Abstract
Barium (Ba) is a non-essential element that can cause toxicity in living organisms and environmental contamination. Plants absorb barium predominantly in its divalent cationic form Ba2+. Sulfur (S) can decrease the availability of Ba2+ in the soil by causing its precipitation as barium sulfate, a compound known for its very low solubility. The objective of this study was to evaluate the effect of soil sulfate supply in soil Ba fractions, as well as on plant growth, and Ba and S uptake by lettuce plants grown in artificially Ba-contaminated soil under greenhouse conditions. The treatments consisted of five Ba doses (0, 150, 300, 450, and 600 mg kg−1 Ba, as barium chloride) combined with three S doses (0, 40, and 80 mg kg−1 S, as potassium sulfate). The treatments were applied to soil samples (2.5 kg) and placed in plastic pots for plant cultivation. The Ba fractions analyzed were extractable-Ba, organic matter-Ba, oxides associated-Ba, and residual-Ba. The results indicate that the extractable-Ba fraction was the main one responsible for Ba bioavailability and phytotoxicity, probably corresponding to the exchangeable Ba in the soil. The dose of 80 mg kg−1 of S reduced extractable-Ba by 30% at higher Ba doses while it increased the other fractions. Furthermore, S supply attenuated the growth inhibition in plants under Ba exposure. Thus, S supply protected the lettuce plants from Ba toxicity by reduction of Ba availability in soil and plant growth enhancement. The results suggest that sulfate supply is a suitable strategy for managing Ba-contaminated areas.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barium (Ba, CAS number 7440-39-3) is a non-essential metal element that may cause harmful effects in living organisms and ecosystems. Chronic exposure to Ba results in hypertension, cardiovascular diseases, and other related health problems in humans and animals (WHO 2022). Furthermore, high Ba concentrations can inhibit plant growth and development (Peana et al. 2021; Sleimi et al. 2021). The recommended environmental safety limit values range from 0.7 to 1.3 mg L−1 Ba in water (USEPA 2004; CONAMA 2005; WHO 2022) and are around 330 mg kg−1 Ba in soils (USEPA 2005), with prevention and investigation threshold concentrations in Brazilian soils being set at 150 and 300 mg kg−1 Ba, respectively (CONAMA 2009). Although Ba has high safety values compared to other contaminants, some studies have reported Ba concentrations above the recommended limits in soils and water bodies contaminated with industrial, mining, and domestic waste (Lamb et al. 2013; Ferreira et al. 2020; Viana et al. 2021). Thus, Ba contamination can be an environmental and public health problem, mainly in waste deposits with high Ba concentrations.
Although Ba has no known benefits for plants, it can be absorbed, probably as Ba2+, a divalent cation similar to nutrients like Ca2+ and Mg2+ (Kabata-Pendias 2010). Indeed, results of sequential extraction experiments indicated that the exchangeable Ba fraction plays an important role in the retention of Ba in a tropical climate (Gong et al. 2020). Barium plant uptake makes phytoremediation a potential strategy for managing Ba-contaminated areas. Phytoremediation consists of a low-cost and environmentally friendly strategy to remove contaminants from soil and water through plant uptake and extraction (Antoniadis et al. 2017). Previous studies have demonstrated that phytoremediation is a suitable strategy for managing Ba contamination (Carvalho et al. 2019; Ferreira et al. 2019). In contrast, Ba plant uptake might increase risks to humans and animals by the possible ingestion of edible parts with high Ba content (Kravchenko et al. 2014). Thus, studies about Ba uptake are important for phytoremediation and risk assessment of contaminated areas (Myrvang et al. 2016). Among the aspects that influence contaminant uptake and extraction, plant nutritional status has a crucial role, affecting the amount of contaminant extracted through the effect on plant growth and antioxidant systems (Anicésio and Monteiro 2022; Cardoso et al. 2022).
