Abstract
To investigate the influence of Moringa seed extract (MSE) on the cerebral Nrf2/NQO1 signaling in TiO2-NPs–induced brain damage, 80 male albino rats were divided into four groups (n = 20); group I was used as a control, group II received TiO2-NPs (500 mg/kg b.w/day orally) for 14 days, group III received MSE (100 mg/kg b.w/day orally) for 30 days, and group IV received MSE an hour before TiO2-NPs administration with the same doses as before. Administration of TiO2-NPs was started on the 17th day for both groups (II) and (IV). Administration of MSE significantly increased the cerebral mitochondrial viability and Nrf2 level with a simultaneous increase of NQO1 mRNA expression. This designates a powerful antioxidant effect of MSE which is indicated by a significant reduction of INOS expression, MDA, TOS, OSI levels, and DNA fragmentation % with a significant increase of GSH concentration, SOD activities, and TAC. MSE possesses an anti-inflammatory effect by a significant reduction of IL-1β and TNF-α levels, and anti-apoptotic effect manifested by a significant reduction of caspase-3 and Fas levels. In harmonization, dopamine, serotonin concentrations, and acetylcholinesterase activities return back to normal as compared to control group. These results were confirmed by the histopathological features which were alleviated with MSE administration. In conclusion, Nrf2 plays a pivotal role in the mechanism of TiO2-NPs cerebral toxicity and MSE as a Nrf2 activator can provide a powerful cerebroprotective effect, whereas MSE increased the Nrf2 expression and consequently restore the antioxidant activity of brain cells by increasing NQO1 gene expression and cerebral mitochondrial viability as well as inhibition of pro-inflammatory and apoptotic mediators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide nanoparticles (TiO2-NPs) possess different physicochemical characters matched to their fine particle analogs, which might modify their bioactivity. Earlier studies have revealed that TiO2-NPs are more toxic than fine particles (Fabian et al. 2008). TiO2-NPs may transport from the gastrointestinal tract and lung to the systemic organs. Oral exposure generally occurs via consumption of food products containing TiO2-NPs additives (Shi et al. 2013). TiO2-NPs is a bright white pigment with very high refractive index. TiO2-NPs are commonly used in industrial and commercial products worldwide (Shukla et al. 2011). TiO2-NPs can be used as a photocatalyst (Gurr et al. 2005), in paints, inks, papers, plastics, food products, toothpaste, pharmaceuticals, cosmetics (Wolf et al. 2003), and sunscreens (Sadrieh et al. 2010). Before accumulating in the brain tissues, TiO2-NPs can be rapidly absorbed via many routes as the olfactory nerve translocation (Wang et al. 2008), the placental barrier (Tsyganova et al. 2014), and blood-brain barrier (Song et al. 2015).
Neurons and glial cells are the most commonly affected cells by TiO2-NPs. TiO2-NPs encourage reactive oxygen species (ROS) production, apoptosis, and inflammation and cause brain dysfunctions, neurodegenerative disorders, and cell death (Long et al. 2007). Inflammation and mitochondrial dysfunction are linked to oxidative stress cascades and are considered as important mechanisms in neurodegeneration. Glial cells with injured mitochondria can generate excess ROS and pro-inflammatory molecules that could be toxic to cells and other neurons in the central nervous system (CNS) (Sun et al. 2007). ROS production can initiate a series of toxic oxidative reactions (Han et al. 2006) accompanied by downregulation of the nuclear erythroid-related factor 2 (Nrf2) genes (Clements et al. 2006; McCoy and Cookson 2011). Nrf2 is considered the main regulator of antioxidant response. Nrf2 activation decreases oxidative stress in the brain and helps manage neurodegenerative diseases through upregulation of antioxidants, inhibition of inflammation, and keeping of mitochondrial function and protein homeostasis (Dinkova-Kostova et al. 2018).
Nrf2 activates the expression of a group of cytoprotective phase II antioxidant enzymes, such as heme-oxygenase-1 (HO-1), superoxide dismutase (SOD), glutathione peroxidase (GPX), and glutathione reductase (GR). One of the most important antioxidant pathways regulated by Nrf2 is the production and regenerating glutathione (GSH) and NAD (P) H: quinone oxidoreductase 1 (NQO1) (Gorrini et al. 2013). NQO1 is a cytosolic homodimeric flavoprotein that catalyzes two-electron depletion and detoxification of highly reactive quinones and its derivatives, protecting cells from the oxidative stress, redox cycling, and neoplastic injury (Dinkova-Kostova and Talalay 2000). Malondialdehyde (MDA) is the major metabolite of arachidonic acid and acts as a reliable biomarker for oxidative stress (IARC 1985). MDA is the best indicator of lipid peroxidation and the consequent oxidative stress.
Medicinal plants are incorporated for centuries into traditional medicine (Sermakkani and Thangapandian 2012). The plants are considered a rich source of secondary metabolites with remarkable biological activities (El-Shemy et al. 2007) and other phytochemicals which can be used in drug synthesis.
Moringa oleifera, belonging to the family of Moringaceae, is rich in nutrients owing to the presence of several important bioactive ingredients in the seeds, leaves, and pods of the plant. In fact, Moringa provides vitamin A, vitamin C, calcium, potassium, iron, and protein (Rockwood et al. 2013). Moringa oleifera has been reported to have neuroprotective (Panda et al. 2013), the powerful antioxidant, anti-inflammatory, and antibacterial properties of its phytochemicals, such as flavonoids and polyphenols (Mbikay 2012). Polyphenols, flavonoids, and terpenoids can motivate key transcription factors like Nrf2 which regulates the antioxidant response and phase II detoxifying enzymes and thereby eliminate toxic ROS (Li et al. 2012). Eugenol that is present in abundance in Moringa seeds is a well-known activator of Nrf2 (Rajappa et al. 2017). Indeed, targeting of the Nrf2 signaling may provide a therapeutic strategy for mediating antioxidant and anti-inflammatory responses which are the underlying mechanisms for the treatment of neurodegenerative disorders. Previous studies have focused on the use of leaves’ extract of moringa and studied its protective mechanism in other tissues rather than the brain. Therefore, the seed extract of moringa (MSE) is used in the current study to evaluate its role in Nrf2/ NQO1 signaling pathway in TiO2-NPs–induced oxidative brain damage.
Materials and methods
Chemicals
TiO2 particles and acetylcholinesterase kit were obtained from Sigma Aldrich/Egypt. Reduced glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), and total antioxidant capacity (TAC) commercial kits were purchased from Biodiagnostic Company for research kits, Egypt. Rat Dopamine ELISA kit (catalog number MBS725908), and Rat 5 Hydroxytryptamine (5-HT) ELISA kit (catalog number MBS725497) were supplied by R&D system, USA. Rat Interleukin-1β (IL-1β) (catalog number K 0331212) ELISA kit was purchased from Komabiotech Company, Korea. Rat TNF-α ELISA kit (catalog number RTAOO-SRTAOO-PRTAOO) was purchased from Quantikine R&D Systems, Inc. USA. Rat Caspase-3 ELISA kit (catalog number PTE-CASP3-D175) and Rat Fas ELISA kit (catalog number ELR-FAS) were purchased from Ray Biotech Inc., Georgia, USA. The other chemicals used in the experiment are of high grade and have been obtained from Sigma, USA.
