Abstract
When diabetes neuropathy occurs, the oxidative stress caused by chronic hyperglycemia may result in chronic neuronal damage. To mitigate the effects of hyperglycemia-induced neuronal damage, it may be beneficial to address oxidative stress and inflammation in the body. The current study evaluated the neuroprotective efficacy of Thuja occidentalis in streptozotocin (STZ)-nicotinamide (NAD)-induced diabetic neuropathy in male Wistar rats. A single dose of STZ (65 mg/kg, i.p.) was used to induce diabetic neuropathy in Wistar rats. Serum insulin, glucose, hyperalgesia, oxidative stress, inflammatory markers, and histopathology of the sciatic nerve were evaluated for neuropathy. Wistar rats were treated with varying doses of hydroalcoholic extracts of Thuja occidentalis (HAETO) and gabapentin for 30 days. Thuja occidentalis considerably corrected the levels of inflammatory markers and oxidative stress caused by hyperglycemia; also, it led to the restoration of neuronal functions, indicating that it is effective in treating diabetic neuropathy. Furthermore, the molecular docking of thujone at the active pockets of various inflammatory mediators (IL-1β, IL-6, TGF-β1, and TNF-α) has shown good interactions with critical amino acid residues. These findings indicate that the hydroalcoholic extract of Thuja occidentalis effectively inhibits the development of diabetic neuropathy. The hypoglycemic, antioxidant, anti-hyperalgesia, and anti-inflammatory properties of Thuja occidentalis are thought to be responsible for the neuroprotective benefit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic neuropathy (DN) is one of the most frequently occurring complications of diabetes, characterized by pain, foot ulcerations, amputations, and mortality. Despite significant feats in managing metabolic disorders, numerous approaches have been yet to explore the various therapeutic strategies for managing pain in diabetes. Additional neuropathy causes include neurotoxic drugs, renal illness, demyelinating neuropathy, vitamin B12 deficiency, hereditary neuropathy, alcoholism, and vasculitis (Alam et al. 2020). Diabetes can result in diabetic neuropathy, a type of nerve damage (Singh et al. 2021a, b, c). Elevated blood sugar (glucose) levels can cause havoc on nerves throughout the body (Kishore et al. 2018). Diabetic neuropathy most frequently affects the nerves in the legs and feet. DM (diabetes mellitus) is a metabolic disorder characterized by hyperglycemia and several associated complications like nephropathy, neuropathy, and retinopathy. (Dewanjee et al. 2018; Singh et al. 2020a, b, c, d, e). This is one of the primary reasons for death globally, affecting nearly 6% of the entire world’s population (Ardeleanu et al. 2020). The rapidly rising incidences of diabetes worldwide have a major impact on people’s health, life expectancy, quality of life, and healthcare systems (Sharma et al. 2020). According to the International Diabetes Federation (IDF), by 2030, more than 80% of people with diabetes will live in low- and middle-income countries (Saeedi et al. 2019). Hyperglycemia is caused by an abnormal hepatic glucose output, a decrease in glucose absorption by skeletal muscles, and a decrease in glycogen synthesis (Simran et al. 2019). Rapidly decreasing body weights, polyphagia, blurred vision, polyuria, and tachycardia are some of the cardinal signs of hyperglycemia (Singh et al. 2020a, b, c, d, e; Rehni et al. 2010). The neuropathy associated with DM is a progressive illness that is frequently asymptomatic. Patients with diabetic neuropathy have been seen to have complications like foot ulcers as the disease progresses (Singh et al. 2020a, b, c, d, e). Although various medications have been successfully researched to treat diabetic neuropathy in the past few decades, there has been a significant prevalence of adverse effects associated with the current pharmacotherapy of DN. Several long non-coding RNAs and micro RNAs (miRNAs) have been developed to improve gene expression, which may facilitate the alleviation of diabetic complications (Fawzy et al. 2020) .
Diabetic-induced oxidative stress results in lipid peroxidation, which damages the cellular organelles and myelin sheath in the sciatic nerve, promoting neuropathy and insulin resistance (Kandeil et al. 2020; Zhang et al. 2020; Singh et al. 2021a, b, c). Excessive free radical production occurs in diabetes due to glucose oxidation, enzymatic degradation of proteins, and other factors (Singh and Singh 2021). A substantial number of studies have confirmed the involvement of numerous inflammatory mediators in developing diabetes complications (Yang et al. 2020). Herbal antioxidants were inversely associated with inflammation markers, implying that inflammation and oxidative stress are related (Kandeil et al. 2011; Hossen et al. 2017; Ekta et al. 2020; Singh et al. 2019). Preclinical and clinical investigations have shown various herbal formulations and phytoconstituents to reduce cytokine and chemokine levels in diabetes (Garg et al. 2022; Grewal et al. 2021). Herbal formulations with anti-inflammatory properties can also be therapeutic agents in diabetic neuropathy. These possible physiological effects of dietary antioxidants have piqued researchers’ interest in functional foods and dietary supplements in recent years (Bakht et al. 2020). Moreover, studies have shown that long-term use of herbal medicines appears to be effective in the management of DN (Tiwari et al. 2019).
Arbor vitae or Thuja occidentalis Linn. (Cupressaceae) is an herbal drug used widely for a long time in the traditional medicine system for the management of various ailments. Thuja occidentalis mainly consists of thujone, thujyl-alcohol, terpinolene, fenchone, limonene, borneol, alpha-terpene, and myricene. Thujone consists of 85% α-thujone and 15% β-thujone is the main compound (Naser et al. 2005; Jasuja et al. 2015; Bagot 2020). Other pharmacological activities represented by Thuja occidentalis include antioxidant activity, antibacterial, antifungal, antiinflammatory, antitumoral, anti-diabetic, hypolipidemic, gastroprotective, antiviral, and immunostimulant (Caruntu et al. 2020; Devi and Krishan 2020; Pradhan and Sarangdevot 2020).
Through streptozotocin (STZ)-induced studies, the present paper explores the merits of using T. occidentalis Linn. in managing and alleviating diabetic neuropathy due to its potent antioxidant potential. To date, there is no preclinical study on the neuroprotective effect of T. occidentalis Linn. on nociceptive behavior in diabetic animals. The present study also explored the mechanistic insight on the role of T. occidentalis in STZ induced DN.
