Abstract
Laboratory experiments were conducted to evaluate the acute toxicity of a widely used textile dye namely Reactive Red 120 (RR 120) on certain aquatic species such as Pseudokirchneriella subcapitata (green alga), Lemna gibba (duck weed), Daphnia magna (water flea) and Oncorhynchus mykiss (Rainbow trout). All experiments were performed as per the OECD Guidelines for Testing of Chemicals. The toxicity end points of EC50, LC50, NOEC and LOEC for RR 120 were determined with 95 % confidence limits using TOX STAT version 3.5. The EC50 of RR 120 for green alga, duck weed and water flea are >100.00, 64.34, 10.40 mg L−1, respectively and LC50 for Rainbow trout is 78.84 mg L−1. Based on the results, the test item RR 120 could be classified as non-toxic to green alga, harmful to duck weed and Rainbow trout, toxic to water flea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Dyes are natural or synthetic, ionizing and aromatic organic colorants possessing characteristics such as solubility, strong color, substansiveness and promptness (Mohan et al. 2002). Eventually, its usage has been terrifically augmented owing to industrialization and man’s desire for color with the world wide annual production of dye over 7 × 105 tons. Synthetic dye stuffs are used comprehensively in textile, paper, printing, leather tanning, plastic, cosmetics, food processing, carpet, pharmaceutical industries and dye houses (Mohan et al. 2002; Aksu and Tezer 2005; Daneshvar et al. 2005; Gupta et al. 2005). Reactive dyes are greatly employed in the textile industry that utilizes about 10,000 different dyes and pigments. They are largely used in dyeing cotton and other cellulose-based fibres. Regardless of their comparatively high price, reactive dyes are used due to their exceptional colour fastness and ease of dyeing procedure (Klemola 2008). Reactive Red 120 (RR 120), a polyaromatic dye is most often stumbled upon in the case of textile dyes. They bind to textile fibers like cotton through covalent bonds and are well-accepted because of their bright color, trouble-free application and water- fast characteristics (Aksu and Tezer 2005).
Dye containing waters have been emerging as a burgeoning challenge (Kumar et al. 2006). The extremely colored effluents released from textile industries into the aquatic ecosystems are of grave ecological concern due to its toxicity, mutagenicity and non-biodegradability which makes waste water treatment extremely intricate (Edris et al. 2013). Disturbed photosynthetic activity due to abridged light penetration and occurrence of aromatics, metals and chlorides adversely affects the food chain organisms that culminate in an ecological imbalance (Pearce et al. 2003).
Owing to the snag associated with textile dyes, awareness is on the rise with regard to the ecological effects of dyes. A toxicant when delivered to its target reacts with it and the ensuing cellular dysfunction manifests itself in toxicity and this is when the toxicity of a compound becomes evident (Klemola 2008). At times, rather than reacting with a definite target molecule, a xenobiotic adversely affects the biological (micro) environment and leads to deleterious effects. (Gregus and Klaassen 2001). Biodepressive effects like mortality is harmful to the ecosystem. Ecotoxicity test is carried out to assess the effects of toxic substances to the biotic system.
RR 120 is an important dye which is widely used in the textile industries. But there has been a dearth of information with regard to the ecotoxicological facet of RR 120. Being an extensively employed dye with regard to textiles, it is indisputably pertinent to generate data on its ecological toxicity which at present is not available. Thus, we have promptly made an attempt to find the ecotoxicological consequences of RR 120 by performing the toxicity study on various aquatic organisms. Toxicity tests on aquatic organisms are crucial because they play the role as a pivotal producer in the aquatic food chain (Mohammed et al. 2011). In the present study, we investigated the acute toxicity of RR 120 dye to the fresh water green alga (Pseudokirchneriella subcapitata), gibbous duck weed (Lemna gibba), cladoceran freshwater water flea (Daphnia magna) and the salmonid fish (Oncorhynchus mykiss).
Materials and Methods
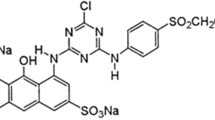
The RR 120 dye (Procion Red HE-3B; C44H24Cl2N14O20S6Na6) was obtained from Sigma-Aldrich Chemicals Co., St. Louis, USA. The dye is available in the form of red uniform powder.