Among the nutrients that enhance plant resistance, sulfur (S) has demonstrated positive effects on plant tolerance to several contaminants (Rabêlo et al. 2018; Gonçalves and Monteiro 2022), including Ba in nutrient solution (de Souza Cardoso and Monteiro, 2021a, b). Some studies indicate that sulfate (SO42−) possibly reduces Ba2+ availability in soils, probably by causing the precipitation of Ba as barium sulfate (Ippolito and Barbarick 2006; Melo et al. 2014; Cappuyns 2018). Thus, S supply may reduce Ba availability in soils, reducing the toxicity risk. In contrast, S can induce Ba accumulation by plant growth improvement (de Souza Cardoso and Monteiro 2021a), enhancing Ba phytoremediation, yet it can also increase Ba content in foods and, thus, toxicity risks. The study of Ba chemical fractions can elucidate the dynamics, mobility, and availability of Ba in soils (Nogueirol and Alleoni 2013); however, the influence of sulfate in Ba fractions was unknown. Here, we evaluated the effect of sulfate supply in Ba chemical soil fractions, as well as on plant growth, and Ba and S uptake by lettuce, an important vegetable and soil pollution bioindicator, cultivated in a tropical soil artificially contaminated with Ba under greenhouse conditions.
Material and methods
Experimental design
The experimental design consisted of a completed randomized design with fifteen treatments and four replicates. The treatments consisted of combinations between five Ba doses (0, 150, 300, 450, and 600 mg kg−1 Ba) and three S doses (0, 40, and 80 mg kg−1 S) in soil, and each experimental unit consisted of one pot with 2.5 kg soil and two plants. The Ba doses were set based on Ba prevention and investigation values established for Brazilian soils (CONAMA 2009), and S doses were determined based on previous studies about S supply in pot experiments (Huang et al. 2021; Cardoso et al. 2022). Barium and S sources were barium chloride (BaCl2·2H2O, CAS number 10326-27-9) and potassium sulfate (K2SO4, CAS number 7778-80-5), respectively, with K and other nutrients being balanced between the treatments (Table S1). The treatments were applied as liquid-solution form along with basic fertilization.
Experimental conditions
Soil characterization
The soil sample was collected in an Oxisol (0–20-cm depth) classified as Rhodic Haplustox under natural vegetation (USDA 1999). The sample was grounded, sieved (4-mm mesh), and mixed for homogenization, and sub-samples were analyzed for chemical and physical attributes. The results are presented in Table S2. For Ba pseudo-total determination, samples (0.5 g) were digested in 10 mL of concentrated hydrochloric and nitric acid solution (HCl/HNO3 ratio 4:1) under heating in a digestion block system (60°C for 180 min, 105°C for 60 min, and 140°C for 30 min) (McGrath and Cunliffe 1985). The analysis quality—QA/QC protocol—was verified with certified soil (NIST SRM 2710a, Montana Soil). After cooling, the extracts were filtered, and the volume was adjusted using deionized water and volumetric flasks (10 mL). The extracts were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES), and Ba concentrations were calculated using a standard curve. The Ba pseudo-total concentration was 3.9 mg kg−1 Ba in soil. For S–SO4 determination, samples (2.5 g) were reacted with 25 mL calcium phosphate (500 mg L−1 P) diluted in an acetic acid solution (2.0 M), and S concentrations were determined by the turbidimetric method (Ajwa and Tabatabai 1993). The S-SO4 concentration was 12 mg kg−1 S in the soil.
Liming and fertilization
Prior to plant cultivation, soil samples were transferred to plastic pots (2.5-L capacity) and received 2.3 g kg−1 of limestone powder (41% CaO and 10.5% MgO), aiming to increase base saturation to 80% for soil acidity correction. Then, the samples were incubated for 20 days. After the incubation period, the samples received the treatments and the following basic fertilization, as liquid-solution form (mg kg−1): 200 N (NH4H2PO4 and NH4NO3), 450 P (NH4H2PO4 and KH2PO4), 300 K (KH2PO4), 0.8 B (H3BO3), 1.3 Cu (CuCl2·2H2O), 3.6 Mn (MnCl2·4H2O), 0.15 Mo (H2MoO4), and 4.0 Zn [(ZnNO3)2] (Table S1). Then, the samples were incubated for 10 days. The soil moisture was maintained at 60% field capacity during the incubation period with deionized water.
