Abstract
The occurrence of pharmaceuticals and illicit drugs in water resources is widely documented in Europe, North America and Asia. However, in South America, these studies are still incipient. The objective of this study was to screen and identify the presence of pharmaceuticals of various therapeutic classes, including illicit drugs such as cocaine and its metabolite benzoylecgonine, in urban drainage channels that flow into the bathing waters of Guarujá city, State of São Paulo, Brazil. Moreover, the ecological potential risks to the aquatic biota were also assessed. The water samples were collected from four beaches of Guarujá in two different points: in the urban drainage channels and in the nearby coast line. A total of 16 compounds were detected using liquid chromatography coupled with tandem mass spectrometry: carbamazepine (0.1–8.0 ng/L), caffeine (33.5–6550.0 ng/L), cocaine (0.2–30.3 ng/L), benzoylecgonine (0.9–278.0 ng/L), citalopram (0.2–0.4 ng/L), acetaminophen (18.3–391.0 ng/L), diclofenac (0.9–79.8 ng/L), orphenadrine (0.2–1.5 ng/L), atenolol (0.1–140.0 ng/L), propranolol (limit of detection: LOD—0.9 ng/L), enalapril (2.2–3.8 ng/L), losartan (3.6–548.0 ng/L), valsartan (19.8–798.0 ng/L), rosuvastatin (2.5–38.5 ng/L), chlortalidone (0.1–0.4 ng/L) and clopidogrel (0.1–0.2 ng/L). The hereby data also showed that five of these compounds, namely caffeine, acetaminophen, diclofenac, losartan and valsartan, could raise moderate to severe risks to aquatic organisms (algae, crustaceans and fishes). This study is the first report of the occurrence of several pharmaceuticals and illicit drugs in urban drainage channels that flow to the bathing waters in South America, and it is the first quantification of rosuvastatin, chlortalidone and clopidogrel in environmental marine waters of Latin America.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Municipal wastewater is being discharged into marine water bodies as world population grows and concentrates in coastal areas (Lolić et al. 2015; Dafouz et al. 2018; Roveri et al. 2020b). These sewers can contain thousands of different chemicals which could compromise the quality of these receiving waters (Quadra et al. 2016; Biel-Maeso et al. 2018; Dafouz et al. 2018). Among these compounds, in the last decade, there has been a growing interest in pharmaceuticals and personal care products (PPCPs), which constitute a group of large numbers of chemical compounds from different therapeutic classes, including pharmaceuticals (e.g. antiepileptics, stimulants, analgesics/anti-inflammatory and antihypertensive drugs) (Celle-Jeanton et al. 2014; Lolić et al. 2015; Peña-Guzmán et al. 2019) and illicit drugs (e.g. cocaine) (Pereira et al. 2016; Parolini et al. 2017; Capaldo et al. 2018). These emerging compounds are considered potentially hazardous to the environment because they are ubiquitous, not easily removed by conventional sewage treatment plants and pseudo-persistent (Archana et al. 2017; Diamanti et al. 2019; Fontes et al. 2019), since they enter continuously into the aquatic compartment, affecting water, sediment and biota (Fabbri and Franzellitti 2015; Cortez et al. 2018; Dafouz et al. 2018). PPCPs are biologically active compounds, and therefore, upon reaching the aquatic ecosystem, they can cause deleterious effects in non-target organisms (Parolini et al. 2016; Pires et al. 2016; Cortez et al. 2018), such as algae (Mano and Okamoto 2016; Watanabe et al. 2016; Tamura et al. 2017), crustaceans (Nunes et al. 2014; Beiras and Tato, 2018; Chen et al. 2019), molluscs (McEneff et al. 2014; Parolini et al. 2016; Cortez et al. 2018) and fishes (Nunes et al. 2015; Parolini et al. 2017; Capaldo et al. 2019). Although concentrations of PPCPs are generally at the ng/L or μg/L levels in surface waters, they can induce severe effects, such as endocrine disruption, inhibition of primary productivity and reduced survival or reproductive success (Fabbri and Franzellitti 2015; Godoy et al., 2015; Godoy and Kummrow, 2017). All these features have made PPCPs a management priority among leading environmental protection agencies, such as the European Commission (EMA, 2006) and the US Environmental Protection Agency (USEPA 2017).
Globally, research on the detection of PPCPs in freshwater surface waters under intense anthropogenic pressure is already well documented in Europe (e.g. Milione et al. 2016; Pereira et al. 2017; Burns et al. 2018), North America (e.g. Klosterhaus et al. 2013; Lara-Martín et al. 2014; Anumol and Snyder, 2015) and Asia (e.g. Hossain et al. 2018; Praveena et al. 2018; Yang et al. 2018). However, in the marine environment, the detection of PPCPs has been neglected for many years under the assumption that dilution would represent a safety factor (Biel-Maeso et al. 2018; Desbiolles et al. 2018). Still, several worldwide monitoring studies to detect the presence of PPCPs have been done in marine environments (e.g. Lolić et al. 2015; Pereira et al. 2016; Dafouz et al. 2018). In South America, specifically in Brazil where the disposal of PPCPs is not regulated, studies aiming to detect the occurrence of PPCPs in coastal waters are, however, limited (Quadra et al. 2016; Godoy and Kummrow, 2017; Peña-Guzmán et al. 2019).
In addition to the scarcity of studies on pharmaceuticals and illicit drugs in South America, as far as it is known, none of these have been dedicated to detect the presence of these emerging pollutants in urban drainage channels that carry the diffuse load into the ocean, along tourist beaches (areas of intense recreation) (Quadra et al. 2016; Starling et al. 2018; Peña-Guzmán et al. 2019). The runoff of these urban surface waters to beaches (popularly known as “black tongues”) (Rocha et al. 2011) has already been identified as a potential threat to the environmental and public health and can be responsible for introducing chemical and biological pollutants related to disease outbreaks in bathing waters (Roveri et al. 2020a, b).
The aim of this study was to investigate, for the first time, the environmental occurrence and toxicity risk assessment of 23 emerging pollutants from different pharmaceutical and illicit drug classes in water samples from the urban drainage channels that flow to four tourist beaches (Tombo, Enseada, Perequê and Iporanga), located in the municipality of Guarujá, coast of São Paulo, Brazil.
