Abstract
Textile dyeing consumes high volumes of water, generating proportional number of colored effluents which contain several hazardous chemical. These contaminants can implicate in significant changes in aquatic environmental, including several adverse effects to organisms in different trophic levels. The present study was developed to assess the ecotoxicological effects of textile effluent samples and reactive Red 239 dye (used in cotton dyeing) to aquatic organisms Vibrio fischeri bacteria, Daphnia similis crustacean, and Biomphalaria glabrata snail (adults and embryos). Chronic assays with lethal and sublethal effects for Daphnia similis were included and performed only for textile effluents samples. The mutagenicity was also evaluated with Salmonella/microsome assay (TA98, TA100, and YG1041 strains). V. fischeri bacteria was the most sensitive to reactive Red 239 dye (EC50 = 10.14 mg L−1) followed by mollusk embryos at all stages (EC50 = 116.41 to 124.14 mg L−1), D. similis (EC50= 389.42 mg L−1), and less sensitive to adult snails (LC50= 517.19 mg L−1). The textile effluent was toxic for all exposed organisms [E(L)C50 < 15%] and B. glabrata embryos showed different responses in the early stages of blastulae and gastrulae (EC50 = 7.60 and 7.08%) compared to advanced development stages trochophore and veliger (EC50 = 21.56 and 29.32%). Developmental and sublethal effects in B. glabrata embryos and D. similis were evidenced. In the chronic assay with effluent, the EC10/NOEC = 3% was obtained. Mutagenic effects were not detected for dye aqueous solutions neither for effluents samples. These data confirmed the importance of evaluating the effects in aquatic organisms from different trophic levels and reinforce the need for environmental aquatic protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical dyes are usually visible in natural waters, even at relatively low concentrations, affecting transparence of water among other negative damages they introduce to the aquatic system, such as changes in photosynthetic activity and toxic effects to living organisms. In 2012, textile production introduced nearly 200 billion liters of colored effluents by year into rivers, representing about 20% of the aquatic pollution in many countries (Kant 2012; Zaharia and Suteu 2012).
The type of dyes and chemicals used in the textile industry differs depending on the fabrics that are manufactured. Reactive, direct, naphthol, and indigo dyes are some of the dyes used to dye cellulose fibers. Cotton, one of the most widely used fibers, is dyed mainly using reactive dyes (Ghaly et al. 2014). Over 630 thousand tons of reactive dyes were consumed in 2017 (Textile Colourant Market 2017).
Reactive azo dyes are the most used in dyeing cotton, accounting for >70% of the global industrial demand, 9 million tons, approximately. They are characterized by nitrogen-to-nitrogen double bonds (-N=N-), good solidity to wet treatments, high photolytic stability, and versatility of application. As examples of reactive dyes that have been widely used in the textile process of dyeing cotton fiber, Red 239, Blue 222, Yellow 145, and Black 5 can be mentioned (Ghaly et al. 2014; Rawat et al. 2016; Rosa et al. 2019).
Textile effluents are complex mixtures of different dyes, surfactants, salts, and peroxides, which are usually toxic to aquatic ecosystems and contribute with important changes to water quality, decreasing dissolved oxygen and severely affecting the dynamics of the ecosystem of the receiving water bodies (Rawat et al. 2016; Yousef et al. 2019; Tkaczyk et al. 2020). Dyes in waters, sediments, and soils have been identified in several regions around the world. In Taiwan, rhodamine 6G and rhodamine B (both basic synthetic dyes) were detected in concentrations of 0.0013 and 0.0048 μg L−1, respectively, in river water samples (Chiang et al. 2012). In Brazil, the disperse dyes, Red 1, Blue 373, and Violet 93, were detected in water samples of São Paulo state, with concentrations up to 3.5 μg L−1 (Zocolo et al. 2015; Vacchi et al. 2017).
Adverse effects in aquatic organisms can include peroxidation of algal cells due to excessive reactive oxygen species related to textile effluents (Cai et al. 2020). Acute and sublethal effects to crustacean Daphnia similis, such as reproduction rate reduction, dye deposition in eggs, and malformations, were also described due to exposure to effluents (Garcia et al. 2020). Physiological alterations in Danio rerio fish embryos, disturbance in swimming activity, included skeletal deformities, and toxic cell effects due the exposure to several types of dyes were reported (Basic Red 51; Disperse Red 60, Disperse Red 73; Disperse Red 78; Congo Red) (Abe et al. 2018; Meireles et al. 2018; Hernández-Zamora and Martínez-Jerónimo 2019).
Other effects were also demonstrated to distinct classes of organisms including Vibrio fischeri luminescent bacteria, Brachionus plicatilis rotifer, Scenedesmus obliquus, and Desmodesmus subspicatus alga (Borrely et al. 2016; Liang et al. 2018; Cai et al. 2020). Mutagenic activity has been observed in surface water and in textile wastewater of industrial areas in different regions of India. Disperse dyes also contributed up to 44% to the observed mutagenicity close to a conglomerate of textile industries in Brazil (Mathur et al. 2005; Vacchi et al. 2017; Khan et al. 2019).
Ecotoxicological data allow the assessment of the impact of hazardous pollutants on aquatic environment. Toxicity assessment using representative organisms from different trophic levels is recommended, allowing a more complete analysis of the impact of pollutants on ecosystems. Bacteria, algae, daphnids, and fishes are examples of aquatic organisms widely used in environmental quality monitoring studies and toxicity assessment of various types of contaminants.
Furthermore, ecotoxicological assays with mollusks, such as gastropods, have been successfully used in recent years as pollution indicators (Tallarico et al. 2016; Oliveira-Filho et al. 2017). Assays with Biomphalaria glabrata were conducted by Tallarico et al. (2014) to assess the toxicity of chromium and natural water samples, and recently the same organism was used for risk assessment of iron oxide nanoparticles in an aquatic ecosystem (Caixeta et al. 2021).
In this context, the present study aimed to evaluate acute toxicity, developmental and sublethal effects of reactive Red 239 dye in aqueous solution, and textile effluents in the aquatic organisms from distinct trophic levels (Vibrio fischeri bacteria, Daphnia similis cladoceran, and Biomphalaria glabrata snail). Mutagenicity for Salmonella typhimurium bacteria was also evaluated herein. The reactive Red 239 dye effects were measured due to its high relevance and use during cotton dyeing.
