Abstract
In the present study, an attempt was made to improve the oxidation stability of biodiesel by adding antioxidants to waste cooking oil biodiesel, and their impact on performance and emissions was analyzed. Two types of antioxidants were chosen for the analysis: an aromatic amine antioxidant, diphenylamine (DPA), and synthetic oxidants, tert-butylhydroxyquinone (TBHQ) and pyrogallol (PY). All the antioxidants were added to the biodiesel at doses of 200 ppm and 500 ppm to evaluate their effect. The oxidation stability was found as per the ASTM standard by mixing 500 ppm antioxidants for all three antioxidant-treated biodiesel blends. DPA yielded similar results as TBHQ, although PY had a better oxidation stability according to the Rancimat test. Gas chromatography and mass chromatography were also performed on the neat biodiesel. Performance and emission tests were performed on the antioxidant-treated biodiesel blends and diesel. The brake thermal efficiency of the tested fuel increased by 9.8%, 6.9%, and 15.88% when the DPA, TBHQ, and PY antioxidants were added to the test fuel compared to that of the test fuel without added antioxidant. The brake specific energy consumption of the test fuel decreased by 9.05% with DPA, 7.03% with TBHQ, and 14.08% with PY compared to that of the test fuel without antioxidant. The NOx emissions of the antioxidant-treated test fuels were reduced by 14.65% with DPA, 11.22% with TBHQ, and 23.10% with PY compared to those of the test fuel without antioxidants. Additionally, the aromatic amine antioxidant (DPA) was found to be effective in enhancing the performance and lowering the exhaust emissions compared to diesel for unmodified diesel engines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prices of conventional fuel are rapidly increasing with rising energy demand. Therefore, to reduce dependency on conventional fuel, there is a need to transform our energy demand from fossil fuel-based energy to renewable and sustainable sources of energy (Pali et al. 2015). The automobile industry is transforming from a conventional fuel-based industry to a minimum emission-based industry to protect the environment from harmful automobile emissions. This transformation will render many diesel-powered machines obsolete. Biodiesel is the best alternative that could provide a smooth transition, as it can be used in traditional diesel engines without any modification. Biodiesel is a renewable, biodegradable, nontoxic source that produces fewer carbon monoxide (CO) and hydrocarbon (HC) emissions than conventional diesel fuel due to its high oxygen content. Biodiesel is a transesterified product produced from vegetable oils, animal fats, and used cooking oil (Kumar et al. 2016; Naresh Kumar Reddy and Marouf Wani 2020).
Biodiesel has similar fuel quality parameters as petrodiesel, but it deteriorates when stored for a long time. Deterioration of biodiesel directly depends on its oxidation, contaminants, temperature, and presence of light. The results of these include changes in the color of biodiesel, the formation of deposits, and changes in the clarity of the biodiesel. The fatty acid chains of the oil do not change during the transesterification process that converts oil into biodiesel (Bharti and Singh 2020).
There is a need to understand the fatty acid composition of the oil to enhance our understanding of the oxidation process. Fatty acids are classified into saturated and unsaturated fatty acids. Saturated fatty acids do not have a double bond between two carbon atoms, whereas carbon–carbon double bonds are present in unsaturated fatty acids. Monounsaturated fatty acids, which have one carbon–carbon double bond, and polyunsaturated fatty acids, which have more than one carbon–carbon double bond, are prone to oxidation (Saluja et al. 2016). Autoxidation of fuel occurs when the fuel reacts with the oxygen present in the air at room temperature. Due to fuel autoxidation, the values of properties such as the peroxide value, number of acidic protons, kinematic viscosity, density, iodine value, and deposit formation increase. Biodiesel oxidation instability can be minimized by preventing the initiation of oxidation by mixing various antioxidants (Kumar Patel and Kumar 2017).
A large quantity of waste cooking oil is generated globally. The disposal of waste cooking oil is difficult to manage because it creates water and land pollution. The UK produces 200,000 t of waste cooking oil per year. EU countries produce 700,000 to 1,000,000 t per year of waste cooking oil. Illegal dumping of this tremendous amount of waste cooking in water and landfills causes severe environmental issues (R U et al. 2011). Conversion of waste cooking oil into biodiesel reduces pollution and will help to reduce the energy crisis (Sonthalia and Kumar 2021). The conversion rate of waste cooking oil biodiesel is high at approximately 92% (Sonthalia et al. 2021). Waste cooking oil biodiesel is found to be a promising source of biodiesel. However, the oxidation stability of waste cooking oil biodiesel is 1.72 h (Nagarajan and Narayanasamy, 2021). The induction of waste cooking oil was evaluated at 2.88 h, which does not meet ASTM criteria (Bharti and Singh 2020). Vegetable biodiesel has poor oxidation stability, so antioxidant additives are added to enhance their oxidation stability (Dueso et al. 2018). Pongamia biodiesel was tested with an antioxidant (PY) to improve its oxidation stability. The author used 20% biodiesel at 300 ppm to maintain the EU 590 standard (Kovács et al. 2015). The most commonly used synthetic antioxidants are BHT, BHA, PG, TBHQ, and PY, and they have been widely used in the literature. Biodiesel transesterified from tilapia oil had enhanced oxidation stability when a natural extract of turmeric and BHT, BHA, and PG were added as synthetic antioxidants. Natural antioxidants can also be used to control the oxidation process (Rodrigues et al. 2020). Jatropha is the most commonly used feedstock for biodiesel production in India. Jatropha biodiesel has an oxidation stability of 3.95 h. TBHQ, BHT, TBP, OBPA, and α-T are synthetic and natural antioxidants used to improve the oxidation stability of Jatropha biodiesel. tert-Butylhydroxyquinone (150 ppm), tert-butyl (200 ppm), tert-butylated phenol derivative (300 ppm), octylated or butylated diphenylamine (300 ppm), and a-tocopherol (200 ppm) were added to Jatropha biodiesel to meet the Rancimat standards of biodiesel. TBHQ was found to be the most effective antioxidant for enhancing oxidation stability. Synthetic antioxidants were more effective than natural antioxidants (Sarin et al. 2010). TBHQ, BHT, PG, and PY antioxidants have been used to analyze oxidation stability. The antioxidant PY (204 ppm), when used in Jatropha biodiesel, led to a higher oxidation stability than TBHQ, BHT, and PG (Supriyono et al. 2015).