Materials and methods
Chemicals
STZ and nicotinamide (NAD) were acquired from M/s Sigma Aldrich, India, and gabapentin from Yarrowchem, India, respectively. Diagnostic kits for biochemical estimates were purchased from Erba Pvt. Ltd., India. All of the other chemicals and solvents utilized were of the highest quality.
Plant material
The aerial part of Thuja occidentalis L. was collected from the city Rajpura, District Patiala, Punjab, India, and authenticated from Sri Venkateshwara University in Tirupati, India, under reference number: SVU/SC/48/99/17–18.
Extraction procedure
The dried shade aerial part of Thuja occidentalis L. was ground into a coarse powder and passed through a sieve with a mesh size of 40. The coarse powdered materials of the plant were subjected to soxhlet apparatus (Kishore et al. 2018). One kilogram of the Thuja occidentalis L. aerial parts was subjected to ethanol in the water ratio of 70:30 (v/v) and was kept at 25 °C for 12 h. The suspension was filtered through Whatman no.4 filter paper. To remove the solvents, the resulting hydroalcoholic solutions were subjected to distillation under a rotary evaporator at 600 °C. After the distillation, the resulting semi-solid was dried in a vacuum desiccator. This extract’s nature and yield were noted. Until further use, the plant extract was stored in a refrigerator at 40 °C, and the extracts were named hydroalcoholic extract of Thuja occidentalis (HAETO). Furthermore, HAETO extracts were dissolved in water or solvent and used to assess in vivo assays.
Fingerprinting of HAETO
Hydroalcoholic extract of Thuja occidentalis analyzed with MS/MS for fingerprinting. For the quantification and detection API 2000 (Applied biosystem/MDS SCIEX, Canada) mass spectrometer in conjunction with electrospray ionization (ESI) source and an LC system has been used (Farooq and Singh 2021).
In vivo evaluation
Animals
Wistar rats (males) weighing 220–250 g were housed in clean polypropylene cages at 24 ± 2 °C, a humidity of 45 ± 5%, and a 12-h day/night cycle maintained on a standard diet with regular water access. The animal experimental protocol was approved as per the guidelines of the Institutional Animal Ethics Committee (IAEC), Chitkara College of Pharmacy, Chitkara University, Punjab, under the registration number: 1181/PO/ReBi/S/08/CPCSEA, and the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India, were followed.
Induction procedure for diabetic neuropathy (DN)
STZ (65 mg/kg) in citrate buffer was administered intraperitoneally 15 min after NAD (230 mg/kg; i.p.) to induce DN in rats. To confirm the development of diabetes, fasting blood glucose (FBG) levels were assessed 72 h after STZ infusion (Kumar et al. 2022). To examine the course of neuropathic diabetes, blood glucose, body weight, insulin, behavioral markers (mechanical and thermal hyperalgesia), and motor nerve conduction velocity (MNCV) were measured at the end of a 60-day trial (Kishore et al. 2018; Tyagi et al. 2019; Farooq and Singh 2021).
Treatment schedule
During the experiment, 48 rats were randomized into 6 groups comprising 8 rats each. Group I was sham group; group II was positive control with DN induced; group III to V consisted of neuropathic animals administered with 50, 100, and 200 mg/kg, p.o. of HAETO; group VI primarily consisted of neuropathy induced rats receiving 30 mg/kg, intraperitoneal injection of gabapentin. Different concentrations of the extracts (50 mg/kg, 100 mg/kg, and 200 mg/kg, p.o.) were finalized based on oral acute toxicity and pilot studies reported in the literature. Treatment with HAETO and gabapentin was started after 30 days of STZ-NAD administration and continued for the next 30 days. Animals were sacrificed under deep anesthesia at the end of the study, and the sciatic nerve was excised surgically and stored at − 70 °C until further use (Kishore et al. 2018; Tyagi et al. 2019; Farooq and Singh 2021).
Blood glucose, body weight, and serum insulin estimation
At the outset of the study, the bodyweight of each animal was measured, and animals of similar weights were grouped. At the start of the treatment protocol, animals were randomly assigned. Throughout the study, each group’s body weight was continually checked. Throughout the study, FBG levels were estimated at a 15-day interval using commercial enzymatic kits purchased from Erba Pvt. Ltd. India. Insulin levels in plasma were measured using an Insulin ELISA kit (Krishgen Biosysytems, India) in blood collected into anticoagulant-coated tubes (Subhasree et al. 2015).
Assessment of behavioral parameters in the form of mechanical and thermal hyperalgesia
Thermal hyperalgesia in DN rats was recorded by performing a tail immersion and hot plate test as the method described by Kishore et al. (2018).
Assessment of mechanical hyperalgesia by Randall-Selitto analgesiometer and allodynia in DN rats
Randall-Selitto analgesiometer was used to determine the hyperalgesia state in diabetic rats, as the method described by Kishore et al. (2018). For assessment of allodynia, Von Frey filaments were used as a method described by Farooq and Singh (2021).
MNCV in DN rats
The MNCV was evaluated in the diabetic animals to assess nerve damage as a method detailed by Kishore et al. (2018).
Estimation of oxidative stress in the sciatic nerve
Homogenate of the sciatic nerve was used to estimate thiobarbituric acid reactive substances (TBARS), catalase, superoxide dismutase (SOD), glutathione (GSH), and nitrite levels in post-mitochondrial supernatant for oxidative stress parameters as methods described by Tekaday et al. (2020).
Estimation of inflammatory mediators in the sciatic nerve
Under deep anesthesia, animals were sacrificed by cervical dislocation, sciatic nerves was extracted, and tissue homogenate was used to determine the presence of various inflammatory mediators such as interleukins (IL-1β, IL-6), transforming growth factor (TGF-β1), and tumor necrosis factor (TNF-α) (Ismail et al. 2018) by ELISA kit protocols (Krishgen Biosystems, India).
