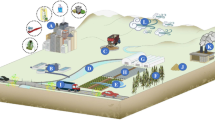
Abstract
Soil and water are two important basic ecosystems for the survival of different organisms. The excessive microplastic pollutants in soil have been directly discharged into the terrestrial ecosystems. Microplastic pollutants (MPs) constitute a ubiquitous global menace due to their durability, flexibility, and tough nature. MPs posed threat to the sustainability of the ecosystem due to their small size and easy transportation via ecological series resulting in the accumulation of MPs in aquatic and terrestrial ecosystems. After being emitted into the terrestrial ecosystem, the MPs might be aged by oxidative degeneration (photo/thermal), reprecipitation (bioturbation), and hetero-accumulation. The mechanism of adsorption, degradation, and breakdown of MPs into unaffected plastic debris is accomplished by using several biological, physical, and chemical strategies. This review presents the importance of ecosystems, occurrence and sources of MPs, its toxicity, and the alteration in the ecology of the ecosystems. The inhibitory impact of MPs on the ecosystems also documents to unveil the ecological hazards of MPs. Further research is required to study the immobilization and recovery efficiency of MPs on a larger scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics (MPs) are solid, synthetic plastic particles that are insoluble in water. They are irregular in form and range in size from 1 μm to 5 mm (Frias and Nash 2019). Food and water have been shown to contain microplastics in the natural environment. Additionally, they are found in human faeces (Mintenig et al. 2019; Schwabl et al. 2019). Globally, the production of plastic was more than 426 MMT (million metric tons) including resins (359 MT), and synthetic fibers (67 MT), according to the PlasticsEurope and The Fiber Year, respectively. The production of plastic is likely to increase in the future to sustain the living standards of the world’s population. Though, approx. 85% of these plastics products are not reprocessed and flow into the ecosystems. Small MPs (< 5 mm) have turned out to be an immense problem illustrating universal alarm as they adsorb contaminants or other organic substances on their surface. MPs could also be consumed and amassed in the food chain by biota resulting in direct exposure of MPs-contaminated ecosystems to humans and threatening worldwide biodiversity.
Terrestrial soil serves as a significant microplastics reservoir (Rillig 2020). After being introduced into the soil, microplastics have the potential to remain there and accumulate, ultimately having an impact on the development, reproduction, and overall biodiversity of soil organisms (Chae and An 2018; de Souza Machado et al. 2018a). The MPs caused a deleterious impact on the terrestrial ecosystem at a physical, cellular, and molecular level. MPs affected the soil aeration and porosity, alter microbial populations, and may result in reduced soil fertility, which can affect agricultural seed germination and seedling growth. Soil acts as a sink for MPs and also enables its transport from one site to groundwater systems through numerous methods such as agricultural practices, soil erosion, surface runoff, and waterlogging further disturbing the groundwater levels and complete aquatic system (Nizzetto et al. 2016a; Rillig et al. 2017a; He et al. 2018a; Wong et al. 2020; Yao et al. 2020). The variation in the MP characteristics in terms of shape, size, type, charge, specificity, density, surface chemistry, and many other environmental attributes have been notified to directly impact their transport as well as the distribution within soils (Zhang et al. 2019). Moreover, the horizontal transfer of microplastics within the soil is impacted by various soil activities, microbiota, and soil physicochemical properties such as soil aggregates and soil pores (Rillig et al. 2017b; Chae and An 2018). Wu et al. (2020) depicted the behavioral transport of polystyrene microspheres in three categories of soils and observed that MPs declined with the presence of higher soil minerals such as Fe/Al oxides. This is mainly attributed to electrostatic interactions among negatively charged MPs and positively charged oxides present in the soil. The hetero-aggregates formed with soil mineral particles and organic matter also induce the transport of MPs.
In aquatic ecosystems, microplastics are reported to be present as suspended sediments in the water column, in sediments, or floating on water surfaces, majorly synthetic plastics that contaminate the aquatic environment include low- and high-density polyethylene (PE), polystyrene (PS), polyethylene terephthalate (PET), polypropylene (PP), and polyvinyl chloride (PVC) (da Costa et al. 2016). The direct ingestion of nanosized and microplastic particles results in physical damage to feeding structures, the digestive tract, and concerned organs (Harmon et al. 2018).
Ubiquitous and highly resistant nature of microplastics (MPs) makes them difficult to eliminate from the environment. Almost all the water bodies have been polluted by MPs, so to regulate the transport of MPs in water some methods should be applied for the degradation of MPs and combat the pollution. The rate of MPs degradation depends upon the type of polymer produced. Polystyrene (EPS) particles are quickly degraded into smaller fragments by mechanical abrasion but polyethylene (PE) and polypropylene (PP) are not completely degraded by this process (Song et al. 2017). In biological methods, the potential organisms are introduced to degrade and eliminate the MPs in the aquatic ecosystems. In a majority of experiments, certain microbial populations are implemented to neutralize these MPs (Harrison et al. 2011). These microbes are capable to disintegrate the complex MPs polymers into monomeric constituents. Aerobic microbe’s degradation actions result in water and CO2 while anaerobic forms result in water, CO2, H2S, and methane as byproducts (Chandra and Singh 2020). The immobilization and degradation of MPs in terrestrial ecosystems was done by using biological, coagulation, agglomeration, and nanomaterials. Corona et al. (2020) evaluated the potential of mushroom coral isolated from Magoodhoo, Maldives, to minimize the pollutants associated with bio-fueled plastics and are found to eliminate 97% of the particles of nearly 200–1000 μm in size in the laboratory environment. Also, scientists are working on the isolation and identification of multiple microorganisms associated with MPs hydrolyzing enzymes, mostly the depolymerases which are effective in the breakdown of MPs are yet to be identified (Wei and Zimmermann 2017). Whereas, other methods adopted by Sturm et al. (2020), follow the step systematic processes of adsorption, agglomeration, and finally filtration was implemented to remove MPs. This method undergoes the application of alkyl trichloro silanes (linear and branched) which efficiently remove the MPs like polypropylene, low and high density- polyethylene, etc. Also, the carbon nanotubes with magnetic potentials show 100% efficiency in removing certain microplastics under marine ecosystems. These tubes effectively adsorb materials like PET, polyamide, and PE and these tubes are recyclable.
Microplastic sources, pathways, and their fate
Multiple sources are accountable for the discharge of microplastics into the environment. Included among these sources are individuals, transportation, and industries. Sources of microplastics are listed in detail in Table 1. When released, microplastics enter the ocean via direct discharge or river transport systems. After release, they accumulate, degrade, and move through the environment, eventually entering the human body by ingestion, inhalation, and skin contact (Prata et al. 2020). Microplastics are introduced into the environment in two distinct forms: primary and secondary. The origins of these major and secondary forms are listed in Table 2.
Air
After being expelled from the atmosphere, these microplastics settle in sediment or soil, where they may pose a threat to human lung health (Chen et al. 2020). Table 1 lists the sources of microplastics in the atmosphere. It has been observed that the concentration of microplastics in indoor air is greater than that in outside air (Dris et al. 2017). It has been noticed that the concentration of outdoor microplastics differs in different locations of the world (Cai et al. 2017). In outdoor conditions, the fate of microplastics relies on wind direction and speed, precipitation, vertical pollutant concentration gradient, and temperature (Prata 2018).
Soil
Soil is an extremely important microplastics reservoir. It has been observed that once these microplastics are introduced into the soil, they persist and accumulate, thereby inhibiting the growth and reproduction of soil-dwelling microorganisms. Consequently, they impact the biodiversity of these microbes (Rillig 2012; Chae and An. 2018). These microplastics also act as pollution carriers, damaging the soil environment (He et al. 2018b). According to research conducted by Wang et al. 2020a, microplastics in the soil also originate from fertilizers and contaminated irrigation water. Additionally, microplastics have been found in agricultural, suburban, urban, coastal, and floodplain soils (Liu et al. 2018).
