Abstract
The feasibility of C3F8 abatement via combining nonthermal plasma with adsorption and/or catalysis is investigated in this study. In terms of the simultaneous combination of plasma, adsorption and catalysis (CPAC), three different configurations including A/C layer (adsorbent layer prior to catalyst layer), C/A layer (catalyst layer prior to adsorbent layer) and A/C mixture (adsorbent and catalyst are mechanically mixed) are adopted. For all the experimental tests conducted in this study, the gas stream consists of 500 ppm C3F8, 2% O2, and balanced N2. The experimental results indicate that C3F8 removal efficiencies depend on what kind of packing material is adopted (adsorbent, catalyst or both) and how the material is packed within the plasma reactor. The removal efficiencies obtained with different reactors are in the order as: CPAC (A/C layer; AC mixture) > CPA (plasma with adsorbent alone) > CPC (plasma with catalyst alone) > CPAC (C/A layer). The indentified products after treatment include CO2, CO, N2O and CF4. The formation of C2F6 is not observed in this study, which is encouraging since the global warming potential of C2F6 is actually higher than that of C3F8.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the characteristics of extremely long lifetimes in the atmosphere and relatively high GWPs (global warming potentials), PFCs are regulated as one of the GHGs (greenhouse gases) in Kyoto Protocol. PFCs are mainly emitted from semiconductor and TFT-LCD manufacturing industries. In terms of PFC abatement, the high temperature-based technologies including catalytic oxidation and thermal decomposition are still the mainstream. Although acceptable removal efficiency could be achieved, these equipments have to be maintained at a certain temperature even during the period without PFC emission. Different from the above-mentioned technologies, nonthermal plasma can be ignited and operated at room temperature, making it a potentially viable technique for PFC abatement. However, prior to further industrial application, the energy efficiency and the products’ selectivities still need to be improved [1, 2].
To resolve these challenges, a novel technique combining the nonthermal plasma, adsorption and catalysis (CPAC) simultaneously is developed in this study. It has been experimentally demonstrated in various studies that introducing catalyst into a nonthermal plasma reactor could effectively improve the abatement of gaseous pollutants [3–9]. Moreover, adsorbent is used to increase the concentration and retention time of pollutant molecules within discharge region, which is believed to be beneficial for energy efficiency and products’ selectivities. C3F8 is chosen as the target pollutant in this study because it has been gradually replacing CF4 and C2F6 as the CVD chamber cleaning gas for semiconductor industry. The main purpose is to demonstrate the feasibility of PFC abatement via CPAC, which is constructed by introducing the same volumes of adsorbent and catalyst in the arrangements of two separate layers or a single layer (i.e. adsorbent and catalyst are mechanically mixed.). In addition to CPAC, the combinations of plasma with catalysis or adsorption alone, which are termed as CPC and CPA, are used as control as well.
Experimental
Physical Properties of the Catalyst and Adsorbents
The γ-Al2O3 and molecular sieves (MS-3A, MS-4A, MS-4A and MS-10A) pellets used as catalyst and adsorbent in this study are commercial products purchased from Alfa Aesae and Merck KGaA, respectively. The γ-Al2O3 and molecular sieve pellets are of spherical and cylindrical shape, respectively. To confirm the catalytic activity of γ-Al2O3, α-Al2O3 pellets are prepared in laboratory by calcinating γ-Al2O3 in air at 1,200°C for 3 h. As shown in Fig. 1, the results of XRD analysis demonstrated calcination of γ-Al2O3 in air could obtain the α-Al2O3 needed for the experiment.
Nitrogen BET measurements were carried out for the materials applied. The results are listed in Table 1. For molecular sieves, the average pore diameter increased as the BET surface areas increased. For instance, the BET surface areas of MS-3A, MS-4A, MS-5A, and MS-10A are 22.50, 290.54, 370.86, and 456.56 m2/g, and the average pore diameters of MS-3A, MS-4A, MS-5A, and MS-10A are 3.52, 4.32, 5.20, and 10.12 Å, respectively. For aluminum oxides, α-Al2O3 is nonporous while γ-Al2O3 is porous. Therefore, the BET surface area of α-Al2O3 was relatively small (c.a. 9.79 m2/g) compared to that of γ-Al2O3, and the average pore diameters of α-Al2O3 was not analyzed.
