Abstract
The worldwide occurrence of pharmaceuticals and personal care products (PPCPs) in aquatic ecosystems is reason for public concern. These emerging micropollutants include a large and diverse group of organic compounds, with continuous input, high environmental persistence and potential threat to biota and human health. The aim of this study was to evaluate, for the first time, the occurrence of twenty-seven PPCPs of various therapeutic classes (including cocaine and its primary metabolite, benzoylecgonine), in the coastal waters of Santa Catarina, southern Brazil. Water samples were taken in November 2020, during the low tide periods, at eight sampling points located along the coast of Santa Catarina, covering its entire geographical extension. Sampling was carried out in triplicate and at different depths of the water column. Nine compounds were detected through liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS): caffeine (12.58–119.80 ng/L), diclofenac (1.34–7.92 ng/L), atenolol (1.13–2.50 ng/L), losartan (0.43–3.20 ng/L), acetaminophen (0.21–10.04 ng/L), orphenadrine (0.07–0.09 ng/L), cocaine (0.02–0.17 ng/L), benzoylecgonine (0.01–1.1 ng/L) and carbamazepine (0.02–0.27 ng/L). The highest occurrence of these compounds was detected in the northern and central coastal region of Santa Catarina, namely in Penha and Palhoça cities. Moreover, the risk assessment showed that almost compounds (atenolol, benzoylecgonine, carbamazepine, cocaine and orphenadrine) presented no ecological risk in the recorded concentrations. However, a few compounds suggest low (caffeine and diclofenac) to moderate (acetaminophen and losartan) risk taking into consideration the acute and chronic effects for the three trophic levels (algae, crustacean and fish) tested. These compounds are usually found in areas with high population density, aggravated by tourism, because of the sanitary sewage and solid waste. Although in low concentrations, the occurrence of these chemical compounds can imply deleterious effects on the environmental health of Santa Catarina coastal zone, and therefore deserve more attention by the public authorities and environmental agencies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals, including illicit drugs, and personal care products (PPCPs) are a unique group of emerging environmental contaminants, with deleterious effects on the aquatic biota ranging from the spread of antimicrobial resistance and species survival to interference in the reproduction of organisms and other related ecotoxicological sublethal effects, such as oxidative stress and lipid peroxidation (Overturf et al. 2015; Ebele et al. 2017; Wang et al. 2021). Pharmaceuticals are prescribed to diagnose, treat or prevent diseases in humans and animals, being physiologically metabolized and eliminated through urine and faeces, in the form of the parental compound or of its metabolites, while personal care products are mainly used to improve the quality of our daily life, but both are later discarded into the aquatic environment mainly through household sewage (Ekpeghere et al. 2018). Moreover, consumption practices such as the self-medication, the excessive and indiscriminate use of drugs, the medicine free-trade and the direct disposal of pharmaceuticals explain the worldwide occurrence of these chemical compounds in the environment (Pereira et al. 2017; Freitas and Radis-Baptista 2021). The ineffectiveness of most wastewater treatment plants (WWTPs) regarding the removal of PPCPs makes these compounds, partially or fully, discarded into receiving waters (Del Rosario et al. 2014; Wang and Wang 2016; Ng et al. 2021). Studies carried out in WWTPs worldwide (Huber et al. 2016; Kosma et al. 2014; Lindholm-Lehto et al. 2016) demonstrated that some compounds are not totally eliminated by the conventional treatments, and that drug residues still remain in the treated effluent (Gurke et al. 2015; Lindholm-Lehto et al. 2016; Zhou et al. 2019). PPCPs can also return to the aquatic compartment by using contaminated surface waters and sewage sludge fertilizers during agricultural practices (Bartrons and Peñuelas 2017; Madikizela et al. 2018). And thus, it may cause risks to human health from exposure to contaminated food (Goldstein et al. 2014; Al-Farsi et al. 2017; Keerthanan et al. 2021), in addition to allowing the transport of these pollutants through runoff to the aquatic environment promoting their accumulation in inland, estuarine and coastal waters (Stewart et al. 2014; Sui et al. 2015; Wilkinson et al. 2017). Furthermore, in the aquatic compartment, PPCPs can undergo biotic and abiotic transformations generating other by-products, besides the potential additive and synergistic effects with other drugs in complex mixtures (Evgenidou et al. 2015; Yuan et al. 2020; Hamid et al. 2021).
In Brazil, including Santa Catarina state, most of the existing WWTPs operate with conventional treatment processes (ANA 2017; Heinz et al. 2020), not suitable for the total removal of the PPCPs (Behera et al. 2011; Wang and Wang 2016; Kumar et al. 2019), which needs more advanced systems to achieve this efficiency (Yang et al. 2017; Tarpani and Azapagic 2018; Rigueto et al. 2020). In addition, there is no regulation that sets specific limits for these compounds in effluents and/or environmental waters (i.e. fresh, brackish or salty waters) (ANA 2017; MMA 2021). In the last decades, a few studies screened the occurrence of PPCPs in aquatic ecosystems off the Brazilian coast, mainly focused in São Paulo and Rio de Janeiro, limited to a few therapeutic classes and mostly published in national journals and reports (Quadra et al. 2017). A few examples can be found here; one of the first published reports was related to the occurrence of PPCPs in the marine sediments of Todos os Santos Bay, Bahia, Brazil (Beretta et al. 2014); in Santos city, São Paulo coastline, cocaine and benzoylecgonine were monitored near the discharge of the submarine outfall in the Santos Bay (Fontes et al. 2019); recently, a few works were published concerning the coastal area of São Paulo, Brazil; 16 PPCPs were detected in the urban drainage channels flowing into the bathing waters of Guarujá (Roveri et al. 2020); 10 PPCPs were found near on the discharge of submarine outfall in the Enseada beach, Guarujá (Roveri et al. 2021a) and 21 PPCPs were recorded in the urban drainage channels that flow to Santos city (Roveri et al. 2021b).
However, despite the existence of several activities related to tourism, agriculture and industries, with a predominance of textile, ceramic and metal-mechanics industries (SEBRAE 2013; FIESC 2021), potentially generating PPCPs in the coastal region of Santa Catarina, and old WWTPs serving the state (ANA 2017), there are no studies on the occurrence and ecological risk of PPCPs in this densely populated coastal area. Santa Catarina is also rich in surface freshwater bodies belonging to five hydrographic regions, where the main and most densely populated cities are located, and where the industrial park of the state is concentrated (IBGE 2020), which flow into the coastal water of the Atlantic Ocean (ANA 2017). Moreover, the occurrence of PPCPs is of greatest relevance for studies of environmental monitoring in Brazil (Chaves et al. 2021), and these compounds were somewhat expected in Santa Catarina as result of the wastewater discharges from industrial and domestic effluents (Krogh et al. 2017; Afsa et al. 2020; Shahriar et al. 2021). Thus, the aim of this study was to evaluate, for the first time, the presence of twenty-seven PPCPs of various therapeutic classes (including cocaine and its primary metabolite, benzoylecgonine) along the coast of the state of Santa Catarina, Brazil, in water samples collected under the influence of the major estuaries. Simultaneously, an ecological risk assessment of the measured maximum concentrations of the different PPCPs in the aquatic biota was carried out. The information obtained here will allow to construct a database on the influence of emerging pollutants on the aquatic ecosystem of the Santa Catarina coast, serving as a support for future decisions by public authorities regarding the use, occupation and management of these coastal zones, particularly for areas with intense human occupation.
