Abstract
Generation of excessive reactive oxygen species (ROS) and advanced glycation end products (AGEs), and cellular apoptosis are implicated in the pathogenesis of diabetic neuropathy. Present study was aimed to explore the effect of Eruca sativa and Kaempferol (KP) on hyperalgesia (thermal and mechanical); tactile allodynia, motor nerve conduction velocity (MNCV) and oxidative–nitrosative stress in streptozotocin (STZ) induced experimental diabetes. Neuropathy developed in diabetic rats was evident from a marked hyperalgesia and allodynia; reduced MNCV associated with excess formation of AGEs and ROS. Chronic treatment with E. sativa hydroalcoholic extract (EHA; 100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) for 30 days starting from the 60th day of STZ administration significantly ameliorated behavioral and biochemical changes linked to diabetic neuropathy. Present study suggested that EHA and KP corrected hyperglycemia and reversed the pain response partially in diabetic rats along via modulating oxidative and nitrosative stress along with reduction of AGEs formation in diabetic rats. Thus E. sativa might be beneficial in chronic diabetes, ameliorate the progression of diabetic neuropathy and may also find application in diabetic neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic peripheral neuropathy is one of the major complications caused by long-standing hyperglycemia and frequently results in clinically significant morbidity. Pathogenesis of diabetic neuropathy (DN) is controversial due to multifactorial underlying causes of the disease. Chronic hyperglycemia remains the main initiator of biochemical changes that contributes to the development of DN (Singh et al. 2014). Hyperglycemia evidently plays a role in the progression of DN along with other microvascular complications. Hyperglycemia activates several biochemical pathways that include activation of the polyol pathway (Kishore et al. 2017), advanced glycation end products (AGEs) and their receptors (Kaur et al. 2017a; Kishore et al. 2016a), activation of protein kinase C (PKC), mitogen-activated protein kinases (MAPK) and inducible nitric oxide synthase (Kishore et al. 2016b, c). Furthermore, hypoxia and ischemia, elevate cytokines such as tumor necrosis factor (TNF-α), and IL-1β nerve growth factor (NGF) deficiency also play significant etiologic role in DN (Kaur et al. 2017b). Reactive oxygen species (ROS) are arbitrator factors involved in all these events (Kaur et al. 2016a; Singh et al. 2013) and activate intracellular signal transduction and transcription cascades, where MAPKs and nuclear factor kappa B (NF-κB) plays significant role (Singh et al. 2014) and damage proteins, lipids, and nucleic acids by oxidation. There is convincing experimental and clinical evidence that the generation of ROS increases in diabetic neuropathy. The disease arises from a combination of as a direct result of prolonged hyperglycemia (Kaur et al. 2016b).
Eruca sativa Mill is a diploid annual herbaceous plant growing up to 80 cm and belongs to the family of mustard (Perry 1978; Yaniv et al. 1998; Rani et al. 2010). The leaves and sprouts of the plant are widely used in salads for their hot pungent taste and can add flavor to any salad (Uphof 1968; Flanders and Abdulkarim 1985; Yaniv et al. 1998). It is known as diuretic, anti-inflammatory, antibacterial (Gulfraz et al. 2011), and also acts as an anticancer agent (Michael et al. 2011). Thiocyanates, isothiocyanates and their precursors, the glucosinolates, have been extensively studied in seeds of E. sativa (Mahran et al. 1992; Kim et al. 2007). Three new quercetin glycosides were isolated from leaves of E. sativa (Mill.) (Weckerle et al. 2001). In a study by Michael et al. (2011), aqueous extract of E. sativa fresh leaves, revealed the presence of nine natural flavonoid compounds (Michael et al. 2011). In terms of antioxidant compounds E. sativa is a good source of vitamins, like vitamin C, carotenoids, and polyphenols, which play a very important role among natural antioxidants (Martinez-Sanchez et al. 2008). E. sativa is known to ameliorate hyperglycemia and also considering the potential flavonoidal moieties present in the plant gives the idea that it can potentially treat diabetes as well as the complications associated with diabetes (El-Missiry and El Gindy 2000). Taking into consideration the promising constituents present in E. sativa and its ameliorating effect on diabetes and oxidative stress, the present study involves the evaluation of E. sativa and its isolate Kaempferol (KP) on diabetic neuropathy.
Materials and methods
Chemicals
Streptozotocin (STZ) was obtained from Sigma-Aldrich, USA, and Nicotinamide (NAD) from Finar India Ltd., and diagnostic kits for the biochemical estimations were obtained from Reckon Diagnostics Pvt. Ltd., India. All the other chemicals used were of analytical grade.
Collection of plant material
Seeds of E. sativa Mill (Brassicaceae) were procured from local supplier and identified by Dr. Sunita Garg, NISCAIR, New Delhi. A voucher specimen (NISCAIR/RHMD/Consult/2013/2296/76) was deposited in the herbarium of NISCAIR, New Delhi for future reference. The botanical name of the plant was verified from published literature and database (The Plant List 2016).
