Abstract
With increasing demand for agricultural production, chemical fertilizers are now being intensively manufactured and used to provide readily available nutrients in larger quantities, which often leach out and contaminate the groundwater source. At the same time, effluents from fertilizer plants also pollute water bodies, when disposed of without proper treatment. The present study evaluates nitrogen and phosphorus removal efficiencies in a single-stage aerobic moving bed bioreactor (MBBR) from diammonium phosphate (DAP)–spiked wastewater containing no organic carbon. To date, no similar study has been undertaken that treats fertilizer plant effluent or agricultural runoff without the aid of external carbon, where organic carbon is hypothesized to be supplied from endogenous degradation of biomass. Both denitrification and phosphorus removal occurs in the anoxic zones of deeper layers of the biofilm. The present investigation demonstrates the feasibility of the processes with the requirement of a two-stage MBBR for effective simultaneous nitrification, denitrification, and phosphorus removal (SNDPr) together with a polishing technology to bring down the phosphorus concentration within limits. A novel bio-carrier designed for efficient SND was used in the study, with a carrier filling ratio of 35% that supported the formation of deep biofilms creating anoxic zones in the inner surface. Identification of the bacterial species reflects the occurrence of simultaneous nitrification, denitrification, and phosphorous removal (SNDPr) in the reactor. A maximum ammonium nitrogen removal efficiency of 98% was recorded with 95% total nitrogen removal, 69% phosphorus removal, and 85% SND efficiency, indicating the applicability of the process with a tertiary phosphorus removal unit to lower the nutrient concentration of effluents prior to disposal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Highly concentrated nitrogenous wastewater with an ammonium concentration between 500 and 2000 mg/L is discharged from several human activities; fertilizer industry effluents, and agricultural runoff being the major contributors (Nancharaiah et al. 2019). The fertilizer industry is an intense water-consuming industrial sector with considerable wastewater generation that can contaminate both water and soil (Singh et al. 2006). Agricultural pollution from non-point sources has long been recognized as another important factor affecting eutrophication levels (Zhang et al. 2004). This is particularly due to the presence of high levels of agrochemicals, including nitrogen and phosphorus (Cui et al. 2002) as a result of widespread application of chemical fertilizers. According to the Indian Standards Guide for Treatment and Disposal of Effluents of Fertilizer Industry (IS 9841:1981, reaffirmed 2005), effluents from both the nitrogenous and phosphatic fertilizer industry contain high amounts of ammonium and nitrates and phosphates together with negligible COD. As per the standard, the ammonium nitrogen content in effluents from ammonium and urea plants varies in the range of 200–1500 and 500–2000 mg/L, respectively. In the case of fertilizer effluents, presence of phosphorus in high concentration also requires proper treatment and biological removal is one of the most cost-effective methods compared to other physico-chemical treatments (Krishnaswamy et al. 2011).
The use of fertilizers for higher crop production is constantly increasing and its demand is estimated to elevate at 1.4% annually from 2016 to 2022 (FAO 2019). Commonly used fertilizers tend to have low uptake efficiency by plants, leading to runoff, leaching, and volatilization (Azeem et al. 2014). Diammonium phosphate [(NH4)2HPO4] is the diammonium salt of phosphoric acid, which is widely used as a fertilizer due to the presence of both nitrogen and phosphorus (Lu et al. 1987). When applied to crops, ammonium and phosphorus leach into agricultural runoff (Ye et al. 2018), contaminating various other water resources. This runoff accounts for up to 52% and 54% of total nitrogen and phosphorus loading in China, respectively (Zhang et al. 2004). Agricultural-intensive places in India have been shown to contain large amounts of urea and DAP (diammonium phosphate) residues in canal, lake, and groundwater, the latter being present in relatively higher concentrations (Divya and Belagali 2012). India is often considered to be the world’s largest consumer of DAP (Maqsood et al. 2022), in addition to being one of the most widely used fertilizers worldwide (Zhou et al. 2020).
In the case of biological treatment of effluents characterized by high levels of ammonium and phosphorus, immobilization or attachment of nitrifying consortia in gel beads or solid surfaces, respectively, avoids the inhibitory effect of several by-products, including free ammonia (NH3), and improves overall nitrogen removal capacity (Liu et al. 2019). Apart from that, these have additional advantages of effective biomass separation from treated effluent, higher biomass retention, effective removal of nutrients and contaminants, and ability to tolerate toxic pollutants and fluctuating loads (Nancharaiahet al. 2019). Attempts have been made to biologically reduce the phosphorus content of fertilizer industry effluents, as reviewed by de Bashan and Bashan (2004). In order to effectively treat the ever-increasing agricultural runoff with biological methods, vegetative drainage ditches can be used, but there remains a need to improve their properties for efficiency optimization (Kumwimba et al. 2018). Runoffs with low TN and TP concentration can be treated using suspended biomass in wetlands, which can be a cost-effective method (Spangler et al. 2019), but for higher loads, attached growth processes have to be installed. Agricultural wastewater with high COD was treated in a packed bed reactor with carriers with a specific surface area of 322 m2/m3 (Cramer et al. 2021). Effluent from ammonium nitrate producing industry was treated using a two-stage reactor consisting of an anaerobic filter and a pond for separate removal of nitrate and ammonium content, respectively (Shivaraman et al. 2001). von Ahnen et al. (2015) attempted to remove urea, ammonium, and nitrite using a moving bed biofilter and determined the kinetics. Nitrate residues in effluent have been successfully treated in a moving bed bioreactor (MBBR) via denitrification (Van Aken et al. 2022).
MBBR is a promising technology for treating a wide variety of effluents and several studies have evaluated the performance of MBBR in removing carbon, nitrogen, and phosphorus. The ratio of carbon, nitrogen, and phosphorous is almost always maintained at around 100:5:1 (Iannacone et al. 2019) or multiple stages of aerobic and anaerobic reactors have been used (Tsuneda et al. 2001). In the process of biological nitrogen and phosphorus removal, the steps of denitrification and dephosphorylation require organic carbon, which is not inherently present in fertilizer industry effluents. Thus, aid of an external carbon source is often necessary which increases the treatment cost. An interesting approach for that would be utilization of internal carbon through endogenous decay of microbial cells of the biofilm, though its rate is comparatively less than that with external sources (Rittmann and McCarty 2001). Proper adjustment of biomass content in the reactor significantly increases the denitrification rate using endogenous carbon (Bhattacharya and Mazumder 2019). Studies have been reported to successfully remove nitrogen from wastewater without organic carbon content by simultaneous nitrification and denitrification in aerobic MBBR where denitrification occurs in the deeper layer of thick biofilms and organic carbon is provided from endogenous biomass degradation (Bhattacharya and Mazumder 2021). In such systems, phosphorus removal can be achieved along with denitrification due to the formation of anoxic zones in deeper biofilm (Iannacone et al. 2020). Although biofilm thickness of 150 µm is required to form anoxic zones due to DO concentration gradient in aerobic biofilm reactors (Van Loosdrecht and Henze 1999), this value depends on bulk liquid DO concentration, hydro-dynamics, and biofilm density. Oxygen penetration depth is thus observed to vary between 50 and 500 µm for significant aerobic nitrification and anoxic denitrification to occur in the same reactor (Liu et al. 2020; Piculell et al. 2016). It has been observed that simultaneous nitrification-endogenous denitrification requires significantly less carbon and oxygen by 45% and 35%, respectively, for successful removal of TN and phosphorus in constructed wetlands (Zaman et al. 2021; Wang et al. 2021).
