Abstract
Experiments were conducted to determine how exogenous abscisic acid (ABA) mediates the tolerance of plants to cadmium (Cd) exposure. Cd stress strongly reduced all the growth parameters of mung bean seedlings. Cd significantly increased ascorbate peroxidase (APX) and catalase (CAT) activities in roots and stems, and peroxidase (POD) activities in roots, stems, and leaves of mung bean seedlings. Cd caused remarkable increases in the levels of leaf chlorophyll and carotenoid, root polyphenols, and malondialdehyde (MDA) and proline in the three organs. However, Cd greatly decreased leaf CAT activity, root and leaf ascorbic acid (AsA) levels, and stem and leaf polyphenol levels. Foliar application of ABA partially alleviated Cd toxicity on the seedlings. ABA could restore most of the changed biochemical parameters caused by Cd, suggesting that ABA played roles in the protection of membrane lipid peroxidation and the modulation of antioxidative defense systems in response to Cd stress. Our results also implied the differential physiological and biochemical responsive patterns of roots, stems, and leaves to Cd and ABA in mung bean seedlings. The great changes in many biochemical parameters in roots suggested that roots were the first to be affected by Cd and play pivotal roles in response to Cd, especially in chelating Cd and reducing Cd absorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal (HM) pollution has been considered to be the most urgent environmental problem in the twenty-first century. Cadmium (Cd) is one of the most toxic HMs. Cd can be absorbed into plant roots firstly from soil through the membrane channels for nutrient elements due to the similarity of chemical and physical properties (Clemens 2006; Verbruggen et al. 2009). When entering root tissues, most of the Cd are accumulated by binding to carboxyl groups of cellulose, lignin, and hemicellulose in the cell wall, as well as proteins and pectin (Parrotta et al. 2015; Dai et al. 2018), and a certain amount are transported to the aboveground part through the xylem (Tanaka et al. 2007). The excessive Cd causes great reduction on the plant growth and productivity (Clemens 2006; Gallego et al. 2012). Cd stress affects stomatal closure, nutrient absorption, transpiration, and photosynthesis in plants (Dong et al. 2006; Hassan et al. 2008). The physiological disorders by Cd stress in plants are attributed to the accumulation of reactive oxygen species (ROS), which lead to cellular oxidative damage (Hassan et al. 2006). The elevations of ROS caused by Cd stress disturb plant metabolism and destroy cell membranes ( Gill and Tuteja 2010; Mahmud et al. 2018). Plants employ a series of detoxification processes to cope with the HM-stressed detrimental impacts in roots, stems, and leaves (Benyó et al. 2016; Shahid et al. 2017; Jiang et al. 2020). The main physiological processes include the chelation and sequestration of HMs (Cobbett 2000; Peng and Gong 2014), and the counteraction of oxidative burst by antioxidative defense systems including enzymatic and non-enzymatic systems ( Gill and Tuteja 2010; Mahmud et al. 2018). Phytochelatins, metallothioneins, and a few organic acids such as amino acid, citric acid, malic acid, and oxalic acid act as chelators participating in the chelation of Cd and other HMs (Cobbett and Goldsbrough 2002; Clemens 2006). The transporters, such as ATP-binding cassette transporter (ABC) family, cation diffusion facilitator (CDF) family, heavy metal ATPase (P1B-ATPase, HMA) family, natural resistance-associated macrophage protein (NRAMP) family, and ZRT-IRT protein (ZIP) family also play an important role in the sequestration and detoxification of HMs (Simões et al. 2012; Menguer et al. 2013; Milner et al. 2013; Singh et al. 2016). Multiple antioxidative enzymes are involved in the plant detoxification of heavy metals, such as dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), glutathione reductase (GR), glutathione peroxidase (GPX), glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX) (Jin et al. 2008; Chen et al. 2010; Li et al. 2014; Jiang et al. 2020). SOD catalyzes the transformation of O2•− to H2O2 and oxygen, playing a pivotal role in the antioxidant defense system. CAT and POD participate in converting H2O2 to oxygen or water to further detoxify the cells (Gill and Tuteja 2010). The activity alterations of these enzymes were detected in plant exposure to Cd. However, the growth inhibition and changes in antioxidative enzyme activities caused by Cd stress vary with plant species and tissues, as well as Cd concentrations (Ding et al. 2014). For example, excessive Cd decreased the CAT activity in spinach and lettuce shoots, POD activity in lettuce shoots and roots, and SOD activity in lettuce shoots, while increased CAT activity in garland chrysanthemum roots and POD activity in spinach shoots (Meng et al. 2019). Cd stress reduced the CAT activity, while increased the SOD, APX, and GST activities in mung bean leaves (Nahar et al. 2016). Another study showed that Cd observably enhanced the activities of SOD and POD in wheat (Guo et al. 2019). Nasibeh et al. (2019) reported that 1 μM CdCl2 caused a significant increase of SOD activity in safflower shoots and roots but 20 μM CdCl2 resulted in a nonsignificant change in SOD activity. Qin et al. (2018) showed that 5 and 50 μM CdCl2 increased APX activities by 19% and 38% in wheat leaves and by 195% and 318% in roots, respectively. The plant antioxidants, such as ascorbic acid (AsA), reduced glutathione (GSH), and phenol compounds, are involved in removing oxidative burst and improving plant tolerance to HM stress (Foyer and Noctor 2011). Proline, a ROS scavenger, acts as a membrane stabilizer and an osmoprotectant during stress (Bandurska 2001; Matysik et al. 2002). Malondialdehyde (MDA) contents are related to the level of plant growth inhibition and used to detect membrane lipid peroxidation (Lin et al. 2007; Ding et al. 2014). These parameters can be exploited for detection of oxidative stress as important indicators in plants.