Preparation of extract
Seeds were powdered and extracted for potential antidiabetic constituents into different solvents (petroleum ether, chloroform, ethanol and hydroalcohol) in order of increasing polarity by soxhlet extraction method (Kaur and Kishore 2012). The extraction procedure was continued until the extract gave no coloration. Extracts obtained were then concentrated under reduced pressure and finally freeze dried.
Phytochemical screening
EHA was subjected to phytochemical screening for the identification of chemical constituents, including alkaloids, carbohydrates, fixed oils and fats, terpenoids, phenols, tannins, glycosides, proteins, saponins amino-acids and flavonoids (Kaur and Kishore 2012).
Determination of total phenol content
Total phenol content in EHA was assessed with Folin–Ciocalteau reagent using gallic acid as a standard phenolic compound. Briefly, aliquot of 1 ml of sample solution was mixed with 1 ml of Folin–Ciocalteau reagent. Three minutes later, 3.0 ml of 2% sodium carbonate was added and the mixture was allowed to stand for 3 h with intermittent shaking. The absorbance of the blue color that developed was measured at 760 nm. The concentration of total phenolic compounds in the extract was determined as milligram of gallic acid equivalent (GAE) per gram dry weight (Liu et al. 2013).
Isolation of the active compound
EHA (10 g) was chromatographed using silica gel (100–200 mesh; Merck). Elution was started with water, followed by increasing concentration of ethanol gradually to obtain 6 fractions. KP was isolated from the last fraction and was characterized using NMR, mass spectroscopy and from literature (Michael et al. 2011).
Experimental procedure
Animals
The male Wistar rats weighing 280–300 gm were kept in clean polypropylene cages and maintained at standard environmental conditions (temperature 24 ± 2 °C, humidity 45 ± 5% and 12 h day/night cycle). The animals were fed with standard pellet diet (Ashirwad Industries, Mohali, Punjab, India) and water ad libitum. The experimental protocol was approved by the Institutional Animals’ Ethics Committee and by the regulatory body of the government (MMCP/IAEC/13/09).
Diabetes induction
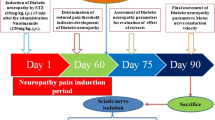
Diabetic mellitus was induced by single intraperitoneal injection of freshly prepared STZ (single dose of 65 mg/kg, b.w.) in 0.1 M citrate buffers (pH 4.5) 15 min after NAD (230 mg/kg, i.p.), in a volume of 1 ml/kg body weight (Bhateja and Singh 2014). After 72 h of STZ administration rats with a fasting blood glucose level of 300–350 mg/dl were selected for the study. At the end of 60 days the body weight, blood glucose, insulin, behavioral parameters (thermal and mechanical hyperalgesia) and motor nerve conduction velocity (MNCV) were measured to assess development of diabetic neuropathy.
Treatment schedule
In the experiment, a total of 48 rats (6 normal; 42 STZ-diabetic rats) divided into eight groups consisting of six rats each were used. Group 1 was normal control; Group 2 was diabetic neuropathy (DN) control; Group 3–5 consisted of DN rats receiving 100, 200 and 400 mg/kg of EHA; Group 6–7 consisted of DN rats receiving 5 and 10 mg/kg of KP; Gabapentin, 30 mg/kg, was used as standard (Group 8). Treatment with extracts, KP and Gabapentin were started after 60 days of STZ-NAD administration and continued to next 30 days (Fig. 1). Animals were sacrificed under deep anesthesia at the end of the study and sciatic nerve was removed and stored at − 70 °C until use.
Body weight, blood glucose and serum insulin estimation
Body weight of each animal was measured at the start of study and animals with similar weight were grouped together. Randomizations of animals were done at the start of treatment protocol. Body weight of each group was measured periodically till end of study. Fasting blood glucose level was estimated at interval of 15 days by using commercial enzymatic kits purchased from Reckon Diagnostics Pvt. Ltd. INDIA throughout the study. Plasma insulin was determined by Insulin ELISA kit (DRG, Germany) in blood collected into tubes with anticoagulant.
Behavioral assessment
Assessment of thermal hyperalgesia
Tail-immersion method. Test was performed by immersing the tail of rat in hot water bath (52.5 ± 0.5 °C) until the animal shows the response of tail withdrawal (flicking response) or signs of struggle were observed (cut-off 12 s). Shortening of the tail withdrawal time indicates hyperalgesia (Kannan et al. 1996).
Hot-plate method. The hyperalgesic response on the hot-plate is considered as a result of both central and peripheral mechanisms (Kannan et al. 1996). Animals were placed individually on a hot-plate (Eddy’s Hot-Plate) with the temperature adjusted to 55 ± 1 °C. The latency to the first sign of paw licking or jump to avoid the heat was taken as an index of the pain threshold (cut-off time was 10 s).
Assessment of mechanical hyperalgesia
Paw pressure thresholds were registered with the paw pressure analgesy meter for the Randall–Selitto test (Ugo Basile, Italy). Increasing pressure was applied to the center of the hind paw at a linear rate of 10 gm/s, with the cut-off of 250 gm to avoid tissue injury. Five tests separated by at least 15 min were performed for each animal, and the mean value of these tests was calculated.