Materials and methods
Study site description
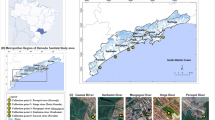
The present study took place in the coastal area of Santa Catarina state (SC), south Brazil. SC has an area of 95,730.684 km2 with a population of 6218.436 (IBGE 2010) and is composed of 295 municipalities politically divided into eight regions (IBGE 2020). SC is delimited by two drainage systems, the Sistema Integrado da Vertente do Interior (SIVI) and the Sistema da Vertente Atlântica (SVA). SVA covers approximately 36,354 km2, which represents 38% of the total area of the state, and also concentrate a large part of the population of the state, around 41%, since the four biggest cities are located in or near the coastal area, namely Joinville (515,288 population), Florianópolis, Capital of Santa Catarina (421,240 population), Blumenau (309,011 population) and Criciúma (192,308 population) (Filho 2003; ANA 2017; SDE 2020). The SVA is composed of five hydrographic regions (HR) [North coast (HR6), Itajaí Valley (HR7), Coast center (HR8), Southern SC (HR9) and Extreme South of SC (HR10)], which drain their waters to the SC coastline (Fig. 1), which is 561 km long (IBGE 2020). The present sampling design included the eight major estuaries, distributed from North to South, on the coast of SC, located in regions that are influenced by the main rivers and the largest cities of the state, and with different anthropogenic contamination profiles, such as agricultural operations, textiles and metallurgic industries, sanitary landfills and energetic and tourism sectors (Table 1). The characterization of the anthropogenic profile of each sampling site was carried out based on the data bases of the State Secretariat for Sustainable Development (SEBRAE 2013) and of the Industries Federation Observatory of the State of Santa Catarina (FIESC) (FIESC 2021).
Map of Brazil with the Santa Catarina State in grey colour (A). Magnification of Santa Catarina showing the neighbour states, Paraná and Rio Grande do Sul (B). Santa Catarina coastal area, showing the hydrographic regions (HR) that belong to the Sistema da Vertente Atlântica (SVA), and identification of the study sampling points: São Francisco do Sul (P1), Balneário Barra do Sul (P2), Penha (P3), Enseada do Brito, Palhoça (P4), Baia de Zimbros, Porto Belo, (P5), Imbituba (P6), Laguna (P7) and Balneário Rincão (P8). For more details, see M&M
Collection of samples
The study was carried out at eight sampling points (labelled as P), along the coast of SC. The Table 1 and Fig. 1 present (i) the details of the selected locations along SC coastline, (ii) the land use and occupation characteristics and (iii) their geographic coordinates. The sampling points of the study area were chosen taking into consideration the coastal area under the influence of the largest rivers of the different hydrographic regions (labelled as HR) (SDE 2020). In marine environments, PPCP levels are expected to be low due to dilution and dispersion by the complex hydrodynamic of coastal zones (Vidal-Dorsch et al. 2012). Moreover, in this study, PPCPs were assumed to be released into seawater through rivers runoff. For this reason, water was collected during the day at low tides. Since it was a preliminary study, a single collection day-window was used (November 9th and 10th, 2020), following a week-end where a peak of illegal drugs consumption is expected due to recreational purposes (González-Mariño et al. 2019). Water was stored in amber bottles, previously cleaned with water and detergent, rinsed with plenty of tap water, ending with solvent application, methanol and, final rinse with distilled water to eliminate any trace of possible contaminants. The water was collected in triplicate, carried out in three different heights, 30 cm, 60 cm and 90 cm from the surface. Equal volume aliquots (500 mL) were mixed and homogenized. From the final mixture, an aliquot of 1 L was used. All samples were kept at 4 °C, and target PPCPs were extracted from water samples within 4 days of collection (ANA 2011).
Preparation and analysis of pharmaceutical compounds
Chemical and standards
Chemicals and analytical reagents such as nitric acid and sulphuric acid were purchased from Merck (Darmstadt, Germany). Grade solvents used in HPLC and LC–MS, such as acetonitrile, methanol and isopropanol, were acquired from Sigma-Aldrich (MA, USA). Mobile phase additives, namely LC–MS grade formic acid and ammonium acetate, were acquired from Sigma-Aldrich and Merck, respectively. Analytical standards of acetaminophen, atenolol, bromazepam, caffeine, carbamazepine, cyproterone, clonazepam, clopidogrel, diclofenac, enalapril, loratadine, losartan, midazolam orphenadrine, propranolol, sildenafil, atorvastatin, ranitidine, diazepam, chlorpheniramine and valsartan were acquired from Sigma-Aldrich. Cocaine and benzoylecgonine were acquired from Cerillant (TX, USA). The other pharmaceuticals were bought in several suppliers: citalopram (Alcytam®: Torrent, Brazil), chlortalidone (Higroton®: Novartis, Swiss), rosuvastatin (Crestor®: AstraZeneca, UK) and generic paroxetine medication (Medley, Brazil). The purity of all the solvents and analytical standards used in this study were ≥ 99% and ≥ 98%, respectively.
Sample preparation
The extraction technique used in the hereby study was modified from Wille et al. (2010) and fully described by Roveri et al. (2021b). Solid phase extraction (SPE) was conducted on a 12 Port Vacuum Extraction Manifold Assy (Phenomenex, USA) equipped with Vacuum Pump (Beco, Germany). Prior to the extraction, the following procedures were adopted: (i) the pH of water samples (7.5 ± 0.1) was adjusted to 7.0 using a hydrochloric acid solution (1.0 M); (ii) samples were filtered through a cellulose filter paper (Whatman® GF/C Glass microfiber filters, diameter 47 mm, particle retention 1.2 μm; Merck, Darmstadt, German); (iii) the filters were washed with 2 mL of methanol (Sigma-Aldrich, St. Louis, USA) and (iv) at the end, the methanol extract collected was combined to the filtered. The solid-phase extraction was performed using SPE Chromabond HR-X cartridges (200 mg, 3 mL; Marcherey-Nagel, Duren, Germany). The cartridges were preconditioned with methanol (5 mL) and ultrapure water (5 mL) (Milli-Q®-Merck, Darmstadt, Germany). Thereafter, there were loaded with 1 L of the filtered sample combined with the methanol from filter washings. The cartridges were then dried under vacuum for 30 min, and the elution was performed twice using 5 mL of methanol and 5 mL of acetone. After the extraction, the samples were dried under a nitrogen flow (at 50 °C) and redissolved with water/acetonitrile (95:5 v/v) prior to mass spectrometry analysis. In the laboratory, each water sample was analysed in triplicate using LC–MS/MS. A concentration factor (1/1000) was used to obtain the final concentrations, and individual average results were expressed in ng/L (Fig. 2).
