Abstract
Biodiesel and single cell oils obtained from oleaginous yeasts grown in industrial waste are attractive alternatives to the conventional fuels. However, there are only few articles dealing with the stability of the microbial biofuels. Hence, this study aimed at characterizing the storage time of biodiesels using Rancimat methods. The microbial oil and the biodiesel obtained from microbial oil have been characterized with storage stability due to various oxidizing and thermal damage. Here, the microbial fuels were subject to Rancimat analysis and found to have high thermal-oxidative stability of 18 and 8.78 h for biodiesel and oil, respectively. The storage stability resulting from storage conditions was extrapolated for biodiesel and oil and has been found to be 1.62 and 0.54 years, respectively. The infrared spectroscopic analysis reveals the degree of oxidation found after the induction time was reached and shows the characteristic peaks for degradation products. Gas chromatography revealed the compounds that were responsible for the stability as well as the amount of degradation products left.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last 50 years has seen the usage of petroleum products go from one to triple that of the usage than previous decades. Most countries rely on oil for their energy needs. The petroleum products are used for various purposes such as energy production, vehicular usage, etc. The energy is mainly obtained from fossil fuels such as coal, petrol, diesel, etc., but the present-day reserves of petroleum are projected to run out of supplies in the next two decades courtesy of demands. Energy is required for a variety of industrial processes, domestic activities, and automobiles as reported by the International Energy Organization (2015) and Khot et al. (2012). There has been a drastic increase in the use of vehicles for the past century. Similarly, industrial activities are also increased owing to meet the needs of the growing population by 2040–2050. Increase in the above activities leads to depletion of energy resources. Another issue relating to usage of fossil fuels is global warming. The carbon dioxide emitted during fuel usage accumulates in the atmosphere and causes an increase in temperature of the earth leading to global warming. One of the emerging solutions for these issues is replacement of fossil fuels by biofuels. According to Khot et al. (2012) and Galafassi et al. (2012), there exist only a few liquid biofuels that can be used for vehicular or industrial activities. Biodiesel is an alternative fuel to the existing liquid fuels which is obtained from various sources such as plant oils (Knothe et al. 1997), animal fats, waste oils, and microbial lipids. It is composed of fatty acid mono-alkyl esters with high concentrations of long-chain monounsaturated and polyunsaturated fatty acids to promote better cold flow properties.
The alternative to plant oils and animal fats is to utilize the oleaginous microbes for lipid as feedstock for biodiesel production. Oleaginous microbes are those microbes which can accumulate triacylglycerols (TAGS) under nutrient-limiting conditions or through genetic manipulations (Gao et al. 2018). The accumulated TAGs can constitute as high as 70% of cell dry weight (Sheehan and Greenfield 1980; Santamauro et al. 2014; Brennan and Owende 2010). The accumulated fatty acids can have a similar composition to plant-derived lipids. The latest generation of biodiesel utilizes lipids from microbial biomass to produce fatty acid methyl esters (FAMEs) in a process called trans-esterification. Oleaginous microbes have the added advantage of being able to grow on any substrate such as wastewaters, cooking wastes, discarded organic materials, wasted juices and oils, and hydrolysates of various lignocellulosic waste materials (Patel et al. 2015; Galafassi et al. 2012; Ling et al. 2014; Xiao-ying et al. 2015). Especially microbes grown on industrial wastes present an attractive option for converting waste into treasure (Selvakumar and Sivashanmugam 2018; Hossain and Salleh 2008; Hu et al. 2008; Gouda et al. 2008). Microbial lipids and biodiesel are composed of mostly oleic and linoleic acids of C16 and C18 chain lengths with 80–85% of total unsaturated components (Gao et al. 2010; Fakas et al. 2009; Knothe and Dunn 2001). Long-chain unsaturated organic acids (lipids and FAMEs) are more susceptible to oxidative damage than saturated compounds of comparable chain lengths. Furthermore, with respect to long-chain FAME, polyunsaturated chains are two or more times as reactive as monounsaturated long-chain compounds as studied by Dunn (2006). Graboski and McCormick (1998) was able to conclude that a study of their oxidative stability is important in the future when microbial biodiesel becomes used in mass transportation.
The most striking disadvantages of biodiesel as researched by Graboski and McCormick (1998) and Hoekman and Robbins (2012) are that it slightly increases nitrogen oxide emissions and possesses relatively poorer cold flow and storage stability characteristics than petrodiesel as per EN14112:2003.
Although microbial biodiesel or lipids do not have the ultimate ability to displace the conventional fuels in use today, they can however be scaled up to match the production through various developments of biomass growth from non-conventional or waste materials. As per Koutinas et al. (2014), the cost of production of biodiesel from oleaginous yeasts grown in bio-refineries is higher and the yield results are mixed. However, the by-products of the biomass growth and extraction processes yield several value-added products such as food additives, which can offset the cost involved in production.
Mechanism of lipid accumulation in oleaginous microbes
Oleaginous microbes are known to accumulate greater amounts of lipids under nitrogen-limiting conditions than non-oleaginous ones. In oleaginous yeasts, the source of acetyl-CoA is citrate, which will be made available in the cytosol after a cascade of biochemical events developed as a response to nutrient-deprived (especially nitrogen) conditions. After citrate enters the cytosol, it is cleaved into oxaloacetate and acetyl-CoA by the ATP-dependent citrate lyase (ACL), an enzyme essential in lipid biosynthesis. Absence or malfunction of ACL can lead to an increase in citrate concentration, which is then moved in the cytosol provoking inhibition of the EMP catabolic pathway. Conversion of cellular polysaccharides into lipids has been reported for both microalgae and yeasts. The acetyl CoA is then absorbed into the fatty acid synthesis via interaction with malonyl CoA synthase (Bellou et al. 2016). This mechanism is most significant in oleaginous microbes than others.
