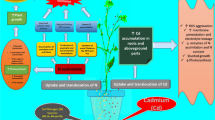
Abstract
Plants used for phytoextraction of metals need to tolerate toxicity conditions. Potassium (K) participates in physiological and biochemical processes that can alleviate toxicity by heavy metals, including cadmium (Cd). This study aimed to evaluate the effect of K on photosynthesis and on the changes in the antioxidant system of tanzania guinea grass [Panicum maximum Jacq. cv. Tanzania (syn. Megathyrsus maximus (Jacq,) B.K. Simon & S.W.L. Jacobs)] under Cd toxicity. Plants were grown in a greenhouse, in nutrient solution, in a randomized complete block design, arranged in a 3 × 4 factorial, with three replications. Plants were supplied with three K levels (0.4 [K deficiency], 6.0, and 11.6 mmol L-1) and exposed to four Cd levels (0.0, 0.5, 1.0, and 1.5 mmol L-1). Two plant growth periods were evaluated. High Cd level (1.5 mmol L-1) led to a reduction in net photosynthesis (76%) by causing low stomatal conductance and losses in quantum efficiency of photosystem II. However, high K supply (11.6 mmol L-1) increased the net photosynthesis by 15% in plants exposed to 1.0 mmol L-1 Cd, due to upregulation of proline synthesis. Cd toxicity resulted in increases in lipid peroxidation and hydrogen peroxide concentration (35 and 50%; 25 and 30%, at first and second harvest, respectively) and reduction by 80–100% in activity of the antioxidant enzymes: superoxide dismutase, catalase, ascorbate peroxidase, guaiacol peroxidase, and glutathione reductase in the shoots of the grass. However, the high K supply (11.6 mmol L-1) increased the activity of these enzymes (about 50–75%) and reduced lipid peroxidation (36%), restoring cellular homeostasis. Moreover, high K supply promoted a 25% increase in spermidine and spermine concentrations in the shoots. Therefore, K reduced the Cd-induced oxidative stress and increased the net photosynthesis in tanzania guinea grass by increasing the activity of antioxidant enzymes and proline and polyamines synthesis, which enhances the tolerance of this grass to Cd.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic environmental pollutant that has high mobility in the soil-plant system and negatively impacts plants and animals. It is a relatively rare metal in nature, but in recent decades, its concentration in the environment has increased due to the intensification of anthropic activities, mainly of industry and mining, but also in agricultural practices (He et al. 2015; Kubier and Pichler 2019). One of the main effects of Cd toxicity on plants is oxidative stress, originated from excessive production of reactive oxygen species (ROS), which results in damage to lipid membranes (lipid peroxidation), proteins, and nucleic acids (Awan et al. 2020). Moreover, Cd stress can influence physiological processes, such as inhibition of photosynthesis in various ways, including reduction in stomatal conductance, chlorophyll content, and quantum yield of photosystem II (Dobrikova et al. 2021). To protect against Cd-induced oxidative stress, plants have antioxidant mechanisms (enzymatic and non-enzymatic), which have the function of eliminating ROS, transforming them into non-toxic compounds (Kaya et al. 2020a).
In recent years, tanzania guinea grass has been studied under conditions of phytotoxicity and recovery of areas contaminated by heavy metals (Cardoso and Monteiro 2021; Silva et al. 2016; Souza Junior et al. 2019). This grass has shown promise for phytoextraction of metals due to its high shoot mass production, fast growth, large root system, and great adaptability in addition to allowing regrowth (Gilabel et al. 2014; Rabêlo et al. 2017). However, the toxic effects of Cd can compromise biomass production and even grass survival (Leite and Monteiro 2019a; Rabêlo et al. 2017). The efficiency of phytoextraction depends on mechanisms of plant resistance to stresses caused by metals (Ke et al. 2021). The optimization of nutritional management positively interferes in the tolerance capacity of plants exposed to Cd (Rabêlo et al. 2018a; Sebastian and Prasad 2016).
Previous studies have shown that K acts on processes, including the protein synthesis, enzymatic activity, and photosynthesis, that can attenuate the severity of heavy metal toxicity in plants (Li et al. 2020; Naciri et al. 2021; Song et al. 2015; Wu et al. 2020). K supplementation can reduce the deleterious effects of Cd toxicity by increasing photosynthesis and promoting chlorophyll synthesis in tomato (Solanum lycopersicum L.) plants (Naciri et al. 2021). Yasin et al. (2018) reported that the application of K alleviated toxic effects of Cd and improved plant growth, photosynthetic pigments, proline content, and activity of antioxidant enzymes in gladiolus (Gladiolus grandiflora L.). However, few studies have investigated the physiological and biochemical processes involved in the role of potassium in alleviating Cd toxicity in plants, especially in grass, such as tanzania guinea grass; therefore, it needs to be further studied. Furthermore, K metabolism is related to the contents of low-molecular-weight organic nitrogen compounds such as putrescine (PUT), spermidine (SPD), and spermine (SPM) polyamines (Santos et al. 2017). Polyamines increase plant tolerance to abiotic stresses, including Cd toxicity (Howladar et al. 2018; Rady and Hemida 2015), by acting primarily as stress signaling molecules that activate the antioxidant system of plants and stabilize lipid membranes (Anjum et al. 2015). Therefore, it is identified a gap in the knowledge of the relationship between K supply and Cd exposure on polyamines accumulation in plants, and particularly in tropical grasses, that needs to be elucidated.
This study was carried out to evaluate the effect of K on photosynthesis and changes in the antioxidant system of tanzania guinea grass under Cd toxicity. The hypothesis is that improving K nutrition can reduce the Cd-induced oxidative stress and enhance photosynthetic activity by upregulating the antioxidant system (antioxidant enzymes, proline, and polyamines), increasing the tolerance of tanzania guinea grass to this heavy metal.
Materials and methods
Experimental conditions
Tanzania guinea grass was grown in nutrient solution in 3.6-L pots filled with ground quartz used as substrate. The pots were arranged in a greenhouse, in randomized complete block design, in a 3 × 4 factorial, with three K levels (0.4, 6.0, and 11.6 mmol L-1) and four Cd levels (0.0, 0.5, 1.0, and 1.5 mmol L-1), with three replications. The nutrient solutions were prepared from the solution of Hoagland and Arnon (1950). K and Cd levels were defined according to Monteiro and Consolmagno Neto (2008) and Rabêlo et al. (2017), respectively.
The grass seeds were germinated in sand washed with deionized water. Fifteen seedlings were transplanted to each pot, and the Hoagland and Arnon nutrient solution (modified to provide the 0.4 mmol L-1 K level) with 20% of ionic strength was immediately used. Seven days after transplantation, a solution with full ionic strength was applied, modified to provide K levels (0.4, 6.0, and 11.6 mmol L-1). The nutrient solution was replaced every two weeks. For aeration of the root system, the nutrient solution was circulated six to three times a day according to the growth of the grass. Deionized water was used every day to complete the volume of nutrient solution lost. During the seedling adaptation period (15 days after transplanting), thinning was carried out and only five plants per pot remained. Other details on seedlings and nutrient solution management were presented by Anicésio and Monteiro (2019).