Material and methods
Study site description and sample collection
This study was carried out in Guarujá city, a micro-region of Santos municipality, São Paulo State, Brazil. The mean annual precipitation and temperature of the sub-basin reach approximately 3000 mm and 22 °C, respectively. Two quite distinct seasonal periods are observed in the municipality: rainy (November to March) and dry (April to October) seasons. The Guarujá city has an area of 143 km2 and 64 km of extension, 36 km2 of which are already completely urbanised. The remaining 107 km2 are made up of environmental protection areas (SMA/CPLEA 2016). Guarujá contains a resident population of approximately 316,000 inhabitants (Ibge 2018). This municipality is one of the main Brazilian tourist destinations (MMA 2017). In the high tourist season during summer, between December and February, the population practically doubles (Cetesb 2017). It is estimated that about 20% of this resident population lives in irregular occupations, commonly known as favela, in areas of relevant ecological interest (such as hills and mangroves) (SMA/SPLA 2018).
The municipality’s economy is mainly driven by the port of Santos (the largest in Latin America), located in the western portion of the island, and also by seasonal tourism along the eight main beaches, located in the eastern and southern portions of the island (Ribeiro and Oliveira 2015). These beaches receive the input of rainwater from superficial runoff, through 43 urban drainage channels. These channels are made of concrete, and none of them has a grated system, meaning that all of their content is discharged directly onto the beaches, on a daily basis, in an area of recreational activities (bathing waters) (Cetesb 2017). Taking into consideration the different characteristics regarding land use and occupation, four of these urbanised beaches were selected for this study: Tombo (an international Blue Flag certification beach), Enseada (a high tourist visitation), Perequê (a fishing community) and Iporanga (a conservation unit). For further details, see Table S1 (online supplementary material).
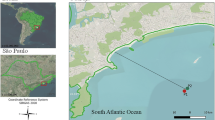
At each beach, two sampling points were selected. One point was located at the mouth of the urban drainage channel (beach sand), without the influence of the tidal regime (identified with the letter A). The second water sample was collected at the mouth of this drainage channel, but in seawater (30 cm deep) at a recreation point (identified with the letter B) (Fig. 1).
Map of the study area showing Brazil, São Paulo State and the metropolitan region of Santos city. Water sampling locations of the current study in the municipality of Guarujá, namely Tombo (point 1), Enseada (point 2), Perequê (point 3) and Iporanga (point 4) beaches. Letters A and B represent the urban drainage channels and the nearby seawater areas, respectively
Because it is known that cultural and festive events occurring in different regions may influence water quality by increasing the presence of sewers containing pharmaceuticals and illicit drugs in the environment (Pereira et al. 2016), it was decided to collect samples on Monday, January 15, 2018, during the Brazilian summer, on the day of the municipal holiday of Santo Amaro. No rainfall was recorded 48 h prior to collection.
Water sampling collection followed a standard procedure (USEPA 2007). Water samples were packaged into 1-L amber glass bottles previously cleaned, transported to the laboratory in an insulated box with ice (< 6 °C), filtered with 0.45 μm pore size membrane to remove suspended solids and kept under refrigeration (− 20 °C). Further chemical processing occurred within 7 days after filtration.
Preparation and analysis of pharmaceutical compounds
Chemical and standards
High purity reagents such as nitric acid and sulphuric acid were purchased from Merck. Organic solvents used in HPLC grade solid phase extraction and LC-MS grade such as acetonitrile, methanol and isopropanol were acquired from Sigma-Aldrich. Mobile phase additives, namely LC-MS grade formic acid and ammonium acetate, were acquired from Sigma-Aldrich and Merck, respectively. Analytical standards of acetaminophen, atenolol, bromazepam, caffeine, carbamazepine, cyproterone, clonazepam, clopidogrel, diclofenac, enalapril, loratadine, losartan, midazolam orphenadrine, propranolol, sildenafil and valsartan were acquired from Sigma-Aldrich. Cocaine and benzoylecgonine were acquired from Cerillant. The other pharmaceuticals were bought in several suppliers: Citalopram (Alcytam®, Torrent by Brazil Limited), Clortalidona (Higroton®, Novarts), rosuvastatin (Crestor®, AstraZeneca) and generic paroxetine medication (Meddley).
Sample preparation
In this study, the extraction technique was adapted from Wille et al. (2010). Before extraction, the pH of each sample (channel and seawater) was adjusted to 7.0 using a hydrochloric acid solution (1 M). Next, 1-L samples were filtered through Whatman® filter paper (GF/C particle retention 1.2 μm, diameter 47 mm; Merck KGaA, Darmstadt, Germany), and to prevent the loss of the compounds of interest, the filters were washed with 2 mL of methanol (Sigma-Aldrich, St. Louis, USA). The methanol extract collected was then combined to the filtered sample. Subsequently, the SPE (solid phase extraction) procedure using spherical, hydrophobic polystyrene-divinylbenzene resin for SPE cartridges Chromabond ® HR-X, (200 mg, 3 mL, Macherey-Nagel GmbH & Co. KG, Düren, Germany) was accomplished as described by Wille et al. (2010) and Ghoshdastidar et al. (2015). The cartridges were preconditioned with methanol (5 mL) and Milli-Q-Water (5 mL) (Milli-Q®-Merck KGaA). Thereafter, they were loaded with 1 L of the filtered sample and the cartridges were rinsed with 5 mL of Milli-Q water (procedure adopted twice). The cartridges were then dried under vacuum for 30 min. The elution was performed using 2 × 5 mL of methanol and 5 mL of acetone. Prior to the analysis, the concentrated eluate was evaporated to dryness under a nitrogen flow (at 50 °C), re-suspended in 1 mL with a solution of water/acetonitrile (95:5, v/v) and then filtered through a 0.45 μm pore size membrane (Merck Millipore). Each resuspension was analysed in triplicate using liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). A concentration factor (1/1000) was used to obtain the measured concentration following LC–MS/MS quantification.