LC–MS/MS analysis
The hereby 27 PPCPs screened were selected taking into account the occurrence, reported annual consumption, expected toxicity and environmental persistence (CMED 2021; Roveri et al. 2021b). LC–MS/MS analytical procedures were validated by Shihomatzu (2015) (see page 87) and fully described by Roveri et al. (2021b) (see details in Table S1a and S1b). Briefly, an aliquot of 10 μL of sample that was subjected to analysis by HPLC Agilent 1260 (Agilent™, Germany) combined with a mass spectrometer hybrid triple quadrupole/LIT instrument (3200QTRAP®-linear ion trap) (ABSciex, Ontario, Canada). The samples were analysed using an Agilent Zorbax Eclipse XDB–C18 column (50 × 4.6 mm ID, 1.8 μm column at 25 °C). The eluent flow was 0.7 mL/min and the mobile phase for positive mode analysis was 0.1% formic acid (Sigma-Aldrich; LC–MS Grade) in water (solvent A) and acetonitrile (solvent B) (J.T. Baker, Philipsburg, NJ, USA). An eluent gradient was used, starting with a mixture of solvent A (95%) and solvent B (5%), but the percentage of solvent A decreased linearly from 95 to 5% over 5 min, and this condition was maintained for 1 min. This mixture was then returned to the initial conditions over 2 min and the analytes were detected and quantified using the electrospray ionization (ESI) and multiple reaction monitoring (MRM), with the selection of a precursor ion and two ion products to quantify and qualify each compound. The data were recorded and processed using the Analyst 1.5.2 software (ABSciex). The MRM parameters for positive and negative ion modes, limit of detection (LOD) and limit of quantification (LOQ) are shown in Table S1.
Ecological risk assessment
The ecological risk assessment followed the work of Roveri et al. (2021b). The risk quotient (RQ) for aquatic species from three trophic levels (algae, crustaceans and fishes) was calculated from the equation RQ = MEC/PNEC, in which MEC is the maximum Measured Environmental Concentration, and PNEC the Predicted No Effect Concentration, both expressed in ng/L. The PNEC values for the acute and chronic toxicity data were obtained from the Ecotoxicology Database (ECOTOX) (USEPA 2019), as well as in other literature sources using the PubMed database (see Table S2). When ecotoxicity laboratory experimentally derived data were not available, PNEC was estimated using the Ecological Structure Activity Relationships Programme (ECOSAR, v 2.0) (USEPA 2017). An attempt was made to compile specifically PNEC data for marine coastal species. However, due to the strong land-sea interaction in this study area and the lack of marine toxicity data, the freshwater species were also taken into consideration in the present study, as followed by Roveri et al. (2021b). The PNEC values for the acute and chronic toxicity data were thereafter calculated by dividing each toxicological endpoint by an assessment factor (AF). For saltwater environments (i.e. salinity during the water collection ranged from 26 to 28 in this study), an AF of 10,000 and 100 should be considered in short- and long-term data sets, respectively. For further details, see the European Chemical Bureau (ECB 2003) and the European Chemicals Agency (ECHA 2008) guidelines. The toxicological endpoints selected for the calculation of the PNECs are shown in Table S2. Finally, RQ was categorised into four levels: no (RQ < 0.01), low (0.01 ≤ RQ < 0.1), moderate (0.1 ≤ RQ < 1.0) and high ecological risk (RQ ≥ 1.0) to aquatic organisms (Hernando et al. 2006).
Results and discussion
Overall occurrence of PPCPs on the coastal waters of Santa Catarina
From the twenty-seven chemical compounds assessed, it was possible to quantitatively (> LOQ – see Fig. 2 and Table S1a) identify nine compounds. Caffeine (12.58–119.80 ng/L), diclofenac (1.40–7.92 ng/L), losartan (0.43–3.20 ng/L), cocaine (0.02–0.17 ng/L) and benzoylecgonine (0.02–1.09 ng/L) were quantified in all sampling points. Carbamazepine (0.02–0.27 ng/L) was quantified in 75% of the samples. Acetaminophen (0.21–10.04 ng/L) was quantified in 60% of the samples. Both atenolol (1.13–2.50 ng/L) and orphenadrine (0.07–0.09 ng/L) were quantified in 25% of the samples. Figure 2 shows the occurrence of each compound, revealing similar profiles for cocaine, caffeine, diclofenac and losartan, with the greatest amounts being consistently reported on the north coast and north-central coast.
Overall, the MEC of these PPCPs, i.e. 119.80 ng/L (caffeine), 10.04 ng/L (acetaminophen), 7.92 ng/L (diclofenac), 3.20 ng/L (losartan), 2.50 ng/L (atenolol), 1.09 ng/L (benzoylecgonine), 0.27 ng/L (carbamazepine) and 0.17 ng/L (cocaine) were lower to the values already reported in other areas of Brazil and worldwide, although the environmental concentrations reported for some aquatic compartments are not directly comparable (e.g. freshwater, brackish water and seawater) (for more details, see Table S2). For instance, high concentrations of the caffeine (MEC: 3000 ng/L) were detected in coastal waters of the Red Sea in Saudi Arabia (Ali et al. 2017). In China, in Xiamen Bay, acetaminophen concentrations ranging from 0.137 to 5.483 ng/L were found (Chen et al. 2021). In Brazil, on the coast of the Guarujá city, São Paulo, the occurrence of caffeine (33.5–6550.0 ng/L), valsartan (19.8–798.0 ng/L), losartan (3.6–548.0 ng/L), acetaminophen (18.3–391.0 ng/L), benzoylecgonine (0.9–278.0 ng/L), atenolol (0.1–140.0 ng/L) and diclofenac (0.9–85.7 ng/L), among other compounds, was also recorded (Roveri et al. 2020). In the coastal waters of Santos, Brazil, losartan was found in similar concentrations (< LOD–3.4 ng/L) (Roveri et al. 2021b), or slightly higher concentrations (0.2–8.6 ng/L) (Cortez et al. 2018) than the values reported hereby. Cocaine consumption in America Latina is reported to be relatively high compared to Europe, here, the prevalence data suggest an overall stable use of it during the last years (González-Mariño et al. 2020), due to its high production and purity level in this area (Huizer et al. 2021). Moreover, cocaine loads underwent an upsurge on week-ends, indicating a high consumption for recreational purposes, and likely a direct disposal in sanitary waters (González-Mariño et al. 2019). However, cocaine and benzoylecgonine levels detected hereby were ecologically irrelevant.
In the existent published studies regarding the coastal area of Brazil (Table S3), the most frequently identified compounds are caffeine, acetaminophen, atenolol, orphenadrine, diclofenac, losartan and carbamazepine. Moreover, a recent review study showed that acetaminophen, caffeine and diclofenac represent the greatest threats to the aquatic environment in Brazil (Chaves et al. 2021), confirming the hereby findings.
Spatial distribution of PPCPs on the coastal waters of Santa Catarina
The spatial distribution of the screened PPCPs in the coastal waters of SC is shown in Fig. 2, which highlight the sampling points with the highest and lowest occurrences, as well as the MEC of the reported compounds. The largest number of PPCPs was detected at points P3 and P5, belonging to the central-north and central coast, respectively. At these points, the highest concentrations of PPCPs were also found (67% of the reported values). Moreover, atenolol and orphenadrine were only recorded here. Caffeine, diclofenac, losartan and cocaine were detected at all sampling points along the coast of SC. The ubiquous presence and environmental persistence of caffeine can be a strong indicator of water contamination by inadequately treated sewage, suggesting that it can be used as a tracer of anthropogenic activity (Li et al. 2020; Rigueto et al. 2020; Roveri et al. 2020). According to the National Water Agency (ANA), SC has a low sewage treatment coverage rate, with only 24% of the population served (ANA, 2021). The occurrence of caffeine in significant amounts in the aquatic ecosystem may be associated with the consumption of coffee and tea, and drinks that are widely appreciated by the Brazilian people (Souza et al. 2013). Although the concentrations detected in this study were low compared to other reports (Table S3) probably because of the estuarine dispersion to the coastal area, in the absence of additional information to safely assess the real influence of this compound in the aquatic environment, precaution is recommended regarding its effects of the aquatic biota (Korekar et al. 2020). The concentrations of acetaminophen (0.21–10.04 ng/L) and diclofenac (1.40–7.92 ng/L) found in this study were similar to the levels found in other areas [e.g. 0.0014 to 1.4000 ng/L (Roveri et al. 2020) and 3.70 to 4.18 ng/L (Ojemaye and Petrik 2021)].
