Abstract
Among the most frequent targets for toxic effects of modern pesticides, namely organophosphates and carbamates, one may find cholinesterases (ChEs). ChEs exist in a wide variety of animals and have been used actively to discriminate among the environmental effects of different pollutant groups, including the aforementioned pesticides. This study had three purposes, namely (i) identifying the ChE forms present in tissues (eyes and walking legs muscle) of two crab species, Carcinus maenas and Pachygrapsus marmoratus; to (ii) determine the in vitro toxicological effects, and (iii) compare the sensitivity of such enzymatic forms towards commonly used anti-ChE pesticides, namely the organophosphate chlorpyrifos and the carbamate carbofuran. Our results showed that there was not a clear preference for any of the tested substrates in any of the tissues from both species. Furthermore, the ChE activity was almost completely suppressed following incubation with eserine and with the specific inhibitor BW284C51 in all tissues from both species. In vitro exposure to chlorpyrifos promoted a significant decrease in ChE activity in both species. Furthermore, the ChE activity was completely suppressed following incubation with carbofuran and chlorpyrifos. These results suggest that the major ChE forms present in tissues of both crab species show intermediate structural properties and activity patterns, halfway between classic acetylcholinesterase and pseudocholinesterases. However, the sensitivity of the found forms towards ChE inhibitors was established, and the responsiveness of such forms towards common anti-ChE chemicals was established. Both tested species seem to be promising test organisms to be used in marine and coastal scenarios of putative contaminations by anti-ChE chemicals, considering the here reported patterns of response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ChEs (ChEs) are classified as esterases and have the capacity of hydrolyzing carboxylic esters. ChEs show a preference for the hydrolysis of choline esters, therefore they can be differentiated from other esterases (Nunes et al. 2005; Rodrigues et al. 2011). ChEs hydrolytic activity can be significantly diminished by exposure to specific chemical agents, such as pesticides, namely those from the organophosphate and carbamate classes. These pesticides form a covalent bond with the ChE’s active site, resulting in a stable enzyme-substrate complex, leading to its inhibition and inactivation (Sanchez-Hernandez and Walker 2000). This toxic effect occurs not only in vertebrates but in other organisms, namely aquatic, that share the same pathway of neurotransmission regulation (Cooper and Bidwell 2006). However, it is essential to have detailed and specific knowledge about the types of ChEs that may simultaneously occur in different species and tissues, and about the diverse responsiveness of such enzymatic forms; only by having this knowledge, it is possible to establish a judicious selection of biomarkers to be used in ecotoxicological assays, especially when considering the use of ChE activity as a biomarker (Jbilo et al. 1994; Nunes et al. 2005; Xuereb et al. 2007; Nunes 2011; Rodrigues et al. 2011). Indeed, ChEs can be divided according to their biochemical affinities and contribution to biological processes: acetylcholinesterases (AChEs) are designated as true ChEs and are involved in the regulation of neurotransmission and neuromuscular functioning; and other ChE forms, named pseudocholinesterases, are butyrylcholinesterases (BChEs) and propionylcholinesterases (PChE) (Jbilo et al. 1994; Nunes et al. 2005; Xuereb et al. 2007; Nunes 2011; Rodrigues et al. 2011).