LC–MS/MS analysis
Based on the reported annual consumption, expected toxicity and environmental persistence (Cmed, 2017), a total of 23 chemical compounds, namely pharmaceuticals and illicit drugs, were analysed using liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) (for further analytical details, see the Table S2-supplementary material- online recourse). LC–MS/MS methodology was described and validated by Shihomatzu (2015). The validation was performed using the parameters of selectivity, matrix effect, dynamic range, linearity, limit of detection (LOD), limit of quantification (LOQ), precision (% RSD), accuracy (% CV), recovery and robustness. An aliquot (10 μL) of each sample was analysed using an Agilent 1260 Infinity HPLC (Agilent™, Germany) combined with a hybrid triple quadrupole/LIT instrument (3200QTRAP®-linear ion trap) mass spectrometer (ABSciex, Ontario, Canada). The conditions used for the liquid chromatography (LC) separation were as follows: an injection volume of 10 μL of each sample was loaded in an Agilent Zorbax Eclipse XDB–C18 column (50 × 4.6 mm ID, 1.8 μm column at 25 °C). The eluent flow rate was 0.7 mL/min, and the mobile phase for positive mode analysis was 0.1% formic acid (Sigma-Aldrich; LC–MS Grade) in solvent A (water) and solvent B (acetonitrile) (J.T. Baker, Philipsburg, NJ, USA). For negative mode analysis, the mobile phase was a 5 mM ammonium acetate buffer (Sigma-Aldrich) with a pH of 4.6 (solvent A) and acetonitrile (solvent B). For both modes of ionisation (negative and positive), a linear gradient of 0.7 mL/min was used, starting with a mixture of solvent A (95%) and solvent B (5%). The solvent A percentage was decreased linearly from 95 to 5% over the course of 5 min and this condition was maintained for 1 min. Over the course of 2 min, the mixture was then returned to the initial conditions. Using electrospray ionisation (ESI: positive and negative modes) and multiple reaction monitoring (MRM mode), analytes were detected and quantified. This procedure was performed with the selection of a precursor ion and two ion products to quantify and qualify each compound. MRM parameters for the positive and negative modes for each chemical compound, LOD and LOQ are shown in Table S2. A sweater matrix-matched external calibration curve was employed, as described by Shihomatzu (2015). LOD and LOQ values were determined, using spiked matrix samples and obtained from seven measurements of the lowest detectable concentration of the calibration curves (with signal-to-noise ratio of at least 10), following the Brazilian Institute of Metrology, Quality and Technology procedures (INMETRO, 2011). Both field and laboratory blanks were below LOD. Data analysis was performed with Analyst® 1.5.2 software (ABsciex). Results, expressed in ng/L, were the average value of the three technical replicates for each concentrated water sample (Table 1).
Ecological risk assessment
The ecological risk assessment was performed calculating the risk quotient (RQ) for three different aquatic organisms (algae, crustaceans and fishes) following the equation RQ = MEC/PNEC, in which MEC is the maximum Measured Environmental Concentration, and PNEC the Predicted No Effect Concentration, both expressed in ng/L. PNEC values were obtained from base-set reliable ecotoxicity data available for the aquatic compartment regarding short-term [lethal concentration 50 (LC50) or median effective concentration (EC50)] and long-term [no observed effect concentration (NOEC)] toxicological endpoints. In the absence of NOEC, the lowest observed effect concentration (LOEC) or, in alternative, the 10% effective concentration (EC10) were used, when available. Since urban drainage channels are already recognised as an important transport mechanism of conventional pollutants (chemical and biological) to Ocean Atlantic (Roveri et al. 2020a, b), it was decided to measure ecological risk through marine species. According to the existent studies and current marine risk assessment practices, a reasonable correlation exists between the ecotoxicological responses of freshwater and saltwater biota, at least for the usual aquatic taxa (i.e. acute and chronic toxicity to algae, crustaceans and fishes) (EMA 2006; Li et al. 2012; Thomaidi et al. 2015). In this context, an attempt was made to compile specifically PNEC data for marine coastal species. When these data were not available, data from freshwater communities were used. In order to collect available ecotoxicity test endpoints, an extensive search was carried out in the Ecotoxicology Database (ECOTOX) from the United States Environmental Protection Agency (USEPA 2019), as well as in other literature sources using the PubMed database. When ecotoxicity laboratory experimentally derived data were not available, short [L(E)C50] and long toxicological endpoints [Chv, geometric mean of NOEC and LOEC, ChV = 10^([log (NOECxLOEC)]/2)] were estimated using the Ecological Structure Activity Relationships Program (ECOSAR, v 2.0) (USEPA 2017). The derived PNEC values for the acute and chronic toxicity data were thereafter calculated by dividing each toxicological endpoint by an assessment factor (AF). For saltwater environments, an AF of 10,000 and 100 should be considered in short- and long-term data sets, respectively. For further details, see the European Chemical Bureau (ECB 2003) and the European Chemicals Agency (ECHA 2008) guidelines. The toxic data that is selected as the toxicological benchmarks for the calculation of the PNECs is shown in Table 2 and Table S3. Finally, the risk (RQ = MEC/PNEC) was categorised into four levels: no (RQ < 0.01), low (0.01 ≤ RQ < 0.1), moderate (0.1 ≤ RQ < 1.0) and high ecological risk (RQ ≥ 1.0) to aquatic organisms (Hernando et al. 2006).
Results and discussion
Occurrence of PPCPs in Guarujá
In light of the lack of data on the occurrence of pharmaceuticals and illicit drugs in tropical coastal zones (Pereira et al. 2016), this study screened and identified, for the first time, the occurrence of PPCPs of various therapeutic classes, including illicit drugs such as cocaine and its metabolite benzoylecgonine, in urban drainage channels that flow into bathing waters of Guarujá City, São Paulo State, Brazil. These channels receive the input of rainwater from urban surface runoff, and none of them has a grating system, meaning that all of their content is daily discharged directly onto the beaches, in an area of extensive recreational activities (Cetesb 2017). In the present study, 16 PPCPs were successfully detected in the beaches of Guarujá, almost covering all the target compounds screened (excluding anxiolytics, contraceptives, antihistamines and sexual stimulants): five antihypertensives, three stimulants and three analgesics/anti-inflammatory, one antiepileptic, one antidepressant, one anticholesteremic, one diuretic and one antiplatelet drug (Table 1).
For the water sampling points located directly at the mouth of the urban drainage channels (1A-4A), the most frequently detected compounds were carbamazepine (4/4: 100%) caffeine (4/4: 100%), cocaíne (4/4: 100%), benzoylecgonine (4/4: 100%), diclofenac (4/4: 100%), valsartan (4/4: 100%), acetaminophen (3/4: 75%), atenolol (3/4: 75%), losartan (3/4: 75%), rosuvastatin (3/4: 75%), clopidogrel (3/4: 75%), citalopram (1/4: 25%), orphenadrine (1/4: 25%), propranolol (1/4: 25%), enalapril (1/4: 25%) and chlorthalidone (2/4: 50%). MEC ranged between 0.2 and 6550 ng/L, such as summarised in Table 1. The concentrations of the PPCPs were typically below 1000 ng/L. However, 50% of the caffeine samples exceeded 1000 ng/L (Table 1 and Fig. 2). Caffeine is found in many consumer products, such as coffee, tea, soft drinks, chocolate and painkillers, and has been reported worldwide with high occurrence (Dafouz et al. 2018; Yang et al. 2018; Griffero et al. 2019). In Guarujá, this product is probably coffee, because of the long tradition of Brazil in the coffee industry (Quadra et al. 2016). In overall, these results were consistent with most of the worldwide published studies on pharmaceuticals and illicit drugs found in surface waters: USA (Klosterhaus et al. 2013; Lara-Martín et al. 2014; Anumol and Snyder, 2015), Europe (Milione et al. 2016; Pereira et al. 2017; Burns et al. 2018), Asia (Hossain et al. 2018; Praveena et al. 2018; Yang et al. 2018) and Latin America (Pereira et al. 2016; Rivera-Jaimes et al. 2018; Griffero et al. 2019). In some cases, MEC, detected in Guarujá, were much higher than those reported in different environmental matrices around the world, with exception of enalapril, chlorthalidone and clopidogrel (Table S4). In Guarujá, the consumption and disposal of these pharmaceuticals and illicit drugs may have been motivated by tourism, since collecting water samples took place in the high summer season. During the summer season, holidays and weekends, the population increases, as well as the consumption of pharmaceuticals and illicit drugs in the cities (Pereira et al. 2016; Fontes et al. 2019).