Assessment of allodynia
Tactile responses were assessed by quantifying the withdrawal threshold of the hind paw in response to stimulation via flexible von Frey filaments (Ilnytska et al. 2006). Rats were placed in individual boxes on a stainless steel mesh floor and were allowed to adapt for at least 20 min. A series of calibrated von Frey filaments (range 0.4–64 gm; Ugo Basile, Italy, Woodland Hills, CA) was applied perpendicularly to the plantar surface of a hind paw with sufficient force to bend the filament for 6 s. Withdrawal or paw flinching was considered as a positive response. The test was repeated four to five times at interval of 5-min on each animal. The mean value was calculated.
Motor nerve conduction velocity (MNCV)
The recording of MNCV was performed in rats according to the method described previously (Morani and Bodhankar 2008). Briefly, rats were anesthetized using thiopental sodium (50 mg/kg, i.p.) for electrophysiological recording. The dorsal side of rat’s paw was shaved and MNCV was recorded by stimulating the sciatic and tibial notch respectively. Electrical current application started with a duration of 1 ms and an intensity of 10 µA produced by an electric stimulator (BIOPAC Systems, Inc., USA). Electric stimulator produced a biphasic current pulse (100 µs pulse width, 20 Hz pulse rate, 1–2 µA amplitude) that was applied to the nerve for five times and results were recorded. The intensity was gradually increased until the supra-maximal stimulation that ensured maximal amplitude was reached (1–2 mA). The recorded signals were digitally converted with MP 30 system (BIOPAC Systems Inc, CA, USA) and the latency period was measured (Coppey et al. 2000).
The value of latency time was taken from terminating point of artifact to peak of very first response after artifact as shown in Fig. 1b. The whole MNCV procedure and calculations are described in Fig. 2. The MNCV was determined using the following formula:
where distance between two stimulation points, i.e., tibial and sciatic notch is measured in mm and MNCV is motor nerve conduction velocity (m/s).
Biochemical analysis
For biochemical estimations, a 10% (w/v) tissue homogenate (sciatic nerve) was prepared in chilled phosphate buffered saline (pH 7.4) using a Teflon homogenizer. The homogenate was centrifuged at 1000 gm for 10 min at 4 °C to remove nuclei and unbroken cells. The pellet was discarded and clear supernatant thus obtained was used to assay thiobarbituric acid reactive substances (TBARS) (Ohkawa et al. 1979) and level of antioxidant enzymes, viz. superoxide dismutase (SOD) (Wang et al. 1998) and reduced glutathione (GSH) (Beutler et al. 1963).
Estimation of superoxide dismutase
The superoxide anion generation was estimated in terms of measuring reduced nitroblue tetrazolium (NBT) (Wang et al. 1998). Weighed amount of tissue (25 mg) was taken in 5 ml phosphate buffered saline containing 100 μM of NBT and incubated at 37 °C for 1.5 h. The NBT reduction was stopped by adding 5 ml of 0.5 M HCl. Then, the left ventricular tissue was taken out and was minced and homogenized in a mixture of 0.1 M sodium hydroxide and 0.1% sodium dodecyl sulphate in water containing 40 mg/l diethylene triamine penta acetic acid. The mixture was centrifuged at 20,000g for 20 min and the resultant pellet was suspended in 1.5 ml of pyridine and kept at 800 °C for 1.5 h to extract formazan, an adduct formed after reaction of NBT with superoxide anions. The mixture was again centrifuged at 10,000 gm for 10 min and absorbance of formazan was determined spectrophotometrically at 540 nm. The amount of reduced NBT was calculated using the formula
where “A” is absorbance, “V” is volume of pyridine (1.5 ml), “T” is time for which the tissue was incubated with NBT (1.5 h), “Wt” is wet weight of tissue (25 mg), “ε” is extinction coefficient (0.72 l/mmol/mm) and “l” is length of light path (1 cm). Results were expressed as reduced NBT picomole per min per mg of wet tissue.
Estimation of reduced glutathione
The reduced glutathione content in tissues was estimated using method of Beutler et al. (1963). The supernatant of homogenate was mixed with trichloroacetic acid (10% w/v) in 1:1 ratio. The tubes were centrifuged at 1000 gm for 10 min at 4 °C. The supernatant obtained (0.5 ml) was mixed with 2 ml of 0.3 M disodium hydrogen phosphate. Then 0.25 ml of 0.001 M freshly prepared DTNB [5,5′-dithiobis (2-nitrobenzoic acid) dissolved in 1% w/v citric acid] was added and absorbance was noted spectrophotometrically at 412 nm. A standard curve was plotted using 5–50 μM of reduced form of glutathione and results were expressed as micromoles of reduced glutathione per mg of protein.
