Abstract
An experimental investigation is conducted on a single-cylinder DI diesel engine, to evaluate the performance, combustion and emission characteristics of Jatropha biodiesel with the addition of antioxidants namely, Succinimide (C4H5NO2), N,N-Dimethyl p-phenylenediamine dihydrochloride (C8H14Cl2N2) and N-Phenyl-p-phenylenediamine (C6H5NHC6H4NH2) at 500, 1000 and 2000 ppm. The performance, combustion and emission characteristic tests are conducted at a constant speed of 1500 rpm, injection pressure of 215 bar, injection timing of 26° before top dead centre for the nine test fuels and the experimental results are compared with neat diesel and neat biodiesel as base fuels. The experimental results show that the addition of antioxidant in biodiesel suppresses the NO emission by quenching the OH radicals that are produced by the reaction of hydrocarbon radicals with molecular nitrogen. The maximum percentage reduction of NO emission by 5, 6 and 7% are observed for N-Phenyl-p-phenylenediamine, N,N-Dimethyl p-phenylenediamine dihydrochloride and Succinimide blended test fuels at 2000 ppm antioxidant addition with biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several experimental strategies have been followed to cut down the engine exhaust emissions to meet the emission standard norms. To achieve those emission norms, engine design modification alone is not enough; fuel formulation techniques that suppress the problem arising during combustion of fuel should be found. Different types of fuel formulations such as fuel blends [1], nanoparticles addition in biodiesel [2], oxygenated additives in biodiesel [3] and antioxidant additives in biodiesel [4–6] are commonly used. Among those additives, antioxidant added to the fuels at ppm (parts per million) levels extends the oxidation stability of the fuel and quenches the free radicals, which are responsible for the NO formation [7–9]. In addition, the antioxidant as additive in biodiesel prevents auto-oxidation when exposed to air, heat, light and metallic contaminants [10]. Usually, antioxidants are used in fuel to improve the oxidative and storage stability of the fuel [1, 11, 12]. Apart from the storage stability, a few experimental investigations were conducted with the addition of antioxidants in the fuel to find the significant reduction of NO emission. Generally, NO was formed by the increased CH radicals present in biodiesel undergoing combustion at high combustion temperatures [13]. An experimental investigation on the effect of two new antioxidants namely, N,N′-diphenyl-1,4-phenylenediamine and N-phenyl-1,4-phenylenediamine at 1000 and 2000 ppm addition in both B100 and B20 blends was carried out in a single cylinder diesel engine and found significant reduction of nitric oxide (NO) with increased smoke, carbon monoxide (CO) and unburned hydrocarbon (HC) emissions [4]. The same team [5] studied the effect of antioxidants such as L-ascorbic acid, a-tocopherol acetate, butylated hydroxytoluene, p-phenylenediamine and ethylenediamine at 0.025% individual addition in Jatropha biodiesel and noticed reduction in the brake specific fuel consumption for ethylenediamine and p-phenylenediamine blended biodiesel as 0.133 and 0.136 kg/kWh, respectively, while for neat biodiesel it was 0.145 kg/kWh. They observed NO reduction efficiency in the order of p-phenylenediamine > ethylenediamine > a-tocopherol > butylated hydroxytoluene > ascorbic acid, among the used test antioxidants and concluded that the addition of antioxidant to the biodiesel reduces NO emission by quenching the free radicals (oxygen molecules, nitric oxides, superoxide ions and hydroxyl radicals), chain breaking reactions, reduction of reactive radical concentration and scavenging of initiating radicals. Ryu [6] carried out the performance and emission characteristic test to examine the effects of the antioxidants namely, tert-butylhydroquinone (TBHQ), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PrG), and the natural antioxidant, tocopherol in soybean biodiesel and found beneficial result for its longer oxidation stability, reduction in both brake specific fuel consumption and the levels of pollutants at the engine exhaust. The addition of antioxidants such as 1,2,3 tri-hydroxy benzene (pyrogallol), 3,4,5-tri hydroxyl benzoic acid (propyl gallate) and 2-tert butyl-4-methoxy phenol (butylated hydroxyanisole) at 1000 ppm in croton megalocarpus biodiesel, resulted in marginal improvement of brake specific fuel consumption for the pyrogallol blended biodiesel [14]. Recent experimental investigation with N,N-diphenyl-1,4-phenylenediamine (DPPD) as additive in Jatropha biodiesel blends had shown a drastic percentage reduction of NO emission by 16.5% with slight increase in CO and HC emissions [15]. Ileri and Kocar [8] conducted experiment using antioxidants; butylated hydroxyanisole, butylated hydroxytoluene, tert-butylhydroquinone and 2-ethylhexyl nitrate at 0, 500, 750 and 1000 ppm individual addition with canola diesel blend (B20) and observed average NOx reduction emission with increased HC and CO emission. Mean while, Fattah, et al [9] blended 2000 ppm of 2(3)-tert-butyl-4-methoxyphenol with 20% of coconut methyl ester diesel blend (B20) and observed 7.8% reduction of NOx emission and 1.7% reduction of brake specific fuel consumption when compared with B20.
