Abstract
Soil pollution due to potentially toxic elements is a worldwide challenge for health and food security. Chelate-assisted phytoextraction along with the application of plant growth regulators (PGRs) could increase the phytoremediation efficiency of metal-contaminated soils. The present study was conducted to investigate the effect of different PGRs [Gibberellic acid (GA3) and indole acetic acid (IAA)] and synthetic chelator (EDTA) on growth parameters and Cd phytoextraction potential of Dysphania ambrosioides (L.) Mosyakin & Clemants grown under Cd-spiked soil. GA3 (10−7 M) and IAA (10−5 M) were applied four times with an interval of 10 days through a foliar spray, while EDTA (40 mg kg−1 soil) was once added to the soil. The results showed that Cd stress significantly decreased fresh biomass, dry biomass, total water contents, and photosynthetic pigments as compared to control. Application of PGRs significantly enhanced plant growth and Cd phytoextraction. The combined application of GA3 and IAA with EDTA significantly increased Cd accumulation (6.72 mg pot−1 dry biomass) and bioconcentration factor (15.21) as compared to C1 (Cd only). The same treatment significantly increased chlorophyll, proline, phenolic contents, and antioxidant activities (CAT, SOD, and POD) while MDA contents were reduced. In roots, Cd accumulation showed a statistically significant and positive correlation with proline, phenolics, fresh biomass, and dry biomass. Similarly, Cd accumulation showed a positive correlation with antioxidant enzyme activities in leaves. D. ambrosioides showed hyperaccumulation potential for Cd, based on bioconcentration factor (BCF) > 1. In conclusion, exogenous application of GA3 and IAA reduces Cd stress while EDTA application enhances Cd phytoextraction and ultimately the phytoremediation potential of D. ambrosioides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil contamination with potentially toxic elements such as heavy metals is a worldwide challenge for health and food security (Ahmad et al. 2020; Heidari et al. 2020; Rizwan et al. 2021). These are ubiquitous in the environment with longer persistence against biodegradation (Naveed et al. 2020; Ullah et al. 2020). Their entrance into the food chain may lead to serious implications (gastrointestinal cancer, lung cancer, disturbances of the nervous system, bone fracture, hypertension, kidney dysfunction, etc.) for human health (Rai et al. 2019). Among heavy metals, cadmium (Cd) is a very toxic metal, enters into the soil from different sources, and bioaccumulates in crop plants (Chen et al. 2019). Cd pollution has been of serious concern in many countries especially in China where its concentrations up to 100 mg kg−1 soil were reported in Cd-contaminated soils (Wang et al. 2019). In Pakistan, there is a large variation of Cd contamination in soils and ranges from 0.02 to 184.0 mg kg−1 under normal and contaminated soils, respectively (Muhammad et al. 2011). The permissible limit of Cd in drinking water is 0.1 mg L−1 (NEQS-Pak 2000). It badly affects plant growth in different ways, i.e., disturbs photosynthesis and respiration processes (Marques et al. 2018; Saleem et al. 2020; Sabir et al. 2020), nutrients uptake (Chibuike and Obiora 2014), and ultimately reduces crop growth and productivity (Mehmood et al. 2018a, 2018b).
Under Cd stress, reactive oxygen species (ROS) are generated due to ionic imbalance (Ijaz et al. 2020; Jan et al. 2017; Yan et al. 2013). To scavenge higher ROS levels, plants utilize an efficient enzymatic (peroxidase, superoxide dismutase, catalase, ascorbate) and nonenzymatic (flavonoids, phenolics, and tocopherols) antioxidant system (Foyer and Noctor 2011; Irshad et al. 2021; Liu et al. 2020). This system protects and stabilizes the membranous structures in the cell against abiotic stress (Imtiaz et al. 2018; Leng et al. 2020; Niamat et al. 2019; Zhang et al. 2018).
To control soil contamination with Cd, various physicochemical and biological methods have been applied. Conventional physicochemical methods require huge technological resources with indirect environmental contamination (Amin et al. 2018; Mehmood et al. 2019). In biological methods, phytoextraction has been an auspicious technique among various phytoremediation techniques to clean metal-contaminated soils due to its cost-effectiveness and in-situ advantages (Ahmad et al. 2016). This technique utilizes certain plants that accumulate higher concentrations of metals from soil in different harvestable parts, thereby reducing metal concentration in the soil (Mahmood 2010). For example, Salix viminalis removed 47 g Cd ha−1 in a field experiment after 2 years from an acidic soil (Hammer et al. 2003). Recently, the application of citric acid significantly decreased the Cd concentration in Cd-contaminated soil (3.68 mg kg−1) by 16.9% via three harvestings of Celosia argentea L. in an 18-month field experiment. Overall, annual Cd extraction and biomass production in Celosia argentea L. was 273 g ha−1 and 8.79 t ha−1, respectively (Yu et al. 2020). From the above discussion, the effectiveness of phytoextraction under field conditions is wholly dependent on the biomass production and bioaccumulation potential of the plant species being used, soil properties, the time required for maximum biomass production, and subsequent removal of metals. Moreover, phytoextraction efficiency increases with the application of certain amendments that enhance the phytoavailability of metals as in the case of Yu et al. (2020).
Under heavy metal stress, plant growth and biomass are severely reduced, posing a new challenge for successful bioremediation. In this regard, researchers have adopted several strategies to increase plant growth and biomass e.g., use of different plant growth regulators (PGRs). In plants, various PGRs are biosynthesized, which enhance tolerance against heavy metal stress (Chen et al. 2020; Mousavi et al. 2020; Santos Neri Soares et al. 2020; Zhang et al. 2020). Among various PGRs, gibberellic acid (GA3) stimulates cell elongation, thereby increasing the dry biomass of the crop plants (Hadi et al. 2010). Similarly, the growth and development of crop plants are enhanced with indole acetic acid (IAA) under abiotic stress (Korver et al. 2018; Zhou et al. 2020).
Wild plants, being an important component of the ecosystem, play an important role in keeping the environment clean from heavy metal contamination. Among wild plants, weeds such as species of the genus Dysphania are commonly found at farmers’ fields and are widely distributed around the world (James et al. 2005). It belongs to the Amaranthaceae family and perennial herb. D. ambrosioides is a herb and its oil extracted from its leaves is very useful from a medicinal point of view. During phytoextraction, most of the accumulation occurs in the root and shoot portion especially in stem and seeds, and a minute concentration in leaves (Qin et al. 2020). Moreover, due to its rapid growth, stress tolerance, and unpalatable nature, D. ambrosioides was selected as a test plant for the present research study. Therefore, it would be safe to use D. ambrosioides as a herb, as well as a phytoextractant.
