Abstract
Organophosphorus pesticides bring significant improvements in agriculture, but their toxicity causes environmental and health negative impacts. The aim of this work was the development of robust biocatalysts to be applied in bioremediation. Four fungi were evaluated as hydrolase sources capable of degrading organophosphorus pesticides: Aspergillus niger, Fusarium sp., Penicillium chrysogenum, and Penicillium nalgiovense. The hydrolysis rates of methyl paraoxon obtained under acidic conditions were in the range of 10 to 21 mg L−1 d−1, which is remarkable since most similar biocatalysts are active under alkaline conditions. Penicillium chrysogenum activity was outstanding, and it was selected to prepare, characterize, and study the applications of its enzymatic extract. It was used to evaluate the bioremediation of apple surfaces at pH 2 in the presence of SDS, achieving complete methyl paraoxon degradation under proposed conditions. These results indicate that this biocatalyst could complement industrialized fruit washing processes for the elimination of organophosphorus pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last decades, the expansion of the world population has led to the development of new technologies to increase the production and quality of food. Among these is the use of pesticides, substances employed to prevent, destroy, repel, or mitigate pests (De Gerónimo et al. 2014; Sidhu et al. 2019). They can be classified according to their chemical structure, and among them, the group of organophosphorus compounds (OPs) is one of the most used for agriculture practices (Casida 2017). Although these pesticides are highly efficient, their widespread use led to the accumulation of OPs in soils, water, and agricultural products. This means a serious impact on public health since OPs are highly toxic by inhibiting acetylcholinesterase in the nervous system, producing serious effects ranging from paralysis to death (Butinof et al. 2017; Abdollahdokht et al. 2021). Pesticide contamination of fruit and vegetables is a concern as they retain their toxic activity when ingested, posing a health hazard to consumers (Pinto et al. 2019; Kazar Soydan et al. 2021). Pesticide residues have been detected in foods such as lettuce, onions, carrots, tomatoes, grapes, and milk, among others. In Argentina, Mac Loughlin et al. (2018) reported the presence of pesticide residues in 65% of 135 fruits and vegetables for domestic consumption, and 56% of the positive samples contained concentrations above the maximum residue limits allowed. In addition, fruits and vegetables are industrially processed to produce juices or frozen and canned foods. In this process, washing is the first step to remove surface contaminants such as dust, wax, heavy metals, pesticide residues, mycotoxins, and microorganisms. It includes the use of various solvents, detergents, salt formulations, or mechanical washers, usually under acidic conditions. Although this methodology is suitable for some cases, it is not effective for some OPs such as diazinon and chlorpyrifos as they are not efficiently removed. Furthermore, this technique generates millions of liters of polluted water (Akram and Mushtaq 2018). Therefore, these deficiencies have promoted the bioremediation process as a suitable alternative (Bhandari et al. 2021).
The use of free enzymes to complement the washing processes constitutes an ecological option to improve the efficiency of fruit decontamination. The use of isolated enzymes offers advantages such as easy handling and storage, and they are active in the presence of organic solvents and under wide ranges of pH, temperature, redox potential, and ionic strength (Torres et al. 2003; Rayu et al. 2012). In particular, different types of enzymes that have the ability to degrade OPs pesticides have been identified and characterized. Among them, phosphotriesterases (PTEs) have been found from bacteria to mammals (Latip et al. 2019). The most studied and characterized PTEs are from bacterial origin, and they are active in alkaline conditions. Since the washing process of fruits and vegetables generally takes place under acidic conditions, it is interesting to find new sources of stable and active organophosphorus hydrolases (OPHs) in acidic media to be used as additives in these washing procedures.
In particular, fungi are used to metabolize harmful chemicals and have the added advantage of producing extracellular enzymes, which facilitates their use as biocatalysts. Although fungal OPHs are potential candidates to develop efficient and economically viable biocatalysts to remove pesticides from polluted food and water, they have been little explored (Jain and Garg 2013; Singh et al. 2019). In this study, four fungal strains were evaluated as biocatalysts for methyl paraoxon degradation under acidic conditions and are proposed for the treatment of apples contaminated with OPs pesticides.
Materials and methods
Reactants and culture media
Reactants and solvents (PA grade) used were from Anedra (Argentina), Merck (Germany), and Biopack (Argentina). Czapek yeast extract medium (CYA: 5 g yeast extract, 30 g sucrose, 1 g K2HPO4, 3 g NaNO3, 0.5 g KCl, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 0.005 g CuSO4.5H2O, 0.01 g ZnSO4.7H2O, 1 L H2O) and Malt extract medium (MEA: 20 g malt extract, 1 g acid casein peptone, 20 g glucose, 1 L H2O) were used.