Total concentrations of 16 pharmaceutical and personal care products (PPCPs) and illicit drugs (cocaine and its metabolite benzoylecgonine) detected on the shoreline of Guarujá, São Paulo, Brazil. At each selected beach (Tombo, Enseada, Perequê and Iporanga), samples were collected in the urban drainage channels (urban surface runoff) and in nearby seawater (bathing waters). Letters A and B represent the urban drainage channels and the nearby seawater areas, respectively
The occurrence and concentration of the PPCPs found in the seawater sampling points (1B-4B) nearby the drainage channels, will depend on the physicochemical properties of the target compounds, degradation processes and water dispersal processes (Biel-Maeso et al. 2018). Ten PPCPs did not show changes in the frequency of occurrence in relation to the respective sampling points of the channels: caffeine (100%), cocaine (100%), benzoylecgonine (100%), diclofenac (100%), valsartan (100%), atenolol (75%), losartan (75%), chlorthalidone (2/4: 50%), citalopram (25%), orphenadrine (25%). These findings suggest therefore that they are ubiquitous and environmentally pseudo-persistent (Diamanti et al. 2019; Fontes et al. 2019). However, six PPCPs showed changes in the frequency of occurrence after the dilution in seawater: carbamazepine (reduction observed in Iporanga: 3/4–75%), acetaminophen (reduction observed in Tombo: 2/4–50%), rosuvastatin (was not detected at any point: 0/4–0%), clopidogrel (reduction observed in Tombo and Enseada: 1/4–25% in both points) and propranolol (reduction observed in Perequê—0/4: 0%) (Table 1 and Fig. 2). Regarding enalapril, unexpectedly, there was an increase in its frequency of occurrence. At Enseada beach, enalapril was not detected in the channels (3A), but was detected at seawater (3B) [enalapril (2/2: 50%)] (Table 1). In this way, it is possible that other similar channels (there are 39 in Guarujá with similar characteristics: Cetesb 2017) are also contributing to the PPCPs (e.g. enalapril) input in the Guarujá seawaters. Moreover, from the eight PPCPs detected in Guarujá seawaters (e.g. caffeine, cocaine, benzoylecgonine, valsartan, losartan, acetaminophen, atenolol and diclofenac), some of them considered bestselling drugs in Brazil (Cmed, 2017), many recorded concentrations above the surface water safety limits (10 ng/L) (Table 1) (EMA, 2006). These high concentrations prove that the supposed dilution of PPCPs into the marine environment does not always represent a safety factor and therefore deserves attention (Fabbri and Franzellitti 2015).
In addition, even some PPCPs that have been detected in low concentrations (≤ 10 ng/L, Table 1), six of them (e.g. diclofenac, losartan, clopidogrel, citalopram, orphenadrine and valsartan) deserve attention because of their high n-octanol/water partition coefficients (log Kow ≥ 3: Table S4), which indicate that they could bioaccumulate and exert toxicity (European Commission 2003; Mendoza et al. 2015; Pereira et al. 2016). The remaining ten PPCPs (e.g. propranolol, rosuvastatin, enalapril, carbamazepine, cocaine, chlorthalidone, benzoylecgonine, acetaminophen, caffeine and atenolol) have low potential for bioaccumulation (log Kow < 3: Table S4) (Mendoza et al. 2015; ECOSAR 2017; Fontes et al. 2019).
The discharge of domestic sewage, which includes waste water containing pharmaceuticals and illicit drugs (original, metabolised or conjugated forms), is the main route by which these emerging pollutants reach the aquatic environment (Fabbri and Franzellitti 2015; Biel-Maeso et al. 2018; Dafouz et al. 2018). Indeed, the highest concentrations (87% of the MEC) were detected mainly in the beaches of Perequê (7 occurrences) and Enseada (6 occurrences) (Table 1 and Fig. 2), both with sanitation deficiencies (Table S1). Historically, sewage discharge stowaways in drainage channels usually occur in these neighbourhoods, which lead to diffuse loads (“black tongues”) flowing directly into the marine environment (Roveri et al. 2020a). However, although in lower concentrations, PPCPs have been also recorded in channels that cross the city’s neighbourhoods and that are served by sanitation networks, such as Tombo (a Blue Flag certified beach served by the municipal sanitation network) and Iporanga (a conservation unit area with its own sewage treatment) (Table S1). Thus, considering the presence of two recognised sewage markers (carbamazepine and caffeine) (Aguirre-Martínez et al. 2015; Dafouz et al. 2018), it is evident that there is a clandestine discharge of sewage into the Tombo and Iporanga channels. This study confirms that urban runoff is a potential driver of emerging pollutants (e.g. pharmaceuticals and illicit drugs) into receiving water bodies (seawater), besides being recognised as an important transport mechanism of conventional pollutants (chemical and biological) (Roveri et al. 2020a, b).
Although the literature suggests that the presence of these compounds can be detected in coastal environments due to discharge of sewage or terrestrial input, their concentrations are generally below the limits of detection in the seawater owing to the degradation processes effect and/or the dilution (Biel-Maeso et al. 2018), which was not the present situation. Moreover, and based on the recent reviews (Starling et al. 2018; Peña-Guzmán et al. 2019), the hereby work seems to be the first report on the occurrence of rosuvastatin, chlortalidone and clopidogrel in the urban water cycle in Latin America, and therefore, studies on the risks of these PPCPs to humans and aquatic biota are required.