TiO2-NPs preparation
TiO2-NPs were prepared by high-energy ball mill (HEBM) technique in Nanoparticles Lab, Faculty of Postgraduate Studies for Advanced Sciences, Beni-Suef University. This is an effective and productive process of grinding solid technique, which is used to obtain nanopowders in a high-energy planetary with an average particle size of less than 100 nm. Obtaining TiO2-NPs nanoparticles was done according to the method that was described by Gusev (2007) and Gusev and Kurlov (2008).
TiO2-NPs characterization by TEM electron microscope
The characterization of TiO2-NPs was done at the National Research Center, Dokki, Giza, Egypt, by using a TEM electron microscope (Model: JEM-2100, JEOL Ltd., Tokyo, Japan). A droplet of TiO2-NPs suspension was dropped onto copper grids and allowed to dry in the air before observation under high-resolution TEM.
Preparation of Moringa seed extract
The seeds of Moringa oleifera were obtained from the Agricultural Research Center, Egypt. The seeds were cleaned, dried in shade, and finely powdered using an electric mixer. The ethanol extract was made from the seed powder according to the procedure designated by Harborne (1973) and Culei (1964) as follows; 1 kg of dried powdered Moringa oleifera seeds was extracted successively with 3 l of 70% ethanol and kept at room temperature for 72 h. The extracts were filtered using Whatman No.1 filter paper. The combined extracts were then evaporated at 40 °C on a water bath and the dried crude extract was kept at 4 °C in a dark sterile container for analysis and use.
Chemical composition of Moringa ethanol extract by gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS is a combination of two different analytical techniques, gas chromatography and mass spectrometry. The chemical composition of MSE samples was performed using Trace GC Ultra-ISQ mass spectrometer (Thermo Scientific, Austin, TX, USA) with a direct capillary column TG-5MS (30 m × 0.25 μm × 0.25 μm film thickness). To be identified, the components retention time and mass spectra were compared with those of WILEY 09 and NIST 11 mass spectral database (NIST, the National Institute of Standards and Technology, UK, 1998) and confirmed by comparing the mass spectra of the peaks and those from the study of Okwu and Ighodaro (2010).
Preliminary quantitative phytochemical analysis
Preliminary quantitative analyses of phytochemical constituents of Moringa oleifera were done using previously reported protocols. Total phenolic content was estimated according to the Folin-Ciocalteau phenol reagent method (Katsube et al. 2004). The total flavonoid content was measured based on the method of Lin and Tang (2006). The antioxidant activity of the extract was assessed by the method described by Prieto et al. (1999). The data existing are average values of 5 measurements for each sample and were expressed as the number of milligram of gallic acid equivalents (GAE)/gram of dried plant extract.
In vitro hydrogen peroxide (H2O2) scavenging activity of MSE
The ability of Moringa oleifera seed extract to scavenge H2O2 was determined according to the method of Nabavi et al. (2008). The percentage of H2O2 scavenged was calculated using the following formula:
Toxicity test of MSE
Acute toxicity was tested in 40 male albino rats (180–250 g). Rats were randomly divided into 4 groups (n = 10) as follows; Control received a saline solution. Group 2 received MSE 100 mg/kg, orally. Group 3 received MSE 200 mg/kg, orally. Group 4 received MSE 500 mg/kg, orally. The signs of toxicity were carefully observed during the first 30 min and hourly for 3 h, then during the first 24 h, and then daily for 14 days for delayed toxicity or mortality.
Experimental animals
The present study was carried out on 80 adult male albino rats ranging between 180 and 250 g body weight. They were obtained from the Helwan Farm of Laboratory Animals, Cairo, Egypt. Rats were kept under observation for 1 week before the onset of the experiment to be acclimatized. Afterward, they were housed in groups in metal cages (each cage contained 5 rats), at room temperature (25 ± 2 °C), humidity (70%) under 12 h light-dark cycle. Rats had free access to diet and water. Body weights were recorded weekly. All experimental measures were performed according to the recommendations for the care and use of laboratory animals and in accordance with the local Animal Care and Use Committee at Beni-Suef University with an approval number (018-8).
Experimental design
The rats were randomly distributed into four equal groups (20 rats each) and treated as follows:
Group I (control)
The rats were given distilled water for 30 days orally by stomach tube.
Group II (TiO2-NPs)
Rats were given TiO2-NPs for 14 days (17th–30th day) at a dose of 500 mg/kg b.w (Rizk et al. 2017). The particles were freshly suspended in distilled water and dispersed by ultrasonic vibration for 15 min then administered orally by stomach tube. The dose selection was based upon the LD50 of TiO2-NPs which exceeded 5000 mg/kg b.w in rats and mice after oral administration (Warheit et al. 2007). In addition, the oral route administration was selected as it is the main route for entry of nanoparticles into the body.
Group III (MSE)
Rats were given MSE (100 mg/kg b.w) for 30 days orally by stomach tube (Chivapat et al. 2012).
Group IV (MSE+ TiO2-NPs)
Rats were given MSE (100 mg/kg b.w) for 30 days, interrupted by TiO2-NPs 500 mg/kg b.w at 17th–30th day orally by stomach tube.
Tissue homogenates for measurement of oxidative/antioxidant parameters and biochemical parameters
To obtain a uniform suspension, 0.5 g of fresh brain tissue sample was homogenized in 5 ml phosphate buffer saline (pH 7) by using a homogenizer (Ortoalresa, Spain). The homogenate was kept at − 80 °C for further biochemical investigations.
Biochemical assays
Determination of dopamine and serotonin (5-HT) by ELISA kits
Dopamine and serotonin concentrations were measured in rat brain tissues by competitive enzyme-linked immunosorbent assay (ELISA) using rat dopamine and Rat 5 hydroxytryptamine (5-HT) ELISA kits according to the manufacturer’s instruction. ELISA is based on the principle of competitive binding according to the method of Tietz (1995).
Determination of acetyl cholinesterase activity
Acetyl cholinesterase activity in brain tissue homogenate was estimated according to the method described by Kovarik et al. (2003).
Determination of brain oxidative/antioxidant parameters
The brain homogenate supernatant was used for the estimation of oxidative/antioxidant levels according to standard procedures: GSH (Beutler et al. 1963), superoxide dismutase (SOD) (Nishikimi et al. 1972), malondialdehyde (MDA) (Buege and Aust 1978), total antioxidant capacity (TAC) (Koracevic et al. 2001), and total oxidative status (TOS) (Erel 2005). The oxidative stress index (OSI) was calculated as the percentage ratio of TOS level to TAC level according to the following formula (Meng et al. 2013):
Assessment of inflammatory markers (IL-1β and TNF-α) and apoptotic markers (Caspase-3 and Fas) in brain tissues
Measurement of IL-1β, TNF-α, Caspase-3, and Fas concentrations was employed in brain tissues by a quantitative sandwich enzyme immunoassay technique using ELISA kits according to the manufacturer’s instruction. ELISA is based on the principle of competitive binding according to the method of Tietz (1995).