Degradation of biodiesel causes deposit formation, hindrance in the fuel injection system, blockage of the fuel filter, and rapid increase in corrosion, all of which can lead to serious engine operation problems. These issues affect engine performance and emissions (Pullen and Saeed 2014). Calophyllum inophyllum methyl ester was tested with antioxidant additives to improve the oxidation and stability characteristics. Ethanox is an antioxidant additive that has been compared with BHT in a 4-stroke diesel engine at constant speed. The authors found that Ethanox resulted in a higher thermal brake efficiency and brake-specific fuel consumption and a significant effect on emission compared to BHT (Alves et al. 2019). The use of antioxidants reduces NOx emission because the addition of antioxidants to biodiesel reduces free radical formation during premixed combustion, which reduces heat release (Rashed et al. 2015). Carbon monoxide (CO) and hydrocarbon (HC) emissions increase slightly when antioxidants are added to a biodiesel blend. However, CO and HC emissions are significantly reduced compared to diesel (Palash et al. 2014). Moreover, some literature has reported that 20% biodiesel blends give better performance than other higher percentage blends (Rashed et al. 2016). p-Phenylenediamine, L-ascorbic acid, and A-tocopherol acetate antioxidants (250 mg) used in an A20 blend of Annona biodiesel are able to mitigate NOx by 25.4% compared to diesel (Rajendran 2020). Antioxidants effectively reduce NOx emissions, as presented in prior studies (Adam et al. 2018; Reddy and Wani 2020; Srinivasan et al. 2021). Performance and emission analysis of NPPD antioxidant was done and compared with titanium oxide nano additives. Antioxidant successfully control NOx emission with increasing BTE and slight increases in other emission. Although, antioxidant with nano additive significantly increases BTE and reduces emission (Reddy and Wani 2021).
Much research has been performed on oxidation stability. Very little literature is available on the effect of antioxidants on engine performance and emission. Most of the work has been done using BHT and BHA antioxidants (Rizwanul Fattah et al. 2014a; Nagappan et al. 2021). In the present work, antioxidant-treated biodiesel fuel blends were evaluated to carry out further analysis. Biodiesel from waste cooking oil was produced via transesterification. The physico-chemical properties were evaluated, and GC analysis of the neat biodiesel was performed. The oxidation stability of pure biodiesel and antioxidant-treated biodiesel were evaluated. The oxidation stability of aromatic (DPA) antioxidants was tested and compared with that of synthetic (TBHQ and PY) antioxidants. Additionally, the effect of antioxidant fuel blends on performance and emission was evaluated.
Material and methods
Waste cooking oil was procured from a local market cafeteria as a feedstock for biodiesel production. All reagents used in biodiesel production, such as methanol (99.9%) and potassium hydroxide (98%), were of analytical grade. The antioxidants, TBHQ, PY, and diphenylamine, were purchased from the market. The TBHQ was 97% pure, PY was 99% pure, and Di-phenylamine was 98% pure. TBHQ and PY are the most commonly used antioxidants reported in the literature (Rodrigues et al. 2020) for enhancing oxidation stability. The properties of the antioxidants are given in Table 1. Antioxidants (200 ppm and 500 ppm) were added to each biodiesel sample. The addition of 200 ppm antioxidant additives to biodiesel does not meet the ASTM standard. When a 500-ppm quantity of antioxidant was added to the biodiesel, the induction period was enhanced for all three antioxidants and met the ASTM D-6751 and EU 14,112 methods. A further increase in ppm of antioxidant had an adverse effect on emissions. All antioxidants fully dissolved in biodiesel at all concentrations.
The waste cooking oil obtained from the local cafeteria was used as a feedstock for the production of biodiesel. Initially, oil was filtered to remove impurities and heated above 100 °C to remove any moisture. The free fatty acid content of the oil was determined according to ASTM-D644. As FFA reached 1.12, only a one-step transesterification process was required to convert WCO into biodiesel. In the transesterification process, a 0.5% (w/w) catalyst solution of potassium hydride and 20% (w/w) methanol of oil was prepared and mixed in WCO at 60 °C and stirred continuously for 1 h. Then, the prepared mixture was poured into a separation funnel to settle.
Glycerol settled in the funnel due to gravity separation. The last step involved washing with water, removing all glycerol from the separation funnel, and mixing hot water with WCO methyl ester so that all unreacted particles were removed. The water was heavier than the other substances, so it settled in the separation funnel and was removed. The biodiesel that remained in the separation funnel was collected and heated above 100 °C to remove moisture. The biodiesel produced from waste cooking oil was analyzed to determine physicochemical properties as per the ASTM standard presented in Table 2. GC–MS analysis of the waste cooking biodiesel was conducted to identify chemical constituents present in the biodiesel.
Preparation of antioxidant-treated biodiesel and fuel blends
Biodiesel obtained from waste cooking oil was blended with petroleum diesel. The splash method was adopted for the preparation of fuel blends. This method required biodiesel and petroleum diesel to be added to one container and stirred for 5–10 min to make a homogenous mixture. In this study, 20% WCO biodiesel was blended with 80% diesel for each fuel blend. Initially, 200 and 500 ppm antioxidant was mixed into the biodiesel. This antioxidant-treated 20% biodiesel quantity was added to 80% petroleum diesel to make the fuel blends. 80D20WCB, 80D20(WCB + 200PY), 80D20(WCB + 500PY), 80D20(WCB + 200TBHQ), 80D20(WCB + 500TBHQ), 80D20(WCB + 200DPA), and 80D20(WCB + 500DPA) fuel blends were considered for analysis through an engine test. Diesel was used as the baseline fuel to compare these test blends.
