Abstract
The aim of this study was to screen and quantify 23 pharmaceutical compounds (including illicit drugs), at two sampling points near the diffusers of the Guarujá submarine outfall, State of São Paulo, Brazil. Samples were collected in triplicate during the high (January 2018) and low (April 2018) seasons at two different water column depths (surface and bottom). A total of 10 compounds were detected using liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). Caffeine (42.3–141.0 ng/L), diclofenac (3.6–85.7 ng/L), valsartan (4.7–14.3 ng/L), benzoylecgonine (0.3–1.7 ng/L), and cocaine (0.3–0.6 ng/L) were frequently detected (75% occurrence). Orphenadrine (0.6–3.0 ng/L) and atenolol (0.1–0.3 ng/L), and acetaminophen (1.2–1.4 ng/L) and losartan (0.7–3.4 ng/L), were detected in 50% and 25% of the samples, respectively. Only one sample (12.5%) detected the presence of carbamazepine (< 0.001–0.1 ng/L). Unexpectedly a lower frequency of occurrence and concentration of these compounds occurred during the summer season, suggesting that other factors, such as the oceanographic and hydrodynamic regimes of the study area, besides the population rise, should be taken into account. Caffeine presented concentrations above the surface water safety limits (0.01 μg/L). For almost all compounds, the observed concentrations indicate nonenvironmental risk for the aquatic biota, except for caffeine, diclofenac, and acetaminophen that showed low to moderate ecological risk for the three trophic levels tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas are of great economic and socio-environmental importance because 50% of the world’s population live within 60 km of a coastline (Roberts et al. 2010). This high concentration of people exposes coastal ecosystems to different anthropogenic pressures, such as the disposal of untreated sewage in the marine environment (Rodgers-Gray et al. 2000). This sewage can contain thousands of chemical substances, such as pharmaceuticals and personal care products (PPCPs) (Moreno-González et al. 2015; Brumovský et al. 2017; Fontes et al. 2019). PPCPs are a vast group of emerging environmental contaminants, such as pharmaceuticals from different therapeutic classes (e.g. antiepileptics, stimulants, analgesics/anti- inflammatory, and antihypertensive drugs) (Moreno-González et al. 2015; Brumovský et al. 2017; Comtois-Marotte et al. 2017), and illicit drugs (e.g. cocaine) (Pereira et al. 2016; Löve et al. 2018; Fontes et al. 2019).
Currently, there is no worldwide regulatory legislation that sets safety limits for these emerging compounds in the environment (Beretta et al. 2014; Machado et al. 2016; Pereira et al. 2016). Consequently, they end up in wastewater treatment plants (WWTPs), which are often inefficient in removing these pollutants (Santos et al. 2009; Behera et al. 2011; Pereira et al. 2016). Pharmaceuticals and illicit drugs are ubiquitous in coastal marine environments at concentrations ranging from ng/L to μg/L (Pereira et al. 2016; Diamanti et al. 2019; Fontes et al. 2019) and could cause harmful effects on the aquatic biota at different trophic levels, namely in molluscs (Aguirre-Martínez et al. 2013b; Almeida et al. 2015; Capolupo et al. 2016), crustaceans (Aguirre-Martínez et al. 2013a; Binelli et al. 2013; Imeh-Nathaniel et al. 2017), and fishes (Ramos et al. 2014; Nunes et al. 2015; Capaldo et al. 2019). Among the various harmful effects, some physiological, biochemical, and behavioural changes at different trophic levels are frequently observed when individuals are chronically exposed to environmentally realistic concentrations (Fabbri and Franzellitti 2015; Godoy et al. 2015a, b; Godoy and Kummrow 2017). Under this scenario, caffeine can cause induction of oxidative stress or metabolism disturbances in molluscs (Binelli et al. 2013; Almeida et al. 2014, 2015); carbamazepine can alter the stability of the lysosomal membrane in crabs (Aguirre-Martínez et al. 2013a); and acetaminophen can cause oxidative stress in fish (Ramos et al. 2014; Nunes et al. 2015), among other effects.
The detection of PPCPs in the coastal and marine environment has been neglected for many years under the assumption that ocean dilution would represent a safety factor (Fabbri and Franzellitti 2015; Desbiolles et al. 2018). Meanwhile, the number of studies on underwater sewage discharge is increasing around the world (Nodler et al. 2010; Afonso-Olivares et al. 2013; Alygizakis et al. 2016). In Brazil (the fifth largest country in the world), where approximately 50 million people live in coastal municipalities (along 8,500 km of coastline) (Quadra et al. 2016; Ibge 2018), only a limited number of' studies have been dedicated to detecting the presence of PPCPs and/or illicit drugs in coastal and marine ecosystems in the last 20 years (Beretta et al. 2014; Pereira et al. 2016; Dos Santos et al. 2018; Fontes et al. 2019). In Brazilian coastal cities, WWTPs with only preliminary levels of treatment (20 systems along the coast) are predominant (Abessa et al. 2012; Ortiz et al. 2016). Each WWTP consists of a series of tanks including a sand filter and a screen, which aims to remove only the solid and floating material from the sewage, followed by a chlorination step in order to eliminate pathogenic microorganisms; but they are not specifically designed to remove PPCPs (Abessa et al. 2012; Ortiz et al. 2016; Pereira et al. 2016). The final destination of the preconditioned sewage is a submarine outfall that disposes the sewage daily into the marine environment (South Atlantic Ocean) (Abessa et al. 2012; Ortiz et al. 2016; Pereira et al. 2016). One such WWTP, which completed 20 years in 2018, is located in the Guarujá municipality (Abessa et al. 2012; Ortiz et al. 2016). Guarujá is a microregion of Santos, São Paulo State, Brazil, where the good climatic conditions (average annual temperature of 22 °C) favour the use of its beaches throughout the year, making the municipality one of the main Brazilian tourist destinations, doubling the population in summer (Ribeiro and Oliveira 2015; Cetesb 2017). However, to the best of the author’s knowledge, no data exist about the occurrence of pharmaceuticals and illicit drugs around this coastal submarine sewage outfall. Therefore, considering that (i) pharmaceuticals and illicit drugs pose a growing risk to marine species and ecosystems (Desbiolles et al. 2018); (ii) marine organisms are exposed to these environmental stressors, mainly in highly urbanised coastal areas and at recreational sites (Fabbri and Franzellitti 2015); (iii) data on marine pollution by these compounds is scarce, namely, in South America, where the consumption of these substances is rapidly increasing (Quadra et al. 2016); and (iv) after the report of PPCPs and illicit drugs in Santos Bay (Pereira et al. 2016), Brazilian environmental agencies showed great concern about their occurrence and associated ecological risks, additional studies on the occurrence and risk assessment of these compounds in the marine environment around the Guarujá sewage outfall are of extreme relevance.
In this context, the objective of this study was, for the first time, to screen and identify the occurrence of 23 pharmaceuticals of various therapeutic classes (including cocaine and its primary metabolite, benzoylecgonine), near the discharge of the outfall of Guarujá on the coast of São Paulo, Brazil. This work also discusses the potential acute and chronic aquatic toxicology and adverse effects of each chemical compound reported hereby to ecologically relevant aquatic species.
Materials and methods
Study site description and sample collection
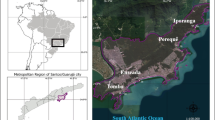
This study was carried out in Guarujá municipality, a microregion of Santos, São Paulo State, Brazil. It is an area of 143 km2 and 64 km of extension, in which 107 km2 are made up of environmental protection areas and 36 km2 of urbanised area (Ribeiro and Oliveira 2015), with 318,000 inhabitants (Ibge 2018). In Guarujá, there are two quite distinct seasonal periods: a rainy season that occurs from November through March and a dry season that occurs from April through October (Cetesb 2017). The annual precipitation of the region ranges between 2500 and 3000 mm and the annual mean temperature is 22 °C (Cetesb 2017). The municipality’s economy is mainly driven by three activities. Two of these activities are non-seasonal and occur in the western portion of the island: trade in the district of Vicente de Carvalho and port-related activities in the port of Santos (the largest port in Latin America). A third activity, which takes place in the eastern and southern parts of the island, is tourism that benefits of the existent 26 beaches. The number of inhabitants almost doubles in the high summer season (between December and February) (Ribeiro and Oliveira 2015; Cetesb 2017).
The municipal sewage of Guarujá is treated through a WWTP with a preliminary treatment (Cetesb 2017). This WWTP consists of a series of tanks including a sand filter and a screen, followed by a chlorination step in order to eliminate pathogenic microorganisms (Ortiz et al. 2016). The final destination of the preconditioned sewage is a submarine outfall 4500 m long and 14 m deep that daily disposes sewage (1.45 m3/s) into the marine environment (Enseada beach) (Cetesb 2017).