Plants were allowed to grow for a month, as such period favors the development of Panicum maximum grown under K rates (Lavres Júnior et al. 2010), and plants can reach complete metabolism, with the antioxidant system able to tolerate the oxidative stress. At 30 days of plant growth, Cd levels were applied and there was a five-day Cd exposure period (until the first harvest).
The plants were grown in two periods. The first harvest (shoots) was done upon the occurrence of severe symptoms of Cd toxicity and occurred at 35 days after transplantation. After the first harvest, the contaminated nutrient solution was replaced, and Cd was not reapplied, because the high Cd levels caused serious toxic effects on the first growth, so its reapplication could lead to the death of plants. Then, Cd response at the second harvest was evaluated based on the residual effect of this metal. The second harvest was done 22 days after the first one, and shoots and roots were collected.
For the metabolic evaluations, in both harvests, the plant material was packed in aluminum bags and frozen in liquid N to cease metabolic reactions, and then stored in a freezer at – 80 °C. Later on, it was ground in a mill containing liquid N.
Gas exchange and fluorescence assessments
At the end of each growth period, the gas exchange parameters were evaluated as net photosynthesis (A) and stomatal conductance (gs), as well as the quantum efficiency of photosystem II (QEP). To perform these determinations, plants were kept for 24 h under controlled conditions of light, temperature, and humidity, in the growth chamber. Readings were taken on the middle third of two newly expanded leaves using the WALZ-GFS-3000 IRGA@ photosynthesis analyzer. The parameters were set at 380 μmol mol-1, 20,000 μmol mol-1, 750 μmol s−1, 1000 μmol m−2 s−1, and 27 °C for CO2 concentration, H2O concentration, flow rate, light, and the cuvette temperature respectively, according to the methods proposed by Long and Bernacchi (2003), Magney et al. (2017) and Rabêlo et al. (2018b), with adaptations.
Lipid peroxidation and hydrogen peroxide
Lipid peroxidation of membranes was measured by malondialdehyde (MDA) concentration (Heath and Packer 1968). Samples of frozen plant tissue (0.2 g) were homogenized with 2 mL of trichloroacetic acid (TCA, 1 g L-1) and polyvinylpolypyrrolidone (PVPP) in an ice bath and then centrifuged at 10,000 × g for 5 min at 4 °C. The supernatant was used to determine MDA and H2O2 concentrations. For determination of MDA concentration, an aliquot of the supernatant was taken and TBA (5 g L-1 TBA diluted with 200 g L-1 TCA) was added. The solution was incubated in a dry bath at 95 °C for 30 min. Spectrophotometer absorbance readings were taken at the wavelengths of 535 and 600 nm. MDA concentration was calculated using the extinction coefficient of 155 mM-1 L-1. For the determination of H2O2 concentration, an aliquot of the supernatant was collected and phosphate buffer (100 mmol L-1, pH 7.5) and potassium iodide (1 mol L-1) were added. The mixture was incubated on ice in the dark for 60 min. The absorbance reading was taken in a spectrophotometer at 390 nm and the hydrogen peroxide concentration was determined based on a standard curve (Velikova et al. 2000).
Extraction and quantification of protein
Frozen plant tissue samples (1 g) were homogenized with 3 or 2 mL (shoots or roots, respectively) of extraction buffer containing 100 mmol L-1 potassium phosphate (pH 7.5), 1 mmol L-1 ethylenediaminetetraacetic acid (EDTA), and 3 mmol L-1 dithiothreitol (DTT) and macerated with polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 10,000 × g for 30 min at 4 °C (Monteiro et al. 2011). The supernatant was collected and divided into aliquots. An aliquot was used for quantification of protein and the others were stored in a freezer at − 80 °C and used to determine the activities of antioxidant enzymes. Soluble protein quantification was performed in accordance with Bradford (1976), using a standard curve prepared with bovine serum albumin (BSA).
Antioxidant enzymes activities
Superoxide dismutase (SOD - EC 1.15.1.1)
To determine superoxide dismutase activity, protein separation by electrophoresis was initially performed using non-denaturing polyacrylamide gels (PAGE), as described by Azevedo et al. (1998). After separation, the gels were incubated for 30 min, in the dark box, in a 100 mmol L-1 potassium phosphate buffer solution (pH 7.8) containing 1 mmol L-1 EDTA, 0.1 mmol L-1 nitroblue tetrazolium chloride (NBT), N, N, N ', N'-tetramethylethylenediamine (TEMED), and 0.05 g L-1 riboflavin. Then, the gels were placed under light for 15 min until the appearance of the achromatic SOD bands on a purple/blue background. The SOD bands for each sample were qualitatively compared with the bovine SOD band (standard).
Catalase (CAT - EC 1.11.1.6)
Catalase activity was determined at 25 °C in a reaction medium composed of 100 mmol L-1 potassium phosphate buffer solution (pH 7.5) containing 300 g L-1 H2O2. The reaction was initiated by the addition of a 25μL aliquot of the protein extract. Reading was performed in a spectrophotometer at 240 nm after 1 min H2O2 decomposition (Monteiro et al. 2011). For the calculation of CAT activity, a molar extinction coefficient of 39.4 M-1 cm-1 was used in the following formula:
Where: Abs = Absorbance, Ɛ = molar extinction coefficient, and volume = volume of protein extract (25 μL).
The results were expressed in μmol/min/mg protein.
Ascorbate peroxidase (APX – EC 1.11.1.11)
The determination of ascorbate peroxidase activity was based on the oxidation of ascorbate at 30 °C (water bath) for 1 min according to Nakano and Asada (1981). In the reaction medium composed of 80 mmol L-1 potassium phosphate buffer (pH 7.0), 5 mmol L-1 ascorbic acid and 1 mmol L-1 EDTA, H2O2 (1 mmol L-1) and the protein extract (40 μL aliquot) were added at the time of reading. The absorbance was measured by spectrophotometer at 290 nm. APX activity was calculated using an extinction coefficient of 2.8 mM-1 cm-1 and the formula:
Where: Abs = Absorbance, Ɛ = molar extinction coefficient, and volume = volume of protein extract (40 μL).
The results were expressed in nmol/min/mg protein.
Guaiacol peroxidase (GPX - EC. 1.11.1.7)
The method described by Matsuno and Uritani (1972) was used to determine guaiacol peroxidase activity. A reaction medium composed of pH 5.0 citrate phosphate buffer [dibasic sodium phosphate (0.2 mmol L-1) plus citric acid (0.1 mmol L-1), guaiacol (5 g L-1) and H2O2 (30 g L-1)] was used. The protein extract (20 μL aliquot) was added, mixed, and incubated at 30 °C (water bath) for 15 min. To stop the reaction, sodium metabisulfite (0.1 mol L-1) was added and the reading was performed in a spectrophotometer at 450 nm. The extinction coefficient of 25.5 mM-1 cm-1 and the formula below were used to calculate GPX activity, which was expressed in μmol/min/mg protein.
Where: Abs = Absorbance, Ɛ = molar extinction coefficient, and volume = volume of protein extract (0.02 mL).