Estimation of the advanced glycation end product (AGEs) in sciatic nerve
An AGEs level in the sciatic nerve was determined using a method established by Kishore et al. (2018).
Histopathology of the sciatic nerve
The sciatic nerve of Wistar rats from various treatment groups was used for histopathological examination. It was performed on renal sections of 5 mm thickness that were produced and stained with hematoxylin and eosin (H & E) dye.
Molecular docking studies
To understand the binding interactions chondroitin sulfate at the active pockets of IL-1β, IL-6, TGF-β1, and TNF-α, the molecular docking simulation was carried out with CHARMm-based docking tool, CDOCKER of Discovery Studio Client v20.1.0.19295 software (Wu et al. 2003). The test compound was sketched and cleaned in Discovery Studio Client v20.1.0.19295 workspace, followed by energy minimization in the “Prepare Ligands” program of Discovery Studio Client at pH 7.4. The binding energy of the hits with proteins was estimated as negative of CDOCKER interaction energy (Shih et al. 2011).
Statistical analysis
The study data was reported as mean ± SEM ANOVA was employed for statistical analysis, followed by Tukey’s post hoc test using sigma stat software. P < 0.05 was chosen as a statistically significant level.
Results
ESI–MS/MS analysis of the crude extracts
The percentage yield of the HAETO extract was 10.56% w/w. The HAETO extracts were subjected to ESI–MS/MS by flow injection analysis to understand the intensity of ionization response of the various compounds present in them by both positive and negative ionization mode. In positive mode (M + 1), seven good intense responses were identified with HAETO (Table 1). The compounds which have identical intense responses and their structures are given.
ESI–MS/MS Fingerprinting of HAETO (Fig. 1)
The effect of Thuja occidentalis extracts on bodyweight
The effect of T. occidentalis hydroalcoholic extract on body weight was determined using an electronic weighing scale. The diabetic control group lost significantly (p ˂ 0.001) more weight than the vehicle control group. After induction of diabetes (after the 30th day), therapy with hydroalcoholic extract of T. occidentalis (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) increased the body weight significantly (p < 0.01) in comparison to the diabetic control group (Fig. 2).
The effect of Thuja occidentalis extracts on glucose levels
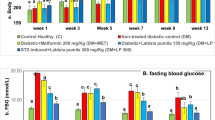
The effect of T. occidentalis hydroalcoholic extract on glucose levels was determined using a glucometer. The diabetic control group had significantly (p ˂ 0.001) higher glucose levels than the vehicle control group. After induction of diabetes (after the 30th day), treatment with HAETO (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) significantly decreased (p < 0.01) the glucose levels in comparison to the diabetic control group (Fig. 3).
Effect of hydroalcoholic extract of Thuja occidentalis on glucose level (mg/dl). Values are represented as mean ± SEM (n = 8); *p ˂ 0.01 vs. vehicle control on 0 day. **p ˂ 0.001 vs. vehicle control on 30th day; ap ˂ 0.0001 vs. vehicle control on 60th day; bp ˂ 0.05 vs. diabetic control on 60th day; cp ˂ 0.01 vs. diabetic control on 60th day
The effect of Thuja occidentalis extracts on insulin levels
The effect of T. occidentalis hydroalcoholic extract on insulin levels was determined using an autoanalyzer. Insulin levels were significantly (p ˂ 0.001) lower in the diabetic control group than the vehicle control group. After induction of diabetes (after the 30th day), therapy with HAETO (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) increased significantly (p < 0.01) in comparison to the diabetic control group (Fig. 4).
Evaluation of hydroalcoholic extract of T. occidentalis on total AGEs
The diabetic control group had significantly (p ˂ 0.001) higher AGEs levels than the vehicle control group. After induction of diabetes (after the 30th day), therapy with HAETO (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) significantly decreased AGEs levels (p < 0.01) compared to the diabetic control group (Fig. 5).
Evaluation of HAETO on mechanical hyperalgesia using randall-selitto analgesiometer
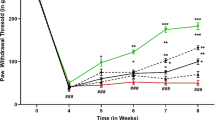
The HAETO was evaluated for hyperalgesia using the randall-selitto analgesiometer. There was a substantial difference between the diabetic control group and the vehicle control group in terms of pain resistance. After induction of diabetes (after the 30th day), therapy with HAETO (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) pain resistance increased significantly (p < 0.01) in comparison to the diabetic control group (Fig. 6).
Evaluation of HAETO on thermal hyperalgesia using hot plate method
The HAETO on hyperalgesia has been evaluated using the hot plate method. There was a significant increase in the frequency of paw licking, jumping, and rearing in the diabetic control group than the vehicle control group. The treatment with HAETO (50, 100, and 200 mg/kg p.o) and gabapentin (30 mg/kg) after induction of diabetes (after the 30th day) showed dose dependent attenuation in thermal hyperalgesia. There was a decrease in the frequency of paw licking, jumping, and rearing (p < 0.01) in comparison to the diabetic control group (Fig. 7).
Effect of hydroalcoholic extract of Thuja occidentalis on thermal hyperalgesia. Values are represented as mean ± SEM (n = 8); *p ˂ 0.001 vs. vehicle control on 30th day; ap ˂ 0.001 vs. vehicle control on 60th day; bp ˂ 0.05 vs. diabetic control on 60th day; cp ˂ 0.01 vs. diabetic control on 60th day
Evaluation of hydroalcoholic extract of T. occidentalis on hyperalgesia using von Frey hair filaments
The HAETO on hyperalgesia has been evaluated using von Frey hair filaments. A significant decrease in pain resistance in the diabetic control group could be seen than in the vehicle control group. The treatment with HAETO (50, 100, and 200 mg/kg p.o) and gabapentin (30 mg/kg) after induction of diabetes (after the 30th day) showed a marked increase in pain resistance (p < 0.01) in comparison to the diabetic control group (Fig. 8).
