Abstract
This work analyses the performance and emission characteristics of biofuelled compression ignition (C.I) engine with the implementation of an antioxidant. Using the transesterification process with sodium hydroxide as a catalyst, the beef tallow methyl ester (BTME) was obtained from the beef tallow oil. Poor physical properties of biodiesel (beef tallow oil (BTO)) namely high viscosity and density cause atomization problems leading to higher smoke, hydrocarbon and carbon monoxide emissions. The purpose of this work is to enhance the performance aspects, to limit smoke emissions from BTO operation and to examine the possibility of direct use of neat BTO in CI engine. This research paves a way of investing the impact of binary blends of BHA and BTO on the research engine. The experiments were conducted on a single-cylinder four-stroke C.I engine using the following fuel compositions: 20% of BTME mixed with 80% diesel (B20), 1000 ppm mono-phenolic antioxidant (butylated hydroxyanisole (BHA)) mixed with the blends of B20 (B20 + BHA), and 100% diesel. Based on the experimental results, it was found that the brake thermal efficiency (BTE) increases by 1.8% and the brake specific fuel consumption (BSFC) decreases by 2.5% for the fuel blend B20 + BHA when compared with that for B20 fuel blend. Compared with the B20 blend, the blend B20 + BHA emits 12.2% lesser nitrogen oxide due to breaking chain reactions, scavenging the initiating radicals and reducing the concentration of reactive radicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diesel fuel among all fossil fuels has played a crucial role in all the major sectors like power, industry, marine, transportation and agriculture owing to fuel economy, reliability and rigidity. The downside is that they emit harmful chemicals like nitrogen oxide (NOx), hydrocarbon (HC), PM and smoke which is too hazardous for the environment. Furthermore, an increase in cost and a drastic reduction in the availability of diesel fuels have paved a way of using biodiesel as a partial alternate. To lower the above-said drawbacks, biodiesel derived from renewable sources has gained attention. In fact, many countries have started employing biodiesel (BD) as a 20% replacement of diesel fuel. Venu and Madhavan (2017), Vellaiyan et al. (2019) and Subramanian et al. (2018) have suggested that blending biodiesel, bio-oil and alcohols above 20% to diesel will result in lower calorific value which affects the overall performance of the blended fuels. Biodiesel (BD) is surmised as a capable alternative to diesel owing to its renewable nature and ease in availability. BD is liquid fuel which has similar properties to diesel. BD shall be produced by many techniques namely pyrolysis, crushing and transesterification. Among which, the transesterification is widely accepted and most trusted way for producing BD (Bharathiraja et al. 2017). This technique requires raw oil (base source), alcohol (ethanol) and catalysts (acid or base). The oil and alcohol are mixed at certain acceptable molar ratio (5–7:1) along with the catalysts at 1–1.5 %wt and heated to a temperature of 50–70 °C. The mixture is then vigorously stirred for homogenous mixing for a time interval (40–60 min). Post heating, this mixture is allowed to settle. This process results in the splitting of two products (methyl/ethyl ester and glycerol). The by-product (glycerol) is removed from an ester by gravity-based separation. Many works tried BD as partial/complete replacement (10–100%) to diesel and found a drastic reduction in tailpipe emissions with lower performance (Kavitha 2019). Many studies attempted with different resources for the progress of substitute resource and some of them were palm oil (Ganesan et al. 2019), rice bran oil (Devarajan et al. 2019a; Kavitha et al. 2019), cashew shell oil (Joy et al. 2019), Punnai oil (Devarajan et al. 2019b), orange peel oil (Siva et al. 2018), corn oil (Ganesan et al. 2018), almond oil (Devarajan 2018), Mahua oil (Sudalaimuthu et al. 2018) and neem oil (Rathinam et al. 2019). From the outcome of the above review, it is quite clear that the BD was a sustainable, promising choice for diesel. Conversely, the outcome also suggested that the usage of BD100 (100% biodiesel) will result and fuel clogging; thus, it was suggested not to use BD more than 20% in a diesel engine. The inference from the above studies concludes that the BD shall be employed as a partial replacement of diesel fuel. Moreover, blending reduces the usage of fossil fuels and lower the emissions associated with the diesel engine. Secondly, the inference also states that very limited works have been tried using waste beef tallow oil (BTO). In other words, scanty researches were recognized for employing beef tallow oil (BTO) as a potential and partial alternate of diesel fuel.
India is one of the world’s largest beef exporter, almost 2100 metric tonnes of beef are exports from India. Beef tallow is one of the main residues from slaughterhouses and these are primarily used in soap industries. If the soap industry is overloaded, these extra fat residues are incinerated and disposed of in the landfill. The use of animal fat residue as biodiesel in compressed ignition (C.I) engine could reduce the burden on petroleum diesel. The market price of the BTO is comparable with conventional diesel. However, the effective use of by-products from the production of animal fat biodiesel could be competitive with the use of commercial petroleum diesel. In general, the oil produced from animal fat (beef tallow or chicken) has similar properties with petroleum diesel except for the viscosity. This is due to the presence of a high amount of saturated fatty acids and this will lead to creating ignition problems. The production of biodiesel from beef tallow oil by the transesterification process was mentioned above. Very few studies were performed in the past decades for the use of animal fatty acids as biodiesel in the diesel engine. Shahir et al. (2017) reported that the use of 30% blends of animal fat biodiesel with conventional diesel lowered carbon monoxide (CO), HC and smoke emissions with inferior performance, higher NO emissions in CRDI engine. John paneer Selvam and Vadivel (2012) analysed the effect of beef tallow biodiesel blends with diesel in a diesel engine and reported that there was a slight decrease in brake thermal efficiency with a considerable reduction in the CO, HC and smoke pollutions for all blends. In addition, the BTO possessed inferior performance and higher NO emissions than neat diesel.
