Abstract
Inflammation is the body’s response against various pathogens and has a critical role in numerous diseases. Zingerone (Zing), a bioactive substance derived from ginger root, has a variety of pharmacological properties, such as reducing inflammation, and antioxidant effects. We aimed to evaluate the beneficial effects of Zing in a carrageenan-induced inflammation model. Paw edema induced by carrageenan (100 μl of 1%) was used to induce acute inflammation in rats. Different doses of Zing (10, 20, and 40 mg/kg) were administered intraperitoneally. Paw tissue levels of MDA, NO, CAT, SOD, GPx, GSH, COX-2, PGE2, TNF-α, and IL-1β were estimated. Our results showed that Zing, especially at the highest dose of 40 mg/kg, significantly reduced paw swelling in carrageenan-injected animals. Zing significantly increased paw enzymatic and nonenzymatic antioxidants except CAT. It also decreased paw levels of MDA, NO, COX-2, PGE2, TNF-α, and IL-1β. The results of this study show that Zing may provide an alternative for the clinical control of inflammation through antioxidant and anti-inflammatory activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is a clinical condition present in most pathologies including toxins, radiation, injury, and infection [8]. Moreover, inflammation is characterized by swelling, redness, heat, and pain. Furthermore, inflammation is one of the main causes of organ dysfunction which requires pharmacological intervention [18]. It is well-documented that inflammation is involved in the pathogenesis of many disorders such as aging and cardiovascular and neurological diseases [20]. Currently, steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs) are the most commonly used medications for treating inflammation. Besides their effectiveness for treatment of this disorder, these drugs have established serious adverse effects such as gastrointestinal ulcers, hepatotoxicity, bleeding, and renal disorders [11]. Therefore, searching for better anti-inflammatory agents with lower side effects is needed, and new biologically active compounds from plants are a promising strategy for discovering these agents [13].
Zingerone (Zing), a bioactive substance of ginger root, has a variety of pharmacological properties such as anticancer, antioxidant, anti-inflammatory, antimicrobial, and antiapoptotic effects [9, 27]. On the other hand, the antioxidant properties of Zing are mediated via increasing the antioxidant capacity and reducing the level of nitric oxide (NO) and lipid peroxidation [3, 35]. The anti-inflammatory effects of Zing have been established in several animal models including sepsis- and cisplatin-induced nephrotoxicity [4, 25]. Zing is a safe substance, and the lethal dose 50% (LD50) for rats is 2580 mg/kg [31].
Although the anti-inflammatory effects of Zing had been shown in different conditions, there is no available study investigating the anti-inflammatory properties of Zing in mitigating rat paw edema induced by carrageenan (Carr). The present investigation was aimed to document more detailed effects of Zing to protect rats from Carr-induced acute inflammation, which was established for evaluating anti-inflammatory agents.
MATERIAL AND METHODS
Drugs and Chemicals
Carr and Zing were obtained from Tocris Co. (Bristol, UK). Indomethacin (Ind) was kindly donated by Iran Daru Pharmaceutical Co. (Tehran, Iran). Carr was dissolved in 0.9% sodium chloride. Zing and Ind were dissolved in 10% DMSO.
Animals
Adult male Wistar rats (200–220 g) were used in this study and were housed at standard conditions (23 ± 2 °C; 12 h/12 h light/dark cycle; 50% humidity). All procedures were conducted according to the Ethical Guideline for Research, Ahvaz University (Ethics code: IR.AJUMS.ABHC.REC.1397.002).
Treatment
Forty-two rats were divided into 6 groups (n = 7). The first group was i.p. injected with DMSO (10 ml/kg of 10%) 30 min prior sodium chloride (100 μl of 0.9%) injection i.pl. in the right paw of the rats. The second group was i.p. injected with DMSO 30 min prior Carr (100 μl of 1%) in the right paw. The third group was i.p. injected with Ind (5 mg/kg in a volume of 10 ml/kg) 30 min prior Carr injection. The fourth, fifth, and sixth groups were i.p. injected with Zing (10, 20, and 40 mg/kg in a volume of 10 ml/kg) 30 min prior Carr injection.