Cerebral mitochondria-enriched fraction
A half gram of cerebrum tissue was homogenized on ice in 10 volumes of isolation medium (10 mM HEPES buffer with pH: 7.0 containing 68 mM sucrose, 220 mM mannitol, 0.1% serum albumin, and 10 mM KCl). After centrifugation of the homogenate at 1000g for 10 min, the supernatant was then centrifuged at 11500g for 10 min to separate a myelin-rich supernatant from a pellet consisting of synaptosomes and free mitochondria. The pellet was resuspended in the isolation medium without albumin. The mitochondria-enriched fractions were preserved on ice for 15 min until the experiments were performed (Franco et al. 2007).
Assessment of cerebral mitochondrial function
Mitochondrial function was evaluated using MTT reduction assay (Mosmann 1983). The reaction proceeds if the mitochondrial preparation is functionally intact. This assay depends on the activity of the mitochondrial dehydrogenases to metabolize 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to a formazan which was quantified with a spectrophotometer at 550 nm. Data were expressed as a percentage of control.
Detection of Nrf2 by western blot technique
Expression of Nrf2 in the brain was determined by western blot. Briefly, brain tissue (50 mg) was homogenized using a Polytron homogenizer in 1.5 ml cold lysis buffer (50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1% NP 40, 0.5% sodium deoxycholate, 0.1% SDS, and 0.5 mmol/L phenylmethyl sulfonyl fluoride). The homogenate was centrifuged for 20 min at 4 °C and the supernatant was collected. Samples were stored at − 80 °C until use. Western immunoblotting is a technique used for the detection of proteins. The method is based on the separation of specific antibody-protein complex from one another according to their size by gel electrophoresis. The gel is placed next to a membrane and the proteins are moved by electrical current to the membrane where they adhere. The membrane is then stained with an antibody that could be detected (Harlow and Lane 1999). Proteins were visualized by enhanced chemiluminescence (ECL plus; Amersham, Arlington Heights, IL, USA) and measured using densitometry and Molecular Analyst Software (Bio-Rad, Richmond, CA, USA). Protein level was expressed relative to beta-actin.
Determination of NQO1 and INOS gene expression by real-time polymerase chain reaction (RT-PCR)
Based on the manufacturer’s instruction, total RNA was isolated from brain tissue homogenates using RNeasy Purification Reagent (Qiagen, Valencia, CA). The RNA was qualified by a UV spectrophotometer. RNA was reverse transcribed into cDNA using high-capacity cDNA reverse transcription kit (Fermentas, USA). Real-time quantitative PCR amplification and analysis were achieved using 2x SYBR Green PCR Master Mix (Applied Biosystem with software version 3.1 (StepOne™, USA). The primers in a Table 1 were designed using the software PRIMER3 and sequence data from the NCBI database. The relative expression of studied genes was calculated in comparison to reference β-actin gene using the comparative threshold cycle method (Livak and Schmittgen 2001).
Assessment of cerebral DNA fragmentation %
The brain DNA fragmentation was measured as described by Burton (1956) depending on colorimetrical quantitation upon staining with diphenylamine (DPA). The DNA fragmentation % was calculated according to the following equation:
Preparation and assessment of histological sections
Left hemispheres of brain samples were fixed in neutral buffered formalin solution for 48 h. Then, they were processed according to Bancroft and Gamble (2002). Five microns tissue thickness of prefrontal cerebral cortex was mounted on clean glass slides and stained by hematoxylin and eosin and examined under a light microscope in a blind manner to avoid examiner bias (Olympus Bx-40, Olympus Optical Co. Ltd., Japan). The following indices were assessed: congestion, pericellular edema, perivascular edema, and pyknosis. Quantitative morphometric estimation was prepared using an image analyzer (Leica Imaging System Ltd., Cambridge, England). Using the measuring field menu, the optical density, area, and area percentage were estimated using the image analyzer. The video images were digitalized using “Lecia Qwin 500C” which is a Leica’s Windows-based image analysis tool kit fitted to an IBM compatible personal computer with a color monitor. Scoring of tissue sample was represented as the mean score ± SE of ten different fields after statistical analysis by one-way ANOVA followed by the Tukey post hoc test for multiple comparisons.
Statistical analysis
All data were expressed in tables and figures as the mean ± standard error (SE). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Tukey post hoc analysis using SPSS software, version 24 (IBM, Armonk. NY, USA). Values of p ≤ 0.05 were considered significant.
Results
TEM characterization of TiO2-NPs
The measurement indicated that the average size of nanoparticles was 90 nm (range 40–140 nm). The TEM revealed that the TiO2-NPs droplets were almost spherical in shape with a homogeneous nanometric size distribution (Fig. 1(a, b)).
Chemical analysis of Moringa ethanol extract by GC-MS
The ethanol extract of the seeds of Moringa oleifera showed 11 major peaks from the chromatogram of the extract (Fig. 2(a)). These peaks indicate the presence of 11 major compounds (1-11) in the extract as listed in Table 2. The molecular formula, weight, and the peak area percentage of constituents were shown in the table. The seeds upon analysis were found to be rich in fatty acids, esters, terpenes, and phenols. The major chemical constituents in the extract included phenolic compounds (eugenol and aceteugenol), anti-inflammatory terpenes, and sesquiterpenes (copaene, humulene, cadinene, ocimene), fatty acids (hexadecanoic acid, trans-13-octadecenoic), and others (Table 2 and Fig. 2(a)).
(a) GC-MS chromatogram of ethanol extract of Moringa oleifera. The ethanol extract of the seeds of Moringa oleifera showed 11 major peaks from the chromatogram of the extract. (b) The constituents of M. oleifera extract (n = 5). The concentrations were expressed as milligrams of gallic acid equivalent (GAE) per gram of powder. (c) H2O2 scavenging activity inhibition % of MSE and ascorbic acid at different concentrations (5, 10, 25, 100 μg/ml). A superscript letter indicates a significant difference at p ≤ 0.05 when each dose of MSE compared to the corresponding dose of ascorbic acid
Preliminary quantitative phytochemical analysis
Results of the preliminary quantitative analyses of phytochemical constituents of MSE were as follows: total phenolic content 22.45 ± 0.12 mg/100 g GAE, total flavonoid content 13.61 ± 0.08 mg/100 g GAE, and total antioxidant capacity 26.96 ± 0.08 mg/100 g GAE (Fig. 2(b)).
In vitro H2O2 scavenging activity of MSE
MSE showed a potent H2O2 scavenging activity as compared with standard ascorbic acid. The maximum antioxidant activity by H2O2 assay was observed at 100 μg/ml. The percentage of inhibition produced by MSE at a concentration of 100 μg/ml was greater than the scavenging activity of ascorbic acid at 5, 10, and 25 μg/ml (Fig. 2(c)).
Toxicity test of MSE
Acute oral administration of MSE up to 500 mg/kg revealed no adverse effects in the experimental animals, including possible alterations such as food intake, unusual body growth, reduced activity, diarrhea, bleeding, or death. Therefore, no lethal dose was determined.
Effect of MSE on behavioral changes in rats
Signs of toxicity such as decreased physical activity, passive behavior, loss of appetite, and tremors were observed in TiO2-NPs group as compared with the control one during the period of the experiment. These behavioral changes were not obvious in MSE+ TiO2-NPs–treated group.