Oxidation stability and their setup
The oxidation stability of biodiesel was determined by the Rancimat method. The ASTM D-6751 and EN-14214 standards ensure the quality of biodiesel. According to the ASTM D-6751 and EN-14112 methods, the minimum required induction periods are 6 h and 3 h, respectively. The Indian standard IS-15607 induction time period is 6 h (Serrano et al. 2013; Narayanasamy et al. 2018). The present study was performed on waste cooking biodiesel and waste cooking biodiesel treated with antioxidants through Rancimat equipment. The 873 biodiesel Rancimat Metrohm apparatus was used according to EN 14,112, as shown in Fig. 3. In the Rancimat method, oxidation was conducted by passing the airstream at 10 L/h through 3 g of biodiesel. The temperature of the instrument was set at 110 °C throughout the biodiesel experiment. A flask was filled with 50 mL of distilled water, and an electrode was installed to measure the thermal conductivity. Vaporization of biodiesel occurs above 100 °C and produces bubbles that travel to the conductivity flask. The conductivity of the distilled water rapidly increases at the end of the induction period. The oxidation stability of the biodiesel was measured by induction period (h) from the conductivity-time graph.
Experimental setup for the engine trial
A 4-stroke, direct injection type, single-cylinder unmodified diesel engine (Kirloskar) was used to conduct the engine trials in this work.
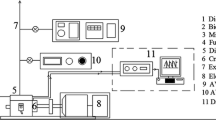
These engines are commonly used for power generation in agricultural areas. The detailed specifications of the engine are highlighted in Table 3. The engine operates at a rated power of 5.9 kW with a constant speed of 1500 rpm. Figure 1 shows a schematic diagram of the diesel engine setup for the experimental trial. The experimental test setup was equipped with an electronic data-controlled acquisition system and computer unit. It monitors, measures, and controls the fuel flow rate, engine load, and temperature at that load. The engine load was applied by a load bank with an electric bulb setup grouped in a parallel circuit. To measure the current, voltage, and revolutions of the engine output shaft per minute, an ammeter, voltmeter, and rpm indicator were positioned on the loading unit. The exhaust gas temperature was measured by a mounted thermocouple at the exhaust pipe of the engine. The exhaust gases released during combustion of fuel at different load conditions were measured using an AVL-1000 gas analyzer and AVL-480 smoke meter used for the smoke opacity measurement.
The engine trials were conducted from no load to 100% loading of the engine in 20% load increments under different loading conditions. The experiments were performed under ambient pressure and temperature conditions. Different doses of antioxidant-treated fuel and diesel biodiesel blend fuel were tested and compared to baseline neat diesel fuel. All tests were conducted at different mean effective pressures of 1.16, 1.76, 2.35, 2.97, 3.59, and 4.21 bar corresponding to engine loadings of 0%, 20%, 40%, 60%, 80%, and 100%, respectively.
Results and discussion
Variations in the physicochemical properties of the WCB and test fuel
Table 1 defines the properties of waste cooking oil biodiesel with their standards and compares them with the properties of petrodiesel. All the measured properties are found as per ASTM standards. The kinematic viscosity of waste cooking oil biodiesel is slightly higher in the given range than ASTM standard for biodiesel. Higher viscosity indicates a longer chain length and the presence of free fatty acids. As cis-oriented chain becomes trans during the production of biodiesel. The trans samples are more viscous than the cis samples (Yaakob et al. 2014). A higher kinematic viscosity implicitly suggests a higher resistance to flow in the pipeline of the fuel injection system, which leads to a delay in the injection timings. However, a higher kinematic viscosity promotes poor fuel atomization (Shahabuddin et al. 2012). Table 4 represents the physicochemical properties of the fuel blends. The viscosity was reduced by making blends with diesel and slightly increased by the addition of antioxidants. Similar trends have been reported in the literature (Rashedul et al. 2017; Jeyakumar and Narayanasamy 2020). However, all measured properties of the test fuel were within the ASTM standard range when an antioxidant was added to the biodiesel.
Oxidation stability of WCB and WCB treated with antioxidant
The oxidation stability of waste cooking oil biodiesel was tested by the Rancimat method. The waste cooking oil biodiesel composition was analyzed by GC–MS. In Table 5, the constituent type and amount in terms of % area present in WCO biodiesel with saturated and unsaturated composition is given. The GC–MS results show that the waste cooking oil biodiesel was highly unsaturated. The unsaturated fatty acid content was 71.67%, and the saturated fatty acid content was 28.33%. GC–MS analysis was conducted on the waste cooking oil biodiesel (Tomar et al. 2020). The GC–MS results showed the compounds responsible for the stability of biodiesel and the degradation product amount left. (Tamilalagan and Singaram 2019). Biodiesel is a mixture of long-chain unsaturated and saturated compounds. The higher degree of unsaturated chains prompts lower oxidation stability. Higher unsaturated compounds and the presence of carbon double bonds make them unstable and cause them to react with oxygen (Kumar 2017). Due to the 71.67% higher level of unsaturated compounds in waste cooking oil, this biodiesel exhibits lower oxidation per induction period of 1.71 h, which was measured by the Rancimat method as shown in Fig. 2. To assess the test’s dependability, the trials were repeated at least thrice. Our results had a variability of less than 3%, which was within the repeatability limit of their respective standard methods. This biodiesel did not meet ASTM D-6751 and EN 14,112 method standards. Similar results of oxidation stability were observed in another study (Zhou et al. 2017) on waste cooking oil at 3.01 h. Therefore, there is a need to increase oxidation stability to meet the ASTM and EN standards. Through the addition of antioxidants, the oxidation stability of biodiesel can be enhanced. Researchers use many synthetic and aromatic antioxidants to increase the oxidation stability. TBHQ, BHT, BHA, PG, and PY were tested in nonedible oil, specifically Karanja oil at 300, 500, 700, and 1000 ppm. Significant improvement was reported in a previous study (Agarwal and Khurana 2013).