Evaluation of Carr-Induced Paw Edema and Inflammation
Immediately before and at 0.5, 1, 2, 3, 4, and 5 h after carrageenan treatment, the volume (ml) of paw edema was quantified by measuring the volume of the paw using a plethysmometer (Ugo Basile Co., Italy).
The changes in edema volume (ml) were evaluated by the following formula:
A and B are the volume (ml) of the right hind paw after (0.5, 1, 2, 3, 4, and 5 h) and before carrageenan administration, respectively.
The percent of inflammation was evaluated by the following formula:
V1 and V2 are the volume (ml) of the right hind paw before and 5 h after carrageenan administration, respectively.
Sample Collection
Immediately after evaluating the paw edema, the animals were sacrificed, and the right hind paws were dissected. An ice-cold 0.1 M Tris-HCl buffer (1/10 w/v) was used for sample homogenization. Then, the samples were spun at 3500 rpm in a centrifuge for 10 min at 4 °C [17]. Measurement of oxidative stress and inflammatory indices were carried out on the supernatants, which was stored at − 80 °C. The total protein concentration in the supernatant was estimated by the method of Bradford [6], using crystalline bovine serum albumin as standard.
Assessment of Oxidative Stress Indices
Catalase (CAT) activity was measured by the method described by Aebi et al. [2]. Superoxide dismutase (SOD) activity was assessed by the method of Martin et al. [30]. The levels of glutathione (GSH) were evaluated by the method of Ellman et al. [14]. The activity of glutathione peroxidase (GPx) was determined by the commercial kit (Randox Labs, Crumlin, UK). The lipid peroxidation was assessed by the measurement of malondialdehyde (MDA) according to the method described by Buege et al. [7]. The levels of NO were evaluated using the Griess method [36].
ELISA Measurements
The tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2) concentrations were evaluated in the paw homogenates. ELISA kit of Abcam Biochemical Co. (Cambridge, UK) for COX-2, ELISA kit of Crystal Day Biotech Co. (Shanghai, China) for PGE2, and ELISA kits of eBiosource International, Inc. (Camarillo, CA) for TNF-α and IL-1β were used.
Statistical Analysis
Data were shown as mean ± SD. Results were statistically compared using one-way analysis of variance (ANOVA) and Tukey post hoc test. A p value ≤ 0.05 was considered statistically significant.
RESULTS
Effects of Zing on Paw Edema and Inflammation
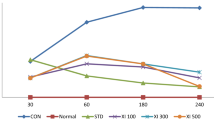
Our results showed that Ind reduced the paw edema of right hind paw in comparison with the Carr group at all evaluated times (p ≤ 0.05) (Fig. 1a). Pretreatment with Zing at the dose of 10 mg/kg reduced the paw edema of right hind paw 3 h after Carr injection as compared with the Carr group (p ≤ 0.05). Moreover, pretreatment with Zing at the doses of 20 and 40 mg/kg decreased the paw edema of right hind paw 2 h after Carr injection compared with the Carr group (p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg; i.p.) and indomethacin (Ind; 5 mg/kg; i.p.) against carrageenan (Carr)-induced paw edema (a) and inflammation (b). Drugs were administrated 30 min before Carr injection. Asterisk indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s test, n = 7).
Also, data obtained from the Ind group revealed a significant reduction in the inflammation of right hind paw compared with the Carr group (p ≤ 0.05) (Fig. 1b). Pretreatment with Zing (20 and 40 mg/kg) 30 min before Carr caused a significant decrease in the inflammation of right hind paw compared with the Carr group (p ≤ 0.05).