The other identified drugs, such as losartan, atenolol, orphenadrine, carbamazepine, valsartan and propranolol, were found in substantially lower concentrations, compared to other studies (Table S3: Cortez et al. 2018; Roveri et al. 2020; Yang et al. 2020). These low values may reflect seawater dilution or environmental degradation processes, which need to be taken into account in the ecological assessment (Biel-Maeso et al. 2018). Although these concentrations were not ecologically relevant and much lower than found elsewhere, other factors such as bioaccumulation and the mixture of compounds in the aquatic environment, generating other metabolites, can magnify the effects of these pollutants on the environment, and therefore these PPCPs should be a matter of concern and frequent monitoring (Cizmas et al. 2015; Yin et al. 2017; Brew et al. 2020). Recently, a hospital wastewater was investigated in south of Brazil, being detected 43 PPCPs and 31 metabolic products; thereafter, a quantitative structure–activity relationship modelation predicted the effect of both parental compounds and metabolites, based on their degradability and bioaccumulative proprieties, revealing that they could be toxic to the environment and human health (Becker et al. 2019). In almost all sampling points, the compounds detected more frequently and in greater quantity were caffeine, diclofenac and losartan, with the maximum values detected in the sampling points located on the north coast (P1: losartan and diclofenac) and centre-north (P3: caffeine and losartan). These sampling points are influenced by large rivers and the largest watershed in the SVA, Itajaí Hydrographic Basin, and nearby two of the largest cities in the state, namely Blumenau (309,011 inhabitants) and Joinville (515.288 inhabitants) (IBGE 2010). The reported values, associated with a greater concentration of people in these regions, allow us to suggest that the presence of PPCPs in coastal waters of SC is mainly due to the contribution of domestic sewage discharged to the ocean through outfalls or due to the freshwater runoff (Dafouz et al. 2018), which are the main routes for contamination of the aquatic environment by these pollutants (Biel-Maeso et al. 2018; Li et al. 2020; Rigueto et al. 2020).
Ecological risk assessment
Table 2 presents the summary data regarding the four chemical compounds that indicated ecological potential risks. The complete data for the nine detected and quantified PPCPs is showed in Table S2. The results show the following trend: (i) in relation to acute toxicity, six of the compounds (acetaminophen, atenolol, benzoylecgonine, carbamazepine, cocaine and orphenadrine) showed no ecotoxicity to algae, crustaceans and fish, while three of them (caffeine, diclofenac and losartan) showed low to moderate ecotoxicity: caffeine and diclofenac had low ecotoxicity to crustacean and fish, respectively; losartan showed moderate toxicity to crustaceans; (ii) regarding the chronic toxicity, seven of the compounds showed no toxicity to algae, crustaceans and fish (diclofenac, losartan, atenolol, benzoylecgonine, carbamazepine, cocaine and orphenadrine), while two of them (caffeine and acetaminophen) showed low and moderate toxicity to algae and fish: caffeine had low toxicity to algae, while acetaminophen had moderate toxicity to fish. The hereby reported compounds with potential, acute or chronic, ecotoxicity can cause deleterious effects in the aquatic environment.
Caffeine in environmentally relevant concentrations can cause oxidative stress, lipid peroxidation and even mortality to aquatic organisms (Li et al. 2020). However, the caffeine concentrations found in this study, in general, do not acutely affect the aquatic environment (Gray et al. 2021). However, the fact that it was detected frequently and in all sampling points, and its direct connection with human activity, suggests the use of caffeine as a potential indicator of the presence of contamination from sanitary effluent discharges (Henderson et al. 2020; Li et al. 2020; Rigueto et al. 2020). Moreover, investigations carried out in recent years have evaluated the presence of caffeine in tissues of organisms such as fish, molluscs and suggesting bioaccumulation (Li et al. 2020; de Sousa et al. 2021). The presence of caffeine in high concentrations in the environment, results in a high-risk quotient for chronic exposure of biota (Dafouz et al. 2018), suggesting a high probability of adverse effects in the aquatic compartment (Pires et al. 2016; Santos-Silva et al. 2018; Godoi et al. 2020).
The occurrence of acetaminophen in the hereby reported concentration suggests an ecological risk considered moderate to fish. The toxicity caused by acetaminophen is generally mediated by reactive oxygen species and can result in multiple effects, ranging from protein denaturation to lipid peroxidation and DNA damage (Antunes et al. 2013), mainly as results of oxidative stress mechanisms (Nunes et al. 2014). Recently, it was show that relatively low levels of this compound can exert adaptive changes with unpredictable consequences in the gastropod Phorcus lineatus (Almeida and Nunes 2019).
Diclofenac presented a low risk to fish. However, existent studies suggest that the presence of this drug and its metabolites may represent a high risk to aquatic biota due to its synergistic interactions with other contaminants (Sathishkumar et al. 2020). Ecotoxicological effects tested through the food chain, using fish (Solea senegalensis) fed with worms (Hediste diversicolor) exposed to environmentally realistic concentrations of diclofenac, showed a significant decrease in the activities of the enzymes catalase and acetylcholinesterase, suggesting that exposure to diclofenac can cause significant physiological and neurotoxic disorders in aquatic organisms (Nunes et al. 2020). Exposure of fish (Danio rerio) embryos and larvae to diclofenac resulted also in oxidative stress problems (Bio and Nunes 2020). Diclofenac tested on mussel embryos (Mitylus galloprivincialis) affected enzymatic processes of gene transcription and promoted malformation of the physical structure of the shell (Balbi et al. 2018). Moreover, the study of the biotransformation processes occurring in aquatic organisms, namely in amphipod crustacean (e.g. Gammarus pulex and Hyalella azteca) exposed to diclofenac, showed that it produces methylated metabolites, more bioaccumulative and toxic than the parental compound, which should be considered for a comprehensive risk assessment (Fu et al. 2020).
Losartan showed moderate acute toxicity risk to crustaceans. Biological effects of losartan have been already reported in mussels Perna perna exposed to concentrations of losartan up to 3000 ng/L resulting in cytotoxic effects on gills and hemocytes (Cortez et al. 2018). But, the hereby reported values were much lower (0.432 to 3.200 ng/L). However, studies carried out in the city of Guarujá, São Paulo, Brazil, detected a significant amounts of losartan (3.6–548.0 ng/L) showing that the compound may present moderate to severe risks for algae, crustaceans and fish (Roveri et al. 2020). Losartan effects were evaluated through inhibition tests with aquatic plants (Lemna minor) (Godoy et al. 2015) and bacteria (Aliivibrio fischeri) (Turek et al. 2020), but no significant toxic effects were observed. Losartan is present in urine in significant concentrations, around 35% (Guateque-Londoño et al. 2020), not being completely removed in WWTPs (Gurke et al. 2015). Regions with high population density and intense agricultural activities represent a potential risk to the local ecosystem (Osório et al. 2016), since it causes cytotoxic effects on aquatic organisms (Cortez et al. 2018).