Crustaceans, particularly crabs, are key components of estuarine and coastal food webs, since they connect primary producers and organic detritus with secondary consumers, promoting nutrient cycling and quality of water (Oliva et al. 2019). In addition, intertidal organisms like Pachygrapsus marmoratus and Carcinus maenas are considered model species by different studies (Madeira et al. 2014; Oliva et al. 2019). However, the number of ecotoxicological studies focusing on the effects of environmental pollutants on crustaceans, is still somewhat scarce, when compared with studies on other taxa, such as bivalves. The macroinvertebrate Carcinus maenas (Linnaeus 1758) is an estuarine and coastal crustacean that has been frequently used in aquatic ecotoxicology and it is known by its common name, European green crab. This choice is supported by the fact that with known biology and ecology, C. maenas is one of the best-studied estuarine species (Rodrigues et al. 2012; Rodrigues and Pardal 2014). Pachygrapsus marmoratus (Fabricius, 1787), generally known as marble crab, is a common species populating the rough shores of the Mediterranean Sea, the Black Sea, and the North-Atlantic Ocean, where the entire intertidal belt is colonized irrespective of its breadth (Oliva et al. 2019). Considering its strong dispersal ability, this species is a good model to be used in ecotoxicological research due to its local abundance and also due to the cumulative knowledge of its growth, ecology, and genetics (Deli et al. 2016; Oliva et al. 2019). The selection of the two crab species to perform this research was focused on their normal occurrence and the large availability, particularly in Ria de Aveiro (Portugal), which contributes to its easy sampling and availability during the entire year, which area decisive considerations supporting their usage in ecotoxicological monitoring and/or testing (Pacheco et al. 2005). Both species of crabs can be subjected to a large range of anthropogenic pollutants but remain abundant (Pedersen et al. 1997). This indicates that compensatory mechanisms that enable these organisms to withstand natural variations in the environment may also grant some resistance to contaminant exposure (Hebel et al. 1997; Brown et al. 2004). Additionally, they have the potential to accumulate diverse pollutants, including heavy metals, PAHs, and PCBs (Pedersen et al. 1998; Orbea et al. 2002) and, consequently, both species can be an effective bioindicator of environmental pollution from such agents.
However, and despite their advantages, the ChEs forms of these crabs were never previously characterized. This is a decisive aspect that must be encompassed before their use as sentinels or test organisms in Ecotoxicology. Consequently, there are still many gaps in the understanding of their possible reaction to anticholinesterasic agents, and the biochemical characterization of their ChEs is now necessary if they are to be actively included in such experiments. Toxic action of pesticides in exposed organisms occurs via the irreversible inhibition of the AChE enzyme; specific pesticides (organophosphates and carbamates, such as chlorpyrifos and carbofuran, respectively), thereby block the hydrolysis of ACH, leading to an excessive accumulation of this neurotransmitter, causing a disruption of nerve function (Peña-Llopis et al. 2003). In addition to AChE inhibition, pesticides also inhibit pseudocholinesterases, like BChE and PChE, which are closely related to enzymes that hydrolyze some xenobiotics and bind to others. Organophosphate and carbamate pesticides are common in coastal environments, such that inhibitory effects on ChEs are likely to occur in the wild (Lionetto et al. 2003; Peña-Llopis et al. 2003; Lionetto et al. 2013). Among these pesticides, chlorpyrifos is a priority substance within the European Water Framework Directive for the protection of aquatic ecosystems (Directive 2008/105/EC) (Franzellitti et al. 2011), and previous studies have reported chlorpyrifos water contamination, with levels up to 17,000 ng/L (Mugni et al. 2012; Bonansea et al. 2013). Another pesticide, the carbamate carbofuran, is used as a broad-spectrum insecticide, acaricide, and nematicide. Vryzas et al. (2011), assessed pesticide loading in drainage canals near the Greek/Bulgarian/Turkish borders and found levels of this pesticide nearing 0.191 mg/L to 0.229 mg/L. Nonetheless, the number of ecotoxicological studies focusing on the effects of these pesticides on both species here presented, and/or other crustacean species, is very scarce, and even studies with other species assessed different biomarkers (Narra et al. 2012).