Phytohormones, such as abscisic acid (ABA), gibberellin, salicylic acid (SA), ethylene, and brassinosteroids (BR), play a fundamental role in associating stress perception with multiple transcriptional responses especially in signaling pathways that stimulate plant adaptation to adverse environments (Song et al. 2014; Sytar et al. 2019). Phytohormones involve in counteracting environmental damage caused by HM toxicity in plants and play a pivotal role in the regulation of plant growth and development (Popova et al. 2013; Wu et al. 2011; Yuan and Wu 2010). ABA is a key abiotic stresses-induced hormone and acts as a regulator of diverse plant stress resistance and developmental processes (Hauser et al. 2017) (Fig. 1). ABA has been known to alleviate several plant stresses such as salinity, nutrient (Osakabe et al. 2014), temperature, and drought (An et al. 2014). ABA functions as a key signaling molecule in plants regulating stomatal closure and promoting the synthesis of stress-resistant proteins to improve the Cd stress tolerance (Adie et al. 2007). In recent years, increasing attentions have been focused on exploring the effects of exogenous ABA on HM stresses. Endogenous ABA has been demonstrated to regulate Cd tolerance in different ways. For example, ABA greatly reduced Cd accumulation in rice shoots and decreased the Cd absorption capacity of rice root (Uraguchi et al. 2009). Exogenous application of ABA has been shown to mediate photosynthesis and transpiration, proline and soluble sugar contents, and antioxidative enzyme activities in plants. For example, hydroponic treatment with ABA enhanced Pb tolerance (Zhao et al. 2009) and decreased Cd transport by reducing transpiration and photosynthesis rates in rice seedlings (Hsu and Kao 2003, 2005). Foliar application of ABA regulated the antioxidant contents and antioxidative enzyme activities (Wang et al. 2013) to alleviate heavy metal–induced toxicity. A recent study using the method of dithizone histochemical staining for Cd distribution in tissues showed that the combination of ABA and SA strongly improved Cd transport from roots to shoots resulting in Cd accumulation in senescent leaves in tall fescue (Zhu et al. 2020). However, the mechanism by which ABA modulates plant tolerance to HMs via the regulation of antioxidative systems has not been fully addressed. Particularly, the differential responses of plant roots, stems, and leaves to abiotic stresses and ABA application remain unclear.
Our previous study has shown that ABA neutralized the changes in GSH and AsA contents and antioxidative enzyme activities induced by Cd and attenuated the Cd inhibition on the adventitious root formation in mung bean plants (Li et al. 2014). However, whether ABA is involved in mediating the redox system to alleviate HM damage throughout the entire plant requires further elucidation. The aims of the present study were to survey how ABA alleviates the Cd-stressed inhibitory effects in mung bean by preventing membrane lipid peroxidation, regulating the antioxidant defense systems, and controlling the contents of proline and photosynthetic pigments under Cd stress. The differences in roots, stems, and leaves of mung bean in response to Cd stress and the alleviation of ABA were comparatively analyzed.
Materials and methods
Plant materials and growth conditions
Mung bean (Vigna radiata (L.) R Wilczek) seeds were cultivated according to the method of our previous study (Li et al. 2014). Briefly, the seeds were incubated in Petri dishes at 25 ± 1 °C in the dark for 36 h until germination, and then sown in seed trays, covered with a thin layer of sterilized perlite. The trays were cultivated in a growth chamber at 25 ± 1 °C with a 14-h photoperiod (PAR of 100 μM m−2 s−1) for 5 days to obtain uniform mung bean seedlings for use in the following experiments.
ABA and cadmium treatments
ABA (Sigma-Aldrich, St. Louis, MO, USA) solutions were prepared in ABA stock solution (10 mM) diluted in ultrapure water and CdCl2·2H2O solutions were prepared in stock solution diluted in Hoagland solution to formulate the desired working concentrations. For the treatments of ABA together with CdCl2 (ABA+Cd), three concentrations of ABA (5 μM, 10 μM, and 15 μM) and two concentrations of CdCl2 (50 μM and 100 μM) were applied. Prior to Cd2+ treatments, equal volumes of ABA solution were uniformly sprayed onto the seedling leaves in the tray respectively. After 2 h, equal volumes of CdCl2 solution were supplied to all the trays. For the control treatments, equal volumes of water were sprayed onto the seedling leaves and Hoagland solutions were applied. Each treatment contained three biological repeats and each repeat contained at least 20 seedlings. All the trays were cultured under the same conditions as described above. To measure the morphological index, the seedlings were cultured for 11 days. The leaves, roots, and stems were respectively harvested after 0, 1, 3, 5, 7, 9 days of incubation for the biochemical assays.
Plant growth analysis and Cd content measurement
The seedlings (at least 20 seedlings each replicate) were collected from the seed trays and washed with distilled water to remove the adhering perlite. After removal the remaining water with filter paper, the seedling height, epicotyl length, hypocotyl length, root length, lateral root number, root fresh weight (RFW), and shoot fresh weight (SFW) were separately measured. The root dry weight (RDW) and shoot dry weight (SDW) of mung beans were recorded after dried in an oven at 60 °C for 24 h. Cd contents were determined as described by Wang et al. (2016) using a 220FS flame atomic absorption spectrophotometer.
Determination of chlorophyll content
Total leaf chlorophyll and carotenoid contents were measured by the methods of Arnon (1949). Leaf discs (0.1 g) were ground in 10 mL of 95% (v/v) ethanol with an appropriate amount of calcium carbonate powder and quartz sand, and centrifuged at 10,000×g for 10 min at room temperature. The ethanol extract absorbance was determined at 665, 649, and 470 nm using a 759S spectrophotometer, and total chlorophyll and carotenoid contents were calculated.
Measurement of antioxidative enzyme activity
For measurement of antioxidative enzyme activities, 0.5 g of the harvested root, stem, and leaf tissues were ground in liquid nitrogen, and then 4.0 mL of potassium phosphate buffer (50 mM, pH 7.8) containing 1.0% (w/v) polyvinyl polypyrrolidone was added and homogenized. The homogenates were collected in 5-mL tubes. After centrifugation for 20 min at 16,500×g and 4 °C, the obtained supernatant was stored at 4 °C for enzymatic assays as the crude enzyme preparation.
APX, POD, and CAT activities were assayed according to the methods of Rao et al. (1997), Polle et al. (1994), and Agarwal et al. (2005). The reaction mixture for APX activity assay contained 2 mL of 0.5 mM ascorbic acid containing 0.1 mM EDTA-Na2, 1 mL of potassium phosphate buffer (50 mM, pH 7.0), 50 μL of 9 mM H2O2, and 100 μL of enzyme extract. The reaction mixture for POD activity assay contained 3 mL of potassium phosphate buffer (50 mM, pH 7.0) mixed with 20 mM of guaiacol, 100 μL of 9 mM H2O2, and 40 μL of enzyme extract. The reaction mixture for CAT activity assay contained 2 mL of potassium phosphate buffer (50 mM, pH 7.0), 1 mL of 15 mM H2O2, and 150 μL enzyme extract. Since the addition of H2O2, the absorbance changes were monitored for 180 s at 290 nm, 470 nm, and 240 nm for calculating the APX, POD, and CAT activities, respectively. One unit of enzyme activity was defined as an increase or decrease in absorbance of 0.01 optical density (OD) units per minute per gram of fresh weight. According to the method of Spychalla and Desborough (1990), the reaction mixture for SOD activity assay consisted of 100 μL enzyme extract, 300 μL of 20 μM riboflavin, 300 μL of 130 mM methionine, 300 μL of 100 μM EDTA-Na2, 300 μL of 750 μM nitro-blue tetrazolium (NBT), and 1.7 mL of potassium phosphate buffer (50 mM, pH 7.8). The absorbance was measured at 560 nm after exposing the mixture to light (350 μM m−2 s−1) for 20 min. One unit of SOD activity was determined as the amount of enzyme needed for effectively inhibition 50% of the NBT photoreduction in comparison with the absence of enzyme.