Carbamazepine, cocaine, benzoylecgonine, orphenadrine and atenolol did not present any risk (RQ < 0.01) for all trophic levels tested, which does not raise immediate concern regarding their toxic effects in the aquatic environment. However, these PPCPs are not alone in the aquatic compartment; they are present in complex mixtures of chemical compounds, where additive or synergistic effects are expected to occur, causing deleterious effects sometime greater than those caused by the individual drug (Fernández et al. 2013; Di Nica et al. 2016). Chronic exposure to a mixture of PPCPs in zebrafish (Danio rerio) altered their metabolism, causing a significant reduction in the hepatosomatic index and histological changes in the liver and intestinal tissues, in addition to other observed effects (Hamid et al. 2021). Similarly, a mixture of PPCPs was tested on freshwater algae (Chlorella vulgaris) observing that the toxic effects of the mixture were greater than the effects of each individual substance (Geiger et al. 2016). Several ecotoxicological tests performed on different aquatic organisms exposed to 10 PPCPs in ecologically relevant concentrations, independently and in mixture, showed that the toxicity was 6.5 times, 100 times and 15,000 times greater than the concentrations of the individual compounds for algae (Pseudokirchneriella subcapitata), daphnia (Ceriodaphia dubia) and fish (Danio rerio), respectively (Watanabe et al. 2016). Other effects of mixtures of PPCPs in water were also observed, such as the increase in antibiotic-resistant bacteria (Cizmas et al. 2015). Moreover, PPCPs present in natural waters, like acetaminophen, which act as drinking water sources, during the chlorination step in conventional water plants could results in the formation of more toxic byproducts, such as trihalomethanes (Ding et al. 2018).
Conclusions
This work intended to evaluate, for the first time, the presence and ecological risk of PPCPs in the marine coastal environment of Santa Catarina, Brazil. Seawater sampling was carried out in November 2020 in eight estuarine points along the entire coast of SC, and the presence of 27 PPCPs was screened. The investigation detected the presence of nine compounds, namely caffeine, acetaminophen, diclofenac, losartan, atenolol, benzoylecgonine, carbamazepine, cocaine and orphenadrine. The spatial distribution, detection frequency and measured concentrations for six of them, e.g. caffeine, diclofenac, losartan, benzoylecgonine, carbamazepine and cocaine, suggested that these compounds are constantly present on the coast of SC. The ecological risk assessment showed that caffeine, diclofenac, losartan and acetaminophen presented low to moderate risks for aquatic organisms acutely and chronically exposed to them. SC is a state whose tourism is constant, especially in the coastal region. The low rate of sewage collection and treatment in the state, associated with traditional WWTPs, explains why such PPCPs are continuously discharged into receiving water bodies, contaminating the inshore environment. Thus, it is recommended to expand and improve the basic sanitation infrastructures, opting for advanced technologies for the removal of PPCPs in the WWTPs. The establishment of periodic monitoring for PPCPs compounds, jointly with other parameters of quality of bathing waters, is also highly recommended. In addition, public awareness actions about the proper use, control and disposal of medicines and hygiene products are extremely important to minimize the discharge of these compounds into the aquatic environment, namely in areas of intense human recreation.
Data availability
Not applicable.
References
Afsa S, Hamden K, Lara Martin PA, Mansour HB (2020) Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ Sci Pollut Res 27(2):1941–1955. https://doi.org/10.1007/s11356-019-06866-5
Al-Farsi RS, Ahmed M, Al-Busaidi A, Choudri BS (2017) Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: a review. Emerg Contam 3(4):132–137. https://doi.org/10.1016/j.emcon.2018.02.001
Ali AM, Rønning HT, Alarif W, Kallenborn R, Al-Lihaibi SS (2017) Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 175:505–513. https://doi.org/10.1016/j.chemosphere.2017.02.095
Almeida F, Nunes B (2019) Effects of acetaminophen in oxidative stress and neurotoxicity biomarkers of the gastropod Phorcus lineatus. Environ Sci Pollut Res 26(10):9823–9831. https://doi.org/10.1007/s11356-019-04349-1
ANA – Agência Nacional de Águas (Brasil)., 2017. Atlas esgotos: despoluição de bacias hidrográficas / Agência Nacional de Águas, Secretaria Nacional de Saneamento Ambiental . -- Brasília: ANA. Available from: <ATLASeESGOTOSDespoluicaodeBaciasHidrograficas-ResumoExecutivo_livro.pdf > Accessed in July 2021.
ANA – Agência Nacional de Águas (Brasil)., 2011. Ministério do Meio Ambiente. Guia Nacional de Coleta e Preservação de Amostras: Água, Sedimento, Comunidades Aquáticas e Efluentes Líquidos, p. 327, 2011. Cetesb, São Paulo, SP, Available from: http://arquivos.ana.gov.br/institucional/sge/CEDOC/Catalogo/2012/GuiaNacionalDeColeta.pdf. Accessed in July 2021.
Antunes SC, Freitas R, Figueira E, Gonçalves F, Nunes B (2013) Biochemical effects of acetaminophen in aquatic species: edible clams Venerupis decussata and Venerupis philippinarum. Environ Sci Pollut Res 20(9):6658–6666. https://doi.org/10.1007/s11356-013-1784-9
Balbi T, Montagna M, Fabbri R, Carbone C, Franzellitti S, Fabbri E, Canesi L (2018) Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci Total Environ 642:601–609. https://doi.org/10.1016/j.scitotenv.2018.06.125
Bartrons M, Peñuelas J (2017) Pharmaceuticals and personal-care products in plants. Trends Plant Sci 22(3):194–203. https://doi.org/10.1016/j.tplants.2016.12.010
Becker RW, Ibáñez M, Lumbaque EC, Wilde ML, da Rosa TF, Hernández F, Sirtori C 2019 Investigation of pharmaceuticals and their metabolites in Brazilian hospital wastewater by LC-QTOF MS screening combined with a preliminary exposure and in silico risk assessment. Sci Total Environ 134218https://doi.org/10.1016/j.scitotenv.2019.1342
Behera SK, Kim HW, Oh J-E, Park H-S (2011) Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci Total Environ 409(20):4351–4360. https://doi.org/10.1016/j.scitotenv.2011.07.015
Beretta M, Britto V, Tavares TM, da Silva SMT, Pletsch AL (2014) Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J Soils Sediments 14(7):1278–1286. https://doi.org/10.1007/s11368-014-0884-6
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA (2018) Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ 612:649–659. https://doi.org/10.1016/j.scitotenv.2017.08.279
Bio S, Nunes B (2020) Acute effects of diclofenac on zebrafish: indications of oxidative effects and damages at environmentally realistic levels of exposure. Environ Toxicol Pharmacol 78:103394. https://doi.org/10.1016/j.etap.2020.103394
Brew DW, Black MC, Santos M, Rodgers J, Henderson WM (2020) Metabolomic investigations of the temporal effects of exposure to pharmaceuticals and personal care products and their mixture in the eastern oyster (Crassostrea virginica). Environ Toxicol Chem 39(2):419–436. https://doi.org/10.1002/etc.4627
de Chaves MJS, Barbosa SC, Primel EG (2021) Emerging contaminants in Brazilian aquatic environment: identifying targets of potential concern based on occurrence and ecological risk. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15245-y
Chen H, Chen W, Guo H, Lin H, Zhang Y (2021) Pharmaceuticals and personal care products in the seawater around a typical subtropical tourist city of China and associated ecological risk. Environ Sci Pollut Res 28(18):22716–22728. https://doi.org/10.1007/s11356-020-12335-1
Cizmas L, Sharma VK, Gray CM, McDonald TJ (2015) Pharmaceuticals and personal care products in waters: occurrence, toxicity, and risk. Environ Chem Lett 13(4):381–394. https://doi.org/10.1007/s10311-015-0524-4
CMED – Câmara de Regulação do Mercado de Medicamentos., 2021. Anuário Estatístico do Mercado Farmacêutico. ANVISA, Brasília http://portal.anvisa.gov.br/. CMED publica o Anuário Estatístico do Mercado Farmacêutico – Edição Comemorativa 2019/2020 — Português (Brasil). Available from: <www.gov.br> Accessed in July 2021.