Effect of hydroalcoholic extract of Thuja occidentalis on hyper algesia level (µU/ml). Values are represented as mean ± SEM (n = 8); *p ˂ 0.001 vs. vehicle control on 30th day; ap ˂ 0.001 vs. vehicle control on 60th day; bp ˂ 0.05 vs. diabetic control on 60th day; cp ˂ 0.01 vs. diabetic control on 60th day
Effect of Thuja occidentalis extract on oxidative stress parameters in sciatic nerve
The effect of Thuja occidentalis hydroalcoholic extract on TBARS in the sciatic nerves has been studied. Compared to the vehicle control group, there was a significant increase (p < 0.001) in the diabetic control group. However, the treatment with HAETO (50 mg/kg, 100 mg/kg, and 200 mg/kg p.o) and gabapentin (30 mg/kg) after induction of diabetes (after the 30th day) decreased the TBARS levels in renal tissue significantly (p < 0.01) when compared to the diabetic control group (Table 3). The effect of T. occidentalis hydroalcoholic extract on catalase, GSH, and SOD levels in the sciatic nerve has been studied. Compared to the vehicle control group, there was a significant decrease in catalase, GSH, and SOD levels (p < 0.01) in the diabetic control group. The treatment with HAETO (50 mg/kg, 100 mg/kg, and 200 mg/kg p.o) and gabapentin (30 mg/kg) after induction of diabetes (after the 30th day) increased the catalase, GSH, and SOD levels in sciatic nerve significantly (p < 0.01) when compared to the diabetic control group (Table 2).
Evaluation of HAETO on nitrite level in the sciatic nerve
The nitrite content of a HAETO was determined. A considerable increase was observed in the diabetic control group to that in the vehicle control group. After induction of diabetes (after the 30th day), therapy with HAETO (50, 100, and 200 mg/kg p.o.) and gabapentin (30 mg/kg) significantly lowered the nitrate levels (p < 0.01) in comparison to the diabetic control group (Fig. 9).
Evaluation of HAETO on inflammatory mediators level in sciatic nerve
The evaluation of hydroalcoholic extract of T. occidentalis on inflammatory mediators has been evaluated. A significant increase (p < 0.001) in levels of IL-1β, IL-6, TGF-β, and TNF-α could be seen in the diabetic control group compared to the vehicle control group. The treatment with HAETO (50, 100 and 200 mg/kg, p.o.) and gabapentin (30 mg/kg) after induction of diabetes (after 30th day) decreased the levels of IL-1β, IL-6, TGF-β, and TNF-α significantly (p < 0.01) in comparison to the diabetic control group (Table 3).
Effect of Thuja occidentalis on histopathological changes in sciatic nerve
In-vehicle control group, the nerve myelin sheath is intact without any degeneration in Schwann cells and edema in endoneural spaces. In the diabetic control group, there is degeneration of myelin sheath and Schwann cells and axonal swelling. In Thuja occidentalis (50, 100, and 200 mg/kg) and gabapentin treatment groups, there is reduced inflammation, accumulation of neutrophils and edema in neural vessels, and normal texture of myelinated fibers without any necrotic changes have seen. A bar scale of 200 µm was used for microscopic examinations (Fig. 10).
Molecular docking studies
In order to discover the interaction patterns of thujone with IL-1β, IL-6, TGF-β1, and TNF-α, docking studies were carried out using PDB IDs 1ITB, 1ALU, 3TZM, and 2AZ5 crystal structures, respectively. The thujone at the active pocket of IL-1β, IL-6, TGF-β1, and TNF-α forms hydrogen bonds, Pi-alkyl, and Van der Waals interactions with the crucial amino acid residues (Table 4).
Discussion
Animal models have been used to answer a wide range of scientific questions, from basic science to the development and testing of new vaccines and therapies. It is acknowledged that major medical breakthroughs such as blood circulation, respiration physiology, and the hormonal system have been used for research purposes on various species of animals resembling human physiology and biology (King 2012). In our study chemically (STZ)-induced diabetic animal model is used to study the effect of HAETO in DN. In chemically induced models of type 1 diabetes, a high percentage of endogenous beta cells are destroyed, resulting in low endogenous insulin production and hyperglycemia (Singh et al. 2022). Chemically induced diabetes is not only a simple and low-cost model of diabetes in rodents, but it can also be used in higher animals. Because of its structural similarity to glucose, glucose can compete with STZ, making fasting animals more vulnerable to STZ (Barré-Sinoussi & Montagutelli 2015). STZ causes clinical features in animals that are similar to those seen in people with diabetes. As a result, STZ-treated animals have been used to investigate diabetogenic mechanisms and for preclinical testing of novel anti-diabetic therapies. STZ-induced type 1 diabetes in rodents is a well-established and widely accepted method for studying diabetes pathogenesis and complications (Singh et al., 2020a, b, c, d, e). The single STZ injection model should continue to be a cost-effective, time-saving, and convenient platform for studying the pathophysiological mitochondrial mechanisms of cell derangement caused by diabetic glucotoxicity in various rodent models (Singh et al. 2022).
Allodynia and hyperalgesia are the clinical features by which DN has been characterized (Rosenberger et al. 2020). Additionally, it is characterized by an increased nociceptive response, decreased neuronal hypoxia, motor nerve condition velocity, and a decreased threshold for painful stimuli. The pathophysiology of progressive nerve fiber loss appears multifaceted, involving the polyol pathway, reactive oxygen species, glycation, and altered protein kinase C activity (Callaghan et al. 2020; Pang et al. 2020). Hyperglycemia, oxidative stress, and inflammation unleash a cascade of events that affect cellular proteins, gene expression, and cell surface receptor expression, ultimately resulting in progressive pathologic changes and subsequent diabetic complications (Tekaday et al. 2020; Bignold and Johnson 2021; Khan et al. 2021). In the present study, STZ-injected rats had significantly higher blood glucose levels, increased food, and water intake, and decreased body weight. The nociceptive threshold was significantly lower than non-diabetic rats, indicated by tactile allodynia thermal and mechanical hyperalgesia in diabetic rats, and results are in line with previous studies (Kishore et al. 2018; Kumar et al. 2022; Singh et al., 2020a, b, c, d, e).