Estimation of TBARS
The quantitative measurement of TBARS, a measure of lipid peroxidation, was assayed by the method of Ohkawa et al. (1979). 0.2 ml of supernatant of homogenate was pipetted out in a test tube, followed by addition of 0.2 ml of 8.1% sodium dodecyl sulphate, 1.5 ml of 30% acetic acid (pH 3.5), 1.5 ml of 0.8% of thiobarbituric acid and the volume was made up to 4 ml with distilled water. The test tubes were incubated for 1 h at 950 °C, then cooled and added 1 ml of distilled water followed by addition of 5 ml of n-butanol-pyridine mixture (15:1 v/v). The tubes were then centrifuged at 4000 gm for 10 min. The absorbance of developed pink color was measured spectrophotometrically at 532 nm. A standard calibration curve was prepared using 1–10 nM of 1,1,3,3-tetra methoxy propane. The TBARS values were expressed as nanomoles per mg of protein.
Nitrite estimation
Nitrite was estimated in the post-mitochondrial supernatant using the Greiss reagent and served as an indicator of nitric oxide production. A measure of 0.5 ml of Greiss reagent (1:1 solution of 1% sulphanilamide in 5% phosphoric acid and 0.1% naphthylamine diamine dihydrochloric acid in water) was added to 0.1 ml of sciatic nerve homogenate and the absorbance was measured at 546 nm (Green et al. 1982). The nitrite concentration was calculated using a standard curve for sodium nitrite. Nitrite levels were expressed as μg/ml.
TNF-α, TGF-β and IL-1β estimation
The quantification of TNF-α, TGF-β and IL-1β were done by the help and instructions provided by R&D Systems Quantikine TNF-α, TGF-β and IL-1β immunoassay kit.
AGEs estimation in sciatic nerve
AGEs levels in the sciatic nerve were determined by a method as previously described by Sensi et al. (1996). Briefly, sciatic nerve was homogenized in 2 ml of 0.25 M sucrose followed by centrifugation at 900 gm at 5 °C and the supernatant was separated. The pellet was resuspended in 2 ml sucrose solution and centrifuged and the supernatant obtained was mixed with the previous one. The proteins present were precipitated by adding equal volume of trichloroacetic acid (TCA). Following centrifugation at 4 °C with 900 gm, the protein pellet obtained was mixed with 1 ml methanol twice to remove the lipid fraction. The insoluble protein, after washing with 10% cooled TCA was centrifuged and the residue was solubilized in 1 ml of 1 M NaOH and the protein concentration was estimated by measuring the absorbance at 280 nm against BSA standard curve. The AGEs content was then measured fluorometrically with an emission at 440 nm and excitation at 370 nm, and the results were expressed as relative fluorescence units (RFU)/mg protein.
Statistical analysis
Statistical analysis was carried out using Graphpad Prism 6. Values were expressed as mean ± SEM and one-way analysis of variance (ANOVA) was used for statistical analysis. ANOVA was followed by Tukey’s as post hoc multiple comparison test. The results were considered significant if p ≤ 0.05.
Results
Phytochemical screening
Preliminary phytochemical screening of EHA showed the presence of carbohydrates, flavonoids, and terpenoids. Total phenol content present in EHA contained 915 mg/gm GAE of phenol content.
Identification of compound
KP was obtained as yellow crystalline powder with M.P. 275–277 °C. Chemical tests of flavonoids were found positive and significant in hydroalcoholic extract of E. sativa. The UV λ max (265 and 280 nm) and IR maxυKBr spectrum exhibited peaks (cm−1) at 32,302 (–OH), 1650 (C=O, aryl ketonic stretch), 1617 (C–C, aromatic ring stretch), 1342 (O–H bending of phenols), 1241, 1210, 1165, 998, 846, 581 have shown positive assignments for flavonoids. The structure of the KP was determined using 1H NMR and 13C NMR (Table 1). The physical and spectral data were in complete agreement with those reported in our previous literature for KP (Sikorska and Matławska 2001).
Effect of Eruca sativa on body weight
Body weight of DN control rats reduced by 16.49% at the end of study as compared to normal control rats. Administration of EHA at a dose of 100, 200 and 400 mg/kg increased the body weight significantly by 2.76, 8.63 and 12.12% respectively Whereas, KP increased the body weight by 8.04 and 11.23% respectively at dose of 5 and 10 mg/kg. But Gabapentin (30 mg/kg), a standard drug for neuropathic pain slightly increased the body weight by 3.11% in diabetic neuropathy rats (Table 2).
Effect of Eruca sativa on blood glucose and serum insulin level
Administration of STZ-NAD intraperitoneally increased serum blood glucose level significantly in 60 days with respect to normal control rats. Treatment with EHA (100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) significantly reduced fasting blood glucose level by the end of study (90th day) compared with DN control rats. A significant decrease in fasting insulin level (7.00 ± 0.12 μIU/ml) was observed in STZ- treated diabetic neuropathy rats in comparison to normal control rats (15.07 ± 0.08 μIU/ml). Administration of 100, 200 and 400 mg/kg of EHA and 5 and 10 mg/kg of KP for 30 days produced significant increase in serum insulin level (Table 2).