Glutathione reductase (GR - EC 1.6.4.2)
To determine glutathione reductase activity, a reaction medium containing 100 mmol L-1 potassium phosphate buffer (pH 7.5) and 1 mmol L-1 2-nitrobenzoic acid (DTNB) was prepared and placed in a water bath at 30 °C. At the time of reading, 0.1 mmol L-1 reduced adenine dinucleotide nicotinamide (NADPH), 1 mmol L-1 oxidized glutathione (GSSG), and protein extract (50 μL aliquot) were added to initiate the reaction. The reading was performed in a spectrophotometer at 412 nm (Azevedo et al. 1998; Smith et al. 1988). A standard curve was prepared from known concentrations of GR. From the GR concentration values, GR activity, expressed in μmol/min/mg protein, was calculated for the ratio of the concentration and the protein content according to the formula:
Where: volume = volume of protein extract (50 μL).
Proline concentration
Proline concentration was determined by colorimetry, according to Bates et al. (1973). Frozen plant tissue samples (0.3 g) were homogenized with 10 mL of sulfosalicylic acid (30 g L-1). The mixture was centrifuged at 10,000 × g at 15 °C for 20 min. Acid ninhydrin solution and glacial acetic acid were added to the supernatant (1 mL). It was placed in a water bath at 100 °C for 60 min. Toluene (2 mL) was added to each sample and mixed. The organic phase (toluene) was collected and placed in glass cuvettes for absorbance reading in a spectrophotometer at 520 nm. Concentrations were obtained using a standard proline curve, inserting the absorbances of the samples. Proline concentration, expressed in μmol g-1 fresh tissue, was calculated according to the following formula:
Where: 115.13 μg/μmol (molar mass of proline) and 10 (DF- dilution factor).
Polyamines concentrations
The putrescine (PUT), spermidine (SPD), and spermine (SPM) polyamines concentrations were determined according to Flores and Galston (1982) with adaptations by Lima et al. (2008). Frozen plant tissue samples (0.2 g) were homogenized with 2 mL of 50 mL L-1 perchloric acid and subsequently centrifuged at 10,000 × g at 4 °C for 10 min. The dansylation reaction was carried out by adding saturated sodium carbonate and dansyl chloride (5-[N, N-dimethylamino] naphthalene-1-sulfonyl chloride) diluted with acetone (5 mg mL-1). The samples were kept at room temperature in the dark for 16 h. Then, proline (100 mg L-1) was added and allowed to stand for a further 30 min in the dark. To extract the dansylated polyamines, toluene was added and the organic phase (dansylated extract) was collected. Polyamines were separated by thin-layer chromatography. Dansylated extract was applied to glass chromatographic plates (20 × 20 cm) covered with 60G-Merck silica gel (250 μm thick) previously activated at 160 °C for 90 min. These plates were placed in chromatography tank and chromatography was performed using chloroform:triethylamine (12:2 v/v) as the mobile phase. A mixture containing the three amine standards was processed parallel to the samples. At the end of chromatography, the dansylated compounds were activated with UV light at 365 nm and fluorescence intensity readings at 507 nm were taken on the GelDoc-It Imaging System UVP photodocumenter using VisionWorksLS Acquisition and Analysis software. Polyamines were quantified by relating the fluorescence intensity of the standards (also applied to the plates) to those of the samples.
Statistical analysis
Results were statistically analyzed by using the “Statistical Analysis System” software (SAS-Institute 2011). Initially, the analysis of variance was performed by the GLM procedure, and when the K levels × Cd levels interaction was significant, the polynomial regression (response surface) was performed by the RSREG procedure. When the K levels × Cd levels interaction was not significant, regression analysis was performed for the Cd levels and means were compared by the Tukey test for K levels. Significance level of 0.05 was used for all analyses.
Results
Gas exchange and fluorescence
At the first harvest, the highest values of stomatal conductance (gs) as well as the net photosynthesis (A) in the leaf blades were observed at K levels of 6.0 and 11.6 mmol L-1 (p ≤ 0.05). The increases were two- to three-fold for gs and three- to four-fold for A compared with the K deficiency conditions (0.4 mmol L-1; Table 1). For the Cd levels, significant (p ≤ 0.05) decreases of 58 and 76% were observed for gs and A, respectively, when comparing the no-Cd application in the nutrient solution with the highest level of this heavy metal (1.5 mmol L-1; Fig. 1a, c).
Net photosynthesis and stomatal conductance in tanzania guinea grass, at the first harvest as related to Cd levels (a and c), at the second harvest as related to K and Cd levels combinations (b and d), and quantum efficiency of photosystem II, at the first and second harvest as related to K and Cd levels combinations (e and f). Bars (a and c) indicate standard errors
At the second harvest, the K levels × Cd levels interaction was significant (p ≤ 0.05) for A and gs (Fig. 1b, d). Despite the deleterious effect of Cd at the first harvest, application of 1.0 mmol L-1 Cd induced increases of 57 and 42%, respectively, in A and gs compared to control (no Cd added). Moreover, plants grown with 1.0 mmol L-1 Cd associated with intermediate and high K availability (6.0 and 11.6 mmol L- 1) had values of A around 15% higher than under conditions of low K availability (0.4 mmol L-1; Fig. 1b).
The interaction of K levels × Cd levels in the nutrient solution showed significant (p ≤ 0.05) effect on the quantum efficiency of photosystem II (QEP), at both harvests (Fig. 1e, f). At the first harvest, the QEP values decreased by 40% with the increase in Cd levels, but by supplying K such problem was alleviated. For plants exposed to 0.5 mmol L-1 Cd, the high availability of K (11.6 mmol L-1) promoted a 35% increase in QEP values, when compared with the low availability of K (0.4 mmol L-1; Fig. 1e). Conversely, at the second harvest, plants exposed to Cd increased the QEP by 35% compared to control. Plants grown at low Cd levels (up to 0.5 mmol L-1) combined with high K levels (6.0 and 11.6 mmol L- 1) in nutrient solution showed values of QEP around 15% higher than those under conditions of low K availability (0.4 mmol L-1; Fig. 1f).
Lipid peroxidation and hydrogen peroxide concentration
In the shoots collected at the first grass harvest, the highest concentration of malondialdehyde (MDA; lipid peroxidation) was observed under K deficiency conditions (0.4 mmol L-1; p ≤ 0.05; Table 1). Exposure to Cd promoted a dose-dependent increase (p ≤ 0.001) in the concentration of MDA in the shoots, with an increase of approximately 35% when comparing the 1.5 mmol L-1 Cd level with no-application of this metal (Fig. 2a). At the second harvest, Cd stress significantly (p ≤ 0.05) increased the concentration of MDA by 50% compared to control; however, the highest K supply (11.6 mmol L-1) led to a 36% reduction in MDA concentrations in the shoots of tanzania guinea grass grown with high Cd level (1.5 mmol L-1) solution (Fig. 2b). There was no significant effect of K and Cd levels on MDA concentration in tanzania guinea grass roots, and the average MDA value was 11.7 nmol g-1 fresh tissue.