Thuja occidentalis L. is a plant rich in flavonoids, glycosides, and triterpenoids (Bagot 2020) and hence arouses tremendous interest in antidiabetic potential (Bhargava et al. 2022), which could be considered a lead to further study effect of this part of the plant on diabetic complications such as neuropathy and nephropathy. T. occidentalis extracts revealed the presence of triterpenoids and flavonoids on further chemical analysis. In the present study, the results evidenced the previous literature reviews that revealed the presence of terpenoids, flavonoids, tannins, and carbohydrates as the major constituents of the plant (Caruntu et al. 2020). Furthermore, MS–MS analysis revealed the presence of different compounds such as catechine, gallocatechin, thujone, and kaempferol in HAETO. Previous literature showed that these compounds mentioned above are an effective dietary strategy for decreasing postprandial glucose responses (Jasuja et al. 2015).
STZ is a nitrosourea analog widely used in experimental animals to induce DM. The STZ action is thought to be a result of its alkylating ability. STZ causes selective pancreatic islet of cell cytotoxicity in experimental animals due to its DNA-alkylating activity mediated by the methyl nitrosourea moiety (Kishore et al. 2018). STZ impairs glucose-stimulated insulin release and increases insulin resistance in rats by rapidly destroying pancreatic cells. STZ impairs glucose-stimulated insulin release and increases insulin resistance in rats by rapidly destroying pancreatic cells (Sharma et al. 2021). This results in a decrease in glucose entry into peripheral tissues, muscle, and adipose tissue, an increase in gluconeogenesis and hepatic glucose synthesis, and a higher blood glucose level (Baeza-Flores et al. 2020). Hyperglycemia has been shown to cause oxidative stress via various mechanisms, including redox imbalances caused by increased aldose reductase activity, increased advanced glycation end products, altered protein kinase C activity, particularly the isoforms, prostanoid imbalances, and mitochondrial superoxide over-production (Nádró et al. 2021). All of these pathways combine to generate oxidative stress, which results in the activation of NF-κβ, TGF-β1, and TNF-α, as well as the stimulation and expression of COX-2 mRNA and gene (Stascheit et al. 2021). In the current study, administration of Thuja occidentalis dose-dependently (p < 0.01) and significantly attenuates the production and release of TNF-α, TGF-β, IL1-β, and IL-6 in diabetic rats and inhibits the growth and exacerbation of chronic DN when compared with diabetic control Wistar rats, and results are in line various studies (Kishore et al. 2018; Singh et al., 2020a, b, c, d, e). These results demonstrated the anti-inflammatory potential of Thuja occidentalis by inhibiting the expression of cytokines.
Hyperglycemia-induced allodynia and hyperalgesia have been linked to functional alterations in sensory and motor neurons (Zeidman 2021). Hyperalgesia, allodynia, enhanced pain perception, and decreased MNCV were seen in the current study. According to previous research, thermal hyperalgesia develops gradually due to persistent hyperglycemia-induced damage and necrotic alterations in myelinated axons (Kishore et al. 2018; Farooq and Singh 2021). In the current study, the treatment of diabetic rats with Thuja occidentalis and gabapentin effectively attenuated DN’s behavioral and functional signs. Thuja occidentalis treatment for 30 days resulted in a significant restoration in the levels of the parameters mentioned above.
The production of AGEs under oxidative stress conditions may result from an interaction between the carbohydrate molecule and the free amino group of proteins (Singh et al., 2020a, b, c, d, e). AGEs are widely implicated in DN via cytokines and growth factors, causing significant diabetes consequences (Yu et al. 2021). The HAETO reduced STZ-induced oxidative stress-induced sciatic nerve injury by increasing antioxidant enzyme levels and decreasing AGE levels, supporting its potential role in reducing hyperglycemia. Increase the antioxidant capacity in chronic oxidative stress. Triterpenoids and flavonoids are well-known antioxidant phytochemicals. Triterpenoids and flavonoids alleviate oxidative stress by inhibiting the production of free radicals, slowing the degradation of GSH, and detoxifying LPO’s mediated reactive products (Garg et al. 2022). In diabetic rats, a considerable rise in lipid peroxides and decreased antioxidant enzyme activity were reported. Thuja occidentalis L. is shown in this study to considerably repair biochemical markers such as lipid peroxidation, GSH, catalase, and SOD activity in the sciatic nerves of diabetic rats. Thuja occidentalis’ antioxidant properties are generally documented, corroborating our current investigation (Kishore et al. 2018).
Nitric oxide (NO) causes protein nitrosylation, lipid peroxidation, protein nitration, and DNA damage that promote cell death, contributing to neuropathy (Singh et al. 2021a, b, c). The presence of various phytoconstituents in HAETO may contribute to its antioxidant property, thus showing inhibition of neurodegeneration in DN (Pang et al. 2020). In diabetes, enhanced levels of TNF-α and interleukins cause microvascular permeability and nerve damage due to phosphorylation of p38, thus promoting the development of micro-angiopathy and polyneuropathy (Dewanjee et al. 2018). In the current study, administration of Thuja occidentalis dose-dependently and significantly attenuates the production and release of TNF-α, TNF-α, TGF-β, and IL-1β in diabetic rats and inhibits the growth and exacerbation of chronic DN when compared with diabetic control Wistar rats (Yang et al. 2020). These results demonstrated the anti-inflammatory potential of Thuja occidentalis by inhibiting the expression of cytokines.
DN causes structural alterations in peripheral nerves. Axonal degeneration causes endoneural edema, decreased blood supply to nerves, and consequently reduced MNCV (Sharma et al. 2020). Administration of hydroalcoholic extract of Thuja occidentalis markedly (p < 0.01) increased MNCV compared to diabetic control rats. Results obtained by treatment of DN rats with hydroalcoholic extract of Thuja occidentalis (50 mg/kg, 100 mg/kg, and 200 mg/kg, p.o.) were prominent compared to the effect of gabapentin (30 mg/kg). These effects may be related to the antihyperglycemic action of Thuja occidentalis, which may be mediated through the alleviation of oxidative/nitrosative stress and the reduction of the production of AGEs (Rosenberger et al. 2020).