Enhanced biological phosphorus removal (EBPR), performed by phosphorus accumulating organisms (PAO) removes phosphorus in alternating anaerobic and aerobic phases (Salehi et al. 2019). Apart from the accumulation of phosphorus, polyphosphate synthesis can take place under aerobic conditions and is usually carried out by denitrifiers (Shi and Lee 2006). Denitrifying P-accumulating bacteria (DPB), also known as denitrifying phosphorus accumulating organisms (DNPAO), use nitrate or nitrite to remove phosphorus (Barak and Van Rijn 2000) even in the absence of organic carbon under anoxic conditions (Tsuneda et al. 2001). A study conducted in MBBR with alternating aerobic and anoxic conditions showed 100%, 75%, and 62% removal efficiencies of dissolved organic carbon, phosphorus, and total nitrogen, respectively, under optimal conditions of DO ranging between 0.2 and 3 mg/L, C/N ratio of 3.6, and HRT of 24 h (Iannacone et al. 2020). Effective combined removal of carbon, nitrogen, and phosphorus via nitrite using aerobic granular sludge has been achieved in municipal wastewaters characterized by low C/N ratio of around 3.8 (Campo et al. 2020).
The aim of this study is to evaluate the occurrence of SND using DAP-spiked wastewater, which would give an idea of the role of MBBR in treating fertilizer industry effluents or agricultural runoff that resemble real-world conditions. One of the challenges in establishing such reactions in the reactor is the absence of carbon in the wastewater, which is not supplied externally. The extent and efficacy of the procedure are compared with the results obtained from previous literatures under sufficient carbon. As the phosphorus content is usually high, an attempt was made to demonstrate the reduction of phosphorus together with SND, resulting in biological nutrient removal from wastewater based on internal carbon. As can be observed from the earlier literature, the treatment of wastewater containing DAP residues using an MBBR has never been investigated, which is an important area in the present aspect that deserves due attention. Establishing kinetics would further aid in the process design of the reactor for treating fertilizer industry effluents and agricultural runoff. In addition, research on removing ammonium from agricultural runoff coupled with high phosphorus content is also extremely limited. This study would be helpful in understanding the process and gaining insight into the efficiency of simultaneous nitrogen and phosphorus removal in a single system for wastewater with limited organic carbon.
Materials and methods
Prepared of DAP-spiked wastewater
Commercial-grade DAP (purity 99%) was procured from a fertilizer store. As observed, DAP is not readily soluble in water and therefore available DAP granules were crushed into fine particles using a pestle and mortar to pass through a 2-mm sieve. The powdered DAP was then dissolved in water to obtain the desired concentration. The solution was dull white or greyish white in colour with a pH in the range of about 7.9 \(\pm\) 0.02. The synthetic wastewater was characterized for the relevant parameters according to the procedures outlined in the American Public Health Association (APHA) Standard Methods (Eaton et al. 2005). The results of the characterization are presented in Table 1. The attached biomass in the carriers was determined according to the procedure followed by Herbert et al. (1971) using Folin-Ciocalteu reagent. Minor modifications were made to the amount of alkali copper reagent to be added for colour development of the protein.
Reactor setup
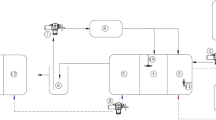
To evaluate nitrogen removal via SND, studies using a laboratory-scale MBBR were conducted at the Environmental Engineering Laboratory of Civil Engineering Department, I.I.E.S.T, Shibpur, India, using DAP-spiked wastewater. An MBBR was designed with a vertical height of 63 cm and a diameter of 20 cm, and cylindrical octahedral polypropylene bio-carriers with fins with a specific surface area of 8.9 cm2/cm3 were used for the study (Bhattacharya and Mazumder 2021). The biomass used in the study was initially obtained from the soil of leguminous plants, later dissolved in water to separate soil particles, and cultured in laboratory-scale reactors for growth and enrichment of the biomass. Biomass was allowed to grow on the surface of these carriers as biofilms in these reactors, and as the biofilm reached an observable thickness of around 1–1.5 mm, the carriers were selected for experimental studies. In the biological treatment of wastewater, use of soil bacteria has been shown to be advantageous and as such bacteria isolated from garden soil have been successfully used to treat ammonium and nitrate fertilizer effluents in fixed film units (Shivaraman et al. 2001). Both batch and continuous studies were conducted to evaluate the performance and the process kinetics of removing nitrogen from DAP-spiked wastewater. Temperature and DO were maintained at 30 ± 2℃ and 5 mg/L, respectively, throughout the studies. The presence of high DO in the reactor should ensure that there is no oxygen limitation (Iannacone et al. 2020).
Experimental conditions for batch study
Batch studies were conducted over a period ranging from 82 to 48 h and parameters were monitored at a 6-h interval. During the study, 25% carrier filling ratio was considered and the initial total equivalent suspended biomass was varied between 200 and 2570 mg/L. pH in the reactor was maintained within 7.8–8.3 without the need for external chemical adjustment, which is considered optimal for the activities of both nitrifiers and denitrifiers (Rittmann and McCarty 2001). During the batch study, the initial operating conditions in the reactor are as shown in Table 2.
Development of Monod’s kinetics
The detailed derivation for evaluating Monod’s kinetic parameters is described in the study by Bhattacharya and Mazumder (2021). To determine the kinetic coefficients of nitrification in DAP containing wastewater, the initial and final NH4-N and biomass concentration and the batch period were taken into account. Accordingly, the values of \(\frac{\uptheta X}{({N}_{O}-N)}\) are plotted against that of \(\frac{1}{N}\) to determine k and KN respectively from the slope and intercept. Similarly, the values of \(\frac{({X}^{\mathrm{^{\prime}}}-X)}{X.\theta }\) are plotted with respect to those of \(\frac{{N}_{o}-N}{X\uptheta }\) to find the values of Y and kd, respectively, from the slope and intercept.
Experimental conditions for continuous study
Continuous study in MBBR was initiated after enrichment of acclimated biomass in the laboratory-scale reactors. Wastewater spiked with DAP with an ammonium concentration of about 500 mg/L was prepared by diluting the stock solution of the same. To investigate the feasibility of SND for the DAP-containing wastewater, the MBBR was operated with varying HRT and attached biomass concentration. The carrier filling ratio (CFR) was maintained at 35% throughout the continuous study; this increase relative to the batch study is due to the absence of any suspended biomass in the reactor. HRT and pH in the reactor were maintained respectively between 18 and 24 h and 7.8 to 8.3. The operating parameters for this continuous study are listed in Table 3, identifying the variation of different parameters in each run. DAP has a high phosphorus content and therefore an attempt was made to examine the removal of phosphorus from the spiked wastewater and to analyse the applicability of MBBR for the complete treatment of DAP-spiked effluent prior to safe disposal. It was observed that the removal of phosphorus after the one-step treatment in MBBR was insignificant. Therefore, a continuous 2nd-stage operation was conducted at similar operating conditions including HRT, using the effluent from the 1st-stage operation for residual phosphorous removal. The operating conditions of the second stage are also listed in Table 3. This was performed to verify that the reactor was efficient for removing both ammonium and phosphorus from low carbon wastewater. A comparison of the results obtained in the case of a DAP-doped sample with those obtained from nitrogenous feed would give an idea of the implementation of the process in practice for effluents with and without high phosphorus content. For all continuous runs, a quasi-steady state was considered, which was resembled by varying two consecutive values within a maximum of 5%.