Prior to the start of experiment, the stability of RR 120 dye was determined in OECD TG 201 medium for alga, 20X-AAP medium for L. gibba, M4 medium for daphnia and blended water for fish using the method of UV–visible spectrophotometry (Shimadzu UV–VIS 1601, UV scanning range 190–900 nm). RR 120 dye spiked with the concentration of 100 mg L−1 and control were used for analysis. The 0th and 72 h sample were analyzed for OECD TG 201 medium. The sampling occasions in the case of 20X AAP medium were day 0, 3, 5 and 7. For stability analysis in M4 medium, the samples at 0 h (within 30 min after spiking), 24 and 48 h were taken. In the case of blended water, samples were taken at 0, 24, 48 and 96 h. The linearity was also determined for the dye RR 120 dissolved in each of the different media. The dye concentrations chosen for the individual medium to determine the linearity range were 0.05, 0.1, 0.5, 1.0, 10.0 and 50.0 and 100.0 mg L−1.
Experiment was done to study the effect of RR 120 dye on the growth of P. subcapitata. The green alga, P. subcapitata Strain No. 61. 81 SAG (formerly known Selenastrum capricornutum) was obtained from SAG: Collection of Algal Cultures, Institute of Plant Physiology, University of Göttingen, Germany. The test was performed based on the OECD Guideline No. 201. Pre-culture of P. subcapitata was done three days prior to start of the experiment. The pre-culture and the studies were performed using the shaker incubator at 100–120 rpm, under continuous illumination of 6000–8000 lux at 22 ± 2°C. The stock solution of 100 mg L−1 was prepared by dissolving 56.62 mg dye in 566.2 mL of OECD TG 201 medium (pH of 8.1 ± 0.1). The P. subcapitata was exposed to different concentrations of dye viz., 9.4, 20.7, 45.5 and 100.0 mg L−1 with a factor of 2.2. Concurrent control group was maintained. Three replicates for concentrations and six replicates for control were maintained. The flasks were inoculated with an initial cell concentration of about 1 × 104 cells per mL. The study was performed for a period of 72 h. The cell count (Number of cells/mL) and appearance of exposed algal cells were recorded using Improved Neubaur’s Haemocytometer under the light microscope at 24, 48 and 72 h after inoculation. During the experiment, all validity criteria were met as per the Guideline. Based on the initial and mean cell count at 24, 48 and 72 h, the percentage inhibition of growth rate (% I r ), yield (% I y ) and biomass (% I b ) were calculated. Based on the inhibition of growth rate, yield and biomass, the toxicity values for E y C 50 (0–72 h), E y C 20 (0–72 h), E y C 10 (0–72 h), E b C 50 (0–72 h), E b C 20 (0–72 h), E b C 10 (0–72 h), E r C 50 (0–72 h), E r C 20 (0–72 h) and E r C 10 (0–72 h) and their 95 % confidence limits were determined. The effective concentration (EC) values, least observable effective concentration (LOEC) and no observable effective concentration (NOEC) were calculated using TOX STAT version 3.5.
The effect of RR 120 dye on the growth of L. gibba was performed based on the OECD Guideline No. 221. The L. gibba culture was obtained from Germany. Young, rapidly growing plants without visible lesions or discoloration (chlorosis) were used for preparation of pre-culture needed for the study. Sufficient colonies of the culture were transferred aseptically into fresh sterile culture medium, 7–10 days before the study initiation. The inoculated flasks were kept in the growth cabinet (slanting position) provided with continuous illumination of light in the range of 6500–10,000 lux using white fluorescent lamps and at temperature of 24 ± 2°C for 7- 10 days. L. gibba was exposed to different concentrations of dye (0.01, 1.0, 50.0, 100 mg L−1) and concurrent control group was also maintained. Three replicates for concentrations and six replicates for control were maintained. Number of fronds and its appearance were recorded on days; 3, 5 and 7. During the experiment, toxic symptoms (e.g. presence of undissolved material and colour) and appearance of the fronds including frond size, necrosis, chlorosis, gibbosity, colony break- up or loss of buoyancy, root length and break down if any were recorded. On day 0 and day 7, colonies/fronds from control and treated beakers were kept in a pre-weighed sterile glass vessel and dried at 60°C in hot air oven for three days to obtain the final dry weight. Based on frond number and dry weight, percentage inhibition of yield (% I y ), growth rate (% I r ) and biomass (% I b ) were calculated. Also, the toxicity values for E y C 50, E y C 20, E y C 10, E b C 50, E b C 20, E b C 10, E r C 50, E r C 20 and E r C 10 and their 95 % confidence limits were determined and the EC values, LOEC and NOEC were calculated using TOX STAT version 3.5.