Plant cultivation and harvest
Lettuce seeds (Lactuca sativa L. cv. Solaris) were germinated in trays with vermiculite under Hoagland solution irrigation (10% ionic strength) under greenhouse conditions (spring season, 25±3°C, 14 h light/10 h dark). Ten days after germination, three seedlings were selected according to uniformity (two 3-cm-length true leaves) and transplanted to the pots containing the treatments. After 7 days, one seedling was removed, and two seedlings remained per pot until the harvest. The soil moisture was maintained at 60% field capacity at plant cultivation with deionized water. Fifteen days after the transplant, a top-dress soil fertilization with 100 mg kg−1 N (NH4NO3) was applied in solution form. The plants were harvested 30 days after transplant. The shoots were harvested, and the roots were washed using sieves and tap water. Then, shoots and roots were rinsed with calcium chloride solution (2 mM) and deionized water. The plant material was dried for 72 h in an oven with air circulation (65°C) and weighed using a digital scale.
Barium and sulfur in plants
Dried plant material was ground, and samples (0.5 g) were digested in 8 mL of concentrated perchloric and nitric acid solution (HClO4/HNO3 ratio 3:1) under heating in a digestion block system (50, 100, and 150°C for 60 min each, and 200°C until translucent extracts, around 30 min). The analysis followed a rigid QA/QC protocol with the use of a certified reference material (NIST SRM 1573a, Tomato Leaves). After cooling, the plant extract volume was adjusted using volumetric flasks and deionized water (25 mL). The extracts were analyzed by ICP-OES, and element concentrations were calculated using standard curves.
Barium indices in plants
Barium bioconcentration (Ba-BCF) and translocation (Ba-TF) factors were calculated according to Antoniadis et al. (2017) using the following equations: 1. Ba − BCF = [Ba in plants]/[Ba in soil], 2. Ba − TF = [Ba in shoots]/[Ba in roots], where [Ba in plants] is the weighted average of Ba concentrations in plant tissues, [Ba in soil] is the Ba dose applied, and [Ba in shoots] and [Ba in roots] are the Ba concentrations in shoots and roots, respectively.
Sequential extraction of Ba fractions
The sequential extraction was carried out according to Nogueirol and Alleoni (2013) with some modifications. Before seedlings transplant, soil samples (50 g) were collected, dried (room temperature), and ground, and sub-samples (0.5 g) were added to centrifuge tubes (50 mL). The fractions were extracted in the following sequence: Ba in extractable fraction (extractable-Ba, 1); Ba bound to organic matter (organic matter-Ba, 2); Ba bound to Al, Fe, and Mn oxides (oxides associated-Ba, 3); and Ba in residual fraction (residual-Ba, 4). Extractable-Ba was obtained with 15 mL calcium chloride solution (0.1 M CaCl2). Samples were shaken in a horizontal shaker at 120 rpm for 2 h (room temperature), centrifuged (1400 g for 20 min), and supernatants were collected, filtered, and transferred to volumetric flasks (25 mL). Then, samples were resuspended with 5 mL of sodium chloride solution (0.1 M NaCl) in a vortex shaker and centrifuged as described. Supernatants were collected, filtered, and transferred to the previous flasks. The volume was adjusted with deionized water. Then, samples were dried overnight in an oven (60°C) and weighed to check soil loss.
Organic matter-Ba was extracted with 5 mL sodium hypochlorite solution (NaOCl 5% v/v, pH 8.5). The samples were kept in a water bath (90–95°C) for 30 min and, after cooling, were centrifuged, resuspended, and dried, and supernatants were collected as described. The oxides associated-Ba was extracted with 20 mL solution (pH 3.0) with ammonium oxalate [0.2 M (NH4)2C2O4], oxalic acid (0.2 M C2H2O4), and ascorbic acid (0.1 M C6H8O6). The samples were kept in a water bath (90–95°C) for 30 min and, after cooling, were centrifuged, resuspended, and dried, and supernatants were collected as described. At last, the samples were transferred to glass digestion tubes for residual-Ba extraction (pseudo-total concentration). The samples were digested in 10 mL of hydrochloric (HCl, 37% v/v) and nitric acid (HNO3, 65% v/v) solution, HCl/HNO3 ratio 4:1, under heating in a digestion block system (60°C for 180 min, 105°C for 60 min, and 140°C for 30 min). After cooling, the extracts were filtered, and the volume was adjusted as described. The extracts were analyzed by ICP-OES, and Ba concentrations were calculated using a standard curve.