The earlier literatures depict that the addition of antioxidants at ppm level in fuel, extends the storage stability of the fuel and also has a good effects on the engine exhaust emission, in particular NO emission. So, to identify the potential of new antioxidants in neat biodiesel, a systematic experimental investigation was conducted and presented in the following next.
Materials and Methods
Preparation of Test Fuels
The antioxidants used for this study are (i) Succinimide (C4H5NO2), (ii) N,N-Dimethyl p-phenylenediamine dihydrochloride (C8H14Cl2N2), and (iii) N-Phenyl-p-phenylenediamine (C6H5NHC6H4NH2). These antioxidants are selected, based on the attempt of adding new additives in neat biodiesel. Antioxidants of 500, 1000 and 2000 ppm are individually weighed, finely powered, added to one litre of neat biodiesel and blended using a high speed mixer of 1500 rpm. The prepared test fuels are investigated for stability analysis and found stable for 48 h without any phase separation. The specifications of the test antioxidants are listed in Table 1 and the properties of test fuels are listed in Table 2.
Test Engine Setup
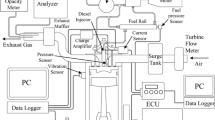
The experiments are conducted in a single cylinder; four-stroke, air-cooled direct injection diesel engine coupled with an ac alternator for loading the engine. The schematic diagram of the engine experimental setup is shown in Fig. 1 and the engine specification is listed in Table 3. The engine is operated at the constant speed of 1500 rpm, injection pressure of 215 bar and injection timing of 26° before top dead centre, producing the rated power of 4.4 kW. The data acquisition system comprising a Kistler piezoelectric pressure sensor, which is flush mounted at the engine cylinder head is used for measuring the in-cylinder pressure and a crank angle indicator is used to determine the crank angle. The combustion characteristics are obtained for fifty consecutive cycles and the mean value is found by averaging the collected data with the help of the data-acquisition system. A calibrated k-type chrome-alumel thermocouple is installed at the immediate exhaust of the engine to measure the exhaust gas temperature and the volumetric fuel flow rate is measured by using a digital stop watch. The levels of pollutants from the engine exhaust such as NO, CO and Unburned HC emissions are measured by using calibrated AVL 444 Di-Gas analyzer and the smoke opacity level is measured by using calibrated AVL 437 smoke meter. The performance parameters such as brake specific fuel consumption (BSFC), brake thermal efficiency (BTE) of the engine, exhaust gas temperature (EGT) and the emission parameters such as carbon monoxide (CO), unburned hydrocarbon (HC), nitric oxide (NO) and smoke opacity are plotted against the brake mean effective pressure (BMEP). The combustion characteristics such as heat release rate and cylinder pressure for the test fuels are plotted against the crank angle at the full load and the ignition delay for the test fuels is plotted against the BMEP. All the tests are carried out for three trails at steady state engine condition and the instruments measuring range and accuracy of the test bed is listed in Table 4.
Results and Discussions
Brake Thermal Efficiency (BTE) and Brake Specific Fuel Consumption (BSFC)
The variation of BSFC and BTE for the test fuel under BMEP is shown in Figs. 2 and 3, respectively. Due to the high viscosity and lower calorific value of neat biodiesel, higher BSFC of 0.318 kg/kWh and lower BTE of 28.6% are observed, when compared with BSFC of 0.263 kg/kWh and BTE of 32.3% for neat diesel. For 500 ppm antioxidant addition in biodiesel, marginal improvement in BTE values are observed for DPD1, NPPD1 and SU1 test fuels as 30.2, 29.8 and 29.3%, respectively and the respective values of BSFC observed for the DPD1, NPPD1 and SU1 test fuels are 0.281, 0.284 and 0.289 kg/kWh. This reduction in BSFC values for the DPD1, NPPD1 and SU1 test fuels are due to the friction reduction qualities of the amines [5]. But for the 1000 and 2000 ppm antioxidants addition in biodiesel, higher BSFC and lower BTE are observed. Increase in BSFC values of 0.325, 0.329, 0.329, 0.334, 0.331 and 0.338 kg/kWh are observed for DPD2, DPD3, NPPD2, NPPD3, SU2 and SU3 test fuels, respectively, which reflected in the reduction of BTE values for DPD2, DPD3, NPPD2, NPPD3, SU2 and SU3 test fuels as 28%, 27.7, 27.7, 27.3, 27.5 and 27.1%, respectively. These reductions in BTE values are due to the higher viscosity of test fuels, resulting in poor atomization of the fuel [16].