Effects of MSE on serotonin and dopamine concentrations and acetyl cholinesterase activity in brain toxicity
The concentration of serotonin was significantly (p < 0.05) increased in the TiO2-NPs group when compared to the control group. The serotonin concentration was significantly (p < 0.05) decreased in MSE+ TiO2-NPs–treated group when compared to the TiO2-NPs group (Fig. 3(a)). Dopamine concentration was significantly (p < 0.05) increased in the TiO2-NPs group as compared to the control group. The dopamine concentration was significantly (p < 0.05) decreased in MSE+ TiO2-NPs–treated group when compared to the TiO2-NPs group (Fig. 3(b)). The activity of acetyl cholinesterase was significantly (p < 0.05) increased in the TiO2-NPs group when compared to the control group. The acetyl cholinesterase activity was significantly (p < 0.05) decreased in MSE+ TiO2-NPs–treated group when compared to the TiO2-NPs group (Fig. 3(c)). MSE has no adverse effect on the brain cells and neurotransmitters as indicated by the non-significant (p < 0.05) difference of serotonin, dopamine concentrations, and acetyl cholinesterase activity in MSE group and control group (Fig. 3).
Effect of treatment with MSE on (a) serotonin, (b) dopamine concentrations, and (c) acetylcholinesterase activity in TiO2-NPs–induced brain toxicity. Values are means ± SE (n = 20). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. a significant difference in comparison with the control group (p < 0.05).b significant difference in comparison with the TiO2-NPs group (p < 0.05)
Effect of MSE on oxidative stress and antioxidant parameters in brain toxicity
The effect of MSE on the concentration of GSH, MDA, TAC, TOS contents, the activity of SOD, and OSI is illustrated in Fig. 4. The GSH content and activity of SOD were significantly (all p < 0.05) decreased in the TiO2-NPs group compared to the control group (Fig. 4(a, b)). Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly increased GSH content and SOD activity compared to the TiO2-NPs group (p < 0.05). Administration of TiO2-NPs significantly increased MDA and decreased TAC with a simultaneous increase of TOS contents and OSI as compared to the control group (all p < 0.05) (Fig. 4(c, d, e, and f)). Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly alleviated these parameters as compared to the TiO2-NPs group. In addition, administration of MSE to normal rats did not change these oxidative/ antioxidant indices compared to rats in the control group.
Effect of treatment with MSE on (a) GSH concentration, (b) SOD activity, (c) MDA, (d) TAC, (e) TOS concentrations, and (f) OSI in TiO2-NPs–induced brain toxicity. Values are means ± SE (n = 20). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. a significant difference in comparison with the control group (p < 0.05).b significant difference in comparison with the TiO2-NPs group (p < 0.05)
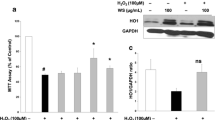
Effect of MSE on cerebral mitochondrial viability, Nrf2, NQO1 mRNA expression, INOS mRNA expression, and DNA fragmentation % in brain toxicity
The effect of MSE on cerebral mitochondrial viability, Nrf2, NQO1 mRNA expression, INOS mRNA expression, and DNA fragmentation % in brain toxicity is illustrated in Fig. 5.
Effect of treatment with MSE on (a) mitochondrial viability %, (b) Nrf2 concentrations and (c) NQO1, (d) INOS mRNA expressions, and (e) DNA fragmentation % in TiO2-NPs–induced brain toxicity. Values are means ± SE (n = 20). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. a significant difference in comparison with the control group (p < 0.05).b significant difference in comparison with the TiO2-NPs group (p < 0.05)
The cerebral mitochondrial viability was significantly (p < 0.05) decreased in the TiO2-NPs group compared to the control group. Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly (p < 0.05) increased the cerebral mitochondrial viability compared to TiO2-NPs group (Fig. 5(a)). Administration of TiO2-NPs significantly reduced Nrf2 concentration and NQO1 mRNA expressions with a simultaneous increase of INOS and DNA fragmentation % compared to the control group (all p < 0.05). Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly increased Nrf2 concentration and NQO1 mRNA expressions and decreased INOS mRNA expressions and DNA fragmentation % compared to TiO2-NPs group (all p < 0.05) (Fig. 5(b, c, d, and e)).
Effect of MSE on anti-inflammatory (IL-1β and TNF-α) and apoptotic (Caspase-3 and Fas) markers in brain toxicity
IL-1β and TNF-α were significantly (p < 0.05) increased in the TiO2-NPs group compared to the control group. Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly (p < 0.05) decreased the concentration of IL-1β and TNF-α compared to TiO2-NPs group (Fig. 6(a, b)). Also, administration of TiO2-NPs significantly increased the levels of Caspase-3 and Fas compared to the control group (all p < 0.05). Pretreatment with MSE at the dose of 100 mg/kg for 30 consecutive days significantly reduced Caspase-3 and Fas levels compared to TiO2-NPs group (all p < 0.05) (Fig. 6(c, d)).
Effect of treatment with MSE on (a) Il-1β level (pg/g tissue), (b) TNF-α concentration (pg/g tissue), (c) Caspase-3 level (pg/g tissue), and (d) Fas concentration (pg/g tissue) in TiO2-NPs–induced brain toxicity. Values are means ± SE (n = 20). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. a significant difference in comparison with the control group (p < 0.05).b significant difference in comparison with the TiO2-NPs group (p < 0.05)
Effect of MSE on cerebrum histopathology
The results showed normal histological structures with most neurons are intact in a cerebrum section of the control group (Fig. 7(a)) (H&E, bar = 200 μm). The brain of a rat treated with TiO2-NPs exhibited the presence of distinct large hemorrhagic area and perivascular and pericellular edema, severe congestion, and moderate pyknosis (Fig. 7(b)) (H&E, bar = 200 μm). MSE-treated rats showed a normal histological structure and most neurons are intact (Fig. 7(c)) (H&E, bar = 200 μm). Cerebrum sections of rats in MSE+ TiO2-NPs group showed normal histological structure; most neurons are intact with mild congestion and perivascular edema (Fig. 7(d)) (H&E, bar = 200 μm).
(a–d) Sections of rat’s cerebral cortex: (a) Control group showed a normal histological structure, and most neurons are intact (H&E, bar = 200 μm). (b) TiO2-NPs group displayed a distinct large hemorrhagic area (black arrows), perivascular and pericellular edema (yellow arrow), and pyknosis (blue arrows) (H&E, bar = 200 μm). (c) MSE group showed a normal histological structure with most neurons are intact (H&E, bar = 200 μm). (d) MSE+ TiO2-NPs group showed nearly normal histological structure, mild congestion, and perivascular edema (black arrows) and most neurons are intact (H&E, bar = 200 μm). Values of the histogram are means ± SE (n = 20). Data were analyzed by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. a significant difference in comparison with the control group (p < 0.05). b significant difference in comparison with the TiO2-NPs group (p < 0.05)
Discussion
The brain is a vital organ with great importance, as it is hard to regenerate from injury. Accordingly, the neurotoxicity of nanoparticles should receive great concerns. For this purpose, we discussed the mechanisms of TiO2 NPs through which they can harm the brain and how can alleviate their neurotoxic hazard. Despite the many advantages of nanoparticles, and the wide applications in the pharmaceutical sciences, drug delivery (Zahin et al. 2019), food industry, agriculture, and medicine (Shi et al. 2013), they may cause dangerous impacts because of their special properties including small size and high surface area. It has been shown that nanoparticles may cause damage to the blood-brain barrier (BBB) leading to increased permeability and entrance of nanoparticles to CNS leading to neurotoxicity (Sharma and Sharma 2010).