Freshwater and ocean water
Microplastics that remain in sludge or are not filtered out during sewage treatment are discharged into freshwater (Horton et al. 2017a). As freshwater provides humans with drinking water, humans can directly inhale microplastics from freshwater (Novotna et al. 2019). According to findings by Fischer et al. (2016), the transmission of microplastics is dependent on wind, water body size, particle density, and current. Additionally, water retention time, urbanization, closeness to urban centers, proximity to a dense human population, sewage spills, and waste management influence the occurrence of microplastics in water systems (Horton et al. 2017b). After entering waterbodies, microplastics create biofilms through the colonization of algae, bacteria, and fungi, which are then consumed by fish, so altering their fate in freshwater (Hoellein et al. 2014).
It has been observed that wastewater treatment plants directly or indirectly contribute to plastic pollution in the ocean (Sun et al. 2019). In the ocean, the fate of microplastics is governed by phytodegradation, biodegradation (external influences), and microplastic characteristics (Li et al. 2016). Microplastics degrade completely in more than 50 years. Michels et al. (2018) reported that when microplastics enter the ocean, they create biofilm which, within 7 to 14 days, is turned into a plastic surface. The altered buoyant density of polymers causes the transfer of microplastics to deeper water and soil.
Uptake of MPs
MPs are being introduced into water bodies and soil on a large scale through different sources as mentioned above. As these MPs are non-biodegradable, they make entry into the living systems in their vicinity through various processes such as absorption, adsorption, ingestion, etc. many research studies supported the uptake of MPs by aquatic animals through their respiratory organs, i.e., gills (Siegfried et al. 2016). The transfer of food and energy as a result of linked food chains in the ecosystem further results in the bio-magnification of these toxic pollutants within the bodies of living organisms at higher trophic levels (Gigault et al. 2016). The plants have been reported to absorb these tiny MPs through their roots from the soil as well as from the microbes with which they interact during their vegetative and reproductive growth. Also, the adsorption or entry of MPs within any living component is inversely linked with the size of the former. The larger the size of MPs will be adsorbed with difficulty in comparison to the small-sized ones (Eriksen et al. 2013; Corcoran et al. 2015). Hence, with different modes of movement through different sources, MPs get accumulated within both terrestrial and aquatic biota (Rillig et al. 2017b; Rodriguez-Seijo et al. 2018; Wang et al. 2019a).
Inhalation is one of the routes of microplastic exposure for humans. Ingestion and cutaneous contact are yet other sources of human exposure. The effects of microplastic exposure on humans are reported in Table 3 (Prata et al. 2020). Microplastics have been detected in the human diet, including seafood, sea salt, sugar, honey, beer, and drinking water (Smith et al. 2018; Kim et al. 2018; Liebezeit and Liebezeit 2013, 2014 and Mintenig et al. 2019). The respiratory tract is thought to be a major pathway for exposure to microplastics. Reports indicate that humans can inhale approximately 272 particles per day from indoor air (Vianello et al. 2019). After the respiratory system, skin contact is the second mode of exposure and is regarded as less significant (Prata et al. 2020). According to Sykes et al. (2014), microplastics (size less than 100 nm) can pass through human skin. Microplastics penetrate human tissues via endocytosis and paracellular absorption. It depends on microplastics’ surface functionalization, size, protein corona formation, surface charge, and hydrophobicity (Wright and Kelly 2017).
Toxicity of MP pollutants
The process of plastic deterioration is extremely sluggish, and it might take more than 50 years for the plastic material to completely degrade (Müller et al. 2001) which further enhanced its toxicity. Direct toxicity of microplastics is caused by the ingestion of microplastics by terrestrial and aquatic creatures. In various aquatic species, direct ingestion of microplastics induces inflammation through the destruction of their filtering mechanisms (von Moos et al. 2012; Anbumani and Kakkar 2018; Wang et al. 2020b), damages the feeding apparatus, digestive tract, lower assimilation capacity, reduced swimming velocity and resistance time (Barboza et al. 2018; Meng et al. 2020), disrupts reproduction cycle, and enters the food chain (Barboza et al. 2018a). In addition to this, microplastics aggregate on the surface of algal cells interrupting gaseous exchange and photosynthesis. In higher plants, microplastic blocks photosynthetic processes, minerals, nutrients, water uptakes and starts various other growth-inhibitory processes.
The fact that microplastics cause indirect toxicity could not be ignored either as these toxicities are much more dangerous and complicated as compared to the direct toxic effects. During the production of plastics goods integration of chemical additives is very frequent such as such as bisphenol A (BPA) and bis (2-ethylhexyl) phthalate, acid scavengers, antistatic agents, antioxidants, flame retardants, plasticizers, lubricants, pigments, and thermal stabilizers that possibly resulting in combined toxicity. These additives are actually added to increase the performance of plastic materials (Hahladakis et al. 2018). Additional research has shown that many plastic-related monomers, oligomers, and other chemicals (for example, di-n-octyl phthalate, di (2-ethylhexyl) phthalate, polybrominated diphenyl ethers, and tetrabromobisphenol A) show adverse effects in humans through various exposure routes, such as through food, air, and water (Wang et al. 2021). Microplastics have been shown to be capable of absorbing heavy metals such as cadmium, zinc, nickel, and lead (Brennecke et al. 2016). As a result, microplastics are now thought of as potential vectors for these co-existing pollutants (Zhao et al. 2020), which raises the hazards associated with them. For example, it has been said that combining organic pollutants (like phenanthrene, 4,4'-DDT, and PBDEs) with microplastics could make them more bioavailable along food chains, which means they could end up in the human body (Zhao et al. 2020; Wang et al. 2021).
Impact of MPs on the terrestrial ecosystem
Terrestrial soil serves as a significant microplastics reservoir (Rillig 2020). After being introduced into the soil, microplastics have the potential to remain there and accumulate, ultimately having an impact on the development, reproduction, and overall biodiversity of soil organisms (Chae and An 2018; de Souza Machado et al. 2018b). In addition, microplastics can serve as vehicles for the transmission of different contaminants to soil biota, causing damage to the soil ecosystem (He et al. 2018a). According to the findings of a study carried out by Liu and colleagues, microplastics can live not only in the topsoil but also in the deeper subsurface soils (Liu et al. 2018). Microplastics have the potential to alter the characteristics of soil as well as its biophysical environment, which may influence the microbiological activity in soil (Wang et al. 2021). The most common way for soil microplastics to get into deep soil and even groundwater is through leaching (Rillig et al. 2020). Furthermore, soil organisms (such as earthworms) have a significant role in determining the accumulation and destiny of microplastics in soil, either by ingesting or excreting them (Wang et al. 2021). Soil organisms may help transfer microplastics across strata (from shallow to deep soil, or vice versa). A recent study showed that terrestrial plants can take in nano-size plastics (55 5 nm and 71 6 nm) depending on their surface charge (Sun et al. 2020). Therefore, remains of plastic, both large and little, have a deleterious impact on the vegetative and reproductive stages, interrupt nutrients, minerals, water uptake, reduce photosynthesis of the plant, alter soil microbial community, and root symbionts. Moreover, it also causes cellular and molecular alterations inside the bodies of terrestrial organisms (also shown in the Fig. 1).