Methyl paraoxon (MPO) was synthetized by oxidative desulfuration from methyl parathion (MP), purchased from Sigma Aldrich (St. Louis, MO, USA) as previously reported (Bielawski and Casida 1988; Santillan et al. 2016).
Fungal culture
Four filamentous fungal strains isolated from Colonia Caroya Salami from Cordoba Argentina –Aspergillus niger, Fusarium sp., Penicillium chrysogenum, and Penicillium nalgiovense – were evaluated as biocatalysts for methyl paraoxon degradation. Fusarium sp., P. chrysogenum, and P. nalgiovense strains were propagated in Petri plates containing MEA agar medium and P. chrysogenum in CYA medium at 28 ℃ until sporulation (7 days). Spores obtained were recovered in 2 mL sterile H2O, counted in a Neubauer chamber using an optical microscope (French and Hebert 1988), and kept at 4 ℃ until their use.
Fungal liquid cultures were obtained by inoculating 1 × 106 spores in 10 mL of MEA medium and incubating at 30 ℃, 130 rpm for 4 days. After this time, 150 µg L−1 methyl paraoxon were added to cultures as inducer and incubated at 30℃, 130 rpm for additional 7 days. Extracellular fractions and complete culture samples were assayed as biocatalysts to determine the degradation of methyl paraoxon.
Enzyme localization
Liquid cultures of 400 mL were performed as in the “Fungal culture” section and centrifuged at 18,000 × g for 20 min. The resulting mycelia and supernatants were separated, and the extracellular fractions were filtered under reduced pressure through a 0.22 µm PVDF filter. The obtained permeated enzyme extracts (EEs) were used as biocatalysts to determine the presence of the enzyme.
Enzyme extract production
Extracellular fractions from liquid cultures (obtained as in the “Enzyme localization” section) were concentrated and partially purified by centrifugation using a 10 kDa cut-off Vivaspin 20 centricon (Sartorius, Germany). The resulting permeates were used as enzyme sources. Subsequently, these concentrated extracts (EEC) were lyophilized. For this purpose, extracellular extracts were frozen for 1 h at − 20 ℃ and kept overnight at − 80 ℃. The lyophilization was carried out using a Freezone 4.5 lyophilizer (LabConco) for 20 h at 133 × 10−3 mBar.
Residual enzyme activities of concentrated extracellular extracts were determined before and after lyophilization. In addition, total proteins were quantified by the Bradford method (Bradford 1976).
Enzymatic activity
The biodegradation of 2 mM methyl paraoxon (494 mg L−1) was evaluated at 30℃, 130 rpm for 30 days using whole cultures, extracellular fractions, concentrated enzyme extracts, and lyophilized purified enzyme extracts as biocatalysts.
Biodegradation employing whole cultures and extracellular fractions as a source of OPHs
Methyl paraoxon biodegradation was evaluated at different pH values (pH 2, 5, and 8) after seven days of enzyme induction. This process started with the addition of 40 µL of a 100 mM methyl paraoxon solution (containing 5% v/v dimethyl sulfoxide) to both the liquid cultures and the extracellular fractions (10 mL final volume).
The reactions were carried out in duplicate, and two control assays were evaluated: one containing only the biocatalyst in MEA medium containing dimethyl sulfoxide and another containing only 2 mM methyl paraoxon supplemented with dimethyl sulfoxide in MEA medium (chemical degradation control).
Biodegradation employing purified enzyme extracts and lyophilized purified enzyme extracts
The determination of the enzyme activity from the EECs obtained in the “Enzyme extract production” section was carried out in 1 mL final volume, diluting 20 µl of the biocatalyst in 2 mM methyl paraoxon solution in an MEA medium containing 1% dimethyl sulfoxide. The pH of these reaction mixtures was adjusted to 2 or 5 when the enzyme extracts were from A. niger and P. nalgiovense or Fusarium sp. and P. chrysogenum, respectively.
In the case of the lyophilized samples, equivalent solid amounts – in terms of total protein content – were taken to determine their activity. These solids were dissolved in 1 mL of 2 mM methyl paraoxon solution in MEA with 1% of dimethyl sulfoxide. The pH was adjusted according to the microorganism, as stated above.