Bioreactor kinetics
The lipid accumulation studies were performed by culturing organisms in a bench top fermenter with a capacity of 3. 5 l. Previous studies were able to predict that the initial C/N was critical for lipid accumulation. Higher C/N ratio led to higher lipid accumulation, but the overall growth was lesser. Lower C/N ratio led to higher biomasses, but the lipid yield was lower overall. Hence, the microbial growth was studied using the growth kinetics as shown in Eq. 1.
where Xt is biomass at time t and Xo is biomass at time 0 td is time for doubling of population
The model developed by Papanikolaou and Aggelis (2011) states that the growth occurs as three stages, viz., biomass formation phase, lipid accumulation phase, and depletion of lipid phase. The growth kinetics for each stage were described and equations arrived at.
In lipid-free biomass formation phase:
In lipid accumulation phase:
The growth of the biomass reached a maximum after 3 days and then addition of extraneous carbon in the form of lignocellulosic hydrolysate was carried out. This caused the rapid uptake of carbon and subsequent accumulation as lipids.
Mechanism of oxidative stability measurement
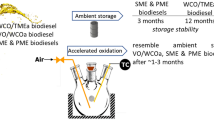
The storage stability of a liquid fuel is defined by Hoekman and Robbins (2012) as resistivity of properties brought by the drastic changes in the environment through thermal and oxidative damage. Furthermore, conformity of the biofuels obtained through the strict quality requirements of standard EN 14214 for biodiesel fuel (EN 14214: 2003; BSI BSIEN14214 2008) was evaluated. As per Marinkovic et al. (2016), destabilizations of biodiesel can be caused by oxidation products such as short-chain carboxylic acids, ketones, and aldehyde compounds. These can lead to the formation of further oxidative compounds, gums that can settle down and cause blockage of fuel pipes attached to them. Oxidation of biofuels can occur due to the presence of water, particulates, soaps, and microbial slimes. This was proved by Marinkovic et al. (2016) and Dunn (2006) that the fuel properties can be altered during long-term storage due to (1) auto-oxidation due to oxygen (C=O) present in the compounds, (2) thermal decomposition from the rise of temperature and associated oxidative reaction, (3) ester degradation due to moisture in tanks and fuel lines or during transportation, and (4) microbial contamination due to dust particles or droplets with microbes that make their way into the fuels via various routes (Marinkovic et al. 2016; Westbrook 2003; Dunn 2006).
Thermal stability
Monitoring the effects of auto-oxidation on biodiesel fuel quality during long-term storage represents a significant concern for usage of biodiesel in conventional engines. The biodiesel contains double bonds in a long chain, and there is a possibility of re-arrangement of double bonds and reaction with other molecules to form di-els that can form greases that can cause problems during combustion as studied by Natarajan (2012). These affect the chain starting from synthesis point and going all the way to the end user. These concerns were addressed by Siddharth and Sharma (2011) as manifesting after blending biodiesel with petroleum diesel. Assessing fuel quality in accordance with relevant standards such as the American Society for Testing and Materials (ASTM) specification D 6751 for biodiesel can be rigorous and time consuming. The work reported herein examines the oxidation component of biodiesel storage stability.
Mechanism of testing
Currently, the European standard for biodiesel quality (EN 14214) uses the method developed by Hardon and Zürcher (1974), also known as the Rancimat method, which consists in exposing the sample to an air flow (10 l h−1) at 110 °C. The oxygen in the air oxidizes the samples and then takes the oxidation products into a conductive cell with deionized water where an electrode measures the change in electrical conductivity of the water throughout the experiment. The variation of the conductivity with respect to time is recorded in a data storage system. Using this method, Giles (2003) was able to observe in real time that, as the reactions of formation of oxidation compounds get elevated, electrical conductivity increased correspondingly. A more accurate way of evaluation of oxidation level is by analysis of transmittance spectra from Fourier-transform infrared spectroscopy (FTIR). Siddharth and Sharma (2011) and Zuleta et al. (2012) observed the possibility to verify the decomposition products that result from biodiesel oxidation. At present, Metrohm manufactures the Rancimat equipment used for this method (Metrohm 2008). Special software is used for finding the induction time (IT) when the maximum increase in electrical conductivity occurs in the cell being measured at the maximum turning point of the time curve against conductivity, determined by the second derivative (Zuleta et al. 2012).
The wastewater and target organism
Ali and Dina (2014) was able to study several oleaginous yeasts and concluded that the usage of oleaginous yeasts eliminates the possibility of food versus fuel debate as they can be grown on inexpensive waste waters. Hence, the media for cultivation and the space for cultivation are eliminated. The target organism is Metschnikowia pulcherrima, a non-Saccharomycetales and a previously non-oleaginous yeast which under special circumstances can be made to reach the oleaginous stage. It tends to grow in sugar-rich environments such as grapes, flowers, and nectars which would otherwise be unsuitable for other oleaginous microbes. Members of Metschnikowia genus are terrestrial while those inhabiting marine environments are pathogenic to echinoderms (Gillian and Alastair 1987).