Effect of Eruca sativa on thermal hyperalgesia
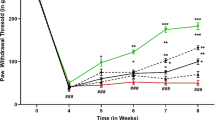
A significant decrease in nociceptive pain threshold was observed in diabetic neuropathy rats tested in both hot plate and tail immersion method. In tail immersion method, oral administration of EHA at doses of 100, 200 and 400 mg/kg for 30 days result in significant increase in reaction time i.e. 7.53 ± 0.20, 9.25 ± 0.08 and 12.12 ± 0.20 s respectively whereas with KP, significant increase in reaction time to 6.00 ± 0.22 and 7.73 ± 0.21 s respectively. Similarly, in hot plate method pain threshold increased by EHA (100, 200 and 400 mg/kg) by 5.01 ± 0.13, 6.21 ± 0.03 and 8.16 ± 0.16 s respectively; KP (5 and 10 mg/kg) increased the pain threshold to 4.40 ± 0.09, 5.18 ± 0.16 s. Hyperalgesia was evident after 60 days of STZ-NAD administration in both hot plate and tail immersion method. Administration of EHA (100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) produced a dose and time dependant increase in pain threshold in comparison to DN control rats (Fig. 3).
Effect of EHA (100, 200 and 400 mg/kg, p.o) and KP (5 and 10 mg/kg, p.o.) treatment for a period of 30 days on pain threshold in (a) hot-plate and (b) tail-immersion assays in diabetic rats. Values are expressed as mean ± SEM (n = 6 in each group). Data were analyzed by using one-way ANOVA followed by Tukey’s multiple test; a vs control; b vs Diabetic control; c vs E. sativa extract 100 mg/kg; d vs E. sativa extract 200 mg/kg.; e vs Gabapentin 30 mg/kg, i.p. * p < 0.001, # p < 0.01, † p < 0.05
Effect of Eruca sativa on mechanical hyperalgesia
On 90th day of study, paw withdrawal threshold in the Randall–Sellito and tactile withdrawal threshold due to light touch was reduced significantly in comparison to DN control. EHA and KP corrected diabetes-induced decrease in paw withdrawal threshold in both the tests in comparison to DN control group (Fig. 4). Paw withdrawal threshold in Randall–Sellitto test was significantly reduced after 60 days of STZ-NAD administration in DN control rats (46.15 ± 2.20 gm) in comparison to normal control rats (158.58 ± 2.43 gm). Treatment with EHA (100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) increase the paw withdrawal threshold i.e. 88.53 ± 1.99, 110.75 ± 1.41 and 126.65 ± 1.86; 68.33 ± 1.52, 79.48 ± 1.61 gm respectively.
Effect of EHA (100, 200 and 400 mg/kg, p.o.) and KP (5 and 10 mg/kg, p.o.) treatment for a period of 30 days on mechanical hyperalgesia in (a) Randell–Selitto and (b) tactile allodynia (c) MNCV in diabetic rats. Values are expressed as mean ± SEM (n = 6 in each group). Data were analyzed by using one-way ANOVA followed by Tukey’s multiple test; a vs control; b vs Diabetic control; c vs E. sativa extract 100 mg/kg; d vs E. sativa extract 200 mg/kg; e vs Gabapentin 30 mg/kg, i.p. * p < 0.001, # p < 0.01, † p < 0.05
Induction of DN with STZ (i.p.) significantly (p < 0.001) reduced paw withdrawal threshold in tactile withdrawal test by 60th days in DPN control rats (16.40 ± 1.26 gm) in comparison to normal control rats (62.50 ± 1.26 gm). Treatment EHA and KP increased the paw withdrawal threshold significantly in a dose dependant manner from 60th to 90th day. Treatment with EHA (100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) increased the tactile withdrawal threshold i.e. 28.68 ± 0.40, 36.50 ± 0.72 and 54.00 ± 1.69; 24.43 ± 0.69, 25.98 ± 0.33 gm, respectively at the end of study.
Effect of Eruca sativa on motor nerve conduction velocity (MNCV)
Motor nerve conduction velocity in diabetic control rats was 20.83 ± 0.80 m/s on day 60th while it was 55.00 ± 0.58 m/s in normal rats. There was no significant change in the motor nerve conduction velocity of normal rats over the time period of 90th day. Whereas, MNCV further decreased significantly to 19.18 ± 0.68 m/s in the diabetic control rats by 90th day of intraperitoneal injection of STZ-NAD as compared to normal rats. Treatment with EHA (100, 200 and 400 mg/kg) for 30 days significantly and dose dependently attenuated (p < 0.001) these decreased motor nerve conduction velocities (37.05 ± 0.701, 42.05 ± 1.93 and 48.33 ± 1.67 m/s respectively) as compared to diabetic control rats. However, treatment with KP (5 and 10 mg/kg)also significantly increased motor nerve conduction velocity (36.76 ± 0.79 and 47.02 ± 0.92 m/s, p < 0.001) as compared to Gabapentin (41.65 ± 0.36 m/s) (Fig. 4).
Effect of Eruca sativa on antioxidant enzymes and lipid peroxidation
The levels of reduced glutathione (GSH) and superoxide dismutase (SOD) was found significantly high after 60 days of STZ-NAD induction in diabetic neuropathic rats as compare to normal control group. Continuous administration of EHA and KP significantly reduced the level of SOD and GSH at end of study (90th day).Moreover, the increased level of TBARS significantly reduced by EHA and KP (Table 2).