There are many reports about the exogenous application of PGRs to enhance abiotic stress tolerance via regulating various physiological and biochemical processes in plants (Ali and Hadi 2015; Bashri and Prasad 2016; Cabello-Conejo et al. 2014; Chen et al. 2020; Hadi et al. 2010; Korver et al. 2018; Mousavi et al. 2020). Certain agents such as ethylene diamine tetraacetic acid (EDTA), Di-iso-propanol-amine (DIPA), bacteria, and mycorrhiza have been used to enhance metal bioavailability (Arshad et al. 2020; Konkolewska et al. 2020; Saffari and Saffari 2020). However, EDTA is a synthetic chelator and has great potential to enhance metal bioavailability and ultimately increased the phytoextraction of the metal (Anning and Akoto 2018; Li et al. 2020; Saffari and Saffari 2020). In this regard, the use of EDTA and PGRs to enhance metal bioavailability and growth under metal stress can be a good strategy for phytoremediation of Cd using D. ambrosioides. Up to our knowledge, no study has been conducted on the phytoextraction potential of D. ambrosioides under Cd stress. Therefore, the present study was designed to explore the impact of exogenously applied PGRs (GA3 and IAA) and a chelating agent (EDTA) in improving the phytoextraction potential of D. ambrosioides under Cd-contaminated soil.
Materials and methods
Soil analysis
The soil was collected from the University Research farm area at depth of 0–15 cm, air-dried, and ground to final particle size (2 mm). After sieving, about 01 kg of soil was put in plastic pots (18 × 15 cm). The soil was analyzed regarding various physicochemical characteristics following standard methods (Estefan et al. 2013). The soil had a water-holding capacity of 21.8% (v/w). From 1:2 (w/v) ratio of soil to water suspension, pH 6.8 and EC 1.5 dS m−1 were measured through a pH meter (InoLab-WTB GmbH; Weilheim, Germany) and an electrical conductivity meter (WTW–330i). The soil was loamy sand in texture with silt (44%), sand (54%), and clay (16%), lime (2.5%), and organic matter (1.6%).
Pot experiment
For Cd contamination, the soil was spiked with cadmium acetate dihydrate (Cd(CH3COO)2∙2H2O, 98.0%; Sigma-Aldrich, St. Louis, MO) at a rate of 100 mg Cd kg−1 and kept for 10 days. Treatments used during current study were C = without Cd; C1 = Cd only; T1 = Cd + EDTA; T2 = Cd + GA3; T3 = Cd + GA3 + EDTA; T4 = Cd + IAA; T5 = Cd + IAA + EDTA and T6 = Cd + GA3 + IAA + EDTA. In the present study, the Cd treatment (100 mg Cd kg−1 soil) used was selected based on the capability of hyperaccumulators to bioaccumulate Cd in biomass, i.e., 100 mg Cd kg−1 DW (Baker and Brooks 1989; Baker et al. 2000). Before seedling transplantation, the pots were well irrigated. After 24h, two uniform seedlings were transferred into each pot. The pots were arranged following a completely randomized design (CRD) in the glasshouse, having five replicates. Tap water was used to irrigate pots twice a week. Recommended agronomic practices such as weeding and hoeing were practiced during the whole study period. The growth conditions in the glasshouse were as follows: temperature = 30±1 °C, relative humidity = 70–80%, and duration of light/dark 14 h/10 h, respectively. After 15 days of seedling transplantation, GA3 (0.1 μM) and IAA (10 μM) were exogenously applied as a foliar spray in four doses at an interval of 10 days. Similarly, aqueous solutions of EDTA (40 mg EDTA kg−1 soil) was added to the pots in a single dose after 15 days of seedling transplantation.
Plant growth and biomass
After 40 days, the plants were harvested from each pot separately. After harvesting, the solution containing EDTA (5 mM) and Tris HCl (5 mM, pH 6.0) was used to wash each plant root to remove surface-bound metal ions (Genrich et al. 2000). Root and shoot length was measured using a common steel scale. For fresh biomass, leaf, stem, and root portions of each plant were separated and fresh biomass of each part was measured using a digital balance. For dry biomass, each part was kept separately in an oven and dried at 70 °C for 2 days until a constant weight was achieved, using a digital balance. For further analysis, the plant samples were crushed into a fine powder using a mortar and pestle.
Proline
Free proline contents in leaf and root samples were estimated following Bates et al. (1973). Briefly, leaf and root samples were thoroughly washing with sterile distilled water. About 200 mg of each sample was homogenized in sulfosalicylic acid (1.5 mL, 3% W/V) and centrifuged at 1300 rpm for 5 min. The supernatant (300 μL) was mixed with acid ninhydrin and glacial acetic acid in a test tube. To stop the reaction, the mixture was first boiled and then immediately dipped in ice. After dipping in ice, toluene (1 mL) was added to the reaction mixture and shaken briskly for 30–40s. The absorbance of the mixture was measured at 520 nm using a spectrophotometer (UV-1700 Shimadzu). Pure toluene was run as blank.
Total phenolics
For total phenolics, leaf and root samples from each treatment were collected and dried in shade at room temperature. Each sample (200 mg) was ground separately into powdered form, added to Eppendorf tubes containing methanol, and kept for 4 h. After centrifugation of the mixture at 1300 rpm for 5 min, the supernatant (0.5 mL) was transferred into fresh Eppendorf tubes containing the Folin–Ciocalteu reagent (2.5 mL, 10% V/V or W/V), NaHCO3 (2.5 mL, 7.5%), and distilled water (2.5 mL) and kept for 1 h in dark. The absorbance of the reaction mixture was measured at 650 nm using a spectrophotometer. The calibration curve was used to calculate the concentration of total phenolic contents and was expressed as mg gallic acid equivalent of phenol g−1 sample (Malik and Singh 1980).
Chlorophyll contents
The photosynthetic pigments in different treatments were estimated following the methods of Arnon (1949). The leaf sample (200 mg) was homogenized with acetone (80%) and ground well. The mixtures were centrifuged, and the supernatant was transferred into a test tube, making volume up to 6 mL with acetone. The absorbance of the mixture was recorded at 645 nm and 663 nm using a spectrophotometer (UV-1700 Shimadzu). The following formula was used to calculate the total chlorophyll contents:
Antioxidant compounds
Sodium phosphate buffer (50 mM, pH 7.8) was mixed with fresh leaves collected separately from each treatment. The mixture was centrifuged, and the supernatant was used to determine various antioxidant enzyme activities such as catalase (CAT) using the methods of Aebi (1984) and superoxide dismutase (SOD) following the method of Dhindsa et al. (1982). For peroxidase (POX) activity, fresh leaves were homogenized in (5 mL) 50 mM sodium phosphate (pH 5.5) containing 0.2 mM EDTA-Na2. The supernatant was used to measure POX activity following Nakano and Asada (1981).
