Abstract
Octafluorocyclobutane (C4F8) with a GWP100 (global warming potential) of 10,000 times of CO2 is listed as potent greenhouse gas. Therefore, development of effective control technologies for reducing C4F8 emissions has become an emerging issue to be addressed. In this study, decomposition of C4F8 was investigated via three systems including catalytic hydrolysis, non-thermal plasma, and plasma catalysis, respectively. Decomposition of C4F8 achieved with catalytic hydrolysis reaches the highest efficiency of 20.1%, being obtained with γ-Al2O3 as catalyst in the presence of 10% H2O(g) and operating temperature of 800 °C. For plasma-based system, the highest C4F8 conversion obtained with non-thermal plasma is 62% at a voltage of 23 kV. As for the plasma catalysis system, 100% C4F8 conversion efficiency can be achieved at an applied voltage of 22–23 kV. The effects of various parameters such as gas flow rate and C4F8 concentration on plasma-based system show that the plasma catalysis also has better resistivity for the high gas flow rate. The highest energy efficiency of 0.75 g/kWh is obtained for the gas flow rate of 500 mL/min, with the C4F8 conversion of 41%. The highest conversion 89% was achieved with the O2 content of 0.5%. Addition of Ar improves the performance of plasma-based system. When Ar is controlled at 20%, C4F8 conversions obtained with plasma catalysis reach 100% at applied voltage of 22–23 kV even in the presence of 5% O2. The main products of the C4F8 conversion include CO2, NOx, and COF2 when O2 is added into the system. As water vapor is added, HF is also formed. This study has confirmed that combined non-thermal plasma with catalyst system to convert C4F8 is indeed feasible and has good potential for further development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluorocarbons (PFCs) are widely used in the semiconductor industry despite their nature as an extremely potent greenhouse gas that contributes to global warming (Kuroki et al. 2005). PFCs are composed of carbon, sulfur, or nitrogen as the central atom bonded with the fluorine atoms such as in CF4, C2F6, C3F8, C4F8, SF6, and NF3 (Lin et al. 2011; Chang and Yu 2001). According to the Taiwan Semiconductor Industry Association (TSIA), the annual usage of PFCs in Taiwan’s semiconductor industry is over 300 metric tons. These PFCs are mostly inert and non-corrosive gases that intensely absorb infrared radiation. Consequently, PFCs are capable of affecting the greenhouse effect of the earth. C4F8 has an atmospheric lifetime of 3200 years (16 times higher than CO2) and GWP100 (global warming potential) of 10,000 (Suzuki et al. 2008). Thus, effective reduction of C4F8 emission has become an emerging issue.
Optimized process with alternative chemicals is commonly applied to reduce the amount of PFCs used in the industry. However, destruction of PFCs via combustion (Jia and Ma 2005), catalytic reduction, and plasma abatement (Lee and Chen 2017) is generally considered as the most effective PFCs reduction strategy. The removal efficiency of PFCs achieved with combustion reaches ≥ 99%, but it has high-energy consumption (Chang and Chang 2006). On the other hand, catalytic hydrolysis is one of the most practical and economical methods for reducing PFCs (Lee and Jeon 2012; Park et al. 2012). Takita et al. (1999) described the decomposition of PFCs via hydrolysis with selected metal phosphate catalysts such as aluminum phosphate (AlPO4). However, the system needs to be operated at high temperatures (≥ 600 °C). Various Al2O3-based catalysts have been investigated for the conversion CF4 through catalytic hydrolysis. For instance, Song et al. (2013) used Ce/Al2O3 as a catalyst for the hydrolysis of CF4. The results show that the highest removal of CF4 reached with Ce/Al2O3 is 63%. Relevant study denotes that alumina-based bimetallic oxides such as Ga-Al2O3 or Ni-Al2O3 could promote the catalytic hydrolysis of CF4 (Takita et al. 2002) . According to Pan et al. (2019) γ-Al2O3 could achieve 72% CF4 conversion at 900 °C, through thermal catalysis. However, the catalyst system needs a high operating temperature (≥ 800 °C) to obtain good performance. Besides, the catalysts are susceptible to poisoning by fluorine, sulfur, and particulate matter (Zhang et al. 2018a).
Non-thermal plasma (NTP) has been demonstrated effective in removing PFCs (Gao et al. 2011). It has the advantages of rapid startup, high operating flexibility, and low equipment cost, but it has the disadvantage of low-energy efficiency (Futamura et al. 2001). Recently, combining non-thermal plasma with catalyst has been investigated as one of viable technologies towards PFC destruction (Futamura and Gurusamy 2005). This technology improves the shortcomings of the plasma system and induces synergistic effects with the catalyst (Chen et al. 2008). So far, plasma catalysis technology has been applied for VOCs removal and hydrocarbon reforming to produce hydrogen (Chen et al. 2008; Kim et al. 2008; Chen et al. 2017). However, relevant studies on PFCs removal are limited. Compared with non-thermal plasma system, plasma catalysis system has the following advantages: high contaminant/reactant conversion efficiency, improved selectivity for harmless by-products, and improved energy efficiency (Chang and Lee 2004).