Effect of Eruca sativa on nitrite level
Nitrite level in sciatic nerve was significantly elevated in the diabetic rat (Table 2). Treatment with EHA (100, 200 and 400 mg/kg) and KP (5 and 10 mg/kg) significantly inhibited this increase in nitric oxide levels in comparison to DN control (p < 0.001).
Effect of Eruca sativa on AGEs in sciatic nerve
Induction of DN in rats led to a significant increase AGEs level in sciatic nerve when compared to normal animals. Administration of EHA (100, 200 and 400 mg/kg) and KP(5 and 10 mg/kg) significantly reduced (p < 0.001) the AGEs levels in sciatic nerve when compared to normal control group. However, administration of Gabapentin (30 mg/kg) also produced significant decrease (p < 0.05) in AGEs levels when compared to control group (Fig. 5).
Effect of EHA (100, 200 and 400 mg/kg, p.o.) and KP (5 and 10 mg/kg, p.o.) on AGEs formation, TNF-α, TGF-1β and IL-1β in sciatic nerve of diabetic rats. Values are expressed as mean ± SEM (n = 6 in each group). Data were analyzed by using one-way ANOVA followed by Tukey’s multiple test; a vs control; b vs Diabetic control; c vs E. sativa extract 100 mg/kg; d vs E. sativa extract 200 mg/kg; e vs Gabapentin 30 mg/kg, i.p. * p < 0.001, # p < 0.01, † p < 0.05
Effect of Eruca sativa on TNF-α, TGF-β and IL-1β
Level of TNF-α, TGF-β and IL-1β in sciatic nerve were markedly increased in diabetic as compared to normal control rats (Fig. 5). EHA and KP produced a dose dependent decrease in TNF-α, TGF-β and IL-1β levels in comparison to DN control rats (p < 0.001).
Discussion
The current study was performed to determine the therapeutic potential of hydro-alcoholic extract of E. sativa seeds and its isolated compound i.e., KP in the treatment of STZ-induced painful DN in Wistar rats. The biochemical parameters studied in the groups treated with EHA and KP were compared with standard drug Gabapentin. It is postulated that the antihyperalgesic action for Gabapentin is mediated by its binding to α2δ subunit on the voltage-dependent calcium channel. Two randomized, double-blind, placebo-controlled studies have been conducted on different doses of Gabapentin have shown significant reduction in DN than placebo. Gabapentin is approved by FDA for treatment of DN (Rice and Maton 2001).
The models of mechanical hyperalgesia and formalin evoked flinching in STZ-induced animals has been demonstrated by many researchers (Kishore et al. 2017; Kaur et al. 2017b). A significant decrease in nociceptive threshold of diabetic rats as observed in hot plate, tail immersion, Randall–Selitto and Von Frey hair test, indicating that diabetic animals exhibit thermal and mechanical hyperalgesia along with tactile allodynia. Treatment with EHA and KP significantly ameliorated thermal and mechanical hyperalgesia in a dose dependent manner. Reduced nerve conduction velocity has been attributed to nerve dysfunction due to pathological changes of arterioles perfusing the sciatic nerve in diabetic neuropathy (Kishore et al. 2017). MNCV was decreased in diabetic animals whereas elevated in a dose dependent manner in EHA and KP treated wistar rats. The decrease of MNCV upon treatment may be implicated to improvement of endoneurial blood flow thereby reversing endoneurial ischemia and ROS-mediated damage in the peripheral nerve tissue (Kishore et al. 2017; Kaur et al. 2017b). In a recent study by Tarozzi et al. (2012) the neuroprotective property of E. sativa was discussed that evidenced our study (Tarozzi et al. 2012).
Hyperglycemia leads to production of AGEs, which damage target cells by mechanisms that plasma proteins modified by AGE precursors bind to AGE receptors on endothelial cells, mesangial cells, microglia and macrophages, inducing receptor-mediated production of reactive oxygen species (ROS). This AGE receptor ligation activates transcription factor nuclear factor-κβ (NF κβ), leading to pro-inflammatory gene expression (Singh et al. 2013, 2014). It includes expression of cytokines and growth factors by macrophages and mesangial cells (IL-1 β, IGF-1, TNF-α, TGF-β), which induces apoptosis in neuronal cells. Uses of agents that suppress cytokine elevation have been advocated to be used to treat diabetic complication (Singh et al. 2013). Chronic treatment with EHA and KP significantly and dose dependently inhibited production and release of these inflammatory cytokines. These results are in line with the observation that ghrelin, a potent anti-inflammatory agent; by inhibiting proinflammatory cytokines such as TNF-α, IL-1β and IL-6, ameliorated neuropathic pain (Singh et al. 2013). It may be postulated that EHA and KP can directly reduce the formation of AGEs, thereby reducing neuronal apoptosis. A recent study by Kim et al. (2014) reported potent anti-inflammatory effects of E. sativa that evidenced the effects produced by EHA and KP against the release of inflammatory cytokines.