Development of overall reaction rate of ammonium oxidation
In case of substrate removal from industrial wastewater, the overall reaction rate can be calculated using the following equation which illustrates the kinetics of oxidation (Eckenfelder and O’Connor 2013).
where
- S o :
-
influent ammonium concentration, mg/L
- S e :
-
effluent ammonium concentration, mg/L
- f b :
-
constant = 1.0 for no influent VSS
- X v :
-
active volatile biomass concentration, mg/L
- t :
-
time, days
- K :
-
overall rate constant, /day
Since the DAP-spiked wastewater resembles effluent from the nitrogenous fertilizer industry, this equation can be used to evaluate the process kinetics from the data obtained from the continuous studies. In the case of SND, the stepwise quantification related to the formation and utilization of nitrate and nitrite is not evaluated from the single reactor and therefore only the reaction rate related to ammonium is considered. The nitrifying biomass responsible for the process is obtained by scraping the outer surface of the carrier and used in the equation as equivalent suspended biomass (mg/L). A graph is plotted with (\(\frac{{S}_{o}-{S}_{e}}{{f}_{b}{X}_{v}t}\)) on the Y-axis with respect to (\(\frac{{S}_{e}}{{S}_{o}}\)) on the X-axis to obtain the rate constant (K) from the slope of the curve. The value of this rate constant K varies with the type of industrial effluent, especially the biodegradable material, and with temperature of the reaction.
Calculation of SND efficiency
From the experimental data obtained, SND efficiency can be calculated by the following equation (Dobbeleers et al. 2017)
where,
- NO2e:
-
nitrite concentration in effluent, mg/L as N
- NO3e:
-
nitrate concentration in effluent, mg/L as N
- NH4i:
-
ammonium concentration in influent, mg/L as N
- NH4e:
-
ammonium concentration in effluent, mg/L as N
Identification of bacterial species
In order to identify the different bacterial species in the biofilm grown on the surface of carriers, metagenomic analysis was conducted. Biomass attached to the carriers was first isolated from the bio-carriers. During individual extraction, randomly selected carriers were washed with sterile water using a sterilized glass pipette. 16S rDNA was used as a gene marker for the analysis as it helps to identify the different responsible genera and species in the biofilm and also to predict the functional potential of the community. PCR was conducted on extracted DNA via barcoding using Ligation Sequencing Kit 1D (SQK-LSK109) and PCR Barcoding Expansion Pack 1–96 (EXP-PBC096). The workflow was designed to BLAST base called sequence against the NCBI 16S bacterial database, which contains 16S sequences from different organisms. This analysis was performed by YaazhXenomics Laboratory, Coimbatore, Tamil Nadu, India.
Result and discussion
Results obtained from the batch study
Batch studies were performed to assess the feasibility of the process of both nitrogen and phosphorus in MBBR under limited organic carbon, along with the establishment of reaction kinetics that would illustrate the relationship between biomass and substrate. Batch studies with different ammonium and phosphorus loadings with different HRTs and biomass concentrations reflect the trend of phosphorus and nitrogen species reduction over time. The experimental data obtained were analysed according to Monod’s approach to evaluate the removal efficiency and kinetic parameters. The CFR and DO concentration of 35% and 5 mg/L were kept constant throughout the study. For MBBR, the DO requirements for nitrification are comparatively higher (about 3–5 mg/L) than those required in suspended growth reactors (1–2 mg/L) (Jenkins and Wanner 2014). Increasing the oxygen content beyond 5 mg/L results in accumulation of nitrate as a by-product of nitrification instead of being converted to nitrogen gas due to inconvenience in denitrification, resulting in poor SND efficiency (Wang et al. 2020).
Concentration profiles for recorded parameters
Several parameters including different nitrogen species, phosphorus, TOC concentration, alkalinity, and pH were regularly monitored to understand the process. Figure 1 shows the change in ammonium concentration with time as observed during studies with variable initial ammonium and phosphorus concentration. It can be observed that the main ammonium conversion for all batches was completed within 55 h from the initiation of the batches and a particular trend can be noticed. NH4-N removal recorded during the study ranged from 86 to 94%, confirming the results obtained with synthetic wastewater without high phosphorus loading (Bhattacharya and Mazumder 2021). Sufficient nitrite and nitrate were present in the reactor during the study showing low total nitrogen removal and hence low SND efficiency. A high DO concentration in the reactor ensured that no DO deficiency hindered the nitrification. This aerobic condition is essential not only for reducing ammonium concentrations, but also for phosphorus, along with establishing a dominance of DNPAOs over DNBs (Jenaet al. 2016). Nitrification process supplies the required nitrate for further phosphorus reduction in anoxic phase. This study is performed with excess phosphorus, similar to that of Kuba et al. (1996) in two-phase systems as it confirms the presence of sufficient phosphorus for nitrifier growth in the reactor.
No organic carbon was added to the influent wastewater spiked with DAP and therefore, the initial TOC concentration obtained from the batches, as shown in Figure S1 (in the supplementary file), is from the microbial cells. In the early hours of the runs, organic carbon utilization was comparatively low due to the delay in commencement of denitrification. Within 12–15 h of the batch runs, the organic carbon reduction rate increased rapidly revealing the use of carbon, thereby exhibiting excellent denitrifying activity. The intercellular carbon was then supplied constantly and a steady TOC concentration was observed in the reactor, indicating a balance between supply and uptake of TOC. Ninety-three to ninety-eight percent of TOC removal was obtained from the batches. Similar results have been observed with biodegradable carriers under organic carbon deficient conditions (Chu and Wang 2011). Similar studies conducted in absence of organic carbon suggest denitrification using intracellular and/or extracellular biopolymers as carbon sources (Cui and Jahng 2004). In the case of external carbon addition, denitrifying activity is higher at a C/N ratio of 4.2, while the highest nitrifying activity is recorded at the lowest C/N ratio used in the study (Iannacone et al. 2019). This rapid decrease in TOC results in a limitation of readily available carbon in the reactor, which would affect denitrification as well as denitrifying dephosphatation for simultaneous nitrogen and phosphorus removal (Iannacone et al. 2020). To achieve complete denitrification, a COD:NO3 ratio between 3 and 6 is often recommended (Van Rijn et al. 2006), and insufficient organic carbon content in the reactor can cause nitrite accumulation (Chu and Wang 2011). This is reflected in the data observed in Fig. 3, which shows higher TN concentration along with low ammonium. When higher ammonium levels are introduced, the COD removal percentages reflect the heterogeneous activity of aerobic bacteria, which is corroborated by a similar acclimation study by Zhang et al. (2021) using fertilizer industry wastewater with gradually replacing synthetic carbon-enriched feed.
The reduction in phosphorus concentration over time during the batch study of treating DAP-spiked wastewater in MBBR is demonstrated in Fig. 2. Denitrifying dephosphatation enables the removal of phosphorus and nitrogen with minimal COD requirements, minimal DO consumption, and the lowest sludge production. DO is only required for nitrification of ammonium (Kuba et al. 1996). In the present study, maximum and minimum phosphorus removal of 56.5% and 37.7%, respectively, was achieved, with removal efficiency decreasing as the load increased. An average total phosphorus removal of 15–55% is generally obtained along with removal of other nutrients in MBBR (Saidulu et al. 2021; Nhut et al. 2020). The main reasons that single-stage MBBR did not achieve high TP removal are longer retention times for the nitrifiers, nitrate inhibition, and competition for carbon sources for denitrification and phosphorus removal (Chen et al. 2020). Iannacone et al. (2020) concluded that under stable aerobic conditions, phosphorus uptake was negligible due to the deficiency of nitrite, which was not the case in the present study. The nitrogen species profile indicated denitrification and thus presence of anoxic zones in deeper biofilm layers (Bhattacharya and Mazumder 2021). Denitrifying PAOs use nitrate as electron acceptors and therefore can be reduced together with denitrification under anoxic conditions (Ma et al. 2013). Under optimized conditions of DO, organic carbon (500 COD mg/L), and phosphorus (12.5 mg PO4-P/L), phosphorus removal of about 95.8% was achieved in a laboratory-scale batch MBBR system (Kermani et al. 2009).