In this study, removal of C4F8 from gas streams through catalytic hydrolysis is first evaluated with γ-Al2O3 as catalyst. As mentioned previously, γ-Al2O3 shows good capability to decompose PFC because its Lewis acid site could break down the strong C-F bond, while the production cost is reasonable and suitable for industrial application. In brief, the plasma-based system used in this study includes non-thermal plasma and plasma catalysis system.
Experimental
Plasma system
The performance of γ-Al2O3 for the removal of C4F8 via catalytic hydrolysis is firstly evaluated. A quartz tube with an inner diameter of 20 mm and the length of 300 mm is employed as reactor. Inlet gas consists of 300 ppm C4F8, 0–10% of H2O(g), and N2 as balance gas. C4F8 and N2 were provided by gas cylinders, while H2O(l) was introduced into the system by a peristaltic pump and evaporated to form H2O(g). The gas flow rate was regulated by mass flow controllers (MFCs) to 100 mL/min, and the gas hourly space velocity (GHSV) was controlled at 6000 h−1, and the system was operated at a temperature ranging from 300 to 800°C.
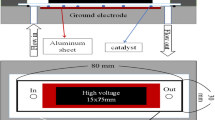
The schematic diagram of the experimental system for the removal of C4F8 via plasma-based systems is shown in Fig. 1. The plasma-based system with a dielectric barrier discharge (DBD)-type reactor was applied to a series of tests. DBD reactor was mainly a quartz tube with the inner diameter of 20 mm and length of 300 mm. The grounded electrode is aluminum foil with length = 94 mm wrapped outside, and stainless-steel rod with outside diameter = 3 mm was used as inner electrode. The total discharge volume was fixed at 27.6 cm3. All plasma-based experiments including non-thermal plasma and plasma catalysis were operated with the inlet C4F8 concentration of 300 ppm, and N2 as carrier gas was imported into the plasma-based systems for reaction. Furthermore, the effects of gas flow rate ranging from 100 to 1,500 mL/min, C4F8 concentration ranging from 300 to 10,000 ppm, and addition of O2 and Ar are evaluated. N2, Ar, and O2 were provided by gas cylinders. An AC power with medium frequency (You-Shang, Taiwan) was used as the power supply, and the highest applied voltage and frequency are 23 kV and 18.5 kHz, respectively. The power consumption was measured via a digital oscilloscope (Tektronix DPO3014, USA) equipped with a current probe (Tektronix TCPA300, USA) and a high-voltage probe (Tektronix P6015A, USA). All by-products were monitored by a Fourier transform infrared spectrophotometer (FTIR, Nicolet 6700, USA Thermo Scientific, USA). All experimental data would be recorded as the reaction reached steady state. For the analysis of the experimental results, conversion efficiency (η) of C4F8 and energy efficiency are shown by Eqs. (1)–(2), respectively:
Catalyst characterization
Commercial γ-Al2O3 applied as catalyst was characterized by transmission electron microscope (TEM), Brunauer-Emmett-Teller (BET) surface areas, and X-ray diffraction (XRD). Transmission electron microscope (JEM2000FX JEOL, Japan) is applied to observe the morphology of catalyst (Fig. S1). ASAP2010 (Micromeritics, USA) was applied for the measurement of the BET surface area (SBET) via the adsorption-desorption process with N2 at – 196 °C. X-ray diffraction (XRD) was performed with X-ray diffractometer (D8AXRD BRUKER, Germany) at 40 kV and 10 mA by using Cu-Kα radiation, and XRD profiles were obtained at a 2θ range of 10–80° with a scanning rate of 6°/min (see Fig. S2). The results of catalyst characterization are presented in Supporting Information (Table S1).