Determination of reduced ascorbic acid and reduced glutathione contents
The contents of AsA and GSH were determined following the methods described by Chen and Gallie (2004) and Tyburski and Tretyn (2010). One gram of the collected root, stem, and leaf tissues were ground in liquid nitrogen, and 4 mL of chilled 5% (w/v) trichloroacetic acid (TCA) solution was added for AsA content determination; 4 mL of chilled 5% (w/v) TCA containing 2 mM EDTA–Na2 was added for GSH content determination. After homogenization and centrifugation for 20 min at 16,500×g and 4 °C, the liquid supernatants were collected for analysis of AsA and GSH. The reaction mixture for AsA content assay consisting of 1 mL of ethanol, 1 mL of supernatant, 1 mL of 0.5% (w/v) 4,7-diphenyl-1,10-phenanthroline (bathophenanthroline), 1 mL of 5% (w/v) TCA, 0.5 mL of 0.03% (w/v) FeCl3, and 0.5 mL of 0.4% (w/v) H3PO4 was kept at 30 °C for 60 min, and the colored solution absorbance was recorded at 525 nm. The reaction mixture for GSH content assay consisting of 1 mL of supernatant, 0.5 mL of 4 mM 5,5-dithio-bis-(2-nitrobenzoic acid), and 1 mL of phosphate buffer (100 mM, pH 7.7) was kept at 25 °C for 10 min, and the colored solution absorbance was recorded at 412 nm.
Determination of total phenol content
Total phenol content was determined according to the method of Pirie and Mullins (1976). One gram of the sampled root, stem, and leaf tissues were ground in 10 mL of distilled water, followed by extraction in a boiling water bath for 40 min. The supernatant was obtained after centrifugation at 5000×g for 10 min. The 3 mL of phosphate buffer (0.1 M, pH 6.8) and 1 mL of 1.5% potassium iron tartrate were added in the tubes with 1 mL of supernatant, and the absorbance of reaction mixture was recorded at 540 nm.
Determination of malondialdehyde content
Malondialdehyde content was measured according to the method of Hodges et al. (1999). One gram of the collected tissues were ground with arenaceous quartz in 5 mL of phosphate buffer (50 mM, pH 7.8), followed by centrifugation at 2000×g for 10 min. Mixed 2 mL of the supernatant with 3 mL of 0.5% (v/v) 2-thiobarbituric acid (TBA) dissolved in 5% (w/v) TCA, and the reaction mixture was maintained at 90 °C for 10 min. Next, the mixture was transferred to an ice bath for rapid cooling, followed by centrifugation for 10 min at 2000×g to remove the residues generated from the heating process. The absorbance at 535 nm was measured with subtraction of the value for nonspecific absorption at 450 and 600 nm and the level of MDA was measured based on the fresh weight (as μg g−1 FW).
Determination of the proline content
Proline content was assayed according to the method of Bates et al. (1973). Fresh leaf, stem, and root tissues (1.0 g) were ground in 5 mL of 3% (w/v) sulphosalicylic acid and boiled in water bath at 90 °C for 10 min to extract the proline. Then, the mixture was rapidly cooled to room temperature and centrifuged for 10 min at 10,000×g. Next, 3 mL of freshly prepared acid-ninhydrin solution and 2 mL of glacial acetic acid were added in the tubes which contained 2 mL of supernatant. The tubes were heated in the boiling water bath for 40 min, followed by extraction of the reaction mixture with 5 mL toluene at room temperature, and rested for 20 min to separate the toluene from aqueous phase, the absorbance of which was recorded at 520 nm. Using a standard curve of analytical grade proline, the proline content was determined and calculated based on the fresh weight (as mg g−1 FW).
Statistical analysis
One-way ANOVA with post hoc tests was used for data analysis. For the comparison of mean values, the least significant difference (LSD test) at p < 0.05 was used to calculate the standard error (SE). SPSS/PC software ver. 14.0 (SPSS Inc., Chicago, Illinois) was used for statistical analysis. The data are expressed as the mean of at least three independent experiments (20 plants per treatment) and as the mean ± SE.
Results
Effect of ABA on Cd toxicity with respect to morphological parameters
Mung bean seedlings treated by Cd demonstrated a remarkable reduction in all morphological parameters. Plant heights were reduced by 26.0% and 46.4% in the 50 μM and 100 μM Cd treatments, respectively, compared to the controls. Cd reduced the plant height mainly by inhibiting epicotyl growth. Root lengths were reduced by 34.8% and 62.0%, and the numbers of lateral roots were reduced by 34.8% and 40.0% in the 50 μM and 100 μM Cd treatments, respectively. The SDW and RDW also showed obvious decreases exposed to Cd (27.1% and 40.0% in SDW, and 10.2% and 26.4% in RDW with 50 and 100 μM Cd, respectively) (Table 1). These results indicated that both 50 μM and 100 μM Cd strongly inhibited the growth of mung bean seedlings, and a highly significant (p < 0.01) inhibitory effect in the treatment with 100 μM Cd was observed. Thus, this Cd concentration was used in subsequent biochemical experiments.
The results obtained from the treatments with ABA+Cd showed that ABA aided in alleviating Cd toxicity to the variable extent in each counterpart alone. Plant height showed an average 14.1% increase (p < 0.05) in the treatments with three concentrations of ABA+100 μM Cd compared to Cd alone (Table 1). Root length showed an 38.6% increase in 10 μM ABA+50 μM Cd relative to 50 μM Cd alone, and 26.0% increase in 15 μM ABA+100 μM Cd relative to 100 μM Cd alone. The 10 μM ABA+100 μM Cd treatment significantly increased the SFW, RFW, SDW, and RDW by 35.4% (p < 0.05), 127.3% (p < 0.05), 19.0% (p < 0.05), and 20.9%, respectively, relative to 100 μM Cd alone. Obviously, the ameliorative effect of ABA on 100 μM Cd–treated seedlings was greater than that on 50 μM Cd–treated. The above results indicate that the pretreatment with three concentrations of ABA clearly ameliorated the negative effects of Cd on the morphological parameters, and the optimal effect was observed in the treatment with 10 μM ABA. Therefore, 10 μM ABA was considered an appropriate concentration for foliar application in subsequent biochemical experiments.