Two field campaigns were carried out: one on 12 January 2018 during the high tourist season (summer/rainy season) and another on 13 April 2018 in the low tourist season (fall/dry season). This study adopted two existing environmental agency (Cetesb) sampling points: P1: 24° 01′ 39, 7″ S; 46° 13′ 27, 5″ W and P2: 24° 01′ 34, 3″ S; 46° 13′ 21, 3″ W. Both points, P1 (southwest) and P2 (northeast), are located about 100 m from the discharge point (Fig. 1). During the sampling work, no rainfall was recorded in the 48 h prior to water collection. The water was collected using a Van Dorn bottle at two different depths: surface (1 m) and bottom (10 m). Samples were labelled according to the sampling points (P1 and P2), collection seasons [high (HS) and low (LS) tourist seasons], and water depths [(surface (S) and bottom (B)]. Thus, a total of eight samples were collected (2 sampling points × 2 water depths × 2 seasons).
Water samples were stored in 1-L amber glass bottles previously cleaned, transported to the laboratory in an insulated box with ice (< 6 °C), filtered with 0.45 μm pore size membrane to remove suspended solids, and kept under refrigeration (− 20 °C) until further processing. The extraction, concentration, and purification of the drugs of interest were performed within 7 days after filtration (USEPA 2007).
Preparation and analysis of pharmaceutical compounds
Chemical and standards
High purity reagents such as nitric acid and sulphuric acid were purchased from Merck. Organic solvents of HPLC or LCMS grade, including acetonitrile, methanol, and isopropanol, were acquired from Sigma-Aldrich. Mobile phase additives, namely LC–MS grade formic acid and ammonium acetate, were acquired from Sigma-Aldrich and Merck, respectively. Analytical standards of acetaminophen, atenolol, bromazepam, caffeine, carbamazepine, cyproterone, clonazepam, clopidogrel, diclofenac, enalapril, loratadine, losartan, midazolam, orphenadrine, propranolol, sildenafil, and valsartan were acquired from Sigma-Aldrich. Cocaine and benzoylecgonine were acquired from Cerillant. Other targeted pharmaceuticals were purchased from several providers: citalopram (Alcytam®, Torrent by Brazil Limited), chlortalidone (Higroton®, Novarts), rosuvastatin (Crestor®, AstraZeneca), and generic paroxetine medication (Medley).
Sample preparation
In this study, the extraction technique was adapted from Wille et al. (2010). Before extraction, the pH of each sample was adjusted to 7.0 using a hydrochloric acid solution (1M). Next, 1 L samples were filtered through Whatman® filter paper (GF/C particle retention 1.2 μm, diameter 47 mm; Merck KGaA, Darmstadt, Germany), and to prevent the loss of the compounds of interest, the filters were washed with 2 mL of methanol (CH3OH) (Sigma-Aldrich, St. Louis, USA). The methanol extract collected was then combined to the filtered sample. Subsequently, the SPE (solid phase extraction) procedure using spherical, hydrophobic polystyrene-divinylbenzene resin for SPE cartridges (Chromabond ® HR-X, 200 mg, 3 mL, Macherey-Nagel GmbH & Co. KG, Düren, Germany) was accomplished as described by Wille et al. (2010) and Ghoshdastidar et al. (2015). The cartridges were preconditioned with methanol (5 mL) and Milli-Q-water (5 mL) (Milli-Q®-Merck KGaA). They were loaded with 1 L of the filtered sample, and the cartridges were rinsed twice with 5 mL of Milli-Q-water. The cartridges were then dried under vacuum for 30 min. The elution was performed using 2 × 5 mL of methanol and 5 mL of acetone. Prior to the analysis, the concentrated eluate was evaporated to dryness under a nitrogen flow (at 50 °C), re-suspended in 1 mL with a solution of water/acetonitrile (C2H3N) (95:5, v/v), and then filtered through a 0.45 μm pore size membrane (Merck Millipore).
LC–MS/MS analysis
Based on the reported annual consumption, expected toxicity, and environmental persistence (CMED 2017), a total of 23 chemical compounds, namely, pharmaceutical and illicit drugs, were analysed using liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) (Table 1). LC-MS/MS methodology was described and validated by Shihomatzu (2015). The validation was performed using the parameters of selectivity, matrix effect, dynamic range, linearity, limit of detection (LOD), limit of quantification (LOQ), precision (% relative standard deviation), accuracy (% coefficient of variation), recovery, and robustness. An aliquot (10 μL) of each sample was analysed using an Agilent 1260 Infinity HPLC (Agilent™, Germany) combined with a hybrid triple quadrupole/LIT instrument (3200QTRAP®-linear ion trap) mass spectrometer (ABSciex, Ontario, Canada). The conditions used for the liquid chromatography (LC) separation were as follows: an injection volume of 10 μL of each sample was loaded in an Agilent Zorbax Eclipse XDB – C18 column (50 × 4.6 mm ID, 1.8 μm column at 25 °C). The eluent flow rate was 0.7 mL/min, and the mobile phase for positive mode analysis was 0.1% formic acid (Sigma-Aldrich; LC–MS grade) in solvent A (water – H2O) and solvent B (acetonitrile – C2H3N) (J.T. Baker, Philipsburg, NJ, USA). For negative mode analysis, the mobile phase was a 5 mM ammonium acetate buffer (Sigma-Aldrich) with a pH of 4.6 (solvent A) and acetonitrile (solvent B). For both modes of ionisation (negative and positive), a linear gradient of 0.7 mL/min was used, starting with a mixture of solvent A (95%) and solvent B (5%). The solvent A percentage was decreased linearly from 95 to 5% over the course of 5 min, and this condition was maintained for 1 min. Over the course of 2 min, the mixture was then returned to the initial conditions. Using electrospray ionisation (ESI: positive and negative modes) and multiple reaction monitoring (MRM mode), analytes were detected and quantified. This procedure was performed with the selection of a precursor ion and two ion products to quantify and qualify each compound. MRM parameters for the positive and negative modes for each chemical compound, LOD, and LOQ are shown in Table 1. A seawater matrix-matched external calibration curve was employed, as described by Shihomatzu (2015). LOD and LOQ values were determined, using spiked matrix samples, and obtained from seven measurements of the lowest detectable concentration of the calibration curves (with signal-to-noise ratio of at least 10), following the Brazilian Institute of Metrology, Quality, and Technology Procedures (INMETRO 2011). Both field and laboratory blanks were below LOD. Data analysis was performed with Analyst® 1.5.2 software (ABsciex). A concentration factor (1/1000) was used to obtain the measured concentration (ng/L) following LC–MS/MS quantification (Table 2).
Ecological risk assessment
The ecological risk assessment for aquatic organisms was performed calculating the risk quotient (RQ) for 3 different trophic levels (algae, crustacean, and fish) following the equation RQ = MEC/PNEC, in which MEC is the maximum measured environmental concentration and PNEC the predicted no effect concentration, both expressed in μg/L. PNEC values were obtained from base-set reliable ecotoxicity data available for the aquatic compartment regarding short-term [lethal concentration 50 (LC50) or median effective concentration (EC50)] and long-term [no observed effect concentration (NOEC)] toxicological endpoints. In the absence of NOEC, the lowest observed effect concentration (LOEC) or, in alternative, the 10% effective concentration (EC10) were used, when available. In the present study, an attempt was made to compile specifically data for coastal marine species. When this information was not available, data from freshwater species were used instead, since a reasonable correlation exists between the ecotoxicological responses of freshwater and saltwater biota, at least for the usual aquatic taxa (i.e. acute and chronic toxicity to algae, crustacean, and fish) (EMA 2006; Li et al. 2012; Thomaidi et al. 2015). In order to collect available aquatic ecotoxicity test endpoints, an extensive search was carried out in the Ecotoxicology Database (ECOTOX) from the United States Environmental Protection Agency (USEPA 2019), as well as in other literature sources using the PubMed database. When ecotoxicity laboratory experimentally derived data were not available, short [L(E)C50] and long toxicological endpoints [Chv, geometric mean of NOEC and LOEC, ChV=10^([log(NOEC × LOEC)]/2)] were estimated using the Ecological Structure Activity Relationships Program (ECOSAR, v 2.0) (USEPA 2017). The derived PNEC values for the acute and chronic toxicity data were thereafter calculated by dividing each toxicological endpoint by an assessment factor (AF). For saltwater environments, an AF of 10,000 and 100 should be considered in short- and long-term data sets. For further details, see the European Chemical Bureau (ECB 2003) and the European Chemicals Agency (ECHA 2008) guidelines. Finally, the risk (RQ = MEC/PNEC) was categorised into four levels: no (RQ < 0.01), low (0.01 ≤ RQ < 0.1), moderate (0.1 ≤ RQ < 1), and high ecological risk (RQ ≥ 1.0) to aquatic organisms (Hernando et al. 2006).