MP inhibitory effects on higher plants
Microplastics cause direct toxicity mainly by limiting plant performance and development by reducing nutrients, minerals, water uptake, blocking photosynthesis, damaging cellular organelles, and suppressing various genes involved in the growth of plants (detailed experimental reports shown in Table 4). Whereas indirect toxicity is caused due to the weak bond between the additives and the basic polymers, as the additives were easily leached and released, causing toxic effects to the organism. Such as microplastic additive leachate from shoe soles hindered the photosynthesisin Vigna radiata (Lee et al. 2022). Similar to this lactic acid, the degradation products of polylactic acid (PLA) cause an adverse effect on Lolium perenneshoot length (Rozman et al. 2021). Polycarbonate (PC) granulate was also reported to inhibit Lepidium sativum seed germination by 60% compared to the control (Pfugmacher et al. 2021). Another investigatory report also shows that polystyrene (PS) microspheres are transported from roots to leaves, where PS microspheres decompose and produce benzene which causes a disruption in chlorophyll and sugar metabolism (Li et al. 2020). Microplastics may indirectly affect plant development by altering soil parameters, soil microorganisms and by affecting other pollutant bioavailability (Li et al. 2022).
MP impact on soil rhizosphere
MPs have been found to modulate the contents of dissolved organic C, N, and P present within the soil thereby affecting its physicochemical properties (Dai et al. 2021; Liu et al. 2017; Machado et al. 2018b). In addition to this, the water holding capacity, microbial activity, and bulk density of soil particles are also adversely affected by major occurring MPs such as polystyrene and polyethylene (Dai et al. 2021; Machado et al. 2019). An increase in the contents of humic and fulvric acid in soils was reported as a result of MPs incidence making them fertility boosters (Wang et al. 2020c; Wong et al. 2020; Zhang and Zhang 2020). These dynamics in soil porosity and moisture caused by MPs pollution affect the process of gaseous exchange between the soil and the microbes flourishing in the rhizospheric zone (Zhou et al. 2020; Rillig et al. 2019; Lu et al. 2020; Imran et al. 2019). Soil microbes which are the foremost component of the rhizospheric soil get incorporated with significant amounts of MPs, owing to their small size and highly adsorbent nature (Horton et al. 2017a; Huerta et al. 2016). In addition to these, the persistence of MPs within soil may lead to the aging of MPs making them active sites for the adsorption of other pollutants within soil especially the heavy metals and organic matter (Nizzetto et al. 2016b).
MP influence on terrestrial organisms
The MPs get accumulated in biotic components of an ecosystem from the soil via plants thereby affecting the organisms at different trophic levels to varying extents depending upon the quantity that is being transferred in the food web. The very minimal concentration of these MPs is excreted by these organisms while most of the accumulated MPs gets retained within their body and pose serious threats to biological and metabolic processes within the organism owing to its indigestible nature (Nizzetto et al. 2016c; Futter et al. 2016). Some of the harmful effects reported in living organisms due to MP uptake are infertility, blockage of the respiratory and digestive tract, and increased mortality (Dris et al. 2016). Another lot of deteriorating symptoms reported in plants as a result of MP accumulation includes poor seed germination, water uptake, root growth, and gaseous exchange limiting the primary metabolic process of photosynthesis (Vickers. 2017; Alam et al. 2018; Balestri et al. 2019). Similarly, the adsorption of MPs over the microbial surfaces leads to biofilm formation altering the species composition of microbial communities in the soil (Dussud et al. 2018; Wang et al. 2017; Jiang et al. 2018). In human beings, the noxious effects of MPs have been reported such as poor reproductive health, skin ailments, decreased immunity, cancer, etc. (Barboza et al. 2018; Peixoto et al. 2019; Schwabl et al. 2019). However, these aspects of MP pollution remain controversial sometimes; hence detailed studies on their persistence and affection need to be carried out.
Impact of MPs on the aquatic ecosystems
The literature serves as a source of many recent reports that highlight the devastating effect of these MPs on the biota of aquatic ecosystems. Ingestion of polyamide fibers by amphipod Gammarus fossarum suffered reduced assimilation efficiency (Blarer and Burkhardt-Holm 2016). Interestingly, altered fish behavior on revelation to polystyrene nanoparticles was documented by Mattsson et al. (2015). His observations included alterations in brain morphology, reduced feeding rates, and disruption in cellular processes. Apart from the direct impacts of MPs, the metals that adhere to their surfaces also increase the toxicity levels (Turner and Holmes 2015; Wang et al. 2017). An experimental setup was used by Brennecke et al. (2016) to demonstrate and study the release of heavy metals such as Cu and Zn from the antifouling paint that got adsorbed to virgin polystyrene beads and polyvinyl chloride fragments in water. Further, we have discussed the effect of MPs in alerting microbial populations and triggering altered gene expressions. Figure 2 represents the diagrammatically injurious effects of MPs on the sustainability of aquatic flora and fauna.
Alters microbial population
Aquatic microbial populations (phytoplankton, zooplankton, algae) are of prime importance for aquatic ecosystems not only due to their autotrophic capabilities, oxygen releasing nature but also because they are primary producers supporting the entire food chain. The theory was proposed and experimentally tested by Bhattacharya et al. (2010). His team exposed Chlorella and Scenedesmus to positively charged plastic nanoparticles, they observed a decline in photosynthetic activity after these particles adhered to the cell surfaces. Zhang et al. (2017) also tested the above-proposed theory by exposing Skeletonema costatum to polyvinyl chloride microspheres and reported the deleterious effect of MPs on photosynthetic efficiency, chlorophyll content, and growth. Many laboratory toxicities studies were conducted to assess the effects of microplastics on algal strains. After the application of Polyethyleneimine PS nanoparticles (0.1–1.0 mg/L for 72 h) on Pseudokirchneriella subcapitata, its growth got constrained (Casado et al. 2013).
Stimulates the gene exchange
Microplastic biofilms are referred to as hot spots of horizontal gene transfer (HGT). These sites have high cell densities resulting in increased interaction levels among the cells (Aminov 2011; Sezonov et al. 2007). The studies conducted by Arias-Andres et al. (2018) describe the impact of microplastics on the ecology of aquatic ecosystems, bacterial evolution, and growing hazards to environmental and human health. They observed that the bacterial population associated with microplastics has a greater frequency of plasmid transfer in comparison to free-living bacterial strains. In addition, they reported that enhanced gene exchange occurs in phylogenetically diverse bacterial communities that were grown on polycarbonate filters. Furthermore, it was observed by Grossart et al. (2003) that under conditions of high dissolved organic carbon, plasmid transfer frequencies increase. Hence, microplastics have an impact on the evolution of aquatic bacteria finally leading to neglected hazards for human health (Fig. 3).
Mechanism of environmental degradation of MPs
The degradation of MPs can be divided into four basic processes mechanical, chemical, and biological. Firstly, macroplastic/synthetic polymer chains are converted into shorter molecular units, i.e., oligomers, dimers, monomers MPs, and then finally degraded into inorganic components (Eubeler et al. 2009).
Mechanical methods
MPs are degraded mechanically by abrasion in which solid particles come in contact with various natural (sediments, debris) and manmade (transportation vehicles, barriers) substances in the terrestrial and aqueous environment (Klein et al. 2018). Small rounded grains with surface textures of grooves and conchoidal cracks produced by abrasion are similar to quartz grains of natural sediments (Corcoran 2022). It was also reported that polymer degradation was increased by mechanical pressure as a bottle with sand containing plastic pieces was continuously rotating for 24 h and the weight of plastic was decreased to 14% indicatingthe abrasion process degrade the polymer (Kalogerakis et al. 2017). Due to wind, wave action, and tidal currents beaches are considered a favorable place for MPs degradation. It was studied that PE microbeads used in facials were found in wastewater and then subjected to shear stress through stirring, pumping, etc. These shear stress forces converted PE microplastic into nanoplastic particles (Enfrin et al. 2020).