Inference from a few kinds of literature using beef tallow and other biodiesel suggested that the NO emissions are on the higher side with a slight reduction in performance aspects while using different BD. Improvement in brake thermal efficiency (BTE) with a significant reduction in NOx emissions shall be achieved by fuel alteration techniques which involve no physical modification in engines. To reduce the NOx emission from diesel-/biodiesel-fuelled engine, numerous attempts have been made by many researchers. Devarajan (2018) tried to modify the fuel properties of almond biodiesel using dimethyl carbonate (DMC) as an antioxidant and reported a noteworthy reduction in NOx emissions. In another study, Ganesan et al. (2018) attempted to mix the DTBP (di-tetra-butyl-peroxide antioxidant) with mustard biodiesel and found a considerable fall in NOx emissions. Joy et al. (2019) formulated the DME (dimethyl-ether) emulsified cashew shell biodiesel and propelled diesel engine. The results show that an increase in DME concentration with biodiesel directly reduces the NOx emissions due to improved properties of DME (antioxidant) during the combustion process. Xiao et al. (2017) attempted the addition of dimethyl carbonate with diesel. The experimental results of diesel show a positive effect in terms of smoke opacity and NOx. Hazar (2017) used the tripropylene glycol as an additive (oxygenated) for diesel-biodiesel blends fuel and recorded a significant improvement in NOx emission characteristics. Teixeira et al. (2010) studied the characteristics of beef tallow biodiesel and reported that the use of mixtures of tallow biodiesel with soybean biodiesel and conventional diesel produces the optimum performance with the minimum emissions. Mei et al. (2017) used the dimethyl carbonate as an additive (oxygenated) for neat diesel and recorded a significant improvement in NOx emission characteristics with a sharp reduction in brake specific fuel consumption (BSFC). Wei et al. (2017) attempted the addition of antioxidants (2,5-dimethylfuran) with biodiesel-diesel blends. Furthermore, the experimental results of formulated biodiesel show a positive effect in terms of NOx and smoke opacity for all the test fuels with improved BTE. A promising solution for improving the oxidation stability and reducing the NOx emission is the treatment of biodiesel with antioxidant additive. The reduction of NOx emission is a result of the hydrogen-free radicals’ reduction, which is responsible for prompt NOx formation during the combustion process (Rashed et al. 2016a, b, c, d, Vellaiyan 2019).
Key findings from the above studies proved that the antioxidants are a promising means to lower the limitations of biodiesel and enhance its usage as fuel in CI engine. Improved properties such as higher cetane number, improved miscibility, higher calorific value, lower pressures (vapour) and flash point are the other positive properties merits of antioxidants. In addition, inference from the literature review also concludes that scanty researches were recognized with beef tallow biodiesel and butylated hydroxyl anisole (BHA) in compression ignition engine. The use of biodiesel in engine reduces emission and provides good lubrication compared with mineral diesel. In general, the biodiesel derived from the animal fat has poor oxidation stability and leads to the formation of hydroperoxides which in turn produce the insoluble gums and sediments that affect the fuel supply systems. The end product may increase the viscosity of the fuel and leads to poor fuel atomization. The addition of antioxidants in biodiesel delays and controls the autoxidation process and decrease the formation of secondary products.
This work aimed to study the performance and emission characteristics of a single-cylinder diesel engine fuelled with beef tallow biodiesel blends integrated with antioxidant butylated hydroxyanisole (BHA). The concentration of the antioxidant additive was limited to 1000 ppm, which resulted in a better induction period compared with other concentrations. The small quantity of beef tallow biodiesel (20%) was mixed with the conventional diesel (80%) and 1000 ppm of antioxidant BHA. The biodiesel prepared by this technique is referred to as B20 + BHA. The comparative performance analyses of the compressed ignition engine fuelled with B20, B20 + BHA, B100 and diesel were studied. In this study, the diesel engine initially experimented with diesel as baseline observation and the obtained results were evaluated with alternative fuel samples. The characteristics of non-edible, widely available and abundant BTO in India and exciting oil content are the major reasons for selecting beef tallow oil. However, no studies have detailed the effect of butylated hydroxyanisole (BHA) to improve the emissions characteristics of BTO. The butylated hydroxyanisole (BHA) antioxidant in biodiesel interrupts the reaction of the formation of secondary products and releases hydrogen atom to a free radical. The BHA is highly soluble in oil and insoluble in water. BHA is a combination of 3-tertiary-butyl-4-hydroxyanisole (90%) and 2-tertiary-butyl-4-hydroxyanisole (10%). BHA helps to protect the hydroxyl (OH) group and improve the thermal stability of animal fats. The properties of BHA are listed in Table 1.
Materials and methods
Biodiesel production
The beef tallow oil (BTO) was bought from the local market in Chennai, Tamil Nadu, India. For the transesterification process, methanol and a base catalyst NaOH pellets were used. Five litres of BTO was procured from Agro Biotech, Chennai, India. The cost of the BTO was 54 INR/l. Five litres of BTO yielded 4340 ml of biodiesel (86.8%) by conventional transesterification procedure. To produce biodiesel from the BTO, a molar ratio of 5:1 (methanol to BTO) and catalysts of 0.3% (wt/wt) to BTO were used in transesterification process adapting standard procedure. A batch reactor with a capacity of 5 l was equipped with a condenser, a magnetic stirrer with a tachometer, a thermometer pocket along with a thermocouple and a stopper to remove samples were used. By means of a constant temperature heating mantle, the reaction temperature was maintained within ± 0.1°. A sufficient amount of BTO was heated in the batch reactor. Methanol and the catalyst NaOH were mixed together in a conical flask and this solution is transferred into the batch reactor containing the preheated BTO. The reaction is allowed to take place for 90 min. After this, the solution is allowed to settle for 12 h in a separating funnel and the lower glycerol layers were removed. The residual methanol was removed using a rotary evaporator. Figure 1 shows the photographic view of the transesterification process. To bring the pH value to normal, the obtained biodiesel crude was then washed multiple times with warm deionized water. Based on previous studies, the small quantity of beef tallow biodiesel (20%) was mixed with the conventional diesel (80%) and 1000 ppm of antioxidant BHA. The biodiesel prepared by this technique is referred to as B20 + BHA. The same methodology has been followed for the preparation B20 blend (80% biodiesel and 20% conventional diesel) without the addition of BHA. The use of 100% biodiesel in the engine is referred to as B100. Figure 2 shows the photographic view of the fuel specimen employed. Physical and chemical properties such as density, viscosity, calorific value, cetane number and flash point are measured for all the base and modified fuels using American Society for Testing Materials (ASTM) test procedures and are compared with diesel.
Table 2 shows the properties of derived biodiesel blends and conventional diesel. ASTM standards are referred and the same testing procedure is performed to find out the desired properties of fuel (Vellaiyan 2020; Raju et al. 2020; Vellaiyan et al. 2020). A DMA 4500 density meter with a range of 0 to 3 g/cm3 and an accuracy of 5 × 10–5 g/cc is used to measure the density of testing fuels. A Brookfield Viscometer Model DV-I+ with UL adapter is employed to measure the viscosity of testing fuels. It consists of a set of seven spindles (RV Spindle Set) with accuracy ± 1%. The flashpoint of biodiesel is measured by a flash point tester which consists of an 80-ml closed copper cup, a heater and a source that gives continuous sparks. A cetane tester SHATOX SX-200 with a range of 20–100 (CN units) and an accuracy of 0.1 °C is used to measure the cetane number. A cloud and pour point analyser (ISL CPP 5Gs) having a range of − 95 to 51 ° C and an accuracy of 0.1 °C is used to measure cloud and pour point of the samples. A stainless-steel bomb jacket water calorimeter equipped with mortised heavy-duty firing unit with immunization requiring 220/203 V and AC supply single-phase ± 10‰ Hz is used to measure the calorific value of test fuels.