Development of kinetics
Nitrification in a biological reactor is quantified using process kinetics, which depend on a number of parameters including substrate and DO concentrations, organic matter, temperature, pH, alkalinity, salinity, and turbulence (Chen et al. 2006). The experimental data obtained from the batch study is used to evaluate kinetic coefficients for ammonium oxidation since ammonium is the rate-limiting substrate for SND (Chen et al. 2006). Evaluating the data using Monod’s kinetics, half saturation coefficient and specific substrate utilization in terms of ammonium nitrogen are evaluated as 231.34 mg/L and 0.74/day, respectively, as obtained from Figure S2 (in the supplementary file), which largely depends on biomass properties and experimental conditions and therefore varies largely when compared to other works (Poduska and Andrews 1975). Figure S3 (in the supplementary file) derives the yield coefficient and endogenous decay rate, respectively, as 0.8466 and 0.002/day. A low endogenous decay coefficient indicates lesser biomass decay, which contributes to a lower organic carbon supply, which explains the low rates of denitrification and phosphorus removal. Kermani et al. (2009) used the Stover-Kincannon model to calculate half saturation constants and maximum specific substrate utilization rates of 43.305 g/L-day and 35.088 g/L-day respectively for nitrogen and 8.50 g/L-day and 7.71 g/L-day for phosphorus removal in anoxic, aerobic, and anaerobic reactor setup. The maximum growth rate (µmax) is calculated to be 0.626/day, which describes the biomass growth and determines both nitrification rate (Barker and Dold 1997) and the minimum required HRT of the reactor (Antoniou et al. 1990).
SND, TN, and TP removal efficiencies
SND, TN, and phosphorus removal efficiencies obtained from the batch studies under different initial concentrations of ammonium and phosphorus are shown in Fig. 3. In all cases, high ammonium nitrogen removal efficiency was obtained while both TN removal efficiency and SND efficiency were found to be considerably low. The reason for this is that the reactor was operated under highly aerobic conditions with DO around 5 mg/L. The biofilm formed on the carriers was not uniformly thick enough to provide anoxic zones and as such denitrification was impaired. Both the suspended biomass and thin layer of attached biofilm on the outer surface and the inner surface attributed to the high ammonium oxidation and thus to high nitrification. Biomass sloughing from the carriers resulted in limited depth in biofilms to support anoxic regions, affecting both denitrification and phosphorus removal. Phosphorus removal efficiency obtained from the study corroborates the results obtained by previous researchers on nutrient removal in MBBR. In order to achieve a better removal and SND efficiency, higher attached biomass needs to be provided for continuous studies. Another reason for the low efficiency can be attributed to the absence of acclimatization of biomass with DAP-spiked wastewater.
Continuous studies using DAP-spiked wastewater
Treating industrial wastewater on a real scale requires a continuous inflow into the system, which requires evaluation of the process in continuous mode. Previous studies have shown that continuous operation in MBBR satisfactorily removes carbon, nitrogen, and phosphorus from municipal wastewater (Leyva-Díaz et al. 2020). Since high removal efficiencies in terms of total nitrogen and phosphorus were not achieved during batch studies, continuous studies in MBBR are evaluated by introducing a second reactor under similar conditions to obtain better removal. Previous SND studies in an aerobic/anoxic MBBR to treat phosphorus and nitrogen showed up to 100%, 68%, and 72% COD, TN, and phosphorus removal, respectively, at a C/N ratio of 4.2 (Iannacone et al. 2019). The present study attempted to investigate the effect of HRT and biomass density on nutrient removal from DAP-spiked wastewater. Phosphorus and SND removal in single-stage MBBR was observed to be low in batch studies and did not meet standard wastewater disposal limits. Therefore, the potential of two-stage MBBR with similar conditions of CFR, pH range, and temperature will be explored during the study.
Concentration profiles recorded during continuous mode of operation
Nitrification in MBBR was particularly efficient with a DO of around 5 mg/L and HRT in the range of 18 to 24 h showed a satisfactory removal between 90 and 96% in all cases, even at a high ammonium concentration of about 500 mg/L, as shown in Fig. 4. The vertical line at 102 h shows the end of the 1st stage of MBBR and commencement of the 2nd stage. However, not much removal was achieved in the second stage as it had already entered a steady state about 100 h after the runs began. At the end of the second stage, about 96–99% ammonium removal was achieved in all cases, confirming the applicability of MBBR for removing ammonium from DAP containing wastewater.
Phosphorus removal in MBBR for DAP-spiked wastewater was observed to be relatively low compared to nitrogen removal, as can be seen in Fig. 5, where a vertical line at 102 h indicates the initiation of the 2nd stage. Several reasons can be attributed to this; the most important one being the biomass, which was initially acclimatized only to a high ammonium concentration. In addition, the C:N:P ratio required for phosphorus removing organisms was not maintained in the reactor, where there was apparently no organic carbon and both ammonium and phosphorus were present in relatively equal concentrations. At the end of the first-stage operation in MBBR, the phosphorus removal efficiency was observed to range from 47 to 56%. As this effluent still contained phosphorus concentration too high to be disposed of, a second-stage MBBR was implemented, similar to the first-stage reactor, to further increase the overall removal efficiency. Phosphorus removal at the end of the two-stage reactor was in the range of about 64–70%, with effluent phosphorus still being too high to dispose. Therefore, a polishing treatment unit is proposed to remove the residual phosphorus from the wastewater. As previously discussed, phosphorus removal can be attributed to PAO and EBPR organisms, which are reported to accumulate phosphorus up to 4–8% in full-scale water treatment plants, compared to only 2% in typical activated sludge (Orhon and Artan 1994). This value can reach up to 10% in laboratory-scale reactors with synthetic wastewater (Crocetti et al. 2000). Limiting organic carbon increases competition between denitrifying heterotrophs and PAOs, often resulting in limited phosphorus removal (Wang et al. 2019). EBPR can also remove 85% phosphorus from domestic wastewater by metabolizing polyhydroxybutyrate (PHB) stored in cells under anoxic conditions (Tchobanoglous et al. 2003).
Removal efficiencies with respect to TN, TP, SND, and ammonium nitrogen
Figure 6 shows ammonium, TN, and phosphorus removal efficiencies along with SND efficiencies obtained from the continuous studies in two consecutive stages. Each bar for each species represents the cumulative removal along with individual efficiencies as marked on the graph. SND efficiency in the first stage varied between 78 and 84.3%, which increased to the range of 88.3% and 95.4% at the end of the two-staged removal system. Total nitrogen removal was also significantly improved at the end of the second stage, ranging from 88 to 95.1%, which at the end of single-staged treatment ranged between 70.5 and 81.4%. This removal efficiency, obtained in both stages, depends directly on the HRT as well as the biomass density in the reactor. The efficiencies were quite high compared to the existing studies, unless external organic carbon was used. It was experimentally reported that an increase in C/N ratio from 4.3 to 7.4 improved the SND efficiency from 55 to 98% (Bueno et al. 2018). Similar results were obtained in the case of SND in SBBR using biodegradable carriers, where TN removal efficiency dropped drastically from 89.2 ± 5.2% to 58.1 ± 3.5% as COD/N decreased from 7.6 to 2.8 (Feng et al. 2018). A comparison of removal efficiencies for biological treatment of fertilizer industry effluents in different reactor systems is presented in Table 4.