Measurement of membrane damage
The MDA (malondialdehyde) contents were estimated following the method of Hodges et al. (1999). The absorbance of the supernatant was measured by spectrophotometer at 440, 532, and 600 nm.
Acid digestion and Cd determination
Before acid digestion, the harvested plant parts were washed with tap water followed by deionized water, oven-dried, and ground into a fine powder. About 0.5 g fine powder was taken and added into 12 mL of HNO3/HClO4 mixtures at the ratio of 3:1. The mixture was kept on a hot plate for 2 h at 300 °C until a clear supernatant was obtained. The clear solution was filtered and finally made volume up to 50 mL using distilled water. Atomic absorption spectrometer (Perkin-Elmer, AAnalyst 800) was used to determine the Cd concentration in different parts of the plant.
Phytoremediation efficiency
Bioconcentration and translocation factors were used to determine Cd accumulation and its translocation into different parts of D. ambrosioides. These factors determine the phytoremediation potential of the plant. Both factors were calculated using the following formula:
Phytoextraction efficiency
The amount of heavy metals accumulated in the dry aboveground biomass of the plants and the plant yields determine the phytoextraction efficiency of plants. In this regard, remediation factor (RF) represents the percentage of an element removed by the plant dry aboveground biomass from the total element content in the soil during one cropping season (Vysloužilová et al. 2003). The following equation was used to calculate the RF during the present study.
where Cdplant is the Cd contents in plant dry aboveground biomass (mg kg−1); Bplant is the plant dry aboveground biomass (g); Cdsoil is the total Cd contents in soil (mg kg−1) and wsoil the amount of soil taken in one pot (g).
Statistical analysis
Statistical analysis of collected data and Pearson’s correlation among different parameters were performed using the GraphPad Prism package, version 8.0 (GraphPad Software, San Diego, CA, USA) and Microsoft Office Excel 2013 (Redmond, WA, USA). The treatment means were compared by Duncan’s multiple range posthoc test (DMRT) at a significance level of P < 0.05.
Results
Plant growth and yield
The data regarding growth and yield parameters of D. ambrosioides showed that Cd stress significantly reduced plant height and fresh and dry biomass (Table 1). However, this stress was significantly reduced with the application of different PGRs (GA3 and IAA) and EDTA. On growth and yield parameters, the impact of GA3 was more pronounced as compared to IAA while EDTA played an important role in increasing Cd availability to the crop plants. The maximum root and shoot length (24.0 and 48.0 cm) were recorded with the combined application of PGRs (GA3 and IAA) and EDTA (T6) while that of the minimum (7.0 and 20.0 cm) was observed under cadmium stress with the application of EDTA under Cd stress (T1). A similar trend was observed in the case of fresh and dry biomass of different portions of D. ambrosioides. In the case of fresh and dry biomasses, the maximum (40.55 and 9.46 g) observed in T6 was 2.14 and 2.95 times more as compared to that of the minimum (18.96 and 3.21 g) observed in T1.
Physiological parameters
Cd stress significantly reduced physiological (chlorophyll contents and relative water contents) parameters of D. ambrosioides (Table 2). In the case of chlorophyll contents, the enhanced Cd availability due to the application of EDTA resulted in the minimum chlorophyll contents (3.69 μg g−1) of D. ambrosioides. Application of different PGRs significantly reduced Cd stress and their effect was more pronounced when application combined compared to their sole application. Combined application of IAA and GA3 significantly enhanced chlorophyll contents to maximum, i.e., 13.03 μg g−1 and it was almost 3 times more as compared to that recorded with the sole application of EDTA. In the case of total water contents, the maximum (25.2 g) was observed with the combined application of IAA, GA3, and EDTA under Cd stress (Table 2).
Antioxidant enzyme activities
The application of PGRs and EDTA under Cd stress had a significant effect on antioxidant enzyme activities (Fig. 1). Sole application of EDTA enhanced Cd availability and ultimately resulted in increased Cd stress. Under Cd stress, the activities of SOD, CAT, and POX were significantly reduced compared to the control treatment. This stress was relieved with the sole or combined application of IAA and GA3. Overall, the combined application of GA3, IAA, and EDTA caused the maximum increase in the activities of all studied antioxidant enzyme activities as compared to control.
Effect of different treatments on antioxidant assays of Chenopodium ambrosioides under Cd-contaminated soil where a catalase (CAT) activity, b superoxide dismutase (SOD) activity, and c peroxidase (POX) activity. Where C = normal soil; C1 = Cd only; T1 = Cd + EDTA; T2 = Cd + GA3; T3 = Cd + GA3 + EDTA; T4 = Cd + IAA; T5 = Cd + IAA + EDTA; and T6 = Cd + GA3 + IAA + EDTA. In all Cd treatments, Cd was applied @ 100 mg Cd kg−1 soil. EDTA, GA3, and IAA were applied @ 40 mg EDTA kg−1 soil, 0.1 μM and 10 μM, respectively
Stress-related metabolites
The effect of different PGRs (IAA and GA3) and EDTA under Cd stress on the biosynthesis of proline and phenolics in leaf and root samples is presented in Fig. 2a, b. Significantly higher free proline and total phenolic contents were recorded in root and leaf samples under all treatments as compared to control. The combined application of GA3 and IAA with EDTA resulted in the highest free proline biosynthesis in roots (85.0 μg g−1) and leaves (71.4 μg g−1) under Cd stress. More proline biosynthesis was observed in roots compared to the leaves. Similarly, the maximum biosynthesis of total phenolic contents in roots (95.0 μg g−1) and leaves (157.0 μg g−1) was observed with the combined application of GA3 and IAA with EDTA under Cd stress. More phenolic contents were biosynthesized in leaves compared to the root samples of D. ambrosioides under all treatments.
Effect of different treatments on stress-related metabolites of Chenopodium ambrosioides under Cd-contaminated soil. a Total phenolics, b proline contents, c MDA contents, and d total soluble proteins. Where C = normal soil; C1 = Cd only; T1 = Cd + EDTA; T2 = Cd + GA3; T3 = Cd + GA3 + EDTA; T4 = Cd + IAA; T5 = Cd + IAA + EDTA; and T6 = Cd + GA3 + IAA + EDTA. In all Cd treatments, Cd was applied @ 100 mg Cd kg−1 soil. EDTA, GA3, and IAA were applied @ 40 mg EDTA kg−1 soil, 0.1 μM and 10 μM, respectively
Under abiotic stress, the production of reactive oxygen species (ROS) results in lipid peroxidation and ultimately damages cellular membranes. The extent of cellular membrane damage is measured through the estimation of MDA contents. The production of MDA contents was enhanced under Cd stress both under sole application of Cd and sole application of EDTA (Fig. 2a). In comparison to the control, exogenous application of GA3 and IAA significantly reduced lipid peroxidation, i.e., MDA contents. Overall, the combined application of GA3 and IAA with EDTA significantly reduced MDA contents compared to the control and other treatments.