Statistical analysis
Data normality and variance analyses were performed using SAS® Studio (SAS® OnDemand for Academics), and treatments were grouped by the Scott-Knott test (p < 0.05) using the SISVAR® software (Ferreira 2019). Pearson correlation and regression analyses were performed using SAS® Studio.
Results
Plant growth and toxicity symptoms
The treatments severely affected plant growth (Fig. 1). Barium exposure inhibited lettuce growth, mainly in plants cultivated without S supply (Fig. 1(b, c)). Conversely, S doses attenuated Ba toxicity, mainly in plants cultivated under the 80 mg kg−1 S supply. The addition of 600 mg kg−1 Ba decreased the shoot and root dry matter production by 85% in plants grown without S supply, compared with the control treatment (40 mg kg−1 S without Ba addition). However, the same Ba dose decreased shoot and root dry matter productions by 21 and 47%, respectively, in plants grown with 80 mg kg−1 S supply compared with the control treatment. Similarly, Ba doses induced leaf chlorosis, mainly in plants grown without S supply, which was less severe in plants cultivated with S supply (Fig. 1(a)). Interestingly, 40 mg kg−1 S dose presented equivalent results to 80 mg kg−1 S until 450 mg kg−1 Ba addition, while 80 mg kg−1 S dose promoted better plant tolerance at 600 mg kg−1 Ba dose.
Barium and sulfur concentrations
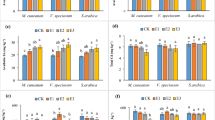
The treatments influenced both Ba and S concentrations in lettuce tissues (Fig. 2). Barium doses increased Ba concentrations in lettuce shoots and roots, mainly in plants grown without S addition. At the same time, this effect was less pronounced under S supply (Fig. 2(a, b)). Plants cultivated under the addition of 600 mg kg−1 without S supply exhibited Ba concentrations of 1.3 g kg−1 Ba in shoots and 3 g kg−1 Ba in roots. In contrast, in plants grown under the same Ba dose with 80 mg kg−1 S supply, the Ba concentrations were 0.5 g kg−1 Ba in shoots and 1.5 g kg−1 Ba in roots. Likewise, in plant growth results, 40 mg kg−1 S dose promoted Ba concentrations in lettuce tissues equivalent to 80 mg kg−1 S until 450 mg kg−1 Ba addition, while 80 mg kg−1 S dose promoted lower Ba concentrations at 600 mg kg−1 Ba. Regarding S in plants, Ba doses decreased S concentrations in shoots and roots. The addition of 600 mg kg−1 Ba decreased S concentrations by 30% in shoots and roots of plants grown with 80 mg kg−1 compared with the same S dose without Ba addition (Fig. 2(c, d)).
Barium indices and accumulation
The treatments influenced the Ba indices and accumulations in lettuce plants (Fig. 3). S supply decreased the Ba accumulation in shoots until 300 mg kg−1 Ba dose, and in roots at 150 mg kg−1 Ba, mainly at 80 mg kg−1 S supply (Fig. 3(a, b)). This S dose decreased the Ba accumulation in shoots by 40% at 300 mg kg−1 Ba and in roots by 54% at 150 mg kg−1 Ba, compared with the same Ba doses without S supply. However, the S supply increased Ba accumulation in plants at higher Ba doses. The 80 mg kg−1 S supply increased the Ba accumulation by 74% in shoots and 6-fold in roots at 600 mg kg−1 Ba dose, compared with the same Ba dose without S supply. Regarding Ba indices, the S supply decreased Ba-BCF, mainly at 80 mg kg−1 S dose (Fig. 3(c)). This S dose decreased the Ba-BCF by 74% in plants grown under 150 mg kg−1 Ba compared with the same Ba dose without S supply. Conversely, the Ba addition increased the Ba-TF by 2.2-fold compared with the control treatment, regardless of Ba and S doses (Fig. 3(d)).