Results and discussion
Catalytic hydrolysis of C4F8 removal
Figure 2 shows the C4F8 conversions obtained with catalytic hydrolysis operated at different temperatures, with γ-Al2O3 as catalyst for the following conditions: [C4F8] = 300 ppm, [H2O(g)] = 0–10%, [GHSV] = 6000 h−1. When the operating temperature is ≤ 500 °C, the C4F8 removal efficiency is 0% for the gas stream containing 0–10% H2O(g). C4F8 conversion increases slightly with increasing temperature, and the highest removal efficiency reaches 5.2% in the absence of H2O(g) at 800 °C. The C4F8 conversion efficiency obtained with γ-Al2O3 reaches 11.3% in the presence of 5% H2O(g) when it is operated at 600 °C. As H2O(g) content is increased to 10%, C4F8 removal efficiency further increases to 20.1% with the operating temperature of 800 °C. Conversion of C4F8 increases with increasing H2O(g) content, implying that increasing H2O(g) content facilitates catalytic hydrolysis of C4F8. Mechanisms of C4F8 hydrolysis with γ-Al2O3 as catalyst are illustrated in Fig. 3 (Kuroki et al. 2005). The first step of catalytic hydrolysis of C4F8 is the adsorption of C4F8 molecules on the active sites of γ-Al2O3. Subsequently, the O atom on metal oxide containing Lewis acid breaks the C-F bond on C4F8 to form M-O-CxFy and M-CxFy. M-O-CxFy is then hydrolyzed to form M-OH and CHF2, while M-CxFy is further hydrolyzed to form M-OH, CxFy-OH, HF, and CO2. Eventually, M-OH that reacted with another M-OH was re-generated to form γ-Al2O3. H2O(g) was released simultaneously as a by-product. On the other hand, CxFy-OH was hydrolyzed until termination. The M (in M-OH) indicates metals, and L (in L-acid) indicates Lewis acid. Under similar operating condition, El-Bahy et al. (2003) apply γ-Al2O3 to decompose CF4, and the main product is CO2.
The acidic site on surface of catalyst plays a significant role in conversion of fluorocarbon (Jie et al. 2008). The results obtained in this study indicate that only moderate C4F8 removal could be obtained by hydrolysis with γ-Al2O3 as a catalyst when operated at a temperature ranging from 600 to 800 °C. However, high PFCs conversion efficiency is difficult to achieve under mild operating conditions because of high C-F bonding energy (543 ± 4 kJ/mol) and chemical stability (Hannus 1999). Song et al. (2013) show that deactivation of catalyst in the decomposition of fluorocarbon is induced by the formation of HF and the transformation of γ-Al2O3 into AlF3. Moreover, high operating temperature also triggers the deactivation of the catalyst. It is interesting to note that 100% PFCs conversion can be achieved when operated at 900 °C even though the phase transformation from γ-Al2O3 into α-Al2O3 is inevitable (Jia et al. 2011).
Performances of plasma-based systems for C4F8 conversion
Performances of plasma-based systems including non-thermal plasma and plasma catalysis are investigated individually for C4F8 conversion with the applied voltage ranging from 12 to 23 kV. As shown in Fig. 4, non-thermal plasma system shows the trend of increasing C4F8 conversion by increasing applied voltage. As the applied voltage is increased, higher electron field is produced, which in turn increases the number of energetic electrons. Hence, the highest C4F8 conversion of non-thermal plasma system is achieved at the highest applied voltage. The C4F8 conversion of 62% can be reached with the applied voltage of 23 kV. In contrast with the non-thermal plasma system, 100% C4F8 conversion is obtained with plasma catalysis when operated with the applied voltage ranging from 22 to 23 kV. These results show that plasma catalysis system has excellent performance for C4F8 conversion and the performance of C4F8 conversion could be greatly improved as γ-Al2O3 is placed into the discharge zone. The combination of the γ-Al2O3 catalyst with plasma can increase the conversion value of C4F8 in terms of both plasma chemistry and the performance of the plasma. Plasma chemistry condition from γ-Al2O3 should occur when the applied voltage was increased. When the applied voltage was increased, more energetic electrons were produced. Eventually, the magnitude of mean electric field should increase too. Inside the plasma system, the collision of energetic electron with C4F8 is considered as the most important mechanism leading to C4F8 conversion. The possible reaction pathways of energetic electron between C4F8 with non-thermal plasma system are given in Fig. 5a. N2(A3\( {\sum}_u^{+}\Big) \) has the characteristic as active species to decompose C4F8 with excitation energy of 6.17 eV (Takita et al. 2002). Hence, it is important to note that N2(A3\( {\sum}_u^{+}\Big) \) plays a crucial role in C4F8 conversion. Addition of O2 into non-thermal plasma produces O radicals, resulting in the collision with CxFy radicals to form CO, CO2, COF2, FO, FO2, and FNO2.