In the case of total soluble proteins, an opposite trend as observed in the case of MDA contents was recorded. Here, minimum total soluble protein production was observed as Cd stress was increased as observed with the sole application of EDTA under Cd stress. The maximum total soluble proteins were produced with the combined application of GA3 and IAA with EDTA under Cd stress.
Cadmium dynamics in D. ambrosioides
As clear from the data presented in Table 3, all treatments significantly increased Cd concentration in root, stem, and samples of D. ambrosioides as compared to the sole application of Cd (C1). Roots followed by leaves accumulated the maximum Cd concentration. Sole application of EDTA under Cd stress resulted in enhanced Cd concentration in the root (334.0 mg kg−1), stem (118.0 mg kg−1), and leaf samples (280.0 mg kg−1). Similarly, foliar application of GA3 and IAA also significantly increased Cd uptake as compared to C1. The maximum Cd contents were found in the root (1521.0 mg kg−1), stem (214.0 mg kg−1), and leaves (449.0 mg kg−1) with the combined application of GA3, IAA, and EDTA. Overall, enhanced bioaccumulation of Cd within plant tissues was recorded under all the treatments. The combined application of GA3, IAA, and EDTA significantly increased Cd accumulation in the root (4.50 mg Cd pot−1 dry biomass), stem (0.64 mg Cd pot−1 dry biomass), leaves (1.58 mg Cd pot−1 dry biomass), and consequently within the entire plant (6.72 mg Cd pot−1 dry biomass) as shown in Table 3. A similar trend was observed in the case of percent accumulation of Cd in different portions of D. ambrosioides. Based on the accumulation pattern of Cd, D. ambrosioides can be regarded as a hyperaccumulator as it accumulated (2.22/6.50)*1000 = 341.5 mg Cd kg−1 dry biomass of shoot.
All treatments either applied sole or in combination showed a significant effect on Cd translocation into different parts of the plant (Table 3). The maximum translocation of Cd was observed from stem to leaves. Similarly, Cd translocation from root to leaves was more as compared to root to stem. Cd translocation from root to stem was found less than 1 under all treatments which showed that Cd concentration in roots was higher compared to the Cd concentration in stem and leaves. The minimum bioconcentration factor, i.e., 1.81 in D. ambrosioides was recorded under Cd stress without application of PGRs or EDTA (C1) as shown in Table 3. All other treatments showed statistically significant and higher Cd bioconcentration values as compared to C1 (Cd only). The highest Cd bioconcentration value (15.21) was recorded with the combined application of GA3, IAA, and EDTA under Cd stress (Table 4).
As clear from Table 3, remediation factor (RF) was calculated to assess the phytoextraction potential of D. ambrosioides. The results showed that the maximum RF value (6.72%) was observed under the combined application of PGRs and EDTA under Cd stress while that of the minimum value (0.49%) was observed without application of PGRs and EDTA under Cd stress.
Correlation analysis
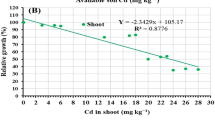
All the parameters measured from the root samples showed a statistically significant and positive correlation with Cd concentration (Tables 4 and 5). As clear from the data presented in Table 4, Cd concentration in root samples showed a positive and statistically significant correlation with total water content (R2 = 0.82), fresh (R2 = 0.75), and dry biomass (R2 = 0.89). Similarly, Cd concentration in the root also showed a highly significant positive correlation with total phenolic (R2 = 0.95) and proline contents (R2 = 0.95). With Cd concentration, almost all parameters measured from leaf samples showed a significant positive correlation. The Cd concentration in leaves showed significant correlation with dry biomass (R2 = 0.88), total water content (R2 = 0.83), SOD (R2 = 0.88), POX (R2 = 0.57) and CAT (R2 = 0.90). Similarly, proline and total phenolic contents also showed a statistically significant correlation (R2 = 0.97 and R2 = 0.65), respectively with Cd concentration (Table 5)
Discussion
Heavy metal (HMs) stress is among one of the abiotic stresses that limit plant growth and metabolism, which eventually reduces crop yield and biomass (Ahmad et al. 2016). Heavy metals after entering the plant body are known to induce toxic effects on photosynthetic pigments, biosynthesis of reactive oxygen species (ROS), and assimilation of heavy metals instead of micronutrients (Irshad et al. 2021; Liu et al. 2020; Mehmood et al. 2018a, 2018b). This situation is very critical especially during the phytoextraction of HMs where the main objective to have larger biomass for the higher accumulation of HMs. In response to HMs stress, plants activate their defense system via the production of various plant growth regulators (PGRs) such as gibberellic acid (GA3) and indole acetic acid (IAA). Recently, various researchers have found that phytohormones play an important role in the amelioration of abiotic stress (Cabello-Conejo et al. 2014; Chen et al. 2020; Ghorbani et al. 2019; Mousavi et al. 2020; Santos Neri Soares et al. 2020; Zhang et al. 2020; Zhou et al. 2020).
In the present investigation, the main objective was not only to enhance Cd phytoextraction but also to increase plant biomass for the maximum accumulation of Cd under Cd stress. Earlier, plants grown under metal-contaminated soil showed arrested growth and biomass which eventually affect their phytoextraction potential (Denton 2007). However, the hyperaccumulators bioaccumulate higher concentrations of metals and produce large biomass (Baker and Brooks 1989; Baker et al. 2000). Reduction in plant growth and biomass under heavy metal stress is due to decreased nutrient uptake (Gopal and Rizvi 2008). In the present study, the application of Cd significantly decreased root and shoot length, biomass, and total water content of D. ambrosioides. Our results are in line with earlier reports (Hasanuzzaman et al. 2017; Saeed et al. 2019). The reduced growth of cabbage under Cd stress was also reported by Kamran et al. (2019). The reduction in growth and metabolism under stress conditions might be due to the suppression of cell division and extended cell cycle. It may also be due to the reduced number of lateral roots and inhibition of various metabolic enzymes that are directly involved in plant growth (Samantary 2002). Reduction in biomass, under Cd stress, is reported for Zea mays L. Parthenium hysterophorus L., Raphanus sativus L., Lycopersicon esculentum Mill. (Ghani et al. 2016; Hadi et al. 2010). Low water and nutrients uptake might be the main cause of growth inhibition due to Cd stress (Ahmad et al. 2016).
In the present study, foliar application of GA3 and IAA, sole or combined increased growth and biomass under Cd stress. Earlier, it has been found that PGRs especially GA3 accelerate cell division in the apex portion of the plant, shoot growth, and increased metal stress resistance due to the dilution effect (Hussain et al. 2019). PGRs are also known to enhance the root growth of the plant, which may lead to enhanced water and nutrient uptake (Bashri and Prasad 2016; Hadi et al. 2010; Zhou et al. 2020). This premise is supported by the increased total water contents of the plants with the application of GA3 and IAA under Cd stress (Table 2).