Barium fractionation
The treatments affected all Ba chemical fractions evaluated (Fig. 4). The Ba doses increased all fractions regardless of S supply, with higher increments in extractable-Ba, residual-Ba, and oxides associated-Ba (Fig. 4(a, c, d)). The addition of 600 mg kg−1 Ba combined with 40 mg kg−1 S yielded 352 mg kg−1 of extractable-Ba, 115 mg kg−1 of residual-Ba, 66 mg kg−1 of oxides associated-Ba, and 14 mg kg−1 of organic matter-Ba. In contrast, the S supply decreased extractable-Ba at Ba doses higher than 150 mg kg−1, mainly at 80 mg kg−1 S, while it increased the other fractions. Both 40 and 80 mg kg−1 S doses decreased extractable-Ba by around 25% at 300 and 450 mg kg−1 Ba, compared with the same Ba doses without S supply (Fig. 4(a)). However, just the higher S dose decreased extractable-Ba at 600 mg kg−1 Ba, decreasing it by 30% compared with the same Ba dose without S supply. Conversely, S doses increased by around 2-fold the residual-Ba, oxides associated-Ba, and organic matter-Ba at 450 and 600 mg kg−1 Ba, compared with the same Ba doses without S supply. Analyzing the relative distribution of Ba fractions, we observed that, at Ba doses higher than 150 mg kg−1, the higher S dose decreased extractable-Ba from 77 to 55%, while increased residual-Ba from 15 to 29%, and oxides associated-Ba from 6 to 14% (Fig. 5).
Relationships between Ba in soil, Ba in plants, and lettuce growth
We observed strong negative correlations between Ba concentrations in lettuce tissues and shoot and root productions (Fig. 6). Regarding Ba concentrations in plant tissues and Ba fractions in soil, we observed strong positive correlations between the extractable-Ba fraction and Ba in shoots and roots (Fig. 7), while moderated positive correlations were observed between organic matter-Ba, oxides associated-Ba, and residual-Ba fractions and Ba concentrations in lettuce tissues.
Correlations between Ba fractions in soil and Ba concentrations in shoot (SDM) and root (RDM) dry matter of lettuce grown under Ba and S doses. Extractable-Ba and Ba in SDM (a) and RDM (b); organic matter-Ba and Ba in SDM (c) and RDM (d); oxides associated-Ba and Ba in SDM (e) and RDM (f); and residual-Ba and Ba in SDM (g) and RDM (h). ***p < 0.001
Discussion
Sulfur supply protected lettuce plants from Ba toxicity (Fig. 1), mainly at the higher S dose (80 mg kg−1 S), which represents twice the standard amount for plant cultivation in soils under greenhouse conditions (Huang et al. 2021; Cardoso et al. 2022). Since we did not observe growth inhibition in plants cultivated without S addition, it seems the S naturally available in soil supplied this nutrient for proper lettuce growth (Table S1). However, the growth inhibition in plants cultivated without S addition under Ba exposure suggests that plants supplied just with S naturally available in soil are more susceptible to Ba’s harmful effects, like inhibition in sulfur and magnesium uptake (Fig. 2(c, d), S1d). These results indicate that high S availability can enhance plant resistance to Ba toxicity, probably due to its role in plant growth and influence in Ba availability in soil.
Sulfur is a nutrient for all organisms and plays several functions in plants, mainly due to its structural role in cysteine and methionine amino acids and other compounds related to plant growth, development, and stress resistance (Capaldi et al. 2015; Kopriva et al. 2019). Thus, many studies have reported that S can enhance plant tolerance to contaminants toxicity (Rabêlo et al. 2018; Siddiqui et al. 2019). Regarding Ba, de Souza Cardoso and Monteiro (2021a) suggested that S can reduce its phytotoxicity by immobilization in roots as barium sulfate, which was a mechanism that probably contributed to lettuce resistance in this study, considering the higher Ba accumulation in roots under S supply (Fig. 3(b, c)). In addition, de Souza Cardoso and Monteiro (2021b) demonstrated that S could enhance the antioxidant system of plants to oxidative stress caused by Ba, which possibly contributed to toxicity alleviation in this study, considering the higher S tissues concentrations in plants grown under S supply (Fig. 2(c, d)).