SOD and GSH are major antioxidant enzymes involved in protection from oxidative stress. Hyperglycemia caused a reduction in the activity of SOD and GSH in sciatic nerve of diabetic animals. Superoxide anions are also believed to cause increase in aldose reductase and protein kinase C activity which are further implicated in pain perception (Kaur et al. 2016b). SOD provides antioxidant protection from superoxide anions by transforming them to H2O2. Furthermore, abnormalities in GSH metabolism in diabetes may also lead to the enhanced TNF-α and IL-1β production (Kaur et al. 2016b; Kishore et al. 2016b, c, 2017). The SOD and GSH levels were significantly lowered in the sciatic nerve of diabetic animals which was restored on treatment with EHA and KP.
Further, total nitric oxide (NO), an indicator of nitrosative stress, is increased in the experimental model of DN. High glucose induces oxidative and nitrosative stress in neurons. NO combines with superoxide to form peroxynitrite, which rapidly causes protein nitration or nitrosylation, DNA damage and cell death and has direct toxic effects on the nerve tissue leading to neuropathic pain (Kishore et al. 2017; Kaur et al. 2017b). Treatment with EHA and KP at varying doses attenuated the increased NO levels.
Eruca sativa is a traditional herb used for the management of diabetes which supported our present study. Numerous studies suggested that E. sativa enriched with flavonoidal moieties (Kim et al. 2014; Bennett et al. 2006). The flavonoids are compounds with antioxidant properties, in addition to their anti-inflammatory and antinociceptive effects (Rice and Maton 2001; Tall and Raja 2004). The actual antidiabetic prospective associated with flavonoids are usually large as a result of their modulatory effects on blood sugar transporter by enhancing insulin secretion, reducing apoptosis and promoting proliferation of pancreatic β-cells, reducing insulin resistance, inflammation and oxidative stress. Previous findings suggested that flavonoids like quercetin, rutin, luteolin etc. (Kaur et al. 2016a, b) can attenuate neuropathic pain associated due to diabetes, which supported our present study. Presence of flavonoids in the hydroalcoholic extract was confirmed through our preliminary phytochemical screening. Moreover, KP (flavonoid moiety) isolated from EHA further evidenced the role of E. sativa in the amelioration of DN. As plants contain a complex blend of phytochemicals that addresses a problem via multiple mechanisms. The full medicinal value of herbs is most likely due to their internal complexity and to the interactions of the different components within the body rather than to one of its specific components. This hypothesis gives an idea that, KP isolated from E. sativa is a potential agent in the amelioration of neuropathic pain but the whole extract holds better activity.
One of the important consequences of chronic hyperglycemia is the enhanced oxidative stress resulting from an imbalance between the production and neutralization of ROS. Therefore, in this study we evaluated whether the mechanism behind the ameliorative action of E. sativa against DN is due to its action on hyperglycemia or inhibition of oxidative stress. The results of present study showed that the repeated treatment with EHA and KP resulted in a decrease of blood glucose level, increased level of antioxidant enzymes, decreased lipid peroxidation and AGE’s demonstrating that the relief of neuropathy occurred via affecting hyperglycemia, oxidative stress and AGE’s formation.
Conclusion
The findings suggest that supplementation with EHA and isolated compound (KP) from E. sativa ameliorates DN by attenuating oxidative stress mediated release of pro-inflammatory cytokines which might be responsible for diabetes-induced nerve damage. However, further studies are warranted to explore the exact mechanism of antinociceptive effect of E. sativa.
References
Bennett R, Rosa S, Mellon F, Kroon P (2006) Ontogenic profiling of glucosinolates, flavonoids and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J Agric Food Chem 54:4005–4015
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bhateja PK, Singh R (2014) Antidiabetic Activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana Polysaccharide on Streptozotocin–Nicotinamide induced diabetic rats. BioMed Res Int 2014:9
Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA (2000) Slowing of motor nerve conduction velocity in streptozotocin induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res 1:131–143
El-Missiry MA, El Gindy AM (2000) Amelioration of alloxan induced diabetes mellitus and oxidative stress in rats by oil of Eruca sativa seeds. Ann Nutr Metab 44:97–100
Flanders A, Abdulkarim SM (1985) The composition of seed and seed oils of Taramira (Eruca sativa). JAOCS 62:1134–1135
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126:131–138
Gulfraz M, Sadiq A, Tariq H, Imran M, Qureshi R, Zeenat A (2011) Phytochemical analysis and antibacterial activity of Eruca SativaSeed. Pak J Bot 43:1351–1359
Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG (2006) Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes 55:1686–1694