Plasma catalysis chemistry is very complicated; it consists of electrons, ions, and exciting species. Possible reactions of energetic electrons with C4F8 via plasma catalysis are given in Fig. 5b. The first step of plasma catalysis is the reaction of C4F8 with the electron in plasma to form CxFy radicals including C3F7, C3F6, C3F5, CF3, CF2, and CF. C4F8 and CxFy radicals were adsorbed on the active sites of γ-Al2O3 catalysts. Subsequently, intermediates (M-O-CxFy) were formed as O atoms on the catalysts attack and break the C-F bond of the adsorbed C4F8. The O atom of other metal oxide or O radical (from addition O2) attacks the C-F bond of M-O-CxFy to form CO, CO2, and CxFy. M-O-CxFy continued to lose its C atom until M-O-Fy is formed. Addition of O2 into plasma-based system produces O radical and leads to the collision with CF3 and CF to form CO, CO2, and COF2 (Hayashi and Satoh 2005; Vasenkov et al. 2004). Besides, N2* and N2(A3\( {\sum}_u^{+}\Big) \) generated by non-thermal plasma are active to convert C4F8. As explained in reactions (3)~(5), N2(A3\( {\sum}_u^{+}\Big) \) plays a crucial role in non-thermal plasma to the removal of gaseous pollutants if N2 is applied as the carrier gas (Radoiu 2004; Choi et al. 2012).
Integration of plasma with catalyst results in the generation of micro-discharge and increased mean electric field due to the contact point between catalysts. One important parameter that affects C4F8 conversion and performance of the plasma is dielectric constant of the catalyst that is placed in the discharge zone. In this study, the dielectric constant of γ-Al2O3 applied is 9.1.
Previous studies confirmed that the value of dielectric constant greatly affects plasma performance as shown in the Eqs. (6)–(8) (Takaki et al. 2004; Zhang et al. 2016). According to the equations, the dielectric constant (εp) can increase the value of the average electrical field (Ex), electron density (ne), and mean electron energy (Ee) which can result in the increase of energy distribution function (EEDF) and electron temperature.
where Ex is the average electrical field, ne is the electron density, Ee is the mean electron energy, and V is the applied voltage. d denotes the separation distance between the electrodes; εg and εp denote the dielectric constants of background gas and packing pellets, respectively. In Eq. (7), α and A denotes the void fraction and cross-sectional area of packed bed reactor, respectively. In addition, e is the electric charge of electrons (equal to 1.6 × 10−19 C), μ0 is the electron mobility at reference electric field, and Vr is reactor volume. E0 and ω are empirical coefficients. Based on Eqs. (6), (7), and (8), we know that εp is linearly proportional to Ee. Thus, when the magnitude of εp is significantly high, the Ee would be enhanced. Carman and Mildren (2000) developed a model using an electron energy distribution function (EEDF) to quantify the plasma kinetics in dielectric barrier discharge. The result shows that the electron energy is directly related to the EEDF. On the other hand, EEDF greatly affects the coefficient of excitation (αex), coefficient of ionization (αi), and electron transport (diffusion De and mobility μe).
Catalyst can provide the contact points on which numerous electrons can collide. The intense collision of electron may increase the discharge zone temperature. A study conducted by Yarahmadi and Alyar (2020) showed that the removal efficiency and the energy efficiency highly are highly dependent on the temperature of the discharge zone. Hence, the increase in the temperature of discharge zone could also increase of C4F8 conversion. The mean electron temperature can be calculated from swarm parameters of electrons in nitrogen as follows (Takaki et al. 2004):
In Eq. (9), β denotes the power law constant, k denotes the Boltzmann constant (1.38 × 10−23 J/K), and De denotes the diffusion constant. Since an experimental value of μe/De is limited, the electron mean temperature can be determined from the plasma neutrality conditions (ne ≒ ni) after computing the total ion density (ni). In our study, the energy efficiency and removal efficiency achieved with the plasma catalysis are 0.6–2 times higher than those achieved with NTP-alone system. Based on the analysis, the addition of a catalyst results in better performance of the plasma kinetic and plasma chemistry.
In plasma-based system, energetic electrons and active N2 species are essential for C4F8 conversions. As previously mentioned, the C4F8 conversions increased with increasing applied voltage; as the applied voltage increases, the mean electric field and electron density should increase as well, resulting in the increase of C4F8 conversion achieved with plasma-based. Figure 6 indicates the energy efficiencies of non-thermal plasma and plasma catalysis system for C4F8 removal (for the same energy input). The removal rate of C4F8 achieved with non-thermal plasma is significantly lower than that obtained with the plasma catalysis system. The energy efficiencies achieved with non-thermal plasma and plasma catalysis are 0.25 g/kWh and 0.53 g/kWh, respectively. Here, the voltage of 12 kV is applied for both systems. As displayed in Fig. 6, the energy efficiency shows gradual decrease with increasing applied voltage. Basically, the increase of applied voltage might increase the power input. According to Eq. (2), increasing power input and conversion manifest different effects on energy efficiency. However, the downtrend of energy efficiency was expected with increasing power input, even though the C4F8 conversion might be increased. The results presented in Fig. 6 indicate that plasma catalysis could reach a higher energy efficiency in C4F8 conversion compared with non-thermal plasma at the same level of energy consumption. Applying density functional theory on the decomposition of c-C4F8 (cyclic-Octafluorocyclobutane), Xiao et al. (2018) reported that the reaction enthalpy required for c-C4F8 decomposition is 420 kJ/mol under trace water conditions. Zhang et al. (2018a, b) reported that the total reaction enthalpy required for c-C4F8 decomposition is 524 kcal/mol based on the ReaxFF MD simulation.