Cortez FS, da Souza LS, Guimarães LL, Almeida JE, Pusceddu FH, Maranho LA, Mota LG, Nobre CR, Moreno BB, de Abessa DMS, Cesar A, Santos AR, Pereira CDS (2018) Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci Total Environ 637–638:1363–1371. https://doi.org/10.1016/j.scitotenv.2018.05.069
Dafouz R, Cáceres N, Rodríguez-Gil JL, Mastroianni N, López de Alda M, Barceló D, de Miguel ÁG, Valcárcel Y (2018) Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci Total Environ 615:632–642. https://doi.org/10.1016/j.scitotenv.2017.09.155
de Sousa ML, dos Santos DYAC, Chow F, Pompêo MLM (2021) Caffeine as a contaminant of periphyton: ecological changes and impacts on primary producers. Ecotoxicology 30(4):599–609. https://doi.org/10.1007/s10646-021-02381-x
Del Rosario KL, Mitra S, Humphrey CP, O’Driscoll MA (2014) Detection of pharmaceuticals and other personal care products in groundwater beneath and adjacent to onsite wastewater treatment systems in a coastal plain shallow aquifer. Sci Total Environ 487:216–223. https://doi.org/10.1016/j.scitotenv.2014.03.135
Di Nica V, Villa S, Finizio A (2016) Toxicity of individual pharmaceuticals and their mixtures to Aliivibrio fischeri: experimental results for single compounds and considerations of their mechanisms of action and potential acute effects on aquatic organisms. Environ Toxicol Chem 36(3):807–814. https://doi.org/10.1002/etc.3568
Ding S, Chu W, Bond T, Wang Q, Gao N, Xu B, Du E (2018) Formation and estimated toxicity of trihalomethanes, haloacetonitriles, and haloacetamides from the chlor(am)ination of acetaminophen. J Hazard Mater 341:112–119. https://doi.org/10.1016/j.jhazmat.2017.07.049
Ebele AJ, Abou-Elwafa Abdallah M, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3(1):1–16. https://doi.org/10.1016/j.emcon.2016.12.004
ECB (2003) Technical guidance document on risk assessment for existing substances. Part II:108–110
ECHA (2008) Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment, pp 7–29
Ekpeghere KI, Sim W-J, Lee H-J, Oh J-E (2018) Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment. Sci Total Environ 640–641:1015–1023. https://doi.org/10.1016/j.scitotenv.2018.05.218
Evgenidou EN, Konstantinou IK, Lambropoulou DA (2015) Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: a review. Sci Total Environ 505:905–926. https://doi.org/10.1016/j.scitotenv.2014.10.021
Fernández C, Carbonell G, Babín M (2013) Effects of individual and a mixture of pharmaceuticals and personal-care products on cytotoxicity, EROD activity and ROS production in a rainbow trout gonadal cell line (RTG-2). J Appl Toxicol 33(11):1203–1212. https://doi.org/10.1002/jat.2752
FIESC – Federação das Indústrias do Estado de Santa Catarina. Observatório (2020) Economia de Santa Catarina. Em linha Available from: https://www.observatoriofiesc.com.br/resumo-executivo. Accessed July 2021
FIESC – Federação das Indústrias do Estado de Santa Catarina. Observatório. Economia de Santa Catarina. Em linha Available from: < https://www.observatoriofiesc.com.br/resumo-executivo> Accessed in July 2021.
Filho NHH 2003 Setorização da Província Costeira de Santa Catarina em base aos aspectos geológicos, geomorfológicos e geográficos. Geosul, 18(35), 71–98. Em linha: Setorização da Província Costeira de Santa Catarina em base aos aspectos geológicos, geomorfológicos e geográficos/Geosul. Available from: <www. ufsc.br> Accessed in July 2021.
Fontes MK, de Campos BG, Cortez FS, Pusceddu FH, Moreno BB, Maranho LA, Lebre DT, Guimarães LL, Pereira CDS (2019) Seasonal monitoring of cocaine and benzoylecgonine in a subtropical coastal zone (Santos Bay, Brazil). Mar Pollut Bull 149:110545. https://doi.org/10.1016/j.marpolbul.2019.110545
de Freitas LAA, Radis-Baptista G (2021) Pharmaceutical pollution and disposal of expired, unused, and unwanted medicines in the Brazilian context. J Xenobiotics 11(2):61–76. https://doi.org/10.3390/jox11020005
Fu Q, Fedrizzi D, Kosfeld V, Schlechtriem C, Ganz V, Derrer S, Rentsch D, Hollender J (2020) Biotransformation changes bioaccumulation and toxicity of diclofenac in aquatic organisms. Environ Sci Technol 54(7):4400–4408. https://doi.org/10.1021/acs.est.9b07127
Geiger E, Hornek-Gausterer R, Saçan MT (2016) Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol Environ Saf 129:189–198. https://doi.org/10.1016/j.ecoenv.2016.03.032
Godoi FGA, Muñoz-Peñuela M, Gomes ADO, Tolussi CE, Brambila-Souza G, Branco GS, Lo Nostro FL, Moreira RG (2020) Endocrine disruptive action of diclofenac and caffeine on Astyanax altiparanae males (Teleostei: Characiformes: Characidae). Comp Biochem Physiol c: Toxicol Pharmacol 231:108720. https://doi.org/10.1016/j.cbpc.2020.108720
Godoy AA, Kummrow F, Pamplin PAZ (2015) Ecotoxicological evaluation of propranolol hydrochloride and losartan potassium to Lemna minor L. (1753) individually and in binary mixtures. Ecotoxicology 24(5):1112–1123. https://doi.org/10.1007/s10646-015-1455-3
González-Mariño I, Estévez-Danta A, Rodil ER, Da Silva KM, Sodré FF, Cela R, Quintana JB (2019) Profiling cocaine residues and pyrolytic products in wastewater by mixed-mode liquid chromatography–tandem mass spectrometry. Drugs Test Anal V11(7):1018–1027. https://doi.org/10.1002/dta.2590
González-Mariño I, Baz-Lomba JA, J.A., et al (2020) Spatio-temporal assessment of illicit drug use at large scale: evidence from 7 years of international wastewater monitoring. Addiction 115(1):109–120. https://doi.org/10.1111/add.14767
Goldstein M, Shenker M, Chefetz B (2014) Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environ Sci Technol 48(10):5593–5600. https://doi.org/10.1021/es5008615
Gray I, Green-Gavrielidis LA, Thornber C (2021) Effect of caffeine on the growth and photosynthetic efficiency of marine macroalgae. Bot Mar 64(1):13–18. https://doi.org/10.1515/bot-2020-0055
Guateque-Londoño JF, Serna-Galvis EA, Ávila-Torres Y, Torres-Palma RA (2020) Degradation of losartan in fresh urine by sonochemical and photochemical advanced oxidation processes. Water 12(12):3398. https://doi.org/10.3390/w12123398
Gurke R, Rößler M, Marx C, Diamond S, Schubert S, Oertel R, Fauler J (2015) Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci Total Environ 532:762–770. https://doi.org/10.1016/j.scitotenv.2015.06.067
Hamid N, Junaid M, Wang Y, Pu S-Y, Jia P-P, Pei D-S (2021) Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish. Environ Pollut 273:116494. https://doi.org/10.1016/j.envpol.2021.116494
Heinz D, Moreno GCL de, Hein N 2020 O saneamento básico nos municípios de Santa Catarina: Uma análise cluster. COLÓQUIO - Revista do Desenvolvimento Regional, 18(1), 1–15. https://doi.org/10.26767/coloquio.v18i1.1888
Henderson A, Ng B, Landeweer S, Quinete N, Gardinali P (2020) Assessment of sucralose, caffeine and acetaminophen as anthropogenic tracers in aquatic systems across Florida. Bull Environ Contam Toxicol 105(3):351–357. https://doi.org/10.1007/s00128-020-02942-6
Hernando MD, Mezcua M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69(2):334–342. https://doi.org/10.1016/j.talanta.2005.09.037
Huber S, Remberger M, Kaj L, Schlabach M, Jörundsdóttir HÓ, Vester J, Arnórsson M, Mortensen I, Schwartson R, Dam M (2016) A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci Total Environ 562:13–25. https://doi.org/10.1016/j.scitotenv.2016.03.063
Huizer M, ter Laak TL, de Voogt P, van Weezel AP (2021) Wastewater-based epidemiology for illicit drugs: a critical review on global data. Water Res 207:117789. https://doi.org/10.1016/j.watres.2021.117789
IBGE – Instituto Brasileiro de Geografia e Estatística. Censo Ibge., 2010. Available from: < https://censo2010.ibge.gov.br> Accessed in July 2021.