Experimental Tests of C3F8 Abatement
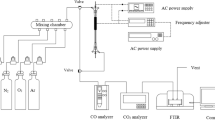
The laboratory-scale experimental system used to evaluate the effectiveness of C3F8 decomposition is schematically shown in Fig. 2. The experimental apparatus was comprised of a gas-generation system, high-voltage power supply system, a plasma reactor, and sampling detection system. The gas-generation system consisted of several gas cylinders, including C3F8/N2, N2, and O2 and was regulated by a set of mass flow meters (MKS-1179A) to control the flow rates of feeding gases. The composition of the gas streams could be accurately controlled by mass flow meters.
The high-voltage power supply system consists of a power meter (Chen-Hwa Co., Model 2100, Power-Win Technology) and a high-voltage transformer (Jui-Hsiang PTY Co. Ltd.). For all experiments carried out in this study, the applied frequency was fixed at 200 Hz and the applied voltage ranged from 12 to 18 kV (rms value). The discharge power was determined by the Lissajous diagram (V-Q plot) [10] which was measured by a digital oscilloscope (Tektronix 3014B) equipped with a high voltage probe (Tektronix P6015A) and a voltage probe (Tektronix 613B). A 10 μF capacitor was connected in series with the reactor for the measurement of the charge.
The laboratory-scale plasma reactor consisting of cylindrical tube, inner electrode, and outer electrode is schematically described in Fig. 3. The cylindrical tube was made of quartz with outer and inner diameters of 2.8 and 2.5 cm, respectively. The inner electrode was composed of a stainless steel rod (diameter, 0.3 cm) and was located along the tube’s centerline. The outer electrode, made of stainless steel mesh, was wrapped around the outside of the crystal quartz tube. The discharge length is 10 cm. For the simultaneous combination of plasma, adsorption and catalysis, three different configurations are tested in this study, including A/C (adsorbent layer prior to catalyst layer), C/A (catalyst layer prior to adsorbent layer) and A/C mixture (adsorbent and catalyst are mechanically mixed). The experimental tests were conducted at different temperatures varying from room to high temperature (ca. 25–250°C) and gas streams were kept at ambient pressure in the plasma reactor for all tests. For all the experimental tests for C3F8 abatement by plasma (including CPC, CPA and CPAC), the gas stream consists of 500 ppm C3F8, 2% O2, and balanced N2. O2 content is fixed at 2% because our experimental results show that C3F8 removal efficiency increases with O2 content up to 2% but further increase in O2 content would lead to a lower removal efficiency instead.
Qualitative and quantitative analysis of C3F8 and associated end products including CO2, CO and CF4 were carried out with FT-IR (Nicolet 6700, Thermo). The C3F8 removal efficiency is calculated by:
Here, [C3F8]inlet and [C3F8]outlet are the concentration of C3F8 at the inlet and the outlet of reactor, respectively. The selectivities of CO2, CO and CF4 are determined by:
where [CO2], [CO] and [CF4] are the concentrations of CO2, CO, and CF4 at outlet of reactor, respectively.
Results and Discussion
Thermal Catalysis Test
The light-off curves for C3F8 removal achieved with molecular sieve (MS-10A) and aluminum oxide (γ-Al2O3, and α-Al2O3) are presented in Fig. 4. The blank test was carried out without packing. Apparently, MS-10A and α-Al2O3 do not have significant catalytic activity for C3F8 abatement while γ-Al2O3 starts showing catalytic activity at 300°C.
Adsorption Test
For all adsorption capacity tests, the adsorbent was packed in a glass tube with an inner diameter of 2.5 cm and the packing height was kept at 2.0 cm. The feeding gases contained 500 ppm C3F8, 2% O2, and balanced N2. The gas flow rate, temperature, and pressure were controlled at 125 sccm, 25°C, and 1 atm, respectively. The adsorption capacity is determined by (5):
where P is the pressure (atm), M.W. is the molecular weight of C3F8 (g/mol), Q is the total gas flow rate (SLPM), m is the mass of adsorbent (g), R is the ideal gas constant (atm-L/mol-K), T is the gas temperature (K), t is the time required to reach adsorption saturation (min), and [C3F8] i and [C3F8] are the inlet and outlet concentrations of C3F8 (ppm), respectively.
The adsorption capacities of molecular sieves (MS-3A, MS-4A, MS-5A, and MS-10A) and aluminum oxides (γ-Al2O3 and α-Al2O3) for C3F8 are presented in Fig. 5. C3F8 adsorption capacity is in the order of MS-10A > MS-5A > MS-4A > MS-3A > γ-Al2O3 > α-Al2O3.