The current study showed that the concentration of Cd and accumulation in various parts of the significantly increased by PGR. Phytohormones affect the synthesis of protein, RNA, and DNA (Broughton and McComb 1971), and the multiplication of polyribosomes (Evins and Varner 1972) might contribute to higher biomass production. Ethylenediaminetetraacetic acid (EDTA) is a renowned metal chelator that enhances metal bioavailability to the crop plants and ultimately increased metal phytoextraction (Anning and Akoto 2018; Li et al. 2020; Saffari and Saffari 2020). Earlier, foliar application of PGRs significantly increased Cd accumulation in plants (Chen et al. 2020; Hadi et al. 2010). In the present study, exogenous application of PGRs enhanced plant growth, biomass, and ultimately enhanced metal stress tolerance. Earlier, it has been found that PGRs enhance the rate of transpiration, which may lead to an increase in metal uptake (Chen et al. 2020). Increased Cd accumulation may also be due to improved plant growth (Khan et al. 2016). The Cd concentration and accumulation in various parts of the plant were in the order of roots > leaves > stems (Table 3). These results are in line with Chen et al. (2019), D’Souza et al. (2013), and Sun et al. (2008).
Cd Stress significantly reduced the photosynthetic pigments in D. ambrosioides as compared to control. The reduction in photosynthetic pigment might be connected with reduced enzyme activities involved in the biosynthesis of photosynthetic pigments (Hussain et al. 2019; Saleem et al. 2020). Cd stress alters chloroplast structure by substitution of Mg with Cd, thereby resulting in reduced photosynthetic pigments (Jibril et al. 2017; Marques et al. 2018). It may also be due to chaotic epidermal and cortical layers cell division in the apex region, which results in leaves chlorosis (Yang et al. 2009).
Under Cd stress, biosynthesis of different stress-related metabolites was enhanced which otherwise protects cells from oxidative stress caused (Chen et al. 2020; Naveed et al. 2020). Stress-related metabolites such as proline and phenolics are compatible solutes and known to reduce the injurious effect of Cd (Ali and Hadi 2015; Khatamipour et al. 2011; Michalak 2006; Sakihama and Yamasaki 2002).
Among metabolites, proline is synthesized in plants during abiotic stress (Jan et al. 2017). The accumulation of free proline in the plant cell is environmental stress indicator, i.e., salinity, drought, metals stress. Proline behaves like a buffer in the cell, providing protection and stability to the cellular macromolecules (Mattioli et al. 2009). Inside the cell, proline is bound with Cd and forms a nontoxic complex of Cd-proline (Khatamipour et al. 2011). Proline plays a vital role in the detoxification of Cd within the plant cell. Its biosynthesis is also reported in several plant species under metal stress, i.e., Triticum aestivum L., Solanum nigrum L., Lycopersicon esculentum Mill., and Silybum marianum L. (Khatamipour et al. 2011; Szabados and Savoure 2010).
Phenolics are phytochemical compounds, which provide defense during abiotic stress conditions (Khan et al. 2016). Different environmental stresses are known to enhance the biosynthesis of phenolic compounds in various plants (Michalak 2006; Sakihama and Yamasaki 2002). Cd stress significantly increased total phenolic contents in D. ambrosioides (Fig. 2). Our results are in line with the finding of Ali and Hadi (2015), who reported a high concentration of phenolics under Cd stress. Phenolic compounds show vital antioxidant activities and scavenge ROS during metals stress (Michalak 2006). Moreover, phenolic compounds are capable of metal chelation and stop superoxide-driven Fenton reactions, which result in their enhanced antioxidant potential against ROS species (Kaur et al. 2008). Exogenous application of PGRs significantly enhanced biosynthesis of total phenolics in the root and leaves of D. ambrosioides in the present study. Ali and Hadi (2015) also reported similar results in the case of P. hysterophorus.
The formation of ROS in the plants is a continuous process by inescapable release of electrons onto oxygen (O2) from chloroplasts, peroxisomes, chloroplasts, or mitochondria (Michalak 2006). It has been found that the accumulation of ROS results in the oxidation of protein (enzymes) and subsequent inactivation, lipid peroxidation, and damage to nuclear structures (Leng et al. 2020; Sabir et al. 2020). To avoid oxidative injury, plants switch on the antioxidant enzymatic system (Irshad et al. 2021; Liu et al. 2020). In the current study, increased antioxidant enzyme levels, i.e., SOD, CAT, and POX were recorded with the application of PGRs. The increase might be linked to encounter oxidative stress and enhanced metal stress tolerance through the application of PGRs (Kamran et al. 2019; Leng et al. 2020; Santos Neri Soares et al. 2020; Zhang et al. 2020). These studies have confirmed that increased levels of SOD, CAT, and POX can decrease the level of active oxygen in the plant cell, thereby help in the detoxification of ROS under stress conditions.
Bioconcentration and translocation factors are important parameters for the evaluation of the phytoextraction potential of a plant species. Earlier, poplar and willow clones accumulated much higher amounts of Cd with the highest BCF values in roots ranging from 84 to 175 (He et al. 2013). The higher BCF might be due to the longer period (90 days) of growth with more biomass compared to the present study (40 days). The BCF values noted in the present study (15.21) were far more compared to Rezapour et al. (2019) who noted the maximum value of 3.1 with a growth period of 230 days. The higher values noted in the present study might be due to the use of EDTA and plant growth regulators, which not only increased Cd availability to the plants but also supported growth. In the case of the translocation factor, the values noted were low (0.14) compared to the values noted in other studies, i.e., 1.24 for He et al. (2013) and 0.22 for Rezapour et al. (2019). The phytoextraction efficiency of the present study is 6.5% and around 1.68 years would be required to gain the permissible safe limit of Cd, i.e., 0.4 mg kg−1 (WHO 2004) in the soil under the present condition of growth by D. ambrosioides. The time required for the complete removal of Cd from the soil is quite low as compared to the other studies. For example, Yu et al. (2020) reported an annual removal of 273 g Cd from the soil which would require around 30 years for complete removal of soil contaminated with Cd, i.e., 3.68 mg kg−1 soil. The time required is low as the calculations were based continuously due to the perennial nature of D. ambrosioides. In the present study, EDTA was used to enhance the phytoextraction potential of D. ambrosioides.
The RF values observed in the current study are in line with Eissa (2016) who observed similar values while using EDTA at 3, 6, and 10 mM kg−1 soil. In another study, Neugschwandtner et al. (2008) observed very low values of RF in maize (Zea mays L.) compared to that of RF values observed in the present study, which might be due to the sole application of EDTA in the former study. The higher values might be due to the additive effect of PGRs and the plant species used (Hammer et al. 2003; Yu et al. 2020).
