Abstract
Microplastics are a global environmental pollution. Due to this fact, new solutions are needed to reduce the amount in various aquatic environments. A new concept introduced by Herbort and Schuhen from the year 2016 describes the agglomeration of microplastics in water using silicon-based precursors. In the study presented here, alkyltrichlorosilanes with different linear and branched alkyl groups and a chain length between 1 and 18 carbon (C-) atoms are investigated for their suitability to fix microplastics (mixtures of polyethylene (PE) and polypropylene (PP)) and to form larger agglomerates. As the alkyl group has a major influence on the reaction rate and agglomeration behavior, we present here the extensive data collection of the evaluation of the best case. The removal efficiency is determined gravimetrically. The reaction behavior and the fixation process are characterized by hydrolysis kinetics. 29Si-MAS-NMR spectroscopy, IR spectroscopy, and thermogravimetry (TGA) are used to characterize the chemical composition of the agglomerates. Finally, the use of optical coherence tomography (OCT) allows the visualization of the formed agglomerates. The results show that the different alkyl groups have a strong impact on the suitability of the alkyltrichlorosilanes for the agglomeration, as they influence the hydrolysis and condensation kinetics in water and the affinity to the microplastics. Best suited for microplastic removal were intermediate chain length between 3 and 5 C-atoms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microplastics in the aquatic environment
Microplastics are currently one of the biggest and most discussed environmental problems. Since the start of mass production of plastic in the 1950s, it has become an integral part of everyday life (Geyer et al. 2017; Thompson et al. 2004). By definition, the collective term “plastic” refers to synthetically produced organic chemical polymer compounds of anthropogenic origin. Large quantities of plastic, including plastic waste, are released into the environment because of improper disposal and lack of waste management systems. A particular problem here is the persistence of plastics against environmental influences (Fig. 1). This results in poor degradability, which means that the plastic remains in the environment for a very long time, fragments over time, and forms ever smaller plastic particles (particles < 5 mm are referred to as microplastics) (Barnes et al. 2009; Thompson et al. 2004). Microplastics can also be introduced directly into the environment, e.g., as an ingredient of cosmetics, as textile fibers or tire abrasion (Herbort et al. 2017; Sundt et al. 2014). Through transport processes, the plastics or microplastics are distributed in the environment and have already been detected in all parts of the environment. Thus, ecosystems and organisms are exposed to microplastics, which they have a negative effect on (Galloway et al. 2017; Law and Thompson 2014; Horton et al. 2017). Due to the continuously increasing inputs and constant fragmentation, there is an ever-increasing burden of microplastics on the environment (Thompson et al. 2004).
Summary of properties, degradation, transport, and effects of microplastics in the environment (Bergmann et al. 2015)
Due to the heterogeneous distribution of microplastics in the environment and the non-standardized sampling and detection methods, the information on the concentrations of microplastics in the environment shows high variations among different studies. For example, studies in the Mediterranean Sea found values ranging from 0.17 ± 0.32 to 3.13 ± 4.95 microplastic particles / m3 (Suaria et al. 2016).
Different studies showed that industrial and municipal wastewater treatment plants are important point sources for the discharge of microplastics into to the environment (Lechner and Ramler 2015; Browne et al. 2011). At the sampling of the effluent of 12 municipal wastewater treatment plants in Germany concentrations between 0.26 and 13.7 microplastic particles/liter (MP/l) were found, while a study in Denmark found 29–447 microplastic particles/l (Mintenig et al. 2016; Simon et al. 2018).
In order to reduce the input into the environment, it is necessary to remove microplastics from the water of point dischargers, such as municipal and industrial wastewater treatment plants (Talvitie et al. 2017). Due to the fact that microplastics can also be a disruptive factor (fouling, contamination, and resulting health risk) in water use processes (e.g., water treatment or sea salt extraction), the particles need to be removed previously (Ma et al. 2019; Amy et al. 2017; Peixoto et al. 2019; Barboza et al. 2018; Iñiguez et al. 2017). Commonly used flocculants turned out to be ineffective and/or cost-intensive for the total sum of microplastic particles, which calls for novel methods for microplastics coagulation (Ma et al. 2019).
How does the novel agglomeration-fixation reaction work?
Inorganic-organic hybrid silica gels are compounds of an inorganic silicon-oxygen compound with one, two, or three organic functionalities (Brinker and Scherer 1990, Iler 2004). In the sol-gel process, an inorganic-organic macromolecule in the form of a highly cross-linked solid is formed by successive hydrolysis and condensation of the precursors (Fig. 2).