Furthermore, we compared various behavioral and biochemical effects of hydroalcoholic extract of Thuja occidentalis with gabapentin to improve the clinical rationality of the current report. By modulating oxidative-nitrosative stress and inflammatory cytokine release in diabetic rats, HAETO reverses neuropathic pain in them.
The molecular docking studies were further carried out to see the interaction pattern of thujone with different protein targets and were found that the test molecule has good interactions with inflammatory markers, i.e., IL-1β, IL-6, TGF-β1, and TNF-α with good binding energies (Dong et al. 2018). The position of thujone with respect to the key residues in the binding site of TNF-alpha, IL-1β, and IL-6 is shown in Fig. 11. The binding of the thujone with some of the essential residues of these target sites may describe one of its anti-inflammatory mechanisms of action.
The Interactions of thujone with the active site of IL-1β (A and B), IL-6 (C and D), TGF-β1 (E and F), TNF-alpha (G and H) pocket. The hydrogen bonds are indicated with green dashed lines, and the van der Waal forces are white. Thus, Thuja occidentalis may find a therapeutic application in treating diabetic patients with neuropathic pain. However, additional research is necessary to elucidate the precise mechanism of Thuja occidentalis antinociceptive potential. To our knowledge, there is no evidence to support the efficacy and potency of Thuja occidentalis in animal models of peripheral DN
Conclusion
T. occidentalis probably exerts its benefit by alleviating inflammatory stress. It is plausible to believe that T. occidentalis’ neuroprotective impact is due, at least in part, to its effect on AGE production and inflammatory condition. T. occidentalis may be advantageous by protecting against diabetic neuronal problems. Taken together, our findings suggest a beneficial role for T. occidentalis hydroalcoholic extract in reversing the progression of neuronal damage, and additional research elucidating the role of T. occidentalis or its bioactive components in molecular pathways may provide insight into the cellular mechanisms underlying diabetic complications and the development of neuropathy. As a result, the presented studies can be generalized to other species and can be followed up with clinical studies to assess Thuja occidentalis efficacy in treating DN in humans as well.
Data availability
Not applicable.
Abbreviations
- Ages:
-
Advanced glycation end products
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic neuropathy
- ESI:
-
Electrospray ionization
- FBG:
-
Fasting blood glucose
- GSH:
-
Glutathione
- H & E:
-
Hematoxylin and eosin
- HAETO:
-
Hydroalcoholic extracts of Thuja occidentalis
- IAEC:
-
Institutional Animal Ethics Committee
- IDF:
-
International Diabetes Federation
- IL:
-
Interleukins
- MNCV:
-
Motor nerve conduction velocity
- NAD:
-
Nicotinamide
- NO:
-
Nitric oxide
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- TBARS:
-
Thiobarbituric acid reactive substances
- TGF-β1:
-
Transforming growth factor-β1
- TNF-α:
-
Tumor necrosis factor-α
References
Alam U, Sloan G, Tesfaye S (2020) Treating pain in diabetic neuropathy: current and developmental drugs. Drugs 80:363–384. https://doi.org/10.1007/s40265-020-01259-2
Ardeleanu V, Toma A, Pafili K, Papanas N, Motofei I, Diaconu CC, Pantea Stoian A (2020) Current pharmacological treatment of painful diabetic neuropathy: a narrative review. Medicina 56:25. https://doi.org/10.3390/medicina56010025
Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, Murbartián J, Torres-López JE, Granados-Soto V (2020) Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol 11:558474. https://doi.org/10.3389/fphar.2020.558474
Bagot JL (2020) How to prescribe Thuja occidentalis in oncology? Analysis of the literature, study of practices and personal experience. Rev Homéopath 11:e26–e32. https://doi.org/10.1016/j.revhom.2020.07.001
Bakht J, Zafar Z, Ahmad J, Khan S (2020) Antibacterial activity of the crude extracts from medicinally important Thuja occidentalis. Pak J Pharm Sci 33:627–630
Barré-Sinoussi F, Montagutelli X (2015) Animal models are essential to biological research: issues and perspectives. Future Sci OA 1(4):FSO63. https://doi.org/10.4155/fso.15.63
Bhargava SK, Singh TG, Mannan A, Singh S, Gupta S (2022) Pharmacological evaluation of Thuja occidentalis for the attenuation of nephropathy in streptozotocin-induced diabetes rats. Obes Med 31:100391. https://doi.org/10.1016/j.obmed.2022.100391
Bignold R, Johnson JR (2021) Effects of cytokine signaling inhibition on inflammation-driven tissue remodeling. Curr Res Pharmacol Drug Discov 2:100023. https://doi.org/10.1016/j.crphar.2021.100023
Callaghan BC, Gallagher G, Fridman V, Feldman EL (2020) Diabetic neuropathy: what does the future hold? Diabetologia 63:891–897. https://doi.org/10.1007/s00125-020-05085-9
Caruntu S, Ciceu A, Olah NK, Don I, Hermenean A, Cotoraci C (2020) Thuja occidentalis L.(Cupressaceae): ethnobotany, phytochemistry and biological activity. Molecules 25:5416. https://doi.org/10.3390/molecules25225416
Devi A, Krishan B (2020) Comparative analysis of antioxidant and antimicrobial properties of Thuja occidentalis and Phyllanthus emblica. Plant Cell Biotechnol Mol Biol 21(61–62):1–15
Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, Manna P (2018) Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol 833:472–523. https://doi.org/10.1016/j.ejphar.2018.06.034
Dong J, Wang N, Yao Z, Zhang L, Cheng Y, Ouyang D, Lu A, Cao D (2018) ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminformatics 10:29. https://doi.org/10.1186/s13321-018-0283-x