IBGE – Instituto Brasileiro de Geografia e Estatística. Cidades e Estados: Santa Catarina., 2020. Available from: <https://www.ibge.gov.br/cidades-e-estados/sc/> Accessed in July 2021.
Keerthanan S, Jayasinghe C, Biswas JK, Vithanage M (2021) Pharmaceutical and personal care products (PPCPs) in the environment: plant uptake, translocation, bioaccumulation, and human health risks. Crit Rev Environ Sci Technol 51(12):1221–1258. https://doi.org/10.1080/10643389.2020.1753634
Korekar G, Kumar A, Ugale C (2020) Occurrence, fate, persistence and remediation of caffeine: a review. Environ Sci Pollut Res 27(28):34715–34733. https://doi.org/10.1007/s11356-019-06998-8
Kosma CI, Lambropoulou DA, Albanis TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466–467:421–438. https://doi.org/10.1016/j.scitotenv.2013.07.044
Krogh J, Lyons S, Lowe CJ (2017) Pharmaceuticals and personal care products in municipal wastewater and the marine receiving environment near Victoria Canada. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00415
Kumar R, Sarmah AK, Padhye LP (2019) Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train. J Environ Manage 233:649–659. https://doi.org/10.1016/j.jenvman.2018.12.062
Li S, He B, Wang J, Liu J, Hu X (2020) Risks of caffeine residues in the environment: necessity for a targeted ecopharmacovigilance program. Chemosphere 243:125343. https://doi.org/10.1016/j.chemosphere.2019.125343
Lindholm-Lehto PC, Ahkola HSJ, Knuutinen JS, Herve SH (2016) Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Environ Sci Pollut Res 23(8):7985–7997. https://doi.org/10.1007/s11356-015-5997-y
Madikizela LM, Ncube S, Chimuka L (2018) Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: a review. Sci Total Environ 636:477–486. https://doi.org/10.1016/j.scitotenv.2018.04.297
MMA – Ministério do Meio Ambiente. Resoluções Conama., 2021. Available from: < https://www2.mma.gov.br/port/conama/> Accessed in July 2021.
Ng B, Quinete N, Maldonado S, Lugo K, Purrinos J, Briceño H, Gardinali P (2021) Understanding the occurrence and distribution of emerging pollutants and endocrine disruptors in sensitive coastal South Florida Ecosystems. Sci Total Environ 757:143720. https://doi.org/10.1016/j.scitotenv.2020.143720
Nunes B, Antunes SC, Santos J, Martins L, Castro BB (2014) Toxic potential of paracetamol to freshwater organisms: a headache to environmental regulators? Ecotoxicol Environ Saf 107:178–185. https://doi.org/10.1016/j.ecoenv.2014.05.027
Nunes B, Daniel D, Canelas GG, Barros J, Correia AT (2020) Toxic effects of environmentally realistic concentrations of diclofenac in organisms from two distinct trophic levels, Hediste diversicolor and Solea senegalensis. Comp Biochem Physiol c: Toxicol Pharmacol 231:108722. https://doi.org/10.1016/j.cbpc.2020.108722
Ojemaye CY, Petrik L (2021) Pharmaceuticals and personal care products in the marine environment around False Bay, Cape Town, South Africa: occurrence and risk-assessment study. Environ Toxicol Chem. https://doi.org/10.1002/etc.5053
Osório V, Larrañaga A, Aceña J, Pérez S, Barceló D (2016) Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci Total Environ 540:267–277. https://doi.org/10.1016/j.scitotenv.2015.06.143
Overturf MD, Anderson JC, Pandelides Z, Beyger L, Holdway DA (2015) Pharmaceuticals and personal care products: a critical review of the impacts on fish reproduction. Crit Rev Toxicol 45(6):469–491. https://doi.org/10.3109/10408444.2015.1038499
Quadra GR, Oliveira de Souza H, dos Costa RS, dos Fernandez MAS (2017) Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ Sci Pollut Res 24(2):1200–1218. https://doi.org/10.1007/s11356-016-7789-4
Pereira AL, de Vasconcelos Barros RT, Pereira SR (2017) Pharmacopollution and Household Waste Medicine (HWM): how reverse logistics is environmentally important to Brazil. Environ Sci Pollut Res 24(31):24061–24075. https://doi.org/10.1007/s11356-017-0097-9
Pires A, Almeida Â, Correia J, Calisto V, Schneider RJ, Esteves VI, Freitas R (2016) Long-term exposure to caffeine and carbamazepine: impacts on the regenerative capacity of the polychaete Diopatra neapolitana. Chemosphere 146:565–573. https://doi.org/10.1016/j.chemosphere.2015.12
Rigueto CVT, Nazari MT, De Souza CF, Cadore JS, Brião VB, Piccin JS (2020) Alternative techniques for caffeine removal from wastewater: an overview of opportunities and challenges. J Water Proc Eng 35:101231. https://doi.org/10.1016/j.jwpe.2020.101231
Roveri V, Guimarães LL, Toma W, Correia AT (2020) Occurrence and ecological risk assessment of pharmaceuticals and cocaine in a beach area of Guarujá, São Paulo State, Brazil, under the influence of urban surface runoff. Environ Sci Pollut Res 27(36):45063–45075. https://doi.org/10.1007/s11356-020-10316-y
Roveri V, Guimarães LL, Toma W, Correia AT (2021a) Occurrence and risk assessment of pharmaceuticals and cocaine around the coastal submarine sewage outfall in Guarujá, São Paulo State, Brazil. Environ Sci Pollut Res 28(9):11384–11400. https://doi.org/10.1007/s11356-020-11320-y
Roveri V, Guimarães LL, Toma W, Correia AT (2021b) Occurrence and ecological risk assessment of pharmaceuticals and cocaine in the urban drainage channels of Santos beaches (São Paulo, Brazil): a neglected, but sensitive issue. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15249-8
Santos-Silva TG, Montagner CC, Martinez CBR (2018) Evaluation of caffeine effects on biochemical and genotoxic biomarkers in the neotropical freshwater teleost Prochilodus lineatus. Environ Toxicol Pharmacol 58:237–242. https://doi.org/10.1016/j.etap.2018.02.002
Sathishkumar P, Meena RAA, Palanisami T, Ashokkumar V, Palvannan T, Gu FL (2020) Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—a review. Sci Total Environ 698:134057. https://doi.org/10.1016/j.scitotenv.2019.134057
SDE – Secretaria do Estado do Desenvolvimento Econômico Sustentável., 2020. Recursos hídricos de Santa Catarina. Available from: <http://www.aguas.sc.gov.br/jsmallfib_top/DHRI/bacias_hidrograficas/bacias_hidrograficas_sc.pdf> Accessed in July 2021.