Some of the disadvantages with culturing the oleaginous organisms are that (1) they are sensitive to other microbes in the environment, which can outcompete them if sterilization is not followed in culturing, and that (2) heterogeneity of the culture media can cause varying levels of growth. Hasenhuetti and Wan (1992) studied M. pulcherrima that had the capacity to inhibit other microbes from contaminating its environment. It is also tolerant to a variety of environmental conditions such as low pH, temperatures, heterogeneity of substrates, and sugar concentrations. Hence, no sterilization maybe required for culturing in distillery spent wash (Santamauro et al. 2014). It also possesses huge potential for genetic manipulation for bulking up on fat. The contents of lipids are expected to get reduced throughout the stationary phase of lipid accumulation, and hence harvesting of the lipids halfway through the stage was necessary. Extended periods of nutrient deprivations ultimately caused the decrease in lipid concentrations of the biomass as well. This is due to the fact that the in absence of nitrogen, the metabolism changes to that of slowing of growth. And after a certain time, the metabolism changes to lipid oxidation of the reserved TAGs. Santamauro et al. (2014) was able to conclude that microbial oils obtained from M. pulcherrima have been found to have molecular composition and other characteristics similar to vegetable oils. The study also found out that under special circumstances of cultivation (temperature, nutrient deprivations, etc.), M. pulcherrima could accumulate up to 37% of its dry weight as lipids and that very high cell densities can be reached. Recently, studies performed at the University of Bath were able to identify M. pulcherrima as being able to generate lipids using lignocellulosic hydrolysates as substrate. The lipid accumulation was also performed on the said microbe. Lipids obtained from M. pulcherrima under the suitable conditions have been shown to have potential to be used on a practical basis as replacement for palm oil (Whiffin 2015). And it has been shown to have huge potential to accumulate amounts of neutral lipids that can then be used for biodiesel preparation (Kolouchová et al. 2016). Hence, the oxidative stabilities of vegetable oils and microbial oils would be highly useful for study of their storage, usage, and transportation conditions. The oxidative stability would also be useful when studies on combustion engines using microbial biodiesel are performed. Hence, the study observed the effect of high temperatures and oxidation of single cell oil (SCO) under temperatures of 110 and 120 °C and studied their characteristics with respect to the end products of oxidation.
This study has focused on the oxidative stability of microbial lipids and FAMEs from oleaginous yeast that were grown in industrial wastewater. The waste water was distillery spent wash which is obtained after distilling of ethanol. It is a highly caramelized waste water which is high in BOD and COD (× 105 mg l−1) which makes it very difficult to treat (Saha et al. 2005; Tewari et al. 2007). And for every 1 l of alcohol produced, 15 l of DSW is generated as waste. However, as observed by Willian et al. (2013), it contains a high amount of sugars which can be utilized for oleaginous microbial biomass growth. And a mixture of DSW with other less polluting wastewaters can be viewed upon as potential media for oleaginous biomass growth.
Materials and methods
Microbial culturing
Obtaining of microbial biodiesel required three steps, viz., culturing, lipid extraction, and trans-esterification. Culturing of oleaginous yeasts onto the wastewater media was performed using sterile techniques under a laminar hood. The target organism was cultured in distillery spent wash (DSW) under special conditions and allowed to grow to maximum biomass for 4 days in a 5-l benchtop fermenter (Dunn 2006). The fermenter had provisions for air supply, temperature control, pH control, and anti-foamin (Hiruta et al. 1997)g as shown in Fig. 1. After the growth period, microbes were harvested from the liquid medium using centrifugation techniques. According to Santamauro et al. (2014), after a certain time, the lipid contents of the microbes had declined and hence the harvesting of the lipids was carried out when the biomass was at its maximum value.
The initial studies on raw DSW yielded non-satisfactory results as done by Anbarasan and Regina (2015). Hence, pre-treatments were performed to increase the carbon-to-nitrogen ratio for higher lipid yields. Table 1 lists the DSW characteristics before and after the various pre-treatments. The waste stream from the most effective pre-treatments was then used for culturing of oleaginous yeasts.
Lipid extraction methods
From the wet biomass, lipid extraction was carried out by two methods, viz., Bligh and Dyer (1959) and Hara and Radin (1978). Extraction from dry biomass is easier and generates higher lipid content, but wet biomass extraction is more cost-effective (Bellou et al 2016). Wet biomass extraction eliminates the need for high-temperature drying that is costly and can potentially destroy the target lipid contents. To obtain intracellular lipids, it is indispensable to break the cell wall before going for lipid extraction. For extraction of lipids from wet biomass, a combination of solvents with varying properties is a requirement.
Since lipids consist of both polar and non-polar types, it is quite difficult to extract all lipid contents. Hence, for extraction, low toxic, low viscous, and highly volatile solvents are preferred. In order to efficiently separate non-polar lipids from other polar compounds, a biphasic solvent mixture is necessary. Hence, the combinations like methanol and chloroform, and hexane and isopropanol are widely used for lipid extractions from oleaginous biomass. This procedure is also advantageous since the lipid-containing layers can easily be separated for purification. Hence, Hara and Radin method was chosen as it gave higher lipid content (Hara and Radin 1978).
Recovering lipids from wet biomass also calls for removal or disruption of the cellular wall or membrane so that the stored lipids are released into the solvents for extraction purposes. There are several methods of disruption such as mechanical disruption, bead milling (Ranjith et al 2015), ultrasonication, pulse electric field, and microwaving (Athenaki et al. 2017). A combination of mechanical disruption followed by bead milling was found to release more lipids than any one single method alone.
The pre-treatment method was done using 0.1 N hydrochloric acid to remove the cellular wall of the yeasts. The wet biomass was treated with HCl and grinded in a high-pressure homogenizer for destruction of outer cell covering. Extraction was achieved by action of the solvents, hexane (non-polar, water immiscible and low-viscous solvent), and isopropanol (polar, water miscible, high viscous solvent) mixed with biomass in the ratio 5:3:1 to extract the lipids from other hydrophilic compounds. Glass beads were added to the solution to further improve cell wall disruption and left at stirring for 2 h in a thermomixer. After the completion of biomass disruption, the formation of two layers of solvents was observed. The hexane layer in which most of the non-polar lipids were extracted to was pipetted out. The solvent hexane was removed from the lipid extract by evaporation in a rotary vacuum evaporator.
Biodiesel conversion
The lipids from the extraction procedure were converted into FAME by means of trans-esterification methods. In situ trans-esterification methods involving the usage of strong alkali agents with a short-chain alcohol were performed (Forough et al. 2014; Mandal et al. 2013; Thliveros et al. 2014). The reagents used included sodium hydroxide and methyl alcohol in the optimum molar ratio to lipid of 6:1.
The mixing and trans-esterification reactions were carried out in a thermomixer by heating to 90 °C and rotating the mixtures at 900 rotations per minute for 90 min. After 90 min of trans-esterification, the FAME layer was pipetted out and washed several times with 0.9 M sodium chloride solution to remove any water-soluble products. The final products were separated and evaporated to get purified FAME (Doshia et al. 2002) in a rotary vacuum evaporator. The obtained FAME was a yellow liquid with the smell of oil.