Chemical degradation
The chemical breakdown of MPs depends on the type of polymer, medium used, chemical composition, and deposition of a particular type of sediment (Gewert et al. 2015; Brandon et al. 2016; Song et al. 2017). MPs absorb a greater amount of UV radiation on beaches as compared to particles hidden under benthic sediments. Chains with smaller molecular units are generated when MPs are photodegraded through exposure to UV radiation and oxygen. But the C-C bonds of PE, PP, and polyvinyl chloride (PVC) do not break completely through photooxidation and require some additives for degradation in comparison to polymer polyethylene terephthalate (PET) (Chamas et al. 2020). After the photodegradation of C-H bonds in PE and PP, the free radicals react with oxygen which leads to the formation of inert products with a low molecular weight of these polymers (Gewert et al. 2015). The generated polymers are then disposed off or mechanical abrasion/biological degradation. The chlorination process is also used to degrade MPs by which old bonds are broken and new ones introduce between chlorine and hydrogen. Big-sized particles of MPs are formed by adding salts of Fe and Al or other coagulants through the processes of agglomeration or flocculation for degradation. One of the advanced oxidation processes for the degradation of contaminated particles is photocatalysis as this green technology uses immeasurable solar energy for the oxidation of microplastics. This process is based on the photocatalytic properties of certain materials such as TiO2 which have been used to transform solar energy into chemical energy to oxidize/reduce pollutants in hydrogen or hydrocarbons. Upon the absorption of UV light, high energy electrons from the valence band are transferred to the conduction band on the TiO2 surface, and then holes are produced in the valence band, therefore both the holes and electrons react with OH., O2, or H2O to generate reactive oxygen species (ROS). These ROS then involved in the process of microplastic degradation (Nakata and Fujishima 2012).
Biological degradation
In biodegradation, a complex community of microorganisms which have the ability to adapt to various environmental fluctuations forma biofilm on the surface of organic pollutants including MPs, and change their properties (Muthukumar et al. 2011). After the formation of biofilm, the polymer structure is disturbed and bonds are weakened. The weakened bonds are then attacked by extracellular enzymatic secretions of microorganisms. At last, the assimilated MPs monomers are completely mineralized by cellular enzymes into smaller components like CO2, H2O, N2, and biomass which are then available as energy sources for microorganisms and then recycle to the atmosphere (Du et al. 2021). The extent of biodegradation depends on the surrounding ecosystems (terrestrial or aquatic), the structure of the polymer (degree of polymerization, branching, chemical bonds, crystallinity, and hydrophobicity), and environmental factors (pH, temperature, moisture) (Klein et al. 2018). Microorganisms such as bacteria (Azotobacter sp. and Pseudomonas sp.), fungi (Aspergillus sp., Penicillium sp.), and actinomycetes (e.g., Amycolatopsis sp., Actinomadura sp.) can degrade both synthetic and natural plastics (Bose 2020). It has been reported that bacterial strains (Bacillus sp. 27 and Rhodococcus sp. 36) obtained from mangrove sediment degrade the polypropylene MPs efficiently as the weight of the polymer was reduced to 7% by Bacillus sp. 27 and 5% by Rhodococcus sp. 36, respectively (Auta et al. 2018). Figure 4 summarizes these three processes of MP degradation.
Immobilization of MPs from terrestrial ecosystem
Polymers like polyethylene (PE), polyvinyl chloride (PVC), and polyethylene terephthalate (PET) are the major plastic particles found in the terrestrial ecosystem. Irradiated or cracked polymers have been degraded under the influence of various microorganisms (Miri et al. 2022). It was observed that earthworms can increase the degradation of MPs as in the gut of earthworms, low molecular polyethylene particle size was reduced within four weeks (Lwanga et al. 2018). It was also stated that using polymers as carbon sources microalgae degrade MPs through the synthesis of some toxins or enzymes and biodegradable plastic can be made using protein and carbohydrate-based polymers by microalgae. The growth of algal cells is faster than in higher plants which can be enhanced through genetic engineering, so algal-based plastics can replace synthetic plastics (Chia et al. 2020).
Immobilization of MPs from aquatic ecosystem
To restrict and eliminate these MPs, advanced approaches should be implemented in combating these pollutants (Fig. 5). Synthetic textile industries are found to generate microfibers of microplastics which are drawn into the surface of waste and act as a pollutant around the globe (Mishra and Ahmaruzzaman 2022).
Immobilization of microplastics using biological methods
Mangrove-derived Bacillus species such as Bacillus gottheilli and Bacillus cereus are typically used in minimizing microplastic polymers like PS, PE, and PP (Auta et al. 2017). However, some other algae and fungal populations are also helpful in minimizing the MPs from the aquatic ecosystems. Paco et al. (2017) found that the application of Zalerion maritimum, a fungus, has a higher potential to degrade or convert these MPs or polyethylene chemically and morphologically. Also, researchers have identified some other MPs sinks for aquatic ecosystems, one among such are organisms like Tridacna maxima generally known as the Red Sea giant clam, some corals, and crustaceans like Euphausia superba (Arossa et al. 2019; Corona et al. 2020).
On the other hand, microalgae are gaining much importance as a biological tool in removing the MPs. Peller et al. (2021) found that the macrophytic algae Cladophora is efficient in the elimination of microplastics due to their high sorption potential as they have a high surface area and shows effective associations among algae and microplastics. Whereas on the other hand Wu et al. (2022b) identified a green-algae namely Chlorella vulgaris which shows its potential in the normalization of the toxic PS-microplastics which are however capable of endocrine disruption like Levofloxacin an antibiotic present in wastewater from aquaculture. Also, the microalgae, Diatoms namely Phaeodactylum tricornutum have been employed to remove multiple microplastics ranging from PVC, PE, PET, and PP, etc. (Song et al. 2020). However, an edible marine sea-weed, Fucus vesiculosus shows effective adsorption of microplastics mainly PS-microplastics on their surfaces. Thus, the algae show an efficiency of up to 94.6% for the PS microplastics as they contain gelatinous compounds like alginates a kind of polysaccharide that helps in PS adsorption (Sundbaek et al. 2018).
Immobilization of microplastics using nanomaterials
Nanomaterials are another breakthrough in the research field and are kept in use over conventional methods, such as microalgae and sponges. Mishra and Ahmaruzzaman (2022) have employed certain iron nanoparticles with hydrophobic, cost-effective, and large surface areas. These nanomaterials are having the ability to interact with microplastics and mediate their removal through ferromagnetic properties with a high potential to act against polymeric microplastics such as PP, PVC, PE, PS, PU, and PET. They have an efficiency of 93% in seawater whereas show 84% of its efficiency to remove PP, PVC, PS, PU, PE, etc. Another nano-catalyst cesium oxide (CeO2) shows excellent adsorption of microplastics which mainly depends on the large surface area, its oxidation state as well the sorption capacity of the microplastics (Ho et al. 2021). Zinc oxide nanorods are applicable in removing low-density PE microplastics (Tofa et al. 2019). Au-doped Ni-TiO2-based micromotors are developed and are well used to eliminate microplastics by using certain UV light irradiations for wastewater treatments. But due to low selectivity, this particular technique is not of much use for wastewater treatments (Wang et al. 2019b). However, Yuan et al. (2020) have developed graphene oxide-based adsorbents having three-dimensional structures that are much more effective against PS microplastics. The π-π bonding between the C- atoms in reduced graphene oxide and the benzene in PS are important to mediate the effective adsorptions of the pollutants. Also, researchers have developed certain nanomaterials like magnetic-nano- Fe3O4 to eliminate marine contaminating magnetized MPs (Shi et al. 2022).