Environmental risk assessment
The ecological risk assessment of pharmaceuticals and illicit drugs released in the aquatic compartment is of great importance to protect the environmental and public health. Thus, considering a worst case scenario in accordance with the Technical Guidance Document on Risk Assessment of the European Union (ECB 2003), a screening level of environmental risk assessment (RQ) was conducted for the hereby reported PPCPs. PNEC were estimated from data available in the scientific peer-reviewed literature or estimated by the ECOSARr program, as described in Table 2 and Table S3. In fact, the shortfall of toxicity data for marine organisms was a major hindrance to the effective risk assessment of these PPCPs resulting in only 30% of the RQ calculations. It means that the present study also adopted toxicity data from freshwater species to calculate the PNEC (Borecka et al. 2015). Moreover, the chronic toxicity data for most of the PPCPs are still scarce (Borecka et al. 2015; Archana et al. 2017). Thus, 60% of the hereby chronic PNEC were estimated using ECOSAR (Table 2 and Table S3) (USEPA 2017). Therefore, the present study reinforces the need for further ecotoxicological studies (especially with tropical marine organisms) to assess the acute and chronic toxicity of these bioactive compounds (Borecka et al. 2015; Biel-Maeso et al. 2018).
Table S3 lists in detail the final RQ values of the 16 PPCPs detected. The results showed the following trend: (i) regarding the acute toxicity, more than 80% of PPCPs showed no or low toxicity for algae, crustaceans and fishes. However, some PPCPs raised great concern. For example, caffeine and valsartan showed moderate toxicity for algae. For crustaceans, caffeine and losartan represented high toxicity. For fishes, diclofenac, together with caffeine and losartan, indicated moderate toxicity; (ii) concerning the chronic toxicity, 93.8%, 81.3%, and 87.5% of PPCPs also are non or low toxic for algae, crustaceans and fishes, respectively. However, caffeine recorded high toxicity for algae. For crustaceans, acetaminophen, losartan and valsartan indicated moderate toxicity, and for fish, losartan and acetaminophen showed moderate and high toxicity, respectively.
Thus, the evidences presented here, suggest that the PPCPs potentially dangerous in Guarujá were especially caffeine, acetaminophen, diclofenac, valsartan and losartan. The risks of these PPCPs were already supported by previous literature. For example, chronic exposure to 500 ng/L of caffeine (less than the concentration found in Guarujá) could alter the regenerative capacity of the annelid Diopatra neapolitana (Pires et al. 2016). Similarly, other authors have found that, in the aquatic environment, acetaminophen may be toxic to crustaceans and fishes (in general, toxicity results from oxidative stress) (Nunes et al. 2014, 2015; Ramos et al. 2014). Previous studies showed that diclofenac, at environmentally realistic concentrations, could cause bioaccumulation in zebra mussel (Dreissena polymorpha) (Daniele et al. 2016) and in Mediterranean mussel (Mytilus galloprovincialis) (Bonnefille et al. 2017). In relation to losartan and valsartan, these antihypertensives also deserve attention because their consumption has increased dramatically in many parts of the world, although studies on the toxicity of these substances are poorly documented (Godoy et al. 2015; Pereira et al. 2016; Desbiolles et al. 2018). A recent study detected cytotoxic effects on gills and hemocytes of the mussel Perna perna exposed to environmental concentrations of up to 300 ng/L of losartan (Cortez et al. 2018), less than the MEC detected in Guarujá.
Although cocaine and benzoylecgonine presented, respectively, no to low toxicity in the detected levels, a precautionary approach is recommended, because of the concentrations found in Guarujá (MEC of 30.3 and 278.0 ng/L, respectively) (Table 1). The low toxicity can be explained by the high PNEC estimated by ECOSAR, due to the scarcity of toxicity data from marine organisms reported in the peer-reviewed literature (Table S2) (USEPA 2017). However, environmentally realistic concentrations of cocaine (20 ng/L) have the potential to cause adverse effects on the European eel (Anguilla anguilla) (Capaldo et al. 2018, 2019). Moreover, cocaine and benzoylecgonine may interact with other therapeutic substances, leading to unexpected pharmacological interactions (Parolini et al. 2016, 2017).
Meanwhile, citalopram, orphenadrine, enalapril and chlorthalidone (100% of RQ < 0.01 for all trophic levels), and carbamazepine, propanolol, rosuvastatin and clopidogrel (more than 75% of RQ < 0.01 for all trophic levels) appear as the less relevant PPCPs in terms of aquatic toxicity effects, due to their high PNEC values and/or low concentrations in the investigated area (Table S3). However, these RQs were estimated for individual compounds, and therefore, one should consider that these PPCPs were detected in the environment usually as chemical mixtures.
Indeed, some studies have shown that toxicity of pharmaceuticals to non-target organisms may occur even at very low environmentally realistic concentrations due to additive or synergic effects (Desbiolles et al. 2018; Sathishkumar et al. 2019). In this regard, a study who analysed the single and combined effect of four drugs at environmental concentrations (including carbamazepine, caffeine and diclofenac) on the metabolism of marine bacteria Aliivibrio fischeri showed that a mixture of pharmaceutical compounds could be more severe than each drug individually (Di Nica et al. 2016). However, the combined toxicity of pharmaceuticals is still poorly known (Biel-Maeso et al. 2018; Desbiolles et al. 2018).
Conclusion
The hereby study showed that according to the ecological risk assessment performed, caffeine, acetaminophen, diclofenac, valsartan and losartan can potentially exert deleterious effects on the aquatic biota. However, the dilution effect of the seawater and the synergetic and/or additive effect of a mixture of chemical compounds cannot be ignored. This research showed that in addition to the sewage disposal (generally over 2 km from the coast), being recognised as vehicles for the transport of PPCPs and illicit drugs to the marine environment, urban drainage channels (which may receive input from underground sewers) are also potential anthropogenic sources of these compounds. Moreover, the present study suggests that these emerging pollutants are ubiquitous in beach areas around the world, which receive urban runoff (e.g. the coast of the state of São Paulo in Brazil has 600 drainage channels flowing to 290 tourist beaches). Thus, in order to maintain the quality of Guarujá beaches and, therefore to prevent environmental and public risks, it is necessary to promote the installation of basic sanitation facilities. It is highly recommended the installation of gate systems in the 43 urban drainage channels of the municipality and interconnection of the urban surface drainage to the sewage collection network (reduction of the first flush effect on the beaches of the municipality). Furthermore, land regularisation of irregularly occupied areas (Perequê beach and Enseada hills) is also recommended. Additional actions need to be taken such as supervision of the trade and residences of the municipality, requiring that they be interconnected to the sewage collection network already established (Tombo, Enseada and Perequê beaches), and increasing awareness, in loco, of users (especially during the summer tourist season), because, during the field work, it was observed that people constantly come into contact with the waters of these channels, demonstrating total ignorance about the health risks of this behaviour.