The deposition of TiO2-NPs in the brain induces changes in the release and metabolism of neurotransmitters. Definitely, TiO2-NPs–induced brain damage was established by the significant upregulation of neurotransmitters (dopamine and serotonin) concentrations and acetyl cholinesterase activity (Fig. 3(b, a, c)) which is an indicator reflecting the availability of acetylcholine. These findings were in agreement with Ma et al. (2010) who reported a significant increase in dopamine in association with neural damage. On the contrary, Hu et al. (2010) found that dopamine concentration decreased in brain tissue in response to TiO2-NPs exposure. In accordance with our findings, Munoz-Castaneda et al. (2006) reported a significant increase of serotonin in order to protect cells against oxidative stress in the brain tissue while Ma et al. (2010) found that the serotonin concentration may decrease or not affect in cerebral damage. Since acetyl cholinesterase is a crucial enzyme in the excitatory neurotransmitter acetylcholine catabolism, its measurement is a logical approach to indicate neural damage as it is responsible for breaking down of acetylcholine into acetic acid and choline at cholinergic synapses (Worek et al. 2002). Oral administrations of TiO2-NPs increased acetyl cholinesterase activity (Wang et al. 2008; Hu et al. 2010). In contrast, some researchers have reported an impaired acetyl cholinesterase activity in neuronal damage (Ma et al. 2010).
In this study, the symptoms of toxicity such as behavioral, physical changes and decreased food intake observed after TiO2-NPs administration are in parallel with the findings of Wang et al. (2007). These symptoms are simultaneously related to our observed results of increased monoamine neurotransmitters (dopamine and serotonin) concentrations and acetyl cholinesterase activity, which are related to the oxidative stress induced by TiO2-NPs administration.
One of the most important mechanisms of TiO2-NPs–induced brain damage is ROS production, which has been implicated in the pathogenesis of neuronal injury (Long et al. 2007). The brain is highly liable to ROS-induced injury because of its high energy demands; low levels of endogenous antioxidants as SOD, catalase, and vitamin C; and an abundance of polyunsaturated fatty acids, proteins, and nucleic acids in the neurons (Huerta-Garcıa et al. 2014). Among oxygen free radicals, superoxide anion (O−2) plays a critical role in the oxidative chain reaction, producing a highly reactive oxidant. These free radicals are removed by antioxidant scavengers, including SOD, glutathione peroxidase, catalase, and GSH. SOD is the first line of the enzymatic antioxidant defense system, which scavenges O−2 to oxygen (O2) and H2O2, then catalase converts the H2O2 into water and oxygen, thus preventing the lipid peroxidation of cell membrane (Niska et al. 2015). Total antioxidant capacity (TAC) is commonly used to evaluate the overall antioxidative status in the cells.
ROS production in the brain is indicated by the increase in MDA level. Overproduction of ROS in TiO2-NPs–treated rats consumes GSH and SOD. Kaya and Akbulut (2015) stated that ROS-induced cellular injury and the damage of cell membrane could be attributed to the lipid peroxidation and the conversion of polyunsaturated fatty acids in the cell membrane to toxic lipid peroxides. This was supported by our findings, which revealed that TiO2-NPs successfully induced lipid peroxidation in brain tissue which is indicated by the increase of MDA and the decrease of GSH concentrations (Fig. 4) in agreement with Hu et al. (2010) and Shi et al. (2015). In accordance with Hu et al. (2011) and Ma et al. (2010), a significant decrease of SOD activity and TAC was detected in the brain of orally exposed rats to TiO2-NPs in response to excess ROS production and induced lipid peroxidation. A dramatic increase in the ROS levels accompanied by the increased levels of TOS and OSI was observed in the brain of orally exposed rats (Fig. 4). Therefore, the synthesis of some enzymes was influenced and the oxidative/antioxidative balance in the brain of TiO2-NPs treated rats was disrupted, resulting in the lipid and protein oxidation.
Mitochondria possess many vital cellular functions, such as regulating the synthesis and breakdown of many metabolites, production of adenosine triphosphate (ATP) by oxidative phosphorylation, and production and getting rid of ROS (Brand and Nicholls 2011). One of the novel results is the toxic effect of TiO2-NPs on cerebral mitochondria. Our results showed that TiO2-NPs decreases mitochondrial viability (Fig. 5(a)). One of the most obvious causes of mitochondrial damage is the generation of mitochondrial ROS (de Moura et al. 2010). Li et al. (2013) concluded that the non-mitochondrial–created ROS can increase the generation of mitochondrial ROS. Deposition of TiO2-NPs in the mitochondria was reported by Huerta-Garcıa et al. (2014). TiO2-NPs–induced mitochondrial damage could be explained by the excess ROS production which opens the mitochondrial membrane permeability transition pore, and subsequently triggers the apoptotic signaling cascade (Fleury et al. 2002). Similarly; several studies have evidenced that ROS have a potent role for induction of mitochondrial dysfunction and subsequent apoptotic cell death (Yoo et al. 2012).
Our results suggest that Nrf-2 has a central role in downregulation of antioxidant defense of the brain cells during TiO2-NPs–induced toxicity and this suggestion was indicated by the significant decrease of Nrf-2 levels (Fig. 5(b)) with a simultaneous decrease in NQO1 expression (Fig. 5(c)) in TiO2-NPs–treated rats when compared to the control rats. This finding was in agreement with McCoy and Cookson (2011) who reported that overproduction of ROS is accompanied by downregulation of Nrf-2 and NQO1 mRNA expressions. The significant low levels of Nrf-2 lead to a significant elevation of INOS mRNA expression (Fig. 5(d)) in accordance with Shih et al. (2005) in TiO2-NPs group. Other study indicated that TiO2-NPs administration increases INOS mRNA expression which is responsible for the overproduction of nitric oxide (NO) in the brain (Chen et al. 2018). NO as a free radical could be oxidized by O−2 to peroxynitrite (ONOO−) radical that causes oxidation of lipids (Wang et al. 2008) and disturbance of the many vital properties of cell resulting in cytolysis and cell death which is a logic fate of the induced lipid peroxidation (Pradeep et al. 2009). The present data revealed that exposure to TiO2-NPs causes DNA damage as evident from the data of DNA fragmentation % illustrated in Fig. 5(e). The increase in DNA fragmentation % could be attributed to the oxidative reactions induced by TiO2-NPs (Trouiller et al. 2009).
Pro-inflammatory mediators are associated with ROS generation (Ansar et al. 2017), which are inconsistent with our results in which the oxidative stress following the exposure to TiO2-NPs increased the brain concentrations of the pro-inflammatory cytokines, IL-1β and TNF-α. These findings may suggest the inflammatory response-induced “neurotoxicity of” TiO2-NPs. DNA fragmentation and damage could be attributed to the release of inflammatory cytokines (Totsuka et al. 2014) and lipid peroxidation (Harangi et al. 2004). Thus, TiO2-NPs genotoxicity is probably due to the exaggerated rise in lipid peroxidation. DNA fragmentation is an important feature of cell apoptosis; therefore, we, next, evaluated the apoptotic markers in TiO2-NPs exposed rats.