During methyl paraoxon hydrolysis, samples were taken at regular time intervals and their pH was adjusted to 8 by appropriate dilution with 50 mM Tris–HCl buffer. Subsequently, the presence of the corresponding hydrolysis product, p-nitrophenol (PNP), was detected by measuring the absorbance at 405 nm in a plate spectrophotometer (Cytation 5, BioTek, USA). Finally, PNP was quantified employing a PNP calibration curve (0 mM, 0.01 mM, 0.025 mM, 0.05 mM, 0.075 mM, and 0.1 mM solutions). One unit of OPH activity (U) was defined as the amount of biocatalyst required to release 1 µmol of PNP per minute under the corresponding reaction conditions.
Apples decontamination
Characterization of P. chrysogenum EECL
Based on the results achieved, the lyophilized concentrated enzyme extract from P. chrysogenum was evaluated as a biocatalyst for apple surface detoxification. For this purpose, the characterization of this biocatalyst under operational or application conditions was carried out previously.
Kinetic parameters
Enzyme kinetic assays were carried out by determining the hydrolysis of methyl paraoxon at different concentrations of this substrate: 0 mM, 0.025 mM, 0.25 mM, 0.5 mM, and 1 mM. P. chrysogenum EECL, containing 5.75 × 10−3 U, were added to 1 mL of methyl paraoxon solutions at pH 2 and 30 ℃. Quantification was performed as mentioned in the Enzymatic activity section. Based on these results, a nonlinear regression analysis was performed using GraphPad Prism 5 to determine KM′ and maximum velocity (Vmax’) values.
Effects of pH and temperature on enzyme activity
The reaction mixtures were composed of P. chrysogenum EECL (5.75 × 10−3 U) and 22.5 µM methyl paraoxon in 1 mL of the corresponding reaction medium. When analyzing the effect of pH on enzymatic activity, the reaction media were (a) distilled water (pH 6), (b) acetic acid solution (10% v/v, pH 2), and (c) Tris–HCl buffer (50 mM pH 8). The effect of temperature on enzymatic activity (20 ℃, 30 ℃, and 40 ℃) was determined using acetic acid solution (10% v/v pH 2) as a reaction medium. In all cases, samples were taken at regular time intervals, analyzing the enzymatic activity was analyzed as previously described for 24 h at 30 ℃. All reactions were performed in duplicate.
Stability
The stability of P. chrysogenum EECL under different conditions was evaluated. The reaction mixtures were composed of P. chrysogenum EECL (5.75 × 10−3 U) and 22.5 µM methyl paraoxon in 1 mL of the corresponding reaction medium. The reactions were conducted for 12 h or 24 h under the following conditions and reaction media: (a) at 30 ℃ using an acetic acid solution (10% v/v pH 2), (b) in the presence of detergent at 30 ℃ using 9.6% p/v SDS water solution at pH 6, and (c) combined variables (equal to the proposed application conditions) at 30 ℃ using an acetic acid solution (10% v/v, pH 2) containing SDS (9.6% p/v). After the corresponding incubation, residual activity toward methyl paraoxon was evaluated, as stated above.
Bioremediation process
The lyophilized concentrated enzyme extract from P. chrysogenum was evaluated as a biocatalyst for apple surface detoxification. Initially, one unit of this fruit (approximately 200 g) was contaminated with 5.6 µmol of methyl paraoxon (approximately 8 mg Kg−1 of apple) and incubated for 1 h to ensure that the OP was impregnated over the fruit (Del Giudice et al. 2016). Subsequently, each contaminated apple was added into different reaction mixtures containing 5.75 × 10−3 U mL−1 of P. chrysogenum EECL in (a) distilled water (pH 6), (b) a 10% v/v acetic acid solution (pH 2), (c) a 9.6% SDS solution (pH 6), and (d) a solution containing 10% acetic acid and 9.6% SDS (Akram and Mushtaq 2018). Moreover, these reaction mixtures but without fruits were used as controls. Also, the OP solutions but without enzyme extract were used as negative controls of each condition. Methyl paraoxon hydrolysis was determined and quantified by PNP release, as mentioned in the “Enzymatic activity” section.