Tanzania guinea grass lipid peroxidation (MDA) in the shoots, at the first harvest as related to Cd levels (a) and at the second harvest as related to the K and Cd levels combinations (b); hydrogen peroxide (H2O2) in the shoots, at the first and second harvests as related to Cd levels (c); superoxide dismutase (SOD) activity in PAGE in the shoots at the first (d) and second (e) harvests and in the roots (f) as related to the K and Cd levels combinations. Arrows (d, e, and f) indicate SOD isoforms identified. S, Standard (Bovine SOD). Bars (a and c) indicate standard errors
At the first harvest, the highest Cd level (1.5 mmol L-1) promoted a significant (p ≤ 0.01) 25% increase in the concentration of hydrogen peroxide (H2O2) in the shoots when compared to the non-availability of Cd in the growth medium. At the second harvest, the H2O2 concentration in the shoots was significantly (p ≤ 0.05) increased non-proportionally with Cd levels. Exposure to Cd increased H2O2 concentration in tanzania guinea grass shoots by 30% compared to unstressed plants (no Cd added; Fig. 2c). The H2O2 concentration in the roots was not significant for the studied factors and showed an average of 1.2 μmol g-1 fresh tissue.
Antioxidant enzymes activities
The superoxide dismutase (SOD) activity in PAGE in the tanzania guinea grass shoots collected at the first harvest showed different band intensities due to K and Cd levels, especially in isoform II (Fig. 2d). It was found, especially through isoforms II and III, that the bands were more intense with the lowest K supply (0.4 mmol L-1). At the K level of 11.6 mmol L-1, the increased availability of Cd caused an increase in SOD activity, which was verified by the increasing intensity of the isoforms I and II bands.
At the second harvest, K level of 11.6 mmol L-1 was responsible for the highest intensities of SOD bands in the three isoforms (and isoform III could only be detected under this condition). The high availability of residual Cd (1.0 and 1.5 mmol L-1) in the nutrient solution promoted an increase in band intensity, especially of isoform II (Fig. 2e). In the roots, only the presence of the isoforms I and II was verified. The intensity differences in SOD bands between K levels and Cd levels were clear in isoform II. Only roots grown under K level of 11.6 mmol L-1 showed clear and non-variable intensity SOD bands for all Cd levels (Fig. 2f).
Catalase (CAT), guaiacol peroxidase (GPX), and ascorbate peroxidase (APX) activities in the grass shoots collected at the first harvest showed no significant effects of K and Cd levels, and the respective average values were 55.08 μmol min-1 mg-1 protein, 29.37 μmol min-1 mg-1 protein, and 54.65 nmol min-1 mg-1 protein. For glutathione reductase (GR) activity, a significant (p ≤ 0.05) response to K levels was found and the high K levels (6.0 and 11.6 mmol L-1) promoted increases of 1.8 and 1.6-fold, respectively, in the activity of this enzyme compared with the K deficiency conditions (0.4 mmol L-1; Fig. 4a).
At the second harvest, a significant (p ≤ 0.05) interaction of K levels × Cd levels was found for CAT, GPX, APX, and GR activities (Fig. 3a, c, d and 4b). Cd stress decreased the activities of these enzymes in the grass shoots by 80–100% compared to control plants. The lowest activities of these enzymes in shoots were obtained with low K availability (0.4 mmol L-1) and high residual Cd (1.5 mmol L-1). Conversely, the increase in the supply of K to plants exposed to Cd promoted an increase in the activities of antioxidant enzymes. For plants exposed to 0.5 mmol L-1 Cd, the high K supply (11.6 mmol L-1) promoted increase in CAT (75%), GPX (50%), APX (66%), and GR (66%) activities.
Tanzania guinea grass activities of catalase (CAT), guaiacol peroxidase (GPX), and ascorbate peroxidase (APX), in the shoots at the second harvest as related to K and Cd levels combinations (a, c, and d); catalase activity in the roots as related to Cd levels (b), and ascorbate peroxidase in the roots as related to K levels (e) and Cd levels (f). Bars (b, e, and f) indicate standard errors
Tanzania guinea grass shoots: glutathione reductase (GR) activity at the first harvest, as related to K levels (a) and, at the second harvest, as related to K and Cd levels combinations (b), and proline concentration at the first harvest, as related to K levels (c) and Cd levels (d) and, at the second harvest, as related to K and Cd levels combinations (e). Bars (a, c, and d) indicate standard errors
APX activity in the roots was increased significantly (p ≤ 0.05) under K deficiency (0.4 mmol L-1; Fig. 3e). For Cd levels, CAT and APX activities in the roots showed a significant (p ≤ 0.01; p ≤ 0.05, respectively) dose-dependent increase, as opposed to the responses in the shoots (Fig. 3b, f). GPX and GR activities in the roots were not significantly affected by K and Cd levels and showed average values of 160.15 and 3.51 μmol min-1 mg-1 protein, respectively.
Proline concentration
At the first harvest, there were significant independent effects of K (p ≤ 0.05) and Cd (p ≤ 0.01) levels on proline concentration. The 0.4 mmol L-1 K level increased proline concentration in shoots of tanzania guinea grass by 2.7-fold when compared to the 11.6 mmol L-1 K level in the nutrient solution (Fig. 4c). Concentration of this amino acid showed a dose-dependent increase with the Cd levels, and an increase of 65% was observed when compared high Cd level (1.5 mmol L-1) with unstressed plants (no Cd added; Fig. 4d).
Conversely, at the second harvest, the interaction K levels × Cd levels was significant (p ≤ 0.05) for the concentration of proline. The increase in the supply of K to plants exposed to Cd promoted an increase in the concentration of proline. Plants grown with high K level (11.6 mmol L-1) and exposed to 1.0 mmol L-1 residual Cd increased their proline concentration, which was 2-fold higher than that observed with the K deficiency (0.4 mmol L-1; Fig. 4e). Proline concentration in the roots was not changed by K and Cd levels and had an average of 0.28 μmol g-1 fresh tissue.
Polyamines concentrations
Significant (p ≤ 0.05) increase was found in the concentration of putrescine in the grass shoots, at the first harvest, in plants under K deficiency (0.4 mmol L-1), which was about 34% higher than in those receiving 6.0 and 11.6 mmol L-1 of this nutrient (Table 2). In the material collected at the second harvest, the interaction K levels × Cd levels was significant (p ≤ 0.05) for the concentration of putrescine, both in shoots and roots. The highest putrescine concentration was found under conditions of low K availability (0.4 mmol L-1) associated with the non-availability of Cd in the nutrient solution. High K supply (11.6 mmol L-1) resulted in low putrescine concentration in shoots and roots (Fig. 5a, b).
At the first harvest of the grass, the concentrations of spermidine and spermine were significantly (p ≤ 0.05) higher in the shoots of plants grown at 6.0 and 11.6 mmol L-1 K levels than at 0.4 mmol L-1 (Table 2). At the second harvest, the concentrations of spermidine and spermine in shoots showed a significant (p ≤ 0.05) interaction K levels × Cd levels. Cd stress decreased spermidine and spermine concentrations in shoots; however, when plants were grown under high K availability (11.6 mmol L-1), a 25% increase was observed (Fig. 5c, d). There was no significant effect of K and Cd levels on spermidine and spermine concentrations in tanzania guinea grass roots, whose averages were 363 and 304 nmols g-1 fresh tissue, respectively.