Material and methods
Preparation of samples
The reactive Red 239 dye (CAS 89157-03-9; C31H19ClN7Na5O19S6; molecular weight 1136.32 g mol−1) in aqueous solution was applied in ecotoxicity and mutagenicity assays. The molecular structure of Reactive Red 239 dye (RR239) was presented at Fig. 1. Dye solutions were prepared in distilled water and natural water (the same used in the culture of each test organism) and used in the dilutions for toxicity assays. The maximum concentration evaluated in acute assays was 564 mg L−1, the same usually found in the final effluent (this value can be calculated according to the percentage of depletion of the dye fixed to the fiber in the dyeing process).
Textile effluents samples were obtained from the cotton dyeing process in a pre-industrial textile laboratory. Samples contained all the compounds used in the dyeing cotton, including auxiliaries, such as surfactants, acids, salts, and reactive dyestuffs and effluents corresponded to all steps of process, such as pre-treatment, dyeing, and after-treatment. Chemicals compounds used in the cotton dyeing: pre-treatment (bleaching) (sulfuric acid 98%, sodium hydroxide 98%, sodium metasilicate, hydrogen peroxide 50%, sequestering, nonionic detergent, and catalase enzyme), dyeing (sodium hydroxide 98%, sodium carbonate 98%, sodium chloride 98%, and reactive dyes (included RR239 dye)), and after-treatment (washing) (acetic acid 98% and sequestering). All auxiliaries and reactive dyes were used with no previous purification and were supplied by Golden Technology and Labsynth. All steps, pre-treatment, dyeing, and after-treatment, were conducted following the literature about cotton dyeing process with reactive dyestuffs (Rosa et al. 2019; Rodrigues et al. 2020).
Bioassays: ecotoxicity and mutagenicity
The assays performed for both RR239 dye and textile effluent samples included acute toxicity assays with Daphnia similis (Claus, 1876) crustacean, Vibrio fischeri (Beijerinck, 1889) bacteria, and Biomphalaria glabrata (Say, 1818) snails. The developmental effects in B. glabrata embryos were also evaluated. The mutagenicity was assessed with Salmonella/microsome assay, with Salmonella enterica ser. Typhimurium TA98, TA100, and YG1041 strains. Chronic assays with lethal and sublethal effects for Daphnia similis were included and performed only for textile effluents samples.
Acute toxicity assays
Vibrio fischeri bacteria were acquired lyophilized and stored in a freezer at −18°C. For the assays, the bacteria were reconstituted in reactivation solution, and the tests were performed in Microtox®, M-500, Microbics system, at a temperature of 3°C ± 0.2. The bacteria luminescence was analyzed before and after 15 min of exposure to the samples; toxicity was evidenced by loss of luminescence.
The concentrations of RR239 in aqueous solution ranged between 2.8 and 23 mg L−1 and for textile effluent between 2.5 and 20.5%, both a dilution factor of 2×. The concentrations were established after preliminary assays. The assays were performed in triplicate, with a minimum of four concentrations. The negative control with diluent solution (NaCl 2%) and a positive control with phenol were also performed. The methodology was in accordance with Brazilian Technical Standard Methods NBR 15411:2012 (Associação Brasileira de Normas Técnicas 2012).
Daphnia similis were maintained at 20°C ± 2, in 16:8 h (light/dark) photoperiod, daily fed with the green algae Pseudokirchneriella subcapitata and a mixture of fish food and yeast. For the ecotoxicity assays, the juvenile individuals (6–24 h) were exposed to the samples for 48 h, with controlled temperature and photoperiod (the same used for maintaining the organisms); no food was added. The observed effect after the exposure period was immobility.
The concentrations of RR239 in aqueous solution ranged between 282 and 564 mg L−1. The textile effluent concentration ranged between 2.5 and 15%. A minimum of five concentrations were evaluated per assay. The concentrations were established after preliminary assays. A negative control only with natural water (the same used in the D. similis culture) and a positive control with potassium chloride were also performed. The assays were carried out in triplicate, with 20 organisms per concentration in each assay. The methodology was in accordance with NBR 12713: 2016 (Associação Brasileira de Normas Técnicasxx 2016).
Acute toxicity results were expressed as EC50, the median concentration to observe an effect for 50% of all exposed organisms, which means that the lower the EC50 number, the higher is the toxicity level. Statistics were applied according to the standard methods recommendation. The linear regression for V. fischeri was used, wherein the values of EC50 (%) were obtained from a statistical procedure based on the gamma effect (γ) and the sample concentration (C%); the gamma value (γ) was calculated from the ratio of lost and remaining luminescence. D. similis data was obtained by the Trimmed Spearman-Karber method (Hamilton et al. 1977).
The significance of differences between average values for the control and the experimental treatments were evaluated by analysis of variance (ANOVA). When the ANOVA revealed significant differences among treatments (p <0.05), a post hoc Dunnett’s test was performed for D. similis and a post hoc Tukey test was performed for V. fischeri.
Chronic assays: lethal and sublethal effects for Daphnia similis
In the present study, the lethal and sublethal effects analyses for Daphnia similis exposed to textile effluents were also carried out. Based on the acute toxicity assays and the EC50 value, the concentrations used for chronic exposure were established in a range between 0.5 and 9%. Ten juveniles (6 to 24 h) were exposed to effluent samples. The organisms were fed daily, the exchange of solutions occurred every two days, and the number of adults and neonates were counted every day. After exposure to samples for 21 days, the survival, reproduction rate, and body length were measured. A negative control only with natural water (the same used in the D. similis culture) was also performed. Chronic assays were carried out in two replicates. The assays were performed according to the OECD guideline 211 (OECD 2012).
Following the performance criteria described by OECD (2012), the chronic assays were validated when the mortality in the control group did not exceed 20% at the end of the test, and the mean number of living offspring produced per parent animal surviving at the end of the test was > 60.
Body length measures were carried out using a Leica MZ95 magnifier (3.2×), Leica DFC 295 camera, and the Leica Application Suite V4.12.0 software. The measurement of organisms, adopted in this study, was performed from the base of the antenna to the beginning of the tail, in millimeters (Fig. 5).