References
Archana G, Dhodapkar R, Kumar A (2017) Ecotoxicological risk assessment and seasonal variation of some pharmaceuticals and personal care products in the sewage treatment plant and surface water bodies (lakes). Environ Monit Assess 189(9):446. https://doi.org/10.1007/s10661-017-6148-3
Aguirre-Martínez GV, DelValls AT, Laura Martín-Díaz M (2015) Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicol Environ Saf 120:142–154. https://doi.org/10.1016/j.ecoenv.2015.05.036
Anumol T, Snyder SA (2015) Rapid analysis of trace organic compounds in water by automated online solid-phase extraction coupled to liquid chromatography–tandem mass spectrometry. Talanta 132:77–86. https://doi.org/10.1016/j.talanta.2014.08.011
Beiras R, Tato T (2018) Marine environmental risk assessment and acute water quality criterion for pentachlorophenol in coastal waters. Ecotoxicol Ecotoxicol 27:803–808. https://doi.org/10.1007/s10646-018-1930-8
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA (2018) Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ 612:649–659. https://doi.org/10.1016/j.scitotenv.2017.08.279
Borecka M, Siedlewicz G, Haliński ŁP, Sikora K, Pazdro K, Stepnowski P, Białk-Bielińska A (2015) Contamination of the southern Baltic Sea waters by the residues of selected pharmaceuticals: method development and field studies. Mar Pollut Bull 94(1–2):62–71. https://doi.org/10.1016/j.marpolbul.2015.03.008
Bonnefille B, Arpin-Pont L, Gomez E, Fenet H, Courant F (2017) Metabolic profiling identification of metabolites formed in Mediterranean mussels (Mytilus galloprovincialis) after diclofenac exposure. Sci Total Environ 583:257–268. https://doi.org/10.1016/j.scitotenv.2017.01.063
Burns EE, Carter LJ, Kolpin DW, Thomas-Oates J, Boxall ABA (2018) Temporal and spatial variation in pharmaceutical concentrations in an urban river system. Water Res 137:72–85. https://doi.org/10.1016/j.watres.2018.02.066
Capaldo A, Gay F, Lepretti M, Paolella G, Martucciello S, Lionetti L, Laforgia V (2018) Effects of environmental cocaine concentrations on the skeletal muscle of the European eel (Anguilla anguilla). Sci Total Environ 640-641:862–873. https://doi.org/10.1016/j.scitotenv.2018.05.357
Capaldo A, Gay F, Laforgia V (2019) Changes in the gills of the European eel (Anguilla anguilla) after chronic exposure to environmental cocaine concentration. Ecotoxicol Environ Saf 169:112–119. https://doi.org/10.1016/j.ecoenv.2018.11.010
Cetesb - Companhia Estadual de Tecnologia e Saneamento Ambiental (2017) Relatório de qualidade das águas costeiras no estado de São Paulo 2016. Série Relatórios/Cetesb. pp178. ISSN 0103-4103
Celle-Jeanton H, Schemberg D, Mohammed N, Huneau F, Bertrand G, Lavastre V, Le Coustumer P (2014) Evaluation of pharmaceuticals in surface water: reliability of PECs compared to MECs. Environ Int 73:10–21. https://doi.org/10.1016/j.envint.2014.06.015
Chen H, Gu X, Zeng Q, Mao Z (2019) Acute and chronic toxicity of carbamazepine on the release of chitobiase, molting, and reproduction in Daphnia similis. Int J Environ Res Public Health 16(2):209. https://doi.org/10.3390/ijerph16020209
CMED - Câmara de Regulação do Mercado de Medicamentos (2017) Anuário Estatístico do Mercado Farmacêutico. ANVISA, Brasília. http://portal.anvisa.gov.br/. (Accessed June 9 2019)
Cortez FS, Souza L d S, Guimarães LL, Almeida JE, Pusceddu FH, Maranho LA, Pereira CDS (2018) Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci Total Environ 637-638:1363–1371. https://doi.org/10.1016/j.scitotenv.2018.05.069
Dafouz R, Cáceres N, Rodríguez-Gil JL, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2018) Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci Total Environ 615:632–642. https://doi.org/10.1016/j.scitotenv.2017.09.155
Daniele G, Fieu M, Joachim S, James-Casas A, Andres S, Baudoin P, Vulliet E (2016) Development of a multi-residue analysis of diclofenac and some transformation products in bivalves using QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Application to samples from mesocosm studies. Talanta 155:1–7. https://doi.org/10.1016/j.talanta.2016.04.016
Desbiolles F, Malleret L, Tiliacos C, Wong-Wah-Chung P, Laffont-Schwob I (2018) Occurrence and ecotoxicological assessment of pharmaceuticals: is there a risk for the Mediterranean aquatic environment? Sci Total Environ 639:1334–1348. https://doi.org/10.1016/j.scitotenv.2018.04.351
Diamanti K, Aalizadeh R, Alygizakis N, Galani A, Mardal M, Thomaidis NS (2019) Wide-scope target and suspect screening methodologies to investigate the occurrence of new psychoactive substances in influent wastewater from Athens. Sci Total Environ 685:1058–1065. https://doi.org/10.1016/j.scitotenv.2019.06.173
Di Nica V, Villa S, Finizio A (2016) Toxicity of individual pharmaceuticals and their mixtures to Aliivibrio fischeri: experimental results for single compounds and considerations of their mechanisms of action and potential acute effects on aquatic organisms. Environ Toxicol Chem 36(3):807–814. https://doi.org/10.1002/etc.3568
ECB (2003) Technical guidance document on risk assessment for existing substances, Part II, pp 108–110
ECHA (2008) Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment, pp7–29
ECOSAR (2017) Ecological Structure - Activity Relationships Program. MS-Windows Version 2.0
EMA - European Medicines Agency, Committee for Medicinal Products for Human use (CHMP) (2006) Guideline on the Environmental Risk Assessment of Medicinal Products for Human use. Doc. Ref.: EMEA/CHMP/SWP/4447/00 corr 1, London, UK
Fabbri E, Franzellitti S (2015) Human pharmaceuticals in the marine environment: focus on exposure and biological effects in animal species. Environ Toxicol Chem 35(4):799–812. https://doi.org/10.1002/etc.3131
Fontes MK, Campos BG, Cortez FS, Pusceddu FH, Moreno BB, Maranho LA, Lebre DT, Guimarães LL, Pereira CDS (2019) Seasonal monitoring of cocaine and benzoylecgonine in a subtropical coastal zone (Santos Bay, Brazil). Mar Pollut Bull 149:110545. https://doi.org/10.1016/j.marpolbul.2019.110545