Daphnia magna immobilization test was done based on the OECD Guideline No. 202. D. magna primary culture was obtained from Marinco Bioassay Aqua culture Lab, United States. Individual beaker culture is maintained in M4 medium. Test conditions such as a photoperiod of 16 h light and 8 h darkness and temperature of 18–22°C were maintained. The physico-chemical parameters such as pH (6–9) and hardness (140–250 mg L−1) of M4 medium were also maintained during the study period. M4 medium was prepared one to two days prior to the commencement of the experiment and aerated before use. Daphnids (<24 h old) were exposed to different dye concentrations; 0.1, 1.0, 10.0, 50.0, 100.0 mg L−1 and Control for 48 h. 50 mL of dye solution at different concentrations was transferred into sterile 100 mL test beakers. Five daphnids were released into the beakers and covered with watch glass. The daphnids were not fed during the experiment. Daphnids were observed for immobility at 24 and 48 h of exposure by placing each beaker on an electric light table/or under an electric lamp/naked eye. Those daphnids not able to swim within few seconds by gentle agitation (swirl) at 24 and 48 h were considered as immobile.
Any abnormal behavior such as pale coloration, floating and lethargy were selected for the criteria to evaluate the toxicity of RR 120 dye. Based on percentage immobility data, the 24 and 48 h EC 50 (with 95 % confidence limits), LOEC and NOEC were calculated using TOX STAT version 3.5.
Fish acute toxicity test was performed based on the OECD Guideline No. 203. Rainbow trout (O. mykiss) was procured from KDHP Plantations, Kerala, India and used for the study. Fishes (ten fishes each) were exposed to different dye concentrations; 1.0, 10.0, 50.0 and 100.0 mg L−1. Concurrent control group was maintained. The fishes were observed on 3, 6, 24, 48, 72 and 96 h after exposure for any mortality and any behavioral abnormalities such as pigmentation, loss of equilibrium, lateral lying at the bottom of the aquaria, rapid opercular movement, excessive mucous production, curved spine, surfacing movements, hyperactivity, lethargy were selected for the criteria to evaluate the toxicity of RR 120 dye. Based on the percentage mortality data, the 24, 48, 72 and 96 h LC 50 (with 95 % confidence limits), LOEC and NOEC were calculated using TOX STAT version 3.5.
Verification of RR 120 dye concentrations for each of the acute toxicity studies was done using the analytical method of UV–visible spectrophotometry (Shimadzu UV–VIS 1601, UV scanning range 190–900 nm). During the algal growth inhibition test, samples of control and RR 120 dye concentrations at 0 and 72 h were analyzed. The samples of control and dye concentrations maintained during the L. gibba growth inhibition test at day 0 and 7 were chosen for dose verification analysis. For D. magna immobilization test, samples were collected at 0 and 48 h. In the case of fish acute toxicity test, 0 and 96 h samples were collected.
Results and Discussion
Pollution of aquatic bionetwork by the emancipation of industrial dyes and effluents is an escalating subject. Even in minimum quantities, dye affects the visual merit and clarity of water and thereby the photosynthetic activity (Robinson et al. 2001). The stability and fade resistant properties of dyes against exposure to water, light, sweat and oxidizing agents make their degradation easier said than done (Aksu and Tezer 2005). This raises question regarding the toxicity of dyes to environment.
In the present study, the stability analysis method has linearity over the dye concentration range of 0.05–50 mg L−1. For each of the media, the calculated correlation co-efficient was 1.000. The limit of quantification (LOQ) and limit of detection (LOD) was determined as 0.05 mg L−1. The dye completely dissolved in all the four media and the stock solution was clear with no insoluble material present. The results of the stability analysis of RR 120 dye in OECD TG 201 medium showed 97.36 % and 95.98 % recovery at 0th and 72 h. In the case of 20X AAP medium, 95.71 %, 95.42 %, 95.33 % and 94.84 % of the dye concentration were recovered at day 0, 3, 5 and 7 respectively. Stability analysis in M4 medium showed dye recovery of 98.12 %, 95.72 % and 95.23 % at 0th, 24 and 48 h. Stability analysis of RR 120 dye in blended water showed a recovery of 98.77 %, 98.54 %, 98.14 % and 94.06 %.