Effects of Zing on Paw Oxidative Stress Indices
The content of GSH and GPx activity was significantly reduced in the Carr group compared with the control group (all p ≤ 0.05) (Fig. 2 a and b). Also, the administration of Ind 30 min before i.pl. injection of Carr significantly elevated these indices in comparison with Carr group. Moreover, pretreatment with Zing at the dose of 40 mg/kg significantly increased the content of GSH and GPx activity in paw tissues as compared with the Carr group (p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg; i.p.) and indomethacin (Ind; 5 mg/kg; i.p.) on GSH content (a) and GPx activity (b) of paw tissue. Drugs were administrated 30 min before Carr injection. Pound indicates versus control group (p ≤ 0.05). Asterisk sign indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s test, n = 7).
The SOD and CAT activities were significantly reduced in the Carr group compared with the control group (all p ≤ 0.05) (Fig. 3 a and b). Also, the administration of Ind 30 min before i.pl. injection of Carr significantly elevated these indices relative to Carr group. Moreover, pretreatment with Zing at doses of 20 and 40 mg/kg only significantly increased the SOD activity in paw tissues as compared with the Carr group (all p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg; i.p.) and indomethacin (Ind; 5 mg/kg; i.p.) on SOD (a) and CAT (b) activities of paw tissue. Drugs were administrated 30 min before Carr injection. Pound indicates versus control group (p ≤ 0.05). Asterisk sign indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s test, n = 7).
The MDA and NO levels were significantly increased in the Carr group in comparison with the control group (all p ≤ 0.05) (Fig. 4 a and b). Also, the administration of Ind 30 min before i.pl. injection of Carr significantly reduced these indices compared with the Carr group. Moreover, pretreatment with Zing at doses of 20 and 40 mg/kg significantly reduced the MDA level in paw tissues compared with the Carr group (all p ≤ 0.05). Furthermore, Zing at all doses (10, 20, and 40 mg/kg) significantly reduced the NO level as compared with the Carr group (all p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg; i.p.) and indomethacin (Ind; 5 mg/kg; i.p.) on MDA (a) and NO (b) levels of paw tissue. Drugs were administrated 30 min before Carr injection. Pound indicates versus control group (p ≤ 0.05). Asterisk sign indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey's test, n = 7).
Effects of Zing on Paw Inflammatory Indices
The levels of COX-2 and PGE2 were significantly increased in the Carr group compared with the control group (all p ≤ 0.05) (Fig. 5 a and b). Also, the administration of Ind 30 min before i.pl. injection of Carr significantly reduced these indices in comparison with the Carr group. Moreover, pretreatment with Zing at doses of 20 and 40 mg/kg significantly reduced the levels of COX-2 and PGE2 in paw tissues compared with the Carr group (p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg; i.p.) and indomethacin (Ind; 5 mg/kg; i.p.) on COX-2 (a) and PGE2 (b) levels of paw tissue. Drugs were administrated 30 min before Carr injection. Pound indicates versus control group (p ≤ 0.05). Asterisk sign indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s test, n = 7).
The IL-1β and TNF-α levels were significantly elevated in the Carr group compared with the control group (all p ≤ 0.05) (Fig. 6 a and b). Also, the administration of Ind 30 min before i.pl. injection of Carr significantly reduced these indices compared with the Carr group. Moreover, pretreatment with Zing at doses of 20 and 40 mg/kg significantly reduced the level of IL-1β in paw tissues in comparison with the Carr group (p ≤ 0.05). Furthermore, Zing at all doses (10, 20, and 40 mg/kg) significantly reduced the TNF-α level compared with the Carr group (all p ≤ 0.05).
Effect of zingerone (Zing; 10, 20, and 40 mg/kg) and indomethacin (Ind; 5 mg/kg) on IL-1β (a) and TNF-α (b) levels of paw tissue. Drugs were administrated 30 min before Carr injection. Pound indicates versus control group (p ≤ 0.05). Asterisk sign indicates versus Carr-treated group (p ≤ 0.05). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s test, n = 7).