Rancidity experiment
The thermal stability of the obtained lipids and biodiesels was checked for their thermo-oxidative stability under various temperatures. A Metrohm Rancimat system running on STABNET software was employed for the purpose (Metrohm 2008). The instrument measured the oxidative stability of the oil or biodiesel sample by analyzing the conductivity of the vapors coming out from the heated oils at specifically set temperatures. The instrument consisted of two blocks each with options for different temperatures.
The oils were heated to the specified temperatures (110 and 120 °C) and purged with air at 15 l h−1. The elevated temperatures caused the oxidation of the oils due to the air supply and the exhaust was sent through an electrical conductivity sensor dipped in deionized water (electrical conductivity was zero). The sensor measured the conductivity of the water as the exhaust air carrying the carboxyl ions entered the water. Thus, the conductivity of water increased slowly and as the oils approached rancidity induction point, there was a spike in the conductivity of the sensor after which the conductivity of the vapors remained stable. This marked the rancidity induction time for the particular sample at the particular temperature (Metrohm 2008). Oxidation stability index (OSI) is defined as the point of maximum rate of oxidation and is determined by analysis of the maximum in the second derivative or by extrapolating of time of onset of oxidation peak. OSI data reported are mean values from replicate analyses of duplicate samples (Robert Dunn 2006).
Five grams of oil or FAME samples was weighed and filled in the heating blocks, and the instrument was set up as shown in Fig. 2. The air flow was started from the start of experiment until the induction time was reached. After that point, the air supply was cut off.
The conductivity versus time graph showing the induction time, oxidation peak, and rancidity was generated as a result. Storage stability times for each samples were calculated individually after the oxidation was completed by means of extrapolation formulae built in to the software and used for generating storage stability times at room temperatures.
Extrapolation of ambient stability
The induction time is an indicator for stability at that temperature and temperatures of 110 and 120 °C were used. All the experiments were performed in duplicate. From the two temperature stability values, the stability time for real-world conditions set at 30 °C were obtained using extrapolation techniques built in to the software. This extrapolation was indicative of the stability time for all samples that were stored in real world conditions (Bondioli et al. 2001).
Fourier-transform infrared spectroscopy
Fourier-transform infrared (FTIR) spectroscopy is a non-invasive and non-destructive technique that gives information on the many biomolecules present in the given sample and the types of bonding present in the molecular chains. This method has been used by Dean and Vongsvivut to determine the lipid contents of microalgae and other microbes (Dean et al. 2010; Vongsvivut et al. 2013).
Fourier-transform spectroscopy utilizes infrared beams for analyzing the vibration characteristics of individual molecular bonds such as bending, stretching, and rotation of individual atoms present in the molecules. The graph showing the IR spectra could be generated from middle infrared (MIR) source. As per Ami et al. (2014), it was possible to identify the FAME and also quantify the most represented classes of the lipids present in the microbial oil samples. Hence, the samples containing the lipid groups can be identified and quantified through their infrared transmission spectra.
A Perkin Elmer spectrum 2 instrument operating in the MIR range (4000–400 cm−1) was employed with the accessory of horizontal attenuated total reflection (HATR) for analyzing the lipid samples (Lucarini et al. 2018). The HATR analysis of the samples was performed both before and after Rancimat analysis to check for the reduction of the target carboxyl groups and oxidation products. The inbuilt library named FLUKA was employed for identifications of molecular products in the samples.
Gas chromatography–mass spectrometry analysis
For fatty acid profile determinations, lipids were trans-esterified and analyzed with a GC instrument equipped with a split/splitless injector. GC instrument was equipped with a DB 35-MS capillary standard non-polar column (30 m, ID 0.25 mm, film 0.25 μm). Helium was employed as the mobile phase carrier gas set at a flow rate of 1.0 ml min−1. The inlet temperature was set at 220 °C. The temperature program was ramped from 70 to 260 °C at the rate of 6 °C per minute. The detector temperature was maintained at 320 °C.
Results and discussions
The effects of temperature on various biodiesels were analyzed for storage stability at elevated temperatures. The storage stability of the oils was found to be thermally stable in meeting the requirements of both ASTM and EN.
Microbial growth kinetics and lipid accumulation kinetics
The Monod growth kinetics followed for the batch culturing of the oleaginous yeasts strains gave the following values for specific growth rate and doubling time. The values for obtained biomass and the extracted lipids are given in Table 2.
From the table, it is clear that the specific growth rate (μ) varies from 0.007 to 0.01 h−1.
And the time for doubling td = 41.36 h.
The maximum growth of biomass and lipid were reached simultaneously at 80 h.
For the calculation of specific lipid accumulation rates, the results are tabulated in Table 3.
The values of maximum biomass, lipid consumption, and lipid converted per biomass and sugar consumed are given in Table 4. Hence, the amount of sugar consumed for biomass formation YX/S (g cell dry weight formed/g of substrate consumed) were found to be about 2.23 g of glucose consumed for growth of 1 g of biomass. And the formation of 1 g of biomass yielded (YL/X) was approximately 0.373 g of lipids. Hence, the value of YL/S was approximately 6.23 g of sugars consumed for the formation of 1 g of lipid. This consumption of carbohydrates for formation of lipids was consistent with the studies done by several authors.
The oxidation stability
Microbial biodiesel
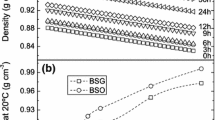
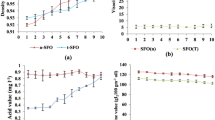
Microbial lipid and FAME were tested at 110 and 120 °C and the stability time for each temperature was found out from the Rancimat instrument analysis. Microbial biodiesel had a thermal stability at 110 °C of about 18 h, as shown in Fig. 3, which is higher than the required time set out by European EN standards.