Xia et al. (2008) defined apoptosis as a process of programmed cell death which regulates cell renovation and get rid of injured cells. Dysregulation of cell apoptosis causes cell death, impairment of tissues, and organ dysfunction (Elmore 2007). There are two major pathways involved in cell apoptosis: The intrinsic (mitochondrial), by the opening of mitochondrial permeability transition pore with the liberation of cytochrome C and the extrinsic (death receptor) by stimulating the death receptor, which is a member of tumor necrosis factor receptors superfamily. Both of them end with the activation of caspase-3 which is the main apoptotic protein (Elmore 2007). Caspase-3 is an inactive cytoplasmic zymogen, which upon activation will trigger cell apoptosis via a signal transduction pathway. Fas is a death receptor that is greatly expressed in several cell types and rapidly mediates apoptosis in response to certain stimuli. In agreement with Yoo et al. (2012), our findings showed that TiO2-NPs induced upregulation of Fas and subsequent caspase-3 activation and apoptotic cell death. Our results suggest that TiO2-NPs activate both the intrinsic pathway of cerebral apoptosis via damaging the cerebral mitochondria and decrease mitochondrial viability, and the extrinsic pathway via Fas activation.
Our results concerning the preliminary quantitative analyses of phytochemicals in MSE indicated the presence of higher contents of total phenolic, total flavonoid, and total antioxidant capacity (Fig. 2(b)). Furthermore, MSE showed a potent H2O2 scavenging activity (Fig. 2(c)). Administration of MSE improves the cerebral functions as manifested by the normal behavior and physical activity of the rat throughout the study as well as the significant decrease of serotonin, dopamine concentrations, and acetyl cholinesterase activity in MSE + TiO2-NPs group when compared to TiO2-NPS group. Chiefly, the administration of MSE considerably alleviated TiO2-NPs cerebral oxidative damage as indicated by the decreased lipid peroxidation MDA, TOS, and OSI levels and the increased GSH concentration, SOD activity, and TAC levels. The neuroprotective effects of MSE might occur partly via the flavonoids (Youdim and Joseph 2001).
The nuclear factor-erythroid-2-related factor 2 (Nrf2), which is an important transcription factor, plays a central role in defense against oxidative stress. Nrf2 is a key leucine zipper transcription factor that regulates the expression of antioxidant enzymes and plays a serious role in protecting the cell against oxidative stress and inflammation (Dinkova-Kostova et al. 2018). In normal cells, Nrf2 is impounded in the cytoplasm by the cytosolic inhibitor Kelch-like ECH associated protein1 (Keap1). In response to oxidants, the release of Nrf2 from the Keap1-Nrf2 complex takes place by disrupting the protein-protein interactions or by promoting the degradation of Keap1 in cells. Once released, the active Nrf2 translocates into the nucleus (Kobayashi et al. 2006). In the nucleus, Nrf2 forms a heterodimer with other transcription factors, such as small Maf protein (musculoaponeurotic fibrosarcoma proteins) (Itoh et al. 1997), that facilitates the interaction of Nrf2 with the antioxidant response element (ARE) located in the promoter region of target genes. Nrf2-ARE increase the transcription of the cytoprotective phase 2 detoxification enzyme genes such as NQO1, GSH, glutathione -S-transferase (GST), haemoxygenase-1 (HO-1), SOD, catalase, GPx, and thioredoxin (Rajappa et al. 2017). In this respect, Nrf2 increases the expression of both γ-glutamylcysteine ligase (GCL) required for the biosynthesis of GSH (Wild et al. 1999), and enzymes involved in the production of NADPH via HMP shunt to maintain GSH in a reduced state (Wu et al. 2011). Furthermore, Nrf2-ARE downregulate the expression of pro-inflammatory mediators such as cyclooxygenase-2 (COX-2) and iNOS (Shih et al. 2005).
NQO1, a member of the NAD(P)H dehydrogenase (quinone) family, is a highly inducible cytosolic homodimeric flavoprotein enzyme-mediated by Nrf2 in response to oxidative stress. NQO1 catalyzes the reduction and detoxification of quinones to hydroquinones, and thus protects cells from oxidative damage (Wu et al. 2013). In addition, NQO1 acts as a component of the redox system producing antioxidant forms of vitamin E and CoQ10 in the plasma membrane (Siegel et al. 1997). Other effective roles of NQO1 include its ability to generate NAD+ (Pink et al. 2000), function as a direct superoxide reductase (Zhu et al. 2007) and protect proteins from proteosomal degradation (Moscovitz et al. 2012).
Our study proved that the administration of MSE has significantly increased Nrf2 level that improves the antioxidant and cytoprotective responses indicated by the subsequent significant increase of NQO1 expression, mitochondrial viability %, and cerebral antioxidant activity with a significant decrease of INOS mRNA levels and DNA fragmentation % in MSE + TiO2-NPs group when compared to TiO2-MPs group. Certainly, MSE has a powerful antioxidant activity (Vongsak et al. 2013) as it is a rich source of antioxidants such as flavonoids and phenolics (Ghiridhari et al. 2011) that proves that MSE could induce Nrf2-mediated antioxidant response. Furthermore, Nrf2 improves mitochondrial function and availability of substrates (NADH and FADH2/succinate) which are essential for the function of Nrf2-upregulated antioxidant enzymes and cellular respiration (Dinkova-Kostova and Abramov 2015).
Nrf2 activation in MSE + TiO2 NPs group caused a significant reduction of IL-1β and TNF-α when compared to TiO2-NPs group. These findings are in accordance with Liu et al. (2013) who reported that Nrf2 decreases toxin-induced liver injury by reducing pro-inflammatory cytokines such as IL-1β and TNF-α.
One of our novel results is the anti-apoptotic effect of MSE on TiO2-NPs–induced cerebral apoptosis which is manifested by a significant reduction of caspase-3 and Fas. This effect could be attributed to the activation of Nrf2 by MSE. Liu et al. (2013) reported that activation of Nrf2 protects the cell from cellular apoptosis by decreasing the mRNA levels of apoptosis executors.
One of the most important phenolic compounds of MSE is eugenol (4-allyl-2-methoxyphenol) (55.15%) (Table 2), which is considered a chain-breaking antioxidant (Fujisawa et al. 2005). A recent study has shown that eugenol is an activator of Nrf2 (Rajappa et al. 2017). It was recorded that the liver was protected by eugenol pretreatment as indicated by prevention of lipid peroxidation, protein oxidation, and DNA strand break and also by decreasing inflammatory markers and improving the antioxidant status in thioacetamide-induced hepatic injury (Yogalakshmi et al. 2010). Other compounds present in MSE which have anti-inflammatory and antioxidant activities are terpenoids and sesquiterpenes including humulene (Fernandes et al. 2007) and cadinene (Kundu et al. 2013). Terpenoids can be considered the most potent Nrf2-inducer (Dinkova-Kostova et al. 2005) through induction of the phase 2 response and suppression of the INOS induction in mouse macrophages (Hond et al. 2002). Alpha-humulene reduced the production of INOS induced by the injection of carrageenan in rats (Fernandes et al. 2007).
Based on our results, the ability of MSE to upregulate Nrf2/ NQO1 pathway in the brain adds a new hypothetic mechanism to MSE as Nrf2 activator.