There are two types of fatty acids in the animal oil, saturated fat and unsaturated fat. Stearic, palmitic and hydroxystearic acids are saturated fatty acids and unsaturated fatty acids are oleic, linoleic, ricinoleic, palmitoleic, linolenic and eicosenoic acids. The composition of fatty acids in the beef tallow oil esters was calculated by the gas chromatograph with a flame ionization detector (GC-FID) system (Shimadzu GD 17A) according to the norms of EN 14103. In general, saturated fatty acids have a very low oxidation rate than unsaturated fatty acids. Fuel stability research is primarily focused on the reactions of unsaturated fatty acids. Table 3 shows the fatty acid contents of the beef tallow methyl esters and it is observed that the oleic acid content in the oil is more compared with other acid contents. The oleic acid is the monosaturated fatty acid and the higher percentage composition of this acid leads to creating the cold plugging problem and thus reduces the ignition quality. The relative oxidation rate of fatty acid series stearic, oleic and linoleic acid is to be in the ratio of 1:100:1200.
The miscibility and stability of BHA antioxidant in BTO was measure by using a laser beam–assisted photonic circuit method which is shown in Fig. 3. The laser beam focused on the photoresistor connected to a multimeter in order to measure the deflection of stability. The absorption properties of BHA-BTO were investigated by the UV absorption spectrum. The photographic view of the UV absorption spectrum for BTO-BHA is shown in Fig. 4. It is seen that the absorption peak was noticed at 375 nm which confirms that the BHA was well dispersed in BTO with uniform size and shape. Also, the gravitational method is followed for evaluating the stability of the beef tallow oil (BTO) and antioxidant BHA mixed test fuel by keeping the samples for a week and the sample shows the least separation of phases (Devarajan et al. 2018). The following relation (Eq. (1)) calculates dispersion stability
Laser beam–assisted photonic circuit (Vellaiyan 2019b)
UV absorption spectrum (Vellaiyan 2019b)
Based on the observations for a week, the dispersion rate and stability of BTO and 1000 ppm BHA were found to be stable.
Experimental setup
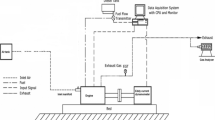
The experiments were performed on a water-cooled, single-cylinder, direct-injection diesel engine; the layout and pictorial view of the engine setup is shown in Figs. 5 and 6. Table 4 shows the test engine specifications. The test engine is coupled to an eddy current type dynamometer for load variations. The AVL Digas 444 five-gas analyser was employed for the measurement of CO, carbon dioxide (CO2), NOx and HC emissions at different load conditions. The diesel engine output shaft is connected with the eddy current dynamometer, which is used to measure the power and torque. The load cell is connected to the dynamometer for varying the load on the engine (0–100%). Table 5 illustrates the details of an exhaust gas analyser. First, the engine is started using diesel and is run for about 15 min to warm it up. At a constant speed of 1500 rpm, the performance parameters and emissions were measured at 4 different engine loads (25%, 50%, 75% and 100%). At the steady-state conditions, the readings were taken. Now, the engine is run on two test fuels (B20 and B20 + BHA) and the results were noted. Engine performance parameters like brake thermal efficiency, brake specific fuel consumption and exhaust gas temperature and emission parameters were analysed in terms of CO, HC and NOx emissions.
Methodology
Initially, the engine is allowed to run for 10 min with diesel to obtain a steady state and at atmospheric conditions. Subsequently, the experimental results are recorded for various fuel blends (B20 and B20 + BHA) at constant engine speed at 1500 rpm and the injection timing and pressure at 17° bTDC and 200 bar respectively. For each fuel sample (B20 and B20 + BHA and diesel), the experiments are repeated 5 times at similar conditions, and the average value is considered for assessment to lessen the experimental errors.
Uncertainty analysis
Every work includes some amount of uncertainty. These uncertainties mainly result in calibration, sensors, observations, test procedure and environmental conditions. In view of uncertainties, the preferred test results can be examined for any work. If a quantity to be measured “R” has a function of many self-regulating variables like the following
let the measured quantity uncertainty be expressed as WR and the independent variables uncertainties are expressed as W1, W2, W3, …Wn.
Subsequently, the uncertainty in the measured quantity is given by
where \( {W}_R=\frac{\delta R}{R} \) ±δR is the error in R. The uncertainty percentage of various measured and calculated parameters are listed in Table 5, and the overall uncertainty percentage of experimental results is calculated as 1.3% using the following equation:
Results and discussion
Performance characteristics
Brake specific fuel consumption
Brake specific fuel consumption (BSFC), expressed in kg/kWh, is defined as the mass of fuel consumed by the engine per unit brake power output. Figure 7 shows the variation of BSFC for B20, diesel and B20 + BHA with the load. BSFC varies from 0.58 to 0.28 kg/kWh for all fuels. BSFC of B20 varies from 0.56 kg/kWh (no load) to 0.31 kg/kWh (full load), whereas it changes from 0.54 kg/kWh (no load) to 0.29 kg/kWh for diesel and 0.58 kg/kWh (no load) to 0.34 kg/kWh for B20. With the increase in load from no to full load, BSFC rates for B20, diesel and B20 + BHA reduce by 22.3, 24.1 and 21.5% respectively. It owes to drop in the ratio between brake power and friction power for tested fuels. BSFC rates for B20 and B20 + BHA are 0.02 and 0.05 kg/kWh higher than diesel. BSFC for diesel is least owing to its approving physicochemical properties. In addition, many researchers describe that an increase in BSFC of biofuels (B20 and B20 + BHA) was due to its lower energy content and high viscosity than diesel. Equally, B20 exhibited higher BSFC than B20 + BHA and diesel because of poor physicochemical properties. However, in comparison with B20, B20 + BHA exhibits lower BSFC of about 0.05 kg/kWh at full load. The kinematic viscosity of B20 + BHA exhibits 17.2% lower viscosity than B20. Furthermore, the oxidation stability of B20 + BHA is 20% higher than B20. Fuel with higher oxidation stability and lower viscosity improves the combustion. The addition of 1000 ppm of BHA to B20 lowers the cohesion between the fuels and disintegrates into finer droplets. There, finer droplets of fuels are surrounded by air in all directions leading to complete combustion and lower BSFC. Furthermore, friction reduction properties of antioxidants are the reason for the reduction in BSFC, which could result in high power output (Pullen and Saeed 2014, Varatharajan and Pushparani 2018). Similar inferences were observed in the different kinds of literature (Vellaiyan, 2020b; Mahalingam et al. 2018; Jayabal et al., 2019; Ganesan et al. 2019; Devarajan et al. 2019a, b; Ravikumar and Saravanan 2016) mentioning that the small viscosity reduction can lower BSFC.