The protective effect of S supply in lettuce under Ba exposure can also be related to its influence on metal availability in soil. Our results indicated that S decreases the Ba availability in soil, as suggested by Melo et al. (2014). The S addition decreased the Ba bioconcentration factor and Ba concentrations in shoots and roots (Figs. 2(a, b) and 3(c)). Furthermore, S supply decreased the Ba accumulation in shoots until 300 mg kg−1 Ba and in roots at 150 mg kg−1 Ba (Fig. 3(a, b)). The lower Ba availability under S supply is also indicated by the reduction in the extractable-Ba fraction (Figs. 4(a) and 5), which was the Ba fraction better correlated with Ba uptake (Fig. 7(a, b)) and growth inhibition (Fig. S2a, b). Thus, the S supply decreased Ba availability and uptake by lettuce plants, protecting the plants from Ba toxicity since the increasing Ba tissue concentrations were related to growth inhibition (Fig. 6(a, b)). Furthermore, the lower Ba uptake by S supply can decrease the Ba toxicity risk for humans and animals by ingesting lettuce cultivated in soil with low Ba concentrations (Fig. S3c).
Many studies have demonstrated the high affinity between Ba2+ cation and SO42− anion in aqueous solutions (Fig. S4), producing barium sulfate or barite (BaSO4), a very-low solubility compost (around 2.5 mg L−1 at 25°C and 1 bar) useful for industrial purposes (He et al. 2014; Corrêa et al. 2022; Ketegenov et al. 2022). This affinity probably was responsible for the decrease in Ba2+ availability, indicated by a reduction of extractable-Ba under S supply, while the BaSO4 produced was probably detected in the less soluble Ba-fractions, mainly the residual-Ba and the oxides associated-Ba (Figs. 4 and 5). Furthermore, the results indicate the potential of CaCl2 0.1 M or similar solutions to evaluate Ba2+ cation or Ba-exchangeable in soils, a fraction that plays a major role in the retention of Ba in soils of tropical climates (Gong et al. 2020). Thus, the extractable-Ba fraction can be used as a Ba toxicity risk indicator, considering that the Ba2+ cation is the main absorbable and toxic Ba form to plants (Llugany et al. 2000; Kabata-Pendias 2010) and the strong positive correlation between extractable-Ba and Ba uptake and toxicity (Fig. 7(a, b), S2a, b).
Although S reduced Ba availability, plants grown at Ba doses higher than 300 mg kg−1 under S supply presented higher Ba accumulations (Fig. 3). This effect is possibly related to the lower Ba tissue concentration due to the higher biomass production in these plants (Fig. 2(a, b)), which resulted in higher Ba accumulations, even presenting lower Ba tissue concentrations and less severe toxicity (Fig. 1). Thus, S supply increased the Ba critical levels in soil and shoots for lettuce growth (Fig. S3a, b). These results indicate the potential of S addition to enhance Ba phytoextraction and phytoremediation through higher plant biomass production due to the S roles in plant growth and development. Thus, S supply can be a suitable low-cost and environmentally friendly strategy to manage Ba-contaminated areas, like the one studied by Viana et al. (2021), due to Ba availability reduction or Ba phytoextraction enhancement.
Conclusions
The extractable-Ba fraction was the main one responsible for Ba bioavailability and phytotoxicity, probably corresponding to the Ba2+ exchangeable cation in the soil. The S supply reduced extractable-Ba at higher Ba doses while it increased the other fractions. Thus, S supply protected the lettuce plants from Ba toxicity by reduction of Ba availability in soil, in addition to plant growth enhancement. Our findings suggest that S supply can be a suitable low-cost and environmentally friendly strategy to manage Ba-contaminated areas due to Ba availability reduction or Ba phytoextraction enhancement. Furthermore, future studies can focus on S doses higher than 80 mg kg−1 S since the results indicate the potential of higher S addition in plant tolerance and Ba availability reduction. However, studies about the S effect in Ba-contaminated soils at field conditions are still necessary.
Data Availability
All data generated or analyzed in this research are included in this article.