Results and discussion
In the vicinity of Guarujá sewage outfall, from the 23 compounds surveyed, 13 were below the LOD (e.g. clonazepam, propranolol, enalapril, citalopram, paroxetine, bromazepam, midazolam, clopidogrel, cyproterone, chlortalidone, rosuvastatin, loratadine, and sildenafil). The other 10 compounds were detected, at least once, according to the different therapeutic classes (Table 2). The detection of PPCPs in the Guarujá outfall mixing zone is a consequence of the significant production (Brazil is the ninth largest producer of medicines in the world) and high consumption of pharmaceuticals in Brazil (CMED 2017). Among the drugs detected in Guarujá, losartan was the second best-selling drug in Brazil in 2017, followed by acetaminophen (sixth), atenolol (twelfth), and diclofenac (fifteenth) (Cmed 2017). In addition, there are problems related to exaggerated drug consumption (self-medication is a common habit among the Brazilian population) (de Loyola Filho et al. 2004) and to the inappropriate disposal of expired and/or unusable drugs into environmental matrices (e.g. household sinks, toilets, and garbage) (WHO 2011). Consequently, these PPCPs and illicit drugs (in parental, metabolised or conjugated forms in human excreta) (Fabbri and Franzellitti 2015; Dafouz et al. 2018) are released indiscriminately into the receiving waters because most of the conventional WWTPs, such as Guarujá, are not efficient in removing these emerging pollutants (Petrie et al. 2015; Dos Santos et al. 2018). Removal of compounds with low octanol-water partition coefficient (i.e. log Kow values < 3.00) generally only occurs in secondary level treatment systems (Behera et al. 2011). This could explain the presence of these PPCPs and illicit drugs in the Guarujá Sea [e.g. carbamazepine (log Kow = 1.51), caffeine (log Kow = − 0.07), cocaine and benzoylecgonine (log Kow < 3.00), diclofenac (log Kow = 0.70), acetaminophen (log Kow = 0.46), and atenolol (log Kow = 0.16) (Behera et al. 2011; Benotti et al. 2012; Fontes et al. 2019).
During the summer season, holidays, and weekends, the population increases such as the consumption of pharmaceuticals and illicit drugs in the cities (Fontes et al. 2019). However, this situation appears to have no interference in the disposal of these compounds in Guarujá, since a higher frequency of occurrence and also the highest concentrations occurred during the low season. It is important to mention that the present study covered a relatively short time period, as the focus was a preliminary assessment occurrence of PPCPs and illicit drugs in Guarujá. Moreover, several factors can influence the occurrence and spatial distribution of these compounds in the marine environment, such as the rain regime, oceanographic conditions, and complex hydrodynamics of the marine environment in coastal areas (Vidal-Dorsch et al. 2012; Arpin-Pont et al. 2014). It is not possible to test these hypotheses with the hereby data. Therefore, monitoring should continue in order to evidence, at a larger time scale, the possible effects of the discharge of Guarujá sewage.
Antiepileptics
In this study, a frequency of occurrence of carbamazepine was observed in only 12.5% of the samples (1/8), being quantified only during the high season at sampling point P2-HS-S (Table 2). Carbamazepine is an anticonvulsant used to treat schizophrenia, epilepsy, and neuralgia (Almeida and Cruciol 2013). It is an anthropogenic indicator and allows us to confirm the presence of sewage (e.g. Guarujá mixing zone) (Donner et al. 2013). Carbamazepine is persistent and resistant to degradation and adsorption, so even secondary and tertiary treatment levels WWTPs (inexistent in Guarujá) show a low rate of carbamazepine depuration (ranging from 5 to 30%, respectively) (Lin et al. 2009; Sui et al. 2010). However, the concentrations found in Guarujá (< 0.01 × 10-3–0.1 × 10-3 μg/L) were much lower than the levels detected in oceanic sewage disposal in the Baltic Sea, Germany (0.026 μg/L) (Nodler et al. 2010), and in San Francisco Bay, USA (0.004 μg/L) (Klosterhaus et al. 2013). Therefore, carbamazepine presented no ecological risk (RQ) regarding the acute and chronic exposures for all trophic levels tested (Table 3). Indeed, reported concentrations of carbamazepine capable of causing toxic effects in different species of marine invertebrates are higher. For example, the mollusks Venerupis decussata, Venerupis philippinarum, and Ruditapes philippinarum, exposed to concentrations between 0.03 and 9.0 μg/L, suffered a dose-related reduction in health status due to the induction of an oxidative stress scenario (Almeida et al. 2014, 2015). The crab Carcinus maenas, after a 28-day exposure to increasing concentrations of carbamazepine (0.1, 1.0, 10.0, and 50.0 μg/L), showed alteration in the stability of the lysosomal membrane (LMS) and activation of glutathione S-transferase (GST) (Aguirre-Martínez et al. 2013a). The polychaeta Hediste diversicolor (Maranho et al. 2015a) and microalgae Isochrysis galbana and Tetraselmis chuii (Maranho et al. 2015b) exposed for 14 days to sediments containing a concentration of 50.0 μg/kg carbamazepine, increased the total lipid content (TLP) and the activity of mitochondrial electron transport (MET) of the polychaeta, in addition to inhibiting the growth of both microalgae.
Stimulants
Caffeine was detected during the high season only at sampling points P1-HS-B and P2-HS-S. During the low season, it was present at all sampling points (frequency of occurrence in 75% of the samples: 6/8) (Table 2). Caffeine is another critical marker of domestic sewage, as its presence is related to the disposal of food and drugs used exclusively by humans. After consumption, caffeine is rapidly metabolised by the liver and converted into one or more metabolites (e.g. paraxanthine) (Machado et al. 2016). Paraxanthine, is the main metabolite in humans, accounting for 80% of the total caffeine excretion in urine and faeces (Almeida and Cruciol 2013; Machado et al. 2016). When submitted to a secondary treatment WWTP, caffeine is rapidly biodegraded through biochemical reactions, with a removal rate of 72 to 98% (Lin et al. 2009; Bueno et al. 2011). Because the Guarujá WWTP is only a primary level system, caffeine was detected at sea (up to 0.141 μg/L) in higher concentrations than those found in discharges to the Baltic Sea, Germany (0.058 μg/L) (Nodler et al. 2010), in San Francisco Bay, USA (0.040 μg/L) (Klosterhaus et al. 2013), and in the Gulf of Saronikos, Greece (0.078 μg/L) (a region that concentrates a population 10 times larger than Guarujá) (Alygizakis et al. 2016). In Guarujá, the RQ for acute (crustaceans and fish) and chronic (algae) tests was between 0.02 and 0.03 (Table 3), signalling a low risk of caffeine for these species. Caffeine usually causes harmful effects in different marine species in slightly higher concentrations. For example, Del Rey et al. (2011) showed that caffeine concentrations of 0.2 μg/L may have an effect in the gill tissue of the mussel Mytilus californianus at a molecular level (positive regulation of Hsp70). Studies have also shown that the crab Carcinus maenas (Aguirre-Martínez et al. 2013a) and mollusks Ruditapes philippinarum (Aguirre-Martínez et al. 2013b) and Mytilus galloprovincialis (Capolupo et al. 2016) have suffered destabilisation of the LMS after exposure to caffeine concentrations of 50.0 μg/L (in the case of Carcinus maenas and Ruditapes philippinarum) and 0.5 μg/L (in the case of Mytilus galloprovincialis). Regarding Ruditapes philippinarum, after the exposure of this mollusk to caffeine for 28 days (0.5, 3.0, and 18.0 μg/L), it was observed that as the concentrations increased, the mollusk lost the ability to prevent lipid peroxidation of cells and also to combat oxidative stress (Cruz et al. 2016).
Cocaine and benzoylecgonine were detected during the high season only at surface sampling points P1-HS-S and P2-HS-S (Table 2). During the low season, both substances were detected at all sampling points at higher concentrations when compared to the high season (both compounds were present in 75% of the samples: 6/8) (Table 2). Some environmental and public health concerns exist regarding cocaine and its metabolite in Brazil (Brazil 2009). It is well-known that Brazil is the main transit route for the cocaine produced in South America, whose final destination is Europe and Asia (Unodc 2016). Five million Brazilians aged 18 and over have used cocaine at least once during their lifetime (Laranjeira et al. 2012). It is known that even WWTP with secondary treatment levels only partially remove cocaine (40–93%) and benzoylecgonine (12–92%) (Zuccato et al. 2008; Domènech et al. 2009). Since the Guarujá WWTP does not serve this purpose, both compounds were detected in the present study raising significant concerns due to their potential effects on biota (Baker and Kasprzyk-Hordern 2013). The concentrations of cocaine (0.0003–0.0006 μg/L) and benzoylecgonine (0.002–0.0003 μg/L) detected in Guarujá are however lower than other studies worldwide. In Santos Bay, Brazil, concentrations of cocaine and benzoylecgonine respectively of 0.537 μg/L and 0.038 μg/L were reported (Pereira et al. 2016; Fontes et al. 2019). In San Francisco Bay, USA, which receives municipal sewage discharge, benzoylecgonine (0.007 μg/L) was also detected (Klosterhaus et al. 2013). Cocaine has a strong pharmacological effect, and its presence in a body of water can produce unpredictable interactions (Zuccato et al. 2008), such as that with chlorine used in sewage treatment. In this sense, the chlorination process performed in a WWTP (e.g. Guarujá) (Ortiz et al. 2016) may interact with cocaine and benzoylecgonine and might generate unwanted transformation products (TPs) (e.g. cocaine – TP:C18H24NO4; and benzoylecgonine – TP:C16H18NO5). The toxicity of these TPs is still unknown (Bijlsma et al. 2013). The RQ for the acute and chronic exposures to cocaine or benzoylecgonine were < 0.01 for all three trophic levels tested (Table 3). These data suggest no environmental risk of these compounds for local aquatic species. However, some studies have already shown that environmentally realistic concentrations of cocaine are capable of producing changes in shellfish metabolism (Dreissena polymorpha) (Binelli et al. 2013), behavioural changes in crustaceans (Orconectes rusticus) (Imeh-Nathaniel et al. 2017), and bioaccumulation in the tissue of the eel (Anguilla anguilla) (Capaldo et al. 2019).