Brake thermal efficiency
The brake thermal efficiency (BTE) is commonly known as fuel conversion efficiency and it is calculated as the ratio between the brake power to energy introduced through fuel injection. Figure 8 represents the BTE variation for Diesel, B20 and B20 + BHA with the load. BTE varies from 15.78 to 30.68% for all fuels. BTE of B20 + BHA varies from 15.78% (at 25% load) to 25.23% (at full load), whereas for B20 fuel, the BTE changes from 15.98 to 27.78%. The engine performance with these fuels is always less than diesel (30.68% BTE) due to the following factors such as shorter ignition delay of biodiesel, higher viscosity, density, low calorific value, high volatility and poor spray properties compared with those of diesel fuel. Madiwale et al. (2020) describe that the biodiesel has a higher viscosity and density leads to poor atomization and fuel vaporization, resulting in uneven combustion compared with that of diesel fuel. Kivevele et al. (2011) investigated that the ratio of friction to brake power decreases due to in reduction in heat loss and an increase in power. Furthermore, the addition of an antioxidant into the B20 fuel lowers the BTE by 1.5%. This is due to partial burning fuel and reduction in combustion chamber pressure by the effect of the addition of antioxidant additive in the B20 fuel. The results obtained by this work are in line with the research work carried out by Dueso et al. (2018) which inferred that the addition of antioxidant in the sunflower biodiesel results in the reduction in BTE by 0.1–0.8%. Furthermore, in another investigation, Ramalingam et al. (2019), experienced the same pattern and reported that the addition of antioxidant p-phenylenediamine in Annona biodiesel (A20 blend) decreases BTE.
Emission characteristics
Carbon monoxide emission
The variation in carbon monoxide (CO) emissions with load is shown in Fig. 9. The most important reason for the formation of CO emission in the exhaust gas is due to incomplete combustion coupled with rich fuel to air ratio and low oxygen concentration. CO emission is produced when the conversion of CO to CO2 is insufficient (Ashok et al. 2017). At full load (100%) conditions, the CO emission values for the fuel blends D (100% diesel), B20 and B20 + BHA were 0.43, 0.31 and 0.35 g/kWh respectively whereas the CO emission changes from 0.15 to 0.21 g/kWh at 25% load. The CO emission for fuel B20 is low by 27.9% compared with that for diesel fuel. This is due to the combined effect of high oxygen content in the chemical structure of biodiesel fuels and high cetane number (Rizwanul Fattah et al. 2014a, b, c; Ileri New York and Kocar 2013; Rashed et al. 2016a, b). The presence of antioxidants in the fuel blend B20 + BHA increases the CO emission by 14.2% compared with that in B20. The antioxidant reduces the conversion of CO to CO2 and resulted in higher CO emission for antioxidant-treated biodiesel blends (Rizwanul Fattah et al. 2014a, b, c). During combustion, peroxyl (HO2) and hydrogen peroxide (H2O2) radicals formed during oxidation are further converted into hydroxyl radicals (OH) by absorbing heat from the combustion chamber. The conversion of CO to CO2 is mostly affected by these OH radicals (Palash et al. 2014; Rashed et al. 2016a, b).
Hydrocarbon emission
The variation in hydrocarbon (HC) emissions with brake power for all fuel blends is shown in Fig. 10. The HC emission values for B20, B20 + BHA and diesel (D) at 25% load (1.25 kW BP) were 0.0468, 0.0527 and 0.06 g/kWh respectively whereas at full load, the HC emission for all fuels changed from 0.0622 to 0.0689 g/kWh. It is seen that the HC emission for all biodiesel blends is low compared with conventional petroleum diesel. HC emission for B20 and B20 + BHA fuel is 20% and 12.17% lower than diesel respectively. The concentration of 20% in the biodiesel in the conventional fuel exhibits more oxygen and enhances the combustion rate. Also, due to the rich oxygen content in the blends of biodiesel fuel, the ignition delay was reduced to a shorter time period and result in the early start of fuel injection and prolonging of the combustion process. The antioxidant in fuel B20 + BHA increases in HC emission by 10.6% compared with that in B20 blend. The presence of antioxidants in the biodiesel reduces peroxyl and hydrogen peroxide radicals, which are responsible for converting CO into CO2 and HC into H2O and CO2, and induces a significant increase in the volume of HC in the exhaust gases. Also, the obtained results are in good agreement with the research work carried by Balaji and Cheralathan (2015), Varatharajan et al. (2011), Radhakrishnan (2017) and Debbarma and Misra (2018).
Nitrogen oxide emission
Higher combustion temperature, longer combustion duration and high oxygen concentration of the fuel are the main reasons for nitrogen oxide (NOx) formation. The variations in NOx emission for B20, B20 + BHA and diesel with respect to different loading conditions are represented in Fig. 11. At low load (25%) condition, NOx emission varies from 1.062 to 1.531 g/kWh for all fuels whereas, at full load (BP 3.55 kW) condition, NOx emissions of fuel diesel, B20 and B20 + BHA are 3.836, 4.685 and 4.128 g/kWh respectively. It is seen that the NOx emission is high for both biodiesel blends compared with diesel. The use of B20 fuel in an engine results in high NOx concentration. This could be correlated to higher exhaust gas temperature for biofuels which is 12% higher than conventional diesel fuel. The mixture of 20% biodiesel and 80% conventional diesel possess rich oxygen content and results in a reduction of ignition delay period and early start of combustion (Velmurugan and Sathiyagnanam 2016, Ramalingam et al. 2016). Due to the early start of injection, modified fuels produce high combustion temperatures and increased formation of NOx (Mueller et al. 2009). The use of antioxidant BHA in biodiesel blend B20 reduces NOx emission by 8.2% than B20 fuel. The catalytic activity of BHA improved the reaction time of combustion and lowered the combustion temperature that results in lower NOx emissions. Different kinds of literature such as Varatharajan and Pushparani (2018), Rashed et al. (2016a, b, c, d) and Rashedul et al. (2015) stated that reactions between molecular nitrogen and hydrocarbon-free radicals (CH, C2, C and CH2) play a role in prompt emission of NOx during biodiesel combustion. Antioxidants can prevent radical reactions and, consequently, reduce the formation of NOx.
Carbon dioxide emission
In general, the complete combustion of fuel leads to the high formation of carbon dioxide (CO2) in a diesel engine, which is always higher when compared with that in gasoline engines. Figure 12 represents the variation in CO2 emission for all test fuels under varying loads. The results show that the fuel B20 and B20 + BHA promote a high magnitude of CO2 emission compared with diesel due to more oxygen available during the combustion process. At lower load, the CO2 emission varies from 1.308 to 1.512 g/kWh whereas at full load condition, the CO2 emissions of fuel diesel, B20 and B20 + BHA are observed as 1.82, 2.936 and 2.063 g/kWh respectively. It is observed that the CO2 emission for B20 fuel is almost 30% higher compared with that for diesel. Oxygen availability in the chemical structure of biodiesel and its lower carbon content strengthens the combustion and increases CO2 formation. The addition of BHA in the blend of biodiesel fuel reduces the formation of CO2 emission by 26.73% compared with B20. In BHA, the oxygen-donating catalysts cause the combustion reaction and lower CO2 emissions (Rashedul et al. 2014; Alagu et al. 2018; Anderson et al. 2008).