Results and discussion
Screening in liquid media of OPH activity
The decontamination ability of Aspergillus niger, Fusarium sp., Penicillium chrysogenum, and Penicillium nalgiovense was evaluated using methyl paraoxon in liquid media due to the simple detection of its hydrolysis product. Furthermore, the filamentous fungi were selected, taking into account their ability to grow in acidic media, which suggests the stability and activity of their enzymes under these conditions. These enzymatic characteristics are interesting for their potential application in the food industry (Akram and Mushtaq 2018). The fungi were cultivated initially in the absence of methyl paraoxon, and after 4 days of growth, this OP was added to the culture to induce enzyme expression, considering that these would not be inherent to the constitutive fungal metabolism. After 7 days, it was verified that the added methyl paraoxon had been consumed and the biodegradation assay was started by adding 494 mg L−1 of this OP. During the following 30 days, pH values and OP hydrolysis percentages were analyzed. Regarding pH, A. niger and P. chrysogenum reached pH 2, while in the case of Fusarium sp. and P. nalgiovense cultures, the final pH was 5. At 30 days, a degradation of 92%, 65%, 64%, and 51% was observed for P. chrysogenum, P. nalgiovense, A. niger, and Fusarium sp., respectively. These degradation percentages were calculated taking into account the chemical hydrolysis observed in the control assays (Fig. 1). The determination of initial rates showed that P. chrysogenum was, among the biocatalysts studied, the most active (21 mg L−1 d−1) for methyl paraoxon degradation (Table 1). Considering that there are scarce reports on the degradation of triester OPs in acidic media, this characteristic suggests its application as an additive in industrialized fruit washing processes (Akram and Mushtaq 2018). This assumption is reinforced by the fact that P. nalgiovense, P. chrysogenum, and A. niger are classified as GRAS (generally recognized as safe) microorganisms; therefore, they could be approved by regulatory agencies such as the FDA (Food and Drug Administration). Both Penicillium strains are used in the maturation and curing of meats, as well as in cheese production (Magistà et al. 2017; Moavro et al. 2019), while A. niger has been used as a source of industrial enzymes such as glucoamylase, pectinase, and glucose oxidase, among others (Graselli 2015).
Since the degradation capacity of xenobiotic microorganisms is highly dependent upon pH conditions (Singh et al. 2003a, b). The ability of the biocatalysts studied in this work to hydrolyze methyl paraoxon at different pH values was evaluated. P. chrysogenum afforded complete degradation of methyl paraoxon (494 mg L−1), while P. nalgiovense and Fusarium sp. reached 74% (366 mg L−1) and 89% (440 mg L−1) hydrolysis, respectively (Fig. 2). As far as we know, there are no reports about fungal methyl paraoxon hydrolysis in basic conditions. Nevertheless, under the alkaline conditions evaluated, A. niger did not exhibit enzymatic activity since the observed degradation rate does not present significant differences compared with the chemical control.
When comparing enzymatic activities under alkaline conditions with respect to acidic conditions, Fusarium sp. exhibited an increase of 37%, but in the case of Penicillium strains, no significant differences were observed.
Formulation of an active fungal enzyme
The extracellular location of fungal OPHs is essential for the development of a simple and robust process for obtaining biocatalysts with potential application in bioremediation. Extracellular enzymes simplify the production and recovery of the biocatalyst compared to bacterial OPHs that are generally associated with the cell membrane (Santillan et al. 2020). In this study, extracellular media (enzyme extract, EE) were obtained from fungal cultures by filtration, and their enzyme activities were determined. It was observed that after 30 days of reaction under acidic conditions, the EE of P. chrysogenum, Fusarium sp., P. nalgiovense, and A. niger produced 80%, 62.5%, 55%, and 45% biodegradation, respectively (Fig. 3). These results correspond to approximately the 80% of the total enzyme activity obtained using the complete culture as enzyme source. Similar results were obtained by Jain et al., who reported extracellular OPHs from fungi of the genus Penicillium and Fusarium, among others (Jain and Garg 2013; Jain et al. 2013). Therefore, these EEs were used for further studies.
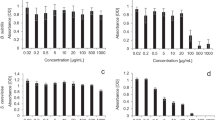
Comparison of methyl paraoxon degradation percentages using as biocatalysts: complete culture (CC), enzyme extract (EE), concentrated enzyme extract (EEC), and lyophilized concentrated enzyme extract (EECL) of A. niger, Fusarium sp., P. chrysogenum, and P. nalgiovense in acidic conditions at 30 days of reaction. The results are expressed as mean ± SD. Two-way ANOVA followed by Bonferroni post hoc test statistical analyses were performed comparing degradation percentages when concentrated enzyme extract and lyophilized concentrated enzyme extract of each fungus were used as biocatalyst (***P < 0.001)
Concentration, purification, and lyophilization of the fungal enzyme extract
The use of pure enzymes is often more expensive than the use of whole cells as biocatalysts, mainly due to the necessary extraction and purification steps (Jain and Garg 2013). Nevertheless, the pure enzyme offers advantages such as greater reproducibility due to cleaner reaction conditions. Furthermore, the degree of enzyme purity depends on their specific application (Graselli 2015). Since the OPHs studied in this work are intended to be used in remediation processes, they require a lower degree of purification compared to enzymes for therapeutic or diagnostic use. Consequently, once determined that the fungal enzymes involved in the degradation of methyl paraoxon were extracellular, concentrated enzyme extracts (EECs) were obtained from the EEs, by centrifugation using 10 KDa Centricon membranes. The enzymatic activity of these EECs was determined under acidic conditions, observing that these biocatalysts kept more than 95% of their efficiency with respect to the EEs, except for the EEC of P. nalgiovense which retained 80% activity (Fig. 3). Moreover, specific activities of EEC and EECL (lyophilized concentrated enzyme extract) were compared, without observing significant difference (Fig. 4). These results indicate that it is possible to produce active lyophilized enzyme extracts without adding cryoprotectors or other substances to maintain OPH activity.