Our results of histopathological examination of the cerebrum section of a rat treated with TiO2-NPs showed large distinct hemorrhagic area, perivascular, pericellular odema, severe congestion, and moderate pyknosis (Fig. 7(b)). The formation and progression of brain injury are attributed to ROS production which is substantially increased by TiO2-NPs. Administration of MSE with TiO2-NPs showed moderate perivascular, pericellular odema, and no hemorrhage nor pyknosis can be seen (Fig. 7(d)). It is meaningful to remark that the administration of MSE only showed no pericellular odema with most neurons are intact (Fig. 7(c)). This shows the harmless effect of MSE in the brain cells. That finding was in confidence with the biochemical parameters which clarified that MSE only caused no significant changes in oxidative/antioxidant parameters, pro-inflammatory and apoptotic markers as compared to the control group.
Conclusion
Observed results in this study have proved that Moringa seed extract is a potential neuroprotectant. The possible underlying mechanism may occur partly via Nrf2 activation. The protective effect of Nrf2 is accompanied by the induction of genes involved in antioxidant defense, increased cerebral mitochondrial viability, and inhibition of pro-inflammatory and apoptotic mediators against TiO2-NPs cerebral toxicity. Further studies concerning other mechanisms of moringa active components are still required.
Change history
31 July 2019
The original publication of this paper contains a mistake. The correct title is shown in this paper.
References
Ansar S, Abudawood M, Hamed SS, Aleem MM (2017) Exposure to zinc oxide nanoparticles induces neurotoxicity and proinflammatory response: amelioration by hesperidin. Biol Trace Elem Res 17:360–366
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th edn. Churchill Livingstone, New York, London, New York & Sydney, pp 377–694
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435(2):297e312
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Burton K (1956) The study of the conditions and mechanisms of the diphenylamine reaction for the calorimetric estimation of deoxyribonucleic acid. Biochem J 62:615–623
Chen Q, Wang N, Zhu M, Lu J, Zhong H, Xue X, Guo S, Li M, Wei X, Tao Y, Yin H (2018) TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: a proteomic and metabolomic insight. Redox Biol 15:266–276
Chivapat S, Sincharoenpokai P, Suppajariyawat P, Rungsipipat A, Phattarapornchaiwat S, Chantarateptawan V (2012) Safety evaluations of ethanolic extract of Moringa oleifera Lam. seed in experimental animals. Thai J Vet Med 42(3):343–352
Clements CM, McNally RS, Conti BJ, Ma TW, Ting JP (2006) DJ-1, a cancer- and Parkinson’s disease–associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A 103:15091–15096
Culei I (1964) Practical manual on the industrial utilization of medicinal plants. University of Bucharest, Romania, pp 56–71
De Moura MB, dos Santos LS, Van Houten B (2010) Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen 51:391e405
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88(B):179–188
Dinkova-Kostova AT, Talalay P (2000) Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med 29(3–4):231–240
Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P (2005) Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A 102:4584–4589
Dinkova-Kostova AT, Kostov RV, Kazantsev AG (2018) The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J 285(19):3576–3590
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
El-Shemy HA, Aboul-Enein AM, Aboul-Enein KM, Fujita K (2007) Willow leaves’ extracts contain anti-tumor agents effective against three cell types. PLoS One 2(1):e178
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, van Ravenzwaay B (2008) Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol 82:151–157
Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, Pianowski LF, Calixto JB (2007) Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol 569(3):228–236
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M (2007) Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol 20:1919–1926
Fujisawa S, Muraoka E, Nakazato Y, Okada N (2005) Effects of visible light irradiation on eugenol-treated oral mucosa. Dent Mater J 24:202–206
Ghiridhari WA, Malhati D, Geetha K (2011) Anti-diabetic properties of drumstick (Moringa oleifera) leaf tablets. Int J Health Nutr 2:1–5
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947
Gurr JR, Wang AS, Chen CH, Jan KY (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73
Gusev AI (2007) Nanomaterials, nanostructures, and nanotechnologies (in Russian) Fizmatlit Moscow 416
Gusev AI, Kurlov AS (2008) Production of nanocrystalline powders by high-energy ball milling: model and experiment. Nanotechnology 19(26):265–302
Han D, Hanawa N, Saberi B, Kaplowitz N (2006) Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol 291(1):G1–G7
Harangi M, Seres I, Varga Z, Emri G, Szilvássy Z, aragh G (2004) Atorvastatin effect on high-density lipoprotein-associated paraoxonase activity and oxidative DNA damage. Eur J Clin Pharmacol 60:685–691
Harborne JB (1973) Phytochemical methods: a guide to modern techniques of plant analysis. Chapman and Hall Ltd, London, pp 49–188
Harlow ED, Lane DP (1999) Using antibodies. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Hond T, Honda Y, Favaloro FG, Gribble GW, Suh N, Place AE, Rendi MH, Sporn MB (2002) A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett 12:1027–1030
Hu R, Gong X, Duan Y, Li N, Che Y, Cui Y, Zhou M, Liu C, Wang H, Hong F (2010) Neurotoxicological effects and the impairment of spatial recognition memory in mice caused by exposure to TiO2 nanoparticles. Biomaterials 31(31):8043–8050
Hu R, Zheng L, Zhang T, Gao G, Cui Y, Cheng Z, Cheng J, Hong M, Tang M, Hong F (2011) Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater 191:32–40
Huerta-Garcıa E, Perez-Arizti JA, Marquez-Ram S, Gırez et al (2014) Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med 73:84–94
IARC (1985) Monographs on the evaluation of the carcinogenic risk of chemicals to humans: allyl compounds, aldehydes, epoxides and peroxides. World Health Organization, International Agency for Research on Cancer, Lyon, pp 163–177
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y (1997) An Nrf2 small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322
Katsube T, Tabata H, Ohta Y, Yamasaki Y, Anuurad E, Shiwaku K, Yamane Y (2004) Screening for antioxidant activity in edible plant products: comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J Agric Food Chem 52:2391–2396
Kaya H, Akbulut M (2015) Effects of waterborne lead exposure in Mozambiquetilapia: oxidative stress, osmoregulatory responses, and tissue accumulation. J Aquat Anim Health 27:77–87. https://doi.org/10.1080/08997659.2014.1001533. https://www.ncbi.nlm.nih.gov/pubmed/25951052
Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M (2006) Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity if Keap1. Mol Cell Biol 26:221–229
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361
Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P (2003) Acetyl cholinesterase active centre and gorge conformations analyzed by combinatorial mutations and enantiomeric phosphonates. Biochem J 1(73):33–40
Kundu A, Saha S, Walia S, Ahluwalia V, Kaur C (2013) Antioxidant potential of essential oil and cadinene sesquiterpenes of Eupatorium adenophorum. Toxicol Environ Chem 95(1):127–137
Li Y, Paonessa JD, Zhang Y (2012) Mechanism of chemical activation of Nrf2. PLoS One 7:e35122
Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF (2013) Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol 2744:6e19
Lin JY, Tang CY (2006) Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 101(1):140–147
Liu J, Wu KC, Lu YF, Ekuase E, Klaassen CD (2013) NRF2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev 2013:305861. https://doi.org/10.1155/2013/305861