Analgesics and anti-inflammatory drugs
Diclofenac was detected during the high season at sampling points P1-HS-S and P1-HS-B (Table 2). During the low season, it was detected at all sampling points at higher concentrations when compared to the high season (frequency of occurrence in 75% of the samples: 6/8) (Table 2). Diclofenac is an analgesic and anti-inflammatory drug (Almeida and Cruciol 2013). Due to its high worldwide consumption and sale (usually without a prescription), it has become one of the most commonly detected PPCPs in aquatic ecosystems and therefore has been rated as high management priority (Sotelo et al. 2014). When submitted to a secondary treatment level WWTP, the percentage of diclofenac removal rate is in the range of 60% (Jelic et al. 2011). However, in Guarujá, the Enseada mixing zone recorded higher concentrations (0.086 μg/L) than those found in other sewage outfalls, namely, in Spain: Gran Canaria Island (0.048 μg/L) and Jinámar (0.028 μg/L) (Afonso-Olivares et al. 2013), Greece: Gulf of Saronikos (0.016 μg/L) (Alygizakis et al. 2016), and Brazil: Santos (0.019 μg/L) (Pereira et al. 2016). Diclofenac is a biologically active compound with a low biodegradation rate and has a rapid photo transformation into new by-products after disposal in the aquatic environment, which can cause deleterious effects in biota at different trophic levels (Lee et al. 2011; Toufexi et al. 2016; Bonnefille et al. 2018). The RQ of diclofenac in the sea of Guarujá was 0.11 for fish acutely exposed (Table 3), indicating a moderate environmental risk. However, the recorded low concentrations are unlikely to cause damage to aquatic biota. For example, diclofenac concentrations of 1 μg/L strongly affected the development of the larvae of this mollusk (after 48-h exposure) (Fabbri et al. 2014) and caused an increase in the DNA breakages of this species (after 1-h exposure) (Mezzelani et al. 2016). Higher concentrations of diclofenac have also caused cytotoxic and genotoxic effects (after exposure to a concentration of 25 μg/L for 14 days) (Toufexi et al. 2016), molecular effects on specific targets (inhibition of prostaglandin E2 synthesis) after exposure to a concentration of 100 μg/L for 3 days (Courant et al. 2017), and potential effects on osmoregulation and reproduction of the mollusk Mytilus galloprovincialis (after exposure to a concentration of 100 μg/L for 7 days) (Bonnefille et al. 2018).
Acetaminophen was not detected at any sampling point during the high season, whereas during the low season, it was detected only at sampling points P2-LS-S and P2-LS-B (frequency of occurrence in 25% of the samples: 2/8) (Table 2). Acetaminophen is a drug with antipyretic and analgesic action (Almeida and Cruciol 2013). Because of its high worldwide consumption (generally without medical prescription), environmental persistence, and significant toxicity to aquatic species, acetaminophen is included in the class II priority pollutants list, requiring future monitoring and the development of specific ecotoxicological studies to address their toxic effects, and therefore must be given priority management (Antunes et al. 2013). When submitted to a secondary treatment level WWTP, the percentage of acetaminophen removal rate is around 90% (Sun et al. 2014). Guarujá concentrations (0.0012–0.0014 μg/L) are well below those detected in the sewage outfall of the island of Gran Canaria, Spain (0.297 μg/L) (Afonso-Olivares et al. 2013), in the Gulf of Saronikos, Greece (0.040 μg/L) (Alygizakis et al. 2016), and in Santos Bay, Brazil (0.035 μg/L) (Pereira et al. 2016). The RQ for acetaminophen was in general < 0.01 (Table 3), with exception of fish chronically exposed showing a low environmental risk. Studies have already demonstrated the ability of environmentally realistic concentrations of acetaminophen (e.g. Guarujá) to bioaccumulate in blue mussels (Mytilus edulis) (Wille et al. 2011) and cause oxidative stress in three species of bivalves, namely, Corbicula fluminea (Brandão et al. 2011), Venerupis decussata, and Venerupis philippinarum (Antunes et al. 2013), and in two species of fish, namely Oncorhynchus mykiss (Ramos et al. 2014) and Anguilla anguilla (Nunes et al. 2015).
Orphenadrine was not quantified at any sampling point during the high season. During the low season, it was detected at all sampling points (frequency of occurrence in 75% of the samples: 4/8) (Table 2). Orphenadrine is a psychoactive drug used as a muscle relaxant anticholinergic drug with low antihistamine activity and is also prescribed to treat Parkinson’s disease (Almeida and Cruciol 2013). Taking into consideration recent reviews (Fabbri and Franzellitti 2015; Quadra et al. 2016; Godoy and Kummrow 2017; Starling et al. 2018), this study seems be the first to report the occurrence of this PPCP in a Latin American submarine sewage outfall. Orphenadrine was reported in Psyttalia Island, Athens, Greece, a country where the drug was widely consumed in 2018 (1.7 mg/day/1000 people) (Diamanti et al. 2019). It was also reported in the Tiber River, Perugia, Italy, but concentrations were not detailed (Milione et al. 2016). The RQ of orphenadrine in Guarujá was < 0.01 for all exposures times and trophic levels (Table 3), thus indicating no risk for aquatic species. Studies have shown that concentrations of 0.014 μg/L orphenadrine (higher than those detected in Guarujá, up to 0.003 μg/L) are capable of bioconcentrating in the blood plasma of rainbow trout (Oncorhynchus mykiss) (Fick et al. 2010). A reduced growth of Lemna minor (duckweed) after exposure to orphenadrine “non-relevant” environmental concentrations of 12.0 mg/L was also found (Kaza et al. 2007).
Antihypertensives
Atenolol was not quantified at any sampling point during the high season. During the low season, it was detected at all sampling points (frequency of occurrence in 50% of the samples: 4/8) (Table 2). The β-blocker atenolol has several therapeutic indications, but it is particularly indicated as an antiarrhythmic and antihypertensive drug in cardiac protection after myocardial infarction (Almeida and Cruciol 2013). In secondary treatment level WWTPs, the atenolol removal rate was reported to be approximately 40% (Papageorgiou et al. 2016). Atenolol is one of the most frequently detected antihypertensives in fresh surface waters in the world (Godoy et al. 2015a, b). In Brazil, there are only a few reports of its presence in fresh surface water (e.g. Billings Reservoir, São Paulo: 0.016 μg/L and São Domingos Stream, Rio de Janeiro: 0.821 μg/L) (Quadra et al. 2016). In the Guarujá sewage outfall, concentrations were very low (up to 0.0003 μg/L). For other similar studies conducted in marine waters, for example, sewage outfalls in Gran Canaria, Spain (Afonso-Olivares et al. 2013), in the Adriatic Sea, Italy (Loos et al. 2013), in Santos, Brazil (Pereira et al. 2016), and on the west coast of the Mediterranean Sea (Brumovský et al. 2017), atenolol was below the detection limit in all samples. The RQ of atenolol in Guarujá was < 0.01 for all trophic levels and both exposure tests (Table 3), thus indicating absence of risk for the aquatic species. However, it has shown that atenolol has an effect on prokaryotic and eukaryotic cells at environmentally relevant exposure levels (ng/L to μg/L) (Pomati et al. 2007). Other studies have also demonstrated that atenolol causes toxic effects in “non-relevant” environmental concentrations. In this context, atenolol concentrations of 2.5, 10.0, and 33.4 mg/L caused larval mortality (LC50-96h) of the fish Danio rerio (Küster et al. 2007) and growth inhibition in the fish larvae of Pimephales promelas (after a 28-day exposure to atenolol) (Winter et al. 2008) and of the crustacean Ceriodaphnia dubia (EC50-48 h) (Fraysse and Garric 2005), respectively. However, Massarsky et al. (2011) argue that new (long-term) experiments are needed with aquatic species (e.g. fish and crustaceans), specifically to test the hypothesis that atenolol is an endocrine disruptor.