In sublethal responses the statistical analyses with ANOVA were applied. When the ANOVA revealed significant differences among treatments (p <0.05), a post hoc Dunnett’s test was performed. Furthermore, the NOEC (no observed effect concentration) and LOEC (lowest observed effect concentration) were calculated for chronic assays with this method.
The EC10, EC20, and EC50% of chronic assays were calculated using regression analysis obtained by a three-parameter-logistic-fit (sigmoidal logistic model) using the software Sigma Plot. Normality was evaluated by Shapiro-Wilk test.
Freshwater mollusks assays
Biomphalaria glabrata specimens were obtained from a population of pigmented wild-type strain, originally from Barreiro de Baixo (Minas Gerais, Brazil), and have been kept under laboratory conditions (Laboratory of Parasitology, Butantan Institute, São Paulo, Brazil). A summary of holding conditions requirements and toxicity assays with snails and embryos was described in Tallarico et al. (2016).
Adult snails and embryos were exposed to reactive Red 239 dye and textile effluent samples for 24 h to determine LC50 values to adults (concentration lethal to 50% of the exposed organisms) and EC50 to embryos (median effective concentration in terms of lethality and malformations). For more assay’s details, see Tallarico et al. (2014) and Tallarico (2015). Concentrations of RR239 dye between 5.64 and 564 mg L−1 and textile effluent samples of 1, 10, 50, and 100% were established to expose adult snails and egg masses. A negative control group was maintained in filtered and dechlorinated tap water, and a positive control group was exposed to sodium dodecyl sulfate, both under the same experimental conditions.
Ten snails per concentration, at least 2 months old and with a shell diameter above 12mm, were used. After exposure, snails were washed and observed daily for 7 days to record the death rate. The death was ascertained by the absence of heart beatings for 2 min and a pale color of the shell as consequence of hemolymph loss. The death values were used for LC50 analyses.
Non-damaged egg masses were collected from transparent plastic sheets (as substrate for oviposition), maintained in Petri dishes with filtered and dechlorinated tap water. Embryos were observed at stereomicroscope and classified according to developmental stage as described by Camey and Verdonk (1970): blastulae (0–15 h after the first egg cleavage), gastrulae (24–39 h), trochophore (48–87 h), and veliger (96–111 h) stages. A minimum of five egg masses, with at least 100 non-damaged embryos of each stage, were exposed to the dye’s dilution. At the end of the exposure, egg masses were washed with dechlorinated tap water and observed daily for sublethal (malformation effects) and lethal effects for 7 days. Effects on embryonic development were classified as described by Tallarico et al. (2014): normal, dead, and malformed (embryos with multiple affected structures with abnormal development, usually classified as teratomorphic or hydropic). The sum of dead and malformed embryos was used to estimate the EC50 values.
The criteria established for classifying the acute toxicity based on LC50 and EC50 values and classifying the intensity of toxic effects based on lethality and embryonic malformations as endpoints were as follows: (1) non-toxic, 100% of effluent; (2) slightly toxic, 99–50%; (3) toxic, 49–20%; and (4) highly toxic, <20% (Tallarico et al. 2014). Statistics were applied using the Trimmed Spearman-Karber method (Hamilton et al. 1977).
Predicted no-effect concentration (PNEC)
The predicted no-effect concentrations (PNEC) for reactive Red 239 was performed according to European guidelines: European Chemicals Agency- ECHA (2008) and European Commission (2011). Based in these guidelines, a short-term quality standard based on acute toxicity data was carried out (PNECacute) by using the deterministic approach.
The most frequently endpoints used for derivation of PNEC are mortality (LC50), growth (ECx or NOEC), and reproduction (ECx or NOEC), and the PNECs are estimated by division of the lowest value for the toxicity with the relevant assessment factor to extrapolate to an environmentally protective concentration. (When only short-term toxicity data are available for three trophic levels organisms an assessment factor of 1000 will be applied to the lowest L(E)C50 of the relevant available toxicity data. The use of a factor of 1000 on short-term toxicity data is a conservative and protective factor and is designed to ensure that substances with the potential to cause adverse effects are identified in the hazard assessment (European Chemicals Agency, 2008).
The PNEC acute evaluated in this study was based on the EC50 values of three trophic levels (V. fischeri, D. similis, and B. glabrata), and the EC50 of the most sensitive organism was used for PNEC determination, with the respective assessment factor (1000). This data was proposed as PNEC of reactive Red 239.
Mutagenicity assay: Salmonella/microsome
The Salmonella/microsome assay consisted in applying certain amount of a liquid sample (RR239 dye in aqueous solution and textile effluents) to a culture of selected mutant strains of Salmonella enterica serovar Typhimurium, which are unable to synthetize histidine. The test was performed in the presence and absence of a liver homogenate and cofactors to provide exogenous metabolism (S9 mix – S9: lyophilized liver homogenate of Sprague-Dawley rats induced with Aroclor 1254, Moltox Ltd., Boone, NC, USA). The test was proceeded according to the classic Ames test protocol (Maron and Ames 1983; Mortelmans and Zeiger 2000). Briefly, bacteria and sample are added into tubes, with and without S9 mix. Molten top agar is then added; the mixture is vortexed and overlaid onto the surface of a minimal agar plate. After 48–72 h at 37°C, mutant cells’ colonies are counted, and the results are compared to a negative (solvent) control. A significant increase of colonies in relation to the control indicates that there are one or more compounds in the tested sample able to induce mutations, like frameshift or base pair substitution, in the Salmonella DNA and restore its capability of synthetizing histidine.