Specific activity of concentrated enzyme extract (EEC) and lyophilized concentrated enzyme extract (EECL) of A. niger, Fusarium sp., P. chrysogenum, and P. nalgiovense in acidic conditions at 30 days of reaction. The results were expressed as mean ± SD. Two-way ANOVA following by Bonferroni post hoc test
Apples decontamination
Several studies have reported a high concentration of pesticides in vegetables, even above the maximum allowed levels. Washing fruits and vegetables is a simple step in their industrial processing, and its efficiency in terms of pesticide removal is around 35–50%. These removal levels depend on physical and chemical characteristics of pesticides, such as polarity, interaction with the surface, and their solubility in the washing solution, thus arising the necessity to improve these processes (Słowik-Borowiec and Szpyrka 2020). In this sense, the removal of paraoxon from surfaces of different materials, such as apples, using a genetically modified SsoxPox enzyme has been reported (Del Giudice et al. 2016). In the present work, the wild-type lyophilized enzyme extract of P. chrysogenum was selected for its characterization and application in the remediation of contaminated apples.
Characterization of P. chrysogenum EECL
As mentioned above, washing fruit and vegetable involves the use of detergents and acid solutions. Therefore, the P. chrysogenum EECL was initially characterized considering the proposed application conditions: 5.75 × 10−3 U mL−1, pH 2, 30 ℃, and 9.6% SDS.
Kinetic parameters
The kinetic parameters of the EECL of P. chrysogenum were determined by nonlinear regression, using the activities obtained at different substrate concentrations. The resulting KM′ value was 0.0409 ± 0.0186 mM, while the Vmax′ was 1.566 ± 0.162 µmol h−1. When these results were compared with the kinetic parameters of the genetically modified SsoxPox enzyme applied to the treatment of paraoxon-contaminated apples at 25℃ (KM: 0.380 ± 0.067 mM), the latter was 10 times higher. This variation could be related to the structural difference of the substrates involved, as it has been reported that the substitution of ethoxy for methoxy groups in the substrate leads to an increase in KM (Santillan et al. 2020).
Effects of pH and temperature on enzyme activity
The effect of pH and temperature on the degradation of methyl paraoxon biocatalyzed by P. chrysogenum EECL was studied. The obtained results showed that there are no significant differences in the degradation rates among the analyzed conditions (Fig. 5a). As mentioned above, OPHs are mostly active under basic conditions (Santillan et al. 2020), with reports of a drastic decrease in OP biodegradation in soils under acidic conditions compared to that obtained under alkaline conditions (Singh et al. 2003a, b). In this sense, the biocatalyst obtained in this work has shown to be versatile in terms of pH, which suggests its potential application not only for the treatment of fruits (under acidic conditions) but also for other systems contaminated with OPs, such as water effluents or contaminated soils. When analyzing the impact of temperature, Fig. 5b shows that the optimal temperature for this biocatalyst under acidic conditions was 30 ℃, decreasing its degradation rates 7 and 2 times when the reaction was carried out at 40 ℃ and 20 ℃, respectively.
Stability
The stability of P. chrysogenum EECL in an acidic medium or in the presence of SDS was assayed after 12 h and 24 h. Figure 6 represents the results obtained, where it is shown that this biocatalyst remained stable (keeping at least 80% of its relative activity) during the first 12 h in both conditions assayed. After 24 h, the stability decreased to 45% in an acidic medium (pH 2) and to 65% in the presence of SDS 9.6%. Two phosphotriesterases applied to the paraoxon degradation, SsoPox and its mutant SsoPox W263F, were stable just for 4 h at lower SDS concentrations (0.1%) (Manco et al. 2018). The effect of both combined variables, pH and presence of SDS in the medium, on the stability of P. chrysogenum EECL was also evaluated. The obtained results were similar to those observed for each of these variables separately. This information suggests the robustness of the P. chrysogenum EECL, for its application in bioremediation processes. Also, these results remark the novelty of this work since there is no reported fungal biocatalyst with high stability under acidic conditions.