References
Ajwa HA, Tabatabai MA (1993) Comparison of some methods for determination of sulfate in soils. Commun Soil Sci Plant Anal 24:1817–1832. https://doi.org/10.1080/00103629309368920
Antoniadis V, Levizou E, Shaheen SM et al (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation–a review. Earth-Science Rev 171:621–645. https://doi.org/10.1016/j.earscirev.2017.06.005
Capaldi FR, Gratão PL, Reis AR et al (2015) Sulfur metabolism and stress defense responses in plants. Trop Plant Biol 8:60–73. https://doi.org/10.1007/s12042-015-9152-1
Cappuyns V (2018) Barium (Ba) leaching from soils and certified reference materials. Appl Geochemistry 88:68–84. https://doi.org/10.1016/j.apgeochem.2017.05.002
CONAMA (Brazilian National Environment Council) (2005) Resolução CONAMA no 357. http://pnqa.ana.gov.br/Publicacao/resolucao_conama_n_357.pdf
CONAMA (Brazilian National Environment Council) (2009) Resolução CONAMA no 420. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=620
da Silva Ferreira M, MPF F, Pacheco AA et al (2020) Risk assessment of trace elements pollution of Manaus urban rivers. Sci Total Environ 709:134471. https://doi.org/10.1016/j.scitotenv.2019.134471
de Anicésio ÉCA, Monteiro FA (2022) Potassium reduces oxidative stress in tanzania guinea grass under cadmium toxicity. Environ Sci Pollut Res 29:1184–1198. https://doi.org/10.1007/s11356-021-15620-9
de Carvalho CFM, Viana DG, Pires FR et al (2019) Phytoremediation of barium-affected flooded soils using single and intercropping cultivation of aquatic macrophytes. Chemosphere 214:10–16. https://doi.org/10.1016/j.chemosphere.2018.09.096
de Souza Cardoso AA, de Lima Gomes FT, JRR A et al (2022) Sulfate availability and soil selenate adsorption alleviate selenium toxicity in rice plants. Environ Exp Bot 201:104971. https://doi.org/10.1016/j.envexpbot.2022.104971
Corrêa LFF, Hao J, Neerup R et al (2022) Review of barium sulphate solubility measurements. Geothermics 104:102465. https://doi.org/10.1016/j.geothermics.2022.102465
de Souza Cardoso AA, Monteiro FA (2021a) Sulfur supply effects on production and mineral nutrition of Tanzania guinea grass under barium levels in nutrient solution. J Plant Nutr 44:801–813. https://doi.org/10.1080/01904167.2021.1871751
de Souza Cardoso AA, Monteiro FA (2021b) Sulfur supply reduces barium toxicity in Tanzania guinea grass (Panicum maximum) by inducing antioxidant enzymes and proline metabolism. Ecotoxicol Environ Saf 208:111643. https://doi.org/10.1016/j.ecoenv.2020.111643
Ferreira AD, Viana DG, Egreja Filho FB et al (2019) Phytoremediation in flooded environments: dynamics of barium absorption and translocation by Eleocharis acutangula. Chemosphere 219:836–844. https://doi.org/10.1016/j.chemosphere.2018.12.074
Ferreira DF (2019) Sisvar: a computer analysis system to fixed effects split plot type designs. Rev Bras Biometria 37:529. https://doi.org/10.28951/rbb.v37i4.450
Gonçalves JM, Monteiro FA (2022) Biomass production and uptake of sulfur, chromium and micronutrients by Tanzania guinea grass grown with sulfur and chromium. Environ Geochem Health. https://doi.org/10.1007/s10653-022-01323-8
Gong Y, Zeng Z, Cheng W et al (2020) Barium isotopic fractionation during strong weathering of basalt in a tropical climate. Environ Int 143:105896. https://doi.org/10.1016/j.envint.2020.105896
He C, Li M, Liu W et al (2014) Kinetics and equilibrium of barium and strontium sulfate formation in marcellus shale flowback water. J Environ Eng 140:B4014001. https://doi.org/10.1061/(asce)ee.1943-7870.0000807
Huang L, Yang X, Xie Z et al (2021) Residual effects of sulfur application prior to oilseed rape cultivation on cadmium accumulation in brown rice under an oilseed rape–rice rotation pot experiment. Ecotoxicol Environ Saf 225:112765. https://doi.org/10.1016/j.ecoenv.2021.112765
Ippolito JA, Barbarick KA (2006) Biosolids affect soil barium in a dryland wheat agroecosystem. J Environ Qual 35:2333–2341. https://doi.org/10.2134/jeq2006.0076
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Ketegenov T, Kamunur K, Batkal A et al (2022) Recent advances in the preparation of barium sulfate nanoparticles: a mini-review. ChemEngineering 6:1–18. https://doi.org/10.3390/chemengineering6020030