Exhaust gas temperature
The exhaust gas temperature (EGT) of all test fuels with respect to load is depicted in Fig. 13. In general, the drop in EGT is not proportional to the drop in fuel quantity. It could be the result of efficient combustion, lower ignition delay period and lower heating value of the fuel (Özener et al. 2014). At full load condition, the EGT of fuel blends D (diesel 100%), B20 and B20 + BHA were 305, 316 and 310 °C respectively. It is obvious that at a lower load level, the EGT is always low compared with that at a higher load and it varied as 180 °C, 184 °C and 186 °C for the use of fuel diesel, B20 + BHA and B20 respectively. From the graphical representation, it is noted that the use of conventional diesel has lower EGT for all loading conditions compared with biodiesel fuel blends B20 and B20 + BHA. The lower ignition delay period and enriched oxygen content in biodiesel result in higher EGT compared with that in diesel. Sathiyamoorthi and Sankaranarayanan (2017), Rizwanul Fattah et al. (2014) and Senthil Ramalingam et al. (2019) describe that higher viscosity of biodiesel blend causes poor atomization resulting in the presence of unburned fuel particles in the premixed combustion phase. This will burn later in the diffusion combustion phase resulting in increased exhaust gas temperature. This phenomenon reflects the loss in heat energy and, in turn, decreases the thermal efficiency. The presence of antioxidants in the fuel blend B20 + BHA reduces the EGT by 1.9% compared with that in B20 and thus, the stable combustion takes place inside the cylinder.
Combustion characteristics
Pressure vs. crank angle
Figure 14 illustrates the variation of cylinder pressure vs. crank angle at the highest load. Emissions and performance analysis shall be correlated with in-cylinder pressure analysis. The extreme pressure values of diesel, B20 and B20 + BHA are 66.114 bar, 65.407 bar and 64.597 bar respectively. Peak cylinder pressure is registered for diesel; this may be owing to extended ignition delay period which paves the way for more mixing time of the blend, causing a rapid premixed burning phase of combustion resultant in augmented highest cylinder pressure. Reduction in pressure is observed for B20 + BHA than B20. The presence of BHA lowered the NOx emissions for B20. The catalytic activity of BHA in B20 increased the reaction time of combustion and lowered the combustion temperature that results in lower NO emissions and peak pressure. The variations in peak pressure are due to high fractions of molecular weight (fatty acids) and other fuel properties, such as high viscosity and cetane number, and low biodiesel volatility (Imtenan et al. 2015). Li et al. (2018) reported that supplying the fuel into the cylinder takes a lengthier ignition delay period and increases the rate of premixed combustion. The antioxidant in biodiesel fuels is very effective in reducing cylinder pressure (Sajjad et al. 2015; Damodharan et al. 2018).
Heat release rate
Figure 15 portraysthe disparity of heat release rate vs. crank angle at the highest load. The extreme heat release rates for diesel, B20 and B20 + BHA are 72.419 J/°, 66.741 J/° and 67.714 J/° respectively. In the beginning, a negative heat release is observed due to the vaporization of the fuel accumulated during an ignition delay, and this heat release becomes positive after the combustion is initiated. The higher cetane number in the biodiesel fuels (B20 and B20 + BHA) leads to shorter ignition delay and early start of combustion (Qi et al. 2009; Mamat et al. 2009). In comparison with B20, the use of fuel B20 + BHA increases the heat release rate from 66.741 to 67.714 J/°. The mixing of antioxidant in the biodiesel provides stable combustion by preventing radical reactions. BHA addition to B20 improves the atomization process and in turn enhances HRR. Zhu et al. (2011) stated that the net combustion period shortened the premixed heat release proportion and highest cylinder pressure increased which results in higher HRR.
Ignition delay
The ignition delay is inferred as the time gap among fuel ignition and injection. Two factors govern ignition delay, one being a physical delay where the fuel atomization, fuel mixing and fuel vapourization is involved. The other is a chemical delay which is controlled by the pre-combustion reaction. Atomization, vaporization and mixing rate of fuel come under physical delay and the cetane number responsible for auto-ignition comes under a chemical delay (Devarajan et al. 2020). Ignition delay for all tested fuel blends is represented in Fig. 16 for all load conditions. At full load conditions, the ignition delay values for the fuel blends D (100% diesel), B20 and B20 + BHA were 15.8, 14.3 and 14.7 respectively. It is also observed that the ignition delay values for both biodiesels blends B20 and B20 + BHA were shorter compared with that for conventional diesel. This is because lower fatty acid methyl esters, compressibility, higher cetane and flashpoints initiate the early combustion for biofuels. The addition of BHA in the B20 fuel blend increases the ignition delay by 2.72% when compared that in the fuel B20 without BHA. This is correlated to lower viscosity, better combustion characteristics and improved fuel atomization, a superior flashpoint of fuel (Varatharajana and Pushparani et al. 2018, Devaraj et al. 2018). Owing to the above-mentioned properties, the combustion starts early for the use of biofuel in a diesel engine.
Conclusion
The combustion, performance and emission characteristics of 20% beef tallow biodiesel, 80% of conventional diesel and 1000 ppm of BHA are detailed in the present study and the results are compared with the performance of the conventional diesel-fuelled engine. The study conclusions are as follows:
-
The physicochemical properties of 20% beef tallow biodiesel, 80% of conventional diesel and 1000 ppm of BHA included fuels are at par with EN 14214 standards and these fuels can be used in existing diesel engine without any modification.
-
The addition of BHA in the blend of B20 (20% beef tallow biodiesel + 80% conventional diesel) results in the reduction in BSFC about 2% than B20 blend; this is due to the friction reduction properties of antioxidants.
-
The use of petroleum diesel in engine always results in higher BTE and the use of 20% beef tallow oil biodiesel in the diesel results lower BTE due to higher viscosity, density, low calorific value, high volatility and poor spray properties. This loss has been recovered by the addition of BHA in the blend B20.
-
The CO emission value for the use of B20 blend in the engine without antioxidant additives was low by 27.9% when compared with diesel fuel. The presence of antioxidant BHA in the blend B20 increases the CO emission by 14.2% when compared with that in B20 without BHA. The antioxidant reduces the conversion of CO to CO2 and resulted in higher CO emission for antioxidant-treated biodiesel blends.
-
NOx emission of B20 was increased by 22% compared with conventional diesel. The use of BHA antioxidant in the blend B20 + BHA emits 12.2% lesser NOx when compared with that in B20. This is due to breaking chain reactions, scavenging the initiating radicals and reducing the concentration of reactive radicals.
-
In addition, the EGT, ignition delay, HC, CO2 and the heat release rate for the use of fuel blend B20 + BHA were controllable to meet the diesel engine performance for all load conditions when compared with B20 blend.