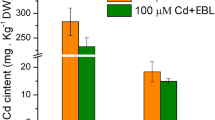
Further analysis showed that, in the ABA+Cd-treated seedlings, ABA significantly (p < 0.05) decreased the Cd contents in roots and shoots by 23.5% and 25.3%, respectively, compared with Cd-treated seedlings (Fig. 2a).
Effect of ABA on Cd content (a), total chlorophyll (b), and carotenoid (c) concentrations in mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
Effect of ABA on leaf pigment concentrations in mung bean seedlings under Cd stress
In comparison with the control, 100 μM Cd resulted in an increment in total chlorophyll and carotenoid contents on average by 5.3% and 18.2%, respectively (Figs. 2b and c). When compared with Cd treatment, ABA+Cd decreased the contents of total chlorophyll and carotenoid at nearly all time points on average by 6.3% and 6.1%, respectively.
Effect of ABA on Cd toxicity in terms of the antioxidative enzyme activities in mung bean seedlings
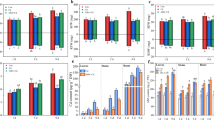
The CAT activities displayed significant differences among the three organs. For example, in the control plants, significantly higher CAT activities were detected in leaves (on average 160.5 U g−1FW) than in stems (on average 54.2 U g−1FW) and roots (on average 25.8 U g−1FW) (Figs. 3a, b, and c). In comparison with the control, Cd stress significantly (p < 0.05) enhanced CAT activity in roots and stems (on average by 79.9% and 78.7%, respectively) from 3 to 9 days, whereas decreased it from 1 to 9 days in leaves (on average by 47.7%) (Figs. 3a, b, and c). In comparison with Cd treatment, ABA+Cd significantly (p < 0.05) decreased CAT activity in roots and stems (on average by 18.3% and 23.5%, respectively) from 3 to 9 days, whereas strongly increased it from 1 to 9 days in leaves (on average by 64.3%).
Effect of ABA on Cd toxicity in terms of the activities of CAT (a, b, c), APX (d, e, f), POD (g, h, i), and SOD (j, k, l) in roots, stems, and leaves of mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
In comparison with the control, Cd stress resulted in a slight increase in APX activity in roots (on average by 9.6%) and a significant (p < 0.05) increment in stems (on average 55.6%), whereas slightly decreased it in leaves (Figs. 3d, e, and f). ABA+Cd weakly reversed the decreased APX activity by Cd only in leaves.
In the control seedlings, the root POD activity (average of 271.5 U) was markedly higher than the stem (average of 86.1 U) and leaf (average of 104.6 U) (Figs. 3g, h, and i). The activity displayed increasing trends in the three organs during the treatment time course. When compared with the controls, both ABA+Cd and Cd treatments significantly (p < 0.05) enhanced POD activities from 1 to 9 days in the three organs. When compared with Cd alone, ABA+Cd further increased POD activities in leaves and stems.
The SOD activities displayed minor differences among the three organs during the treatment time course (Figs. 2j, k, and l). In comparison with the control, Cd stress slightly decreased SOD activity from 1 to 9 days in roots, which was restored by ABA in the ABA+Cd treatment (Fig. 3j). However, ABA+Cd treatment significantly (p < 0.05) reduced the activities in stems (on average by 33.4%) and leaves (on average by 14%), compared with Cd alone (Figs. 3k and l).
Effect of ABA on Cd toxicity in terms of the levels of AsA, GSH, and polyphenols in mung bean seedlings
The AsA content in the control leaves (average of 212.6 μg g−1 FW) was significantly (p < 0.05) higher than those in roots (average of 85.6 μg g−1 FW) and stems (average of 83.1 μg g−1 FW) (Figs. 4a–c). When compared with the control, Cd reduced AsA contents in roots (on average by 15.9%) and leaves (on average by 14.9%) from 1 to 9 days. In comparison with Cd alone, ABA+Cd significantly (p < 0.05) increased AsA contents in roots (on average by 22.0%), stems (on average by 13.0%), and leaves (on average by 16.2%) from 1 to 9 days, suggesting that ABA pretreatment restored the decreased AsA content caused by Cd in the three organs.
Effect of ABA on Cd toxicity in terms of AsA content in roots, stems, and leaves of mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
The average contents of GSH remained relatively stable among the three organs (Figs. 5a, b, and c). In comparison with the control, the leaf GSH content showed unobvious change in Cd treatment, but the root and stem GSH contents showed a significant (p < 0.05) increase at 3 days and 7 days and at 7 days, respectively. ABA+Cd completely reversed the enhanced GSH contents by Cd in roots.
Effect of ABA on Cd toxicity in terms of GSH content in roots, stems, and leaves tissues in mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
The polyphenol contents displayed gradual increases during the treatment time course in the control and Cd-treated roots and stems, whereas it showed a sharp decrease in leaves (Figs. 6a, b, and c). Cd stress significantly (p < 0.05) enhanced the root polyphenol contents from 3 to 9 days (on average by 107.1%) (Fig. 6a), whereas significantly (p < 0.05) reduced the stem polyphenol content from 1 to 9 days (on average by 26.1%) (Fig. 6b) and leaf polyphenol content after 3 days (on average by 19.3%) (Fig. 6c). In comparison with Cd alone, ABA+Cd significantly (p < 0.05) decreased the root polyphenol content at 3 days and 7 days, and the stem polyphenol content from 3 to 9 days (on average by 34.8%), whereas increased the leaf polyphenol content from 1 to 9 days (on average by 23.9%), indicating that ABA completely reversed the decreased polyphenol content by Cd in leaves.
Effect of ABA on Cd toxicity in terms of polyphenol content in roots, stems, and leaves in mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
Effect of ABA on MDA and proline levels in mung bean seedlings under Cd stress
The MDA contents showed a gradual decline from 0 to 9 days in the roots of control seedling and Cd- and ABA+Cd–treated seedlings (Fig. 7a). MDA content showed a slight change during the time course in stems and leaves (Figs. 7b and c). Cd stress significantly (p < 0.05) elevated MDA content relative to the control from 3 to 9 days in roots (on average by 45.8%), from 3 to 5 days in stems (on average by 15.6%), and from 5 to 9 days in leaves (on average by 44.8%), suggesting that Cd stress strongly triggered the membrane lipid peroxidation, resulting in the obvious increases in MDA levels in roots, stems, and leaves. The highly increased MDA contents by Cd were decreased by ABA+Cd in roots (on average by 14.6%) and leaves (on average by 21.8%), but was not decreased in stems, suggesting that ABA alleviated the membrane lipid peroxidation caused by Cd in the roots and leaves.