The same induction time measurement which was carried out at 120 °C saw a rapid reduction of thermal-oxidative stability to 9.7 h as shown in Fig. 4. This is a 50% reduction of the stability observed at 110 °C. This observation of stability reduction is characteristic of biological samples which can remain stable only up to 135 °C, after which there is destruction. The oxidation of the biodiesel can affect the acidity index, the viscosity, and the cetane number (Dunn 2008; McCormick et al. 2007) of the fuel when they are fueled into engines whether as blends with conventional diesel (B10, B20, etc.) or as pure biodiesel (B100).
Initially, the study of oxidation stability was made with biodiesel of M. pulcherrima oil. Biodiesel has a thermo-oxidative stability higher than other biodiesels with a stability time of 18 h at 110 °C as observed by Zuleta et al. (2012). The stability of the biodiesel requires a minimum of 3 to 8 h stability at room temperature as per ASTM or EN14112.
Microbial oil
From the values of stability time, it is observed that the stability of M. pulcherrima oil reaches above 8 h which is more than the minimum requirement of 8 h laid down by EN 14214:2012. Figure 5 shows the induction time curve for the stability at 110 °C. The stability times for the temperature of 120 °C shows a drop of nearly 50% of the time reached for 110 °C. Figure 6 shows the conductivity curve for 120 °C. This is again consistent with those values observed for microbial FAME.
The stability times for the same oil and biodiesel at room temperatures of 30 °C were extrapolated using extrapolation techniques. The stability times obtained from 110 and 120 °C were used for extrapolating the stability time for the storage temperature taken to be 30 °C. The results are consistent with the presence of linoleic acids which act as natural antioxidants as observed by Loha et al. (2006).
Air exposure sample
The samples exposed for atmosphere under light conditions were also analyzed for stability under the same conditions of temperatures.
The exposed samples had lowered induction times with the same temperatures of 5 and 3.52 h at 110 and 120 °C, respectively. These values are shown in Figs. 7 and 8, respectively. This can be due to the effect of light and air exposure that forms oxidation products that accumulate over time.
The comparative values of induction times for each sample at each temperature are tabulated in Table 4 .
Extrapolation of stability time
Extrapolation of stability time at storage temperature was done by taking the ambient temperature to be 30 °C. The values are obtained by feeding the values obtained at previous values (110 and 120 °C).
The extrapolation time was calculated by Arrhenius equation.
where A is the regression coefficient, B is the Arrhenius coefficient, and T is temperature. Figure 9 shows the stability time for microbial biodiesel as extrapolated to 30 °C from 110 and 120 °C.
The time for microbial FAME was found to have reached nearly 1.6 years indicating a highly stable FAME. The R2 values are consistent with the linearity of the calculated values (Fig. 10).
Regression coefficients
Extrapolation time = 14,221 h or 1.62 years
Similarly for microbial oil stability, Fig. 9 shows the stability time as extrapolated to 30 °C from 110 and 120 °C.
Extrapolation time = 4775 h or 0.54 years
FTIR spectral analysis
The level of oxidation end products at the end of the Rancimat experiment is a good indicator of oxidative instability as well as viscosity of the lipid and FAME. Oxidative attack targets the long alkyl chains and also ester bonds present in the lipid molecules. Their spectra reveal the difference between them at the molecular level. Hence, an oxidized sample has less of esters and more of aldehydes, ketones, and carboxylic acids than fresh samples. Both of the effects can be assessed in the sample by reading the spectral signatures in their respective wavenumbers that correspond to different molecular groups.
The FTIR spectra obtained from fresh samples possess deep ester peaks which indicate a higher number of unoxidized ester molecules and lower aldehyde and ketone products as seen in Fig. 11. A decrease of the ester peak is seen in rancid samples indicating oxidation as compared to fresh samples. On the other hand, the rancid samples show shallower peaks at the wavenumber bands of 1740–1690 cm−1 which correspond to the presence of aldehyde and ketone groups in the molecules. The rancidized samples show reduction of the ester peak or disappearance or a shift from the ester band range indicating alteration of replacement of the ester molecules by aldehyde or ketone groups. The depth of the peaks indicates the amount of degradation products and the likely molecules associated with the peaks. Comparison of samples before and after Rancimat experiment has revealed the oxidation products.
As seen from Fig. 11, it is evident that the lipid samples had undergone deep oxidation and the peaks were moving from the fresh samples to new wavenumbers. The new wavenumbers are characteristic of short-chain degradation products of long-chain fatty acids (aldehydes and ketones). The extent of reduction of peak depth of oil samples is shown in Table 5.
In lipid samples, the ester peaks at 1750 cm−1 are seen to be shifted toward the aldehyde and ketone end nearer to 1735 cm−1. At the rancidity experiment conducted for 120 °C, the ester peak vanishes and replaced by alkene bonds. Those of hydroxyl peaks at 3500–2800 cm−1 have reduced considerably (~ 40%) and those of long-chain peaks at 725 cm−1 are reduced by nearly 60%. So there is considerable amount of oxidative damage to the lipid sample under rancidity conditions. Hence, it can be inferred that the inherent instability of pure microbial oil to oxidation is relatively higher than trans-esterified oils.
The oxidation products included aldehydes, ketones, and carboxylic acids, and the peaks corresponding to them are given in Table 6.
From Fig. 12, it can be seen that the fresh samples possess deep ester peaks and the rancid samples have reduced peaks of esters, aldehydes, and ketones. The region of IR in the range of 2000–1200 cm−1 is generally referred to as the fingerprint region which is very specific for each and every molecular compound. The ester peaks at 1750 cm−1 have gone down by about 35.68% as compared to fresh samples implying a reduction of the ester groups. Hydroxyl groups at wavenumbers of 3359–3387 cm−1 are also reduced by 50%. Also, the long-chain groups associated with wavenumbers at 725 cm−1 have been reduced by 40% indicating oxidative attack had broken the esters down into smaller oxidation products such as carboxylic acids, aldehydes, and ketones. The aldehyde C–H stretching seen at ~ 2850 cm−1 confirms the presence of aldehydes in the rancidized sample. Those of the C–C stretching of ketones seen at vibrations of 1300–1100 cm−1 are confirmative of the presence of ketone molecules in the samples. The oxygen attacked and thereby nicked the chains and hence a reduction in the peaks of long-chain compounds was observed. The breakage of long chains can be inferred from the shallowing of the methylene peaks at 725 cm-1.