Effects of gas flow rate, inlet concentration, oxygen and argon contents on C4F8 removal
Figure 7 shows the effects of gas flow rate on C4F8 conversion obtained with plasma-based systems; gas flow rates of 100 mL/min, 500 mL/min, 1,000 mL/min, and 1,500 mL/min are imported individually into the plasma-based systems for C4F8 conversion. The plasma-based system is operated at applied voltage and frequency of 23 kV and 18.5 kHz, respectively, [C4F8] = 300 ppm and carrier gas = N2. As shown in Fig. 7, as Q = 500 mL/min, the C4F8 conversion obtained with non-thermal plasma is 23.1% at applied voltage of 23 kV. Compared with Q = 100 mL/min, the C4F8 conversion obtained with non-thermal plasma is significantly lower. As the gas flow rate is further increased to 1500 mL/min (with the gas residence time of 1.1 second), the C4F8 conversion approaches 0%; the highest conversion is achieved at a flow rate 100 mL/min, corresponding to the gas residence time of 16.5 s. Overall, the highest energy efficiency with the plasma catalysis is 0.75 g/kWh at the gas flow rate of 500 mL/min, and conversion of 41 % is achieved with the gas residence time of 3.3 s. At the same power consumption, the conversion efficiency and gas flow rate are proportional to the energy efficiency. As shown in Fig. 7, increasing gas flow rate leads to decreasing residence time, resulting in lower C4F8 conversion. Figure 8 shows that the C4F8 conversion efficiency reaches the highest value at a low concentration (300 ppm), and the conversion also increases with increasing applied voltage, i.e., from 36.8% at 12 kV to 72.6% at 23 kV. However, as C4F8 concentration increases, the C4F8 conversion efficiency decreases significantly. The C4F8 conversion efficiencies achieved are less than 20% for the inlet C4F8 concentrations ranging from 6000 to 10,000 ppm. The overall trend of the plasma catalysis system is similar to that of the non-thermal plasma system, except that higher conversion efficiency is obtained. Figure 9 shows the performance of plasma catalysis evaluated with the addition of O2 content varying from 0 to 5% O2. The purpose of adding oxygen to the gas stream is to increase the generation of active species which are beneficial to C4F8 oxidation. In fact, if oxygen is absent, lattice oxygen from Al2O3 and O species from etching quartz (SiO2) could help oxidize C4F8. However, the results show that the addition of 0.5% oxygen can increase C4F8 conversion and then decrease if too much O2 is added. These phenomena could explain that O2 molecules themselves will turn into active species such O+, O-, O2+, and O2-. These active species will react with C4F8 to form CO2, CO, and COF2. However, too much oxygen in the plasma system may reduce C4F8 conversion because O2 is an electronegative gas to which electrons would attach. As a consequence, the electron density may be reduced, resulting in lower C4F8 conversion. Similar trends were observed in the decomposition of fluorinated compounds with non-thermal plasma as reported by Gandhi and Mok (2012) and Wallis et al. (2007). The best C4F8 conversion reached 89% with the O2 content of 0.5% at applied voltage of 23 kV. Furthermore, addition of oxygen into N2-CXFY gas mixture may form several products such as CO, CO2, COF2, OF2, NO, NO2, N2O, FO, FO2, FNO, FNO2, and FONO2. Downward trend of C4F8 conversion with increasing O2 is partly attributed to the reactions of O2 with some N2* and N2(A3\( {\sum}_u^{+}\Big) \) to form NOx, resulting in lower C4F8 conversion. Kim et al. (2008) and Xie et al. (2009) indicate that energetic electrons react with excess oxygen; the collision might occur to form other compounds, which in turn, decrease the PFCs decomposition efficiency. O3 formation might occur in the non-thermal plasma system when O2 is added into the system. Since the reaction rate constant is relatively low as reported by Vasenkov et al. (2004), the presence of O3 has a minor effect on C4F8 removal; the mechanism regarding C4F8 removal via the reactions with O+, O-, O2+, O2-, and O3. could be described in reactions (10)–(18):