Immobilization of microplastics using coagulation and agglomeration
These effective methods are employed to deal with the enlarged MPs in aquatic ecosystems. The contaminants are captured by the Fe and Al salt-based catalysts through ligand-based interactions. Arzia-Tarazona et al. (2019) demonstrated the elimination of certain PE microplastics by employing the Fe and Al-based salt catalysts through ultrafiltration and coagulation mechanisms. In different studies, the Al3+ ions were found to be more effective with respect to Fe3+ ions to eliminate the MPs. However, an alteration in the pH of the solution did not change the activities of the Al coagulation with a size of 0.5 mm MP particles, whereas an increase in pH impacts and limits the elimination of MPs below 0.5 mm in diameter. However, Zhou et al. (2021b) have developed a method that utilizes ferric chloride and polyaluminum chloride as coagulants to treat the MPs from the wastewater. These +vely charged coagulants are made to interact with the -vely charged microplastics and other pollutants which finally get settled at the surfaces via gravity. Furthermore, the sediments were collected and the supernatants were allowed to undergo mechanisms like filtration and drying. Finally, the characterizations of the MPs’ flocs were done. On the other hand, Akarsu et al. (2021) implemented electrocoagulation techniques to eliminate the PE -microplastics from the reactors containing sludge which are mainly used in wastewater treatments and are thought to be more cost and energy efficient. The researchers emphasized the factor stabilization of suspended microplastics through effective van der Waals forces in action under electrocoagulation techniques (Akbal and Camci 2011). This technique is found to be 90% efficient to trap microplastics suspended on surfaces.
Conclusion
MPs are found to have high persistence and a very slow biodegradable nature. They pose direct physical and nutritional complications post ingestion, also the presence of plasticizers associated with these MPs often aggravates the toxicity. Also, if these plastic pieces get smaller, i.e., nanosized, they have more surface area, which means they can absorb more chemicals and change chemically on the outside, which could make them more dangerous and gain scientific attention. However, the recent studies accompanying MP transport through bioturbation in regard to various soil fauna may not reflect real-world conditions due to the fact that experimentation is being conducted using model organisms in laboratory conditions. Therefore, the transport pathway of MPs in diverse organisms and their impact on the entire soil ecosystem is considered in the near future research. There is an urgent need of investigating the behavior and mechanism of microplastic degradation in terrestrial and aquatic ecosystems because it will not be feasible to evaluate the risk of MPs to human health and the environment.
Future perspectives
The microplastic pollution in the environment and its long-standing effects are less implicit. The reported documentation of MPs in the aquatic ecosystem should address the abundance of polymers like PE, PET, PE, PP, and PS. It is important to examine their fate in the environment. There is a knowledge gap in understanding the exact nature and long-term effects of MPs on both ecosystems. Competent and reliable ecosystem models should be developed to evaluate the fate of free-floating and plummeting MP waste in aquatic systems. The mechanism of migration and degradation of MPs into other products is still unclear for which the generalized approach should be developed. There is doubt about the volume, configuration, and diversity of MPs penetrating the environment because there is no quantified data about the release rate of MPs either accidentally or purposely. However, MP litter and its accidental discharge is considered as one of the utmost uncertainties for discharge predictions. This review documented to understand the subtleties and effects of MPs as a pollutant, especially in a terrestrial and aquatic ecosystems context. There is a need of extensive study to determine how MPs in wastewater leached out in the cropland and hence enter the food chain of the ecosystem. MP effects on the formation of biofilms and the expansion of infective microbes mainly with potable sources of water need to be discovered. Field research on the environmental effects of MPs presently remains at the level of species which demands research at the level of ecosystem also. More investigations are needed to study the direct MP impressions on the ecosystems’ food chain flow and distribution. It is necessary to follow and relate existing crumbled data to advance the knowledge gap about impacts of MPs on various processes like sequestration of carbon, nutrient cycling, etc. This study reviewed some research extents associated with MP impacts and degradation that further needs an urgent advancement to understand the possible environmental risks and offers some references to recover and control the MP management system into the environment.
Data availability
Not applicable.
References
Akarsu C, Kumbur H, Kideys AE (2021) Removal of microplastics from wastewater through electrocoagulation-electroflotation and membrane filtration processes. Water Sci Technol 84(7):1648–1662
Akbal F, Camcı S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269(1-3):214–222
Alam O, Billah M, Yajie D (2018) Characteristics of plastic bags and their potential environmental hazards. Resour Conserv Recycl 132:121–129. https://doi.org/10.1016/j.resconrec.2018.01.037
Alomar C, Sureda A, Capo X, Guijarro B, Tejada S, Deudero S (2017) Micro-plastic ingestion by Mullussurmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ Res 159:135–142
Aminov RI (2011) Horizontal gene exchange in environmental microbiota. Front Microbiol 2:158. https://doi.org/10.3389/fmicb.2011.00158
Anbumani S, Kakkar P (2018) Ecotoxicological effects of microplastics on biota: a review. Environ Sci Pollut Res 25(15):14373–14396
Arias-Andres M, Klümper U, Rojas-Jimenez K, Grossart HP (2018) Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut 237:253–261. https://doi.org/10.1016/j.envpol.2016.06.074
Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V, Siligardi C, Cedillo-González EI (2019) New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram Int 45(7):9618–9624
Arossa S, Martin C, Rossbach S, Duarte CM (2019) Microplastic removal by Red Sea giant clam (Tridacna maxima). Environ Pollut 252:1257–1266
Auta HS, Emenike CU, Fauziah SH (2017) Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int 102:165–176
Auta HS, Emenike CU, Jayanthi B, Fauziah SH (2018) Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar Pollut Bull 127:15–21
Balestri E, Menicagli V, Ligorini V, Fulignati S, Galletti AM, Lardicci C (2019) Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol Indic 102:569–580. https://doi.org/10.1016/j.ecolind.2019.03.005
Barboza LG, Vethaak AD, Lavorante BR, Lundebye AK, Guilhermino L (2018) Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar Pollut Bull 133:336–348. https://doi.org/10.1016/j.marpolbul.2018.05.047
Barboza LGA, Vieira LR, Guilhermino L (2018a) Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchuslabrax): changes in behavioural responses and reduction of swimming velocity and resistance time. Environ Pollut 236:1014–1019
Bhattacharya P, Lin S, Turner JP, Ke PC (2010) Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem 114(39):16556–16561. https://doi.org/10.1021/jp1054759
Blaesing M, Amelung W (2018) Plastics in soil: analytical methods and possible sources. Sci Total Environ 612:422–435
Blarer P, Burkhardt-Holm P (2016) Microplastics affect assimilation efficiency in the freshwater amphipod Gammarus fossarum. Environ Sci Pollut Res 23:23522–23532