Phytoplankton is the algae which form the basic food for the aquatic fauna. The growth of the algae is a recommended model system for assessing possible effects of chemicals on primary production in water. The regeneration times for unicellular algal species are measured in hours. The relatively short test of alga growth inhibition can determine effects over several generations. In the treated flasks, cells appeared normal in shape and green in colour. Subsequent decrease in cell count was observed with increasing dye concentrations. The cell concentration in the control flask was 202 × 104 cells (mL)−1 which indicates 202 times increase in cell count during the 72 h experiment. Highest percentage inhibition of growth rate, yield and biomass was observed for 100.00 mg L−1 dye concentration. Also the E y C 50, E r C 50 and E b C 50 were determined as >100.00 mg L−1. The level of significance was accepted at p ≤ 0.05. The determined NOEC was 9.4 mg L−1 (Table 1). Hence, the dye appears to be toxic only at concentrations greater than 100.00 mg L−1. These results suggest that the selected dye concentrations are not toxic to P. subcapitata. Dye removal from industrial effluents is of immense significance to the aquatic ecosystems for which bioaccumulation and biodegradation designs are in the vanguard today as against the conventional treatment methods (Çelekli et al. 2013). Aksu (2005) and Crini (2006) have mentioned the importance of various functional chemical groups of algal cell wall in capturing dye from wastewaters and reports affirm the biosorbent property of algae (Kumar et al. 2006; Dönmez and Aksu 2002). This concept throws light regarding the relevance of screening P. subcapitata as an important biological entity for further dye decolorization and degradation studies.
Duckweeds are widespread free floating aquatic plants ranging in the world from tropic to temperate zones. They are a food source for waterfowl and small animals and provide shade and shelter for fish. The plants also serve as physical support for a variety of small invertebrates. Thus it is essential to understand the effect of dyes on these aquatic plants.
In the case of L. gibba growth inhibition study, the observations made on days; 3, 5 and 7 showed no toxic symptoms and change in appearance of the fronds. Based on frond number and dry weight, the percent inhibition of growth rate, yield and biomass were calculated. The highest percent inhibition of growth rate, yield and biomass were observed with 100 mg L−1 dye concentration. The NOEC and LOEC were determined as 1.0 and 50.0 mg L−1, respectively (Table 1). This indicates that the dye would be harmful to L. gibba at concentration >50.00 mg L−1.
Invertebrates occupy a key position as intermediate consumer in pelagic as well as in the benthic food chain of aquatic ecosystems. The reasons for the selection of Daphnids for routine use in toxicity testing are both scientific and practical, since they are broadly distributed in fresh water bodies. In acute immobilization test using D. magna, the Daphnids treated with various concentrations of dye showed no abnormal behavior at 24 and 48 h. 100 % immobilization was observed with the higher dye concentrations of 50.0 and 100.0 mg L−1. The 24 and 48 h EC 50 (with 95 % confidence limits) was calculated as 51.10 and 10.40 mg L−1 respectively. The NOEC and LOEC were determined as 1.0 and 10.0 mg L−1 (Table 1). Based on the results obtained the dye concentrations above 10.0 mg L−1 would be toxic to daphnia.
Fish toxicity test in ecotoxicology is a logical consequence of ecological and economic factors. Although there may be other aquatic organisms in the aquatic environment with greater sensitivity to xenobiotics, fish constitutes as one of the bio-indicator organism. The fishes after treatment with dye did not show any behavioral abnormalities at 3, 6, 24, 48, 72 and 96 h. Fish acute toxicity study revealed the highest percent mortality at 96 h as 80 % for 100.0 mg L−1 dye concentration. The calculated LC 50 was 74.84 and the NOEC and LOEC was determined at 50.0 and 100.0 mg L−1 (Table 1) which indicates that the dye would be toxic to fish at concentrations above 75.0 mg L−1.
For verification of RR 120 dye concentrations, samples of control and RR 120 dye concentrations were analyzed for each of the acute toxicity studies. The results of analysis are presented in Table 2. The recovery of RR 120 dye for different concentrations in the four studies was greater than 90 %. Therefore, biological results are calculated based on nominal concentrations.