In general, the BET surface area and average pore diameter are the important parameters in determining adsorption capacity, suggesting that it can be used as an indicator for selecting the appropriate adsorbent. Figure 6a and b shows the relationships between the BET surface area and average pore diameter and the adsorption capacity obtained with all the materials tested in this study. If one just focuses on the results obtained with molecular sieves, it seems that the adsorption capacity is strongly related to the average pore size. For instance, the average pore diameter of MS-3A, MS-4A, MS-5A and MS-10A are 3.52, 4.32, 5.2, 10.12 Å and the corresponding adsorption capacities are 0.92, 0.93, 0.98, 13.90 mg/g, respectively. Since the size of C3F8 molecule is 5.450 Å [11], the adsorption capacity should be low when the average pore size is smaller than 5.450 Å, which is consistent with the experimental results. However, it needs to be emphasized that the average pore size seems not the only factor dominating the adsorption capacity. For instance, the average pore size of γ-Al2O3 is actually greater than the molecule size of C3F8, however, the adsorption capacity for C3F8 is still insignificant. Further study on this topic is needed to establish a suitable indicator to provide useful information for the selection of an appropriate adsorbent.
Relationship between BET surface area (a) and average pore diameter (b) and C3F8 adsorption capacity for various materials tested in this study. Experimental conditions are the same as that described in Fig. 5
Comparison of C3F8 Abatement Via CPC, CPA and CPACs
γ-Al2O3 and MS-10A are selected for the experimental tests for CPC and CPA because of their good adsorption capacity and catalytic activity for C3F8, respectively. The dependence of C3F8 removal efficiency on the operating temperature of the system combining plasma with catalyst or adsorbent is presented in Fig. 7.
As shown in Fig. 7, the performance of CPA is better than that of CPC when the operating temperature is between 50 and 150°C. The C3F8 removal efficiencies of α-Al2O3 and CPC were almost the same, suggesting that catalysis is ineffective for C3F8 abatement at this temperature window. The reason is mainly attributed to the low activity of catalysts when the temperature is controlled at the range of 50–150°C. On the contrary, CPC could achieve better performance instead as the temperature is raised to 250°C. Higher C3F8 removal efficiency achieved with CPC results from the higher activity of catalysts. The C3F8 removal efficiency of CPA decreases with increasing operating temperature because higher temperature is unfavorable for physical adsorption.
In order to combine the advantages of the two types of packing materials tested in this study, the co-operation of adsorption and catalysis was studied. MS-10A was mainly applied to enhance the adsorption, whereas γ-Al2O3 was used as the catalytically active component. The combination was realized by introducing the same volumes of adsorbent and catalyst in the arrangement of separate layers (A/C layer or C/A layer) or a layer of mechanical mixture (A/C mixture). Here, A/C layer represents the adsorbent is placed upstream of the catalyst while C/A layer stands for the location of catalyst is ahead of adsorbent.
Dependence of C3F8 removal efficiency on the operating temperature achieved with CPACs with different configurations is presented in Fig. 8. When the operating temperature is below 150°C, the performance of C3F8 abatement is in the order of A/C layer ≈ A/C mixture > C/A layer at a fixed temperature. The purpose of packing adsorbent within the plasma reactor is to increase C3F8 concentration and retention time in the discharge zone. In the configuration of C/A layer, C3F8 is immediately adsorbed once the gas stream enters the plasma reactor. Therefore, compared with CPAC (C/A layer), it can be expected that the probability of the collision between C3F8 and the active species generated in plasma would be higher in CPAC (A/C layer). As for CPAC (A/C mixture), it can be considered that half of the adsorbent is placed in the first of the plasma reactor with the assumption that adsorbent and catalyst are homogeneously mixed. As a result, it is not surprising that CPAC (A/C mixture) achieved better performance than CPAC (C/A layer). Nevertheless, it needs to be noticed the differences between the C3F8 removal efficiencies achieved with different CPACs are less significant as the temperature is increased.
On the other hand, by comparing the results presented in Figs. 7 and 8, it can be concluded that C3F8 removal efficiencies obtained with different plasma reactors are in the order as: CPAC (A/C layer; AC mixture) > CPA (plasma with adsorbent alone) > CPC (plasma with catalyst alone) > CPAC (C/A layer), confirming the benefits of the simultaneous combination of adsorption, catalysis and plasma for C3F8 removal.