Ekta GM, Kaur A, Singh TG, Bedi O (2020) Pathobiological and molecular connections involved in the high fructose and high fat diet induced diabetes associated nonalcoholic fatty liver disease. Inflamm Res 69:851–867. https://doi.org/10.1007/s00011-020-01373-7
Farooq SA, Singh R (2021) Inhibitory potential of Murraya koenigii (L.) and Ficus carica L. extracts against aldose reductase (ALR), advanced glycation end products (AGEs) formation and sorbitol accumulation. Serb J Exp Clin Res 22:125–130. https://doi.org/10.2478/sjecr-2020-0056
Fawzy MS, Abu AlSel BT, Al Ageeli E, Al-Qahtani SA, Abdel-Daim MM, Toraih EA (2020) Long non-coding RNA MALAT1 and microRNA-499a expression profiles in diabetic ESRD patients undergoing dialysis: a preliminary cross-sectional analysis. Arch Physiol Biochem 126(2):172–182. https://doi.org/10.1080/13813455.2018.1499119
Garg N, Singh TG, Khan H, Arora S, Kaur A, Mannan A (2022) Mechanistic interventions of selected Ocimum species in management of diabetes, obesity and liver disorders: transformative developments from preclinical to clinical approaches. Bioint Res Appl Chem 12:1304–1323. https://doi.org/10.33263/BRIAC121.13041323
Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, Najda A, Walasek-Janusz M, Kamel M, Albadrani GM, Akhtar MF, Saleem A, Abdel-Daim MM (2021) Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother 140:111729. https://doi.org/10.1016/j.biopha.2021.111729
Hossen MS, Ali MY, Jahurul M, Abdel-Daim MM, Gan SH, Khalil MI (2017) Beneficial roles of honey polyphenols against some human degenerative diseases: a review. Pharmacol Rep 69(6):1194–1205. https://doi.org/10.1016/j.pharep.2017.07.002
Ismail CAN, Aziz CBA, Suppian R, Long I (2018) Imbalanced oxidative stress and pro-inflammatory markers differentiate the development of diabetic neuropathy variants in streptozotocin-induced diabetic rats. J Diabetes Metab Disord 17:129–136. https://doi.org/10.1007/s40200-018-0350-x
Jasuja ND, Sharma S, Choudhary J, Joshi SC (2015) Essential oil and important activities of Thuja orientalis and Thuja occidentalis. J Essent Oil-Bear Plants 18:931–949. https://doi.org/10.1080/0972060X.2014.884774
Kandeil MA, Amin KA, Hassanin KA, Ali KM, Mohammed ET (2011) Role of lipoic acid on insulin resistance and leptin in experimentally diabetic rats. J Diabetes Complications 25(1):31–38. https://doi.org/10.1016/j.jdiacomp.2009.09.007
Kandeil MA, Mohammed ET, Hashem KS, Aleya L, Abdel-Daim MM (2020) Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res 27:19169–19184. https://doi.org/10.1007/s11356-019-05514-2
Khan H, Gupta A, Singh TG, Kaur A (2021) Mechanistic insight on the role of leukotriene receptors in ischemic-reperfusion injury. Pharmacol Rep 73(5):1240–1254. https://doi.org/10.1007/s43440-021-00258-8
King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166(3):877–894. https://doi.org/10.1111/j.1476-5381.2012.01911.x
Kishore L, Kaur N, Singh R (2018) Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 26:993–1003. https://doi.org/10.1007/s10787-017-0416-2
Kumar A, Aswal S, Chauhan A, Semwal RB, Singh R, Andola HC, Semwal DK (2022) Antidiabetic effect of aqueous-ethanol extract from the aerial parts of Artemisia roxburghiana. Nat Prod Res 36(5):1300–1305. https://doi.org/10.1080/14786419.2020.1858414
Nádró B, Lőrincz H, Molnár Á, Szentpéteri A, Zöld E, Seres I, Páll D, Paragh G, Kempler P, Harangi M, Sztanek F (2021) Effects of alpha-lipoic acid treatment on serum progranulin levels and inflammatory markers in diabetic neuropathy. J Int Med Res 49:3000605211012213. https://doi.org/10.1177/03000605211012213
Naser B, Bodinet C, Tegtmeier M, Lindequist U (2005) Thuja occidentalis (Arbor vitae): a review of its pharmaceutical, pharmacological and clinical properties. eCAM 2:69–78. https://doi.org/10.1093/ecam/neh065
Pang L, Lian X, Liu H, Zhang Y, Li Q, Cai Y, Ma H, Yu X (2020) Understanding diabetic neuropathy: focus on oxidative stress. Oxid Med Cell Longev 2020:9524635. https://doi.org/10.1155/2020/9524635
Pradhan P, Sarangdevot YS (2020) Evaluation of antidiabetic activity of aerial parts Of Thuja Occidentalis. Plant Arch 20(1):957–962
Rehni AK, Singh TG, Singh N, Arora S (2010) Tramadol-induced seizurogenic effect: a possible role of opioid-dependent histamine H1 receptor activation-linked mechanism. Naunyn Schmiedebergs Arch Pharmacol 81:11–19. https://doi.org/10.1007/s00210-009-0476-y
Rosenberger DC, Blechschmidt V, Timmerman H, Wolff A, Treede RD (2020) Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Trans 127:589–624. https://doi.org/10.1007/s00702-020-02145-7
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, IDF Diabetes Atlas Committee (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843
Sharma VK, Singh TG (2020) Chronic stress and diabetes mellitus: interwoven pathologies. Curr Diabetes Rev 16:546–556. https://doi.org/10.2174/1573399815666191111152248
Sharma VK, Singh TG, Singh S (2020) Cyclic nucleotides signaling and phosphodiesterase inhibition: defying Alzheimer’s disease. Curr Drug Targets 21(13):1371–1384. https://doi.org/10.2174/1389450121666200727104728
Sharma T, Kaur D, Grewal AK, Singh TG (2021) Therapies modulating insulin resistance in Parkinson’s disease: a cross talk. Neurosci Lett 749:135754. https://doi.org/10.1016/j.neulet.2021.135754
Shih KC, Shiau CW, Chen TS, Ko CH, Lin CL, Lin CY, Hwang CS, Tang CY, Chen WR, Huang JW (2011) Pharmacophore modeling and virtual screening to identify potential RET kinase inhibitors. Bioorg Med Chem Lett 21:4490–4497. https://doi.org/10.1016/j.bmcl.2011.06.003