Discussion
Cd-induced plant toxicity interferes with various plant physiological and biochemical processes, causing damage to cell structure and reducing photosynthetic rate due to the oxidative stress caused by this metal (He et al. 2015). Few studies show the positive interference of K in plants’ tolerance to this heavy metal. In a previous study, it was found that K promotes high growth and shoot dry mass production of tanzania guinea grass exposed to Cd, and therefore, K increases extraction of Cd from the growing medium (Anicésio and Monteiro 2019). However, there was no information on the physiological and biochemical mechanisms responsible for increasing Cd phytoextraction capacity of this grass supplied with high K levels.
At the end of the first growth period, a short period of exposure (five days) to Cd led to low stomatal conductance (gs) and quantum efficiency of photosystem II (QEP), thus resulting in low net photosynthesis (A) in tanzania guinea grass. These results corroborate those found by Zhang et al. (2014) in Pennisetum americanum (L.) K. Schum. x Pennisetum purpureum Schum., by Ahanger et al. (2020) in Vigna angularis (L.), and by Imran et al. (2021) in rice (Oryza sativa L.). The reduction in photosynthetic activity caused by Cd may be related to stomatal and non-stomatal limitations (Varone et al. 2012). There was a decrease in gs, possibly due to stomatal closure, which resulted in lower CO2 diffusion (Rabêlo et al. 2018a). Non-stomatal limitations are related to the change caused by Cd in chloroplasts structure, in chlorophyll content, in the composition of thylakoid membranes, and in the electron transport, resulting in low QEP (Araújo et al. 2017; Kaya et al. 2020b). These non-stomatal limitations under Cd toxicity conditions occur when there is an excess in accumulation of ROS (demonstrated by high H2O2 and MDA concentrations), which cause oxidative stress in chloroplasts, one of the main organelles where ROS are produced (Kaya et al. 2019; Kaya et al. 2020a).
At the second harvest, there were increases in A, gs, and QEP in plants exposed to Cd (up to 1.0 mmol L-1), which suggest a eustress state, as previously evidenced by Leite and Monteiro (2019b). This could be related to the high proline concentration and its protective role in photosynthesis. Among the functions of proline in plants are osmotic regulation and prevention against the degradation of photosynthetic pigments induced by Cd (Zouari et al. 2016). This beneficial effect of proline on the photosynthetic activity has been previously reported in date palm (Phoenix dactylifera L.) (Zouari et al. 2016) and eggplant (Solanum melongena L.) (Singh et al. 2015). However, high K availability in nutrient solution further improved A under Cd toxicity (1.0 mmol L-1). Interestingly, plants exposed to 1.0 mmol L-1 Cd and supplied with high K levels showed the same value of A (15 μmol m-2 s-1) as control plants, at the first harvest. These results indicate the positive role of K in increasing the photosynthetic efficiency of plants under Cd toxicity. These findings agree with previous reports that K supplementation improved photosynthesis, enhanced chlorophyll synthesis, and maintained chlorophyll fluorescence in plants under Cd stress (Naciri et al. 2021). This could be associated with the high proline concentration and upregulation of antioxidant enzymes, due to the important role of this amino acid and of these enzymes in reducing oxidative stress and increasing photosynthetic activity (Gill et al. 2012; Zhang et al. 2020).
Oxidative stress in tanzania guinea grass due to Cd toxicity increased H2O2 and MDA concentrations in its shoots. Cd is a non-redox metal, so it cannot produce hydroxyl radicals (OH-) through the Haber-Weis and Fenton reactions (Clemens 2006). However, this heavy metal indirectly induces oxidative stress by accumulating other types of ROS through its interference with electron transport chains (blocking) and its negative relationship with compounds of the antioxidant defense system (inhibition of antioxidant enzymes due to the high Cd affinity for thiol groups) (Gallego et al. 2012). Cd inhibited the antioxidant enzymes activities in tanzania guinea grass and may be responsible for the oxidative stress. However, high K supply reduced the concentration of MDA and thus the oxidative stress under Cd toxicity. One reason for the reduction caused by K in lipid peroxidation might be due to enhanced antioxidant enzymes activities as previously reported by Yasin et al. (2018).
Three SOD isoforms were detected in the grass tissue under Cd stress. The changes observed in the profile of this enzyme indicate its involvement in the detoxification mechanisms of tanzania guinea grass. Plants grown under the highest K level (11.6 mmol L-1) maintained high SOD activity in the tissue collected at the second harvest, even under high Cd levels. This suggests high efficiency in converting O2- into H2O2 (Clemens 2006), that was eliminated by peroxidases, which also had high activities under high K availability.
In the plant tissue collected at the second harvest, CAT and GPX peroxidases as well as enzymes of the AsA-GSH cycle (APX and GR) were inhibited at high Cd levels, which resulted in high H2O2 accumulation and lipid peroxidation. Therefore, they play a crucial role in the resistance to Cd-induced stress. This metal causes inhibition of the enzymes because of its high affinity with cysteine residues (thiol groups) of their structures, as well as compromising the transcription and translation of genes responsible for their synthesis (DalCorso et al. 2013). The decrease in APX and GR activities due to Cd toxicity may also be due to disturbances in chloroplasts, where these enzymes are found (Lou et al. 2017). Wu et al. (2017) found low activity of CAT, APX, and GR and Wang et al. (2013) found low activity of CAT and APX in mustard (Brassica campestris L.) and ryegrass (Lolium perenne L.) plants, respectively, exposed to Cd. However, the supply of K to plants under Cd toxicity increased the activity of antioxidant enzymes SOD, CAT, and GPX as well as AsA-GSH cycle enzymes (APX and GR) in shoots of tanzania guinea grass, reducing lipid peroxidation (demonstrated by the low MDA concentration). Positive K responses in the antioxidant system of plants exposed to Cd toxicity were also found by Ahmad et al. (2016b), who reported high activity of the enzymes SOD, APX, and GR in chickpea (Cicer arietinum L.) and by Zaheer et al. (2018), who reported increase in CAT and APX activities in gladiolus (Gladiolus grandiflora L.). Increases in antioxidant enzymes activity may be due to the recognized role of K as enzyme activator (Marschner 2012). Under stress conditions, K plays an important role in the synthesis of proteins, such as thioredoxins, glutaredoxins, among others. These proteins act as reducing agents, regenerating the catalytically active form of peroxiredoxins, which participate in reducing ROS formation in plants under environmental stress (Tripathi et al. 2009). Increasing antioxidant capacity is especially important for the survival of plants grown in metal-contaminated environments (Habiba et al. 2015). Therefore, the application of K increases the tolerance of this grass to Cd stress, improving the enzymatic antioxidant defense system. Thus, K promoted high growth and shoot dry mass production in tanzania guinea grass under Cd toxicity, as verified by Anicésio and Monteiro (2019).