Reaction | Rate constant (cm3 s−1) | |
O+ +C4F8 → C4F8+ +O | 1.22 × 10−9 | (10) |
O2+ +C4F8 → C4F8+ +O2 | 1.55 × 10−9 | (11) |
O+ +C4F8 → C3F5+ + CF3+ +O | 0.76 × 10−9 | (12) |
O+ +C4F8 → C4F7+ + F+ +O | 0.28 × 10−9 | (13) |
O2+ +C4F8 → C2F4+ + C2F4 + O2 | 4.48 × 10−10 | (14) |
O2+ +C4F8 → C3F5+ + CF3 + O2 | 1.15 × 10−9 | (15) |
O2- +C4F8 → C4F8- +O2 | 4.60 × 10−10 | (16) |
O- +C4F8 → C4F8- +O | 1.0 × 10−10 | (17) |
O3 + C4F8 → Adduct | 1.83 × 10−18 | (18) |
To evaluate the effect of Ar addition on C4F8 conversion, various Ar contents ranging from 1 to 20% are introduced into the system. In general, formation of active species in plasma is a very complicated process; it consists of electrons, ions, and exciting species. Ar is considered as a good carrier gas in the plasma process because it can easily be excited to metastable state (Ar*). Compared with N2, Ar* has several advantages such as a high threshold energy of 13 eV and lower dielectric strength. The effect of Ar contents on the performance of plasma catalysis for C4F8 removals with the presence of 5% O2 is shown in Fig. 10, indicating that the adverse effects caused by O2 on C4F8 removal is mitigated with increasing Ar content. Specifically, C4F8 conversions obtained with plasma catalysis reaches 100% as Ar content is controlled at 20%, with the applied voltage of 22 and 23 kV even in the presence of 5% O2. According to Vasenkov et al. (2004), the mechanism regarding C4F8 removal via the reactions with Ar+ or Ar* could be described in reactions (19)–(23):
Conclusion
In this study, decomposition of C4F8 was investigated with three systems, i.e., catalytic hydrolysis, non-thermal plasma, and plasma catalysis. Decomposition of C4F8 obtained with catalytic hydrolysis reaches the highest efficiency of 20.1%, being obtained with γ-Al2O3 as catalyst in the presence of 10% H2O(g) and operating temperature of 800 °C. A non-thermal plasma system was established to investigate the efficiency of converting C4F8. As N2 is applied as carrier gas in the non-thermal plasma system, the conversion efficiencies of C4F8 are up to 72%. Regarding the influence of the gas flow rate, as the non-thermal plasma system is operated at 500 mL/min, the C4F8 conversion decreases significantly, indicating that appropriate gas residence time is needed for non-thermal plasma system towards effective removal of C4F8. As the system is operated at 22 kV, C4F8 conversion also reaches 100% achieved via plasma catalysis. The effect of O2 content on plasma catalysis shows that the conversion of C4F8 decreases as 1 to 5% O2 is added into the system. This is due to the reaction of O2 with N2* and N2(A3\( {\sum}_u^{+}\Big) \); furthermore, O2 gas has electronegative property due to the electron attachment to the oxygen molecules, resulting in lower C4F8 conversion. As the O2 content is reduced to 0.5%, the results show that C4F8 conversions increase. It is because other species formed in the plasma reaction combine with O2 to inhibit re-combinations of C4F8. In terms of the influence of Ar content, the results show that the conversion efficiency of C4F8 increases significantly with increasing Ar content, indicating that the addition of appropriate Ar content into the plasma system poses a positive effect on C4F8 removal.