Schematic representation of the reaction course of the sol-gel process (Bögershausen 2004)
In the first reaction step, leaving groups are hydrolyzed to highly reactive silanol groups, which connect the precursors in the second reaction step to each other by forming siloxane bonds. This leads to the growth of a three-dimensional network, a so-called hybrid silica. By adjusting the sol-gel conditions such as the proportion of water, the solvent, the temperature, the pH value, the addition of catalysts and the precursor/solvent ratio, the reaction can be controlled in terms of reaction speed and product formation, among other things (Iler 2004, Sakka 2008).
The choice of the leaving group (e.g., -Cl, -OR, -OH) is decisive for the consideration of hydrolysis and condensation kinetics (Perchacz et al. 2015; Corriu and Nguyên 2009; Brinker 1988; Hench and West 1990). Depending on the choice of the leaving group and the influence of the organic part, the reactivity of the precursors and thus the structure of the hybrid silica gel and its inclusion behavior toward particles, e.g., microplastics, can be influenced (Herbort and Schuhen 2016; Herbort et al. 2018a, b). The reason for this behavior lies in steric effects, inductive effects, resonance effects, and their polarizability (Hook 1996; Loy et al. 2000). The organic unit can additionally induce a self-organization process. The pre-organizing function is generated by van der Waals interactions, hydrogen bonds, and/or dipole-dipole interactions (Moreau et al. 2004; Hoffmann et al. 2006).
Silicon-based precursors for microplastic reduction (Wasser 3.0 PE-X)
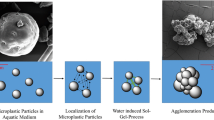
A conceptual approach of Herbort and Schuhen from the year 2016 describes the use of silicon-based precursors for microplastic elimination and was adapted for the technical application in 2018 (Herbort and Schuhen 2016, Schuhen 2016, Herbort et al. 2018a, b). The concept consists of two process steps, taking place during the sol-gel process of the silicon-based precursors in water: particle localization and immediate fixation. When added to water, the organosilanes attached to the surface of the microplastic particles due to the affinity of their organic groups to the surface of the microplastics by van-der-Waals interactions (self-organization). Subsequently, the water-induced sol-gel process described in the previous section leads to the linkage of the silicon-based precursors to each other. Thus, colliding microplastic particles attach to each other and collect in large agglomerates (localization), which are chemically bound by the ongoing sol-gel process (fixation). This results in the formation of large agglomerates (> 1 cm) which can be removed using a simple and, therefore, more cost-effective filtration process (Herbort et al. 2018b). Initially, the precursors were alkoxy-silyl-functionalized derivatives with a large organic-chemical residue containing different heteroatoms (e.g., O, N) (Fig. A1). In the course of adapting the concept of agglomeration fixation to polymer mixtures, the use of different alkyltrichlorosilanes provides the best results (scheme 1).
Materials and methods
Chemicals and reagents
The company LyondellBasell (Basell Polyolefine GmbH, Frankfurt am Main, Germany) provided PE (LDPE, HDPE) and PP polymers with a size of 1 μm to 1 mm. The alkyltrichlorosilanes used are purchased from the company abcr GmbH (Karlsruhe, Germany) (Table 1).
Overview of analytical methods and processes
The microplastic-free hybrid silica gel samples used for the analysis were produced analogously to the experimental setup for determining the quantitative determination of polymer removal (2.3) without the addition of polymers.
IR spectra (4500–600 cm−1, resolution 1 cm−1) of the aggregates and hybrid silica gels were recorded with the ATR-FTIR spectrometer Vertex 70, Bruker, Ettlingen, Germany. The aggregates and gels had previously been dried for 24 h at 105 °C.
FK-NMR spectra were recorded with the device Avance 400 WB, Bruker, Ettlingen, Germany. For the 29Si SP MAS measurement, a 4 mm MAS-NMR sample head with 4 mm zirconium dioxide rotors and a rotation frequency of 3 kHz was used. The spectra were obtained at 79.5 MHz and π/2 pulse (5 ms) and recycle delay of 30 s. Hexamethyldisiloxane was used as the standard. The aggregates and hybrid silica gels had previously been dried for 24 h at 105 °C. The peaks are divided into T-peaks according to the number of siloxane bonds (Hook 1996; Loy et al. 2000).