In this study, DPA antioxidant was used, and for comparative analysis of their results, TBHQ and PY were the most commonly used antioxidants. DPA, PY, and TBHQ were initially added at 200 ppm to determine the effect of antioxidants on the oxidation stability. The induction period was enhanced by 2.61 h, 3.75 h, and 8.39 h for DPA, TBHQ, and PY, respectively, as DPA and PY did not meet the ASTM D-6751 standard. Furthermore, 500 ppm antioxidant was added to the biodiesel, and the induction period was enhanced by 6.76 h, 6.38 h, and 12.28 h with DPA, PY, and TBHQ, respectively. All biodiesel samples treated with 500 ppm antioxidant met the ASTM D-6751 and EN 14,112 standards. The induction periods of waste cooking oil biodiesel at doses of 200 ppm and 500 ppm are shown in Fig. 3. Similar trends have been reported in previous reports (Rashed et al. 2016) and (Zhou et al. 2017).
Performance and emission error with uncertainty analysis
Uncertainty is inherent in every work. Error analysis is a term used to describe a thorough examination of the uncertainties that arise during physical measurements. The calibrators of instruments, sensors, observations, test procedures, and environmental conditions are all affected by these uncertainties. The detailed uncertainties and accuracies of the various instruments employed are given in Table 6. The overall level of uncertainty in the current study is 2.0326%.
Overall uncertainty in this study:
= square root of [(uncertainty of BSEC)2 + (uncertainty of BTE)2 + (uncertainty of brake power)2 + (uncertainty of HC)2 + (uncertainty of CO)2 + (uncertainty of NOx)2 + (uncertainty of smoke)2 + (uncertainty of EGT)2].
= square root of [(0.3)2 + (0.25)2 + (0.05)2 + (0.2)2 + (0.01)2 + (1.3)2 + (1)2 + (0.2)2] = 2.9251.
Engine performance characteristics
The performance characteristics of the CI engine were evaluated in terms of brake thermal efficiency and brake specific energy consumption at different loads for WCB- and antioxidant-treated biodiesel blends and pure diesel.
Brake thermal efficiency
The brake thermal efficiency (BTE) describes the conversion of chemical energy into mechanical energy. The full 100% of the chemical energy did not convert into mechanical energy output; some of it was wasted through exhaust gases, cooling heat dissipation, and mechanical friction. Figure 4 shows the variation in brake thermal efficiency with the brake mean effective pressure (BMEP) for different fuel samples. 80D20WCB shows lower BTE due to its low calorific value, higher viscosity, and poor spray properties, leading to higher fuel consumption (Kumar and Tomar 2019). When antioxidant-treated biodiesel-diesel fuel was used, the blends with antioxidants showed an increase in BTE compared to blends without antioxidants. Moreover, the 500-ppm antioxidant-treated biodiesel sample had a better BTE than the 200-ppm antioxidant-treated biodiesel sample. At full load, the BTE increased for the 80D20(WCB + 200DPA), 80D20(WCB + 200TBHQ), 80D20(WCB + 500TBHQ), 80D20(WCB + 500DPA), 80D20(WCB + 200PY), and 80D20(WCB + 500PY) samples, and the D100 values were 0.78%, 2.16%, 5.99%, 8.56%, 9.68%, and 12.81% that of 80D20(WCB). The combined effect of a lower BSEC and an increased power output resulted in a higher BTE (Rizwanul Fattah et al. 2014b). Similar trends were found in previous studies (Rizwanul Fattah et al. 2014b; Rashedul et al. 2015). Petrodiesel has higher brake thermal efficiency than all other fuel blends due to its lower density, viscosity, and higher calorific value at lower fuel consumption (Vijay Kumar et al. 2018). At higher loads, the BTE slightly decreases due to a decrease in the air–fuel ratio.
Brake-specific energy consumption
Brake-specific energy consumption (BSEC) is also considered a performance-measured parameter, as the BSEC measures the amount of fuel consumed per the produced power. Figure 5 illustrates the brake-specific energy consumption with varying brake mean effective pressures for different test fuel blends. The D100 shows lower brake-specific energy consumption because of its higher volatility, low viscosity, and higher calorific value, and adding 20% biodiesel to diesel results in a higher BSEC. This investigation has shown that the BSEC decreases for all blends with antioxidants compared to those without antioxidants. The BSEC of the 80D20(WCB + 200DPA), 80D20(WCB + 200TBHQ), 80D20(WCB + 500TBHQ), 80D20(WCB + 500DPA), 80D20(WCB + 200PY), 80D20(WCB + 500PY), and D100 are reduced by 0.79%, 2.2%, 6.3%, 9.3%, 10.72%, 14.7%, 17.67%, respectively in comparison with 80D20(WCB). The reduction in BSEC might be due to the friction reduction properties of amines, which improve the ignition quality (Palash et al. 2014). The addition of antioxidants reduces the calorific value and the BSEC (Rizwanul Fattah et al. 2014b). Similar trends were observed in previous studies (Adam et al. 2018; Jeyakumar and Narayanasamy 2020).
Effect of antioxidants on the exhaust gas temperature
Figure 6 illustrates the exhaust gas temperature with varying brake mean effective pressures for different test fuel blends. It has been seen that increasing the load on the engine increases the exhaust gas temperature (EGT) (Reddy and Wani 2019). The EGT is a result of the waste heat released during the combustion of fuel that cannot be utilized further. A higher combustion temperature increases the waste heat release. The main reason for NOx production is high oxygen content, high combustion temperature, and longer combustion duration (Velmurugan and Sathiyagnanam 2016). Exhaust gas temperature depends on combustion cylinder temperature, affected by NOx formation. In all the test blends, pure diesel had a lower EGT, and the biodiesel-diesel (B20) test blends had a higher EGT. A higher EGT results from better combustion due to a sufficient supply of oxygen, proper mixing of air–fuel, and higher ignition probability (Tomar et al. 2020). When antioxidants are added, the EGT slightly decreases by 2.78%, 8.7%, 10.4%, 12.08%, 14.12%, 15.79%, and 17.28% for 80D20(WCB + 500PY), 80D20(WCB + 200PY), 80D20(WCB + 500DPA), 80D20(WCB + 500TBHQ), 80D20(WCB + 200TBHQ), 80D20(WCB + 200DPA), and D100 in comparison to 80D20WCB at full load. All the antioxidant-treated biodiesel-diesel fuel blends showed slightly higher EGTs than pure diesel. Similar results were reported in a previous study (Alagu et al. 2018); the authors stated that the addition of antioxidants BHA and BHT to rice bran biodiesel blends (B20) reduced the EGT by 5.25% and 3.74%, respectively, because the antioxidant additives hindered the conversion of fuel during combustion. The addition of BHA antioxidant to a beef tallow biodiesel blend of B20 reduced the EGT by 1.9% compared to the B20 blend (Nagappan et al. 2021).