Proline concentration in the shoots of tanzania guinea grass was high when Cd increased in the nutrient solution, indicating that Cd stress stimulates osmoprotectant production. This may be due to proline, which, besides acting on the plant water balance, eliminates the excess of ROS (avoiding the degradation of photosynthetic pigments), stabilizes the structure of cell membranes, among other functions that help plants survive under Cd stress (Zouari et al. 2016). Higher proline concentration due to Cd stress has also been observed in mustard (Brassica juncea L.) (Ahmad et al. 2016a) and wheat (Triticum aestivum L.) (Repkina et al. 2019). At the second harvest, Cd toxicity (up to level 1.0 mmol L-1) also increased the concentration of proline but it was observed that the high K level (11.6 mmol L-1) further improved the concentration this amino acid. The reason might be that proline is derived from precursors like glutamic acid, arginine, and ornithine (Fichman et al. 2015). Genes related to enzymes involved in the biosynthesis of proline from glutamic acid showed a slight increase with K (Li et al. 2020). Moreover, K enhanced the conversion of arginine into proline, possibly due to increased arginase activity (Nageswararao et al. 1981). K supplementation also enhanced the concentration of proline in Brassica oleracea L. (Ahmad et al. 2019) and Gossypium hirsutum L. (Zahoor et al. 2017), stressed by salt and drought, respectively.
Overall, Cd stress causes disruption in polyamines’ homeostasis due to increased PUT concentration and decreased SPD and SPM, impairing plant development (Qiao et al. 2015). However, it was found that Cd stress reduced SPD, SPM and PUT concentrations. This can be attributed to high proline synthesis under Cd toxicity, as it occurs through activation of precursors in common with PUT (arginine and ornithine) (Szabados and Savoure 2010), so the increase in proline synthesis resulted in low PUT concentration in the grass. The decrease in PUT concentration resulted in low SPD and SPM concentrations because PUT is a precursor molecule of these polyamines (SPD and SPM). In addition, polyamine oxidase (PAO) activity was possibly increased in plants with Cd supply, and consequently SPD and SPM concentrations were low. However, high K supply (11.6 mmol L-1) to plants under Cd stress resulted in high SPD and SPM concentrations, reaching values close to those found in control plants and showing the importance of K in the synthesis of polyamines. The reason might be that the biosynthesis of SPD and SPM needs S adenosyl methioninamine (SAE), which is produced from S-adenosyl methionine (SAM) decarboxylation, and K+ acts as a cofactor for SAM synthetase (Takusagawa et al. 1996). SPD and SPM polyamines play an important protective role in plants exposed to stress by regulating cell redox homeostasis, by acting as metal chelators and components of stress signaling pathways, by eliminating ROS, and by activating the antioxidant defense system of the cells (Anjum et al. 2015; Kebert et al. 2017). Thus, the decrease in MDA concentration promoted by K may be a result of the increases in SPD and SPM concentrations caused by this nutrient in plants exposed to Cd. K also can alleviate Cd stress by increasing the polyamines (SPD and SPM) concentrations in tanzania guinea grass.
Conclusions
Cd reduced photosynthetic activity in tanzania guinea grass by causing low stomatal conductance and losses in quantum efficiency of photosystem II. However, the increase in proline synthesis under 1.0 mmol L-1 Cd and high K level conditions resulted in a eustress state that restored the net photosynthesis of this grass. Cd increased lipid peroxidation by inhibiting antioxidant enzymes activities and reducing polyamines concentrations. However, the abundant supply of K can reduce the Cd-induced oxidative stress in tanzania guinea grass, which is evidenced by the decrease in MDA concentration in the shoots. Mitigation of Cd toxicity by increasing K availability results from increased antioxidant enzymes activities and proline and polyamines (SPD and SPM) synthesis. Therefore, K increases tanzania guinea grass tolerance to Cd.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Ahanger MA, Aziz U, Sahli AA, Alyemeni MN, Ahmad P (2020) Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 10:1–21. https://doi.org/10.3390/biom10010147
Ahmad P, Abd Allah EF, Hashem A, Sarwat M, Gucel S (2016a) Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J Plant Growth Regul 35:936–950. https://doi.org/10.1007/s00344-016-9632-z
Ahmad P, Latef AAA, Abd Allah EF, Hashem A, Sarwat M, Anjum NA, Gucel S (2016b) Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:1–12. https://doi.org/10.3389/fpls.2016.00513
Ahmad W, Ayyub CM, Shehzad MA, Ziaf K, Ijaz M, Sher A, Abbas T, Shafi J (2019) Supplemental potassium mediates antioxidant metabolism, physiological processes, and osmoregulation to confer salt stress tolerance in cabbage (Brassica oleracea L.). Hortic Environ Biotechnol 60:853–869. https://doi.org/10.1007/s13580-019-00172-2
Anicésio ÉCA, Monteiro FA (2019) Potassium affects the phytoextraction potential of Tanzania guinea grass under cadmium stress. Environ Sci Pollut Res 26:30472–30484. https://doi.org/10.1007/s11356-019-06191-x
Anjum NA, Hasanuzzaman M, Hossein MA, Thangavel P, Roychoudhury A, Gill SS, Rodrigo MAM, Adam V, Fujita M, Kizek R, Duarte AC, Pereira E, Ahmed I (2015) Jacks of metal/metalloid chelation trade in plants-an overview. Front Plant Sci 6:1–17. https://doi.org/10.3389/fpls-2015.00192
Araújo RP, Almeida AAF, Pereira LS, Mangabeira PAO, Souza JO, Pirovani CP, Ahnert D, Baligar VC (2017) Photosynthetic, antioxidative, molecular and ultrastructural responses of young cacao plants to Cd toxicity in the soil. Ecotoxicol Environ Saf 144:148–157. https://doi.org/10.1016/j.ecoenv.2017.06.006
Awan SA, Ilyas N, Khan I, Raza MA, Rehman AU, Rizwan M, Rastogi A, Tariq R, Brestic M (2020) Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants 9:1–14. https://doi.org/10.3390/plants9070878
Azevedo RA, Alas RM, Smith RJ, Lea PJ (1998) Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase-deficient mutant of barley. Physiol Plant 104:280–292. https://doi.org/10.1034/j.1399-3054.1998.1040217.x
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cardoso AAS, Monteiro FA (2021) Sulfur supply reduces barium toxicity in Tanzania guinea grass (Panicum maximum) by inducing antioxidant enzymes and proline metabolism. Ecotoxicol Environ Saf 208:111643. https://doi.org/10.1016/j.ecoenv.2020.111643
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719. https://doi.org/10.1016/j.biochi.2006.07.003
DalCorso G, Manara A, Furini A (2013) An overview of heavy metal challenge in plants: from roots to shoots. Metallomics 5:1117–1132. https://doi.org/10.1039/c3mt00038a
Dobrikova AG, Apostolova EL, Hanć A, Yotsova E, Borisova P, Sperdouli I, Adamakis I-DS, Moustakas M (2021) Cadmium toxicity in Salvia sclarea L.: an integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol Environ Saf 209:111851. https://doi.org/10.1016/j.ecoenv.2020.111851
Fichman Y, Gerdes SY, Kovács H, Szabados L, Zilberstein A, Csonka LN (2015) Evolution of proline biosynthesis: enzymology, bioinformatics, genetics, and transcriptional regulation. Biol Rev 90:1065–1099. https://doi.org/10.1111/brv.12146
Flores HE, Galston AW (1982) Analysis of polyamines in higher-plants by high-performance liquid-chromatography. Plant Physiol 69:701–706. https://doi.org/10.1104/pp.69.3.701
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. https://doi.org/10.1016/j.envexpbot.2012.04.006
Gilabel AP, Nogueirol RC, Garbo AI, Monteiro FA (2014) The role of sulfur in increasing guinea grass tolerance of copper phytotoxicity. Water Air Soil Pollut 225:1–10. https://doi.org/10.1007/s11270-013-1806-8
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120. https://doi.org/10.1016/j.plantsci.2011.04.018
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res 22:1534–1544. https://doi.org/10.1007/s11356-014-3431-5
He SY, He ZL, Yang XE, Stoffella PJ, Baligar VC (2015) Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv Agron 134:135–225. https://doi.org/10.1016/bs.agron.2015.06.005
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. California Agriculture Experimental Station, Berkeley.