References
Carman RJ, Mildren RP (2000) Electron energy distribution functions for modeling the plasma kineticts in dielectrical barrier discharges. J Phys D: Appl 33:L99–L103. https://doi.org/10.1088/0022-3727/33/19/101/meta
Chang MB, Chang JS (2006) Abatement of PFCs from semiconductor manufacturing processes by nonthermal plasma technologies. A critical review. Ind Eng Chem Res 45:4101–4109. https://doi.org/10.1021/ie051227b
Chang MB, Lee HM (2004) Abatement of perfluorocarbons with combined plasma catalysis in atmospheric-pressure environment. Catal Today 89:109–115. https://doi.org/10.1016/j.cattod.2003.11.016
Chang MB, Yu SJ (2001) An atmospheric-pressure plasma process for C2F6 removal. Environt Sci Techno 35:1587–1592. https://doi.org/10.1021/es001556p
Chen HL, Lee HM, Chen SH, Chao Y, Chang MB (2008) Review of plasma catalysis on hydrocarbon reforming for hydrogen production—interaction, integration, and prospects. Appl Catal B Environ 85:1–9. https://doi.org/10.1016/j.apcatb.2008.06.021
Chen JX, Pan KL, Yu SJ, Yen SJ, Chang MB (2017) Combined fast selective reduction using Mn-based catalysts and nonthermal plasma for NOx removal. Environ Sci Pollut Res 24:21496–21508. https://doi.org/10.1007/s11356-017-9785-8
Choi S, Hong SH, Lee HS, Watanabe A (2012) A comparative study of air and nitrogen thermal plasmas for PFCs decomposition. Chem Eng J 185:193–200. https://doi.org/10.1016/j.cej.2012.01.077
El-Bahy Z, Ohnishi R, Ichikawa M (2003) Hydrolysis of CF4 over alumina-based binary metal oxide catalysts. Appl Catal B Environ 40:81–91. https://doi.org/10.1016/S0926-3373(02)00143-1
Futamura S, Gurusamy A (2005) Synergy of nonthermal plasma and catalysts in the decomposition of fluorinated hydrocarbons. J Electrost 63:949–954. https://doi.org/10.1016/j.elstat.2005.03.067
Futamura S, Einaga H, Zhang A (2001) Comparison of reactor performance in the nonthermal plasma chemical processing of hazardous air pollutants. IEEE Trans Ind Appl 37:978–985. https://doi.org/10.1109/28.936387
Gandhi MS, Mok YS (2012) Decomposition of trifluoromethane in a dielectric barrier discharge non-thermal plasma reactor. J Environ Sci 24:1234–1239 S1001074211609352
Gao SH, GaoLH ZKS (2011) Super-hydrophobicity and oleophobicity of silicone rubber modified by CF4 radio frequency plasma. Appl Surf Sci 257:4945–4950. https://doi.org/10.1016/j.apsusc.2011.01.001
Hannus I (1999) Adsorption and transformation of halogenated hydrocarbons over zeolites. Appl Cataly A Gen 189:263–276. https://doi.org/10.1016/S0926-860X(99)00283-5
Hayashi N, Satoh S (2005) Treatment of a perfluorocarbon using nonthermal plasma produced by atmospheric streamer corona discharge. IEEE Trans Plasma Sci 33:274–275. https://doi.org/10.1109/TPS.2005.845003
Jia L, Ma S (2005) The experimental study on high temperature air combustion and CF4 decomposition. Heat Transf Summer Conf 1:705–708. https://doi.org/10.1115/HT2005-72440
Jia W, Jin L, Wang Y, Lu J, Luo M (2011) Fluorination of dichlorodifluoromethane to synthesize tetrafluoromethane over Cr2O3–AlF3 catalyst. J Ind Eng Chem 17:615–620. https://doi.org/10.1016/j.jiec.2011.05.005
Jie FAN, Xiu-Feng XU, Xian-Jun NIU (2008) Decomposition of CF4 over Al2O3-based metal oxides. Acta Physico-Chimica Sin 24:1271–1276. https://doi.org/10.3866/pku.whxb20080725
Kim HH, Ogata A, Futamura S (2008) Oxygen partial pressure-dependent behavior of various catalysts for the total oxidation of VOCs using cycled system of adsorption and oxygen plasma. Appl Catal B Environ 79:356–367. https://doi.org/10.1016/j.apcatb.2007.10.038
Kuroki T, Mine J, Odahara S, Okubo M, Yamamoto T, Saeki N (2005) CF4 decomposition of flue gas from semiconductor process using inductively coupled plasma. IEEE Trans Ind Appl 41:221–228. https://doi.org/10.1109/TIA.2004.840954
Lee HM, Chen SH (2017) Thermal abatement of perfluorocompounds with plasma torches. Energy Procedia 142:3637–3643. https://doi.org/10.1016/j.egypro.2017.12.256
Lee YC, Jeon JK (2012) A study on catalytic process in pilot plant for abatement of PFC emission. Clean Technol 18:216–220. https://doi.org/10.7464/ksct.2012.18.2.216
Lin BY, Chang MB, Chen HL, Lee HM, Yu SJ, Li SN (2011) Removal of C3F8 via the combination of non-thermal plasma, adsorption and catalysis. Plasma Chem Plasma Process 31:585–594. https://doi.org/10.1007/s11090-011-9303-6
Pan KL, Chen YS, Chang MB (2019) Effective removal of CF4 by combining nonthermal plasma with γ-Al2O3. Plasma Chem Plasma Process 39:877–896. https://doi.org/10.1007/s11090-019-09990-9