In this study, three different Salmonella strains were used: TA98 (detects frameshift mutations), TA100 (detects base pair substitutions), and YG1041, a TA98 derivative with high levels of nitroreductase and acetyltransferase activities. We used TA98 and TA100 strains, because they are used in the majority of environmental studies and sensitive enough to detect 93% of the mutagens compared with the five strains recommended by OECD TG471 (Ohe et al, 2004; Williams et al. 2019). We included YG1041 strain to enhance the detection of the azo dye tested. Multiple doses of pure dye using ultrapure water as solvent and negative control were tested initially in duplicate plates. Eight doses varying from 0.28 up to 33.8 mg per plate were evaluated along with negative and positive controls. Subsequently, five doses of effluent samples were tested with the same tester strains in triplicates. Before testing the effluent samples, organic extraction of volumes up to 1 L of effluent were performed using solid phase extraction cartridges (Oasis®, Waters). Elution of extracts was carried out with methanol. Evaporation of the extracts was performed using ultrapure nitrogen gas. One, 2.5, 5, 10, and 20 mL equivalent of effluent per plate were evaluated along with negative and positive controls. Dimethyl sulfoxide (Merck) was used as solvent and negative control. Positive controls were standardized in all assays, 0.5 μg/plate of 4-nitroquinoline-1-oxide (4NQO, Alfa Aesar) for TA98 and TA100 without S9, 10 μg/plate of 4-nitro-o-phenylenediamine (4NOP, Sigma-Aldrich) for YG1041 without S9, and 2-aminoanthracene (2AA, Sigma-Aldrich) for all strains tested with S9, 2.5 μg/plate for TA98 and TA100, and 0.0625 μg/plate for YG1041.
The mutagenicity was assessed using analysis of variance (ANOVA) for confirmation of statistical differences among the negative control and tested doses. Samples were considered positive when a significant ANOVA and a dose response were observed (p < 0.05) along with the ratio greater than 2 between any tested doses in relation to the negative control. Calculations were done using the Salanal computer program (Integrated Laboratory Systems, Research Triangle Park, NC).
Physico-chemical parameters
The physico-chemical parameters of samples (effluents and dye in aqueous solution) and natural water were measured by specific electrodes: dissolved oxygen, HQ40d, Hach, and pH-Micronal B474. Total organic carbon (TOC) analyses in effluents samples were performed using a Shimadzu TOC-5000A equipment, and the results were based on total carbon and inorganic carbon values. Chemical oxygen demand (COD) was assessed by a colorimetric method using a spectrophotometer.
Results and discussion
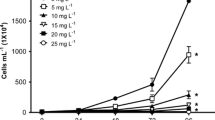
Acute toxicity effects, expressed by EC50 means (median effective concentration), of reactive Red 239 dye and textile effluents to V. fischeri, D. similis, and B. glabrata (adults and embryos) are reported in Table 1. D. similis immobility (%) values of negative control and different concentrations to RR239 and textile effluent were reported in Fig. 2A–B, while bioluminescence inhibition (%) in V. fischeri were reported in Fig. 3A–B. Physico-chemical parameters of samples were displayed in Table 2. Developmental effects for snails are showed in Fig. 4, and lethal and sublethal effects for Daphnia similis after 21 days of exposure to textile effluent are summarized in Table 3 and Fig. 5.
Biomphalaria glabrata exposed to textile effluents. Adults and embryos in static assay (24-h exposure) observed daily for 8 days. A Snail exposed to 50% of effluent (during first 24 h), and B animal after exposure with penile overexposure (48 h) (pe) penis. C Trochophore stage (48 h), D veliger stage (72 h) exposed to effluent, and E veliger stage exposed to reactive Red 239 dye, (ad) abnormal development, (sm) shell malformation, and (de) died embryo
The mutagenicity results of Salmonella enterica ser. Typhimurium are reported in Table 4, and all the results data are presented in the Supplementary Material (SM1).
Acute toxicity results about RR239 dye displayed in Table 1 showed that V. fischeri bacteria was the most sensitive, with EC50 values between 8.57 and 12.06 mg L−1 (EC50 means= 10.14 mg L−1 ± 1.76), followed by D. similis crustacean with EC50 = 389.42 mg L−1 ± 3.77 (average of three assays) and B. glabrata snails LC50 =517.19 mg L−1. Acute effects in B. glabrata embryos were also detected, with EC50 between 116.41 mg L−1 in gastrulae stage and 124.14 mg L−1 in blastulae.
The immobility (%) for D. similis (Fig. 2A) was higher with the increase in RR239 concentration. The RR239 did not significantly induce toxic effect comparing to control until 282 mg L−1, while at 100% immobility was obtained with 479.4 mg L−1 (p <0.05). And the bioluminescence inhibition (%) for V. fischeri (Fig. 3A) was also verified with increasing concentration: 2.87 mg L−1 resulted in 22% bioluminescence inhibition while 23 mg L−1 more than 75% of inhibition (p <0.05).
Similar results have been shown when comparisons are made among distinct trophic levels of organisms: when studying toxic effects of azo direct dye, Congo Red Hernández-Zamora and Martínez-Jerónimo (2019) observed that D. magna was tolerant to high dye concentrations, LC50= 322.9 mg L−1, but for cladoceran Ceriodaphnia rigaudi, LC50 was significantly lower 62.92 mg L−1, and P. subcapitata was the most sensitive species to IC50 = 3.11 mg L−1. The same pattern was obtained in a study with Solvent Yellow 34 Auramine dye; Raphidocelis subcapitata alga was the most sensitive (EC50= 0.3 mg L−1) when compared to Daphnia similis and Danio rerio (Azevedo et al. 2020).
To compare the toxic effects of textile dyes, Table 5 shows a compilation of toxicity data reported in literature and in the present study for several dye classes. These data and our results confirm that the multiple toxic effects depend on the type of dye and species studied, as well as the interaction dye-organism. Note that the acute toxicity for the same dye class widely varies according to the organism test. Croce et al. (2017) investigated the aquatic toxicity of 42 commercial dye formulations, and only 9 formulations were toxic for daphnids, with LD50 values lower than 100 mg L−1, while 30 dyes were toxic for algae. Furthermore, the same study showed that most of the dyes have high similarity to other dyes of the same chemical class.
Furthermore, a short-term PNECacute of 10.14 μg L−1 was proposed for reactive Red 239, based on the lowest EC50 (V. fischeri = 10.14 mg L−1). In this case, the factor 1000 was applied, since there were only acute toxicity results for the three trophic levels for reactive Red 239, (following the proposed for European Chemicals Agency 2008 and European Commission 2011). A PNEC of 2.3 μg L−1 for azo dyes and 1 μg L−1 for non-sulfonated dyes (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7) using the deterministic approach were determined by the Canadian Government guidelines (Government of Canada 2016, 2020). A PNECacute of 1.8 μg L−1 (D. similis) was proposed for Disperse Red 1 by Vacchi et al. (2016). PNEC chronic values of 0.92 μg L−1 for Auramine and 4.6 μg L−1 for Auramine O (R. subcapitata) and for 0.06 μg L−1 for Disperse Red 1 were described by Azevedo et al. (2020) and Vacchi et al. (2016), respectively.