Kannan SA, Saade NE, Haddad JJ, Abdelnoor AM, Atweh SF, Jabbur SJ, Safieh-Garabedian B (1996) Endotoxin induced local inflammation and hyperalgesia in rats mince, a new model for inflammatory pain. Pain 66:373–379
Kaur N, Kishore L (2012) Antioxidant activity of methanolic extract of Phaseolus trilobus root powder. IJPPS 4:271–275
Kaur N, Kishore L, Singh R (2016a) Antidiabetic effect of new chromane isolated from Dillenia indica L. leaves in streptozotocin induced diabetic rats. J Func Foods 22:547–555
Kaur N, Kishore L, Singh R (2016b) Attenuating diabetes: what really works? Curr Diabetes Rev 12:259–278
Kaur N, Kishore L, Singh R (2017a) Chromane isolated from leaves of Dillenia indica improves the neuronal dysfunction in STZ-induced diabetic neuropathy. J Ethnopharmacol 206:19–30
Kaur N, Kishore L, Singh R (2017b) Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE’s and oxidative stress. J Food Sci Technol 54:408–421
Kim SJ, Kawaharada C, Jin S, Hashimoto M, Ishi G, Yamauchi H (2007) Structurtal elucidation of 4-(Cysteinyl-S-yl) butyl glucosinolate from the leaves of Eruca sativa. Biosci Biotechnol Biochem 71:114–121
Kim B, Choi YE, Kim HS (2014) Eruca sativa and its flavonoid components, quercetin and isorhamnetin, improve skin barrier function by activation of peroxisome proliferator-activated receptor (PPAR)-α and suppression of inflammatory cytokines. Phytother Res 28:1359–1366
Kishore L, Kaur N, Singh R (2016a) Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. J Food Drug Anal. https://doi.org/10.1016/j.jfda.2016.08.009 (in press)
Kishore L, Kaur N, Singh R (2016b) Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren Fail 38:1528–1544
Kishore L, Kaur N, Singh R (2016c) Distinct biomarkers for early diagnosis of diabetic nephropathy. Curr Diabetes Rev. https://doi.org/10.2174/1573399812666161207123007 (In Press)
Kishore L, Kaur N, Singh R (2017) Bacosine isolated from aerial parts of Bacopa monnieri improves the neuronal dysfunction in Streptozotocin-induced diabetic neuropathy. J Func Foods 34:237–247
Liu J, Jia L, Kan J, Jin CH (2013) In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 51:310–316
Mahran GH, Kadry HA, Thabet CK, Olemy MM, Al-Azizi MM, Schi PL, Wong LK (1992) GC/MS analysis of volatile oil from Eruca sativa seeds. Int J Pharmacog 30:135–137
Martinez-Sanchez A, Gil-Izquierdo A, Gil MI, Ferreres F (2008) A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J Agric Food Chem 56:2330–2340
Michael HN, Shafik RE, Rasmy GE (2011) Studies on the chemical constituents of fresh leaf of Eruca sativaextract and its biological activity as anticancer agent in vitro. J Med Plant Res 5:1184–1191
Morani A, Bodhankar S (2008) Neuroprotective effect of vitamin E acetate in models of mononeuropathy in rats. Neuroanatomy 7:33–37
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Perry LM (1978) Medicinal plants of East and Southeast Asia: attributed properties and uses. MIT Press, Cambridge
Rani I, Akhund S, Suhail M, Abro H (2010) Antimicrobial potential of seed extract of Eruca Sativa. Pak J Bot 42:2949–2953
Rice ASC, Maton S (2001) Postherpetic Neuralgia Study Group. Gabapentin in post-therapeutic neuralgia: a randomised, double blind, placebo controlled study. Pain 94:215–224
Sensi M, Pricci F, Pugliese G, De Rossi MG, Petrucci AF, Cristina A, Morano S, Pozzessere G, Valle E, Andreani D, Di Mario U (1996) Role of advanced glycation end-products (AGE) in late diabetic complications. Diabetes Res Clin Pract 28:9–17
Sikorska M, Matławska I (2001) Kaempferol, isorhamnetin and their glycosides in the flowers of Asclepias syriaca L. Acta Pol Pharm 58:269–272
Singh R, Kaur N, Kishore L, Gupta GK (2013) Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol 150:51–70
Singh R, Kishore L, Kaur N (2014) Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 80:21–35
Tall JM, Raja SN (2004) Dietary constituents as novel therapies for pain. Clin J Pain 20:19–26
Tarozzi A, Morroni F, Bolondi C, Sita G, Hrelia P, Djemil A, Cantelli-Forti G (2012) Neuroprotective effects of Erucin against 6-hydroxydopamine-induced oxidative damage in a dopaminergic-like neuroblastoma cell line. Int J Mol Sci 13:10899–10910
The Plant List (2016) A working list of all plant species. Webpage at: https://www.theplantlist.org
Uphof JCT (1968) Dictionary of Economic Plants, 2nd edn. Verlag Von J. Cramer Publ, New York, p 591
Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA (1998) Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res 82:810–818
Weckerle B, Michel K, Balazs B, Schreier P, Toth G (2001) Quercetin 3,30,40-tri-O-b-d-glucopyranosides from leaves of Eruca sativa (Mill.). Phytochemistry 57:547–551
Yaniv Z, Schafferman D, Amar Z (1998) Tradition, uses, and biodiversity of rocket (Eruca sativa) in Israel. Econ Bot 52:394–400
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Kishore, L., Kaur, N. & Singh, R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacol 26, 993–1003 (2018). https://doi.org/10.1007/s10787-017-0416-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0416-2