DISCUSSION
The protective role of Zing on Carr-induced acute inflammation in the rat paw was investigated in this study. The present study shows that Zing, especially at the highest dose of 40 mg/kg, is capable of mitigating Carr-induced rat paw edema in a dose-dependent manner. Upon oxidative stress and inflammatory measurement, we also noted that treatment with Zing resulted in a reduction of the inflammatory and oxidative reaction in the rat paw.
It has been described that Carr induced an inflammation with biphasic phenomenon (early and delayed phases). The early phase (0 to 1 h after injection) is dominantly associated with the serotonin, bradykinin, and histamine. In this phase, the local blood flow and capillary permeability increased, resulting in edema initiation [34]. The delayed phase (after 1 h) is related with leukocyte migration and prostaglandins that considered the key elements for its maintenance. Inflammation induced by Carr has been applied as a useful model to identify the new anti-inflammatory agents [37].
In inflammatory conditions, over production of reactive oxygen species (ROS) is responsible for organ and cellular damages [16, 26]. On the other hand, high ROS causes the production of other oxidative stress markers such as MDA and NO [15, 21]. Moreover, inflammatory conditions reduced the level and/or activity of antioxidant defense mediators such as CAT, SOD, GPx, and GSH [5]. On the other hand, previous reports have shown that i.pl. administration of Carr increases the level of MDA and NO and reduces the level and/or activity of these antioxidant parameters [13, 19, 37]. In agreement with these reports, we also found similar effects from i.pl. administration of Carr. As potent NSAIDs, Ind attenuated all of these deleterious effects of Carr in paw tissue. Our results revealed that the treatment of animals with Zing (especially at dose of 40 mg/kg) before Carr significantly reduces the paw MDA and NO compared with the Carr group while causing a significant elevation in paw GSH, SOD, and GPx. Zing has potent direct and indirect antioxidative effects. Zing has scavenging activity against ROS via its phenolic group which reduces MDA and NO production [9]. Moreover, Zing indirectly reduces the oxidative stress via increasing the level and/or activity of antioxidant parameters [27]. It has been shown that Zing reduces the mouse testicular damage induced by zinc oxide nanoparticles via reducing the levels of MDA and increasing SOD and CAT activities [32]. According to the reports of Soliman, Zing attenuated radiation oxidative stress in the heart tissue of rats [35]. In another study, Zing reduced the oxidative stress in the acute vancomycin-induced kidney damages [22]. They found that Zing acts through decreasing MDA level and elevating the activity of SOD, GPx, and CAT.
In the present study, we found a significant increase in the levels of inflammatory mediators such as TNF-α, IL-1β, COX-2, and PGE2 in the paw of Carr-administered animals. Ind and Zing (especially at the doses of 20 and 40 mg/kg) decreased the levels of inflammatory mediators such as TNF-α, IL-1β, COX-2, and PGE2 in the paw after the carrageenan injection. Cytokines such as TNF-α and IL-1β are regulatory mediators produced by a different kind of immune cells. It is well-established that these cytokines lead signs of inflammation and cellular injuries [10]. As a pro-inflammatory cytokine, TNF-α further regulates the production of other inflammatory mediators [29]. Moreover, TNF-α stimulates the expression of COX-2 and subsequently releases prostaglandins such as PGE2 [1]. Prostaglandins are important contributors of inflammatory response. On the other hand, the COX-2/PGE2 pathway has a critical role in different inflammatory disorders [33]. IL-1β is a prototypical pro-inflammatory cytokine that stimulates both local and systemic immune responses [38]. This cytokine induces the production of various enzymes, such as COX-2 and inducible nitric oxide synthase (iNOS), leading to the production of the inflammatory mediators PGE2 and NO [19]. On the other hand, it has been demonstrated that the NO production could influence the prostaglandin levels, so that the reduction of NO levels declines the expression of COX-2 and production of prostaglandin [12]. The results of present study are in agreement with the results of previous reports about anti-inflammatory effects of Zing in different inflammatory conditions. Kandemir et al. reported that Zing reduces the levels of COX-2, TNF-α, and IL-1β in nephrotoxicity induced by cisplatin [23]. Zing was able to attenuate the levels of the inflammatory markers TNF-α, IL-1β, COX-2, and iNOS in cisplatin-induced ovarian and uterine damage [24]. In another study, it was found that Zing reduces the expression of COX-2 and TNF-α in ethanol-induced hepatotoxicity [28]. Hence, it seems that the reduction of TNF-α and IL-1β concentration and COX-2 and PGE2 levels in the paw can be responsible for the anti-inflammatory effects of Zing.