The FAME samples spectra retain the ester and long-chain peaks implicating their higher resistivity to oxidation than the lipid samples. This is due to the presence of the terminal methyl groups on the FAME which confer resistivity to the samples. Also, the resistivity can be attributed to the presence of oleic acids in the sample which act as an antioxidant and prevent oxidative radical damage to lipid samples.
Gas chromatography–mass spectrometry analysis
The GC-MS analysis of the FAME before and after Rancimat experiment reveals the differences between the two and the degree of oxidative damage done by Rancimat experiment. The analysis is a definitive confirmation of the presence of compounds such as aldehydes and ketones in the oxidized sample, whose presence confirms oxidation products after the oxidation events as compared with fresh samples.
In the initial runs, the FAME can be seen to possess more unsaturated FAME such as methyl oleate (C18:1ω−9, 27.63%), methyl palmitoleate (C16:1ω−7, 17.03%), methyl linoleate (C18:3ω−3, 41.3%), and methyl ricinoleate (3.37%) Falk and Meyer-Pittroff 2004).
All of the mentioned esters belong to the long-chain unsaturated fatty acids, which is typical of SCOs from oleaginous microbes. The amount of esters in the sample before Rancimat analysis is calculated to exceed 85% of the total composition which sits well with the FTIR analysis of the samples which show a spectral library match of 88% with various methyl esters. The characteristic methyl esters of oleic acid and linoleic acid together compose 68% of the sample, which is common in the FAME obtained from microbial TAGs (Bucy et al. 2012; Ramos et al. 2009; Stefania et al. 2003).
The time-aged samples resulted in the oxidation of long alkyl chains via chain radical attack and resulting in a number of aldehydes and carboxylic acids as shown in Table 6 These compounds belonged to alkanals, 2-alkenals, alkanes, alcohols, ketones, and furans derived from lipids and one aromatic hydrocarbon (Sakthivel et al. 2018). In the samples analyzed, the presence of aldehyde molecules like hexanal, heptanal, octanal, nonanal, and decanal was observed. These carbonyl compounds are known to result from the oxidation of polyunsaturated fatty acids, which takes place in foods rich in fat (Klensporf and Jeleñ 2005).
After the thermal oxidation at 120 °C, the oxidation products were analyzed with GC-MS and the compounds that are present have been tabulated in Table 6, and the results are consistent with the results from the FTIR results. The aldehyde compounds have been found to have a decrease in the amount of the sample than in the fresh samples (Rajamohan and Kasimani 2018). The decrease in the aldehyde and ketones is a steep 25% when compared with fresh samples. And the carboxylic acids have been found to increase after the Rancimat (Peer et al. 2017). The residue left behind has been shown to contain a multitude of compounds that were either formed as di-els or as hemi-acetals (Natarajan 2012). Their accumulation was attributed to the breaking of the long chain and the ester groups of the constituent molecule due to their oxidation. The double bonds are especially vulnerable to oxidative attack due to the pi bond electrons in the C=C radicals (Pullen and Saeed 2012) (Tables 7 and 8).
Conclusions
Microbial lipids and the biodiesel produced from microbial biomass that were grown on industrial waste possess the dual advantages of waste reduction and energy generation. In effect, the pollution is converted into useful products in the form of liquid fuel, food additives, antioxidants, and a variety of other compounds. And the stabilities are also higher. The fresh samples of microbial biodiesel and lipids reportedly are found to have higher stability time values of 18.62 and 8.68 h at 110 °C. The higher temperature study at 120 °C reveals a shorter time than at 110 °C. The arrived stability times indicate that the microbial-derived biodiesel is more stable than the conventional biodiesels. Two samples exposed to ambient air conditions for 6 months have lowered stability times of 5 and 3.52 h after Rancimat experiment. The storage stability extrapolation to room temperature 30 °C was able to arrive at a value of 14,221 and 4775 h which far exceed the times for fuel storage requirements at room temperature. The GC-MS results identified the presence of oleic acid esters that were responsible for the stability of FAME and their concentrations before and after the Rancimat experiment. The FTIR spectrum also was able to corroborate the GC results by showing the presence of oleic acids and other esters that make up microbial biodiesel. The end products were identified at the molecular level with their spectral signatures and also through direct chromatographic analysis of the FAMEs before and after the experiment. Hence, the study established the superior stability of SCO when compared to existing biodiesel fuel. Hence, storing, transporting, and ultimate usage of the microbial FAME as everyday fuel can be approached upon on a practical basis. Future studies involving the emission characteristics of the biodiesel used as blends in a CI diesel engine are to be carried out and corroborated with the spectral characteristics and are planned upon for further studies.
References
Ali T, Dina H (2014) El-Ghonemy. Optimization of culture conditions for the highest lipid production from some oleaginous fungi for biodiesel preparation. Asian J Appl Sci 02(05)
Ami D, Posteri R, Mereghetti P, Porro D, Doglia SM, Branduardi P (2014) Fourier transform infrared spectroscopy as a method to study lipid accumulation in oleaginous yeasts. Biotech for Biofuels 7:12
Anbarasan T, Regina Y (2015) Investigation on synthesis of biodiesel from distillery spent wash using oleaginous yeast Metschnikowia pulcherrima. Inter J of Appl Engg Res 10(67):310–314 (2015) Research India Publications;
Athenaki M, Gardeli C, Diamantopoulou P, Tchakouteu SS, Sarris D, Philippoussis A (2017) Lipids from yeasts and fungi: physiology, production and analytical considerations. https://doi.org/10.1111/jam.13633
Bellou S, Triantaphyllidou I, Aggeli D, Elazzazy AM, Baeshen MN, Aggelis G (2016) ScienceDirect Microbial oils as food additives : recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr Opin Biotechnol 37:24–35. https://doi.org/10.1016/j.copbio.2015.09.005
Bligh, Dyer (1959) Neutral lipid extraction by the method of Bligh–Dyer. Can J Biochem Physiol 37:922
Bondioli P, Gasparoli A, Lanzani A, Fedeli E, Veronese S, Sala M (2001) Storage stability of biodiesel. Ibid 72:699
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
BSI, BSEN14214 (2008) Automotive fuels—fatty acid methyl esters (FAME) for diesel engines—requirements and test methods. 2008.