P. chrysogenum EECL stability analysis under different conditions: (a) acetic acid solution (10% v/v pH 2), (b) SDS water solution (9.6% p/v pH 6), and (c) acetic acid solution (10% v/v, pH 2) containing SDS (9.6% p/v). Reactions were conducted for 12 h or 24 h at 30℃. Residual activity toward methyl paraoxon was evaluated. The results are expressed as mean ± SD, two-way ANOVA, followed by the Bonferroni post hoc test. **P < 0.01 and *P < 0.05 significances when comparing the incubation times for each condition
Bioremediation process
In the present work, the wild-type lyophilized enzyme extract of P. chrysogenum was selected for the remediation of contaminated apples (with 8.5 mg kg−1 of methyl paraoxon). These fruits were submerged in a water solution containing 5.75 × 10−3 U mL−1 of the enzyme (pH 6), which caused the OP degradation at a catalytic rate of 2.5 nmol min−1 (Fig. 7). It was reported that using 0.15 U mL−1 of the genetically modified SsoxPox enzyme, a degradation rate of 5 nmol min−1 (at 25 ℃, pH 7) was achieved (Del Giudice et al. 2016). Considering the final concentration of the biocatalysts employed in each case, the rates obtained here mean a higher efficiency of the wild-type P. chrysogenum biocatalyst compared to the recombinant SsoxPox enzyme (Del Giudice et al. 2016). These results indicate that this biocatalyst could be an additive that enhances the fruit washing process.
Methyl paraoxon (MPO) degradation from apples. The reaction mix evaluated were (a) EECL (lyophilized concentrated enzyme extract) + MPO + distillate water (pH 6), (b) EECL + MPO + 9.6% SDS + distilled water (pH 6), (c) EECL + MPO + 10% acetic acid + distilled water (pH 2), and (d) EECL + MPO + 9.6% SDS + 10% acetic acid + distilled water (pH 2)
Currently, to improve the efficiency of pesticide removal during industrial fruit processing, industries have incorporated detergents and other chemicals into washing solutions (Akram and Mushtaq 2018). In this order of ideas, the methyl paraoxon removal from the apple surface was evaluated using the EECL in solutions containing acetic acid or SDS. When acetic acid was added to the reaction mixture (pH 2), the maximum degradation rate increased to 3 nmol min−1. This result suggests that the reaction carried out in acidic conditions decreases the time required to achieve OP degradation to levels below the maximum residue limits (MRL approximately 200 µg kg−1 apple) (CASAFE 2015; Ferré et al. 2018) (Fig. 7).
When SDS was added to the enzyme solution, an increase in OP degradation was also observed, reaching a rate of 3.5 nmol min−1. These results could be related to those reported by D’auria et al. (2001) and Del Giudice et al. (2016), who proposed that the addition of an ionic detergent contributes to the flexibilization of the active site, thus facilitating the degradation. In addition, the higher polarity of methyl paraoxon compared to other OPs could contribute to its solubilization in the presence of SDS (Santillan et al. 2020). Finally, the joint use of acetic acid and SDS in the reaction mixture – currently applied to industrial washing procedures – showed a synergistic effect, achieving a methyl paraoxon degradation rate of 6.3 nmol min−1. This combination provided a 2.5-fold increase in degradation rates compared to the enzyme in pure water. This biocatalyst generated an OP removal to concentrations below the MRL concentration allowed in Argentina.
Furthermore, the production of larger quantities of EECL from P. chrysogenum is a viable alternative due to the simple process involved in its preparation. This would allow the use of more concentrated formulations of this biocatalyst in the washing process and thus optimize process times. These results demonstrate that it is possible to produce an efficient enzymatic product that is easy to store, economical, and with potential application in industrial fruit washing processes.
Conclusion
Fruit and vegetable processing includes a washing step that is usually done under acidic conditions. This methodology generates millions of liters of contaminated water, not always being effective for the removal of all types of OPs. As an alternative to solve these deficiencies, the present study led to the detection of new fungal biocatalysts with OPH activity that showed versatility in different pH conditions, particularly in acidic conditions. This characteristic makes them especially useful to be used as additives for the bioremediation of OP pesticides in the processing of fruits and vegetables. In particular, this work shows the degradation by the EECL of P. chrysogenum of the methyl paraoxon present on the surface of apples, which suggests the potential application of this methodology to the elimination of other OP pesticides, commonly used in agriculture.
In addition, these biocatalysts are produced by simple and economically viable processes that generate easily manipulated formulations, starting mainly from GRAS microorganisms, characteristics that make them exceptional for these applications.