Losartan was not quantified at any sampling point during the high season. During the low season, it was quantified only at sampling points P1-LS-S and P1-LS-B (frequency of occurrence in 25% of the samples: 2/8) (Table 2). Valsartan was detected, during the high season, only at sampling points P2-HS-S and P2-HS-B. During the low season, it was detected at all sampling points (frequency of occurrence in 75% of the samples: 6/8) (Table 2). Losartan and valsartan are prescribed drugs to treat hypertension and are generally consumed by the elderly population (Almeida and Cruciol 2013). Guarujá has an estimated population of 316,000 (about 27,000 elderly) (Ibge 2018). When submitted to a WWTP (activated sludge system), the percentage of losartan and valsartan removed is as high as 90% (Oosterhuis et al. 2013). Losartan concentrations (up to 0.0034 μg/L) were lower than those found in the Mediterranean Sea, Spain (0.004 μg/L) (Gros et al. 2012), and in Santos Bay, Brazil (0.032 μg/L) (Pereira et al. 2016). RQ < 0.01 was recorded for losartan (Table 3). Valsartan concentrations detected in Guarujá (up to 0.0143 μg/L) were higher than those found in the Lesser Sea lagoon, Spain (0.004 μg/L) (Moreno-González et al. 2015), and in the Saronikos Gulf, Greece (0.003 μg/L) (Alygizakis et al. 2016) but lower than those reported in Santos Bay, Brazil (0.075 μg/L) (Pereira et al. 2016). For valsartan, the RQ was < 0.01 for all trophic levels and exposures times (Table 3). These results indicate that losartan and valsartan does not represent any risk for the aquatic species in the hereby reported seawater concentrations. However, these antihypertensives deserve attention, as their consumption has increased in many parts of the world, and because studies on the toxicity of these substances are still poorly documented (Godoy et al. 2015a, b; Pereira et al. 2016; Desbiolles et al. 2018). Bayer et al. (2014) did not observe inhibition of Desmodesmus subspicatus algae growth after 72-h exposure at 120.0 mg/L of valsartan; Yamamoto et al. (2014) found embryo/larval alteration of the sea urchin Lytechinus variegatus exposed to concentrations of valsartan 25.0 mg/L; and Cortez et al. (2018) detected cytotoxic effects on gills and haemocytes of Pernaperna mussel, exposed at environmental realistic concentrations of up to 0.3 μg/L of losartan. However, the previous tested concentrations of both antihypertensives were much higher than the concentrations found in Guarujá.
Conclusion
The detection of emerging pollutants such as carbamazepine, caffeine, cocaine, benzoylecgonine, diclofenac, acetaminophen, orphenadrine, atenolol, losartan, and valsartan in the Guarujá outfall reinforces the worldwide concern about the disposal of pharmaceuticals and illicit drugs via primary-level treatment sewage outlets in the marine coastal areas. More rigorous standards for oceanic sewage disposal in Brazil need to be imposed. There is also evidence for the need to resize sewage treatment along the Brazilian coastal zone (total of 20 outfalls), including a level of treatment capable of removing, at least partially, PPCPs and illicit drugs. In order to understand what happens to these PPCPs after ocean disposal, a long-term monitoring programme would be necessary because Guarujá Island has strong hydrodynamic conditions, and therefore, these contaminants are likely to be dispersed along the coastal zone. Although most of the screened drugs do not present environmental risks in the hereby reported concentrations, the present study reinforces the need for further ecotoxicological studies (especially with tropical marine organisms) to assess the long-term toxicity of these bioactive compounds.
Data availability
Not applicable.
References
Abessa DMS, Rachid BRF, Moser GAO, Oliveira AJFC (2012) Efeitos ambientais da disposição oceânica de esgotos por meio de emissários submarinos: uma revisão. Mundo da Saúde 36(4):643–661
Afonso-Olivares C, Torres-Padrón M, Sosa-Ferrera Z, Santana-Rodríguez J (2013) Assessment of the presence of pharmaceutical compounds in seawater samples from coastal area of Gran Canaria Island (Spain). Antibiotics 2(2):274–287. https://doi.org/10.3390/antibiotics2020274
Aguirre-Martínez GV, Del Valls TA, Martín-Díaz ML (2013a) Early responses measured in the brachyuran crab Carcinus maenas exposed to carbamazepine and novobiocin: Application of a 2-tier approach. Ecotoxicol Environ Saf 97:47–58. https://doi.org/10.1016/j.ecoenv.2013.07.002
Aguirre-Martínez GV, Buratti S, Fabbri E, DelValls AT, Martín-Díaz ML (2013b) Using lysosomal membrane stability of haemocytes in Ruditapes philippinarum as a biomarker of cellular stress to assess contamination by caffeine, ibuprofen, carbamazepine and novobiocin. J Environ Sci 25(7):1408–1418. https://doi.org/10.1016/s1001-0742(12)60207-1
Almeida JRC, Cruciol JM (2013) Farmacologia e Terapêutica Clínica, 1st edn. Atheneu, São Paulo, p 712
Almeida Â, Calisto V, Esteves VI, Schneider RJ, Soares AMVM, Figueira E, Freitas R (2014) Presence of the pharmaceutical drug carbamazepine in coastal systems: Effects on bivalves. Aquat Toxicol 156:74–87. https://doi.org/10.1016/j.aquatox.2014.08.002
Almeida Â, Freitas R, Calisto V, Esteves VI, Schneider RJ, Soares AMVM, Figueira E (2015) Chronic toxicity of the antiepileptic carbamazepine on the clam Ruditapes philippinarum. Comp Biochem Physiol Part C Toxicol Pharmacol 172-173:26–35. https://doi.org/10.1016/j.cbpc.2015.04.004
Alygizakis NA, Gago-Ferrero P, Borova VL, Pavlidou A, Hatzianestis I, Thomaidis NS (2016) Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci Total Environ 541:1097–1105. https://doi.org/10.1016/j.scitotenv.2015.09.145
Antunes SC, Freitas R, Figueira E, Gonçalves F, Nunes B (2013) Biochemical effects of acetaminophen in aquatic species: edible clams Venerupis decussata and Venerupis philippinarum. Environ Sci Pollut Res 20(9):6658–6666. https://doi.org/10.1007/s11356-013-1784-9
Arpin-Pont L, Bueno MJM, Gomez E, Fenet H (2014) Occurrence of PPCPs in the marine environment: a review. Environ Sci Pollut Res 23(6):4978–4991. https://doi.org/10.1007/s11356-014-3617-x
Baker DR, Kasprzyk-Hordern B (2013) Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments. Sci Total Environ 454-455:442–456. https://doi.org/10.1016/j.scitotenv.2013.03.043
Bayer A, Asner R, Schüssler W, Kopf W, Weiß K, Sengl M, Letzel M (2014) Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ Sci Pollut Res 21(18):10830–10839. https://doi.org/10.1007/s11356-014-3060-z
Behera SK, Kim HW, Oh J-E, Park H-S (2011) Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci Total Environ 409(20):4351–4360. https://doi.org/10.1016/j.scitotenv.2011.07.015
Benotti MJ, Song R, Wilson D, Snyder SA (2012) Removal of pharmaceuticals and endocrine disrupting compounds through pilot- and full-scale riverbank filtration. Water Sci Technol Water Supply 12(1):11–23. https://doi.org/10.2166/ws.2011.068
Beretta M, Britto V, Tavares TM, Silva SMT, Pletsch AL (2014) Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J Soils Sediments 14(7):1278–1286. https://doi.org/10.1007/s11368-014-0884-6
Bijlsma L, Boix C, Niessen WMA, Ibáñez M, Sancho JV, Hernández F (2013) Investigation of degradation products of cocaine and benzoylecgonine in the aquatic environment. Sci Total Environ 443:200–208. https://doi.org/10.1016/j.scitotenv.2012.11.006
Binelli A, Marisa I, Fedorova M, Hoffmann R, Riva C (2013) First evidence of protein profile alteration due to the main cocaine metabolite (benzoylecgonine) in a freshwater biological model. Aquat Toxicol 140-141:268–278. https://doi.org/10.1016/j.aquatox.2013.06.013
Blaise C, Gagné F, Eullaffroy P, Férard JF (2006) Ecotoxicity of selected pharmaceuticals of urban origin discharged to the Saint-Lawrence river (Québec, Canada): a review. Ecotoxicity of selected pharmaceuticals of urban origin discharged to the Saint-Lawrence river (Québec, Canada): a review. Braz J Aquat Sci Technol 10(2):29–51