Park NK, Park HG, Lee TJ, Chang WC, Kwon WT (2012) Hydrolysis and oxidation on supported phosphate catalyst for decomposition of SF6. Catal Today 185:247–252. https://doi.org/10.1016/j.cattod.2011.08.008
Radoiu MT (2004) Studies on atmospheric plasma abatement of PFCs. Radiat Phys Chem 69:113–120. https://doi.org/10.1016/S0969-806X(03)00455-9
Song JY, Chung SH, Kim MS, Seo MG, Lee YH, Lee KY, Kim JS (2013) The catalytic decomposition of CF4 over Ce/Al2O3 modified by a cerium sulfate precursor. J Mol Catal A Chem 370:50–55. https://doi.org/10.1016/j.molcata.2012.12.011
Suzuki K, Ishihira Y, Sakoda K, Shirai Y, Teramoto A, Hirayama M, Ohmi T, Watanabe T, Ito T (2008) High-efficiency PFC abatement system utilizing plasma decomposition and Ca(OH)2/CaO immobilization. IEEE Trans Semiconductor Manuf 21:668–675. https://doi.org/10.1109/TSM.2008.2005400
Takaki K, Chang JS, Kostov KG (2004) Atmospheric pressure of nitrogen plasmas in a ferro-electric packed bed barrier discharge reactor part I: modeling. IEEE Trans Dielect Elect Insul 11:481–490. https://doi.org/10.1109/TDEI.2004.1306726
Takita Y, Ninomiya M, Miyake H, Wakamatsu H, Yoshinaga Y, Ishihara T (1999) Catalytic decomposition of perfluorocarbons Part II. Decomposition of CF4 over AlPO4-rare earth phosphate catalysts. Phys Chem Chem Phys 1:4501–4504. https://doi.org/10.1039/a904311j
Takita Y, Tanabe T, Ito M, Ogura M, Muraya T, Yasuda S, Nishiguchi H, Ishihara T (2002) Decomposition of CH2FCF3 (134a) over metal phosphate catalysts. Ind Eng Chem Res 41:2585–2590. https://doi.org/10.1021/ie0106229
Vasenkov AV, Li X, Oehrlein GS, Kushner MJ (2004) Properties of c-C4F8 inductively coupled plasmas. II. Plasma chemistry and reaction mechanism for modeling of Ar/c-C4F8/O2 discharges. J Vac Sci Technol A Vacuum, Surfaces ,Films 22:511–530. https://doi.org/10.1116/1.1697483
Wallis AE, Whitehead JC, Zhang K (2007) Plasma-assisted catalysis for the destruction of CFC-12 in atmospheric pressure gas streams using TiO2. Catatysis Lett 113:29–33. https://doi.org/10.1007/s10562-006-9000-x
Xiao S, Li Y, Zhang X, Zhuo R, Wang D, Tang J, Zhang J, Chen Q (2018) Influence of trace water on decomposition mechanism of c-C4F8 as environmental friendly insulating gas at high temperature. AIP Adv 8:125202. https://doi.org/10.1063/1.5044751
Xie HD, Sun B, Zhu XM, Liu YJ (2009) Influence of O2 on the CF4 decomposition by atmospheric microwave plasma. Int J Plasma Environ Sci Technol 3:39–42. https://doi.org/10.1088/0022-3727/47/35/355205
Yarahmadi R, Alyar S (2020) A Laboratory study of low-temperature co removal from mobile exhaust gas using in-plasma catalysis. Emiss Control Sci Technol 6:17–27. https://doi.org/10.1007/s40825-020-00154-2
Zhang X, Xiao H, Hu X, Gui Y (2016) Effects of background gas on sulfur hexafluoride removal by atmospheric dielectric barrier discharge plasma. AIP Adv 6:115005. https://doi.org/10.1063/1.4967277
Zhang X, Zhang G, Wu Y, Song S (2018a) Synergistic treatment of SF6 by dielectric barrier discharge/γ-Al2O3 catalysis. AIP Adv 8:125109. https://doi.org/10.1063/1.5054729
Zhang Y, Li Y, Zhang X, Xiao S, Tang J (2018b) Insights on decomposition process of c-C4F8 and c-C4F8/N2 mixture as substitutes for SF6. R Soc Open Sci 5:81104. https://doi.org/10.1098/rsos.181104
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Funding
This study was financially supported by TSMC (Taiwan Semiconductor Manufacturing Company).
Author information
Authors and Affiliations
Contributions
Ya Sheng developed and designed the methodology of this experiment and prepared the original draft. Kuan Lun reviewed and edit the published work by those from the original research group. Moo Been Chang supervised the project and had the oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team. All authors read and approved the final manuscript."
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Consent to Publish
Not applicable
Competing interests
Not applicable
Additional information
Responsible Editor: Santiago V. Luis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 267 kb)
Rights and permissions
About this article
Cite this article
Chen, Y.S., Pan, K.L. & Chang, M.B. Application of plasma catalysis system for C4F8 removal. Environ Sci Pollut Res 28, 57619–57628 (2021). https://doi.org/10.1007/s11356-021-14649-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14649-0