Images of the formed aggregates (in wet state) were taken by optical coherence tomography (OCT) (GANYMEDE I, Thorlabs GmbH, Lübeck, Germany). An area of 6.5 × 7 × 1 mm was scanned with a resolution of 10 μm on the x- and y-axis and 2.08 μm on the z-axis.
Thermogravimetry (TGA) was measured with a Q5000 IR from TA Instruments. Starting temperature was at 45 °C, purge gas 1: nitrogen 5.0; 25 ml/min with a heating rate of 20 °C/min. The gas switching temperature was at 800 °C, purge gas 2: oxygen 5.0; 0.25 ml/min with a heating rate of 20 °C/min and final temperature of 950 °C. A platinum crucible was used.
Evaluation of polymer removal efficiency from water
To determine the removal efficiency of the polymers, 1 L of distilled water was placed in a 2 L beaker (high form), mixed with 100 mg polymer (PE, PP) and stirred for 5 min at RT. Then 300 μl of the respective alkyltrichlorosilane were added. After 20 min, the entire contents were filtered through an analysis sieve (stainless steel, mesh size 1 mm, diameter 20 cm) to remove the aggregates > 1 mm. All aggregates larger than 1 mm remain in the sieve and are classified as removed polymer agglomerates, as first pilot plant tests showed that particles > 1 mm can be removed easily with low technical effort. The filtrate was discharged into a filter crucible (porosity 4, max. pore size 16 μm). Beaker and sieve were thoroughly rinsed with distilled water. The filter crucible was rinsed with isopropanol to dissolve possible organosilicon-based adhesions, dried for 24 h at 105 °C and weighed. By subtracting the tare weight, the mass of the polymer in the crucible, which is considered as free polymer, can be determined. Figure 3 illustrates the test procedure. The experiments were repeated three times. In addition, for economic and ecological reasons, the tests for the alkyltrichlorosilanes with the best removal efficiencies (> 95%) were carried out with a reduced application quantity of 100 μl to distinguish which alkyltrichlorosilanes has the best performance at a lower concentration.
Determination of hydrolysis rates
To determine the hydrolysis rates, 1 L of distilled water was placed in a 2 L beaker (high form), 300 μl of the respective organosilane added and stirred for 20 min at RT. The conductivity is measured at defined intervals with a WTW Cond 3110, Xylem Analytics Germany Sales GmbH & Co. KG, WTW, Weilheim, Germany. The change in conductivity is due to the HCl formed during the hydrolysis of the chlorosilane groups (Si-Cl). Based on the change in conductivity, a 1st order reaction kinetics can be adapted for hydrolysis and the reaction constant (k) determined (Eq. 1). The half-life (t1/2) was determined according to Eq. 2. The experiments were performed simply for all precursors, in addition, isobutyltrichlorosilanes and n-octadecyltrichlorosilanes were analyzed three times in order to be able to give an error estimate.
Results and discussion
Determination of removal efficiencies
After addition to the water, the hydrophobicity of the alkyl group causes the alkyltrichlorosilanes to adhere to the polymer particles suspended in the water and bond them to agglomerates via self-organization processes (localization). The agglomerates formed are then chemically bound in a water-induced sol-gel process to form an inclusion compound of hybrid silica gels and polymers (fixation).
The starting point of the reaction is the hydrolysis of the chlorosilanes and the formation of silanols with the release of HCl (Scheme 1) (Pietschnig and Spirk 2016; Herbort et al. 2018b). The formation of silanols also increases water solubility and supports the agglomeration (localization) of polymer particles (Hurkes et al. 2014). Silanols have a high reactivity and cross-link through condensation, which is additionally catalyzed by the previously formed HCl, to three-dimensional hybrid silica gels, which fix the localized polymer particles.
For optimal removal, the gel formation must take place in a time window that enables a large quantity of polymer particles to be localized in the agglomerates and fixed in the course of gel formation. If the gel formation takes place too quickly, there is not enough time for the localization of the particles. If the gel formation is too slow, the particles cannot be fixed. Both lead to poor removal efficiencies and poor reproducibility. Table 2 and Fig. A2, A3 summarizes the measured removal efficiencies.
Besides the removal efficiencies, also the standard deviation is a good indicator to evaluate the fixation process. The standard deviations are caused by uncontrollable factors of the experiment as attachment of plastic particles to the glass wall and turbulent flow conditions. The turbulent flow conditions cause a random movement and collision of the microplastic particles and agglomerates. Additionally, already formed aggregates are repeatedly drawn into the agitator and broken up. Alkyltrichlorosilanes with suitable reaction kinetics can compensate for these random factors, as they leave enough time for the agglomerates to collect all particles and reunite when broken up. For this reason, alkyltrichlorosilanes with high removal efficiencies show lower standard deviations as the ones with low removal efficiencies. As PE and PP are both completely nonpolar and with a similar density, the differences between both polymers are caused by uncontrollable factors of the experiment.