Effect of ABA on Cd toxicity in terms of the levels of MDA in roots, stems, and leaves in mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
The proline levels showed obvious differences in roots (average of 13.2 μg g−1 FW), stems (average of 35.3 μg g−1 FW), and leaves (average of 43.9 μg g−1 FW). In the controls, the proline contents in the three organs displayed decrease trends during the time course (Figs. 8a, b, and c). Cd stress significantly (p < 0.01) increased the proline contents on average by 354.2%, 115.7%, and 43.4% in roots, stems, and leaves, respectively, relative to the controls. In comparison with Cd alone, ABA+Cd significantly (p < 0.01) decreased the content on average by 72.5% in roots, suggesting that ABA completely reversed the enhanced proline content by Cd in roots. However, ABA+Cd further increased the content on average by 20.0% in both stems and leaves.
Effect of ABA on Cd toxicity in terms of the levels of proline in roots, stems and leaves in mung bean seedlings. The values shown for each treatment are the means ± SE (n = 20) of three different experiments. Mean values with different letters above the bars indicate significant differences between treatment and control plants (LSD test) at p < 0.05
Discussion
Many previous studies have demonstrated that excessive Cd greatly inhibited the plant growth and biomass (Clemens 2006; Gallego et al. 2012; Ali et al. 2014; Sadia et al. 2019). A similar result was observed in the present study, showing that both 50 and 100 μM CdCl2 greatly inhibited the growth and biomass of mung bean seedlings. ABA considerably counteracted the Cd-caused negative effect on mung bean growth. An appropriate concentration of ABA eliminated the Cd-induced reduction in the morphology indexes, i.e., root length, plant height, and biomass of shoots and roots of mung bean seedlings. The alleviative effect of exogenous ABA on Cd stress was also observed in rice and Solanum photeinocarpum (Uraguchi et al. 2009; Wang et al. 2013, 2016). Notably, Cd caused increases in chlorophyll and carotenoid contents (Fig. 2). Similar results were observed in Abelmoschus manihot (Wu et al. 2018) and Lonicera japonica (Jia et al. 2015) exposure to Cd stress. Our result showed that ABA reversed the enhanced chlorophyll and carotenoid contents by Cd. A recent study showed that ABA alleviated the inhibitory effect of Cd on the photosynthesis in lettuce (Tang et al. 2020). These results imply that exogenous ABA is involved in the regulation of photosynthesis in plant response to Cd stress.
Environmental stresses have been known to cause the generation and overproduction of ROS in plants, which are toxic molecules that can trigger oxidative damage to cell constituents including cell membranes, proteins, DNA, and lipids (Suzuki et al. 2012). The damages of cell membranes are accompanied by the accumulations of MDA in plants (Farooq et al. 2019). ROS-scavenging mechanisms play a vital role in protecting plants against environmental stresses. The antioxidants such as AsA, GSH, and proline, and ROS-scavenging enzymes such as SOD, APX, CAT, POD, and GPX are directly involved in ROS homeostasis and detoxification in plant cells (Suzuki et al. 2012). Our results showed that Cd stress triggered great increments in MDA levels in roots, stems, and leaves of mung bean seedlings (Fig. 7), suggesting the occurrence of membrane lipid peroxidation in the entire plants. The membrane damage further disrupts various biochemical and metabolic processes in plants (Hu et al. 2016). In our study, the enhanced MDA content in roots caused by Cd was much higher than those in stems and leaves, suggesting that Cd toxicity caused a greater damage to roots than to stems and leaves (Wu et al. 2004). The highest and lowest proline contents were detected in leaves and roots, respectively. This result is consistent with the report by Verbruggen and Hermans (2008). Cd stress caused continuous increases in the proline levels in roots, stems, and leaves during treatment time course. The maximal values were detected in roots. Therefore, the proline accumulation in roots, stems, and leaves effectively improved Cd tolerance of plants. Of the three organs, roots played a leading role in plant response to Cd stress via accumulating a higher proline level (Yang et al. 2009; Szabados and Savoure 2010).
Many studies have shown that Cd stress activates the antioxidative systems in plants, leading to the increased Cd tolerance (Zhang et al. 2007; Ekmekc et al. 2008; Hassan et al. 2008; Jin et al. 2008; Smeets et al. 2008; Chen et al. 2010). However, some conflicting results were obtained. For example, Cd stress caused the reduction of CAT and APX activities in Brassica napus and slight changes in SOD and CAT activities in Brassica juncea (Nouairi et al. 2009). Cd stress decreased the activities of POD, SOD, and CAT in Thlaspi (Pongrac et al. 2009) and mung bean seedlings during adventitious rooting (Li et al. 2014, 2018). The present study showed that Cd strongly increased CAT activity in both roots and stems, whereas decreased the activity in leaves. The POD activity was strongly increased in the three organs, whereas the SOD and APX activities were less changed in the three organs by Cd, except APX activity in stems which was highly increased. These results suggest CAT and POD play a dominant role in maintaining redox homeostasis in roots, stems, and leaves in response to Cd stress.
The antioxidants such as AsA, GSH, and polyphenols play a major role in the stress response in plants (Bashandy et al. 2010; Foyer and Noctor 2011). GSH is the substrate for the synthesis of phytochelatins (PCs). PCs directly bind cytoplasmic Cd, playing a crucial role in Cd detoxification in plants (Ammar et al. 2008). Cd caused a significantly decrease in the GSH levels in roots at 3 days and stems from 3 to 5 days, suggesting that a large amount of GSH was utilized to synthesize PCs in roots and then in stems at early stage of Cd exposure (Semane et al. 2007). The significant increases in GSH contents in roots from 5 to 9 days and in stems at 7 days implied that a large amount of GSH was synthesized in roots at secondary stage of Cd exposure. The great change in GSH level in roots further denoted that roots play a greater role in the chelation of Cd (Freeman et al. 2004). Polyphenol level is considered to be an indicator for plant resistance to abiotic stress because of the accumulation occurring under various abiotic stresses including Cd (Pereira 2016; Sharma et al. 2019). Polyphenols play a role in scavenging free radicals produced by heavy metals and make a great contribution to protect plant cells from damaging effect of oxidative stress (Jaleel et al. 2009). Due to the presence of carboxyl and hydroxyl groups with binding capacity of metal ions, polyphenols have high tendency to be a chelator which is conducive to decrease the harmful radical levels (Jun et al. 2003). Notably, the polyphenol content was strongly increased in roots and stems with an increasing treatment time, suggesting that this secondary metabolite is accumulated in cells with the growth of plant. Interestingly, the polyphenol content was strongly increased in roots, whereas it was decreased in stems and leaves by Cd stress, suggesting that polyphenols play a crucial role in alleviating Cd-induced oxidative stress in roots (Fig. 6).