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdollahdokht D, Asadikaram G, Abolhassani M, Pourghadamyari H, Abbasi-Jorjandi M, Faramarz S, Nematollahi M (2021) Pesticide exposure and related health problems among farmworkers’ children: a case-control study in southeast Iran. Environ Sci Pollut Res 28:57216–57231. https://doi.org/10.1007/s11356-021-14319-1
Akram S, Mushtaq M (2018) Techniques to detect and detoxify organophosphorus pesticides from fruit juices. In: Rajauria G, Tiwari BK (eds) Fruit juices: extraction, composition, quality and analysis, 1st edn. Elsevier Inc., London, pp 363–389
Bhandari S, Poudel DK, Marahatha R, Dawadi S, Khadayat K, Phuyal S, Shrestha S, Gaire S, Basnet K, Khadka U, Parajuli N (2021) Microbial enzymes used in bioremediation. J Chem 2021https://doi.org/10.1155/2021/8849512
Bielawski J, Casida JE (1988) Phosphorylating intermediates in the peracid oxidation of phosphorothionates, phosphorothiolates, and phosphorodithioates. J Agric Food Chem 36:610–615. https://doi.org/10.1021/jf00081a052
Bradford MM (1976) A rapid and sensitive method for the quiantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/j.cj.2017.04.003
Butinof M, Fernández R, Muñoz S, Lerda D, Blanco M, Lantieri M, Antolini L, Gieco M, Ortiz P, Filippi I, Franchini G, Eandi M, Montedoro F, Díaz M (2017) Valoración de la exposición a plaguicidas en cultivos extensivos de Argentina y su potencial impacto sobre la salud. Rev Argentina Salud Pública 8:8–15
CASAFE (2015) Buenas Prácticas Agrícolas: Directivas y requisitos para cultivos intensivos. Red buenas prácticas Agric 1–55
Casida JE (2017) Organophosphorus xenobiotic toxicology. Annu Rev Pharmacol Toxicol 57:309–327. https://doi.org/10.1146/annurev-pharmtox-010716-104926
D’Auria S, Di Cesare N, Gryczynski I, Rossi M, Lakowicz J (2001) On the effect of sodium dodecyl sulfate on the structure of β-galactosidase from Escherichia coli. A fluorescence study. J Biochem 130:13–18. https://doi.org/10.1093/oxfordjournals.jbchem.a002951
De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa J (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431. https://doi.org/10.1016/j.chemosphere.2014.01.039
Del Giudice I, Coppolecchia R, Merone L, Porzio E, Carusone T, Mandrich L, Worek F, Manco G (2016) An efficient thermostable organophosphate hydrolase and its application in pesticide decontamination. Biotechnol Bioeng 113:724–734. https://doi.org/10.1002/bit.25843
French E, Hebert T (1988) Métodos de Investigación de fitopatología. In: De la Cruz M (ed) Instituto Interamericano de Ciencias Agrícolas, 1t edn. IICA, Costa Rica
Ferré DM, Quero AAM, Hernández AF, Hynes V, Tornello MJ, Lüders C, Gorla N (2018) Potential risks of dietary exposure to chlorpyrifos and cypermethrin from their use in fruit/vegetable crops and beef cattle productions. Environ Monit Assess 190https://doi.org/10.1007/s10661-018-6647-x
Graselli M (2015) Proteínas puras. Entre el laboratorio y la industria. In: Golombek D (ed) Colección nuevos enfoques en ciencia y tecnología, 1st edn. Editorial UNQ, Buenos Aires
Jain R, Garg V (2013) Enzymatic degradation of monocrotophos by extracellular fungal OP hydrolases. Appl Biochem Biotechnol 171:1473–1486. https://doi.org/10.1007/s12010-013-0438-1
Jain R, Garg V, Dangwal K, Lily MK (2013) Comparative purification and characterization of two distinct extracellular monocrotophos hydrolases secreted by penicillium aculeatum and fusarium pallidoroseum isolated from agricultural fields. Biosci Biotechnol Biochem 77:961–965. https://doi.org/10.1271/bbb.120907
Kazar Soydan D, Turgut N, Yalçın M, Turgut C, Karakus P (2021) Evaluation of pesticide residues in fruits and vegetables from the Aegean region of Turkey and assessment of risk to consumers. Environ Sci Pollut Res 28:27511–27519. https://doi.org/10.1007/s11356-021-12580-y
Latip W, Knight VF, Halim NA, Ong KK, Kassim N, Yunus W, Noor S, Ali M (2019) Microbial phosphotriesterase: structure, function, and biotechnological applications. Catalysts 9:1–11. https://doi.org/10.3390/catal9080671