After phytoextraction, the plant material could be pretreated through pyrolysis, composting, and compaction and finally discarded or incinerated (Sas-Nowosielskaa et al. 2004). Overall, the present study confirmed that D. ambrosioides had much potential for bioremediation of Cd-contaminated soils with a shorter growth period.
Conclusions
With the exogenous individual or combined application of plant growth regulators, i.e., GA3 and IAA, growth parameters of D. ambrosioides were significantly enhanced which otherwise were reduced under Cd stress. GA3 and IAA sole or in combination significantly increased Cd accumulation. The combined application of GA3 and IAA significantly enhanced the biosynthesis of stress-related metabolites such as proline, phenolics, SOD, CAT, POX, and MDA. Proline, phenolics, and antioxidant enzymes showed a statistically significant correlation with Cd accumulation in plants. D. ambrosioides showed higher accumulation potential of Cd as clear from the bioconcentration factor, i.e., BCF > 1. Moreover, it also acted as a phytostabilizer as clear from the values of the translocation factor, i.e., TF < 1. In conclusion, the combined application of plant growth regulators (GA3 and IAA) and chelating agent (EDTA) improves the phytoextraction potential of D. ambrosioides under Cd stress.
Data Availability
All the data and tools/models used for this work are publicly available.
References
Aebi H (1984) Catalase in vitro. In: Lester P (ed) Methods in enzymology. Academic Press, Cambridge, pp 121–126
Ahmad A, Hadi F, Ali N, Jan AU (2016) Enhanced phytoremediation of cadmium polluted water through two aquatic plants Veronica anagallis-aquatica and Epilobium laxum. Environ Sci Pollut Res 23(17):17715–17729
Ahmad I, Tahir M, Daraz U, Ditta A, Hussain MB, Khan ZUH (2020) Responses and tolerance of cereal crops to metals and metalloids toxicity. In: Hassanuzzaman M (ed) Agronomic crops. Springer, Singapore, pp 235–264. https://doi.org/10.1007/978-981-15-0025-1_14
Ali N, Hadi F (2015) Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ Sci Pollut Res 22:13305–13318
Amin H, Arain BA, Jahangir TM, Abbasi MS, Amin F (2018) Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): profitable phytoremediation with biofuel crops. Geol Ecol Landsc 2(1):51–60
Anning AK, Akoto R (2018) Assisted phytoremediation of heavy metal contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol Environ Saf 148:97–104. https://doi.org/10.1016/j.ecoenv.2017.10.014
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. Plant Physiol 24:1–15
Arshad M, Naqvi N, Gul I, Yaqoob K, Bilal M, Kallerhoff J (2020) Lead phytoextraction by Pelargonium hortorum: comparative assessment of EDTA and DIPA for Pb mobility and toxicity. Sci Total Environ 748:141496. https://doi.org/10.1016/j.scitotenv.2020.141496
Baker AJM, Brooks RR (1989) Terrestrial higher plants which accumulate metallic elements: a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biological resource for phytoremediation of metal polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of Contaminated Soils and Waters. CRC Press LLC, Boca Raton, pp 85–107
Bashri G, Prasad SM (2016) Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate glutathione cycle. Ecotoxicol Environ Saf 132:329–338. https://doi.org/10.1016/j.ecoenv.2016.06.015
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Broughton WJ, McComb AJ (1971) Changes in the pattern of enzyme development in gibberellin-treated pea internodes. Ann Bot 35:213–228
Cabello-Conejo MI, Prieto-Fernández Á, Kidd PS (2014) Exogenous treatments with phytohormones can improve growth and nickel yield of hyperaccumulating plants. Sci Total Environ 494–495:1–8. https://doi.org/10.1016/j.scitotenv.2014.06.102
Chen H, Li Y, Ma X, Guo L, He Y, Ren Z, Kuang Z, Zhang X, Zhang Z (2019) Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci Rep 9:1–13
Chen L, Long C, Wang D, Yang J (2020) Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 242:125112
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:12. https://doi.org/10.1155/2014/752708
D’Souza R, Varun M, Pratas J, Paul MS (2013) Spatial distribution of heavy metals in soil and flora associated with the glass industry in North Central India: implications for phytoremediation. Soil Sediment Contam Int J 22(1):1–20
Denton B (2007) Advances in phytoremediation of heavy metals using plant growth promoting bacteria and fungi. Basic Biotechnol 3:1–5
Dhindsa RS, Plumb-Dhindsa PL, Reid DM (1982) Leaf senescence and lipid peroxidation: effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol Plant 56:453–457
Eissa MA (2016) Effect of sugarcane vinasse and EDTA on cadmium phytoextraction by two saltbush plants. Environ Sci Pollut Res 23:10247–10254. https://doi.org/10.1007/s11356-016-6261-9
Estefan G, Sommer R, Ryan J (2013) Methods of soil, plant, and water analysis: a manual for the West Asia and North Africa Region, 3rd edn. International Center for Agricultural Research in the Dry Areas (ICARDA), Beirut
Evins WH, Varner JE (1972) Hormonal control of polyribosome formation in barley aleurone layers. Plant Physiol 49:348–352
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Genrich I, Burd D, George D, Glick BR (2000) Plant growth promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbial 46:237–245
Ghani A, Hussain M, Ikram M, Hameed T, Ahmad I, Imran M, Iftikhar M, Farooq M, Muhammad N, Fatima H, Arshad M (2016) Effect of nickel toxicity on the growth of Raphanus sativus (L.). Int J Basic Appl Chem Sci 6:6–9
Ghorbani A, Omran VOG, Razavi SM, Pirdashti H, Ranjbar M (2019) Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep 38:1151–1163
Gopal R, Rizvi AH (2008) Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 70(9):1539–1544
Hadi F, Bano A, Fuller MF (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80:457–462
Hammer D, Kayser A, Keller C (2003) Phytoextraction of Cd and Zn with Salix viminalis in field trials. Soil Use Manag 19:187–192
Hasanuzzaman M, Nahar K, Gill SS, Alharby HF, Razafindrabe BH, Fujita M (2017) Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: an intrinsic study on antioxidant defense and glyoxalase systems. Front Plant Sci 8:115
He J, Ma C, Ma Y, Li H, Kang J, Liu T, Polle A, Peng C, Luo ZB (2013) Cadmium tolerance in six poplar species. Environ Sci Pollut Res 20:163–174