An optimal reaction process and the best results for polymer elimination (PE, PP) can be observed with n-propyltrichlorosilane, n-butyltrichlorosilane, isobutyltrichlorosilane, and pentyltrichlorosilane (isomers). Here, consistently good removal efficiencies are achieved with mean values of more than 95% and with low fluctuations with regard to the reproducibility of the values.
For methyltrichlorosilane, n-octyltrichlorosilane and n-decyltrichlorosilane, n-undecyltrichlorosilane, n-dodecyltrichlorosilane, n-hexadecyltrichlorosilane, n-octadecyltrichlorosilane (experiments at higher concentration (300 μl/l), a rapid gel formation can be observed within 5 to 20 s after addition to water. The fast gel formation and fixation does not leave enough time for the microplastics to be localized in the agglomerates. The gel is in the form of white flocks, which float finely distributed in the water and do not interact with microplastic particles after the gelation. This causes an entry of those hybrid silica gel particles smaller than 1 mm into the filter crucible, which cannot be completely removed by rinsing with isopropanol and additionally increases the standard deviations. This leads to poor removal efficiencies, high standard deviations, and poor reproducibility. Due to the shortest alkyl residue and the associated lower affinity to the polymer particles used, methyltrichlorosilane shows the lowest removal efficiencies of all alkyltrichlorosilanes in this series with 20.6 ± 14.9% for PE and 7.7 ± 7.6% for PP.
Isopropyltrichlorosilane and isobutyltrichlorosilane show worse removal efficiencies as the alkyltrichlorosilanes with respective linear alkyl groups. The stabilizing effects of the branching cause a slower condensation as they stabilize the formed silanols via steric and inductive effects and slow down the condensation process (Hurkes et al. 2014). This leads to incomplete fixation and the formation of soft and unstable aggregates. A incomplete fixation can also be observed with n-hexyltrichlorosilane, where the hexyl groups have a stabilizing effect on the silanols.
The effect of too slow fixation is evens stronger with thexyltrichlorosilane and t-butyltrichlorosilane, as they have a stronger branching and the dimethyl group is directly after the silicon atom inhibits the condensation process by steric protection (Hurkes et al. 2014). t-Butyltrichlorosilane dissolves in the water after 1 min, as it forms a water stable silanol. The formed agglomerates are not stable and disintegrate continuously. Thexyltrichlorosilane remains liquid during the entire reaction and, due to the strong Si-Si interactions, is deposited on the glass wall, where it collects the polymer particles due to the hydrophobicity of the thexyl group. Thus, it is not detected as a free polymer.
An exception was 3,3-dimethylbutylchlorosilane. Due to its well suitable reaction kinetics, a reasonable agglomeration fixation of PE or PP could be observed. However, due to the high density of the hybrid silica gel formed, the aggregates formed sink and are broken up by the agitator continuously. This leads to a lower removal efficiency and higher standard deviations.
In the experiments with 4 alkyltrichlorosilanes at lower concentrations (Table 3, Fig. A4) only n-butyltrichlorosilane achieves a good removal efficiency of 98.3 ± 1.0%. It is, therefore, best suited for an economically efficient polymer removal from water. It is followed by isobutyltrichlorosilane with 91.4 ± 1.4%. This is caused by the stabilizing effect of the branching in the isobutyl group, which slows down the condensation, respectively with the fixation of polymers. n-Propyltrichlorosilane with 68.5 ± 10.1% and pentyltrichlorosilane(isomers) with 47.3 ± 6.8% performed significantly worse, as they form unstable and small aggregates at lower concentrations.
Hydrolysis rates and NMR analytics
When determining the hydrolysis rates, it must be taken into account that the alkyltrichlorosilanes are not water-soluble and are present in the water in drop form. This results in a phase transition which, in addition to the actual reactivity of the alkyltrichlorosilanes, influences the reaction rate (Kim et al. 1991; Levy and Zayat 2015). The size of the droplets and thus of the interface depends, on the one hand, on the surface tension of the alkyltrichlorosilane but is also influenced by the addition of the alkyltrichlorosilanes and the flow conditions. Immediately after addition, the droplets retain their size and shape, then unite in the flow after the collision or get into the agitator, and are broken up into fine droplets. Some of the droplets remain on the glass wall, where they can form smaller droplets or larger aggregations. The addition by hand and the turbulent flow conditions result in relatively large standard deviations between 21.3% (isobutyltrichlorosilane) and 24.7% (n-octadecyltrichlorosilane). Table A1 summarizes the results of the determination of the hydrolysis rates.