Exhaust emission characteristics
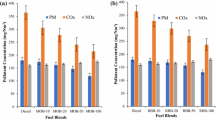
Effect of antioxidants on nitrogen oxides
The majority of exhaust emissions coming out of diesel engines are nitrogen oxides. The most common oxides of nitrogen are nitric oxide (NOx) and nitrogen dioxide (NO2), formed by the Zeldovic mechanism. NOx emissions increase during each stage of combustion. NOx emission is affected by many parameters, such as adiabatic flame temperature, physiochemical properties, ignition delay time, combustion chamber geometry, injection timing, and equivalence ratio (Dueso et al. 2018). Figure 7 illustrates the nitrogen oxide emissions with varying brake mean effective pressures for different test fuel blends. It is observed in the figure that when the load is increased, NOx emissions increase because of the higher combustion chamber temperature at higher loads. The 80D20(WCB) blend emitted more NOx because of its physicochemical properties and the molecular structure of biodiesel, and combustion was improved as the in-cylinder temperature increased. When antioxidants were added, the NOx decreased by 2.32%, 5.24%, 6.5%, 8.0%, 12.0%, 15.7%, and 19.65% for 80D20(WCB + 500PY), 80D20(WCB + 200PY), 80D20(WCB + 500DPA), 80D20(WCB + 500TBHQ), 80D20(WCB + 200TBHQ), 80D20(WCB + 200DPA), and D100 in comparison to 80D20WCB at full load. Similar trends were observed in previous literature (Rizwanul Fattah et al. 2014b). During the oxidative reaction of biodiesel, peroxyl-free radicals are continually created, which are responsible for the creation of nitric oxide. When antioxidant-treated biodiesel-diesel fuel blends are used, NOx emissions significantly decrease because of the reaction of aromatic amine, which reduces free radicals and unsaturation in the fuel blends. Hydrogen peroxide and peroxyl radicals are produced during the oxidation process and further converted into hydroxyl radicals by absorbing the heat inside the combustion chamber (Palash et al. 2014). Diphenylamine reacts rapidly with free radicals and produces diphenyl nitric oxides as shown in Eqs. 1 and 2. Nitric oxides have considered as scavenging agent because of their rapid reaction with free radicals like nitrogen-cantered and carbon radicals. The radical quenching nature of antioxidants helps to minimize NOx formation.
Effect of antioxidants on carbon monoxide
Carbon monoxide also contributes to a large percentage of emissions as exhaust of diesel engines. CO emissions are formed due to the incomplete combustion of fuel. Figure 8 illustrates carbon monoxide emissions with varying brake mean effective pressures for different test fuel blends. CO emissions are found to increase as the load increases. Up to half of the loading, the rate of increase in CO emissions is not so rapid, but after the half loading point, emissions increase rapidly due to the higher fuel availability, and incomplete combustion increases the CO emissions at peak loading (Tomar and Kumar 2020). The 80D20(WCB) blend had lower CO emissions than D100 due to the supply of available oxygen in the biodiesel. D100 emits more CO than all blends because diesel has insufficient oxygen for combustion. The CO emissions from antioxidant-treated biodiesel-diesel blends are decreased by 1.61%, 7.78%, 9.2%, 11.1%, 16.27%, 19.21%, and 21.13% for 80D20(WCB + 500PY), 80D20(WCB + 200PY), 80D20(WCB + 500DPA), 80D20(WCB + 500TBHQ), 80D20(WCB + 200TBHQ), 80D20(WCB + 200DPA), and 80D20WCB compared to D100 at full load. These results are similar to those of a prior study (Adam et al. 2018), in which the authors claimed that the addition of DPPD, NPPD, and BHA antioxidants to palm biodiesel blends of PB30 increased CO emissions by 8.41 to 17.58%. Similar results were shown in another study (Nagappan et al. 2021), where a 14.2% increase in CO was observed when BHA was added to B20 (beef tallow biodiesel blend). When antioxidant-treated biodiesel-diesel fuel blends were used, CO emissions were significantly reduced for all the blends compared to diesel. OH (hydroxyl) radicals formed during combustion are prone to CO oxidation (Rizwanul Fattah et al. 2014a). During oxidation process, the hydrogen peroxide (H2O2) and peroxyl radicals (HO2) are continuously formed. By absorbing heat these, radicals converted into hydroxyl radicals (OH) shown in Eqs. (3), (4), and (5). Conversion of CO into CO2 depends on these OH radicals.
Use of antioxidant in biodiesel decreases the concentration of hydrogen peroxide and peroxyl radicals. Due to reduction in free radicals, formation of hydroxyl radicals also reduces and increases CO.
Effect of antioxidants on hydrocarbons
Another form of emission is hydrocarbon emission caused by the incomplete combustion of fuel within the combustion chamber. These unburnt fuel particles result from a deficiency of oxygen, as oxygen is required for complete combustion (Pali et al. 2015). Figure 9 illustrates the hydrocarbon emissions with varying brake mean effective pressures for different test fuel blends. At lower loads, the emissions are lower, and they increase as the load increases. At higher loads due to the rich mixture availability, HC emissions slightly increase. All the tested blends had similar HC emissions. The 80D20(WCB) blend emitted fewer HCs as the oxygen content in the blend was increased through the addition of biodiesel (Dueso et al. 2018). At the maximum load for antioxidant-treated biodiesel blends, HCs were reduced by 5%, 8.6%, 15%, 18.96%, 20.6%, 24.13%, and 29.31% for 80D20(WCB + 500PY), 80D20(WCB + 200PY), 80D20(WCB + 500DPA), 80D20(WCB + 500TBHQ), 80D20(WCB + 200TBHQ), 80D20(WCB + 200DPA), and 80D20WCB compared to D100. Antioxidant-treated fuel blends have reduced amount of hydrogen peroxide and peroxyl radicals. As they contain fewer free radicals, leading to slight increases in HC emissions similar to CO emission. OH radicals formation required for oxidation of hydrocarbon (Eqs. 6 and 7). Similar trends were reported in previous literature (Rizwanul Fattah et al. 2014c, a; Rashedul et al. 2017). The addition of BHA and BHT to a B20 blend of rice bran biodiesel increased HC emissions by 10.6% and 11.2%, respectively, compared to those of B20 (Alagu et al. 2018). All the test blends exhibited significantly lower HC emissions than diesel.