Overall, it can be concluded that the addition of BHA antioxidant in the beef tallow biodiesel blend B20 has the potential to promote beef tallow biodiesel as a greener alternative fuel for existing diesel engines. To increase the percentage concentration of beef tallow biodiesel with diesel, the modified exhaust gas after-treatment systems have to be considered.
Abbreviations
- ASTM:
-
American Society for Testing Materials
- BD100:
-
neat biodiesel
- B20:
-
20% of biodiesel + 80% neat diesel
- B20 + BHA:
-
20% of biodiesel + 80% neat diesel + 1000 ppm of antioxidant BHA
- NOx :
-
Oxides of nitrogen emission
- BSFC:
-
Brake specific fuel consumption
- HC:
-
Unburned Hydrocarbon
- BTE:
-
Brake thermal efficiency
- CO:
-
Carbon monoxide
- DI:
-
Direct injection
- IMEP:
-
Indicated mean effective pressure
- NOx :
-
Oxides of nitrogen (NOx)
- EGT:
-
Exhaust gas temperature
References
Alagu K, Nagappan B, Jayaraman J, Arul A (2018) Impact of antioxidant additives on the performance and emission characteristics of C.I engine fuelled with B20 blend of rice bran biodiesel. Environ Sci Pollut Res 25(18):17634–17644
Anderson K, Bows A, Mander S (2008) From long-term targets to cumulative emission pathways: reframing UK climate policy. Energy Policy 36(10):3714–3722. https://doi.org/10.1016/j.enpol.2008.07.003
Ashok B, Nanthagopal K, Jeevanantham AK, Bhowmick P, Malhotra D, Agarwal P (2017) An assessment of Calophyllum inophyllum biodiesel fuelled diesel engine characteristics using novel antioxidant additives. Energy Convers Manag 148:935–943
Balaji G, Cheralathan M (2015) Experimental investigation of antioxidant effect on oxidation stability and emissions in a methyl ester of neem oil fueled DI diesel engine. Renew Energy 74:910–916
Bharathiraja B, Jayamuthunagai J, Sudharsanaa T, Bharghavi A, Praveenkumar R, Chakravarthy M, Yuvaraj D (2017) Biobutanol – an impending biofuel for future: a review on upstream and downstream processing techniques. Renew Sust Energ Rev 68:788–807. https://doi.org/10.1016/j.rser.2016.10.017
Damodharan D, Sathiyagnanam AP, Kumar BR, Ganesh KC (2018) Cleaner emissions from a DI diesel engine fueled with waste plastic oil derived from municipal solid waste under the influence of n-pentanol addition, cold EGR, and injection timing. Environ Sci Pollut Res 25(14):13611–13625. https://doi.org/10.1007/s11356-018-1558-5
Debbarma S, Misra RD (2018) Effects of iron nanoparticle fuel additive on the performance and exhaust emissions of a compression ignition engine fueled with diesel and biodiesel. J Thermal Sci Eng Appl 10(4):041002
Devaraj A, Yuvarajan D, Vinoth Kanna I (2018) Study on the outcome of a cetane improver on the emission characteristics of a diesel engine. Int J Ambient Energy:1–4. https://doi.org/10.1080/01430750.2018.1492452
Devarajan Y (2018) Experimental evaluation of combustion, emission and performance of research diesel engine fuelled di-methyl- carbonate and biodiesel blends. Atmos Pollution Res 10(3):795–801. https://doi.org/10.1016/j.apr.2018.12.007
Devarajan Y, Munuswamy DB, Radhakrishnan S, Mahalingam A, Nagappan B (2018) Experimental testing and evaluation of neat biodiesel and heptanol blends in diesel engine. J Test Eval 47(2):20170307. https://doi.org/10.1520/jte20170307
Devarajan Y, Choubey G, Mehar K (2019a) Ignition analysis on neat alcohols and biodiesel blends propelled research compression ignition engine. Energy Sources A: Recovery Utilization Environ Effects:1–12. https://doi.org/10.1080/15567036.2019.1618998
Devarajan Y, Munuswamy D, Nagappan B, Subbiah G (2019b) Experimental assessment of performance and exhaust emission characteristics of a diesel engine fuelled with Punnai biodiesel/butanol fuel blends. Pet Sci 16:1471–1478. https://doi.org/10.1007/s12182-019-00361-9
Devarajan Y, Beemkumar N, Ganesan S, Arunkumar T (2020) An experimental study on the influence of an oxygenated additive in diesel engine fuelled with neat papaya seed biodiesel/diesel blends. Fuel 268:117254. https://doi.org/10.1016/j.fuel.2020.117254
Dimitrios N, Tziourtzioumis, Anastassios M, Stamatelos (2017) Experimental investigation of the effect of biodiesel blends on a DI Diesel engine’s injection and combustion. Energies 2017(10):970. https://doi.org/10.3390/en10070970
Dueso C, Muñoz M, Moreno F, Arroyo J, Gil-Lalaguna N, Bautista A, Gonzalo A, Sánchez JL (2018) Performance and emissions of a diesel engine using sunflower biodiesel with a renewable antioxidant additive from bio-oil. Fuel 234:276–285. https://doi.org/10.1016/j.fuel.2018.07.013
Ganesan S, Sivasubramanian R, Sajin JB, Ganesan S (2018) Performance and emission study on the effect of oxygenated additive in neat biodiesel fueled diesel engine. Energy Sources A: Recovery Utilization Environ Effects 41(16):2017–2027. https://doi.org/10.1080/15567036.2018.1549148
Ganesan S, Dineshbabu M, Prabhu A, Arunkumar T, Yuvarajan D (2019) Effect of EGR & nanoparticles on performance and emission characteristics of a diesel engine fuelled with palm biodiesel and diesel blends. J Oil Palm Res 31:130–137. https://doi.org/10.21894/jopr.2018.0065
Hazar H (2017) Investigation of the effects of tripropylene glycol addition to diesel fuel on combustion and exhaust emissions at an isolated diesel engine. Energy Convers Manag 142:62–68. https://doi.org/10.1016/j.enconman.2017.02.082
Ileri New York E, Kocar G (2013) Effects of antioxidant additives on engine performance and exhaust emissions of a diesel engine fueled with canola oil methyl ester–diesel blend. Energy Convers Manag 76:145–154
Imtenan S, Masjuki H, Varman M, Fattah IR (2015) Evaluation of n-butanol as an oxygenated additive to improve combustion–emission–performance characteristics of a diesel engine fuelled with a diesel-Calophyllum inophyllum biodiesel blend. RSC Adv 5:17160–17170
Jayabal R, Thangavelu L, Velu C (2019) Experimental investigation on the effect of ignition enhancers in the blends of sapota biodiesel/diesel blends on a CRDi engine. Energ Fuel:12431–12440
Joy N, Yuvarajan D, Beemkumar N (2019) Performance evaluation and emission characteristics of biodiesel-ignition enhancer blends propelled in a research diesel engine. Int J Green Energy 16(4):277–283. https://doi.org/10.1080/15435075.2018.1561455