In the present study, we made an attempt to demonstrate the acute toxicity of RR 120 dye to certain aquatic test species. Based on the results of our study, it could be concluded that RR 120 dye is not a highly toxic chemical to aquatic species. The intensity of toxicity levels varied from species to species which might be due to their behavioral responses and habitation. Further studies on long term effect of RR 120 dye on aquatic test systems are warranted, as such data stand as an imperative necessity.
References
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026. doi:10.1016/j.procbio.2004.04.008
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40:1347–1361. doi:10.1016/j.procbio.2004.06.007
Çelekli A, Yavuzatmaca M, Bozkurt H (2013) Binary adsorption of Reactive Red 120 and Yellow 81 on Spirogyra majuscule. Middle East J Sci Res 13:740–748
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Daneshvar N, Aleboyeh A, Khataee AR (2005) The evaluation of electrical energy per order (EE0) for photooxidative decolorization of four textile dye solutions by the kinetic model. Chemosphere 59:761–767
Dönmez G, Aksu Z (2002) Removal of chromium(VI) from wastewaters by Dunaliella species. Process Biochem 38:751–762
Edris B, Ferdos KM, Ali RH, Ataola RK, Amir HM (2013) Decolorisation of Reactive Red 120 Dye by using single-walled carbon nanotubes in aqueous solutions. Eur J Chem (Online) ISSN 2090-9071. doi:10.1155/2013/938374
Gregus Z, Klaassen CD (2001) Mechanisms of toxicity. In: Casarett and Doull`s toxicology: the basic science of poisons. 6th edn. The McGraw, Hill Companies, pp 35–78
Gupta VK, Suhas IA, Saini VK, Garven VT, Van Der Bruugen B, Vandecasteele C (2005) Removal of dyes from wastewater using bottom ash. Ind Eng Chem Res 44:3655–3664
Klemola K (2008) Textile toxicity: cytotoxicity and spermatozoa motility inhibition resulting from reactive dyes and dyed fabrics. Kuopio Univ Publ C Nat Environ Sci 241:1–67
Kumar KV, Ramamurthi V, Sivanesan S (2006) Biosorption of malachite green cationic dye onto Pithophora sp., a fresh water alga. Dyes Pigm 69:74–79
Mohammed SI, Sunandan P, Chandrasekaran N, Amitava M (2011) Studies on toxicity of aluminium oxide nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J Nanopart Res 13:3287–3299
Mohan SV, Roa CN, Prasad KK, Karthikeyan J (2002) Treatment of simulated reactive yellow 22 (Azo) dye effluents using Spirogyra species. Waste Manage 22:575–582
OECD Guidelines for Testing of Chemicals (No. 201, Adopted: 23rd March 2006) Freshwater Alga and Cyanobacteria, Growth Inhibition Test. http://www.oecd-ilibrary.org/environment/test-no-201-alga-growth-inhibition-test_9789264069923-en;jsessionid=3jojqb4879lug.x-oecd-live-01
OECD Guidelines for Testing of Chemicals (No. 202, Adopted: 13th April 2004) Daphnia sp., Acute Immobilization Test. http://www.oecd-ilibrary.org/environment/test-no-202-daphnia-sp-acute-immobilisation-test_9789264069947-en
OECD Guidelines for Testing of Chemicals (No. 203, Adopted: 17th July 1992) Fish, Acute Toxicity Test. http://www.oecd-ilibrary.org/environment/test-no-203-fish-acute-toxicity-test_9789264069961-en
OECD Guidelines for Testing of Chemicals (No. 221, Adopted: 23rd March 2006) Lemna sp. Growth Inhibition Test. http://www.oecd-ilibrary.org/environment/test-no-221-lemna-sp-growth-inhabition-test_9789264016194-en
Pearce CI, Lioyd JR, Guthric JT (2003) The removal of colour from textile wastewater using whole bacterial cell: a review. Dyes Pigm 58:179–196
Robinson T, Mcmullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technol 77:247–255
Acknowledgments
We are grateful to Dr. P. Balakrishna Murthy, Former Director, IIBAT, for his valuable support during the study. Also, we extend our sincere thanks to Dr. T.N. Sathya, Department of Genetic Toxicology, IIBAT for her valuable inputs in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darsana, R., Chandrasehar, G., Deepa, V. et al. Acute Toxicity Assessment of Reactive Red 120 to Certain Aquatic Organisms. Bull Environ Contam Toxicol 95, 582–587 (2015). https://doi.org/10.1007/s00128-015-1636-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1636-z