Effect of antioxidants on smoke opacity
Smoke opacity describes the particles that are expelled from the combustion chamber as exhaust. Smoke opacity is due to incomplete combustion, insufficient oxygen content, and a rich air–fuel mixture during combustion. Figure 10 illustrates the smoke opacity with varying brake mean effective pressures for different test fuel blends. Pure diesel shows a higher smoke opacity for all loads because it has insufficient oxygen. As the load increases, smoke opacity increases because of the increasing fuel supply at higher loads. All the tested fuel blends showed similar trends (Sidharth and Kumar 2020; Tomar and Kumar 2020). The 80D20(WCB) diesel–biodiesel blend had a lower smoke opacity due to the addition of biodiesel into the diesel. The presence of biodiesel increased the oxygen content in the fuel blends, which helped to reduce emissions. At the maximum load for antioxidant-treated biodiesel blends, the smoke opacity is 1.79%, 3.83%, 6.90%, 10.99%, 16.62%, 18.67%, and 20.46% lower for 80D20(WCB + 500PY), 80D20(WCB + 200PY), 80D20(WCB + 500DPA), 80D20(WCB + 500TBHQ), 80D20(WCB + 200TBHQ), 80D20(WCB + 200DPA), and 80D20WCB than that of D100. Similar test results were obtained in previous literature (Rashedul et al. 2015). By using antioxidant-treated biodiesel-diesel fuel blends, a slight increase in smoke opacity was observed. An increase in smoke is due to a reduction in available oxygen, higher aromatic contents, and a greater number of C–C bonds; such factors are a result of the addition of antioxidants into the fuel blend. Although these antioxidant-treated biodiesel fuel blends lie between pure diesel and diesel–biodiesel blends, they show a significant reduction in smoke opacity compared to diesel (Rashed et al. 2016).
Conclusion
In the present research, DPA, TBHQ, and PY antioxidant tests were used to evaluate their performance and emissions in biodiesel blends. Oxidation stability of WCB did not meet the ASTM standard, so antioxidants were added at different concentrations to evaluate the oxidation stability. All the test blends of 500 ppm and 200 ppm, antioxidant PY showed oxidation stability as per the ASTM standard. A 500 ppm concentration of DPA antioxidant was more effective than synthetic antioxidants, i.e., TBHQ and PY. However, PY was the most oxidation-stable. The addition of 500 ppm antioxidants improved the brake thermal efficiency at peak load by 14.45% for PY, 6.61% for TBHQ, and 9.53% for DPA compared to antioxidant. In contrast, the BTE decreased by 2.45% for PY, 9.13% for TBHQ, and 6.64% for DPA compared to diesel. The addition of 500 ppm antioxidants decreased the brake specific energy consumption at peak loads of 12.62% for PY, 6.20% for TBHQ, and 8.70% for DPA in comparison to the absence of antioxidant. In contrast, the BSEC increased by 2.51% for PY, 10.05% for TBHQ, and 7.12% for DPA compared to diesel. The NOx emissions were reduced significantly by the addition of antioxidants compared to those without the antioxidant blend. The average reduction in NOx emission at full load was 19.72% for PY, 12.77% for TBHQ, and 15.46% for DPA compared to biodiesel without antioxidant treatment. However, HC and CO emissions of all the test blends treated with antioxidants increased compared to those without antioxidant blends. All antioxidant-treated biodiesel blends still had lower HC, CO, and smoke emissions than diesel, which may be considered an achievement of added antioxidants. It can be concluded that antioxidants (both aromatic and synthetic) were effective in improving the performance and emissions of biodiesel. However, an exhaust gas treatment system can be used for treating the increased exhaust.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- TBHQ:
-
tert-Butylhydroxyquinone
- PY:
-
pyrogallol
- DPA:
-
diphenylamine
- PG:
-
propyl gallate
- BHT:
-
tert-butylated
- BHA:
-
butyl-hydroxyanisole
- DPPD:
-
N,N′-diphenyl-p-phenylenediamine
- NPPD:
-
phenylenediamine
- EN:
-
European standard
- WCB:
-
waste cooking oil biodiesel
- FFA:
-
free fatty acid
- GC–MS:
-
gas chromatography and mass chromatography
- BTE:
-
brake thermal efficiency
- BSEC:
-
brake specific energy consumption
- NOx:
-
nitrogen oxide
- HC:
-
hydro carbon
- CO:
-
carbon mono oxide
- EGT:
-
exhaust gas temperature
- % Vol:
-
percentage volume
References
Adam IK, Heikal M, Aziz ARA, Yusup S (2018) Mitigation of NOx emission using aromatic and phenolic antioxidant-treated biodiesel blends in a multi-cylinder diesel engine. Environ Sci Pollut Res 25:28500–28516. https://doi.org/10.1007/s11356-018-2863-8
Agarwal AK, Khurana D (2013) Long-term storage oxidation stability of Karanja biodiesel with the use of antioxidants. Fuel Process Technol 106:447–452. https://doi.org/10.1016/j.fuproc.2012.09.011
Alagu K, Nagappan B, Jayaraman J, Arul Gnana Dhas A (2018) Impact of antioxidant additives on the performance and emission characteristics of CI engine fuelled with B20 blend of rice bran biodiesel. Environ Sci Pollut Res 25:17634–17644. https://doi.org/10.1007/s11356-018-1934-1
Alves SM, Dutra-pereira FK, Bicudo TC (2019) Influence of stainless steel corrosion on biodiesel oxidative stability during storage. Fuel 249:73–79. https://doi.org/10.1016/j.fuel.2019.03.097