Concerning predicted environmental concentration (PEC), aquatic exposure analyses were conducted for scenarios representing potential major environmental releases due to industrial activities, and the likelihood of the PECs exceeding the predicted no-effect concentrations (PNECs) for azo direct dyes and azo reactive dyes was found to be low (Department of the Environment, Canada 2015).
Regarding the results of textile effluent samples, the acute toxicity results displayed in Table 1 showed high toxicity level for assessed organisms, with EC50s below 15%. For bacteria, the EC50 values were between 6.79 and 8.89% (EC50 means = 8.18% ± 1.20); for D. similis, about three assays, the values were 8.97, 9.28, and 9.61%; EC50 means= 9.28% ± 0.32. The samples were also toxic to B. glabrata adults, LC50 = 14.05% and for embryos, with EC50 values around 7% to blastulae and gastrulae stages, and 20% to trochophore and veliger. The immobility (%) values of D. similis displayed in Fig. 2B showed an increase in effect with increasing concentration; 2.5 and 5% concentrations did not result in an effect for D. similis, while with 15%, it was possible to observe immobility in 100% of the organisms (p <0.05). When analyzing the bioluminescence inhibition (%) in V. fischeri (Fig. 3B), the lowest concentration (2.55%) indicated 20% of inhibition, approximately, while in the highest tested concentration, this bioluminescence inhibition was 77.4% (p <0.05).
Acute effects for different organisms when exposed to textile effluents were described by Castro et al. (2019), EC50 = 7.2% for V. fischeri, 24.2% for D. magna exposed to a textile effluent from a Portuguese textile manufacturer, and Liang et al. (2018), EC50 = 4.70% for D. subspicatus alga when assessing textile wastewater in China. Borrely et al. (2016) evaluated the textile effluents containing reactive Blue 222 dye and evidenced acute effects to B. plicatilis rotifer EC50 = 8.73% and D. similis EC50 = 9.81%.
Physico-chemical parameters were determined for water, effluent, and RR239 (Table 2). Regarding our data set, some main parameters were measured only for textile effluent: total organic carbon, 287.85 mg L−1; chemical oxygen demand, 430 mg L−1 and pH 7.35. Dissolved oxygen and pH were monitored during daphnids assays for all running concentrations and negative control tubes (natural water was used for negative control, dilutions, and D. similis cultivation into laboratory). The values are following environmental regulations, confirming that toxicity and negative effects to organisms evaluated are related to critical characteristics of studied samples.
Different effects were observed in survival, body length, and reproduction on D. similis exposed for 21 days to the effluent samples (Table 3, Fig. 5). The survival (parental organisms) (%) was lower with the increase on the effluent concentration, and with 5% concentration, 100% of mortality was observed. Body length values increased in 1.5 and 3% concentrations when compared to the control (mean = 1.64 mm ± 0.21), and 2.09±0.06 (3%). In relation to reproduction rate, the average number of neonates obtained in the lower concentrations in textile effluent samples (0.5% and 1.5%) was higher than the control, and with higher concentration (3%), the reproduction rate was lower. And in the same concentration (3%), egg malformations were observed in 50% of the survival parental organisms after 21 days of exposure. These results can be explained by the higher concentration of organic matter in the effluent samples. The NOEC and EC10 values for textile effluent was 3%, and the other parameters calculated were also similar, EC20 = 3.03% and EC50 = 2.99%. When comparing the values of EC50% to acute and chronic assays, it was possible to observe that the EC50% chronic was three times smaller than EC50% acute. These results confirm the importance of chronic assessments since they demonstrate long-term effects on populations.
Effects on D. similis reproduction in chronic assays were also demonstrated for Solvent Auramine, Auramine O, and Disperse Red 1 dyes. For Auramine dyes, 6- to 10-fold increase in toxicity from the acute test to the chronic test (Azevedo et al. 2020) and reactive Red 1 observed an almost a 50-fold increase in toxicity from the acute test to the chronic test (Vacchi et al. 2016). Furthermore, previous work showed other adverse effects due to the exposure to textile effluents for D. similis, including egg malformations and dye deposition in their filter system (Garcia et al. 2020). These data and the observed effects in present study (Table 3) confirmed the relevance of sublethal effects in daphnids as an indicator of chronic toxicity.
Data was also obtained with B. glabrata adults and embryos exposed to textile effluents (Fig. 4). B glabrata adults snails exposed to 50% of the effluent remained lethargic, releasing large amounts of hemolymph, with exposed reproductive system, penis erection maintained during the entire exposure (Fig. 4A), and even after water replacement (Fig. 4B) until death. A long-lasting effect on reproduction may result in reduced reproductive capacity, for example, reduced copulation even with erect penises. This event was observed in snails exposed to methiothepin, with preputium erection that contains the penis (Muschamp and Fong 2001).
Shell malformation and abnormal development after 72 h were observed. Embryos at blastula and gastrula stages died within the first 24 h after exposure to 100 and 50% of textile effluent. Trochophore died within the first 24 h and at 50% became malformed with impaired hatching (Fig. 4C) and died after 48 h, without hatching. Veliger stage first became malformed within 24 h and fails to hatch at concentrations of 100 and 50% (Fig. 4D). It is important to note that the toxic effect observed with the red dye for the embryos was different from that with the effluents. In the dye assays, we could observe high deposition of the substance under the spawning capsule, and although of the embryos develop over the period of analysis, they did not hatch (Fig. 4E). Adverse effects like this can occur with the presence of the dye, causing smothering organisms leading to death.
Adverse effects and malformations were also observed in clawed frog embryos (Silurana tropicalis) exposed to Bismarck Brown Y dye, including asymmetric eye formation, tails with axial abnormalities, heart malformation, head, and face deformities (concentration as from of 100 ppm) (Soriano et al. 2014). For zebrafish embryos, exposure to Congo Red dye induced skeletal deformities, stopped larvae hatching and caused lack of heart beating between 6.25 and 500 mg L−1 (Hernández-Zamora and Martínez-Jerónimo 2019).