Howladar SM, Al-Robai SA, Al-Zahrani FS, Howladar MM, Aldhebiani AY (2018) Silicon and its application method effects on modulation of cadmium stress responses in Triticum aestivum (L.) through improving the antioxidative defense system and polyamine gene expression. Ecotoxicol Environ Saf 159:143–152. https://doi.org/10.1016/j.ecoenv.2018.05.004
Imran M, Hussain S, Rana MS, Saleem MH, Rasul F, Ali KH, Potcho MP, Pan S, Duan M, Tang X (2021) Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol Environ Saf 211:111911. https://doi.org/10.1016/j.ecoenv.2021.111911
Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P (2019) Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225:627–638. https://doi.org/10.1016/j.chemosphere.2019.03.026
Kaya C, Akram NA, Ashraf M, Alyemeni MN, Ahmad P (2020a) Exogenously supplied silicon (Si) improves cadmium tolerance in pepper (Capsicum annuum L.) by up-regulating the synthesis of nitric oxide and hydrogen sulfide. J Biotechnol 316:35–45. https://doi.org/10.1016/j.jbiotec.2020.04.008
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020b) The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by upregulating the ascorbate-glutathione cycle. Ecotoxicol Environ Saf 196:110483. https://doi.org/10.1016/j.ecoenv.2020.110483
Ke T, Zhang J, Tao Y, Zhang C, Zhang Y, Xu Y, Chen L (2021) Individual and combined application of Cu-tolerant Bacillus spp. enhance the Cu phytoextraction efficiency of perennial ryegrass. Chemosphere 263:127952. https://doi.org/10.1016/j.chemosphere.2020.127952
Kebert M, Rapparini F, Neri L, Bertazza G, Orlović S, Biondi S (2017) Copper-induced responses in poplar clones are associated with genotype- and organ-specific changes in peroxidase activity and proline, polyamine, ABA, and IAA levels. J Plant Growth Regul 36:131–147. https://doi.org/10.1007/s00344-016-9626-x
Kubier A, Pichler T (2019) Cadmium in groundwater — a synopsis based on a large hydrogeochemical data set. Sci Total Environ 689:831–842. https://doi.org/10.1016/j.scitotenv.2019.06.499
Lavres Júnior J, Santos JDG, Monteiro FA (2010) Nitrate reductase activity and SPAD readings in leaf tissues of guinea grass submitted to nitrogen and potassium rates. Rev Bras Ciênc Solo 34:801–809. https://doi.org/10.1590/s0100-06832010000300022
Leite TS, Monteiro FA (2019a) Nitrogen form regulates cadmium uptake and accumulation in Tanzania guinea grass used for phytoextraction. Chemosphere 236:1–9. https://doi.org/10.1016/j.chemosphere.2019.07.055
Leite TS, Monteiro FA (2019b) Partial replacement of nitrate by ammonium increases photosynthesis and reduces oxidative stress in tanzania guinea grass exposed to cadmium. Ecotoxicol Environ Saf 174:592–600. https://doi.org/10.1016/j.ecoenv.2019.02.090
Li W-L, Wang J-F, Lv Y, Dong H-J, Wang L-L, He T, Li Q-S (2020) Improving cadmium mobilization by phosphate-solubilizing bacteria via regulating organic acids metabolism with potassium. Chemosphere 244:125475. https://doi.org/10.1016/j.chemosphere.2019.125475
Lima GPP, Rocha SA, Takaki M, Ramos PRR, Ono EO (2008) Comparison of polyamine, phenol and flavonoid contents in plants grown under conventional and organic methods. Int J Food Sci Technol 43:1838–1843. https://doi.org/10.1111/j.1365-2621.2008.01725.x
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401. https://doi.org/10.1093/jxb/erg262
Lou L, Kang J, Pang H, Li Q, Du X, Wu W, Chen J, Lv J (2017) Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione metabolism. Int J Mol Sci 18:1628–1628. https://doi.org/10.3390/ijms18081628
Magney TS, Frankenberg C, Fisher JB, Sun Y, North GB, Davis TS, Kornfeld A, Siebke K (2017) Connecting active to passive fluorescence with photosynthesis: a method for evaluating remote sensing measurements of Chl fluorescence. New Phytol 215:1594–1608. https://doi.org/10.1111/nph.14662
Marschner H (2012) Marschner’s mineral nutrition of higher plants. Academic Press, London.
Matsuno H, Uritani I (1972) Physiological behavior of peroxidase isoenzymes in sweet potato root tissue injured by cutting or with black rot. Plant Cell Physiol 13:1091–1101. https://doi.org/10.1093/oxfordjournals.pcp.a074815
Monteiro FA, Consolmagno Neto D (2008) Tanzania guineagrass root system as related to potassium and magnesium fertilization. Rev Bras Zootec 37:810–818. https://doi.org/10.1590/s1516-35982008000500005 (in portuguese)
Monteiro CC, Carvalho RF, Gratão PL, Carvalho G, Tezotto T, Medici LO, Peres LEP, Azevedo RA (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ Exp Bot 71:306–320. https://doi.org/10.1016/j.envexpbot.2010.12.020
Naciri R, Lahrir M, Benadis C, Chtouki M, Oukarroum A (2021) Interactive effect of potassium and cadmium on growth, root morphology and chlorophyll a fluorescence in tomato plant. Sci Rep 11:5384. https://doi.org/10.1038/s41598-021-84990-4
Nageswararao RC, Krishnasastry KS, Udayakumar M (1981) Role of potassium in proline metabolism. I. Conversion of precursors into proline under stress conditions in K-sufficient and K-deficient plants. Plant Sci Lett 23:327–334. https://doi.org/10.1016/0304-4211(81)90044-4
Nakano Y, Asada K (1981) Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Qiao X, Wang P, Shi G, Yang H (2015) Zinc conferred cadmium tolerance in Lemna minor L. via modulating polyamines and proline metabolism. Plant Growth Regul 77:1–9. https://doi.org/10.1007/s10725-015-0027-0
Rabêlo FHS, Azevedo RA, Monteiro FA (2017) Proper supply of S increases GSH synthesis in the establishment and reduces tiller mortality during the regrowth of Tanzania guinea grass used for Cd phytoextraction. J Soils Sediments 17:1427–1436. https://doi.org/10.1007/s11368-016-1429-y
Rabêlo FHS, Lux A, Rossi ML, Martinelli AP, Cuypers A, Lavres J (2018a) Adequate S supply reduces the damage of high Cd exposure in roots and increases N, S and Mn uptake by Massai grass grown in hydroponics. Environ Exp Bot 148:35–46. https://doi.org/10.1016/j.envexpbot.2018.01.005
Rabêlo FHS, Silva BKA, Borgo L, Keunen E, Rossi ML, Borges KLR, Santos EF, Reis AR, Martinelli AP, Azevedo RA, Cuypers A, Lavres J (2018b) Enzymatic antioxidants—relevant or not to protect the photosynthetic system against cadmium-induced stress in Massai grass supplied with sulfur? Environ Exp Bot 155:702–717. https://doi.org/10.1016/j.envexpbot.2018.08.020
Rady MM, Hemida KA (2015) Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf 119:178–185. https://doi.org/10.1016/j.ecoenv.2015.05.008