Kopriva S, Malagoli M, Takahashi H (2019) Sulfur nutrition: impacts on plant development, metabolism, and stress responses. J Exp Bot 70:4069–4073. https://doi.org/10.1093/jxb/erz319
Kravchenko J, Darrah TH, Miller RK et al (2014) A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health 36:797–814. https://doi.org/10.1007/s10653-014-9622-7
Lamb DT, Matanitobua VP, Palanisami T et al (2013) Bioavailability of barium to plants and invertebrates in soils contaminated by barite. Environ Sci Technol 47:4670–4676. https://doi.org/10.1021/es302053d
Llugany M, Poschenrieder C, Barceló J (2000) Assessment of barium toxicity in bush beans. Arch Environ Contam Toxicol 39:440–444. https://doi.org/10.1007/s002440010125
McGrath SP, Cunliffe CH (1985) A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J Sci Food Agric 36:794–798. https://doi.org/10.1002/jsfa.2740360906
Melo LCA, da Silva EB, Alleoni LRF (2014) Transfer of cadmium and barium from soil to crops grown in tropical soils. Rev Bras Ciência do Solo 38:1939–1949. https://doi.org/10.1590/S0100-06832014000600028
Myrvang MB, Bleken MA, Krogstad T et al (2016) Can liming reduce barium uptake by agricultural plants grown on sandy soil? J Plant Nutr Soil Sci 179:557–565. https://doi.org/10.1002/jpln.201600104
Nogueirol RC, Alleoni LRF (2013) Sequential extraction and speciation of Ba, Cu, Ni, Pb and Zn in soil contaminated with automotive industry waste. Chem Speciat Bioavailab 25:34–42. https://doi.org/10.3184/095422913X13584417355199
Peana M, Medici S, Dadar M et al (2021) Environmental barium: potential exposure and health-hazards. Arch Toxicol 95:2605–2612. https://doi.org/10.1007/s00204-021-03049-5
Rabêlo FHS, da Silva BKDA, Borgo L et al (2018) Enzymatic antioxidants—relevant or not to protect the photosynthetic system against cadmium-induced stress in Massai grass supplied with sulfur? Environ Exp Bot 155:702–717. https://doi.org/10.1016/j.envexpbot.2018.08.020
Siddiqui MH, Alamri S, Alsubaie QD et al (2019) Potential roles of melatonin and sulfur in alleviation of lanthanum toxicity in tomato seedlings. Ecotoxicol Environ Saf 180:656–667. https://doi.org/10.1016/j.ecoenv.2019.05.043
Sleimi N, Kouki R, Hadj Ammar M et al (2021) Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci Nutr 9:2086–2094. https://doi.org/10.1002/fsn3.2177
USDA (United States Department of Agriculture) (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. U.S. Dep. Agric. Handb, USA, pp 436–886
USEPA (United States Environmental Protection Agency) (2004) National recommended water quality criteria. https://www.epa.gov/sites/default/files/2015-06/documents/nrwqc-2004.pdf
USEPA (United States Environmental Protection Agency) (2005) Ecological soil screening levels for barium. https://rais.ornl.gov/documents/eco-ssl_barium.pdf
Viana DG, Egreja Filho FB, Pires FR, et al (2021) In situ barium phytoremediation in flooded soil using Typha domingensis under different planting densities. Ecotoxicol Environ Saf 210:0–7. https://doi.org/10.1016/j.ecoenv.2021.111890
WHO (World Health Organization) (2022) Chemical fact sheets: barium. https://www.who.int/publications/m/item/chemical-fact-sheets%2D%2Dbarium
Acknowledgements
The authors thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support for the research project and scholarship to the first author [155319/2017-5]. They also thank FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for their funding support, as well as the participants of the Plant Nutrition Study Group (GENP-UFLA), for help in the research project.
Funding
This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) [155319/2017-5], FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
Conceptualization: AASC, VF, MLSS. Methodology: AASC, LRGG, VF, MLSS. Validation: all authors. Investigation: all authors. Formal analysis: AASC. Visualization: AASC, AAL. Writing—original draft: AASC. Resources: LRGG, VF, MLSS. Supervision: LRGG, VF, MLSS. Project administration: MLSS. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza Cardoso, A.A., Nunes, A.P.P., Batista, É.R. et al. Sulfate supply decreases barium availability, uptake, and toxicity in lettuce plants grown in a tropical Ba-contaminated soil. Environ Sci Pollut Res 30, 53938–53947 (2023). https://doi.org/10.1007/s11356-023-25960-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25960-3