Furthermore, other alterations reported in literature include planarian Girardia tigrina newborns exposed to Disperse Red 1 that presented uncoordinated movements, irregular twists, colored skin, and increased mucous production (Ribeiro and Umbuzeiro 2014). Zebrafish larvae exposed to dye Basic Red 51 showed decrease in consumption of energy and swimming activity (Abe et al. 2018). In S. tropicalis larvae, Azo Dye Disperse Yellow 7 induced cellular stress and interfered with both androgen signaling and biosynthesis in developing (Mathieu-Denoncourt et al. 2014).
Assays with the effluent showed more accentuated effects (Table 1; Fig. 4). With B. glabrata embryos exposure, we observed malformation induction and death in the first days of development. The differential sensitivity of the organisms to the dye and effluent may be related to the compounds that exist in the effluent, with more complex compounds that can interact with the organisms, as is the case of the surfactant present in effluent samples (fatty alcohol ethoxylate non-ionic). Surfactants and other auxiliaries are applied in different steps of dyeing. Usually for dyeing 1 kg of cotton fiber, at least 1g L−1 of nonionic surfactant is added to the process in addition to other auxiliary products, such as dispersant and sequestering agents, which contain surfactants in their formulations (Rosa et al. 2019; Garcia et al. 2020).
Previous studies reported acute toxic effects of surfactants to different aquatic organisms including algae, daphnids, and bacteria (Lechuga et al. 2016; Fernández Serrano et al. 2014). For V. fischeri, for example, values of EC50 mg L−1 = 0.35 (sodium dodecyl sulfate) and 1.92 (amine-oxide-based surfactant AO-R12) were described by Romanelli et al. (2004) and Fernández Serrano et al. (2014).
Mutagenicity was not detected in the Salmonella/microsome assay either with the pure dye or the effluent samples (Table 4, Supplementary Material, SM1). However, it should be noted that toxicity was observed for the effluent sample at dose 20 mL to Salmonella enterica ser. Typhimurium YG1041 strain. Although mutagenicity was not detected with the specific conditions of this study, the literature shows that several textile dyes are mutagenic for Salmonella strains TA98 and YG1041, such as Acid Black 210, Disperse Blue 373, and Disperse Red 13, as well as mutagenic activity observed in surface waters and textile wastewaters (Chequer et al. 2015; Rocha et al. 2017; Vacchi et al. 2017; Khan et al. 2019).
Conclusion
The ecotoxicological data indicated acute and developmental effects due the exposure to textile effluents and reactive dye (RR239) for different trophic level species. It is important to highlight that the acute effects varied according to the interaction of the species with RR239, following decreasing levels of effects from bacteria to B. glabrata snail (sensibility/acute effects: V. fischeri > D. similis > B. glabrata). Furthermore, we also detected high acute toxicity level of textile effluents and sublethal effects, such as body length increased to D. similis and malformation in B. glabrata embryos exposed to effluent samples. The results obtained herein are important tools for understanding the biological effects related to these hazardous mixtures of chemical substances, as observed by comparing acute data among dye solutions and effluent samples. The ecotoxicological analyses presented in this study are important to understand the behavior of these contaminants (RR239 and textile effluent) in aquatic ecosystems and can contribute for the pollution control, water quality needs and treatment, and environmental protection.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abe FR, Soares AMVM, Oliveira DP, Gravato C (2018) Toxicity of dyes to zebrafish at the biochemical level: cellular energy allocation and neurotoxicity. Environ Pollut 235:255–262. https://doi.org/10.1016/j.envpol.2017.12.020
Associação Brasileira de Normas Técnicas (2012) Ecotoxicologia aquática – Determinação do efeito inibitório de amostras de água sobre a emissão de luz de Vibrio fischeri. ABNT NBR 15411, Rio de Janeiro.
Associação Brasileira de Normas Técnicas (2016) Ecotoxicologia aquática – Toxicidade Aguda- Método de ensaio com Daphnia spp (Crustacea, Cladocera). ABNT NBR 12713, Rio de Janeiro.
Azevedo CCJ, Oliveira R, Suares-Rocha P, Sousa-Moura D, Li AT, Grisolia CK, Umbuzeiro GA, Montagner CC (2020) Auramine dyes induce toxic effects to aquatic organisms from different trophic levels: an application of predicted non-effect concentration (PNEC). Environ Sci Pollut Res 28:1866–1877. https://doi.org/10.1007/s11356-020-10462-3
Borrely SI, Morais AV, Rosa JM, Badaró-Pedroso C, Pereira MC, Higa MC (2016) Decoloration and detoxification of effluents by ionizing radiation. Radiat Phys Chem 124:198–202. https://doi.org/10.1016/j.radphyschem.2015.11.001
Cai H, Liang J, Ning X, Lai X, Li Y (2020) Algal toxicity induced by effluents from textile dyeing wastewater treatment plants. J Environ Sci 91:199–208. https://doi.org/10.1016/j.jes.2020.01.004
Caixeta MB, Araújo PS, Rodrigues CC, Gonçalves BB, Araújo OA, Bevilaqua GB, Malafaia G, Silva LD, Rocha TL (2021) Risk assessment of iron oxide nanoparticles in an aquatic ecosystem: a case study on Biomphalaria glabrata. J Hazard Mater 401:123398. https://doi.org/10.1016/j.jhazmat.2020.123398
Camey T, Verdonk NH (1970) The early development of the snail Biomphalaria glabrata (Say) and the origin of the head organs. Neth J Zool 20:93–121. https://doi.org/10.1163/002829670X00097
Castro AM, Nogueira V, Lopes I, Santos TR, Pereira R (2019) Evaluation of the potential toxicity of effluents from the textile industry before and after treatment. Appl Sci 9(18):3804. https://doi.org/10.3390/app9183804
Chequer FMD, Lizier TM, Felício R, Zanoni MVB, Debonsi HM, Lopes NP, Oliveira DP (2015) The azo dye Disperse Red 13 and its oxidation and reduction products showed mutagenic potential. Toxicol in Vitro 29(7):1906–1915. https://doi.org/10.1016/j.tiv.2015.08.001
Chiang TL, Wang YC, Ding WH (2012) Trace determination of Rhodamine B and Rhodamine 6G dyes in aqueous samples by solid-phase extraction and high-performance liquid chromatography coupled with fluorescence detection. J Chin Chem Soc 59:515–519. https://doi.org/10.1002/jccs.201100318
Croce R, Cinà F, Crispeyn ALG, Cappelli CI, Vian M, Maiorana S, Benfenati E, Baderna D (2017) Aquatic toxicity of several textile dye formulations: acute and chronic assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol Environ Saf 144:79–87. https://doi.org/10.1016/j.ecoenv.2017.05.046
Darsana R, Chandrasehar G, Deepa V, Gowthami Y, Chitrikha T, Ayyappan S, Goparaju A (2015) Acute Toxicity Assessment of Reactive Red 120 to Certain Aquatic Organisms. Bulletin of Environmental Contamination and Toxicology 95:582–587. https://doi.org/10.1007/s00128-015-1636-z
Department of the Environment, Canada (2015) Summary of the screening assessment of azo metal complexes and other substances https://canadagazette.gc.ca/rp-pr/p1/2015/2015-04-04/html/notice-avis-eng.html#ne3
European Chemicals Agency- ECHA (2008) Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterization of dose [concentration]-response for environment. https://echa.europa.eu/guidance-documents/guidance-on-information-requirements-and-chemical-safety-assessment Accessed 10 April 2021
European Commission (2011) Common implementation strategy for the Water Framework Directive (2000/60/EC). Guidance Document No. 27. Technical guidance for deriving Environmental Quality Standards. European Union. 204 pp.