The half-lives of the alkyltrichlorosilanes, which show the best removal efficiencies (n-propyltrichlorosilane, n-butyltrichlorosilane, isobutyltrichlorosilane, and pentyltrichlorosilane (isomers)), are between 18 and 73 s. Other alkyltrichlorosilanes also lie in this range but show worse removal efficiencies (e.g., methyltrichlorosilanes, (3,3-dimethylbutyl)trichlorosilanes, or n-hexadecyltrichlorosilanes). This shows that, in addition to hydrolysis kinetics, condensation kinetics and preorganization also play a decisive role in the fixation process. The hydrolysis rate gives only limited information about the second reaction step in the sol-gel process, condensation (Iler 2004).
Therefore, the poor fixation of the polymer particles by isopropyltrichlorosilane, n-hexyltrichlorosilane, t-butyltrichlorosilane, with a half-life ranging from 7 and 53 s, indicates slow condensation and thus slow fixation. This can be explained by the stabilizing effects of the alkyl groups on the formed silanols through steric and inductive effects.
Thexyltrichlorosilane has the longest half-life of 162 min. The reason for this is that the strongly branched thexyl residue prevents hydrolysis due to its steric hindrance. Due to the slow hydrolysis, no gelation takes place within the standardized reaction time of 20 min selected in our study. This observation explains why in Section 3.1 no solid agglomerates could be observed.
Since n-undecyltrichlorosilane and n-octadecyltrichlorosilane have a half-life of 493 s and 798 s, respectively, the absence of hydrolysis slows down the gelation process. The fact that they form gels within a reaction time of 180 to 600 s indicates a low stability of the silanols formed and a direct condensation. The large hydrophobic alkyl groups lead to low water solubility and strong aggregation of formed silanols. Due to the spatial proximity, condensation can, therefore, take place directly (Loy et al. 2000). Despite the slow hydrolysis, this complicates the interaction with the polymers used and their agglomeration.
Table 4 summarizes the data of the 29Si-MAS-NMR measurements. Since the signals of the spectra cannot be clearly assigned (wide signals, poor S/N ratio), the range of the peak is also given. All spectra show two wide signals in the range around 50 ppm and 60 ppm. The width can be explained by the fact that the chemical environment of the respective silicon atoms varies greatly due to the heterogeneous gel network. This leads to an overlapping of peaks. The signals at 50 ppm are assigned to the T1 units (δ = −48 to −52 ppm) (Hook 1996; Loy et al. 2000). These can be dimers or simply precursor units connected to the hybrid silica gel network. Since the peaks at 60 ppm are also very wide (10–12 ppm), this is a superposition of the stronger T2 units (δ = − 57 to − 59 ppm) and weaker T3 units (δ = − 64 to − 67 ppm). This suggests a mixture of less condensed hybrid silica gel with long-chain structural (intramolecular) units or intermolecular aggregation (Iler 2004).
In the pH range of 3–7, which was present in the investigations, only a small percentage of the silanol groups formed during hydrolysis are protonated. Thus, the catalytic influence of the HCl released can be classified as weak and the condensation is only accelerated marginally. In addition, this supports the formation of long-chained oligomers with low surface charge, which promotes the preorganization with the uncharged surfaces of the microplastic particles and the aggregation of formed aggregates. (Brochier Salon and Belgacem 2011; Hook 1996; Hurkes et al. 2014; Pietschnig and Spirk 2016).
Characterization of agglomerates by OCT, IR spectroscopy, and TGA
The OCT images in Fig. 4 illustrate the compact agglomeration of the microplastic. PP particles have a round shape (left); PE particles are irregularly shaped (right). It can be seen that the cavities between the polymer particles are filled with hybrid silica gel, which fixes it.