Ghoshdastidar AJ, Fox S, Tong AZ (2015) The presence of the top prescribed pharmaceuticals in treated sewage effluents and receiving waters in Southwest Nova Scotia, Canada. Environ Sci Pollut Res 22(1):689–700. https://doi.org/10.1007/s11356-014-3400-z
Godoy AA, Kummrow F (2017) What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit Rev Environ Sci Technol 47(16):1453–1496. https://doi.org/10.1080/10643389.2017.1370991
Godoy AA, Kummrow F, Pamplin PAZ (2015) Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment - a review. Chemosphere 138:281–291. https://doi.org/10.1016/j.chemosphere.2015.06.024
Griffero L, Alcántara-Durán J, Alonso C, Rodríguez-Gallego L, Moreno-González D, García-Reyes JF, Pérez-Parada A (2019) Basin-scale monitoring and risk assessment of emerging contaminants in South American Atlantic coastal lagoons. Sci Total Environ 134058:134058. https://doi.org/10.1016/j.scitotenv.2019.134058
Hernando MD, Mezcua M, Fernandez-Alba AR, Barcelo D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69(2):334–342. https://doi.org/10.1016/j.talanta.2005.09.037
Hossain A, Nakamichi S, Habibullah-Al-Mamun M, Tani K, Masunaga S, Matsuda H (2018) Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ Res 165:258–266. https://doi.org/10.1016/j.envres.2018.04.030
Ibge – Instituto brasileiro de Geografia e Estatística (2018) Estimativa da população brasileira. Rio de Janeiro. Brazil. http://ftp.ibge.gov.br/Estimativas_de_Populacao/Estimativas_2018/Estimativa_dou_2018_20181019.pdf, (Accessed June 11 2019)
INMETRO (2011) Instituto Nacional de Metrologia, Normalização e Qualidade Industrial. Orientação sobre validação de métodos de ensaios químicos. Rio de Janeiro, Brasil. DOQ-CGCRE-008. <http://www.inmetro.gov.br/Sidoq/Arquivos/CGCRE/DOQ/DOQ-CGCRE-8_04.pdf>
Klosterhaus SL, Grace R, Hamilton MC, Yee D (2013) Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ Int 54:92–99. https://doi.org/10.1016/j.envint.2013.01.009
Lara-Martín PA, González-Mazo E, Petrovic M, Barceló D, Brownawell BJ (2014) Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanized Long Island Sound Estuary (NY). Mar Pollut Bull 85(2):710–719. https://doi.org/10.1016/j.marpolbul.2014.01.022
Li Y, Zhang X, Li W, Lu X, Liu B, Wang J (2012) The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ Monit Assess 185(3):2211–2220. https://doi.org/10.1007/s10661-012-2702-1
Lolić A, Paíga P, Santos LHMLM, Ramos S, Correia M, Delerue-Matos C (2015) Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: occurrence and environmental risk. Sci Total Environ 508:240–250. https://doi.org/10.1016/j.scitotenv.2014.11.097
Mano H, Okamoto S (2016) Preliminary ecological risk assessment of 10 PPCPs and their contributions to the toxicity of concentrated surface water on an algal species in the Middle Basin of Tama River. J Water Environ Technol 14(6):423–436. https://doi.org/10.2965/jwet.15-045
McEneff G, Barron L, Kelleher B, Paull B, Quinn B (2014) A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci Total Environ 476-477:317–326. https://doi.org/10.1016/j.scitotenv.2013.12.123
Mendoza A, Aceña J, Pérez S, López de Alda M, Barceló D, Gil A, Valcárcel Y (2015) Pharmaceuticals and iodinated contrast media in a hospital wastewater: a case study to analyse their presence and characterise their environmental risk and hazard. Environ Res 140:225–241
Milione S, Mercurio I, Troiano G, Melai P, Agostinelli V, Nante N, Bacci M (2016) Drugs and psychoactive substances in the Tiber River. Australian J For Sci 49(6):679–686. https://doi.org/10.1080/00450618.2016.1212270
MMA - Ministério do Meio Ambiente (2017) Instituto do Meio Ambiente e dos Recursos Naturais Renováveis Projeto orla: fundamentos para gestão integrada / Ministério do Meio Ambiente. Ministério do Planejamento, Orçamento e Gestão, Brasília 82 p
Nunes B, Antunes SC, Santos J, Martins L, Castro BB (2014) Toxic potential of paracetamol to freshwater organisms: a headache to environmental regulators? Ecotoxicol Environ Saf 107:178–185. https://doi.org/10.1016/j.ecoenv.2014.05.027
Nunes B, Verde MF, Soares AMVM (2015) Biochemical effects of the pharmaceutical drug paracetamol on Anguilla anguilla. Environ Sci Pollut Res 22(15):11574–11584. https://doi.org/10.1007/s11356-015-4329-6
Parolini M, Ghilardi A, Della Torre C, Magni S, Prosperi L, Calvagno M, Binelli A (2017) Environmental concentrations of cocaine and its main metabolites modulated antioxidant response and caused cyto-genotoxic effects in zebrafish embryo cells. Environ Pollut 226:504–514. https://doi.org/10.1016/j.envpol.2017.04.046
Parolini M, Magni S, Castiglioni S, Binelli A (2016) Genotoxic effects induced by the exposure to an environmental mixture of illicit drugs to the zebra mussel. Ecotoxicol Environ Saf 132:26–30. https://doi.org/10.1016/j.ecoenv.2016.05.022
Peña-Guzmán C, Ulloa-Sánchez S, Mora K, Helena-Bustos R, Lopez-Barrera E, Alvarez J, Rodriguez-Pinzón M (2019) Emerging pollutants in the urban water cycle in Latin America: a review of the current literature. J Environ Manag 237:408–423. https://doi.org/10.1016/j.jenvman.2019.02.100
Pereira AMPT, Silva LJG, Laranjeiro CSM, Meisel LM, Lino CM, Pena A (2017) Human pharmaceuticals in Portuguese rivers: the impact of water scarcity in the environmental risk. Sci Total Environ 609:1182–1191. https://doi.org/10.1016/j.scitotenv.2017.07.200
Pereira CDS, Maranho LA, Cortez FS, Pusceddu FH, Santos AR, Ribeiro DA, Cesar A, Guimarães LL (2016) Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci Total Environ 548-549:148–154. https://doi.org/10.1016/j.scitotenv.2016.01.051
Pires A, Almeida Â, Correia J, Calisto V, Schneider RJ, Esteves VI, Freitas R (2016) Long-term exposure to caffeine and carbamazepine: impacts on the regenerative capacity of the polychaete Diopatra neapolitana. Chemosphere 146:565–573. https://doi.org/10.1016/j.chemosphere.2015.12.035