Bonnefille B, Gomez E, Alali M, Rosain D, Fenet H, Courant F (2018) Metabolomics assessment of the effects of diclofenac exposure on Mytilus galloprovincialis: Potential effects on osmoregulation and reproduction. Sci Total Environ 613-614:611–618. https://doi.org/10.1016/j.scitotenv.2017.09.146
Brain RA, Johnson DJ, Richards SM, Hanson ML, Sanderson H, Lam MW, Solomon KR (2004) Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic macrophytes Lemna gibba and Myriophyllum sibiricum. Aquat Toxicol 70(1):23–40. https://doi.org/10.1016/j.aquatox.2004.06.011
Brandão FP, Pereira JL, Gonçalves F, Nunes B (2011) The impact of paracetamol on selected biomarkers of the mollusc species Corbicula fluminea. Environ Toxicol 29(1):74–83. https://doi.org/10.1002/tox.20774
Brandhof EJ, Montforts M (2010) Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol Environ Saf 73(8):1862–1866. https://doi.org/10.1016/j.ecoenv.2010.08.031
Brazil – Senad: Secretaria Nacional de Políticas Sobre Drogas (2009) Relatório Brasileiro sobre Drogas. IME/USP, Brasília, p 364 ISBN 978-85-60662-29-6
Brumovský M, Bečanová J, Kohoutek J, Borghini M, Nizzetto L (2017) Contaminants of emerging concern in the open seawaters of the Western Mediterranean. Environ Pollut 229:976–983. https://doi.org/10.1016/j.envpol.2017.07.082
Bueno MJM, Uclés S, Hernando MD, Fernadéz-Alba AR (2011) Development of a solvent-free method for the simultaneous identification/quantification of drugs of abuse and their metabolites in environmental water by LC–MS/MS. Talanta 85(1):157–166. https://doi.org/10.1016/j.talanta.2011.03.051
Calleja MC, Persoone G, Geladi P (1994) Comparative acute toxicity of the first 50 multicentre evaluation of in vitro cytotoxicity chemicals to aquatic non-vertebrates. Arch Environ Contam Toxicol 26(1):69–78. https://doi.org/10.1007/bf00212796
Capaldo A, Gay F, Laforgia V (2019) Changes in the gills of the European eel (Anguilla anguilla) after chronic exposure to environmental cocaine concentration. Ecotoxicol Environ Saf 169:112–119. https://doi.org/10.1016/j.ecoenv.2018.11.010
Capolupo M, Valbonesi P, Kiwan A, Buratti S, Franzellitti S, Fabbri E (2016) Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus galloprovincialis. Sci Total Environ 563-564:538–548. https://doi.org/10.1016/j.scitotenv.2016.04.125
Cetesb - Companhia Estadual de Tecnologia e Saneamento ambiental (2017) Relatório de qualidade das águas costeiras no estado de São Paulo 2016. Série Relatórios/Cetesb, p 178 ISSN 0103-4103
Claessens M, Vanhaecke L, Wille K, Janssen CR (2013) Emerging contaminants in Belgian marine waters: single toxicant and mixture risks of pharmaceuticals. Mar Pollut Bull 71(1-2):41–50. https://doi.org/10.1016/j.marpolbul.2013.03.039
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142(3):185–194. https://doi.org/10.1016/s0378-4274(03)00068-7
CMED - Câmara de Regulação do Mercado de Medicamentos (2017) Anuário Estatístico do Mercado Farmacêutico. ANVISA, Brasília http://portal.anvisa.gov.br/. (Accessed Jun 9 2019)
Comtois-Marotte S, Chappuis T, Vo Duy S, Gilbert N, Lajeunesse A, Taktek S, Sauvé S (2017) Analysis of emerging contaminants in water and solid samples using high resolution mass spectrometry with a Q Exactive orbital ion trap and estrogenic activity with YES-assay. Chemosphere 166:400–411. https://doi.org/10.1016/j.chemosphere.2016.09.077
Cortez FS, Souza L d S, Guimarães LL, Almeida JE, Pusceddu FH, Maranho LA, Pereira CDS (2018) Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci Total Environ 637-638:1363–1371. https://doi.org/10.1016/j.scitotenv.2018.05.069
Courant F, Arpin-Pont L, Bonnefille B, Vacher S, Picot-Groz M, Gomez E, Fenet H (2017) Exposure of marine mussels to diclofenac: modulation of prostaglandin biosynthesis. Environ Sci Pollut Res 25(7):6087–6094. https://doi.org/10.1007/s11356-017-9228-6
Cruz D, Almeida Â, Calisto V, Esteves VI, Schneider RJ, Wrona FJ, Freitas R (2016) Caffeine impacts in the clam Ruditapes philippinarum: alterations on energy reserves, metabolic activity and oxidative stress biomarkers. Chemosphere 160:95–103. https://doi.org/10.1016/j.chemosphere.2016.06.068
Dafouz R, Cáceres N, Rodríguez-Gil JL, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2018) Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci Total Environ 615:632–642. https://doi.org/10.1016/j.scitotenv.2017.09.155
de Loyola Filho AI, Lima-Costa MF, Uchôa E (2004) Bambuí Project: a qualitative approach to self-medication. Cad Saúde Pública 20(6):1661–1669. https://doi.org/10.1590/s0102-311x2004000600025
Del Rey ZR, Granek EF, Buckley BA (2011) Expression of HSP70 in Mytilus californianus following exposure to caffeine. Ecotoxicology 20(4):855–861. https://doi.org/10.1007/s10646-011-0649-6
DeLorenzo ME, Fleming J (2007) Individual and mixture effects of selected pharmaceuticals and personal care products on the marine Phytoplankton species Dunaliella tertiolecta. Arch Environ Contam Toxicol 54(2):203–210. https://doi.org/10.1007/s00244-007-9032-2
Desbiolles F, Malleret L, Tiliacos C, Wong-Wah-Chung P, Laffont-Schwob I (2018) Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci Total Environ 639:1334–1348. https://doi.org/10.1016/j.scitotenv.2018.04.351
Diamanti K, Aalizadeh R, Alygizakis N, Galani A, Mardal M, Thomaidis NS (2019) Wide-scope target and suspect screening methodologies to investigate the occurrence of new psychoactive substances in influent wastewater from Athens. Sci Total Environ 685:1058–1065. https://doi.org/10.1016/j.scitotenv.2019.06.173
Domènech X, Peral J, Muñoz I (2009) Predicted environmental concentrations of cocaine and benzoylecgonine in a model environmental system. Water Res 43(20):5236–5242. https://doi.org/10.1016/j.watres.2009.08.033
Donner E, Kosjek T, Qualmann S, Kusk KO, Heath E, Revitt DM, Andersen HR (2013) Ecotoxicity of carbamazepine and its UV photolysis transformation products. Sci Total Environ 443:870–876. https://doi.org/10.1016/j.scitotenv.2012.11.059
Dos Santos DM, Buruaem L, Gonçalves RM, Williams M, Abessa DMS, Kookana R, de Marchi MRR (2018) Multiresidue determination and predicted risk assessment of contaminants of emerging concern in marine sediments from the vicinities of submarine sewage outfalls. Mar Pollut Bull 129(1):299–307. https://doi.org/10.1016/j.marpolbul.2018.02.048
ECB (2003) Technical guidance document on risk assessment for existing substances. Part II:108–110
ECHA (2008) Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment, pp 7–29
EMA - European Medicines Agency, Committee for Medicinal Products for Human use (CHMP) (2006) Guideline on the environmental risk assessment of medicinal products for guman use. Doc. Ref.: EMEA/CHMP/SWP/4447/00 corr 1, London, UK
Fabbri E, Franzellitti S (2015) Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environ Toxicol Chem 35(4):799–812. https://doi.org/10.1002/etc.3131
Fabbri R, Montagna M, Balbi T, Raffo E, Palumbo F, Canesi L (2014) Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in Mytilus galloprovincialis. Mar Environ Res 99:1–8. https://doi.org/10.1016/j.marenvres.2014.05.007
FDA – U.S. Food and Drug Administration (2002) Center for drug evaluation and research. Approach Package for: Application number 20-386/S-019 and 029. Environment Assessment/ Fonsi, p 8
Ferrari B, Paxéus N, Giudice RL, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol Environ Saf 55(3):359–370. https://doi.org/10.1016/s0147-6513(02)00082-9
Fick J, Lindberg RH, Parkkonen J, Arvidsson B, Tysklind M, Larsson DGJ (2010) Therapeutic levels of levonorgestrel detected in blood plasma of fish: results from screening rainbow trout exposed to treated sewage effluents. Environ Sci Technol 44(7):2661–2666. https://doi.org/10.1021/es903440m
Fontes MK, Campos BG, Cortez FS, Pusceddu FH, Moreno BB, Maranho LA, Lebre DT, Guimarães LL, Pereira CDS (2019) Seasonal monitoring of cocaine and benzoylecgonine in a subtropical coastal zone (Santos Bay, Brazil). Mar Pollut Bull 149:110545. https://doi.org/10.1016/j.marpolbul.2019.110545
Fraysse B, Garric J (2005) Prediction and experimental validation of acute toxicity of β-blockers in Ceriodaphinia dubia. Environ Toxicol Chem 24(10):2470–2476. https://doi.org/10.1897/04-541r.1