In summary, pretreatment with Zing before Carr injection has the following effects: (i) produces anti-inflammatory effect in an acute inflammatory model; (ii) produces an attenuated effect in TNF-α, IL-1β, COX-2, and PGE2 levels; and (iii) produces a mitigated effect in activities of CAT, SOD, and GPx and level of GSH. This could provide future directions for studying the anti-inflammatory properties of Zing in inflammatory disorders.
References
Abdelazeem, A.H., S.A. Abdelatef, M.T. El-Saadi, H.A. Omar, S.I. Khan, C.R. McCurdy, and S.M. El-Moghazy. 2014. Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. European Journal of Pharmaceutical Sciences 62: 197–211. https://doi.org/10.1016/j.ejps.2014.05.025.
Aebi, Hugo. 1984. Catalase in vitro. Methods in Enzymology 105: 121–126.
Ahmad, B., M.U. Rehman, I. Amin, M.U.R. Mir, S.B. Ahmad, A. Farooq, S. Muzamil, I. Hussain, M. Masoodi, and B. Fatima. 2018. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation: probable role of NF-kB activation. Saudi Pharmaceutical Journal 26 (8): 1137–1145. https://doi.org/10.1016/j.jsps.2018.07.001.
Alibakhshi, T., M.J. Khodayar, L. Khorsandi, M. Rashno, and L. Zeidooni. 2018. Protective effects of zingerone on oxidative stress and inflammation in cisplatin-induced rat nephrotoxicity. Biomedicine & Pharmacotherapy 105: 225–232. https://doi.org/10.1016/j.biopha.2018.05.085.
Arulselvan, P., M.T. Fard, W.S. Tan, S. Gothai, S. Fakurazi, M.E. Norhaizan, and S.S. Kumar. 2016. Role of antioxidants and natural products in inflammation. Oxidative Medicine and Cellular Longevity 2016: 5276130–5276115. https://doi.org/10.1155/2016/5276130.
Bradford, Marion M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72 (1): 248–254.
Buege, John A., and S.D. Aust. 1978. Microsomal lipid peroxidation. Methods in Enzymology 52: 302–310.
Chen, L., H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang, and L. Zhao. 2018. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9 (6): 7204–7218. https://doi.org/10.18632/oncotarget.23208.
Choi, J.G., S.Y. Kim, M. Jeong, and M.S. Oh. 2018. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacology & Therapeutics 182: 56–69. https://doi.org/10.1016/j.pharmthera.2017.08.010.
Chou, T.C. 2003. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. British Journal of Pharmacology 139 (6): 1146–1152. https://doi.org/10.1038/sj.bjp.0705360.