Kavitha, Kutuva R, Nagappan B, Rajendiran R (2019) Experimental investigation of diesel engine performance fuelled with the blends of Jatropha curcas, ethanol, and diesel. Environ Sci Pollut Res 26:8633–8639
Kishore Pandian A, Munuswamy DB, Radhakrishana S, Bathey Ramakrishnan RB, Nagappan B, Devarajan Y (2017) Influence of an oxygenated additive on emission of an engine fueled with neat biodiesel. Pet Sci 14:791–797. https://doi.org/10.1007/s12182-017-0186-x
Kivevele TT, Kristóf L, Bereczky Á, Makame M (2011) Mbarawa Engine performance, exhaust emissions and combustion characteristics of a CI engine fuelled with croton megalocarpus methyl ester with antioxidant. Fuel 90:2782–2789
Li G et al (2018) Effects of dilute gas on combustion and emission characteristics of a common-rail diesel engine fueled with isopropanol-butanol-ethanol and diesel blends. Energy Convers Manag 165:373–381
Madiwale S, Alagu K, Bhojwani V (2020) Investigation of cottonseed oil biodiesel with ethanol as an additive on fuel properties, engine performance, combustion and emission characteristics of a diesel engine. Therm Sci 24(1 Part A):27–36. https://doi.org/10.2298/tsci180604235m
Mahalingam A, Devarajan Y, Radhakrishnan S, Vellaiyan S, Nagappan B (2018) Emissions analysis on mahua oil biodiesel and higher alcohol blends in diesel engine. Alex Eng J 57(4):2627–2631. https://doi.org/10.1016/j.aej.2017.07.009
Mamat R, Abdullah NR, Xu H, Wyszynski ML, Tsolakis A (2009) Effect of fuel temperature on performance and emissions of a common rail diesel engine operating with rapeseed methyl ester (RME). SAE Technical Paper
Mei D, Yue S, Zhao X, Hielscher K, Baar R (2017) Effects of center of heat release on combustion and emissions in a PCCI diesel engine fuelled by DMC-diesel blend. Appl Therm Eng 114:969–976. https://doi.org/10.1016/j.applthermaleng.2016.12.064
Mueller CJ, Boehman AL, Martin GC (2009) An experimental investigation of the origin of increased NOx emissions when fueling a heavy-duty compression ignition engine with soy biodiesel. SAE paper 01–1792
Özener O, Yüksek L, Ergenç AT, Özkan M (2014) Effects of soybean biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 115:875–883. https://doi.org/10.1016/j.fuel.2012.10.081
Palash SM, Kalam MA, Masjuki HH, Arbab MI, Masum BM, Sanjid A (2014) Impacts of NOx reducing antioxidant additive on performance and emissions of a multi-cylinder diesel engine fueled with Jatropha biodiesel blends. Energy Convers Manag 77:577–585
Prabhu A, Jayaprabakar J, Beemkumar N, Yuvarajan D (2018) Effect of injection timing on performance and emission characteristics of palm biodiesel and diesel blends. J Oil Palm Res 30:674–681. https://doi.org/10.21894/jopr.2018.0057
Pullen J, Saeed K (2014) Factors affecting biodiesel engine performance and exhaust emissions – Part I: Review. Energy 72(C):1–16
Qi DH, Geng LM, Chen H, Bian YZ, Liu J, Ren XC (2009) Combustion and performance evaluation of a diesel engine fueled with biodiesel produced from soybean crude oil. Renew Energy 34:2706–2713
Radhakrishnan K, Kalyanasundharam S, Ravichandran N, Thiyagarajan S, Thilagaraj WR (2018) A novel method of unburned hydrocarbons and NOx gases capture from vehicular exhaust using natural biosorbent. Sep Sci Technol 53(1):13–21. https://doi.org/10.1080/01496395.2017.1380046
Raju VD, Venu H, Subramani L, Reddy SR (2020) Comparative assessment of various nanoadditives on the characteristic diesel engine powered by novel tamarind seed-methyl ester blend. Advances in Mechatronics and Mechanical Engineering:138–158. https://doi.org/10.4018/978-1-7998-2539-5.ch007
Ramalingam S, Rajendran S, Ganesan P (2016) Improving the performance is better and emission reductions from Annona biodiesel operated diesel engine using 1,4-dioxane fuel additive. Fuel 185:804–809
Ramalingam S, Rajendran S, Ganesan P, Govindasamy M (2018) Effect of operating parameters and antioxidant additives with biodiesels to improve the performance and reducing the emissions in a compression ignition engine – a review. Renew Sust Energ Rev 81:775–788
Ramalingam S, Ganesan P, Murugasan E (2019) Effect of antioxidant additives on performance and emission behavior of biodiesel fueled DI diesel engine. Energy Sources A: Recov Utilization Environ Effects 42:1085–1096. https://doi.org/10.1080/15567036.2019.1602219
Rashed MM, Kalam MA, Masjuki HH, Habibullah M, Imdadul HK, Shahin MM, Rahman MM (2016a) Improving oxidation stability and NOX reduction of biodiesel blends using aromatic and synthetic antioxidant in a light duty diesel engine. Ind Crop Prod 89:273–284
Rashed MM, Masjuki HH, Kalam MA, Alabdulkarem A, Rahman MM, Imdadul HK, Rashedul HK (2016b) Study of the oxidation stability and exhaust emission analysis of Moringa olifera biodiesel in a multi-cylinder diesel engine with aromatic amine antioxidants. Renew Energy 94:294–303
Rashed MM, Kalam MA, Masjuki HH, Habibullah M, Imdadul HK, Shahin MM, Rahman MM (2016c) Improving oxidation stability and NOx reduction of biodiesel blends using aromatic and synthetic antioxidant in a light duty diesel engine. Ind Crop Prod 89:273–284
Rashed MM, Masjuki HH, Kalam MA, Alabdulkarem A, Rahman MM, Imdadul HK, Rashedul HK (2016d) Study of the oxidation stability and exhaust emission analysis of Moringa biodiesel in a multi-cylinder diesel engine with aromatic amine antioxidants. Renew Energy 94:294–303
Rashedul HK, Masjuki HH, Kalam MA, Ashraful AM, Ashrafur Rahman SM, Shahir SA (2014) The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine. Energy Convers Manag 88:348–364
Rashedul HK, Masjuki HH, Kalam MA, Teoh YH, How HG, Rizwanul Fattah IM (2015) Effect of antioxidant on the oxidation stability and combustion–performance–emission characteristics of a diesel engine fueled with diesel–Biodiesel blend. Energy Convers Manag 106:849–858. https://doi.org/10.1016/j.enconman.2015.10.024
Rathinam S, Balan K, Subbiah G, Sajin J (2019) Emission study of a diesel engine fueled with higher alcohol-biodiesel blended fuels. Int J Green Energy 16(9):667–673. https://doi.org/10.1080/15435075.2019.1617001