Galus M, Kirischian N, Higgins S, Purdy J, Chow J, Rangaranjan S, Wilson JY (2013) Chronic, low concentration exposure to pharmaceuticals impacts multiple organ systems in zebrafish. Aquat Toxicol 132-133:200–211. https://doi.org/10.1016/j.aquatox.2012.12.021
Ghoshdastidar AJ, Fox S, Tong AZ (2015) The presence of the top prescribed pharmaceuticals in treated sewage effluents and receiving waters in Southwest Nova Scotia, Canada. Environ Sci Pollut Res 22(1):689–700. https://doi.org/10.1007/s11356-014-3400-z
Godoy AA, Kummrow F (2017) What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit Rev Environ Sci Technol 47(16):1453–1496. https://doi.org/10.1080/10643389.2017.1370991
Godoy AA, Kummrow F, Pamplin PAZ (2015a) Ecotoxicological evaluation of propranolol hydrochloride and losartan potassium to Lemna minor L. (1753) individually and in binary mixtures. Ecotoxicology 24(5):1112–1123. https://doi.org/10.1007/s10646-015-1455-3
Godoy AA, Kummrow F, Pamplin PAZ (2015b) Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment - a review. Chemosphere 138:281–291. https://doi.org/10.1016/j.chemosphere.2015.06.024
Gros M, Rodríguez-Mozaz S, Barceló D (2012) Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A 1248:104–121. https://doi.org/10.1016/j.chroma.2012.05.084
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69(2):334–342. https://doi.org/10.1016/j.talanta.2005.09.037
Ibge – Instituto brasileiro de Geografia e Estatística (2018) Estimativa da população brasileira. Rio de Janeiro. Brazilhtftp://ftp.ibge.gov.br/Estimativas_de_Populacao/Estimativas_2018/Estimativadou_2018_20181019.pdf. Accessed 24 Oct 2020
Imeh-Nathaniel A, Rincon N, Orfanakos VB, Brechtel L, Wormack L, Richardson E, Nathaniel TI (2017) Effects of chronic cocaine, morphine and methamphetamine on the mobility, immobility and stereotyped behaviors in crayfish. Behav Brain Res 332:120–125. https://doi.org/10.1016/j.bbr.2017.05.069
INMETRO (2011) Instituto Nacional de Metrologia, Normalização e Qualidade Industrial. Orientação sobre validação de métodos de ensaios químicos, Rio de Janeiro DOQ-CGCRE-008. <http://www.inmetro.gov.br/Sidoq/Arquivos/CGCRE/DOQ/DOQ-CGCRE-8_04.pdf>
Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barcelo D (2011) Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res 45(3):1165–1176. https://doi.org/10.1016/j.watres.2010.11.010
Kaza M, Nałęcz-Jawecki G, Sawicki J (2007) The toxicity of selected pharmacelticals to the aquatic plant Lemna minor. Fresenius Environ Bull 16(5):524–531
Kim Y, Choi K, Jung J, Park S, Kim P-G, Park J (2007) Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ Int 33(3):370–375. https://doi.org/10.1016/j.envint.2006.11.017
Kim J-W, Ishibashi H, Yamauchi R, Ichikawa N, Takao Y, Hirano M, Arizono K (2009) Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J Toxicol Sci 34(2):227–232. https://doi.org/10.2131/jts.34.227
Klosterhaus SL, Grace R, Hamilton MC, Yee D (2013) Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ Int 54:92–99. https://doi.org/10.1016/j.envint.2013.01.009
Küster A, Alder AC, Escher B, Duis K, Fenner K, Garric J, Knacker T (2007) Environmental risk assessment of human pharmaceuticals in the European Union - a case study with the β-blocker atenolol. Integr Environ Assess Manag 1. https://doi.org/10.1897/ieam_2009-050.1
Laranjeira R, Madruga CS, Pinsky I, Caetano R, Ribeiro M, Mitsuhiro S (2012) II Levantamento Nacional de Álcool e Drogas - Consumo de Álcool no Brasil: Tendências entre 2006/2012. INPAD, Brazil, São Paulo http://inpad.org.br (Accessed 11 Jun 2019)
Lee J, Ji K, Lim Kho Y, Kim P, Choi K (2011) Chronic exposure to diclofenac on two freshwater cladocerans and Japanese medaka. Ecotoxicol Environ Saf 74(5):1216–1225. https://doi.org/10.1016/j.ecoenv.2011.03.014
Li Y, Zhang X, Li W, Lu X, Liu B, Wang J (2012) The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ Monit Assess 185(3):2211–2220. https://doi.org/10.1007/s10661-012-2702-1
Lin AY-C, Yu T-H, Lateef SK (2009) Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. J Hazard Mater 167(1-3):1163–1169. https://doi.org/10.1016/j.jhazmat.2009.01.108
Loos R, Tavazzi S, Paracchini B, Canuti E, Weissteiner C (2013) Analysis of polar organic contaminants in surface water of the northern Adriatic Sea by solid-phase extraction followed by ultrahigh-pressure liquid chromatography–QTRAP® MS using a hybrid triple-quadrupole linear ion trap instrument. Anal Bioanal Chem 405(18):5875–5885. https://doi.org/10.1007/s00216-013-6944-8
Löve ASC, Baz-Lomba JA, Reid MJ, Kankaanpää A, Gunnar T, Dam M, Thomas KV (2018) Analysis of stimulant drugs in the wastewater of five Nordic capitals. Sci Total Environ 627:1039–1047. https://doi.org/10.1016/j.scitotenv.2018.01.274
Machado KC, Grassi MT, Vidal C, Pescara IC, Jardim WF, Fernandes AN, Severo FJR (2016) A preliminary nationwide survey of the presence of emerging contaminants in drinking and source waters in Brazil. Sci Total Environ 572:138–146. https://doi.org/10.1016/j.scitotenv.2016.07.210
Maranho LA, André C, DelValls TA, Gagné F, Martín-Díaz ML (2015a) Toxicological evaluation of sediment samples spiked with human pharmaceutical products: energy status and neuroendocrine effects in marine polychaetes Hediste diversicolor. Ecotoxicol Environ Saf 118:27–36. https://doi.org/10.1016/j.ecoenv.2015.04.010
Maranho LA, Garrido-Pérez MC, DelValls TA, Martín-Díaz ML (2015b) Suitability of standardized acute toxicity tests for marine sediment assessment: pharmaceutical contamination. Water Air Soil Pollut 226(3). https://doi.org/10.1007/s11270-014-2273-6
Massarsky A, Trudeau VL, Moon TW (2011) β-blockers as endocrine disruptors: the potential effects of human β-blockers on aquatic organisms. J Exp Zool A Ecol Genet Physiol 315A(5):251–265. https://doi.org/10.1002/jez.672
Mezzelani M, Gorbi S, Da Ros Z, Fattorini D, d’Errico G, Milan M, Regoli F (2016) Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis. Mar Environ Res 121:31–39. https://doi.org/10.1016/j.marenvres.2016.03.005
Milione S, Mercurio I, Troiano G, Melai P, Agostinelli V, Nante N, Bacci M (2016) Drugs and psychoactive substances in the Tiber River. Aust J Forensic Sci 49(6):679–686. https://doi.org/10.1080/00450618.2016.1212270
Minguez L, Pedelucq J, Farcy E, Ballandonne C, Budzinski H, Halm-Lemeille M-P (2014) Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ Sci Pollut Res 23(6):4992–5001. https://doi.org/10.1007/s11356-014-3662-5
Moore MT, Greenway SL, Farris JL, Guerra B (2008) Assessing caffeine as an emerging environmental concern using conventional approaches. Arch Environ Contam Toxicol 54(1):31–35. https://doi.org/10.1007/s00244-007-9059-4
Moreno-González R, Rodriguez-Mozaz S, Gros M, Barceló D, León VM (2015) Seasonal distribution of pharmaceuticals in marine water and sediment from a mediterranean coastal lagoon (SE Spain). Environ Res 138:326–344. https://doi.org/10.1016/j.envres.2015.02.016
Nodler K, Licha T, Bester K, Sauter M (2010) Development of a multi-residue analytical method, based on liquid chromatography–tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J Chromatogr A 1217(42):6511–6521. https://doi.org/10.1016/j.chroma.2010.08.048
Nunes B, Verde MF, Soares AMVM (2015) Biochemical effects of the pharmaceutical drug paracetamol on Anguilla anguilla. Environ Sci Pollut Res 22(15):11574–11584. https://doi.org/10.1007/s11356-015-4329-6
Oosterhuis M, Sacher F, ter Laak TL (2013) Prediction of concentration levels of metformin and other high consumption pharmaceuticals in wastewater and regional surface water based on sales data. Sci Total Environ 442:380–388. https://doi.org/10.1016/j.scitotenv.2012.10.046
Ortiz JP, Braulio A, Yanes JP (2016) Wastewater marine disposal through outfalls on the coast of São Paulo State Brazil: an overview. Revista DAE 64:29–46. https://doi.org/10.4322/dae.2016.015
Papageorgiou M, Kosma C, Lambropoulou D (2016) Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci Total Environ 543:547–569. https://doi.org/10.1016/j.scitotenv.2015.11.047