Fernández Serrano M, Jurado E, Fernández Arteaga A, Ríos F, Lechuga M (2014) Ecotoxicological assessment of mixtures of ether carboxylic derivative and amine oxide based nonionic surfactants on the aquatic environment. J Surfactant Deterg 17:1161–1168. https://doi.org/10.1007/s11743-014-1621-2
Garcia VSG, Rosa JM, Borrely SI (2020) Toxicity and color reduction of a textile effluent containing reactive red 239 dye by electron beam irradiation. Radiat Phys Chem 172:108765. https://doi.org/10.1016/j.radphyschem.2020.108765
Ghaly AE, Ananthashankar R, Alhattab M, Ramakrishnan VV (2014) Production, Characterization and Treatment of Textile Effluents: A Critical Review. J Chem Eng Process Technol 5:1–18. https://doi.org/10.4172/2157-7048.1000182
Government of Canada (2016) Screening assessment aromatic azo and benzidine-based substance grouping certain azo solvent dyes. Environment and Climate Change Canada Health Canada. http://www.publications.gc.ca/site/eng/9.804137/publication.html Accessed 10 April 2021
Government of Canada (2020) Screening Assessment Triarylmethanes Group. Environment and Climate Change Canada Health Canada. https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/screening-assessment-triarylmethanes-group.html Accessed 10 April 2021
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11(7):714–719. https://doi.org/10.1021/es60130a004
Hernández-Zamora M, Martínez-Jerónimo F (2019) Congo red dye diversely affects organisms of different trophic levels: a comparative study with microalgae, cladocerans, and zebrafish embryos. Environ Sci Pollut Res 26:11743–11755. https://doi.org/10.1007/s11356-019-04589-1
Kant R (2012) Textile dyeing industry and environmental hazard. Nat Sci 4:22–26. https://doi.org/10.4236/ns.2012.41004
Khan S, Anas M, Malik A (2019) Mutagenicity and genotoxicity evaluation of textile industry wastewater using bacterial and plant bioassays. Toxicol Rep 6:193–201. https://doi.org/10.1016/j.toxrep.2019.02.002
Lechuga M, Fernández-Serrano M, Jurado E, Núñez-Olea J, Ríos F (2016) Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol Environ Saf 125:1–8. https://doi.org/10.1016/j.ecoenv.2015.11.027
Liang J, Ning X, Sun J, Song J, Lu J, Cai H, Hong Y (2018) Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand. Ecotoxicol Environ Saf 166:56–62. https://doi.org/10.1016/j.ecoenv.2018.08.106
Maron DM, Ames BN (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215. https://doi.org/10.1016/0165-1161(83)90010-9
Mathieu-Denoncourt J, Martyniuk CJ, Solla SR, Balakrishnan VK, Langlois VS (2014) Sediment contaminated with the azo dye disperse yellow 7 alters cellular stress- and androgen-related transcription in Silurana tropicalis larvae. Environ Sci Technol 48(5):2952–2961. https://doi.org/10.1021/es500263x
Mathur N, Bhatnagar P, Nagar P, Bijarnia MK (2005) Mutagenicity assessment of effluents from textile/dye industries of Sanganer, Jaipur (India): a case study. Ecotoxicol Environ Saf 61:105–113. https://doi.org/10.1016/j.ecoenv.2004.08.003
Meireles G, Daam MA, Sanches ALM, Zanoni MVB, Soares AMVM, Gravato C, Oliveira DP (2018) Red disperse dyes (DR 60, DR 73 and DR 78) at environmentally realistic concentrations impact biochemical profile of early life stages of zebrafish (Danio rerio). Chem Biol Interact 292:94–100. https://doi.org/10.1016/j.cbi.2018.07.007
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455:29–60. https://doi.org/10.1016/s0027-5107(00)00064-6
Muschamp JW, Fong PP (2001) Effects of the serotonin receptor ligand methiothepin on reproductive behavior of the freshwater snail Biomphalaria glabrata: reduction of egg laying and induction of penile erection. J Exp Zool 289(3):202–207
OECD (Organization for Economic Co-operation and Development) (2012) Test No. 211: Daphnia magna reproduction test. OECD Guidelines for the Testing of Chemicals, Section 2. https://doi.org/10.1787/9789264185203-en
Ohe T, Watanabe T, Wakabayashi K (2004) Mutagens in surface waters: a review. Mutat Res 567:109–149. https://doi.org/10.1016/j.mrrev.2004.08.003
Oliveira-Filho EC, Nakano E, Tallarico LF (2017) Bioassays with freshwater snails Biomphalaria sp.: from control of hosts in public health to alternative tools in ecotoxicology. Invertebr Reprod Dev 61(1):49–57. https://doi.org/10.1080/07924259.2016.1276484
Rawat D, Mishra V, Sharma RS (2016) Detoxification of azo dyes in the context of environmental processes. Chemosphere 155:591–605. https://doi.org/10.1016/j.chemosphere.2016.04.068
Ribeiro AR, Umbuzeiro GA (2014) Effects of a textile azo dye on mortality, regeneration, and reproductive performance of the planarian, Girardia tigrina. Environ Sci Eur 26(22):1–8. https://doi.org/10.1186/s12302-014-0022-5
Rocha OP, Cesila CA, Christovam EM, Barros SBM, Zanoni MVB, Oliveira DPO (2017) Ecotoxicological risk assessment of the “Acid Black 210” dye. Toxicology 376:113–119. https://doi.org/10.1016/j.tox.2016.04.002