Reaction rate of overall ammonium nitrogen oxidation
To determine the overall ammonium oxidation rate, the specific ammonium removal rates (\(\frac{{S}_{o}-{S}_{e}}{{f}_{b}{X}_{v}t}\)) for various continuous runs are plotted with respect to ammonium nitrogen remaining fraction (\(\frac{{S}_{e}}{{S}_{o}}\)) as shown in Figure S3 (in the supplementary file). The slope of best-fit line in this graph is calculated as 7.641/day, which represents the overall removal rate constant for ammonium nitrogen oxidation in the DAP-spiked wastewater. The value of overall ammonium oxidation rate indicates moderate removal of ammonium nitrogen over the time period. However, it is observed to be about a third of the overall removal rate for effluents with high organic nitrogen (K = 22.2) at 22 \(^\circ{\rm C}\) as mentioned by Eckenfelder and O'Connor (2013). However, effluents spiked with DAP did not contain organic nitrogen and the data evaluated are representative of wastewater with high inorganic nitrogen and phosphorus. The overall reaction rate is also largely temperature dependent and therefore for more comprehensive applications of this parameter, the effect of temperature on microbial kinetics must be considered in process design (Leong et al. 2011).
Bacteriological analysis of the biofilm
The carriers used in the study had thick biofilms on the inner surface and thinner biofilm on the outer surface exposed to hydraulic shear. For the inner surface biofilm, thickness of more than 1 mm would be thick enough to form anoxic zones for denitrification and phosphorus removal. Identification of bacterial species on the surface carriers, as shown in Figure S4 (in the supplementary file), reflected the dominance of aerobic nitrifying species on the outer carrier surface and more species diversity on the thick inner biofilm, protected from hydraulic shear. The abundance of various dominant bacteria genera in respective inner and outer surface biofilm growing on the carrier is shown in the figure. For both biofilms, proteobacteria was the most predominant group with gamma-proteobacteria dominating others which is commonly observed to play a crucial role in plant-biome interaction (Prasedya et al. 2022). Stenotrophomonas under the family Xanthomonadaceae is the dominant genus observed in the biofilm growing on the outer surface of the carrier which has shown biofilm forming (Ulrich et al. 2021) and nitrifying abilities including heterotrophic nitrification and partial nitrification (Qiao et al. 2020). Pelomonas is often predominant in nitrifying reactors (Zhou et al. 2016), often supporting high nitrogen removal in membrane reactors (Zhong et al. 2017) and biofilm reactors undergoing SND (Wu et al. 2020). Bosea has denitrification genes (nir, nor, and nos) and actively responsible for nitrate reduction in heterotrophically hypoxic conditions (Dandie et al. 2007). Dokdonella is reported to be responsible for removing nitrogenous compounds, mainly through denitrification (Palma et al. 2018; Zhang et al. 2021). Nitrococcus, found mainly on the outer surface biofilm, is a well-known nitrifier, specifically an ammonium oxidizing bacterial genus (Arp et al. 2007). Ralstonia, isolated from outer surface of the carriers, is another plant growth–promoting rhizobacteria (PGPR) with phosphate solubilizing and denitrifying properties, extensively found in cultivation soils (Han et al. 2012).
Among the dominant bacteria in the inner surface biofilm of the carrier, Rhodanobacter, commonly, a denitrifying bacterial genus (van den Heuvel et al. 2010), has been reported to undergo complete denitrification (Green et al. 2010). Chujaibacter has been reported to play a crucial role in denitrification (Prasedya et al. 2022) with potential genus for ammonium oxidation in MBBR-MBR systems (Rodriguez-Sanchez et al. 2018). The species of Mycolicibacter obtained in the biofilm is categorized under Terrae clade and are commonly identified as major players in denitrification (Mun et al. 2008). Species of Luteibacter has been reported to convert nitrate to nitrite under aerobic conditions (Wang et al. 2011). Tetrasphaera is a dominant polyphosphate-accumulating genus that has been isolated from wastewater treatment plants undergoing EBPR (Nguyen et al. 2011; Nielsen et al. 2019). This genus is often abundantly found in full-scale biological treatment plants with significant phosphorus removal. Frateuria is commonly identified as a potassium-solubilizing bacteria in soil and might be associated with simultaneous nitrogen fixation though the exact pathway is yet to be determined (Subhashini 2015). All these bacterial genera isolated from both outer and inner surface of biofilm carriers are characterized for exhibiting properties for nitrification, denitrification, and phosphorus removal. It is, however, interesting to note that majority of the species are attributed towards nitrogen removal and that of phosphorus removal is particularly limited. This corroborates with the experimental results of restricted phosphorus removal in the system.
Conclusion
With the increasing use of fertilizers for production, effluents from fertilizer industry and agricultural runoff are characterized by high phosphorus and ammonium content. Since both are nutrients, biological treatment processes for these effluents are particularly efficient and cost-effective, although the lack of sufficient organic carbon for the biological processes requires external addition of carbon sources, which in turn increases treatment costs. In an attempt to solve this challenge, the effectiveness of MBBR when organic carbon is supplied from endogenous degradation of the biomass is investigated. With a filling ratio of 35%, novel bio-carriers were used to support the growth of thicker biofilms on the inner surface. Batch studies performed with DAP-spiked wastewater demonstrate the feasibility of treatment in MBBR with approximately 95% ammonium reduction for a batch period of around 85 h. Denitrification and phosphorus removal occurs in the deeper layers of the biofilm, which are anoxic due to DO depletion. However, denitrification and phosphorus removal was not efficient due to low organic carbon content, resulting in low SND efficiency. With the aim of improving the treatment efficiency, continuous studies were conducted using a two-stage aerobic MBBR, which achieved approximately 90% SND efficiency with over 60% phosphorus removal. The effluent after two-stage treatment still contained high levels of phosphorus, which could not be safely disposed of without any tertiary treatment. The study shows successful application of MBBR to treat high DAP-containing wastewater as a biological treatment unit, which can be followed in conjunction with a polishing step. The present study investigated the biological speciation of the biofilms which further illustrated the occurrence of the biochemical processes in the reactor system.
Data availability
Not applicable.