Conforti, Filomena, Silvio Sosa, Mariangela Marrelli, Federica Menichini, Giancarlo A. Statti, Dimitar Uzunov, Aurelia Tubaro, and Francesco Menichini. 2009. The protective ability of Mediterranean dietary plants against the oxidative damage: the role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chemistry 112 (3): 587–594.
de Aquino, P.E., T.R. Magalhaes, L.A. Nicolau, L.K. Leal, N.C. de Aquino, S.M. Dos Santos, K.R. Neves, E.R. Silveira, and G.S. Viana. 2017. The anti-inflammatory effects of N-methyl-(2S,4R)-trans-4-hydroxy-l-proline from Syderoxylon obtusifolium are related to its inhibition of TNF-alpha and inflammatory enzymes. Phytomedicine 24: 14–23. https://doi.org/10.1016/j.phymed.2016.11.010.
Dharmasiri, M.G., J.R. Jayakody, G. Galhena, S.S. Liyanage, and W.D. Ratnasooriya. 2003. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. Journal of Ethnopharmacology 87 (2-3): 199–206. https://doi.org/10.1016/s0378-8741(03)00159-4.
Ellman, George L. 1959. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82 (1): 70–77.
Germolec, D.R., K.A. Shipkowski, R.P. Frawley, and E. Evans. 2018. Markers of Inflammation. Methods in Molecular Biology 1803: 57–79. https://doi.org/10.1007/978-1-4939-8549-4_5.
Ghaznavi, H., I. Fatemi, H. Kalantari, S.M.T. Hosseini Tabatabaei, M. Mehrabani, B. Gholamine, M. Kalantar, S. Mehrzadi, and M. Goudarzi. 2018. Ameliorative effects of gallic acid on gentamicin-induced nephrotoxicity in rats. Journal of Asian Natural Products Research 20 (12): 1182–1193. https://doi.org/10.1080/10286020.2017.1384819.
Goudarzi, M., M.A. Mombeini, I. Fatemi, A. Aminzadeh, H. Kalantari, A. Nesari, H. Najafzadehvarzi, and S. Mehrzadi. 2019. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurological Research 41 (5): 419–428. https://doi.org/10.1080/01616412.2019.1576319.
Henschke, Nicholas, Steven J Kamper, and Chris G Maher. 2015. The epidemiology and economic consequences of pain. Mayo Clinic Proceedings: Elsevier.
Houshmand, G., M.T. Mansouri, B. Naghizadeh, A.A. Hemmati, and M. Hashemitabar. 2016. Potentiation of indomethacin-induced anti-inflammatory response by pioglitazone in carrageenan-induced acute inflammation in rats: role of PPARgamma receptors. International Immunopharmacology 38: 434–442. https://doi.org/10.1016/j.intimp.2016.06.027.
Hunter, Philip. 2012. The inflammation theory of disease. EMBO Reports 13 (11): 968–970.
Javadi, Iraj, Mohammadreza Rashidi Nooshabadi, Mehdi Goudarzi, and Rahimeh Roudbari. 2015. Protective effects of celery (Apium graveloens) seed extract on bleomycin-induced pulmonary fibrosis in rats. Journal of Babol University of Medical Sciences 17 (1): 70–76.
Kandemir, F.M., S. Yildirim, S. Kucukler, C. Caglayan, A. Mahamadu, and M.B. Dortbudak. 2018. Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomedicine & Pharmacotherapy 105: 981–991. https://doi.org/10.1016/j.biopha.2018.06.048.
Kandemir, F.M., S. Yildirim, C. Caglayan, S. Kucukler, and G. Eser. 2019. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environmental Science and Pollution Research International 26 (22): 22562–22574. https://doi.org/10.1007/s11356-019-05505-3.
Kaygusuzoglu, E., C. Caglayan, F.M. Kandemir, S. Yildirim, S. Kucukler, M.A. Kilinc, and Y.S. Saglam. 2018. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female Wistar rats. Biomedicine & Pharmacotherapy 102: 517–530. https://doi.org/10.1016/j.biopha.2018.03.119.
Lee, B.S., C. Lee, S. Yang, S.K. Ku, and J.S. Bae. 2019. Renal protective effects of zingerone in a mouse model of sepsis. BMB Reports 52 (4): 271–276.