The IR spectra of the hybrid silica gels (Fig. 5) show the typical peaks for the siloxane bonds at vasSi–O–Si 1200 cm−1 and a wide peak vasSi–O–Si of 1100–1000 cm−1 (Al-Oweini and El-Rassy 2009). The peaks of the silanol groups can be recognized as broad, flat bands at 3500 cm−1 due to the hydrogen bonds. The peaks of the deformation oscillations of the alkyl groups (δ -CH, δ = CH2) can be seen in the range of 1270–1470 cm−1. The stretching vibrations of the alkyl groups (v -CH) can be found in the range 2680–2850 cm−1. Compared to pure silica, the agglomerate shows much stronger peaks of the alkyl groups at 1375 cm−1 (δ -CH) ,1453 cm−1 (δ -CH), and 2950–2838 cm−1 (v -CH), induced by the polypropylene contained in the agglomerate. In addition, the Si–O–Si peaks of the hybrid silica are clearly visible in the agglomerate. The IR spectra thus confirm the fixation of the polymer particles (e.g., PP) in the hybrid silica gel network.
Thermogravimetry coupled with differential scanning calorimetry (TGA-DSC) is suitable for characterizing microplastics > 12 μm more closely. For example, the most common plastics PE and PP can be detected accurately. However, it is unsuitable for use in multi-substance wastewater because of strong interference (Majewsky et al. 2016).
The TGA analysis of our agglomeration-fixation products compared to starting materials shows a strong weight loss between 380 °C and 500 °C for the pure plastic particles (99.96%, PE)/99.97%, PP), which consist almost exclusively of organic material. The inorganic component (0.16% (PE)/0.04% (PP)) due to additives and impurities is negligible (Table 5).
The TGA curve for the hybrid silica (C1, C4, C18) shows a slight decrease with increasing temperatures. The strongest is in the range between 500 °C and 625 °C. At these temperatures, the organic ingredient (e.g., 14.35%, C1) of the hybrid silica gel is expelled as CO2. The remainder (e.g., 84.21%, C1) remains as an inorganic ingredient, which is substantially higher than the organic constituent.
For the products of our agglomeration-fixation process (e.g., C1 PE, C1 PP), the organic constituents are significantly higher than in the hybrid silica gel without microplastic inclusion. Accordingly, the inorganic component is much lower. The TGA analysis finally confirms that a mixed compound of hybrid silica and microplastic was formed.
Conclusion
In summary, it can be said that there are characteristic differences between the various alkyltrichlorosilanes with respect to their suitability for localization and fixation of polymer particles and their reaction behavior in water. This is due to the influence of the alkyl group on the reaction kinetics in the sol-gel process via steric and inductive effects. Both hydrolysis and condensation kinetics have a decisive influence. Pre-organizing effects of the alkyl groups also have an effect on reaction behavior and the locating of polymer particles.
Particularly well suited for polymer particle fixation are n-propyltrichlorosilane, n-butyltrichlorosilane, isobutyltrichlorosilane, and pentyltrichlorosilane (isomers), whereby n-butyltrichlorosilanes provide the best removal efficiencies even at lower alkyltrichlorosilane concentrations.
Branches of the alkyl group show a slowing effect on the hydrolysis and condensation kinetics. This is particularly strong in the case of the strongly branched thexyl and t-butyl groups. Isopropyltrichlorosilane also provides worse results with regard to polymer elimination than n-propyltrichlorosilane due to slower condensation. With n-butyltrichlorosilane and isobutyltrichlorosilane, this effect is also visible at the lower concentration of alkyltrichlorosilane.
Long alkyl groups of 8 or more carbon atoms and the short-chain methyl group proved to be unsuitable because the microplastic particles are not localized sufficiently here, resulting in significantly lower removal efficiencies and high fluctuations within the process. The reasons for this include rapid condensation and the increase in substituent effects.
In future studies, this process is transferred to environmental samples. The first applications are in wastewater and seawater. Simultaneously, a scale up on pilot plant scale is implemented, which allows to run tests at environmental microplastics concentrations and to optimize the process for a large-scale applications.