Bucy HB, Baumgardner ME, Marchese AJ (2012) Chemical and physical properties of algal methyl ester biodiesel containing varying levels of methyl eicosapentaenoate and methyl docosahexaenoate. Algal Res 1(1):57–69
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
Doshi VA, Vuthaluru HB, Bastow T (2005) Investigations into the control of odour and viscosity of biomass oil derived from pyrolysis of sewage sludge. Fuel Process Technol 86(8):885–97
Dunn RO (2006) Oxidative Stability of Biodiesel By Dynamic Mode Pressurized−Differential Scanning Calorimetry (P−Dsc). Am Soc Agric Biol Eng 49(5):1633–1641
Engines (2013) Requirements and test methods data on cold flow properties pour points. fuel
Fakas S, Papanikolaou S, Batsos A, Panayotoumg, Malloucho A, Aggelis G (2009) Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy 33:573–580
Falk O, Meyer-Pittroff R (2004) The effect of fatty acid composition on biodiesel oxidative stability. Eur J Lipid Sci Technol 106(12):837–843
Fame En 14214 (2012) + a1 : 2014. 2014;14214.
Forough GN, Thomas-Hall SR, Ratnam RD, Pratt S, Schenk PM (2014) Comparative effects of biomass pre-treatments for direct and indirect transesterification to enhance micro algal lipid recovery. Front. Energy Res https://doi.org/10.3389/fenrg.2014.00057
Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C (2012) Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol 111:398–403
Gao C, Zhai Y, Ding Y, Wu Q (2010) Application of sweet sorghum for biodiesel production by heterotrophic Chlorella protothecoides. Appl Egy 87:756–761
Gao Q, Cao X, Huang YY, Yang JL, Chen J, Wei LJ, Hua Q (2018) ACS. Overproduction of fatty acid ethyl esters by the oleaginous yeast Yarrowia lipolytica through metabolic engineering and process optimization. Synth Biol 7(5):1371–1380
Giles HH (2003) Methods for assessing stability and cleanliness of liquid fuels. In: Rand SJ (ed). Significance of Tests for Petroleum Products, 7th edn. American Society for Testing and Materials, West Conshohocken, pp 108−117
Gouda MK, Omar SH, Aouad LM (2008) Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotech 24:1703–1711
Graboski MS, McCormick RL (1998) Combustion of fat and vegetable oil derived fuels in diesel engines. Prog Egy Combust Sci 24:125–164
Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90(1):420–426
Hardon H, K Zürcher Dtsch. Lebensm.-Rudsch (1974) 70:57
Hasenhuetti GL, Wan PJ (1992) Temperature effects on the determination of oxidative stability with the Metrohm Rancimat. J Am Oil Chem Soc 69:525
Hiruta O, Yamamura K, Takebe H, Futamura T, Iinuma K, Tanaka H (1997) Application of Maxblend fermenter for microbial processes. J Ferment Bioeng 83(1):79–86
Hoekman SK, Robbins C (2012) Review of the effects of biodiesel on NOx emissions. Fuel Process Technol 96:237–249
Hossain ABMS, Salleh A (2008) Biodiesel fuel production from algae as renewable energy. Am J Biochem Biotech 4(3):250–254
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Micro algal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
International Energy Organization 2013 and 2016 (2015)
Khot M, Kamat S, Zinjarde S, Pant A, Chopade B, Ravi Kumar A (2012) Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microbe Cell Fact 11:71
Klensporf D, Jeleñ HH (2005) Analysis of volatile aldehydes in oat flakes by SPME-GC/MS. Pol J Food Nutr Sci 14/55(4):389–395
Knothe G, Dunn RO (2001) Biofuels derived from vegetable oils and fats, in oleo chemical manufacture and applications. pp. 106–163
Knothe G, Dunn RO, Bagby MO (1997) Biodiesel: the use of vegetable oils and their derivatives as alternative diesel fuels. In: Saha BC, Woodward J (eds) ACS Symposium Series No. 666: Fuels and Chemicals from Biomass. ACS, Washington, DC, pp 172–208
Kolouchová, Maťátková O, Sigler K, Masák J, Řezanka T (2016) Production of palmitoleic and linoleic acid in oleaginous and nonoleaginous yeast biomass. Int J Anal Chem 2016:1–8
Koutinas AA, Chatzifragkou A, Kopsahelis N, Papanikolaou S, Kookos IK. (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. FUEL 116:566–77. https://doi.org/10.1016/j.fuel.2013.08.045
Ling J, Nip S, Cheok WL, de Toledo RA, Shim H (2014) Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol 173:132–139
Loha S, Chewb S, Chooa Y (2006) Oxidative stability and storage behavior of fatty acid methyl esters derived from used palm oil. J Am Oil Chemists’ Soc 83(11):947–952
Lucarini M, Durazzo A, Sánchez del Pulgar J, Gabrielli P, Lombardi-Boccia G (2018) Determination of fatty acid content in meat and meat products: the FTIR-ATR approach. Food Chem 267:223–230
Mandal S, Patnaik R, Singh AK, Mallick (2013) Comparative assessment of various lipid extraction protocols and optimization of transesterification process for micro algal biodiesel production. Env Tech 34(13–16):2009–2018
Marinkovic DM, Stankovic MV, Velickovic AV, Avramovic JM, Miladinovic MR, Stamenkovic OO, Veljkovic VB, Jovanovic DM (2016) Calcium oxide as a promising heterogeneous catalyst for biodiesel production: current state and perspectives. Renew Sus Egy Rev 56:1387–1408
McCormick RL, Ratcliff M, Moens L, Lawrence R (2007) Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Proces Tech 88(7):651–657
Metrohm AG (2008). Quality control of biofuels