Bosker T, Bouwman LJ, Brun NR, Behrens P, Vijver MG (2019) Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 226:774–781
Brandon J, Goldstein M, Ohman MD (2016) Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar Pollut Bull 15:299–308
Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J (2016) Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci 178:189–195. https://doi.org/10.1016/j.ecss.2015.12.003
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Envrion Sci Technol 42:5026–5031
Cai L, Wang J, Peng J, Tan Z, Zhan Z, Tan X, Chen Q (2017) Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ Sci Pollut R 24:24928–24935
Casado MP, Macken A, Byrne HJ (2013) Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery. Environ Int 51:97–105. https://doi.org/10.1016/j.envint.2012.11.001
Chae Y, An YJ (2018) Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ Pollut 240:387–395
Chamas A, Moon H, Zheng J, Qui Y, Tabassum T, Jang JH, Abu-Omar M, Scott SL, Suh S (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8:3494–3511
Chandra P, Singh DP (2020) Microplastic degradation by bacteria in aquatic ecosystem. In: In Microorganisms for sustainable environment and health. Elsevier, pp 431–467
Chen G, Feng Q, Wang J (2020) Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ 703:135504
Chia WY, Tang DY, Khoo KS, Lup AN, Chew KW (2020) Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ Sci Technol 4:100065
Cole M, Coppock R, Lindeque PK, Altin D, Reed S, Pond DW, Sørensen L, Galloway TS, Booth AM (2019) Effects of nylon microplastic on feeding, lipid accumulation, and moulting in a coldwater copepod. Environ Sci Technol 53:7075–7082
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Corcoran PL (2022) Degradation of microplastics in the environment. In: Handbook of Microplastics in the Environment. Springer, Cham, pp 531–542
Corcoran PL, Norris T, Ceccanese T, Walzak MJ, Helm PA, Marvin CH (2015) Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record. Environ Pollut 204:17–25. https://doi.org/10.1016/j.envpol.2015.04.009
Corona E, Martin C, Marasco R, Duarte CM (2020) Passive and active removal of marine microplastics by a mushroom coral (Danafungiascruposa). Front Mar Sci 7:128. https://doi.org/10.3389/fmars.2020.00128
da Costa JP, Santos PS, Duarte AC, Rocha-Santos T (2016) (Nano) plastics in the environment–sources, fates and effects. Sci Total Environ 566:15–26. https://doi.org/10.1016/j.scitotenv.2016.05.041
Dai Y, Shi J, Zhang N, Pan Z, Xing C, Chen X (2021) Current research trends on microplastics pollution and impacts on agro-ecosystems: A short review. Sep Sci Technol 57(4):656–669. https://doi.org/10.1080/01496395.2021.1927094
de Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC (2019) Microplastics can change soil properties and affect plant performance. Environ Sci Technol 6044-52. https://doi.org/10.1021/acs.est.9b01339
de Souza Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC (2018a) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 9656-65. https://doi.org/10.1021/acs.est.8b02212
de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC (2018b) Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol 24(4):1405–1416
Dong Y, Gao M, Song Z, Qiu W (2020) Microplastic particles increase arsenic toxicity to rice seedlings. Environ Pollut 259:113892
Dris R, Gasperi J, Saad M, Mirande C, Tassin B (2016) Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull 104(1-2):290–293. https://doi.org/10.1016/j.marpolbul.2016.01.006
Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B (2017) A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut 221:453–458
Du H, Xie Y, Wang J (2021) Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J Hazard Mater 418:126377
Dussud C, Meistertzheim AL, Conan P, Pujo-Pay M, George M, Fabre P, Coudane J, Higgs P, Elineau A, Pedrotti ML, Gorsky G (2018) Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ Pollut 236:807–816. https://doi.org/10.1016/j.envpol.2017.12.027
Enfrin M, Lee J, Gibert Y, Basheer F, Kong L, Dumée LF (2020) Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J Hazard Mater 384:121393
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull 77(1-2):177–182. https://doi.org/10.1016/j.marpolbul.2013.10.007
Eubeler JP, Zok S, Bernhard M, Knepper TP (2009) Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trends Anal Chem 28(9):1057–1072. https://doi.org/10.1016/j.trac.2009.06.007
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments–a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657
JPGL F, Nash R (2019) Microplastics: finding a consensus on the definition. Mar Pollut Bull 138:145–147
Futter MN, Erlandsson MA, Butterfield D, Whitehead PG, Oni SK, Wade AJ (2016) PERSiST: a flexible rainfall-runoff modelling toolkit for use with the INCA family of models. Hydrol Earth Syst Sci 18(2):855–873. https://doi.org/10.5194/hess-18-855-2014
Gao M, Xu Y, Liu Y, Wang S, Wang C, Dong Y, Song Z (2021) Effect of polystyrene on di-butyl phthalate (DBP) bioavailability and DBP-induced phytotoxicity in lettuce. Environ Pollut 268:115870
GESAMP, (2016). Reports and studies: Sources, fate and effects of microplastics in the marine environment: Part two of a global assessment. Journal series gesamp reports and studies, London
Gewert B, Plassman MM, MacLeod M (2015) Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts 17:1513
Gigault J, Pedrono B, Maxit B, Ter Halle A (2016) Marine plastic litter: the unanalyzed nano-fraction. Environ Sci: nano 3(2):346–350. https://doi.org/10.1039/C6EN00008H
Grossart HP, Kiørboe T, Tang K, Ploug H (2003) Bacterial colonization of particles: growth and interactions. Appl Environ Microbiol 69(6):500–3509. https://doi.org/10.1128/AEM.69.6.3500-3509.2003
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P (2018) An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199
Harmon SM (2018) The effects of microplastic pollution on aquatic organisms. In cc (pp. 249-270). : https://doi.org/10.1007/s11356-021-13184-2
Harrison JP, Sapp M, Schratzberger M, Osborn AM (2011) Interactions between microorganisms and marine microplastics: a call for research. Mar Technol Soc J 45(2):12–20
He D, Luo Y, Lu S, Liu M, Song Y, Lei L (2018a) Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal Chem 109:163–172
He L, Wu D, Rong H, Li M, Tong M, Kim H (2018b) Influence of nano-and microplastic particles on the transport and deposition behaviors of bacteria in quartz sand. Environ Sci Technol 52(20):11555–11563
Ho WK, Leung KSY (2021) The crucial role of heavy metals on the interaction of engineered nanoparticles with polystyrene microplastics. Water Res 201:117317
Hoellein T, Rojas M, Pink A, Gasior J, Kelly J (2014) Anthropogenic litter in urban freshwater ecosystems: distribution and microbial interactions. PLoS One 9:e98485
Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E (2017a) Large microplastic particles in sediments of tributaries of the River Thames, UK–Abundance, sources and methods for effective quantification. Mar Pollut Bull 114(1):218–226. https://doi.org/10.1016/j.marpolbul.2016.09.004
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017b) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141
Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, Van Der Ploeg M, Geissen V (2016) Microplastics in the terrestrial ecosystem: implications for Lumbricusterrestris (Oligochaeta, Lumbricidae). Environ Sci Technol 50(5):2685–2691
Imran M, Das KR, Naik MM (2019) Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere 215:846–857. https://doi.org/10.1016/j.chemosphere.2018.10.114
Jeong CB, Kang HM, Lee MC, Kim DH, Han J, Hwang DS, Souissi S, Lee SJ, Shin KH, Park HG, Lee JS (2017) Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci Rep 7:1–11
Jiang P, Zhao S, Zhu L, Li D (2018) Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci Total Environ 624:48–54. https://doi.org/10.1016/j.scitotenv.2017.12.105