References
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J Mol Struct 919(1–3):140–145. https://doi.org/10.1016/j.molstruc.2008.08.025
Amy G, Ghaffour N, Li Z, Francis L, Linares RV, Missimer T, Lattemann S (2017) Membrane-based seawater desalination: present and future prospects. Desalination 401:16–21. https://doi.org/10.1016/j.desal.2016.10.002
Barboza LGA, Dick Vethaak A, Lavorante BRBO, Lundebye A-K, Guilhermino L (2018) Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar Pollut Bull 133:336–348. https://doi.org/10.1016/j.marpolbul.2018.05.047
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B Biol Sci 364(1526):1985–1998. https://doi.org/10.1098/rstb.2008.0205
Bergmann M, Gutow L, Klages M (2015) Marine anthropogenic litter. https://doi.org/10.1007/978-3-319-16510-3
Bögershausen A (2004) Sol-Gel-Synthese und Poreneigenschaften von anorganisch organischen Hybridgelen für die kontrollierte Wirkstofffreisetzung. Westfälische Wilhelms-Universität Münster, Münster
Brinker CJ (1988) Hydrolysis and condensation of silicates: effects on structure. J Non-Cryst Solids 100(1–3):31–50. https://doi.org/10.1016/0022-3093(88)90005-1
Brinker CJ, Scherer GW (1990) Sol-gel science. The physics and chemistry of sol-gel processing. Academic, Boston
Brochier Salon M-C, Belgacem MN (2011) Hydrolysis-condensation kinetics of different Silane coupling agents. Phosphorus Sulfur Silicon Relat Elem 186(2):240–254. https://doi.org/10.1080/10426507.2010.494644
Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R (2011) Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ Sci Technol 45(21):9175–9179. https://doi.org/10.1021/es201811s
Corriu R, Nguyên TA (2009) Molecular chemistry of sol-gel derived nanomaterials. Wiley, Chichester
Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5):116. https://doi.org/10.1038/s41559-017-0116
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3(7):e1700782. https://doi.org/10.1126/sciadv.1700782
Hench LL, West JK (1990) The sol-gel process. Chem Rev 90(1):33–72. https://doi.org/10.1021/cr00099a003
Herbort AF, Schuhen K (2016) A concept for the removal of microplastics from the marine environment with innovative host-guest relationships. Environ Sci Pollut Res 24(12):1–5. https://doi.org/10.1007/s11356-016-7216-x
Herbort AF, Sturm MT, Hiller C, Schuhen K (2017) Ökologische Chemie von Mikroplastik – Ab wann werden Alltagshelfer zum Umweltproblem? Nachhaltiger Gewässerschutz durch die Implementierung einer Partikelelimination in der Kläranlage. GWF Abwasser/Wasser:1–9
Herbort AF, Sturm MT, Fiedler S, Abkai G, Schuhen K (2018a) Alkoxy-silyl induced agglomeration: a new approach for the sustainable removal of microplastic from aquatic systems. J Polym Environ 62(8):1–13. https://doi.org/10.1007/s10924-018-1287-3
Herbort AF, Sturm MT, Schuhen K (2018b) A new approach for the agglomeration and subsequent removal of polyethylene, polypropylene, and mixtures of both from freshwater systems - a case study. Environmental science and pollution research international (25(15)):15226–15234. https://doi.org/10.1007/s11356-018-1981-7
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based Mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed 45(20):3216–3251. https://doi.org/10.1002/anie.200503075
Hook RJ (1996) A 29Si NMR study of the sol-gel polymerisation rates of substituted ethoxysilanes. J Non-Cryst Solids 195(1–2):1–15. https://doi.org/10.1016/0022-3093(95)00508-0
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ 586:127–141. https://doi.org/10.1016/j.scitotenv.2017.01.190
Hurkes N, Ehmann HMA, List M, Spirk S, Bussiek M, Belaj F, Pietschnig R (2014) Silanol-based surfactants: synthetic access and properties of an innovative class of environmentally benign detergents. Chemistry (Weinheim an der Bergstrasse, Germany) 20(30):9330–9335. https://doi.org/10.1002/chem.201402857
Iler RK (2004) The chemistry of silica. In: Solubility, polymerization, colloid and surface properties, and biochemistry, [Nachdr]. Wiley, New York
Iñiguez ME, Conesa JA, Fullana A (2017) Microplastics in Spanish table salt. Sci Rep 7(1):8620. https://doi.org/10.1038/s41598-017-09128-x
Kim K, Jang KY, Upadhye RS (1991) Hollow silica spheres of controlled size and porosity by sol-gel processing. J Am Ceram Soc 74(8):1987–1992. https://doi.org/10.1111/j.1151-2916.1991.tb07819.x
Law KL, Thompson RC (2014) Oceans. Microplastics in the seas. Science (New York, NY) 345(6193):144–145. https://doi.org/10.1126/science.1254065