SEBRAE – Serviço Brasileiro de Apoio às Micro e Pequenas Empresas. Santa Catarina em números. Florianópolis, 2013. Available from: <www.sebrae.com.br)>. Accessed in July 2021.
Shahriar A, Tan J, Sharma P, Hanigan D, Verburg P, Pagilla K, Yang Y (2021) Modeling the fate and human health impacts of pharmaceuticals and personal care products in reclaimed wastewater irrigation for agriculture. Environ Pollut 276:116532. https://doi.org/10.1016/j.envpol.2021.116532
Shihomatzu HM 2015 Desenvolvimento e Validação de Metodologia SPE-LC-MS/MS para determinação de Fármacos e Droga de Abuso nas Águas da Represa Guarapiranga, São Paulo/SP, Brasil. IPEN/USP. https://doi.org/10.11606/T.85.2015.tde-28042015-095207
de Souza AM, Pereira RA, Yokoo EM, Levy RB, Sichieri R (2013) Alimentos mais consumidos no Brasil: Inquérito Nacional de Alimentação 2008–2009. Rev Saude Publica 47:190s–199s. https://doi.org/10.1590/S0034-89102013000700005
Stewart M, Olsen G, Hickey CW, Ferreira B, Jelić A, Petrović M, Barcelo D (2014) A survey of emerging contaminants in the estuarine receiving environment around Auckland, New Zealand. Sci Total Environ 468–469:202–210. https://doi.org/10.1016/j.scitotenv.2013.08.039
Sui Q, Cao X, Lu S, Zhao W, Qiu Z, Yu G (2015) Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: a review. Emerg Contam 1(1):14–24. https://doi.org/10.1016/j.emcon.2015.07.001
Tarpani RR, Azapagic A (2018) Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J Environ Manage 215:258–272. https://doi.org/10.1016/j.jenvman.2018.03.047
Turek M, Różycka-Sokołowska E, Owsianik K, Marciniak B, Bałczewski P (2020) Modification of the Microtox® basic solid phase test: a new application for the ecotoxicological studies on poorly soluble antihypertensive drugs. J Hazard Mater 399:122839. https://doi.org/10.1016/j.jhazmat.2020.122839
USEPA – United States Environmental Protection Agency, 2017. Ecological Structure-Activity Relationship Model (ECOSAR) Class Program. MS-Windows Version 2.0. https://www.epa.gov/tsca732screening-tools/ecological-structure-activity-relationships-ecosarcpredictive-model.
USEPA – United States Environmental Protection Agency, 2019. ECOTOX User Guide: Ecotoxicology Database System, Version 4.0. http://www.epa.gov/ecotox/.
Vidal-Dorsch DE, Bay SM, Maruya K, Snyder SA, Trenholm RA, Vanderford BJ (2012) Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ Toxicol Chem 31:2674–2682. https://doi.org/10.1002/etc.2004
Wang H, Xi H, Xu L, Jin M, Zhao W, Liu H (2021) Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: a review. Sci Total Environ 788:147819. https://doi.org/10.1016/j.scitotenv.2021.147819
Wang J, Wang S (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manage 182:620–640. https://doi.org/10.1016/j.jenvman.2016.07.049
Watanabe H, Tamura I, Abe R, Takanobu H, Nakamura A, Suzuki T, Hirose A, Nishimura T, Tatarazako N (2016) Chronic toxicity of an environmentally relevant mixture of pharmaceuticals to three aquatic organisms (alga, daphnid, and fish). Environ Toxicol Chem 35(4):996–1006. https://doi.org/10.1002/etc.3285
Wilkinson J, Hooda PS, Barker J, Barton S, Swinden J (2017) Occurrence, fate and transformation of emerging contaminants in water: an overarching review of the field. Environ Pollut 231:954–970. https://doi.org/10.1016/j.envpol.2017.08.032
Wille K, Noppe H, Verheyden K, Vanden Bussche J, De Wulf E, Van Caeter P, Vanhaecke L (2010) Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal Bioanal Chem 397(5):1797–1808. https://doi.org/10.1007/s00216-010-3702-z
Yang L, Zhou Y, Shi B, Meng J, He B, Yang H, Yoon SJ, Kim T, Kwon B-O, Khim JS, Wang T (2020) Anthropogenic impacts on the contamination of pharmaceuticals and personal care products (PPCPs) in the coastal environments of the Yellow and Bohai seas. Environ Int 135:105306. https://doi.org/10.1016/j.envint.2019.105306
Yang Y, Ok YS, Kim K-H, Kwon EE, Tsang YF (2017) Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ 596–597:303–320. https://doi.org/10.1016/j.scitotenv.2017.04.102
Yin L, Wang B, Yuan H, Deng S, Huang J, Wang Y, Yu G (2017) Pay special attention to the transformation products of PPCPs in environment. Emerg Contam 3(2):69–75. https://doi.org/10.1016/j.emcon.2017.04.001
Yuan X, Hu J, Li S, Yu M (2020) Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Environ Pollut 266:115340. https://doi.org/10.1016/j.envpol.2020.115340
Zhou Y, Meng J, Zhang M, Chen S, He B, Zhao H, Li Q, Zhang S, Wang T (2019) Which type of pollutants need to be controlled with priority in wastewater treatment plants: traditional or emerging pollutants? Environ Int 131:104982. https://doi.org/10.1016/j.envint.2019.104982
Acknowledgements
Authors want to acknowledge Daniel Temponi Lebre from the Centro de Espectrometria de Massa Aplicada — Instituto de Pesquisas Energéticas e Nucleares (CEMSA, IPEN, São Paulo, Brazil) for technical support regarding the LC–MS/MS analyses. Thanks also to Professor Ítalo Braga de Castro, from Instituto do Mar, Universidade Federal de São Paulo (IMAR-UNIFESP, São Paulo, Brazil) for technical support with the Manifold.
Funding
Correia AT was supported by national funds through FCT — Foundation for Science and Technology within the scope of UIDB/04423/2020 and UIDP/04423/2020.
Author information
Authors and Affiliations
Contributions
AMP: Conceptualization, sampling acquisition, data analysis and writing of the original draft. VR: Laboratorial work and review and editing of the MS. LLG: Laboratorial work and review and editing. TMNO: Review and editing and co-supervision. ATC: Sampling design, funding acquisition, supervision, writing-original draft and writing-review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pisetta, AM., Roveri, V., Guimarães, L.L. et al. First report on the occurrence of pharmaceuticals and cocaine in the coastal waters of Santa Catarina, Brazil, and its related ecological risk assessment. Environ Sci Pollut Res 29, 63099–63111 (2022). https://doi.org/10.1007/s11356-022-20312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20312-z