The variation of cylinder pressure and heat release rate under crank angle for the test fuels at the full load is shown in Figs. 4 and 5. Higher cylinder pressure characteristic is attained for neat biodiesel due to the presence of rich oxygen molecules, resulting in complete combustion [17]. On the other hand, the incorporation of antioxidants in the biodiesel resulted in reduction of both cylinder pressure and heat release rate. At the full load, the cylinder pressure observed for the neat biodiesel is 73.3 bar, whereas it is 69.5, 68.7, 68.3, 69.7, 68.1, 68.4, 69.6, 69.1 and 67.7 bar for SU1, SU2, SU3, NPPD1, NPPD2, NPPD3, DPD1, DPD2 and DPD3 test fuels, respectively. Due to the reduced cylinder temperature and pressure, the chemical delay is increased for the antioxidant test fuel resulting in lower heat release rate. At full load, the heat release rate observed for neat biodiesel is 20.9 J/deg CA whereas it is 20.1, 18.9, 18.6, 18.7, 18.3, 16.0, 19.5, 16.6 and 15.8 J/deg CA for SU1, SU2, SU3, NPPD1, NPPD2, NPPD3, DPD1, DPD2 and DPD3 test fuels respectively. The variation of ignition delay for the test fuels under BMEP is shown in Fig. 6. The time duration between the start of injection and the start of detectable heat release is denoted by ignition delay. With the addition of antioxidants to neat biodiesel, longer ignition delay is observed, due to the extension of the combustion process, lower cylinder pressure [18] and increased viscosity of fuel, resulting in poor atomization of fuel and slower mixing of air fuel mixture [19]. Higher viscosity of test fuels results in larger fuel droplets at the spray nozzle delivery, high spray jet penetration and angle of spray, which affects the combustion process by poor atomization of fuel (solid stream instead of spray of small droplet), resulting in uneven fuel distribution and poor air fuel mixing, affecting proper burning of fuel inside the combustion chamber. At the full load, the ignition delay observed for the neat biodiesel is 5.8 deg CA, whereas it is 6.1, 7.2, 7.3, 6.2, 6.6, 6.8, 6.3, 7.5 and 7.8 deg CA for SU1, SU2, SU3, NPPD1, NPPD2, NPPD3, DPD1, DPD2 and DPD3 test fuels respectively. The presence of oxygen molecule in biodiesel is quenched by the addition of antioxidants to biodiesel [4, 5], which delayed the early start of combustion resulting in longer ignition delay for the antioxidants dispersed test fuels.
Exhaust Gas Temperature and NO
The variation of EGT for the test fuels under BMEP is shown in Fig. 7. It is observed that the exhaust gas temperature (EGT) for the neat biodiesel (339°C) is higher when compared with that of neat diesel (325°C) because of the rich oxygen content of biodiesel, causing sudden burning of fuel resulting in higher combustion temperature. The lowest exhaust gas temperature observed for DPD1, DPD2, DPD3, NPPD1, NPPD2, NPPD3, SU1, SU2 and SU3 test fuels are 333, 331, 325, 339, 327, 321, 332, 326 and 319°C respectively due to the suppression of oxygen availability in biodiesel by the addition of antioxidants [4]. The variation of NO for the test fuels under BMEP is shown in Fig. 8. In general, the oxygenated nature of neat biodiesel promotes higher NO than the diesel fuel, due to the reaction of hydrocarbon radicals (CH. CH2, C2, C and C2H) with nitrogen at higher temperature [20]. The peroxyl and hydrogen peroxide radicals, that are formed by absorbing heat during combustion gets converted into hydroxyl radicals [21], which further undergoes series of gas phase reaction with nitrogen at higher temperature forming higher NO emission. Reduction in NO emission is observed for the test fuels, when compared with neat biodiesel of 1390 ppm and neat diesel of 1320 ppm. The observed values of NO emission for the test fuels DPD1, DPD2, DPD3, NPPD1, NPPD2, NPPD3, SU1, SU2 and SU3 are 1358, 1349, 1320, 1390, 1328, 1301, 1357, 1325 and 1290 ppm, respectively. At 2000 ppm antioxidant addition in biodiesel, maximum percentage reduction of NO by 5, 6 and 7% are observed for DPD3, NPPD3 and SU3 test fuels respectively when compared with neat biodiesel. The addition of antioxidants in biodiesel quenches the free radical formation and inhibits the participation of gas phase reaction between the free radicals and nitrogen [14], resulting in the reduction of NO emission.