Simran GAK, Arora S, Singh TG (2019) Role of protein kinase C in diabetic complications. J Pharma Technol Res Manag 7:87–95. https://doi.org/10.15415/jptrm.2019.72011
Singh S, Singh TG (2021) Emerging perspectives on mitochondrial dysfunctioning and inflammation in epileptogenesis. Inflamm Res 70(10–12):1027–1042. https://doi.org/10.1007/s00011-021-01511-9
Singh H, Mehta M, Khurana N, Sharma N, Vyas M, Singh TG, Mahajan S, Satija S (2019) Recent patent technologies of Tinospora cordifolia for anti-diabetic potential: a review. Plant Arch 19(2):994–999
Singh HP, Singh TG, Singh R (2020a) Attenuation of cisplatin–induced nephrotoxicity by p-coumaric acid through peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonism in male rats. Research J Pharm Tech 13(11):5270–5276. https://doi.org/10.5958/0974-360X.2020.00922.1
Singh R, Kaur HR, Singh TG (2020b) Advanced glycated end products (ages) in diabetes and its complications: an insight. Plant Arch 20(1):3838–3841
Singh R, Rao HK, Singh TG (2020c) Advanced glycated end products (ages) in diabetes and its complications: an insight. Plant Arch 20:3838–3841. https://doi.org/10.4196/kjpp.2014.18.1.1
Singh R, Rao HK, Singh TG (2020d) Neuropathic pain in diabetes mellitus: challenges and future trends. Obes Med 18:100215. https://doi.org/10.1016/j.obmed.2020.100215
Singh TG, Sharma R, Kaur A, Dhiman S, Singh R (2020e) Evaluation of renoprotective potential of Ficus religiosa in attenuation of diabetic nephropathy in rats. Obes Med 19:100268. https://doi.org/10.1016/j.obmed.2020.100268
Singh HP, Singh TG, Singh R (2021a) Evaluation of the renoprotective effect of syringic acid against nephrotoxicity induced by cisplatin in rats. J Appl Pharm Sci 11:80–85. https://doi.org/10.7324/JAPS.2021.11s109
Singh R, Rao HK, Singh TG (2021b) Comparison of safety and efficacy of pregabalin, duloxetine and their combination with epalrestat in diabetic neuropathy: a prospective, doubleblind, randomized, controlled trial. J Appl Pharm Sci 11:71–79. https://doi.org/10.7324/JAPS.2021.11s108
Singh S, Singh TG, Rehni AK, Sharma V, Singh M, Kaur R (2021c) Reviving mitochondrial bioenergetics: a relevant approach in epilepsy. Mitochondrion 58:213–226. https://doi.org/10.1016/j.mito.2021.03.009
Singh R, Farooq SA, Mannan A, Singh TG, Najda A, Grażyna Z, Albadrani GM, Sayed AA, Abdel-Daim MM (2022) Animal models of diabetic microvascular complications: relevance to clinical features. Biomed Pharmacother 145:112305. https://doi.org/10.1016/j.biopha.2021.112305
Stascheit F, Hotter B, Klose S, Meisel C, Meisel A, Klehmet J (2021) Calprotectin in chronic inflammatory demyelinating polyneuropathy and variants-A potential novel biomarker of disease activity. Front Neurol 12:723009. https://doi.org/10.3389/fneur.2021.723009
Subhasree N, Kamella A, Kaliappan I, Agrawal A, Dubey GP (2015) Antidiabetic and antihyperlipidemic activities of a novel polyherbal formulation in high fat diet/streptozotocin induced diabetic rat model. Indian J Pharmacol 47:509–513. https://doi.org/10.4103/0253-7613.165200
Tekaday D, Antony R, Jain S (2020) Antimicrobial, antioxidant and phytochemical investigation of Thuja occidentalis (Arbor vitae) leave extract. GSC Biol Pharm Sci 12:108–116. https://doi.org/10.30574/gscbps.2020.12.3.0292
Tiwari R, Siddiqui MH, Mahmood T, Bagga P, Ahsan F, Shamim A (2019) Herbal remedies: a boon for diabetic neuropathy. J Diet Suppl 16(4):470–490. https://doi.org/10.1080/19390211.2018.1441203
Tyagi CK, Porwal P, Mishra N, Sharma A, Chandekar A, Punekar R, Anghore D (2019) Antidiabetic activity of the methanolic extracts of Thuja occidentalis twings in alloxan-induced rats. Curr Trad Med 5:138–143. https://doi.org/10.2174/2215083805666190312153743
Wu G, Robertson DH, Brooks CL, Vieth M (2003) Detailed analysis of grid-based molecular docking: a case study of CDOCKER — a CHARMm - based MD docking algorithm. J Comput Chem 24:1549–1562. https://doi.org/10.1002/jcc.10306
Yang H, Sloan G, Ye Y, Wang S, Duan B, Tesfaye S, Gao L (2020) New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol 10:929. https://doi.org/10.3389/fendo.2019.00929
Yu MX, Lei B, Song X, Huang YM, Ma XQ, Hao CX, Yang WH, Pan ML (2021) Compound XiongShao Capsule ameliorates streptozotocin-induced diabetic peripheral neuropathy in rats via inhibiting apoptosis, oxidative - nitrosative stress and advanced glycation end products. J Ethnopharmacol 268:113560. https://doi.org/10.1016/j.jep.2020.113560
Zeidman LA (2021) Advances in the management of small fiber neuropathy. Neurol Clin 39:113–131. https://doi.org/10.1016/j.ncl.2020.09.006
Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y (2020) Oxidative stress and diabetes: antioxidative strategies. Front Med 14(5):583–600. https://doi.org/10.1007/s11684-019-0729-1
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India, for providing the necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Contributions
Conceptualization, conceived, and designed the experiments: TGS. Analyzed the data: TGS and SG. Wrote the manuscript: SKB, SS, and AM. Software: MS. Editing of the manuscript: SKB and SG. Critically reviewed the article: TGS. Supervision: TGS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhargava, S.K., Singh, T.G., Mannan, A. et al. Pharmacological evaluation of Thuja occidentalis for the attenuation of neuropathy via AGEs and TNF-α inhibition in diabetic neuropathic rats. Environ Sci Pollut Res 29, 60542–60557 (2022). https://doi.org/10.1007/s11356-022-20106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20106-3