Lechner A, Ramler D (2015) The discharge of certain amounts of industrial microplastic from a production plant into the river Danube is permitted by the Austrian legislation. Environmental pollution (barking, Essex: 1987) 200:159–160. https://doi.org/10.1016/j.envpol.2015.02.019
Levy D, Zayat M (2015) The sol-gel handbook. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Loy DA, Baugher BM, Baugher CR, Schneider DA, Rahimian K (2000) Substituent effects on the sol−gel chemistry of organotrialkoxysilanes. Chem Mater 12(12):3624–3632. https://doi.org/10.1021/cm000451i
Ma B, Xue W, Hu C, Liu H, Qu J, Li L (2019) Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J 359:159–167. https://doi.org/10.1016/j.cej.2018.11.155
Majewsky M, Bitter H, Eiche E, Horn H (2016) Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci Total Environ 568:507–511. https://doi.org/10.1016/j.scitotenv.2016.06.017
Mintenig SM, Int-Veen I, Loder MGJ, Primpke S, Gerdts G (2016) Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. https://doi.org/10.1016/j.watres.2016.11.015
Moreau JJE, Pichon BP, Man MWC, Bied C, Pritzkow H, Bantignies J-L, Dieudonné P, Sauvajol J-L (2004) A better understanding of the self-structuration of bridged silsesquioxanes. Angew Chem Int Ed 43(2):203–206. https://doi.org/10.1002/anie.200352485
Peixoto D, Pinheiro C, Amorim J, Oliva-Teles L, Guilhermino L, Vieira MN (2019) Microplastic pollution in commercial salt for human consumption: a review. Estuar Coast Shelf Sci 219:161–168. https://doi.org/10.1016/j.ecss.2019.02.018
Perchacz M, Beneš H, Kobera L, Walterová Z (2015) Influence of sol–gel conditions on the final structure of silica-based precursors. J Sol-Gel Sci Technol 75(3):649–663. https://doi.org/10.1007/s10971-015-3735-z
Pietschnig R, Spirk S (2016) The chemistry of organo silanetriols. Coord Chem Rev 323:87–106. https://doi.org/10.1016/j.ccr.2016.03.010
Sakka S (2008) Sol–gel technology as representative processing for nanomaterials: case studies on the starting solution. J Sol-Gel Sci Technol 46(3):241–249. https://doi.org/10.1007/s10971-007-1651-6
Schuhen K (2016) Hybridkieselsäurematerial, insbesondere zur Fixierung anthropogener Verunreinigungen aus einem aquatischen Umfeld WO2016166219 (A1).
Simon M, van Alst N, Vollertsen J (2018) Quantification of microplastic mass and removal rates at wastewater treatment plants applying focal plane Array (FPA)-based Fourier transform infrared (FT-IR) imaging. Water Res 142:1–9. https://doi.org/10.1016/j.watres.2018.05.019
Suaria G, Avio CG, Mineo A, Lattin GL, Magaldi MG, Belmonte G, Moore CJ, Regoli F, Aliani S (2016) The Mediterranean plastic soup: synthetic polymers in Mediterranean surface waters. Sci Rep 6:37551. https://doi.org/10.1038/srep37551
Sundt P, Schulze P-E, Syversen F (2014) Sources of microplastics-pollution to the marine environment. http://www.miljodirektoratet.no/Documents/publikasjoner/M321/M321.pdf. Accessed 6 Dec 2016
Talvitie J, Mikola A, Koistinen A, Setälä O (2017) Solutions to microplastic pollution - removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res 123:401–407. https://doi.org/10.1016/j.watres.2017.07.005
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG (2004) Lost at sea: where is all the plastic? Science 304(5672):838. https://doi.org/10.1126/science.1094559
Funding
The research projects of Wasser 3.0 (www.wasserdreinull.de) are conducted by means of the financial support by the German Federal Ministry for Economic Affairs and Energy through the provision of ZIM (Central Innovation Program for SME) project funds. The enterprise abcr GmbH (www.abcr.de) from Karlsruhe (GERMANY) is directly involved in the project as an industrial partner for the material science scale-up. Michael Sturm thanks the German Federal Environmental Foundation (DBU) for the support with a PhD scholarship (reference number: 80018/174). The authors thank Prof. Dr. Gisela Guthausen from Karlsruhe Institute of Technology (KIT) / Pro2NMR for her contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 204 kb)
Rights and permissions
About this article
Cite this article
Sturm, M.T., Herbort, A.F., Horn, H. et al. Comparative study of the influence of linear and branched alkyltrichlorosilanes on the removal efficiency of polyethylene and polypropylene-based microplastic particles from water. Environ Sci Pollut Res 27, 10888–10898 (2020). https://doi.org/10.1007/s11356-020-07712-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07712-9