ABA plays an important role in regulating plant responses to adverse environmental stresses, such as drought, cold, salinity, and heavy metals. Under stresses, ABA reduced lipid peroxidation by reducing the accumulation of ROS production (Ozfidan et al. 2012). It is well known that environmental stresses cause an increase in ABA content in plants and exogenous ABA addition results in a rapid increase in endogenous ABA levels (Huang et al. 2008). Exogenous application of ABA could alleviate Cd-induced toxicity by reducing Cd contents and ROS accumulation and activating antioxidant systems (Fan et al. 2014; Shen et al. 2017). Several previous studies have shown that pretreatment with ABA attenuated Pb-induced toxicity in rice (Zhao et al. 2009), and enhanced Cd and Pb tolerance in rice seedlings and Atractylodes macrocephala leaves (Hsu and Kao 2003, 2005; Wang et al. 2013). Consistent with the result obtained in rice seedlings, exogenous ABA reduced Cd accumulation in mung bean seedlings (Fig. 2a). ABA application prevented ROS production and counteracted Cd-stressed toxicity symptoms via promoting the activities of antioxidative enzymes (Shen et al. 2017). The present study demonstrated that ABA mitigated the toxic effects of Cd by restoring the antioxidative enzyme activities and the antioxidant levels. At the physiological and biochemical levels, the changes in all the measured parameters caused by Cd were partially or completely restored by ABA in the treatment with ABA+Cd, indicating that ABA was involved in the regulation of physiological processes in the plants to cope with Cd stress. ABA reversed the changed CAT activities in roots, stems, and leaves. Of the four kinds of enzymes, CAT appeared to be the pivotal enzyme that was involved in detoxifying excess H2O2 during Cd stress and ABA application. Although APX and CAT serve the same function of detoxifying, their different affinities suggest the roles of APX in modulating H2O2 for signaling while CAT in modulating H2O2 for detoxifying (Mittler 2002). The POD activities were strongly induced by Cd and ABA+Cd in roots, stems, and leaves, suggesting that POD played a critical role in ROS elimination (Abbas et al. 2017).
Studies have shown that, under osmotic and drought stresses, exogenous ABA significantly increased SOD, CAT, and APX activity, which in turn scavenge the overaccumulation of ROS and protect membranes from oxidative damage by ROS (Ozfidan et al. 2012), suggesting that ABA overcome the stress damage by upregulating the activity of antioxidative enzymes (Ozfidan et al. 2012). Exogenous ABA also led to increases in antioxidant contents such as AsA, GSH, carotenoid, and α-tocopherol (Jiang and Zhang 2001). ABA involved in the regulation of proline biosynthesis and accumulation (Cao et al. 2020). In the present study, ABA reversed the decreased AsA levels by Cd in roots and leaves, the increased GSH, polyphenol, and proline levels by Cd in roots. The Cd caused increases in MDA levels in roots and leaves were decreased by ABA in the ABA+Cd treatment, suggesting that ABA could alleviate the membrane lipid peroxidation in roots and stems triggered by Cd-caused oxidative stress. The same result was observed in lettuce plant (Tang et al. 2020). Obviously, ABA modulated the activities of antioxidative enzymes, mainly CAT and POD, and the antioxidant levels, leading to the amelioration of membrane lipid peroxidation to cope with Cd stress. ABA prevented Cd absorption and caused a decline in the Cd contents in roots and shoots. Thus, ABA triggered the physiological and biochemical changes in response to Cd stress was at least partly attributed to the reduction in Cd content in the seedlings.
A recent study showed that ABA (10 μM) differentially mediated the activities of NADPH oxidase (NOX), SOD, POD, APX, and CAT in roots and shoots of mung bean seedlings under water stress (Das and Kar 2018). The present study revealed the different biochemical and physiological responsive patterns of mung bean roots, stems, and leaves to Cd and ABA. For example, the CAT activity in leaves was much higher than those in roots and stems, and the change trends in Cd- and ABA+Cd–treated leaves were distinct from those in roots and stems. The stem APX activity positively responded to Cd and ABA+Cd but the root and leaf APX did not. The stem and leaf SOD activities negatively responded to ABA+Cd but the root SOD did not. The root GSH levels were strongly influenced by Cd and ABA+Cd, whereas the leaf GSH was less affected. The root polyphenol levels were induced by both Cd and ABA+Cd but the stem and leaf polyphenol levels were repressed. The root proline levels were strongly induced by Cd but did not respond to ABA+Cd. The stem and leaf proline levels were strongly induced by Cd and ABA+Cd. We conclude that roots play a primary role in Cd detoxification, though stems and leaves are involved in this process. In the root cells, Cd induced the PCs synthesis from GSH, triggered the accumulation of GSH, proline, and polyphenols, and further upregulated CAT and POD activities to cope with the oxidative burst caused by Cd. ABA can easily cross biological membranes and might be transported to target cells through the plant phloem and xylem (Hartung et al. 2002; Kuromori et al. 2014). Our results suggested that the foliar application of ABA was then transferred from leaves to roots to modulate the multiple processes of roots, stems, and leaves in response to Cd stress (Jahan et al. 2016; Naimah and Jahan 2017).
In conclusion, the present study reveals the physiological and biochemical mechanisms underlying ABA-mediated the alleviation of Cd stress to plants. We focused on the differential responses of roots, stems, and leaves of entire plant to Cd stress and the beneficial effects of ABA application on alleviating Cd toxicity. Cd stress caused a significant reduction in plant growth parameters and great changes in POD, CAT, and APX activities and MDA, proline, AsA, GSH, and polyphenol levels. Foliar application of ABA alleviated the Cd-stressed inhibitory effect on plant growth by modifying the antioxidant defense systems. ABA partially or completely recovered the changed enzyme activities, antioxidant levels, and photosynthetic pigment levels by Cd stress. Our study also reveals the differential physiological and biochemical responsive patterns of mung bean roots, stems, and leaves to Cd and ABA. Many of the detected biochemical indexes implied that roots played pivotal roles in response to Cd, especially in chelating Cd and reducing Cd absorption. Our study helps to elucidate the mechanisms by which ABA regulates plant tolerance to heavy metals, and provides new technical method and insight for phytoremediation of soil heavy metal pollution. Further researches are needed to explore the molecular mechanisms underlying ABA-mediated the alleviation of heavy metal toxicity to plant and the effect of the combination of ABA and other phytohormones on the alleviation of heavy metal toxicity.