Heidari J, Amooaghaie R, Kiani S (2020) Impact of chitosan on nickel bioavailability in soil, the accumulation and tolerance of nickel in Calendula tripterocarpa. Int J Phytoremed 22:1175–1184. https://doi.org/10.1080/15226514.2020.1748564
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hussain A, Nazir F, Fariduddin Q (2019) Polyamines (spermidine and putrescine) mitigate the adverse effects of manganese-induced toxicity through improved antioxidant system and photosynthetic attributes in Brassica juncea. Chemosphere 236:124830
Ijaz M, Rizwan MS, Sarfraz M, Ul-Allah S, Sher A, Sattar A, Ali L, Ditta A, Yousaf B (2020) Biochar reduced cadmium uptake and enhanced wheat productivity in alkaline contaminated soil. Int J Agric Biol 24:1633–1640. https://doi.org/10.17957/IJAB/15.1605
Imtiaz M, Ashraf M, Rizwan MS, Nawaz MA, Rizwan M, Mehmood S, Yousaf B, Yuan Y, Mumtaz MA, Ditta A, Ali M, Mahmood S, Tu S (2018) Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: effects on cell death, ROS and antioxidative systems. Ecotoxicol Environ Saf 158:139–144. https://doi.org/10.1016/j.ecoenv.2018.04.022
Irshad S, Xie Z, Mehmood S, Nawaz A, Ditta A, Mahmood Q (2021) Insights into conventional and recent technologies for arsenic bioremediation: a systematic review. Environ Sci Pollut Res 28:18870–18892. https://doi.org/10.1007/s11356-021-12487-8
James TK, Rahman A, Mellsop JM (2005) Fathen (Chenopodium album): a biotype resistant to dicamba. N Z Plant Prot 58:152–156
Jan AU, Hadi F, Midrarullah NMA, Rahman K (2017) Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol Biochem 116:139–149
Jibril SA, Hassan SA, Ishak CF, Wahab PEM (2017) Cadmium toxicity affects phytochemicals and nutrient elements composition of lettuce (Lactuca sativa L.). Adv Agri 2017:7. https://doi.org/10.1155/2017/123683
Kamran M, Malik Z, Parveen A, Zong Y, Abbasi GH, Rafiq MT, Shaaban M, Mustafa A, Bashir S, Rafay M, Mehmood S, Ali M (2019) Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J Environ Manag 250:1–12
Kaur R, Arora S, Singh B (2008) Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss Bioresour Technol 99(16):7692–7698
Khan MS, Zaka M, Abbasi BH, Shah A (2016) Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles. IET Nanobiotechnol 10:359–366. https://doi.org/10.1049/iet-nbt.2015.0050
Khatamipour M, Piri E, Esmaeilian Y, Tavassoli A (2011) Toxic effect of cadmium on germination, seedling growth and proline content of milk thistle (Silybum marianum). Ann Biol Res 2(5):527–532
Konkolewska A, Piechalak A, Ciszewska L, Antos-Krzemińska N, Skrzypczak T, Hanć A, Sitko K, Małkowski E, Barałkiewicz D, Małecka A (2020) Combined use of companion planting and PGPR for the assisted phytoextraction of trace metals (Zn, Pb, Cd). Environ Sci Pollut Res 27:13809–13825 1-17
Korver RA, Koevoets IT, Testerink C (2018) Out of shape during stress: a key role for auxin. Trends Plant Sci 23(9):783–793
Leng Y, Li Y, Ma Y-H, He L-F, Li S-W (2020) Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ Sci Pollut Res 28:6030–6043. https://doi.org/10.1007/s11356-020-10843-8
Li F, Yang F, Chen Y, Jin H, Leng Y, Wang J (2020) Chemical reagent-assisted phytoextraction of heavy metals by Bryophyllum laetivirens from garden soil made of sludge. Chemosphere 253:126574. https://doi.org/10.1016/j.chemosphere.2020.126574
Liu YZ, Imtiaz M, Ditta A, Rizwan MS, Ashraf M, Mehmood S, Aziz O, Mubeen F, Ali M, Elahi NN, Ijaz R, Lelel S, Shuang C, Tu S (2020) Response of growth, antioxidant enzymes and root exudates production towards As stress in Pteris vittata and Astragalus sinicus colonized by arbuscular mycorrhizal fungi. Environ Sci Pollut Res 27:2340–2352. https://doi.org/10.1007/s11356-019-06785-5
Mahmood T (2010) Phytoextraction of heavy metals: the process and scope for remediation of contaminated soils. Soil Environ 29:91–109
Malik CP, Singh MB (1980) Extraction and estimation of amino acids and keto acids. In: lant enzymology and histo-enzymology. Kalyani Publishers, New Delhi-Lud Hana, p 286
Marques DM, Júnior VV, da Silva AB, Mantovani JR, Magalhaes PC, de Souza TC (2018) Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L. (Caesal pinioideae). Water Air Soil Pollut 229:138
Mattioli R, Falasca G, Sabatini S, Altamura MM, Costantino P, Trovato M (2009) The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plant 137:72–85
Mehmood S, Imtiaz M, Bashir S, Rizwan M, Irshad S, Yuvaraja G, Ikram M, Aziz O, Ditta A, Rehman SU, Shakeel Q, Mumtaz MA, Ahmed W, Mahmood S, Chen D, Tu S (2019) Leaching behaviour of Pb and Cd and transformation of their speciation in co-contaminated soil receiving different passivators. Environ Eng Sci 36(6):749–759. https://doi.org/10.1089/ees.2018.0503
Mehmood S, Rizwan M, Bashir S, Ditta A, Aziz O, Yong LZ, Dai Z, Akmal M, Ahmed W, Adeel M, Imtiaz M, Tu S (2018b) Comparative effects of biochar, slag and ferrous–Mn ore on lead and cadmium immobilization in soil. Bull Environ Contam Toxicol 100(2):286–292. https://doi.org/10.1007/s00128-017-2222-3
Mehmood S, Saeed DA, Rizwan M, Khan MN, Aziz O, Bashir S, Ibrahim M, Ditta A, Akmal M, Mumtaz MA, Ahmed W, Irshad S, Imtiaz M, Tu S, Shaheen A (2018a) Impact of different amendments on biochemical responses of sesame (Sesamum Indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol Biochem 132:345–355. https://doi.org/10.1016/j.plaphy.2018.09.019
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15(4):523–530
Mousavi SR, Niknejad Y, Fallah H, Tari DB (2020) Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep 39:1041–1060. https://doi.org/10.1007/s00299-020-02547-7
Muhammad S, Shah MT, Khan S (2011) Heavy metal concentrations in soil and wild plants growing around Pb-Zn sulfide terrain in the Kohistan region, northern Pakistan. Microchem J 99(1):67–75
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Naveed M, Bukhari SS, Mustafa A, Ditta A, Alamri S, El-Esawi MA, Rafique M, Ashraf S, Siddiqui MH (2020) Mitigation of nickel toxicity and growth promotion in sesame through the application of a bacterial endophyte and zeolite in nickel contaminated soil. Int J Environ Res Public Health 17(23):8859. https://doi.org/10.3390/ijerph17238859
NEQS (2000) National Environmental Quality Standards, S.R.O, 549 (I)/2000, Ministry of Environment, Local Government, and Rural Development, Islamabad, Pakistan.