Bharti R, Singh B (2020) Green tea (Camellia assamica) extract as an antioxidant additive to enhance the oxidation stability of biodiesel synthesized from waste cooking oil. Fuel 262. https://doi.org/10.1016/j.fuel.2019.116658
Dueso C, Muñoz M, Moreno F et al (2018) Performance and emissions of a diesel engine using sunflower biodiesel with a renewable antioxidant additive from bio-oil. Fuel 234:276–285. https://doi.org/10.1016/j.fuel.2018.07.013
Nagarajan J, Narayanasamy B (2021) Effects of natural antioxidants on the oxidative stability of waste cooking oil biodiesel. Biofuels 12:485–494. https://doi.org/10.1080/17597269.2019.1711320
Jeyakumar N, Narayanasamy B (2020) Effect of Basil antioxidant additive on the performance, combustion and emission characteristics of used cooking oil biodiesel in CI engine. J Therm Anal Calorim 140:457–473. https://doi.org/10.1007/s10973-019-08699-3
Kovács A, Tóth J, Isaák G, Keresztényi I (2015) Aspects of storage and corrosion characteristics of biodiesel. Fuel Process Technol 134:59–64. https://doi.org/10.1016/j.fuproc.2015.01.014
Kumar N (2017) Oxidative stability of biodiesel: causes, effects and prevention. Fuel 190:328–350. https://doi.org/10.1016/j.fuel.2016.11.001
Kumar N, Tomar M (2019) Influence of nanoadditives on ignition characteristics of Kusum (Schleichera oleosa) biodiesel. Int J Energy Res 43:3223–3236. https://doi.org/10.1002/er.4446
Kumar P, Sharma MP, Dwivedi G (2016) Impact of ternary blends of biodiesel on diesel engine performance. Egypt J Pet 25:255–261. https://doi.org/10.1016/j.ejpe.2015.06.010
Kumar Patel H, Kumar S (2017) Experimental analysis on performance of diesel engine using mixture of diesel and bio-diesel as a working fuel with aluminum oxide nanoparticle additive. Therm Sci Eng Prog 4:252–258. https://doi.org/10.1016/j.tsep.2017.09.011
Nagappan B, Devarajan Y, Kariappan E et al (2021) Influence of antioxidant additives on performance and emission characteristics of beef tallow biodiesel-fuelled CI engine. Environ Sci Pollut Res 28:12041–12055. https://doi.org/10.1007/s11356-020-09065-9
Narayanasamy B, Jeyakumar N, Manoharan DK (2018) Effect of natural antioxidants on the oxidation stability of methyl ester of rubber seed oil. Energy Sources, Part A Recover Util Environ Eff 40:680–687. https://doi.org/10.1080/15567036.2018.1454549
Naresh Kumar Reddy S, Marouf Wani M (2020) Engine performance and emission studies by application of nanoparticles as additive in biodiesel diesel blends. Mater Today Proc 43:3631–3634. https://doi.org/10.1016/j.matpr.2020.09.832
Palash SM, Kalam MA, Masjuki HH et al (2014) Impacts of NOx reducing antioxidant additive on performance and emissions of a multi-cylinder diesel engine fueled with Jatropha biodiesel blends. Energy Convers Manag 77:577–585. https://doi.org/10.1016/j.enconman.2013.10.016
Pali HS, Kumar N, Alhassan Y (2015) Performance and emission characteristics of an agricultural diesel engine fueled with blends of Sal methyl esters and diesel. Energy Convers Manag 90:146–153. https://doi.org/10.1016/j.enconman.2014.10.064
Pullen J, Saeed K (2014) Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process Technol 125:223–235. https://doi.org/10.1016/j.fuproc.2014.03.032
Biodiesel from household/restaurant waste cooking oil (WCO). J ChemEng Process Technol 02.https://doi.org/10.4172/2157-7048.1000112
Rajendran S (2020) Effect of antioxidant additives on oxides of nitrogen (NOx) emission reduction from Annona biodiesel operated diesel engine. Renew Energy 148:1321–1326. https://doi.org/10.1016/j.renene.2019.10.104
Rashed MM, Kalam MA, Masjuki HH et al (2015) Stability of biodiesel, its improvement and the effect of antioxidant treated blends on engine performance and emission. RSC Adv 5:36240–36261. https://doi.org/10.1039/c4ra14977g
Rashed MM, Kalam MA, Masjuki HH et al (2016) Improving oxidation stability and NOX reduction of biodiesel blends using aromatic and synthetic antioxidant in a light duty diesel engine. Ind Crops Prod 89:273–284. https://doi.org/10.1016/j.indcrop.2016.05.008
Rashedul HK, Kalam MA, Masjuki HH et al (2017) Attempts to minimize nitrogen oxide emission from diesel engine by using antioxidant-treated diesel-biodiesel blend. Environ Sci Pollut Res 24:9305–9313. https://doi.org/10.1007/s11356-017-8573-9
Rashedul HK, Masjuki HH, Kalam MA et al (2015) Effect of antioxidant on the oxidation stability and combustion-performance-emission characteristics of a diesel engine fueled with diesel-biodiesel blend. Energy Convers Manag 106:849–858. https://doi.org/10.1016/j.enconman.2015.10.024
Reddy SNK, Wani MM (2020) A comprehensive review on effects of nanoparticles-antioxidant additives-biodiesel blends on performance and emissions of diesel engine. Appl Sci Eng Prog 13:285–298. https://doi.org/10.14416/J.ASEP.2020.06.002
Reddy SNK, Wani MM (2019) Engine performance and emission studies by application of antioxidant as additive in biodiesel diesel blends. AIP Conf Proc 2200.https://doi.org/10.1063/1.5141185
Reddy SNK, Wani MM (2021) An investigation on the performance and emission studies on diesel engine by addition of nanoparticles and antioxidants as additives in biodiesel blends. Int Rev Appl Sci Eng 12:111–118. https://doi.org/10.1556/1848.2020.00157
Rizwanul Fattah IM, Masjuki HH, Kalam MA et al (2014a) Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers Manag 79:265–272. https://doi.org/10.1016/j.enconman.2013.12.024