References
Abbas G, Saqib M, Akhtar J, Murtaza G (2017) Physiological and biochemical characterization of Acacia stenophylla and Acacia albida exposed to salinity under hydroponic conditions. Can J For Res 47:1293–1301
Adie BAT, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681
Agarwal S, Sairam RK, Srivastava GC, Tyagi A, Meena RC (2005) Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidative enzymes induction in wheat seedlings. Plant Sci 169:559–570
Ali B, Qian P, Jin R, Ali S, Khan M, Aziz R, Tian T, Zhou W (2014) Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol Plant 58:131–138
Ammar WB, Mediouni C, Tray B, Ghorbel MH, Jemal F (2008) Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biol Plant 52:314–320
An Y, Zhou P, Liang J (2014) Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two Lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci 65:274–286
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Bandurska H (2001) Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiologiae Plantarum 23:483–490
Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, Reichheld JP (2010) Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 22:376–391
Bates L, Waldren RP, Teare JD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Benyó D, Horváth E, Németh E, Leviczky T, Takács K, Lehotai N, Feigl G, Kolbert Z, Ördög A, Gallé R, Csiszár J, Szabados L, Erdei L, Gallé Á (2016) Physiological and molecular responses to heavy metal stresses suggest different detoxification mechanism of Populus deltoides and P. canadensis. J Plant Physiol 201:62–70
Cao X, Wu L, Wu M, Zhu C, Jin Q, Zhang J (2020) Abscisic acid mediated proline biosynthesis and antioxidant ability in roots of two different rice genotypes under hypoxic stress. BMC Plant Biol 20:198
Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16:1143–1162
Chen F, Wang F, Wu FB, Mao WH, Zhang GP, Zhou MX (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Dai M, Liu W, Hong H, Lu H, Liu J, Jia H, Yan C (2018) Exogenous phosphorus enhances cadmium tolerance by affecting cell wall polysaccharides in two mangrove seedlings Avicennia marina (Forsk.) Vierh and Kandelia obovata (S., L.) Yong differing in cadmium accumulation. Mar Pollut Bull 126:86–92
Das S, Kar RK (2018) Abscisic acid mediated differential growth responses of root and shoot of Vigna radiata (L.) Wilczek seedlings under water stress. Plant Physiol Biochem 123:213–221
Ding Y, Feng R, Wang R, Guo J, Zheng X (2014) A dual effect of Se on Cd toxicity: evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 375:289–301
Dong J, Wu F, Zhang G (2006) Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 64:1659–1666
Ekmekçi Y, Tanyolaç D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611
Fan SK, Fang XZ, Guan MY, Ye YQ, Lin XY, Du ST, Jin CW (2014) Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front Plant Sci 5:721
Farooq MA, Niazi A, Akhtar J, Saifullah FM, Souri Z, Karimi N, Rengel Z (2019) Acquiring control: the evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem 141:353–369
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Freeman JI, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thalpsi nickel hyperaccumulator. Plant Cell 16:2176–2191
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Guo JJ, Qin SY, Reng Z, Gao W, Nie ZJ, Liu HG, Li C, Zhao P (2019) Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol Environ Saf 172:380–387
Hartung W, Sauter A, Hose E (2002) Abscisic acid in the xylem: where does it come from, where does it go to? J Exp Bot 53(366):27–32
Hassan MJ, Shao G, Zhang G (2006) Influence of Cadmium Toxicity on Growth and Antioxidant Enzyme Activity in Rice Cultivars with Different Grain Cadmium Accumulation. Journal of Plant Nutrition 28:1259–1270
Hassan MJ, Shafi M, Zhang G, Zhu Z, Qaisar M (2008) The growth and some physiological responses of rice to Cd toxicity as affected by nitrogen form. Plant Growth Regul 54:125–132
Hauser F, Li Z, Waadt R, Schroeder JI (2017) SnapShot: abscisic acid signaling. Cell 171:1708–1708e0
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124:71–80
Hu ZB, Cools T, De Veylder L (2016) Mechanisms used by plants to cope with DNA damage. Annu Rev Plant Biol 67:439–462
Huang D, Wu W, Abrams SR, Cutler AJ (2008) The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J Exp Bot 59:2991–3007
Jahan MS, Nozulaidi M, Khairi M, Mat N (2016) Light-harvesting complexes in photosystem II regulate glutathione-induced sensitivity of Arabidopsis guard cells to abscisic acid. J Plant Physiol 195:1–8
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi H, Zhao CX, Shao HB, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Jia L, Liu Z, Chen W, Ye Y, Yu S, He X (2015) Hormesis effects induced by cadmium on growth and photosynthetic performance in a hyperaccumulator, Lonicera japonica thunb. J Plant Growth Regul 34:13–21
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jiang MY, Cai XY, Liao JR, Yang YX, Chen QB, Gao SYXF, Luo ZH, Lei T, Lv BY, Liu SL (2020) Different strategies for lead detoxification in dwarf bamboo tissues. Ecotoxicol Environ Saf 193:110329
Jin X, Yang X, Mahmood Q, Islam E, Liu D, Li H (2008) Response of antioxidant enzymes, ascorbate and glutathione metabolism towards cadmium in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii H. Environ Toxicol 23:517
Jun M, Fu HY, Hong J, Wan X, Yang CS, Ho CT (2003) Comparison of antioxidant activities of isoflavones from kudzu root (Pueraria lobata Ohwi). J Food Sci 68:2117–2122
Kuromori T, Sugimoto E, Shinozaki K (2014) Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol 164:1587–1592
Li SW, Leng Y, Feng L, Zeng XY (2014) Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ Sci Pollut Res 21:525–537
Li SW, Zeng XY, Leng Y, Feng L, Kang XH (2018) Indole-3-butyric acid mediates antioxidative defense systems to promote adventitious rooting in mung bean seedlings under cadmium and drought stresses. Ecotoxicol Environ Saf 161:332–341
Lin R, Wang X, Luo Y, Du W, Guo H, Yin D (2007) Effects of soil cadmium on growth, oxidative stress and antioxidant system in wheat seedlings (Triticum aestivum L.). Chemosphere 69:89–98
Mahmud JA, Hasanuzzaman M, Nahar K, Bhuyan MHMB, Fujita M (2018) Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol Environ Saf 147:990–1001
Matysik J, Alia BB, Mohanty P (2002) Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Meng Y, Zhang L, Wang LQ, Zhou CJ, Shangguan YX, Yang Y (2019) Antioxidative enzymes activity and thiol metabolism in three leafy vegetables under Cd stress. Ecotoxicol Environ Saf 173:214–224