Natarajan E (2012) Stability studies of biodiesel. IJES 2(4):152–155
Papanikolaou S, Aggelis G (2011) Review article lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. 1031–1051. https://doi.org/10.1002/ejlt.201100014
Patel D, Sindhu K, Arora N, Singh RP, Pruthi V, Pruthi PA (2015) Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 197:91–98
Peer MS, Kasimani R, Rajamohan S, Ramakrishnan P (2017) Experimental evaluation on oxidation stability of biodiesel/diesel blends with alcohol addition by Rancimat instrument and FTIR spectroscopy†, 31(1), 455–463. https://doi.org/10.1007/s12206-016-1248-5
Pullen J, Saeed K (2012) An overview of biodiesel oxidation stability. Renew Sust Energ Rev 16(8):5924–5950
Rajamohan S, Kasimani R (2018) Analytical characterization of products obtained from slow pyrolysis of Calophyllum inophyllum seed cake: study on performance and emission characteristics of direct injection diesel engine fuelled with bio-oil blends. 25(10), 9523–9538. https://doi.org/10.1007/s11356-018-1241-x
Ramos MJ, Maria Fernandez CM, Casas A, Rodriguez L (2009) Perez A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268
Ranjith Kumar R, Hanumantha Rao P, Arumugam M (2015) Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front Energy Res [Internet] 2(January):1–9. Available from: http://journal.frontiersin.org/article/10.3389/fenrg.2014.00061/abstract
Robert O. Dunn (2008) Antioxidants for improving storage stability of biodiesel. Biofuels, Bioproducts and Biorefining 2 (4):304–318
Saha NK, Balakrishnan M, Batra VS (2005) Improving industrial water use: case study for an Indian distillery. Res. Conserv. Recycl. 43:163–174
Sakthivel R, Ramesh K, Shameer PM, Purnachandran R (2018) Experimental investigation on improvement of storage stability of bio-oil derived from intermediate pyrolysis of Calophyllum inophyllum seed cake, (5). J Energy Inst xxx 2018. https://doi.org/10.1016/j.joei.2018.02.006
Santamauro F, Whiffin FM, Scott RJ, Chuck CJ (2014) Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotech for Biofuels 7:34
Selvakumar P, Sivashanmugam P (2018) Study on lipid accumulation in novel oleaginous yeast Naganishia liquefaciens NITTS2 utilizing pre-digested municipal waste activated sludge: a low-cost feedstock for biodiesel production. Appl Biochem Biotech 186(3):731–749. https://doi.org/10.1007/s12010-018-2777-4
Sheehan GJ, Greenfield PF (1980) Utilization, treatment and disposal of distillery wastewater. Water Res 14(3):257–277
Siddharth J, Sharma MP (2011) Study of oxidation stability of Jatropha curcas biodiesel/diesel blends. Int J Egy Env 2(3):533–542
Stefania V, Castellote AI, Pizzale L, Conte LS, Buxaderasb S, Lo’pez-Tamames E (2003) Analysis of virgin olive oil volatile compounds by headspace solid-phase micro extraction coupled to gas chromatography with mass spectrometric and flame ionization detection. J Chromatography A 983:19–33
Tewari PK, Batra VS, Balakrishnan M (2007) Water management initiatives in sugarcane molasses based distilleries in India. Res Conserv Recycl 52:351–367
Thliveros P, Uçkun Kiran E, Webb C (2014) Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour Technol. 157:181-7. https://doi.org/10.1016/j.biortech.2014.01.111
Turton GC, Wardlaw AC (1987). Pathogenicity of the marine yeasts Metschnikowia zobelli and Rhodotorula rubra for the sea urchin Echinus esculentus. Aquaculture. 67(1–2):199–202.
Vongsvivut J, Heraud P, Gupta A, Puri M, McNaughton D, Barrow CJ (2013) FTIR micro spectroscopy for rapid screening and monitoring of polyunsaturated fatty acid production in commercially valuable marine yeasts and protists. Analyst 138:6016–6031
Westbrook SR (2003) Fuels for land and marine diesel engines and for non-aviation gas turbines. In: Significance of tests for petroleum products, 7th edn. ASTM International, West Conshohocken, PA, pp 63–81 11
Whiffin F (2015). A palm oil substitute and care product emulsions from a yeast cultivated on waste resources. Thesis (doctor of philosophy (PhD)). University of Bath. http://opus.bath.ac.uk/view/person_id/6097.html
Willian TW, Bariccatti RA, Martins GI, Secco D, de Souza SNM, Rosa HA, Chaves LI (2013) Study of the methyl crambe (Crambe abyssinica Hochst) and soybean biodiesel oxidative stability. Ind Crops Prod 43:207–212
Xiao-ying LI, Xiao-an NIE, Jie C, Yi-gang W (2015) The development tendency and research statues of microbial oil for biodiesel production. 4(4):137–43
Zuleta EC, Baena L, Riosa LA, Calderón JA (2012) The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: a review. J Braz Chem Soc 23(12):2159–2175
Acknowledgements
The authors would like to thank the Centre of Excellence for environmental studies for supplying Rancimat for performing studies. Special thanks to Mechanical Research Scholar R. Sakthivel for his timely inputs on article preparation and gas chromatography interpretations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Tamilalagan, A., Singaram, J. Oxidation stability of yeast biodiesel using Rancimat analysis: validation using infrared spectroscopy and gas chromatography–mass spectrometry. Environ Sci Pollut Res 26, 3075–3090 (2019). https://doi.org/10.1007/s11356-018-3619-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3619-1