References
Antoniou P, Hamilton J, Koopman B, Jain R, Holloway B, Lyberatos G, Svoronos SA (1990) Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Res 24:97–101. https://doi.org/10.1016/0043-1354(90)90070-M
Arp DJ, Chain PS, Klotz MG (2007) The impact of genome analyses on our understanding of ammonia-oxidizing bacteria. Annu Rev Microbiol 61:503–528. https://doi.org/10.1146/annurev.micro.61.080706.093449
Azeem B, KuShaari K, Man ZB, Basit A, Thanh TH (2014) Review on materials & methods to produce controlled release coated urea fertilizer. J Control Release 181:11–21. https://doi.org/10.1016/j.jconrel.2014.02.020
Barak Y, Van Rijn J (2000) A typical polyphosphate accumulation by the denitrifying bacterium Paracoccus denitrificans. Appl Environ Microbiol 66:1209–1212. https://doi.org/10.1128/AEM.66.3.1209-1212.2000
Barker PS, Dold PL (1997) General model for biological nutrient removal activated-sludge systems: model presentation. Water Environ Res 69:969–984. https://doi.org/10.2175/106143097X125669
Bhattacharya R, Mazumder D (2019) Determination of rate and order of denitrification of nitrified effluent using activated sludge. J Indian Chem Soc 96:487–492
Bhattacharya R, Mazumder D (2021) Evaluation of nitrification kinetics for treating ammonium nitrogen enriched wastewater in moving bed hybrid bioreactor. J Environ Chem Eng 9:104589. https://doi.org/10.1016/j.jece.2020.104589
Bueno RF, Piveli RP, Campos F, Sobrinho PA (2018) Simultaneous nitrification and denitrification in the activated sludge systems of continuous flow. Environ Technol 39:2641–2652. https://doi.org/10.1080/09593330.2017.1363820
Campo R, Sguanci S, Caffaz S, Mazzoli L, Ramazzotti M, Lubello C, Lotti T (2020) Efficient carbon, nitrogen and phosphorus removal from low C/N real domestic wastewater with aerobic granular sludge. Bioresour Technol 305:122961. https://doi.org/10.1016/j.biortech.2020.122961
Çeçen F (1996) Investigation of partial and full nitrification characteristics of fertilizer wastewaters in a submerged biofilm reactor. Water Sci Technol 34:77–85. https://doi.org/10.1016/S0273-1223(96)00823-2
Chen S, Ling J, Blancheton JP (2006) Nitrification kinetics of biofilm as affected by water quality factors. Aquacult Eng 34:179–197. https://doi.org/10.1016/j.aquaeng.2005.09.004
Chen W, Lu Y, Jin Q, Zhang M, Wu J (2020) A novel feedforward control strategy for simultaneous nitrification and denitrification (SND) in aerobic granular sludge sequential batch reactor (AGS-SBR). J Environ Manage 260:110103. https://doi.org/10.1016/j.jenvman.2020.110103
Chu L, Wang J (2011) Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chem Eng J 170:220–225. https://doi.org/10.1016/j.cej.2011.03.058
Cramer M, Schelhorn P, Kotzbauer U, Tränckner J (2021) Degradation kinetics and COD fractioning of agricultural wastewaters from biogas plants applying biofilm respirometry. Environ Technol 42:2391–2401. https://doi.org/10.1080/09593330.2019.1701570
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182. https://doi.org/10.1128/AEM.66.3.1175-1182.2000
Cui R, Jahng D (2004) Nitrogen control in AO process with recirculation of solubilized excess sludge. Water Res 38:1159–1172. https://doi.org/10.1016/j.watres.2003.11.013
Cui N, Cai M, Zhang X, Abdelhafez AA, Zhou L, Sun H, Chen G, Zou G, Zhou S (2002) Runoff loss of nitrogen and phosphorus from a rice paddy field in the east of China: effects of long-term chemical N fertilizer and organic manure applications. Glob Ecol Conserv 22:01011. https://doi.org/10.1016/j.gecco.2020.e01011
Dandie CE, Burton DL, Zebarth BJ, Trevors JT, Goyer C (2007) Analysis of denitrification genes and comparison of nosZ, cnorB and 16S rDNA from culturable denitrifying bacteria in potato cropping systems. Syst Appl Microbiol 30:128–138. https://doi.org/10.1016/j.syapm.2006.05.002
De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246. https://doi.org/10.1016/j.watres.2004.07.014
Divya J, Belagali SL (2012) Impact of chemical fertilizers on water quality in selected agricultural areas of Mysore district, Karnataka, India. Int J Environ Sci 2:1449–1458. https://doi.org/10.6088/ijes.00202030030
Dobbeleers T, D’aes J, Miele S, Caluwé M, Akkermans V, Daens D, Geuens L, Dries J (2017) Aeration control strategies to stimulate simultaneous nitrification-denitrification via nitrite during the formation of aerobic granular sludge. Appl Microbiol Biotechnol 101:6829–6839. https://doi.org/10.1007/s00253-017-8415-1
Eaton AD, Clesceri LS, Rice EW (2005) Greenberg AE (2005) American Public Health Association (APHA) Standard methods for the examination of water and wastewater. American Water Works Association; Water Pollution Control Federation, Washington
Eckenfelder WW, O'Connor DJ (2013) Biological waste treatment. Elsevier. https://books.google.co.in/books?hl=en&lr=&id=3fUbBQAAQBAJ&oi=fnd&pg=PP1&dq=Eckenfelder+WW,+O%27Connor+DJ+(2013)+Biological+waste+treatment.+&ots=HK8gkqCbFf&sig=Z2FO0ftXTXnvsKJAPn-6t69klCM&redir_esc=y#v=onepage&q=Eckenfelder%20WW%2C%20O'Connor%20DJ%20(2013)%20Biological%20waste%20treatment.&f=false
FAO (2019) World fertilizer trends and outlook to 2022. Rome, Italy. http://faostat3.fao.org
Feng LJ, Jia R, Zeng Z, Yang GF, Xu XY (2018) Simultaneous nitrification denitrification and microbial community profile in an oxygen-limiting intermittent aeration SBBR with biodegradable carriers. Biodegradation 29:473–486. https://doi.org/10.1007/s10532-018-9845-x
Green SJ, Prakash O, Gihring TM, Akob DM, Jasrotia P, Jardine PM, Watson DB, Brown SD, Palumbo AV, Kostka JE (2010) Denitrifying bacteria isolated from terrestrial subsurface sediments exposed to mixed-waste contamination. Appl Environ Microbiol 76:3244–3254. https://doi.org/10.1128/AEM.03069-09
Han C, Wright GS, Fisher K, Rigby SE, Eady RR, Hasnain SS (2012) Characterization of a novel copper-haem c dissimilatory nitrite reductase from Ralstonia pickettii. Biochem J 444:219–226. https://doi.org/10.1042/BJ20111623
Herbert D, Phipps PJ, Strange RE (1971) Chapter III chemical analysis of microbial cells. Methods Microbiol 5:209–344. https://doi.org/10.1016/S0580-9517(08)70641-X
Iannacone F, Di Capua F, Granata F, Gargano R, Pirozzi F, Esposito G (2019) Effect of carbon-to-nitrogen ratio on simultaneous nitrification denitrification and phosphorus removal in a microaerobic moving bed biofilm reactor. J Environ Manag 250:109518. https://doi.org/10.1016/j.jenvman.2019.109518
Iannacone F, Di Capua F, Granata F, Gargano R, Esposito G (2020) Simultaneous nitrification, denitrification and phosphorus removal in a continuous-flow moving bed biofilm reactor alternating microaerobic and aerobic conditions. Bioresour Technol 310:123453. https://doi.org/10.1016/j.biortech.2020.123453
Jena J, Kumar R, Saifuddin M, Dixit A, Das T (2016) Anoxic–aerobic SBR system for nitrate, phosphate and COD removal from high-strength wastewater and diversity study of microbial communities. Biochem Eng J 105:80–89. https://doi.org/10.1016/j.bej.2015.09.007