Mac Loughlin TM, Peluso ML, Etchegoyen MA, Alonso L, de Castro MC, Percudani MC, Marino D (2018) Pesticide residues in fruits and vegetables of the argentine domestic market: occurrence and quality. Food Control 93:129–138. https://doi.org/10.1016/j.foodcont.2018.05.041
Magistà D, Susca A, Ferrara M, Logrieco A, Perrone G (2017) Penicillium species: crossroad between quality and safety of cured meat production. Curr Opin Food Sci 17:36–40. https://doi.org/10.1016/j.cofs.2017.09.007
Manco G, Porzio E, Suzumoto Y (2018) Enzymatic detoxification: a sustainable means of degrading toxic organophosphate pesticides and chemical warfare nerve agents. J Chem Technol Biotechnol 93:2064–2082. https://doi.org/10.1002/jctb.5603
Moavro A, Stenglein S, Delfederico L, Wagner J, Ludemann V (2019) Novel use of Penicillium nalgiovense on stuffed semi–hard and hard cheeses. Lwt 110:255–261. https://doi.org/10.1016/j.lwt.2019.04.074
Pinto GDA, Castro IM, Miguel MAL, Koblitz MGB (2019) Lactic acid bacteria – promising technology for organophosphate degradation in food: a pilot study. Lwt 110:353–359. https://doi.org/10.1016/j.lwt.2019.02.037
Rayu S, Karpouzas DG, Singh BK (2012) Emerging technologies in bioremediation: constraints and opportunities. Biodegradation 23:917–926. https://doi.org/10.1007/s10532-012-9576-3
Santillan JY, Dettorre LA, Lewkowicz ES, Iribarren AM (2016) New and highly active microbial phosphotriesterase sources. FEMS Microbiol Lett 363:1–5. https://doi.org/10.1093/femsle/fnw276
Santillan JY, Muzlera A, Molina M, Lewkowicz ES, Iribarren AM (2020) Microbial degradation of organophosphorus pesticides using whole cells and enzyme extracts. Biodegradation 31:423–433. https://doi.org/10.1007/s10532-020-09918-7
Sidhu GK, Singh S, Kumar V, Dhanjal D, Datta S, Singh J (2019) Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit Rev Environ Sci Technol 49:1135–1187. https://doi.org/10.1080/10643389.2019.1565554
Singh BK, Walker A, Morgan JAW, Wright DJ (2003a) Effects of soil pH on the biodegradation of chlorpyrifos and isolation of a chlorpyrifos-degrading bacterium. Appl Environ Microbiol 69:5198–5206. https://doi.org/10.1128/AEM.69.9.5198-5206.2003
Singh BK, Walker A, Morgan JAW, Wright DJ (2003b) Role of soil pH in the development of enhanced biodegradation of fenamiphos. Appl Environ Microbiol 69:7035–7043. https://doi.org/10.1128/AEM.69.12.7035-7043.2003
Singh RK, Tripathi R, Ranjan A, Srivastava AK (2019) Fungi as potential candidates for bioremediation. In: Singh P, Kuma A, Borthakur A (eds) Abatement of environmental pollutants: trends and strategies, 1st edn. Elsevier Inc., Netherlands, pp 177–191
Słowik-Borowiec M, Szpyrka E (2020) Selected food processing techniques as a factor for pesticide residue removal in apple fruit. Environ Sci Pollut Res 27:2361–2373. https://doi.org/10.1007/s11356-019-06943-9
Torres E, Bustos-Jaimes I, Le Borgne S (2003) Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B Environ 46:1–15. https://doi.org/10.1016/S0926-3373(03)00228-5
Acknowledgements
The authors acknowledge Professor Vanesa Ludemann from the Food Mycology Laboratory, Quilmes National University, for kindly providing the fungal strains used in this investigation.
Funding
This study was supported by grants from Universidad Nacional de Quilmes and ANPCyT (PICT 2016–0861). NLR, ESL, and AMI are members of the Scientific Researcher Career of CONICET. JYS is a CONICET fellow.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this work. The preparation of the material, data collection, and analysis were in charge of Julia Yamila Santillan and Natalia Lorena Rojas. The manuscript was written by Julia Yamila Santillan and Natalia Lorena Rojas, and all the authors reviewed and modified it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santillan, J.Y., Rojas, N.L., Lewkowicz, E.S. et al. Novel fungal organophosphorus hydrolases in acidic media: an application to apples decontamination. Environ Sci Pollut Res 30, 10803–10811 (2023). https://doi.org/10.1007/s11356-022-22854-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22854-8