Rodrigues J, Hatami T, Rosa JM, Tambourgi EB, Mei LHI (2020) Photocatalytic degradation using ZnO for the treatment of RB 19 and RB 21 dyes in industrial effluents and mathematical modeling of the process. Chem Eng Res Des 153:294–305. https://doi.org/10.1016/j.cherd.2019.10.021
Romanelli MF, Moraes MCF, Villavicencio ALCH, Borrely SI (2004) Evaluation of toxicity reduction of sodium dodecyl sulfate submitted to electron beam radiation. Rad Physics and Chem 71:411–413. https://doi.org/10.1016/j.radphyschem.2004.03.038
Rosa JM, Garcia VSG, Boiani NF, Melo CG, Pereira MCC, Borrely SI (2019) Toxicity and environmental impacts approached in the dyeing of polyamide, polyester and cotton knits. J of Environ Chem Engin 7:102973. https://doi.org/10.1016/j.jece.2019.102973
Soriano JJ, Mathieu-Denoncourt J, Norman G, Solla SR, Langlois VS (2014) Toxicity of the azo dyes Acid Red 97 and Bismarck Brown Y to Western clawed frog (Silurana tropicalis). Environ Sci Pollut Res 21:3582–3591. https://doi.org/10.1007/s11356-013-2323-4
Tallarico LF (2015) Freshwater gastropods as a tool for ecotoxicology assessments in Latin America. Am Malacol Bull 33(2):1–7. https://doi.org/10.4003/006.033.0220
Tallarico LF, Borrely SI, Hamada N, Grazeffe VS, Ohlweiler FP, Okazaki K, Granatelli AT, Pereira IW, Pereira CA, Nakano E (2014) Developmental toxicity, acute toxicity and mutagenicity testing in freshwater snails Biomphalaria glabrata (Mollusca: Gastropoda) exposed to chromium and water samples. Ecotoxicol Environ Saf 110:208–215. https://doi.org/10.1016/j.ecoenv.2014.09.005
Tallarico LF, Miyasato PA, Nakano E (2016) Rearing and maintenance of Biomphalaria glabrata (Say,1818): adults and embryos under laboratory conditions. Ann Aquac Res 3(1):1013
Textile Colourant Market (2017) Technical textiles application segment expected to expand at a moderate CAGR over the forecast period: Global Industry Analysis and Opportunity Assessment, 2016-2026. REP-GB-3776. https://www.futuremarketinsights.com/reports/textile-colourant-market. Accessed 2 Feb 2021
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Scien of the Total Environ 717:137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Vacchi FI, Ohe PCV, Albuquerque AF, Vendemiatti JAS, Azevedo CCJ, Honório JG, Silva BF, Zanoni MVB, Henry TB, Nogueira AJ, Umbuzeiro GA (2016) Occurrence and risk assessment of an azo dye - the case of Disperse Red 1. Chemosphere 156:95–100. https://doi.org/10.1016/j.chemosphere.2016.04.121
Vacchi FI, Vendemiatti JAS, Silva BF, Zanoni MVB, Umbuzeiro GA (2017) Quantifying the contribution of dyes to the mutagenicity of waters under the influence of textile activities. Sci Total Environ 601-602:230–236. https://doi.org/10.1016/j.scitotenv.2017.05.103
Williams RV, DeMarini DM, Stankowski LF Jr, Escobar PA, Zeiger E, Howe J, Elespuru R, Cross KP (2019) Are all bacterial strains required by OECD mutagenicity test guideline TG471 needed? Mutat Res Gen Tox En 848:503181. https://doi.org/10.1016/j.mrgentox.2019.503081
Yousef S, Tatariants M, Tichonovas M, Sarwar Z, Jonuškienė I, Kliucininkas L (2019) A new strategy for using textile waste as a sustainable source of recovered cotton. Resour Conserv Recycl 145:359–369. https://doi.org/10.1016/J.RESCONREC.2019.02.031
Zaharia C, Suteu D (2012) Textile organic dyes – characteristics, polluting effects and separation/elimination procedures from industrial effluents – a critical overview. In: Puzyn T (ed) Organic Pollutants Ten Years After the Stockholm Convention - Environmental and Analytical Update, pp 55–86. https://doi.org/10.5772/32373
Zocolo GJ, Pilon dos Santos G, Vendemiatti J, Vacchi FI, Umbuzeiro Gisela de Aragão, Zanoni MVB (2015) Using SPE-LC-ESI-MS/MS Analysis to Assess Disperse Dyes in Environmental Water Samples. J Chromatogr Sci 53:1257–1264
Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes) and the International Atomic Energy Agency (IAEA) for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization, VSGG and SIB; formal analysis, VSSG, JMR, SIB, EN, LFT, DAR, and CFS; funding acquisition, SIB, EN, JMR, and DAR; investigation, VSGG, LFT, and CFS; methodology, VSGG, LFT, DAR, and CFS; project administration, SIB; resources, SIB, EN, JMR, and DAR; supervision, SIB; visualization, VSGG and SIB; writing (original draft), VSGG and LFT; writing (review and editing), VSGG, LFT, EN, CFS, DAR, JMR, and SIB.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Garcia, V.S.G., de Freitas Tallarico, L., Rosa, J.M. et al. Multiple adverse effects of textile effluents and reactive Red 239 dye to aquatic organisms. Environ Sci Pollut Res 28, 63202–63214 (2021). https://doi.org/10.1007/s11356-021-15115-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15115-7