Jenkins D, Wanner J (2014) Activated sludge-100 years and counting. IWA publishing. https://books.google.co.in/books?hl=en&lr=&id=J7cDBAAAQBAJ&oi=fnd&pg=PP1&dq=Jenkins+D,+Wanner+J+(2014)+Activated+sludge-100+years+and+counting.+&ots=fqGBSD__CX&sig=Ol_nzGY4ZOQuqJZtHCyoKa8lCL4&redir_esc=y#v=onepage&q=Jenkins%20D%2C%20Wanner%20J%20(2014)%20Activated%20sludge-100%20years%20and%20counting.&f=false
Kermani M, Bina B, Movahedian H, Amin MM, Nikaein M (2009) Biological phosphorus and nitrogen removal from wastewater using moving bed biofilm process. Iran J Biotechnol 7:19–27
Krishnaswamy U, Muthuchamy M, Perumalsamy L (2011) Biological removal of phosphate from synthetic wastewater using bacterial consortium. Iran J Biotechnol 9:37–49
Kuba TMCM, Van Loosdrecht MCM, Heijnen JJ (1996) Phosphorus and nitrogen removal with minimal COD requirement by integration of denitrifying dephosphatation and nitrification in a two-sludge system. Water Res 30:1702–1710. https://doi.org/10.1016/0043-1354(96)00050-4
Kumwimba MN, Meng F, Iseyemi O, Moore MT, Zhu B, Tao W, Liang TJ, Ilunga L (2018) Removal of non-point source pollutants from domestic sewage and agricultural runoff by vegetated drainage ditches (VDDs): design, mechanism, management strategies, and future directions. Sci Total Environ 639:742–759. https://doi.org/10.1016/j.scitotenv.2018.05.184
Leong SK, Chang SH, Kassim NA, Nasuha N (2011) Determination of treatability constant and reaction-rate constant for an attached-growth upflow fixed-film reactor on pulp and paper wastewater treatment. Int J Chem Eng Appl 2:14–19
Leyva-Díaz JC, Monteoliva-García A, Martín-Pascual J, Munio MM, García-Mesa JJ, Poyatos JM (2020) Moving bed biofilm reactor as an alternative wastewater treatment process for nutrient removal and recovery in the circular economy model. Bioresour Technol 299:122631. https://doi.org/10.1016/j.biortech.2019.122631
Liu W, Chen W, Yang D, Shen Y (2019) Functional and compositional characteristics of nitrifiers reveal the failure of achieving mainstream nitritation under limited oxygen or ammonia conditions. Biores Technol 275:272–279. https://doi.org/10.1016/j.biortech.2018.12.065
Liu T, He X, Jia G, Xu J, Quan X, You S (2020) Simultaneous nitrification and denitrification process using novel surface-modified suspended carriers for the treatment of real domestic wastewater. Chemosphere 247:125831. https://doi.org/10.1016/j.chemosphere.2020.125831
Lu DQ, Chien SH, Henao J, Sompongse D (1987) Evaluation of short-term efficiency of diammonium phosphate versus urea plus single superphosphate on a calcareous soil. Agron J 79:896–900. https://doi.org/10.2134/agronj1987.00021962007900050028x
Ma B, Wang S, Zhu G, Ge S, Wang J, Ren N, Peng Y (2013) Denitrification and phosphorus uptake by DPAOs using nitrite as an electron acceptor by step-feed strategies. Front Environ Sci Eng 7:267–272. https://doi.org/10.1007/s11783-012-0439-2
Maqsood MA, Ashraf I, Rasheed N (2022) Sources of nitrogen for crop growth: Pakistan’s case. In Nitrogen assessment 13–28, Academic Press. https://doi.org/10.1016/B978-0-12-824417-3.00005-8
Mun HS, Park JH, Kim H, Yu HK, Park YG, Cha CY, Kook YH, Kim BJ (2008) Mycobacterium senuense sp. nov., a slowly growing, non-chromogenic species closely related to the Mycobacterium terrae complex. Int J Syst Evol Microbiol 58:641–646. https://doi.org/10.1099/ijs.0.65374-0
Nancharaiah YV, Mohan SV, Lens PNL (2019) Removal and recovery of metals and nutrients from wastewater using bioelectrochemical systems. In Microbial Electrochemical Technology 693–720, Elsevier. https://doi.org/10.1016/B978-0-444-64052-9.00028-5
Nguyen HTT, Le VQ, Hansen AA, Nielsen JL, Nielsen PH (2011) High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol Ecol 76:256–267. https://doi.org/10.1111/j.1574-6941.2011.01049.x
Nhut HT, Hung NTQ, Sac TC, Bang NHK, Tri TQ, Hiep NT, Ky NM (2020) Removal of nutrients and organic pollutants from domestic wastewater treatment by sponge-based moving bed biofilm reactor. Environ Eng Res 25:652–658. https://doi.org/10.4491/eer.2019.285
Nielsen PH, McIlroy SJ, Albertsen M, Nierychlo M (2019) Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr Opin Biotechnol 57:111–118. https://doi.org/10.1016/j.copbio.2019.03.008
Orhon D, Artan N (1994) Modeling of activated sludge systems. Technomic Publ. Co., Lancaster
Palma TL, Donaldben MN, Costa MC, Carlier JD (2018) Putative role of Flavobacterium, Dokdonella and Methylophilus strains in paracetamol biodegradation. Water Air Soil Pollut 229:1–23. https://doi.org/10.1007/s11270-018-3858-2
Piculell M, Welander P, Jönsson K, Welander T (2016) Evaluating the effect of biofilm thickness on nitrification in moving bed biofilm reactors. Environ Technol 37:732–743. https://doi.org/10.1080/09593330.2015.1080308
Poduska RA, Andrews JF (1975) Dynamics of nitrification in the activated sludge process. J (Water Pollution Control Federation) 47:2599–2619
Prasedya ES, Kurniawan NSH, Kirana IAP, Ardiana N, Abidin AS, Ilhami BTK, Jupri A, Widyastuti S, Sunarpi H, Nikmatullah A (2022) Seaweed fertilizer prepared by EM-fermentation increases abundance of beneficial soil microbiome in paddy (Oryzasativa L.) during vegetative stage. Fermentation 8:46. https://doi.org/10.3390/fermentation8020046
Qiao Z, Sun R, Wu Y, Hu S, Liu X, Chan J, Mi X (2020) Characteristics and metabolic pathway of the bacteria for heterotrophic nitrification and aerobic denitrification in aquatic ecosystems. Environ Res 191:110069. https://doi.org/10.1016/j.envres.2020.110069
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill Education. https://www.accessengineeringlibrary.com/content/book/9781260440591
Rittstieg K, Robra KH, Somitsch W (2001) Aerobic treatment of a concentrated urea wastewater with simultaneous stripping of ammonia. Appl Microbiol Biotechnol 56:820–825. https://doi.org/10.1007/s002530100696
Rodriguez-Sanchez A, Muñoz-Palazon B, Hurtado-Martinez M, Rivadeneyra MA, Poyatos JM, Gonzalez-Lopez J (2018) Maximum influent salinity affects the diversity of mineral-precipitation-mediating bacterial communities in membrane biofilm of hybrid moving bed biofilm reactor-membrane bioreactor. Water Air Soil Pollut 229:1–17. https://doi.org/10.1007/s11270-018-4020-x
Saidulu D, Majumder A, Gupta AK (2021) A systematic review of moving bed biofilm reactor, membrane bioreactor, and moving bed membrane bioreactor for wastewater treatment: comparison of research trends, removal mechanisms, and performance. J Enviro Chem Eng 9:106112. https://doi.org/10.1016/j.jece.2021.106112
Salehi S, Cheng KY, Heitz A, Ginige MP (2019) Simultaneous nitrification, denitrification and phosphorus recovery (SNDPr) - an opportunity to facilitate full-scale recovery of phosphorus from municipal wastewater. J Environ Manage 238:41–48. https://doi.org/10.1016/j.jenvman.2019.02.063