Repkina N, Talanova V, Ignatenko A, Titov A (2019) Involvement of proline and non-protein thiols in response to low temperature and cadmium stresses in wheat. Biol Plant 63:70–77. https://doi.org/10.32615/bp.2019.009
Santos EF, Macedo FG, Zanchim BJ, Lima GPP, Lavres Júnior J (2017) Prognosis of physiological disorders in physic nut to N, P, and K deficiency during initial growth. Plant Physiol Biochem 115:249–258. https://doi.org/10.1016/j.plaphy.2017.04.001
SAS-Institute (2011) SAS/STAT 9.3 User’s guide. SAS:Institute, Carry
Sebastian A, Prasad MNV (2016) Modulatory role of mineral nutrients on cadmium accumulation and stress tolerance in Oryza sativa L. seedlings. Environ Sci Pollut Res 23:1224–1233. https://doi.org/10.1007/s11356-015-5346-1
Silva EB, Fonseca FG, Alleoni LRF, Nascimento SS, Grazziotti PH, Nardis BO (2016) Availability and toxicity of cadmium to forage grasses grown in contaminated soil. Int J Phytoremediation 18:847–852. https://doi.org/10.1080/15226514.2016.1146225
Singh M, Singh VP, Dubey G, Prasad SM (2015) Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol Environ Saf 117:164–173. https://doi.org/10.1016/j.ecoenv.2015.03.021
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione-reductase in crude tissue-homogenates using 5,5'-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413. https://doi.org/10.1016/0003-2697(88)90564-7
Song ZZ, Duan CL, Guo SL, Yang Y, Feng YF, Ma RJ, Yu ML (2015) Potassium contributes to zinc stress tolerance in peach (Prunus persica) seedlings by enhancing photosynthesis and the antioxidant defense system. Genet Mol Res 14:8338–8351. https://doi.org/10.4238/2015.July.27.22
Souza Junior JC, Nogueirol RC, Monteiro FA (2019) Nitrate and ammonium proportion plays a key role in copper phytoextraction, improving the antioxidant defense in Tanzania guinea grass. Ecotoxicol Environ Saf 171:823–832. https://doi.org/10.1016/j.ecoenv.2019.01.013
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Takusagawa F, Kamitori S, Markham GD (1996) Structure and function of S-adenosylmethionine synthetase: crystal structures of S-adenosylmethionine synthetase with ADP, BrADP, and PPi at 28 angstroms resolution. Biochemistry 35:2586–2596. https://doi.org/10.1021/bi952604z
Tripathi BN, Bhatt I, Dietz KJ (2009) Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 235:3–15. https://doi.org/10.1007/s00709-009-0032-0
Varone L, Ribas-Carbo M, Cardona C, Galle A, Medrano H, Gratani L, Flexas J (2012) Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: different response to water stress. Environ Exp Bot 75:235–247. https://doi.org/10.1016/j.envexpbot.2011.07.007
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wang Q, Liang X, Dong Y, Xu L, Zhang X, Hou J, Fan Z (2013) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20. https://doi.org/10.1007/s10725-012-9742-y
Wu Z, Liu S, Zhao J, Wang F, Du Y, Zou S, Li H, Wen D, Huang Y (2017) Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 133:1–11. https://doi.org/10.1016/j.envexpbot.2016.09.005
Wu Q, Zhu X, Zhao X, Shen R (2020) Potassium affects cadmium resistance in Arabidopsis through facilitating root cell wall Cd retention in a nitric oxide dependent manner. Environ Exp Bot 178:104175. https://doi.org/10.1016/j.envexpbot.2020.104175
Yasin NA, Zaheer MM, Khan WU, Ahmad SR, Ahmad A, Ali A, Akram W (2018) The beneficial role of potassium in Cd-induced stress alleviation and growth improvement in Gladiolus grandiflora L. Int J Phytoremediation 20:274–283. https://doi.org/10.1080/15226514.2017.1374337
Zaheer MM, Yasin NA, Ahmad SR, Khan WU, Ahmad A, Ali A, Rehman SU (2018) Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J Plant Nutr 41:461–476. https://doi.org/10.1080/01904167.2017.1385808
Zahoor R, Zhao W, Dong H, Snider JL, Abid M, Iqbal B, Zhou Z (2017) Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L.). Plant Physiol Biochem 119:21–32. https://doi.org/10.1016/j.plaphy.2017.08.011
Zhang XF, Zhang XH, Gao B, Li ZA, Xia HP, Li HF, Li J (2014) Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of an energy crop, king grass (Pennisetum americanum x P. purpureum). Biomass Bioenergy 67:179–187. https://doi.org/10.1016/j.biombioe.2014.04.030
Zhang G, Yan Z, Wang Y, Feng Y, Yuan Q (2020) Exogenous proline improve the growth and yield of lettuce with low potassium content. Sci Hortic 271:109469. https://doi.org/10.1016/j.scienta.2020.109469
Zouari M, Ben Ahmed C, Zorrig W, Elloumi N, Rabhi M, Delmail D, Ben Rouina B, Labrousse P, Ben Abdallah F (2016) Exogenous proline mediates alleviation of cadmium stress by promoting photosynthetic activity, water status and antioxidative enzymes activities of young date palm (Phoenix dactylifera L.). Ecotoxicol Environ Saf 128:100–108. https://doi.org/10.1016/j.ecoenv.2016.02.015
Acknowledgements
Thanks to the participants of the Plant Mineral Nutrition Research Group at ESALQ-USP for the help in all phases of the research project.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP [Grant no. 20624-4, 2015] and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Doctoral fellowship was granted to the first author (Grant no. 142316/2015-6) and research fellowship to the second author (Grant no. 307030/2017-2) by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP [Grant no. 20624-4, 2015] and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Doctoral fellowship was granted to the first author (Grant no. 142316/2015-6) and research fellowship to the second author (Grant no. 307030/2017-2) by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
Éllen Cristina Alves de Anicésio: Conceptualization, Formal analysis, Investigation, Writing-Original Draft, Visualization.
Francisco Antonio Monteiro: Conceptualization, Methodology, Validation, Resources, Writing-Review & Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• K increases proline synthesis, so restores the net photosynthesis under Cd stress.
• K reduces Cd-induced lipid peroxidation by upregulating antioxidative system.
• High polyamines concentrations provided by high K rates alleviate the Cd stress.
• K supply enhances the tolerance of tanzania guinea grass to Cd toxicity.
Rights and permissions
About this article
Cite this article
de Anicésio, É.C.A., Monteiro, F.A. Potassium reduces oxidative stress in tanzania guinea grass under cadmium toxicity. Environ Sci Pollut Res 29, 1184–1198 (2022). https://doi.org/10.1007/s11356-021-15620-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15620-9