Ravikumar J, Saravanan S (2016) Performance and emission analysis on blends of diesel, restaurant yellow grease and n-pentanol in direct-injection diesel engine. Environ Sci Pollut Res 24(6):5381–5390. https://doi.org/10.1007/s11356-016-8298-1
Rizwanul Fattah IM, Masjuki HH, Kalam MA, Mofijur M, Abedin MJ (2014) Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers Manag 79:265–272. https://doi.org/10.1016/j.enconman.2013.12.024
Rizwanul Fattah IM, Masjuki HH, Kalam MA, Mofijur M, Abedin MJ (2014a) Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers Manag 79:265–272
Rizwanul Fattah IM, Masjuki HH, Kalam MA, Wakil MA, Ashraful AM, Shahir SA (2014b) Experimental investigation of performance and regulated emissions of a diesel engine with Calophyllum inophyllum biodiesel blends accompanied by oxidation inhibitors. Energy Convers Manag 83:232–240
Rizwanul Fattah IM, Masjuki HH, Kalam MA, Wakil MA, Rashedul HK, Abedin MJ (2014c) Performance and emission characteristics of a CI engine fueled with Cocos nucifera and Jatropha curcas B20 blends accompanying antioxidants. Ind Crop Prod 57:132–140
Sajjad H, Masjuki H, Varman M, Kalam M, Arbab M, Imtenan S et al (2015) Influence of gas-to-liquid (GTL) fuel in the combined blend of Jatropha biodiesel and diesel: an analysis of engine combustion–performance–emission parameters. RSC Adv 5:29723–29733
Sathiyamoorthi R, Sankaranarayanan G (2017) The effects of using ethanol as additive on the combustion and emissions of a direct injection diesel engine fuelled with neat lemongrass oil-diesel fuel blend. Renew Energy 101:747–756. https://doi.org/10.1016/j.renene.2016.09.044
Selvam JP, Vadivel K (2012) Performance and emission analysis of di diesel engine fuelled with methyl esters of beef tallow and diesel blends. Procedia Eng 38:342–358
Shahir VK, Jawahar CP, Suresh PR, Vinod V (2017) Experimental Investigation on performance and emission characteristics of a common rail direct injection engine using animal fat biodiesel blends. Energy Procedia 117:283–290
Siva R, Munuswamy DB, Yuvarajan D (2018) Emission and performance study emulsified orange peel oil biodiesel in an aspirated research engine. Pet Sci 16:180–186. https://doi.org/10.1007/s12182-018-0288-0
Subramanian T, Varuvel EG, Ganapathy S, Vedharaj S, Vallinayagam R (2018) Role of fuel additives on reduction of NO X emission from a diesel engine powered by camphor oil biofuel. Environ Sci Pollut Res 1-10
Sudalaimuthu G, Rathinam S, Munuswamy DB, Thirugnanasambandam A (2018) Testing and evaluation of performance and emissions characteristics of water- biodiesel aspirated research engine. J Test Eval 48(5):20180306. https://doi.org/10.1520/jte20180306
Teixeira, Leonardo SG, Couto MB, Souza GS, Filho MA, Assis JCR, Guimarães PRB, Pontes LAM, Almeida SQ, Teixeira JSR (2010) Characterization of beef tallow biodiesel and their mixtures with soybean biodiesel and mineral diesel fuel, bio mass and bio energy. 34:438–441
Varatharajan K, Pushparani DS (2018) Screening of antioxidant additives for biodiesel fuels, renewable and sustainable energy Reviews 82(3):2017-2028. https://doi.org/10.1016/j.rser.2017.07.020
K. Varatharajan, D.S. Pushparanib (2018) Renew Sust Energ Rev, Volume 82, Part 3, Pages 2017-2028, https://doi.org/10.1016/j.rser.2017.07.020.
Varatharajan K, Cheralathan M, Velraj R (2011) Mitigation of NOx emissions from a jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel 90(8):2721–2725
Vellaiyan S (2019) Enhancement in combustion, performance, and emission characteristics of a diesel engine fueled with diesel, biodiesel, and its blends by using nanoadditive. Environ Sci Pollut Res 26(10):269561e–2269573e
Vellaiyan S, Subbiah A, Chockalingam P (2019) Multi-response optimization to improve the performance and emissions level of a diesel engine fueled with ZnO incorporated water emulsified soybean biodiesel/diesel fuel blends. Fuel 237:1013–1020. https://doi.org/10.1016/j.fuel.2018.10.057
Vellaiyan S, Subbiah A, Chockalingam P (2019b) Effect of titanium dioxide nanoparticle as an additive on the working characteristics of biodieselwater emulsion fuel blends. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. https://doi.org/10.1080/15567036.2019.1634776
Vellaiyan S (2020a) Effect of cerium oxide nanoadditive on the working characteristics of water emulsified biodiesel fueled diesel engine; an experimental study. Therm Sci 24(1A):231–241
Vellaiyan S (2020b) Enhancement in combustion, performance, and emission characteristics of a biodiesel-fueled diesel engine by using water emulsion and nanoadditive. Renew Energ 145:2108–2120
Vellaiyan S, Subbiah A, Chockalingam P (2020) Effect of titanium dioxide nanoparticle as an additive on the exhaust characteristics of diesel-water emulsion fuel blends. Pet Sci Technol 38(3):194–202
Velmurugan K, Sathiyagnanam AP (2016) Impact of antioxidants on NOx emissions from a mango seed biodiesel powered DI diesel engine. Alexandria Eng J 55(1):715–722
Venu H, Madhavan V (2017) Influence of diethyl ether (DEE) addition in ethanol-biodiesel-diesel (EBD) and methanol-biodiesel-diesel (MBD) blends in a diesel engine. Fuel 189:377–390. https://doi.org/10.1016/j.fuel.2016.10.101
Wei M, Li S, Liu J, Guo G, Sun Z, Xiao H (2017) Effects of injection timing on combustion and emissions in a diesel engine fueled with 2,5-dimethylfuran-diesel blends. Fuel 192:208–217. https://doi.org/10.1016/j.fuel.2016.11.084
Xiao H, Hou B, Zeng P, Jiang A (2017) Combustion and emissions characteristics of a diesel engine fueled with blends of diesel and DMF. Chin Sci Bull 62(30):3506–3513. https://doi.org/10.1360/n972017-00020
Zhu R, Cheung CS, Huang Z (2011) Particulate emission characteristics of a compression ignition engine fueled with diesel–dmc blends. Aerosol Sci Technol 45.2:137–147
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagappan, B., Devarajan, Y., Kariappan, E. et al. Influence of antioxidant additives on performance and emission characteristics of beef tallow biodiesel-fuelled C.I engine. Environ Sci Pollut Res 28, 12041–12055 (2021). https://doi.org/10.1007/s11356-020-09065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09065-9