Shi HP, Lee CM (2006) Combining anoxic denitrifying ability with aerobic–anoxic phosphorus-removal examinations to screen denitrifying phosphorus-removing bacteria. Int Biodeterior Biodegrad 57:121–128. https://doi.org/10.1016/j.ibiod.2006.01.001
Shivaraman N, Vaidya AN, Waghmare SV, Paunikar WN, Tankhiwale A, Padoley K (2001) A two-stage biological treatment system for ammonium-nitrate-laden wastewater. World J Microbiol Biotechnol 17:447–453. https://doi.org/10.1023/A:1011996323850
Singh PP, Mall M, Singh J (2006) Impact of fertilizer factory effluent on seed germination, seedling growth and chlorophyll content of gram (Cicer aeritenum). J Environ Biol 27:153–156
Spangler JT, Sample DJ, Fox LJ, Owen JS Jr, White SA (2019) Floating treatment wetland aided nutrient removal from agricultural runoff using two wetland species. Ecol Eng 127:468–479. https://doi.org/10.1016/j.ecoleng.2018.12.017
Subhashini DV (2015) Growth promotion and increased potassium uptake of tobacco by potassium-mobilizing bacterium Frateuriaaurantia grown at different potassium levels in vertisols. Commun Soil Sci Plant Anal 46:210–220. https://doi.org/10.1080/00103624.2014.967860
Tchobanoglous G, Burton FL, Stensel HD (2003) Wastewater engineering treatment and reuse (No. 628.3 T252s). McGraw-Hill Higher Education, Boston, US
Tsuneda S, Park S, Hayashi H, Jung J, Hirata A (2001) Enhancement of nitrifying biofilm formation using selected EPS produced by heterotrophic bacteria. Water Sci Technol 43:197–204. https://doi.org/10.2166/wst.2001.0374
Ulrich K, Kube M, Becker R, Schneck V, Ulrich A (2021) Genomic analysis of the endophytic Stenotrophomonas strain 169 reveals features related to plant-growth promotion and stress tolerance. Front Microbiol 12:1542. https://doi.org/10.3389/fmicb.2021.687463
Van Aken P, Lambert N, Appels L (2022) Low temperature moving bed bioreactor denitrification as mitigation measure to reduce agricultural nitrate losses. Sci Total Environ 810:152110. https://doi.org/10.1016/j.scitotenv.2021.152110
Van Loosdrecht MC, Henze M (1999) Maintenance, endogeneous respiration, lysis, decay and predation. Water Sci Technol 39:107–117. https://doi.org/10.1016/S0273-1223(98)00780-X
Van Rijn J, Tal Y, Schreier HJ (2006) Denitrification in recirculating systems: theory and applications. Aquacult Eng 34:364–376. https://doi.org/10.1016/j.aquaeng.2005.04.004
van den Heuvel RN, van der Biezen E, Jetten MSM, Hefting MM, Kartal B (2010) Denitrification at pH 4 by a soil-derived Rhodanobacter dominated community. Environ Microbiol 12:3264–3271. https://doi.org/10.1111/j.1462-2920.2010.02301.x
von Ahnen M, Pedersen LF, Pedersen PB, Dalsgaard J (2015) Degradation of urea, ammonia and nitrite in moving bed biofilters operated at different feed loadings. Aquac Eng 69:50–59. https://doi.org/10.1016/j.aquaeng.2015.10.004
Wang L, Wang GL, Li SP, Jiang JD (2011) Luteibacterjiangsuensis sp. nov.: a methamidophos-degrading bacterium isolated from a methamidophos-manufacturing factory. Curr Microbiol 62:289–295. https://doi.org/10.1007/s00284-010-9707-1
Wang X, Zhao J, Yu D, Chen G, Du S, Zhen J, Yuan M (2019) Stable nitrite accumulation and phosphorous removal from nitrate and municipal wastewaters in a combined process of endogenous partial denitrification and denitrifying phosphorus removal (EPDPR). Chem Eng J 355:560–571. https://doi.org/10.1016/j.cej.2018.08.165
Wang J, Rong H, Cao Y, Zhang C (2020) Factors affecting simultaneous nitrification and denitrification (SND) in a moving bed sequencing batch reactor (MBSBR) system as revealed by microbial community structures. Bioprocess Biosyst Eng 43:1833–1846
Wang J, Xia L, Chen J, Wang X, Wu H, Li D, Wells GF, Yang J, Hou J, He X (2021) Synergistic simultaneous nitrification-endogenous denitrification and EBPR for advanced nitrogen and phosphorus removal in constructed wetlands. Chem Eng J 420:127605. https://doi.org/10.1016/j.cej.2020.127605
Wu Z, Zhu W, Liu Y, Peng P, Li X, Zhou X, Xu J (2020) An integrated threedimensional electrochemical system for efficient treatment of coking wastewater rich in ammonia nitrogen. Chemosphere 246:125703. https://doi.org/10.1016/j.chemosphere.2019.125703
Ye Y, Ngo HH, Guo W, Liu Y, Chang SW, Nguyen DD, Liang H, Wang J (2018) A critical review on ammonium recovery from wastewater for sustainable wastewater management. Biores Technol 268:749–758. https://doi.org/10.1016/j.biortech.2018.07.111
Zaman M, Kim M, Nakhla G (2021) Simultaneous nitrification-denitrifying phosphorus removal (SNDPR) at low DO for treating carbon-limited municipal wastewater. Sci Total Environ 760:143387. https://doi.org/10.1016/j.scitotenv.2020.143387
Zhang WL, Wu SX, Ji HJ, Kolbe H (2004) Estimation of agricultural non-point source pollution in China and the alleviating strategies I. Estimation of agricultural non-point source pollution in China in early 21 century. Scientiaagriculturasinica 37:1008–1017
Zhang X, Zhang H, Chen Z, Wei D, Song Y, Ma Y, Zhang H (2021) Achieving biogas production and efficient pollutants removal from nitrogenous fertilizer wastewater using combined anaerobic digestion and autotrophic nitrogen removal process. Bioresour Technol 339:125659. https://doi.org/10.1016/j.biortech.2021.125659
Zhong L, Lai CY, Shi LD, Wang KD, Dai YJ, Liu YW, Ma F, Rittmann BE, Zheng P, Zhao HP (2017) Nitrate effects on chromate reduction in a methane based biofilm. Water Res 115:130–137. https://doi.org/10.1016/j.watres.2017.03.003
Zhou Q, Zhang L, Chen J, Luo Y, Zou H, Sun B (2016) Enhanced stable long-term operation of biotrickling filters treating VOCs by low-dose ozonation and its affecting mechanism on biofilm. Chemosphere 162:139–147. https://doi.org/10.1016/j.chemosphere.2016.07.072
Zhou J, Ji L, Gong C, Lu L, Cheng X (2020) Ceramsite vegetated concrete with water and fertilizer conservation and light weight: effect of w/c and fertilizer on basic physical performances of concrete and physiological characteristics of Festucaarundinacea. Constr Build Mater 236:117785. https://doi.org/10.1016/j.conbuildmat.2019.117785
Acknowledgements
The authors are thankful to the Ministry of Education, Government of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis along with preparation of the first draft were performed by Roumi Bhattacharya. Debabrata Mazumder commented on previous versions of the manuscript and supervised the work. Both the authors have approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Both authors would like to consent to publish all information provided in the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhattacharya, R., Mazumder, D. Performance evaluation of moving bed bioreactor for simultaneous nitrification denitrification and phosphorus removal from simulated fertilizer industry wastewater. Environ Sci Pollut Res 30, 49060–49074 (2023). https://doi.org/10.1007/s11356-023-25708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25708-z