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25(4):402–408
Long TM, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, Hester S, Lowry GV, Veronesi B (2007) Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect 115(11):1631–1637
Ma L, Liu J, Li N, Wang J, Duan Y, Yan J, Liu H, Wang H, Hong F (2010) Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials 31:99–105
Mbikay M (2012) Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol 3:24
McCoy MK, Cookson MR (2011) DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy 7:531–532
Meng B, Li J, Cao H (2013) Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des 19:2101–2113
Moscovitz O, Tsvetkov P, Hazan N, Michaelevski I, Keisar H, Ben-Nissan G, Shaul Y, Sharon M (2012) A mutually inhibitory feedback loop between the 20S proteasome and its regulator, NQO1. Mol Cell 47:76–86
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Munoz-Castaneda JK, Montilla P, Padillo FJ, Bujalance I, Munoz MC, Muntane J, Tunez I (2006) Role of serotonin in cerebral oxidative stress in rats. Acta Neurobiol Exp 66(1):1–6
Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Hamidinia A, Bekhradnia AR (2008) Determination of antioxidant activity, phenol and flavonoids content of Parrotia persica Mey. Pharmacol Online 2:560–567
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Niska K, Pyszka K, Tukaj C, Wozniak M, Radomski MW, Inkielewicz-Stepniak I (2015) Titanium dioxide nanoparticles enhance production of superoxide anion and alter the antioxidant system in human osteoblast cells. Int J Nanomedicine 4(10):1095–1107
Okwu ED, Ighodaro UB (2010) GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstoniaboonei. De Wild, Der Pharma Chemica 2(1):261–262
Panda S, Kar A, Sharma P, Sharma A (2013) Cardioprotective potential of N,alpha-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: in vivo and in vitro studies. Bioorg Med Chem Lett 23:959–962
Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA (2000) NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem 275:5416–5424
Pradeep HA, Khan S, Ravikumar K, Ahmed MF, Rao MS, Kiranmai M, Reddy DS, Ahamed SR, Ibrahim M (2009) Hepatoprotective evaluation of Anogeissus latifolia: in vitro and in vivo studies. World J Gastroenterol 15(38):4816–4822
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Rajappa R, Bovilla V, Madhunapantula SV (2017) Naturally occurring Nrf2 activators in the management of diabetes. Nutr Food Sci Int J 2(4):1–10
Rizk MZ, Ali SA, Hamed MA, El-Rigal NS, Aly HF, Salah HH (2017) Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed Pharmacother 90:466–472
Rockwood JL, Anderson BG, Casamatta DA (2013) Potential uses of Moringa oleifera and an examination of antibiotic efficacy conferred by M. oleifera seed and leaf extracts using crude extraction techniques available to underserved indigenous populations. Int J Phytotherapy Res 3:61–71
Sadrieh N, Wokovich AM, Gopee NV, Zheng J, Haines D, Parmiter D, Siitonen PH, Cozart CR, Patri AK, McNeil SE, Howard PC, Doub WH, Buhse LF (2010) Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci 115:156–166
Sermakkani M, Thangapandian V (2012) GC-MS analysis of Cassia italic leaf methanol extract. Asian J Pharm Clin Res 5(2):90–94
Sharma HS, Sharma A (2010) Conference scene: nanoneuroprotection and nanoneurotoxicity: recent progress and future perspectives. Nanomedicine 5:533–537 https://www.futuremedicine.com/doi/abs/10.2217/nnm.10.25
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15
Shi Z, Niu Y, Wang Q, Shi L, Guo H, Liu Y et al (2015) Reduction of DNA damage induced by titanium dioxide nanoparticles through Nrf2 in vitro and in vivo. J Hazard Mater 15(298):310–319
Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH (2005) Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280(24):22925–22936
Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A (2011) ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol in Vitro 25:231–241
Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D (1997) The reduction of alpha-tocopherol quinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherol hydroquinone as a cellular antioxidant. Mol Pharmacol 52:300–305
Song B, Liu J, Feng X, Wei L, Sha L (2015) A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res Lett 10:342
Sun GY, Horrocks LA, Farooqui AA (2007) The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem 103(1):1–16
Tietz NW (1995) Clinical guide to laboratory tests (ELISA), 3rd edn. W.B. Saunders, Co, Philadelphia, pp 22–23
Totsuka Y, Ishino K, Kato T, Goto S, Tada Y, Nakae D, Watanabe M, Wakabayashi K (2014) Magnetite nanoparticles induce genotoxicity in the lungs of mice via inflammatory response. Nanomaterials 4:175–188
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69:8784–8789
Tsyganova NA, Khairullin NA, Terentyuk GS, Khlebtsov BN, Bogatyrev VA, Dykman LA et al (2014) Penetration of pegylated gold nanoparticles through rat placental barrier. Bull Exp Biol Med 157(3):383–385
Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W (2013) Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crop Prod 44:566–571
Wang JX, Zhou GQ, Chen CY, Yu HW, Wang TC, Ma YM, Jia G et al (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185
Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F et al (2008) Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett 183(1–3):72–80
Warheit RA, Hoke DB, Finlay C, Donner EM, Reed KL, Sayes CM (2007) Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol Lett 171:99–110
Wild AC, Moinova HR, Mulcahy RT (1999) Regulation of gamma- glutamyl cysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 274:33627–33636
Wolf R, Matz H, Orion EJ (2003) Lipozencic, sunscreens—the ultimate cosmetic. Acta Dermatovenerol Croat 11(3):158–162
Worek F, Reiter G, Eyer P, Szinicz L (2002) Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol 76:523–529
Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123:590–600
Wu Y, Wang D, Peng X, Chen Y, Zheng D, Chen W et al (2013) Epigenetic silencing of NAD(P)H: quinone oxidoreductase 1 by hepatitis B virus X protein increases mitochondrial injury and cellular susceptibility to oxidative stress in hepatoma cells. Free Radic Biol Med 65:632–664
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2:2121–2134
Yogalakshmi B, Viswanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 168:204–212
Yoo KC, Yoon CH, Kwon D, Hyun KH, Woo SJ, Kim RK, Lim EJ, Suh Y, Kim MJ, Yoon TH, Lee SJ (2012) Titanium dioxide induces apoptotic cell death through reactive oxygen species-mediated Fas upregulation and Bax activation. Int J Nanomedicine 7:1203–1214
Youdim KA, Joseph JA (2001) A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med 30(6):583–594
Zahin N, Anwar R, Tewari D et al (2019) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-05211-0
Zhu H, Jia Z, Mahaney JE, Ross D, Misra HP, Trush MA, Li Y (2007) The highly expressed and inducible endogenous NAD(P)H:quinone oxidoreductase 1 in cardiovascular cells acts as a potential superoxide scavenger. Cardiovasc Toxicol 7:202–211
Acknowledgments
We thank Dr. Rawya G. Abd El-Wahab, Department of Biochemistry, Beni-Suef University, Egypt, for helping in rearing of animals and sampling. The authors thank all staff members in the Biochemistry and Pathology Departments, Beni-Suef University, Egypt, for their help and advices.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article was revised: The original publication of this paper contains a mistake. The correct title is shown in this paper.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Kandeil, M.A., Mohammed, E.T., Hashem, K.S. et al. Moringa seed extract alleviates titanium oxide nanoparticles (TiO2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res 27, 19169–19184 (2020). https://doi.org/10.1007/s11356-019-05514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05514-2