Neugschwandtner RW, Tlustoš P, Komárek M, Száková J (2008) Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: laboratory versus field scale measures of efficiency. Geoderma 144(3-4):446–454
Niamat B, Naveed M, Ahmad Z, Yaseen M, Ditta A, Mustafa A, Rafique M, Bibi R, Minggang X (2019) Calcium-enriched animal manure alleviates the adverse effects of salt stress on growth, physiology and nutrients homeostasis of Zea Mays L. Plants 8(11):480. https://doi.org/10.3390/plants8110480
Qin L, Zhao M, Li B, Li Y, Li M (2020) Effects of root exudation on the accumulation of Cd by Chenopodium ambrosioides L. and maize in intercropping systems. IOP Conf Ser: Earth Environ Sci 446:032066. https://doi.org/10.1088/1755-1315/446/3/032066
Rai PK, Lee SS, Zhang M, Tsang YF, Kim K (2019) Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ Int 125:365–385. https://doi.org/10.1016/j.envint.2019.01.067
Rezapour S, Atashpaz B, Moghaddam SS, Kalavrouziotis IK, Damalas CA (2019) Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 231:579–587
Rizwan MS, Imtiaz M, Zhu J, Yousaf B, Hussain M, Ali L, Ditta A, Ihsan MZ, Huang G, Ashraf M, Hu H (2021) Immobilization of Pb and Cu by organic and inorganic amendments in contaminated soil. Geoderma 385:114803. https://doi.org/10.1016/j.geoderma.2020.114803
Sabir A, Naveed M, Bashir MA, Hussain A, Mustafa A, Kamran M, Ditta A, Saeed Q, Qadeer A (2020) Cadmium mediated phytotoxic impacts in Brassica napus: managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17 J Environ Manage 265:110522
Saeed Z, Naveed M, Imran M, Bashir MA, Sattar A, Mustafa A, Hussain A, Xu M (2019) Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS One 14(3):213–216
Saffari VR, Saffari M (2020) Effects of EDTA, citric acid, and tartaric acid application on growth, phytoremediation potential, and antioxidant response of Calendula officinalis L. in a cadmium-spiked calcareous soil. Int J Phytoremed. https://doi.org/10.1080/15226514.2020.1754758
Sakihama Y, Yamasaki H (2002) Lipid peroxidation induces by phenolic in conjunction with aluminum ions. Biol Plant 45:249–254
Saleem MH, Kamran M, Zhou Y, Parveen A, Rehman M, Ahmar S, Malik Z, Mustafa A, Anjum RMA, Wang B, Liu L (2020) Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J Environ Manag 257:109994
Samantary S (2002) Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47:1065–1072
Sas-Nowosielskaa A, Kucharski R, Malkowski E, Pogrzeba M, Kuperberg JM, Krynski K (2004) Phytoextraction crop disposal—an unsolved problem. Environ Pollut 128:373–379
Santos Neri Soares TF, dos Santos Dias DCF, Oliveira AMS, Ribeirob DM, dos Santos Dias LA (2020) Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol Environ Saf 193:110296. https://doi.org/10.1016/j.ecoenv.2020.110296
Sun Y, Zhou Q, Diao C (2008) Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour Technol 99:1103–1110
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Ullah I, Ditta A, Imtiaz M, Mehmood S, Rizwan M, Rizwan MS, Jan AU, Ahmad I (2020) Assessment of health and ecological risks of heavy metal contamination: a case study of agricultural soils in Thall, Dir-Kohistan. Environ Monit Assess 192:786. https://doi.org/10.1007/s10661-020-08722-3
Vysloužilová M, Tlustoš P, Száková J (2003) Cadmium and zinc phytoextraction potential of seven clones of Salix spp. planted on heavy metal contaminated soils. Plant Soil Environ 49:542–547
Wang F, Huang C, Chen Z, Bao K (2019) Distribution, ecological risk assessment, and bioavailability of cadmium in soil from Nansha, Pearl River Delta, China. Int J Environ Res Public Health 16(19):3637
World Health Organization (WHO) (2004) Guidelines for drinking water quality. World Health Organization (WHO), vol 1, 3rd edn. World Health Organization, Geneva
Yan L, Li X, He M, Zeng F (2013) Behavior of native species Arrhenatherum Elatius (Poaceae) and Sonchus Trans caspicus (Asteraceae) exposed to a heavy metal-polluted field: plant metal concentration, phytotoxicity, and detoxification responses. Int J Phytoremed 15:924–937
Yang Y, Zhang FS, Li HF, Jiang RF (2009) Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J Environ Manag 90:1117–1122
Yu G, Jiang P, Fu X, Liu J, Sunahara GI, Chen Z, Xiao H, Lin F, Wang X (2020) Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: a long-term field study. Environ Pollut 266:115408. https://doi.org/10.1016/j.envpol.2020.115408
Zhang C, He Q, Wang M, Gao X, Chen J, Shen C (2020) Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotoxicol Environ Saf 190:110090. https://doi.org/10.1016/j.ecoenv.2019.110090
Zhang E, Yuan Y, Qian Z, Fei G, Ditta A, Mehmood S, Rizwan MS, Mustaq MA, Rizwan M, Aziz O, Ijaz R, Afzal J, Imtiaz M, Tu S (2018) Seed priming with selenium to affect seed germination, seedling growth, and electrolyte leakage in rice under vanadium and cadmium stress. J Environ Agri 3(1):262–273
Zhou J, Cheng K, Huang G, Chen G, Zhou S, Huang Y, Zhang J, Duan H, Fan H (2020) Effects of exogenous 3-indoleacetic acid and cadmium stress on the physiological and biochemical characteristics of Cinnamomum camphora. Ecotoxicol Environ Saf 191:109998. https://doi.org/10.1016/j.ecoenv.2019.109998
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Amin Ullah Jan, Muhammad Tariq, and Muhammad Asif Nawaz. Data analysis and supervision were performed by Fazal Hadi and Allah Ditta. The first draft of the manuscript was written by Amin Ullah Jan. All the authors including Fazal Hadi, Abdullah Shah, Allah Ditta, Muhammad Asif Nawaz, and Muhammad Tariq critically reviewed and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. This manuscript does not involve researching about humans or animals.
Consent to participate
All of the authors consented to participate in the drafting of this manuscript.
Consent to publish
All of the authors consented to publish this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jan, A.U., Hadi, F., Shah, A. et al. Plant growth regulators and EDTA improve phytoremediation potential and antioxidant response of Dysphania ambrosioides (L.) Mosyakin & Clemants in a Cd-spiked soil. Environ Sci Pollut Res 28, 43417–43430 (2021). https://doi.org/10.1007/s11356-021-13772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13772-2