Li, S., Y. Wang, M. Zhao, J. Wu, and S. Peng. 2015. BPIC: a novel anti-tumor lead capable of inhibiting inflammation and scavenging free radicals. Bioorganic & Medicinal Chemistry Letters 25 (5): 1146–1150. https://doi.org/10.1016/j.bmcl.2014.12.013.
Mahomoodally, M.F., M.Z. Aumeeruddy, K.R.R. Rengasamy, S. Roshan, S. Hammad, J. Pandohee, X. Hu, and G. Zengin. 2019. Ginger and its active compounds in cancer therapy: from folk uses to nano-therapeutic applications. Seminars in Cancer Biology. https://doi.org/10.1016/j.semcancer.2019.08.009.
Mani, V., S. Arivalagan, A.I. Siddique, and N. Namasivayam. 2016. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Molecular and Cellular Biochemistry 421 (1-2): 169–181. https://doi.org/10.1007/s11010-016-2798-7.
Mark, K.S., W.J. Trickler, and D.W. Miller. 2001. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. The Journal of Pharmacology and Experimental Therapeutics 297 (3): 1051–1058.
Martin, Joseph P., Michael Dailey, and Elliott Sugarman. 1987. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Archives of Biochemistry and Biophysics 255 (2): 329–336.
Opdyke, D.L., and C. Letizia. 1982. Fragrance raw materials monographs. Food and Chemical Toxicology 20 (6): 637–852. https://doi.org/10.1016/S0015-6264(82)80217-4.
Rafiee, Z., L. Khorsandi, and F. Nejad-Dehbashi. 2019. Protective effect of zingerone against mouse testicular damage induced by zinc oxide nanoparticles. Environmental Science and Pollution Research International 26 (25): 25814–25824. https://doi.org/10.1007/s11356-019-05818-3.
Seibert, K., Y. Zhang, K. Leahy, S. Hauser, J. Masferrer, W. Perkins, L. Lee, and P. Isakson. 1994. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proceedings of the National Academy of Sciences of the United States of America 91 (25): 12013–12017. https://doi.org/10.1073/pnas.91.25.12013.
Sengar, N., A. Joshi, S.K. Prasad, and S. Hemalatha. 2015. Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. Journal of Ethnopharmacology 160: 140–148. https://doi.org/10.1016/j.jep.2014.11.039.
Soliman, A.F., L.M. Anees, and D.M. Ibrahim. 2018. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn-Schmiedeberg's Archives of Pharmacology 391 (8): 819–832. https://doi.org/10.1007/s00210-018-1506-4.
Tracey, W. Ross, Joel Linden, Michael J. Peach, and Roger A. Johns. 1990. Comparison of spectrophotometric and biological assays for nitric oxide (NO) and endothelium-derived relaxing factor (EDRF): nonspecificity of the diazotization reaction for NO and failure to detect EDRF. Journal of Pharmacology and Experimental Therapeutics 252 (3): 922–928.
Vinegar, R., W. Schreiber, and R. Hugo. 1969. Biphasic development of carrageenin edema in rats. The Journal of Pharmacology and Experimental Therapeutics 166 (1): 96–103.
Yazdi, A.S., and K. Ghoreschi. 2016. The interleukin-1 family. Advances in Experimental Medicine and Biology 941: 21–29. https://doi.org/10.1007/978-94-024-0921-5_2.
Funding
This study was funded by the Deputy of Research, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (grant number: MPRC-9707).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were conducted according to the Ethical Guideline for Research, Ahvaz University (Ethics code: IR.AJUMS.ABHC.REC.1397.002).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehrzadi, S., Khalili, H., Fatemi, I. et al. Zingerone Mitigates Carrageenan-Induced Inflammation Through Antioxidant and Anti-inflammatory Activities. Inflammation 44, 186–193 (2021). https://doi.org/10.1007/s10753-020-01320-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01320-y