Pereira CDS, Maranho LA, Cortez FS, Pusceddu FH, Santos AR, Ribeiro DA, Guimarães LL (2016) Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci Total Environ 548-549:148–154. https://doi.org/10.1016/j.scitotenv.2016.01.051
Perrodin Y, Orias F (2017) Ecotoxicity of hospital wastewater. Hospital Wastewaters, 33–47. Springer, Cham
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27. https://doi.org/10.1016/j.watres.2014.08.053
Pomati F, Orlandi C, Clerici M, Luciani F, Zuccato E (2007) Effects and interactions in an environmentally relevant mixture of pharmaceuticals. Toxicol Sci 102(1):129–137. https://doi.org/10.1093/toxsci/kfm291
Quadra GR, Oliveira de Souza H, Costa R d S, Fernandez MA d S (2016) Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ Sci Pollut Res 24(2):1200–1218. https://doi.org/10.1007/s11356-016-7789-4
Ramos AS, Correia AT, Antunes SC, Gonçalves F, Nunes B (2014) Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: detoxification mechanisms, oxidative defence system and peroxidative damage. Environ Toxicol Pharmacol 37(3):1221–1228. https://doi.org/10.1016/j.etap.2014.04.005
Ribeiro ALPM, Oliveira RC (2015) Baixada Santista: uma contribuição à análise geoambiental, 1st edn. UNESP, São Paulo, p 255
Roberts PJW, Salas JH, Reiff FM, Libhaber M, Labbe A, Thomsom JC (2010) Marine wastewater outfalls and treatment systems. IWA Publishing, London
Rodgers-Gray TP, Jobling S, Morris S, Kelly C, Kirby S, Janbakhsh A, Harries JE, Waldock MJ, Sumpter JP, Tyler CR (2000) Long-term temporal changes in the estrogenic composition of treated sewage effluent and its biological effects on fish. Environ Sci Technol 34(8):1521–1528. https://doi.org/10.1021/es991059c
Santos JL, Aparicio I, Callejón M, Alonso E (2009) Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J Hazard Mater 164(2-3):1509–1516. https://doi.org/10.1016/j.jhazmat.2008.09.073
Shihomatzu HM (2015) Desenvolvimento e Validação de Metodologia SPE-LC-MS/MS para determinação de Fármacos e Droga de Abuso nas Águas da Represa Guarapiranga. IPEN/USP, São Paulo. https://doi.org/10.11606/T.85.2015.tde-28042015-095207
Sotelo JL, Ovejero G, Rodríguez A, Álvarez S, Galán J, García J (2014) Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem Eng J 240:443–453. https://doi.org/10.1016/j.cej.2013.11.094
Starling MCVM, Amorim CC, Leão MMD (2018) Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J Hazard Mater 372:17–36. https://doi.org/10.1016/j.jhazmat.2018.04.043
Sui Q, Huang J, Deng S, Yu G, Fan Q (2010) Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res 44(2):417–426. https://doi.org/10.1016/j.watres.2009.07.010
Sun Q, Lv M, Hu A, Yang X, Yu C-P (2014) Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J Hazard Mater 277:69–75. https://doi.org/10.1016/j.jhazmat.2013.11.056
Thomaidi VS, Stasinakis AS, Borova VL, Thomaidis NS (2015) Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study. J Hazard Mater 283:740–747. https://doi.org/10.1016/j.jhazmat.2014.10.023
Toufexi E, Dailianis S, Vlastos D, Manariotis ID (2016) Mediated effect of ultrasound treated Diclofenac on mussel hemocytes: first evidence for the involvement of respiratory burst enzymes in the induction of DCF-mediated unspecific mode of action. Aquat Toxicol 175:144–153. https://doi.org/10.1016/j.aquatox.2016.03.017
UNODC - United Nations Office on Drugs and Crime (2016) World drug report. United Nations publication, Sales No. E.16.XI.7, New York http://www.unodc.org. (Accessed Jun 12 2019)
USEPA - United States Environmental Protection Agency (2007) Method 1684: pharmaceuticals and personal care products in water, soil sediment, and biosolids by HPLC/MS/MS. Washington
USEPA - United States Environmental Protection Agency (2017) Ecological structure-activity relationship model (ECOSAR) class program. MS-Windows Version 2.0. https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosarcpredictive-model. Accessed 24 Oct 2020
USEPA - United States Environmental Protection Agency (2019) ECOTOX user guide: ecotoxicology database system, Version 4.0. http://www.epa.gov/ecotox/. Accessed 24 Oct 2020
Vidal-Dorsch DE, Bay SM, Maruya K, Snyder SA, Trenholm RA, Vanderford BJ (2012) Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ Toxicol Chem 31(12):2674–2682. https://doi.org/10.1002/etc.2004
Who – World Health Organization (2011) World Health Day 2011: policy briefs. Regulate and promote rational use of medicines, including in animal husbandry, and ensure proper patient care. World Health Organization, Geneva
Wille K, Noppe H, Verheyden K, Vanden Bussche J, De Wulf E, Van Caeter P, Vanhaecke L (2010) Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal Bioanal Chem 397(5):1797–1808. https://doi.org/10.1007/s00216-010-3702-z
Wille K, Kiebooms JAL, Claessens M, Rappé K, Vanden Bussche J, Noppe H, Vanhaecke L (2011) Development of analytical strategies using U-HPLC-MS/MS and LC-ToF-MS for the quantification of micropollutants in marine organisms. Anal Bioanal Chem 400(5):1459–1472. https://doi.org/10.1007/s00216-011-4878-6
Winter MJ, Lillicrap AD, Caunter JE, Schaffner C, Alder AC, Ramil M, Hutchinson TH (2008) Defining the chronic impacts of atenolol on embryo-larval development and reproduction in the fathead minnow (Pimephales promelas). Aquat Toxicol 86(3):361–369. https://doi.org/10.1016/j.aquatox.2007.11.017
Yamamoto NS, Pereira CDS, Cortez FS, Pusceddu FH, Santos AR, Toma W, Guimarães LL (2014) Avaliação dos efeitos biológicos adversos dos fármacos anti-hipertensivos Losartan e Valsartan em ouriço-do-mar Lytechinus variegatus (Echinodermata: Echinoidea). Unisanta BioScience 3:27–32
Zuccato E, Castiglioni S, Bagnati R, Chiabrando C, Grassi P, Fanelli R (2008) Illicit drugs, a novel group of environmental contaminants. Water Res 42(4-5):961–968. https://doi.org/10.1016/j.watres.2007.09.010
Acknowledgements
The authors want to acknowledge Daniel Temponi Lebre from the Centro de Espectrometria de Massa Aplicada - Instituto de Pesquisas Energéticas e Nucleares (CEMSA, IPEN, São Paulo, Brazil) for the technical support regarding the LC–MS/MS analyses. Thanks also to Vinicius Roveri of Pulsar pictures Ltda, Apiacás St., 934, São Paulo, Brazil, for the licence to reproduce images of the beaches of Guarujá (contract n° 27918).
Funding
This research was supported by the national funds through FCT - Foundation for Science and Technology within the scope of UIDB/04423/2020 and UIDP/04423/2020.
Author information
Authors and Affiliations
Contributions
Vinicius Roveri: Conceptualisation, data curation, formal analysis, methodology, writing - original draft. Luciana Lopes Guimarães: Co-supervision, writing - review and editing. Walber Toma: Writing - review. Alberto Teodorico Correia: Funding acquisition, supervision, writing - original draft, writing - review and editing
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
We declare that this work has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Consent to participate
Not applicable.
Consent to publish
All authors authorise the publication of this manuscript following its final acceptance.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• First report of the presence of pharmaceutical compounds and illicit drugs in the mixture zone of the Guarujá submarine outfall (State of São Paulo, Brazil).
• First record of the occurrence of orphenadrine near a Latin American submarine sewage outfall.
• All detected compounds presented concentrations below the surface water safety limits (0.01 μg/L), except for caffeine.
• For almost all compounds, the observed concentrations indicate nonenvironmental risk for the aquatic biota.
• Only acetaminophen, diclofenac, and caffeine showed low to moderate ecological risk.
Rights and permissions
About this article
Cite this article
Roveri, V., Guimarães, L.L., Toma, W. et al. Occurrence and risk assessment of pharmaceuticals and cocaine around the coastal submarine sewage outfall in Guarujá, São Paulo State, Brazil. Environ Sci Pollut Res 28, 11384–11400 (2021). https://doi.org/10.1007/s11356-020-11320-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11320-y