Products Selectivity and Carbon Balance
The products’ selectivities obtained with CPACs (A/C layer and AC mixture) are shown in Fig. 9. The major end products identified by FT-IR include CO2, CO, N2O and CF4. The concentrations of N2O ranged from 150 to 300 ppm, depending on the reactor configuration and operating temperature. Moreover, it is worth noticing that both NO and NO2 are not detected in this study; however, the reasons for this interesting phenomenon is still unclear and further study is needed. The carbon balance ranges from 95 to 100%, meaning that most of the carbon from C3F8 removal has been transformed to CO2, CO, and CF4. Furthermore, it needs to be emphasized that the formation of C2F6 is not observed in this study, indicating that the novel technique proposed is promising for C3F8 abatement in that the GWP of C2F6 is higher than that of C3F8.
Conclusions
Several novel plasma reactors, including CPC, CPA and CPACs (A/C layer, C/A layer and A/C mixture) are experimentally tested for C3F8 abatement in this study. The dependences of C3F8 removal efficiencies obtained with CPC and CPA on the temperature are completely different. For CPC, increasing temperature is beneficial for C3F8 decomposition due to the increased catalytic activity. On the contrary, increasing temperature is unfavorable for C3F8 abatement in CPA because higher temperature would result in a lower adsorption capacity. Moreover, the experimental results show that CPAC could achieve better performance than CPC and CPA. However, one must be careful in choosing the right configuration. For example, CPAC (C/A layer) would lead to poorer performance compared with CPC and CPA. On the other hand, it needs to be mentioned that the global warming potential of C2F6 is actually higher than that of C3F8. Therefore, the plasma treatment would be meaningless if most of the C3F8 molecules decomposed are converted into C2F6. Fortunately, the generation of C2F6 is not observed in this study, suggesting the feasibility of C3F8 abatement via CPAC.
References
Holzer F, Roland U, Kopinke F-D (2002) Combining of nonthermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds Part 1. Accessibility of the intra-particle volume. Appl Catal B-Environ 38:163–181
Kim HH, Ogata A, Futamura S (2006) Effect of different catalysts on the decomposition of VOCs using flow-type plasma-driven catalysis. IEEE Trans Plasma Sci 34:984–995
Kim HH (2004) Nonthermal plasma processing for air-pollution control: A historical review, current issues, and future prospects. Plasma Process Polym 1:91–100
Chang MB, Lee HM (2004) Abatement of perfluorocarbons with combined plasma catalysis in atmospheric-pressure environment. Catal Today 89:109–115
Magureanu M, Mandache NB, Gaigneaux E, Panu C, Parvulescu VI (2006) Toluene oxidation in a plasma-catalytic system. J Appl Phys 99:123301
Chavadej S, Saktrakool K, Rangsunvigit P, Lobban LL, Sreethawong T (2007) Oxidation of ethylene by a multistage corona discharge system in the absence and presence of Pt/TiO2. Chem Eng J 132:345–353
Subrahmanyam Ch, Magureanu M, Renken A, Kiwi-Minsker L (2006) Catalytic abatement of volatile compounds assisted by non-thermal plasma Part 1. A novel dielectric barrier discharge reactor containing catalytic electrode. Appl Catal B Environ 65:150–156
Harling AM, Demidyuk V, Fischer SJ, Whitehead JC (2008) Plasma-catalysis destruction of aromatics for environmental clean-up: Effect of temperature and configuration. Appl Catal B-Environ 82:180–189
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Removal of volatile organic compounds (VOCs) by single-stage and two-stage plasma catalysis systems: a review of the performance enhancement mechanisms, current status and suitable applications. Environ Sci Technol 43:2216–2227
Manley TC (1943) The electrical characteristic of the ozonator discharge. Trans Electrochem Soc 52:5820–5828
Gindulytè A, Mass L, Banks BA, Miller SKR (2002) Direct C-C bond breaking in the reaction of O (3P) with flouropolymers in low earth orbit. J Phys Chem A 106:5463–5467
Acknowledgments
The authors gratefully acknowledge the financial support provided by Industrial Technology Research Institute of Taiwan (grant number 985914).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, B.Y., Chang, M.B., Chen, H.L. et al. Removal of C3F8 Via the Combination of Non-Thermal Plasma, Adsorption and Catalysis. Plasma Chem Plasma Process 31, 585–594 (2011). https://doi.org/10.1007/s11090-011-9303-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-011-9303-6