Praveena SM, Shaifuddin SNM, Sukiman S, Nasir FAM, Hanafi Z, Kamarudin N, Aris AZ (2018) Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): occurrence and potential risk assessments. Sci Total Environ 642:230–240. https://doi.org/10.1016/j.scitotenv.2018.06.058
Quadra GR, Oliveira de Souza H, Costa R d S, Fernandez MA d S (2016) Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ Sci Pollut Res 24(2):1200–1218. https://doi.org/10.1007/s11356-016-7789-4
Ramos AS, Correia AT, Antunes SC, Gonçalves F, Nunes B (2014) Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: detoxification mechanisms, oxidative defence system and peroxidative damage. Environ Toxicol Pharmacol 37(3):1221–1228. https://doi.org/10.1016/j.etap.2014.04.005
Ribeiro, A.L.P.M.; Oliveira, R.C (Orgs), 2015. Baixada Santista: uma contribuição à análise geoambiental. UNESP. São Paulo. 1° ed. pp. 255. ISBN 978-85-68334-55-3
Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, López de Alda M (2018) Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: occurrence and environmental risk assessment. Sci Total Environ 613-614:1263–1274. https://doi.org/10.1016/j.scitotenv.2017.09.134
Rocha S, Pinto RMF, Floriano AP, Teixeira LH, Bassili B, Martinez A, Caseiro MM (2011) Environmental analyses of the parasitic profile found in the sandy soil from the Santos municipality beaches, SP, Brazil. Rev Inst Med Trop Sao Paulo 53(5):277–281. https://doi.org/10.1590/s0036-46652011000500007
Roveri V, Guimarães LL, Correia AT, Demoliner M, Spilki FR (2020a) Occurrence of human adenoviruses in a beach area of Guarujá, São Paulo. Brazil Water Environ Res. https://doi.org/10.1002/wer.1338
Roveri V, Guimarães LL, Correia AT (2020b) Temporal and spatial variation of benthic macroinvertebrates on the shoreline of Guarujá, São Paulo, Brazil, under the influence of urban surface runoff. Reg Stud Mar Sci 36:36–101289. https://doi.org/10.1016/j.rsma.2020.101289
Sathishkumar P, Meena RAA, Palanisami T, Ashokkumar V, Palvannan T, Gu FL (2019) Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota - a review. Sci Total Environ 134057
Shihomatzu, H. M., 2015. Desenvolvimento e Validação de Metodologia SPE-LC-MS/MS para determinação de Fármacos e Droga de Abuso nas Águas da Represa Guarapiranga, São Paulo/SP, Brasil. IPEN/USP. https://doi.org/10.11606/T.85.2015.tde-28042015-095207
SMA/CPLA – Secretaria de Meio Ambiente do Estado de São Paulo/Coordenação de Planejamento Ambiental (2018) Zona Costeira Paulista: Relatório de Qualidade Ambiental. Org. Organizadores Nádia Gilma Beserra de Lima e Tatiana Camolez Morales Ferreira. SMA/CPLA, São Paulo. Brasil
SMA/CPLEA – Secretaria do Meio Ambiente do Estado de São Paulo/Coordenadoria de Planejamento e Educação Ambiental (2016) Zoneamento Ecológico - Econômico – Baixada Santista, São Paulo, Brasil, 55 p
Starling MCVM, Amorim CC, Leão MMD (2018) Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J Hazard Mater 372:17–36. https://doi.org/10.1016/j.jhazmat.2018.04.043
Tamura I, Yasuda Y, Kagota K, Yoneda S, Nakada N, Kumar V, Yamamoto H (2017) Contribution of pharmaceuticals and personal care products (PPCPs) to whole toxicity of water samples collected in effluent-dominated urban streams. Ecotoxicol Environ Saf 144:338–350. https://doi.org/10.1016/j.ecoenv.2017.06.032
Thomaidi VS, Stasinakis AS, Borova VL, Thomaidis NS (2015) Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study. J Hazard Mater 283:740–747. https://doi.org/10.1016/j.jhazmat.2014.10.023
USEPA - United States Environmental Protection Agency (2007) Method 1694: pharmaceuticals and personal care products in water, soil sediment, and biosolids by HPLC/MS/MS. Washington
USEPA - United States Environmental Protection Agency (2017) Ecological structure-activity relationship model (ECOSAR) class program. MS-Windows Version 2.0. https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosarcpredictive-model. (Accessed June 12 2019)
USEPA - United States Environmental Protection Agency (2019) ECOTOX User Guide: Ecotoxicology Database System, Version 4.0. http://www.epa.gov/ecotox/. (Accessed January 10 2019)
Watanabe H, Tamura I, Abe R, Takanobu H, Nakamura A, Suzuki T, Tatarazako N (2016) Chronic toxicity of an environmentally relevant mixture of pharmaceuticals to three aquatic organisms (alga, daphnid, and fish). Environ Toxicol Chem 35(4):996–1006. https://doi.org/10.1002/etc.3285
Wille K, Noppe H, Verheyden K, Vanden Bussche J, De Wulf E, Van Caeter P, Vanhaecke L (2010) Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal Bioanal Chem 397(5):1797–1808. https://doi.org/10.1007/s00216-010-3702-z
Yang Y-Y, Zhao J-L, Liu Y-S, Liu W-R, Zhang Q-Q, Yao L, Ying G-G (2018) Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci Total Environ 616-617:816–823. https://doi.org/10.1016/j.scitotenv.2017.10.241
Acknowledgments
Authors want to acknowledge Daniel Temponi Lebre from the Centro de Espectrometria de Massa Aplicada - Instituto de Pesquisas Energéticas e Nucleares (CEMSA, IPEN, São Paulo, Brazil) for technical support regarding the LC–MS/MS analyses. Thanks also to Pulsar pictures Ltda, Apiacás St., 934, São Paulo, Brazil, for the licence to reproduce the images of the beaches of Guarujá (contract no 27918). Correia AT was supported by national funds through FCT - Foundation for Science and Technology within the scope of UIDB/04423/2020 and UIDP/04423/2020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 437 kb)
Rights and permissions
About this article
Cite this article
Roveri, V., Guimarães, L.L., Toma, W. et al. Occurrence and ecological risk assessment of pharmaceuticals and cocaine in a beach area of Guarujá, São Paulo State, Brazil, under the influence of urban surface runoff. Environ Sci Pollut Res 27, 45063–45075 (2020). https://doi.org/10.1007/s11356-020-10316-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10316-y