Menguer PK, Farthing E, Peaston KA, Ricachenevsky FK, Fett JP, Williams LE (2013) Functional analysis of the rice vacuolar zinc transporter OsMTP1. J Exp Bot 64:2871–2883
Milner MJ, Seamon J, Craft E, Kochian LV (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot 64:369–381
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nahar K, Hasanuzzamanc M, Alam MM, Rahman A, Suzukid T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255
Naimah N, Jahan MS (2017) Signaling behaviour of abscisic acid on physiological activities in plants under stress. Pertanika J Trop Agric Sci 40:485–496
Nasibeh P, Tommy L, Parviz E, Maria G (2019) Different response to Cd stress in domesticated and wild safflower (Carthamus spp.). Ecotoxicol Environ Saf 171:321–328
Nouairi I, Ammar WB, Youssef NB, Miled DDB, Ghorbal MH, Zarrouk M (2009) Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol Plant 31:237–247
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202:35–49
Ozfidan C, Turkan I, Sekmen AH, Seckin B (2012) Abscisic acid-regulated responses of aba2-1 under osmotic stress: the abscisic acid-inducible antioxidant defence system and reactive oxygen species production. Plant Biol 14:337–346
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:133
Peng JS, Gong JM (2014) Vacuolar sequestration capacity and long-distance metal transport in plants. Front Plant Sci 5:19
Pereira A (2016) Plant abiotic stress challenges from the changing environment. Front Plant Sci 7:1123
Pirie A, Mullins MG (1976) Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol 58:468–472
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Piceaabies L.). Plant Physiol 106:53–60
Pongrac P, Zhao FJ, Razinger J, Zrimec A, Regvar M (2009) Physiological responses to Cd and Zn in two Cd/Zn hyperaccumulating Thlaspi species. Environ Exp Bot 66:479–486
Popova L, Maslenkova L, Yordanova R, Krantev A, Szalai G, Janda T (2013) Salicylic acid protects photosynthesis against cadmium toxicity in pea plants. Bulg J Plant Physiol 34:133–148
Qin HY, Liu HG, Nie ZJ, Gao W, Li C, Lin YH, Zhao P (2018) AsA–GSH cycle and antioxidant enzymes play important roles in Cd tolerance of wheat. Bull Environ Contam Toxicol 101:684–690
Rao MV, Paliyath G, Ormrod DP, Murr DO, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative, stress, and H2O2-metabolizing enzymes. Plant Physiol 115:137–149
Sadia R, Ghulam A, Muhammad S, Muhammad S, Abu BUF, Munawar H, Behzad M, Muhammad A, Muhammad AN, Amjad F (2019) Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: implications for phytoremediation. Ecotoxicol Environ Saf 171:146–153
Semane B, Cuypers A, Smeets K, Van BF, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528
Shahid M, Dumat C, Khalid S, Schreck E, Xiong TT, Niazi NK (2017) Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J Hazard Mater 325:36–58
Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng BS (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24:2452
Shen GM, Niu JK, Deng ZX (2017) Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk (Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol Biochem 118:471–478
Simões CC, Melo JO, Magalhaes JV, Guimarães CT (2012) Genetic and molecular mechanisms of aluminum tolerance in plants. Genet Mol Res 11:1949–1957
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and lonomics. Front Plant Sci 6:1143
Smeets K, Ruytinx J, Semane B, Belleghem FV, Remans T, Sanden SV, Vangronsveld J, Cuypers A (2008) Cadmium-induced transcriptional and enzymatic alteration related to oxidative stress. Environ Exp Bot 63:1–8
Song WY, Yang HC, Shao HB, Zheng AZ, Brestic M (2014) The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. Clean-Soil Air Water 42:88–97
Spychalla JP, Desborough SL (1990) Superoxide dismutase, catalase and alpha tocopherol content of stored potato tubers. Plant Physiol 94:1214–1218
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2019) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tanaka K, Fujimaki S, Fujiwara T, Yoneyama T, Hayashi H (2007) Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants. Soil Sci Plant Nutr 53:72–77
Tang Y, Wang L, Xie Y, Yu X, Li H, Lin L, Liao MA, Wang Z, Sun G, Wang X, Liang D, Xia H, Tu L (2020) Effects of exogenous abscisic acid on the growth and cadmium accumulation of lettuce under cadmium-stress conditions. Int J Environ Anal Chem 100:720–731
Tyburski J, Tretyn A (2010) Glutathione and glutathione disulfide affect adventitious root formation and growth in tomato seedling cuttings. Acta Physiol Plant 32:411–417
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 2:364–372
Wang J, Chen J, Pan K (2013) Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut Res 20:1441–1449
Wang J, Lin LJ, Luo L, Liao MA, Lv XL, Wang ZH, Liang D, Xia H, Wang X, Lai YS, Tang Y (2016) The effects of abscisic acid (ABA) addition on cadmium accumulation of two ecotypes of Solanum photeinocarpum. Environ Monit Assess 188:182
Wu FB, Chen F, Wei K, Zhang GP (2004) Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L.) differing in cadmium tolerance. Chemosphere 57:447–454
Wu K, Wu ZH, Tai FJ, Han Y, Xie BE, Yuan ZL (2011) Effects of cadmium on the contents of phytohormones, photosynthetic performance and fluorescent characteristics in tobacco leaves. Acta Ecol Sin 31:4517–4524
Wu M, Luo Q, Zhao Y, Long Y, Liu S, Pan Y (2018) Physiological and biochemical mechanisms preventing Cd toxicity in the new hyperaccumulator Abelmoschus manihot. J Plant Growth Regul 37:709–718
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699
Yuan ZL, Wu ZH (2010) Effect of cadmium on antioxidative capability and phytohormone level in tobacco roots. Acta Ecol Sin 30:4109–4118
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhao L, Xiong J, Li LP, Zhu C (2009) Low concentration of exogenous abscisic acid increases lead tolerance in rice seedlings. Biol Plant 53:728–732
Zhu HH, Chen L, Xing W, Ran SM, Wei ZH, Amee M, Wassie M, Niu H, Tang DY, Sun J, Du DY, Yao J, Hou HB, Chen K, Sun J (2020) Phytohormones-induced senescence efficiently promotes the transport of cadmium from roots into shoots of plants: a novel strategy for strengthening of phytoremediation. J Hazard Mater 388:122080
Funding
This work was supported through funding from the National Natural Science Foundation of China (31760110 and 31560121) and Young Scholars Science Foundation of Lanzhou Jiaotong University (2020048).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Leng, Y., Li, Y., Ma, YH. et al. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ Sci Pollut Res 28, 6030–6043 (2021). https://doi.org/10.1007/s11356-020-10843-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10843-8