Unburned HC, CO and Smoke Emission
The variation of unburned HC for the test fuels under BMEP is shown in Fig. 9. The observed Unburned HC emission for neat biodiesel (18 ppm) is less than that of neat diesel (25 ppm), because of plenteous oxygen molecule in biodiesel favouring better combustion. On the other hand, addition of antioxidants to the neat biodiesel increases the unburned HC values for the DPD2, DPD3, NPPD2, NPPD3, SU1, SU2 and SU3 test fuels as 20, 22, 19, 23, 19, 22 and 24 ppm, respectively. The variation of CO emission for the test fuels under BMEP is shown in Fig. 10. The rich oxygen content of biodiesel enhances the combustion process [16] and results in lower CO emission (0.05% vol), when compared with neat diesel (0.09% vol). Higher CO emission values are observed for DPD1, DPD2, DPD3, NPPD1, NPPD2, NPPD3, SU1, SU2 and SU3 test fuels as 0.06, 0.09, 0.10, 0.06, 0.08, 0.09, 0.07, 0.09 and 0.11% vol, respectively, due to the suppression of OH radicals which inhibits the conversion of CO into CO2 and HC into H2O and CO2 [4]. The variation of smoke opacity for the test fuels under BMEP is shown in Fig. 11. It is observed that the smoke opacity for neat biodiesel is 37.6% and for neat diesel is 43.5%. Higher smoke opacity values are observed for DPD1, DPD2, DPD3, NPPD1, NPPD2, NPPD3, SU1, SU2 and SU3 test fuels as 38.9, 40.4, 40.8, 37.7, 38.4, 39.2, 37.8, 38.6 and 39.4%, respectively, due to the trade-off between smoke and NOx emission [6].
Conclusions
The current study investigates the influence of using antioxidants in neat biodiesel that affects the performance, combustion and emission characteristics of the engine. Based on the observed experimental results for the test fuels, the following conclusions are drawn:
-
Maximum percentage reduction of NO emission by 5, 6 and 7%, are observed for NPPD3, DPD3 and SU3 test fuels respectively, due to the free radical termination process by the addition of antioxidants in biodiesel.
-
Ignition delay increases for the antioxidants dispersed test fuels due to quenching of free radicals by addition of antioxidants. As a result of insufficient free radical concentration, reduced heat release rate and cylinder pressure characteristics are observed for antioxidants dispersed test fuels.
-
The efficiency of the antioxidants on NO emission is observed in the order of succinimide > N,N-Dimethyl p-phenylenediamine dihydrochloride > N-Phenyl-p-phenylenediamine.
-
DPD1, NPD1 and SU1 test fuels shows marginal improvement in the brake thermal efficiency when compared with neat biodiesel.
-
Addition of antioxidants to the biodiesel increases CO, Unburned HC and smoke opacity for the test fuels, due to the scavenging effect of the OH radicals resulting in inhibition of oxidation process.
Abbreviations
- DPD1:
-
Jatropha biodiesel + 500 ppm N-Phenyl-p-phenylenediamine
- DPD2:
-
Jatropha biodiesel + 1000 ppm N-Phenyl-p-phenylenediamine
- DPD3:
-
Jatropha biodiesel + 2000 ppm N-Phenyl-p-phenylenediamine
- NPPD1:
-
Jatropha biodiesel + 500 ppm N,N-Dimethyl p- phenylenediamine dihydrochloride
- NPPD2:
-
Jatropha biodiesel + 1000 ppm N,N-Dimethyl p-phenylenediamine dihydrochloride
- NPPD3:
-
Jatropha biodiesel + 2000 ppm N,N-Dimethyl p-phenylenediamine dihydrochloride
- SU1:
-
Jatropha biodiesel + 500 ppm Succinimide
- SU2:
-
Jatropha biodiesel + 1000 ppm Succinimide
- SU3:
-
Jatropha biodiesel + 2000 ppm Succinimide
References
S. Jain, M.P. Sharma, Oxidation stability of blends of Jatropha biodiesel with diesel. Fuel, 90, 3014–3020 (2011)
J.S. Basha, R.B. Anand, The influence of nano additive blended biodiesel fuels on the working characteristics of a diesel engine. J. Braz. Soc. Mech. Sci. Eng., 35, 257–264 (2013)
S. Sivalakshmi, T. Balusamy, Effect of biodiesel and its blends with diethyl ether on the combustion, performance and emissions from a diesel engine. Fuel, 106, 106–110 (2013)