Jiang X, Chen H, Liao Y, Ye Z, Li M, Klobučar G (2019) Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ Pollut 250:831–838
Kalogerakis N, Karkanorachaki K, Kalogerakis GC, Triantafyllidi EI, Gotsis AD, Partsinevelos P, Fava F (2017) Microplastics generation: onset of fragmentation of polyethylene films in marine environment mesocosms. Front Mar Sci 4:84
Kim JS, Lee HJ, Kim SK, Kim HJ (2018) Global pattern of microplastics (mps) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ Sci Technol 52:12819–12828
Klein S, Dimzon IK, Eubeler J, Knepper TP (2018) Analysis, occurrence, and degradation of microplastics in the aqueous environment. In: Wagner M, Lambert S (eds) Freshwater microplastics. SpringerLink, pp 51–67
Kumar M, Xiong X, He M, Tsang DC, Gupta J, Khan E et al (2020) Microplastics as pollutants in agricultural soils. Environ Pollut 265:114980
Lee TY, Kim L, Kim D, An S, An YJ (2022) Microplastics from shoe sole fragments cause oxidative stress in a plant (Vigna radiata) and impair soil environment. J Hazard Mater 429:128306
Li C, Busquets R, Campos LC (2020) Assessment of microplastics in freshwater systems: A review. Sci Total Environ 707:135578
Li J, Qu X, Su L, Zhang W, Yang D, Kolandhasamy P, ... Shi H (2016) Microplastics in mussels along the coastal waters of China. Environ Pollut 214:177–184
Li J, Yu S, Yu Y, Xu M (2022) Effects of microplastics on higher plants: a review. Bull Environ Contam Toxicol 109(2):241–265
Lian J, Liu W, Meng L, Wu J, Chao L, Zeb A, Sun Y (2021b) Foliar-applied polystyrene nanoplastics (PSNPs) reduce the growth and nutritional quality of lettuce (Lactuca sativa L.). Environ Pollut 280:116978
Lian J, Liu W, Meng L, Wu J, Zeb A, Cheng L et al (2021a) Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J Clean Prod 318:128571
Liebezeit G, Liebezeit E (2013) Non-pollen particulates in honey and sugar. Food Addit Contam A 30:2136–2140
Liebezeit G, Liebezeit E (2014) Synthetic particles as contaminants in German beers. Food Addit Contam A 31:1574–1578
Liu H, Yang X, Liu G, Liang C, Xue S, Chen H, Ritsema CJ, Geissen V (2017) Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 185:907–917. https://doi.org/10.1016/j.chemosphere.2017.07.064
Liu M, Lu S, Song Y, Lei L, Hu J, Lv W et al (2018) Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ Pollut 242:855–862
Lu XM, Lu PZ, Liu XP (2020) Fate and abundance of antibiotic resistance genes on microplastics in facility vegetable soil. Sci Total Environ 709:136276. https://doi.org/10.1016/j.scitotenv.2019.136276
Lwanga EH, Thapa B, Yang X, Gertsen H, Salánki T, Geissen V, Garbeva P (2018) Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci Total Environ 624:753–757
Mattsson K, Ekvall MT, Hansson LA, Linse S, Malmendal A, Cedervall T (2015) Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ Sci Technol 49(1):553–561. https://doi.org/10.1021/es5053655
Meng Y, Kelly FJ, Wright SL (2020) Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective. Environ Pollut 256:113445
Michels J, Stippkugel A, Lenz M, Wirtz K, Engel A (2018) Papid aggregation of biofilm-covered microplastics with marine biogenic particles. P Roy Soc B-Biol Sci 285:20181203
Mintenig SM, Löder MGJ, Primpke S, Gerdts G (2019) Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ 648:631–635
Miri S, Saini R, Davoodi SM, Pulicharla R, Brar SK, Magdouli S (2022) Biodegradation of microplastics: better late than never. Chemosphere 286:131670
Mishra SR, Ahmaruzzaman M (2022) Microplastics: identification, toxicity and their remediation from aqueous streams. Sep Purif Rev 1–22. https://doi.org/10.1080/15422119.2022.2096071
Müller RJ, Kleeberg I, Deckwer WD (2001) Biodegradation of polyesters containing aromatic constituents. J Biotechnol 86(2):87–95
Muthukumar T, Aravinthan A, Lakshmi K, Venkatesan R, Vedaprakash L, Doble M (2011) Fouling and stability of polymers and composites in marine environment. Biodeterior biodegrad 65:276–284
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J PhotochemPhotobiol 13:169–189
Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG (2016a) A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ Sci Process Impacts 18(8):1050–1059. https://doi.org/10.1039/C6EM00206D
Nizzetto L, Butterfield D, Futter M, Lin Y, Allan I, Larssen T (2016b) Assessment of contaminant fate in catchments using a novel integrated hydrobiogeochemical-multimedia fate model. Sci Total Environ 544:553–563. https://doi.org/10.1016/j.scitotenv.2015.11.087
Nizzetto L, Langaas S, Futter M (2016c) Pollution: do microplastics spill on to farm soils?. Nature 537(7621):488–488
Novotna K, Cermakova L, Pivokonska L, Cajthaml T, Pivokonsky M (2019) Microplastics in drinking water treatment – current knowledge and research needs. Sci Total Environ 667:730–740
P Bose (2020) Microbial degradation of plastic waste and the PETase enzyme, https://www.azom.com/article.aspx?ArticleID=19280
Paço A, Duarte K, da Costa JP, Santos PS, Pereira R, Pereira ME et al (2017) Biodegradation of polyethylene microplastics by the marine fungus Zalerionmaritimum. Sci Total Environ 586:10–15
Peixoto D, Pinheiro C, Amorim J, Oliva-Teles L, Guilhermino L, Vieira MN (2019) Microplastic pollution in commercial salt for human consumption: A review. Estuar Coast Shelf Sci 219:161–168. https://doi.org/10.1016/j.ecss.2019.02.018
Peller J, Nevers MB, Byappanahalli M, Nelson C, Babu BG, Evans MA et al (2021) Sequestration of microfibers and other microplastics by green algae, Cladophora, in the US Great Lakes. Environ Pollut 276:116695
Pflugmacher S, Tallinen S, Kim YJ, Kim S, Esterhuizen M (2021) Ageing affects microplastic toxicity over time: Effects of aged polycarbonate on germination, growth, and oxidative stress of Lepidium sativum. Sci Total Environ 790:148166
Prata JC (2018) Airborne microplastics: consequences to human health?. Environ Pollut 234:115–126
Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T (2020) Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ 702:134455
Qi Y, Yang X, Pelaez AM, Lwanga EH, Beriot N, Gertsen H, Garbeva P, Geissen V (2018) Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci Total Environ 645:1048–1056
Rillig MC, Lehmann A, de Souza Machado AA, Yang G (2019) Microplastic effects on plants. New Phytol 223(3):1066–1070. https://doi.org/10.1111/nph.15794
Rillig MC, Ziersch L, Hempel S (2017a) Microplastic transport in soil by earthworms. Sci Rep 7(1):1–6. https://doi.org/10.1038/s41598-017-01594-7
Rillig MC, Lehmann A (2020) Microplastic in terrestrial ecosystems. Science 368(6498):1430–1431
Rillig MC, Ingraffia R, de Souza Machado AA (2017b) Microplastic incorporation into soil in agroecosystems. Front Plant Sci 8:1805
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46:6453–6454
Rodríguez-Seijo A, Santos B, da Silva EF, Cachada A, Pereira R (2018) Low-density polyethylene microplastics as a source and carriers of agrochemicals to soil and earthworms. Environ Chem 16(1):8–17. https://doi.org/10.1071/EN18162
Rozman U, Turk T, Skalar T, Zupančič M, Korošin NČ, Marinšek M et al (2021) An extensive characterization of various environmentally relevant microplastics–Material properties, leaching and ecotoxicity testing. SciTotal Environ 773:145576
Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B (2019) Detection of various microplastics in human stool: a prospective case series. Ann Intern Med 171(7):453–457. https://doi.org/10.7326/M19-0618
Sezono G, Joseleau-Petit D, d'Ari R (2007) Escherichia coli physiology in Luria-Bertani broth. Int. J Bacteriol 189(23):8746–8749. https://doi.org/10.1128/JB.01368-07
Sharma S, Chatterjee S (2017) Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ Sci Pollut R 24:21530–21547
Shi X, Zhang X, Gao W, Zhang Y, He D (2022) Removal of microplastics from water by magnetic nano-Fe3O4. Sci Total Environ 802:149838
Siegfried M, Gabbert S, Koelmans AA, Kroeze C, Löhr A, Verburg C (2016) River export of plastic from land to sea: a global modeling approach. InEGU General Assembly Conference Abstracts (pp. EPSC2016-11507). 2016EGUGA.1811507S