Rizwanul Fattah IM, Masjuki HH, Kalam MA et al (2014b) Performance and emission characteristics of a CI engine fueled with Cocos nucifera and Jatropha curcas B20 blends accompanying antioxidants. Ind Crops Prod 57:132–140. https://doi.org/10.1016/j.indcrop.2014.03.022
Rizwanul Fattah IM, Masjuki HH, Kalam MA et al (2014c) Effect of antioxidants on oxidation stability of biodiesel derived from vegetable and animal based feedstocks. Renew Sustain Energy Rev 30:356–370. https://doi.org/10.1016/j.rser.2013.10.026
Rodrigues JS, do Valle CP, Uchoa AFJ et al (2020) Comparative study of synthetic and natural antioxidants on the oxidative stability of biodiesel from Tilapia oil. Renew Energy 156:1100–1106. https://doi.org/10.1016/j.renene.2020.04.153
Saluja RK, Kumar V, Sham R (2016) Stability of biodiesel—a review. Renew Sustain Energy Rev 62:866–881. https://doi.org/10.1016/j.rser.2016.05.001
Sarin A, Singh NP, Sarin R, Malhotra RK (2010) Natural and synthetic antioxidants: influence on the oxidative stability of biodiesel synthesized from non-edible oil. Energy 35:4645–4648. https://doi.org/10.1016/j.energy.2010.09.044
Serrano M, Martínez M, Aracil J (2013) Long-term storage stability of biodiesel: influence of feedstock, commercial additives and purification step. Fuel Process Technol 116:135–141. https://doi.org/10.1016/j.fuproc.2013.05.011
Shahabuddin M, Kalam MA, Masjuki HH et al (2012) An experimental investigation into biodiesel stability by means of oxidation and property determination. Energy 44:616–622. https://doi.org/10.1016/j.energy.2012.05.032
Sidharth, Sidharth N (2020) Performance and emission studies of ternary fuel blends of diesel, biodiesel and octanol. Energy Sources, Part A Recover Util Environ Eff 42:2277–2296. https://doi.org/10.1080/15567036.2019.1607940
Sonthalia A, Garg S, Sharma R et al (2021) Effect of electrostatic precipitator on exhaust emissions in biodiesel fuelled CI engine. Environ Sci Pollut Res 28:11850–11859. https://doi.org/10.1007/s11356-019-07359-1
Sonthalia A, Kumar N (2021) Comparison of fuel characteristics of hydrotreated waste cooking oil with its biodiesel and fossil diesel. Environ Sci Pollut Res 28:11824–11834. https://doi.org/10.1007/s11356-019-07110-w
Srinivasan SK, Kuppusamy R, Krishnan P (2021) Effect of nanoparticle-blended biodiesel mixtures on diesel engine performance, emission, and combustion characteristics. Environ Sci Pollut Res 28:39210–39226. https://doi.org/10.1007/s11356-021-13367-x
Supriyono SH, Almeida MF, Dias JM (2015) Influence of synthetic antioxidants on the oxidation stability of biodiesel produced from acid raw Jatropha curcas oil. Fuel Process Technol 132:133–138. https://doi.org/10.1016/j.fuproc.2014.12.003
Tamilalagan A, Singaram J (2019) Oxidation stability of yeast biodiesel using Rancimat analysis: validation using infrared spectroscopy and gas chromatography–mass spectrometry. Environ Sci Pollut Res 26:3075–3090. https://doi.org/10.1007/s11356-018-3619-1
Tomar M, Jain A, Pujari PC, et al (2020) Potentials of waste plastic pyrolysis oil as an extender fuel for diesel engine. Arab J Geosci 13https://doi.org/10.1007/s12517-020-05574-6
Tomar M, Kumar N (2020) Effect of multi-walled carbon nanotubes and alumina nano-additives in a light duty diesel engine fuelled with schleichera oleosa biodiesel blends. Sustain Energy Technol Assessments 42:100833. https://doi.org/10.1016/j.seta.2020.100833
Velmurugan K, Sathiyagnanam AP (2016) Impact of antioxidants on NOx emissions from a mango seed biodiesel powered di diesel engine. Alexandria Eng J 55:715–722. https://doi.org/10.1016/j.aej.2015.10.004
Vijay Kumar M, Veeresh Babu A, Ravi Kumar P (2018) The impacts on combustion, performance and emissions of biodiesel by using additives in direct injection diesel engine. Alexandria Eng J 57:509–516. https://doi.org/10.1016/j.aej.2016.12.016
Yaakob Z, Narayanan BN, Padikkaparambil S et al (2014) A review on the oxidation stability of biodiesel. Renew Sustain Energy Rev 35:136–153. https://doi.org/10.1016/j.rser.2014.03.055
Zhou J, Xiong Y, Liu X (2017) Evaluation of the oxidation stability of biodiesel stabilized with antioxidants using the Rancimat and PDSC methods. Fuel 188:61–68. https://doi.org/10.1016/j.fuel.2016.10.026
Acknowledgements
The author would like to thank to the researchers who have worked in the area of oxidation stability of biodiesel and evaluated their different parameters. This research article compiles the previously available data provided by researchers to get objective of this study.
Author information
Authors and Affiliations
Contributions
KY had written the complete paper which was supervised by NK and RC. All the experiments were performed by KY.
NK conceptualized the idea of working on antioxidants and their effects on performance and emission. The complete paper was also supervised.
RC conceptualized the idea of working on oxidation stability of synthetic and aromatic antioxidants. The complete paper was also supervised.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yadav, K., Kumar, N. & Chaudhary, R. Effect of synthetic and aromatic amine antioxidants on oxidation stability, performance, and emission analysis of waste cooking oil biodiesel. Environ Sci Pollut Res 29, 27939–27953 (2022). https://doi.org/10.1007/s11356-021-18086-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18086-x