K. Varatharajan, M. Cheralathan, Effect of aromatic amine antioxidants on NOx emissions from a soybean biodiesel powered DI diesel engine. Fuel Process. Technol., 106, 526–532 (2013)
K. Varatharajan, M. Cheralathan, R. Velraj, Mitigation of NOx emissions from a Jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel, 90, 2721–2725 (2011)
K. Ryu, The characteristics of performance and exhaust emissions of a diesel engine using a biodiesel with antioxidants. Biores. Technol., 101, 78–82 (2010)
M.A. Hess, M.J. Haas, T.A. Foglia, W.N. Marmer, The effect of antioxidant addition on NOx emissions from biodiesel. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem., 49(2), 852 (2004)
E. Ileri, G. Kocar, Experimental investigation of the effect of antioxidant additives on NOx emissions of a diesel engine using biodiesel. Fuel, 125, 44–49 (2014)
I.M.R. Fattah, M.H. Hassan, M.A. Kalam, A.E. Atabani, M.J. Abedin, Synthetic phenolic antioxidants to biodiesel: path toward NOx reduction of an unmodified indirect injection diesel engine. J. Clean. Prod., 79, 82–90 (2014)
R. de Guzman, H. Tang, S. Salley, K.Y.S. Ng, Synergistic effects of antioxidants on the oxidative stability of soybean oil-and poultry fat-based biodiesel. J. Am. Oil Chem. Soc., 86, 459–467 (2009)
S.S. Damasceno, N.A. Santos, I.M.G. Santos, A.L. Souza, A.G. Souza, N. Queiroz, Caffeic and ferulic acids: an investigation of the effect of antioxidants on the stability of soybean biodiesel during storage. Fuel, 10, 641–646 (2013)
M. Mittelbach, S. Schober, The influence of antioxidants on the oxidation stability of biodiesel. J. Am. Oil Chem. Soc., 80, 817–823 (2003)
S. Garner, R. Sivaramakrishnan, K. Brezinsky, The high-pressure pyrolysis of saturated and unsaturated C7 hydrocarbons. Proc. Combust. Inst., 32, 461–467 (2009)
T.T. Kivevele, L. Kristof, A. Bereczky, M.M. Mbarawa, Engine performance, exhaust emissions and combustion characteristics of a CI engine fuelled with croton megalocarpus methyl ester with antioxidant. Fuel, 90, 2782–2789 (2011)
S.M. Palash, M.A. Kalam, H.H. Masjuki, M.I. Arbab, B.M. Masum, A. Sanjid, Impacts of NOx reducing antioxidant additive on performance and emissions of a multi-cylinder diesel engine fuelled with Jatropha biodiesel blends. Energy Convers. Manag., 77, 577–585 (2014)
B.S. Chauhan, N. Kumar, Y.D. Jun, K.B. Lee, Performance and emission study of preheated Jatropha oil on medium capacity diesel engine. Energy, 35, 2484–2492 (2010)
T. Belachew, M. Rakesh, G. Fengshou, B. Andrew, Combustion characteristics of CI engine running with biodiesel blends, Proceedings of International Conference on Renewable Energies and Power Quality (ICREPQ’11), Las Palmas de Gran Canaria, Spain (2011)
R. Prakash, R.K. Singh, S. Murugan, Experimental studies on combustion, performance and emission characteristics of diesel engine using different biodiesel bio oil emulsions. J. Energy Inst., 88, 64–75 (2015)
E. Buyukkaya, Effects of biodiesel on a DI diesel engine performance emission and combustion characteristics. Fuel, 89, 3099–3105 (2010)
C. Fenimore, Formation of nitric oxide in premixed hydrocarbon flames, The Combustion Institute 13th Symposium (International) on Combustion 13, 373–380 (1971)
I.M.R. Fattah, H.H. Masjuki, M.A. Kalam, M.A. Wakil, A.M. Ashraful, S.A. Shahir, Experimental investigation of performance and regulated emissions of a diesel engine with Calophyllum inophyllum biodiesel blends accompanied by oxidation inhibitors. Energy Convers. Manag., 83, 232–240 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arockiasamy, P., Ramachandran Bhagavathiammal, A. Influence of Antioxidant Addition in Jatropha Biodiesel on the Performance, Combustion and Emission Characteristics of a DI Diesel Engine. J. Inst. Eng. India Ser. C 99, 207–216 (2018). https://doi.org/10.1007/s40032-017-0350-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40032-017-0350-5