Smith M, Love DC, Rochman CM, Neff RA (2018) Microplastics in seafood and the implications for human health. Curr Environ Health Rpt 5:375–386
Song YK, Hong SH, Jang M, Han GM, Jung SW, Shim WJ (2017) Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol 18:4368–4376
Song C, Liu Z, Wang C, Li S, Kitamura Y (2020) Different interaction performance between microplastics and microalgae: The bio-elimination potential of Chlorella sp. L38 and Phaeodactylumtricornutum MASCC-0025. Sci Total Environ 723:138146
Sturm MT, Herbort AF, Horn H, Schuhen K (2020) Comparative study of the influence of linear and branched alkyltrichlorosilanes on the removal efficiency of polyethylene and polypropylene-based microplastic particles from water. Environ Sci Pollut Res 27(10):10888–10898
Sun H, Lei C, Xu J, Li R (2021) Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J Hazard Mater 416:125854
Sun J, Dai X, Wang Q, van Loosdrecht MC, Ni BJ (2019) Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res 152:21–37
Sun XD, Yuan XZ, Jia Y, Feng LJ, Zhu FP, Dong SS et al (2020) Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat Nanotechnol 15(9):755–760
Sundbæk KB, Koch IDW, Villaro CG, Rasmussen NS, Holdt SL, Hartmann NB (2018) Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J Appl Phycol 30(5):2923–2927
Sundt P, Schulze PE, Syversen F, (2014) Sources of microplastic-pollution to the marine environment. Nor Environ Agency 1-86. Mepex 1032
Sykes EA, Dai Q, Tsoi KM, Hwang DM, Chan WC (2014) Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nat Commun 5:1–8
Tan H, Yue T, Xu Y, Zhao J, Xing B (2020) Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ Sci Technol 54:12285–12294
Tofa TS, Kunjali KL, Paul S, Dutta J (2019) Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ Chem Lett 17(3):1341–1346
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12(5):600–610
Urbina MA, Correa F, Aburto F, Ferrio JP (2020) Adsorption of polyethylene microbeads and physiological effects on hydroponic maize. Sci Total Environ 741:140216
Vianello A, Jensen RL, Liu L, Vollertsen J (2019) Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci Rep 9:8670
Vickers NJ (2017) Animal communication: when I’m calling you, will you answer too? Curr Bio 27(14):R713–R715. https://doi.org/10.1016/j.cub.2017.05.064
von Moos N, Burkhardt-Holm P, Kohler A (2012) Uptake and effects of microplastics on cells and tissue of the blue 1059 mussel Mytilusedulis L. after an experimental exposure. Environ Sci Technol 46:11327–11335
Wang C, Zhao J, Xing B (2021) Environmental source, fate, and toxicity of microplastics. J Hazard Mater 407:124357
Wang L, Li H, Shi G, Hong J, Chen Z, Jin C, Sun C, Yao B (2017) Synthesis of SiOH-functionalized composite particles with buckled surface by seeded emulsion polymerization. Colloid Polym Sci 295(3):471–478. https://doi.org/10.1007/s00396-017-4026-8
Wang HT, Ding J, Xiong C, Zhu D, Li G, Jia XY, Zhu YG, Xue XM (2019a) Exposure to microplastics lowers arsenic accumulation and alters gut bacterial communities of earthworm Metaphire californica. Environ Pollut 251:110–116. https://doi.org/10.1016/j.envpol.2019.04.054
Wang T, Li B, Zou X, Wang Y, Li Y, Xu Y, Mao L, Zhang C, Yu W (2019b) Emission of primary microplastics in mainland China: Invisible but not negligible. Water Res 162:214–224
Wang F, Zhang X, Zhang S, Zhang S, Sun Y (2020a) Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 254:126791. https://doi.org/10.1016/j.chemosphere.2020.126791
Wang W, Ge J, Yu X, Li H (2020b) Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci Total Environ 708:134841
Wang X, Zheng H, Zhao J, Luo X, Wang Z, Xing B (2020c) Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelusmoara) through disrupting hepatic lipid homeostasis. Environ Sci Technol 54(10):6202–6212
Wei R, Zimmermann W (2017) Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol 10(6):1308–1322
Wong JKH, Lee KK, Tang KHD, Yap PS (2020) Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci Total Environ 719:137512
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51:6634–6647
Wu J, Liu W, Zeb A, Lian J, Sun Y, Sun H (2021) Polystyrene microplastic interaction with Oryza sativa: toxicity and metabolic mechanism. Environ Sci Nano 8(12):3699–3710
Wu X, Zhao X, Chen R, Liu P, Liang W, Wang J et al (2022a) Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: a critical review. Water Res 221:118825
Wu X, Hou H, Liu Y, Yin S, Bian S, Liang S et al (2022b) Microplastics affect rice (Oryza sativa L.) quality by interfering metabolite accumulation and energy expenditure pathways: a field study. J Hazard Mater 422:126834
Wu X, Lyu X, Li Z, Gao B, Zeng X, Wu J, Sun Y (2020) Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci Total Environ 707:136065
Xu G, Liu Y, Yu Y (2021) Effects of polystyrene microplastics on uptake and toxicity of phenanthrene in soybean. Sci Total Environ 783:147016
Yao L, Hui L, Yang Z, Chen X, Xiao A (2020) Freshwater microplastics pollution: Detecting and visualizing emerging trends based on Citespace II. Chemosphere 245:125627
Yu H, Zhang X, Hu J, Peng J, Qu J (2020) Ecotoxicity of polystyrene microplastics to submerged carnivorous Utricularia vulgaris plants in freshwater ecosystems. Environ Pollut 265:114830
Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y (2018) Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol 200:28–36
Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F (2020) Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ 715:136968
Zhang GS, Zhang FX (2020) Variations in aggregate-associated organic carbon and polyester microfibers resulting from polyester microfibers addition in a clayey soil. Environ Pollut 258:113716. https://doi.org/10.1016/j.envpol.2019.113716
Zhang GS, Zhang FX, Li XT (2019) Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci Total Environ 670:1–7
Zhang C, Chen X, Wang J, Tan L (2017) Toxic effects of microplastic on marine microalgae Skeletonemacostatum: interactions between microplastic and algae. Environ Pollut 220:1282–1288. https://doi.org/10.1016/j.envpol.2016.11.005
Zhang Q, Zhao M, Meng F, Xiao Y, Dai W, Luan Y (2021) Effect of polystyrene microplastics on rice seed germination and antioxidant enzyme activity. Toxics 9(8):179
Zhao L, Rong L, Xu J, Lian J, Wang L, Sun H (2020) Sorption of five organic compounds by polar and nonpolar microplastics. Chemosphere 257:127206
Zhou Y, Liu X, Wang J (2020) Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J Hazard Mater 392:122273. https://doi.org/10.1016/j.jhazmat.2020.122273
Zhou J, Wen Y, Marshall MR, Zhao J, Gui H, Yang Y et al (2021a) Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci Total Environ 787:147444
Zhou J, Gui H, Banfield CC, Wen Y, Zang H, Dippold MA et al (2021b) The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil BiolBiochem 156:108211
Zhu F, Zhu C, Wang C, Gu C (2019) Occurrence and ecological impacts of microplastics in soil systems: a review. B Environ Contam Tox 102:741–749
Author information
Authors and Affiliations
Contributions
Kamini Devi, Arun Dev Singh, Shalini Dhiman, Jaspreet Kour, Tamanna Bhardwaj, Neerja Sharma, Isha Madaan, Kanika Khanna: methodology and writing; Puja Ohri, Amrit Pal Singh, Geetika Sirhindi, Vinod Kumar: reviewing and editing; Renu Bhardwaj: editing and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors of this paper consent to participate.
Consent for publication
All authors of this manuscript have consented to its publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, ., Singh, A.D., Dhiman, S. et al. Current studies on the degradation of microplastics in the terrestrial and aquatic ecosystem. Environ Sci Pollut Res 30, 102010–102026 (2023). https://doi.org/10.1007/s11356-023-29640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29640-0