Characterization of the ChEs forms present in a tissue relies on the differential measurement of the hydrolytic activity of all ChE forms, by using different substrates and specific inhibitors. Eserine sulfate inhibits ChEs in general, providing a clear indication of the contribution of non-specific esterases to the measured activity (Eto 1974). True AChE is strongly inhibited by 1,5-bis-(4-allyldimethyl-ammoniumphenyl)-pentan-3-one dibromide (BW284C51) at concentrations in the mM range. Tetramonoisopropyl pyrophosphortetramide (iso-OMPA) inhibits BChE (Eto 1974; Nunes et al. 2005; Rodrigues et al. 2011; Ramos et al. 2012; Nunes and Resende 2017; and Pereira et al. 2019). In light of the significance of ChEs as environmental biomarkers and the importance of Carcinus maenas and Pachygrapsus marmoratus as test organisms and environmental sentinels in ecotoxicological monitoring, the present study envisioned to characterize the ChE forms present in tissues (eyes and walking legs muscle) of both species and determine the in vitro toxicological effects and comparison of the sensitivity of different ChEs towards commonly used pesticides, namely the organophosphate chlorpyrifos, and the carbamate carbofuran.
Materials and methods
Chemicals
AChE iodide (≥ 98%; CAS 1866-15-5), BChE iodide (≥ 98%; CAS 1866-16-6), propionylthicholine iodide (≥ 98%; CAS 1866-73-5), 5,5′-dithio-bis (γ-nitrobenzoic acid) (DTNB; ≥ 98%; CAS 69–78-3), physiostigmine salicylate (98%; CAS 57–64-7), 1,5-bis (4-allyldimethyl ammoniumphenyl)–pentan–3-one dibromide (BW284C51; 97%; CAS 402-40-4), tetraisopropylpyrophosphoramide (iso-OMPA; 100%; CAS 513- 00-8), ethanol absolute (≥ 99.8%; CAS 64-17-5), bovine γ-globulin (≥ 99%; CAS 9007-83-4), acetone (≥ 99.5%; CAS 67-64-1), chlorpyrifos (CAS 2921-88-2), carbofuran (CAS 1563-66-2) were purchased from Sigma-Aldrich™, USA. Bradford reagent was purchased from BIO-RAD (Watford, UK).
Test organisms, sample processing, and enzymatic analysis
Organisms of the two species were manually collected during low tide in Ria de Aveiro (Aveiro, Portuguese Littoral-Centre), From the Barra area, Ria de Aveiro, Portugal (40° 38′ 34.5″ N 8° 44′ 07.7″ W). This location refers to a protected coastal lagoon away from direct sea action and is the initial portion of the Mira channel, which is primarily subjected to naval traffic (Oliveira et al. 2009). Consequently, the known input of anti-ChE substances does not substantially impact it.
Approximately 15 individuals (males) of each species were euthanized by hypothermia, and each individual’s eyes and walking legs (skeletal) muscle were isolated and homogenized in phosphate buffer (0.1 M, pH = 7.2), and centrifuged with a Thermo Scientific Heraeus Megafuge 8R centrifuge at 3300g for 3 min. The supernatants were retrieved and obtained separately to provide a sample of homogenized tissues to be included in all testing procedures. The enzymatic assay for the assessment of ChE activity was based on the quantification of ChEs activity according to Ellman’s protocol (Ellman et al. 1961). This enzymatic assay involves monitoring ChEs activity for 15 min, at room temperature (25 ± 1 °C), by the formation of a complex by conjugation of thiocholine (resulting from the hydrolytic degradation of AChE by ChEs) with DTNB (5,5′-dithio-bis (γ-nitrobenzoic acid). This complex absorbs at a wavelength of 414 nm, and the increase in absorbance is proportional to the enzyme’s activity. The concentration of total soluble protein was calculated by the process described by Bradford (1976), adapted to the microplate. pH = 7.2).
Characterization of ChEs
Characterization of ChEs was based on the procedures described by Nunes et al. (2005), Rodrigues et al. (2011), Ramos et al. (2012), Nunes and Resende (2017), and Pereira et al. (2019). These studies focused on the in vitro study of hydrolytic sensitivity of ChEs to various substrates, as well as the use of general and specific enzyme inhibitors. For this purpose, samples of both tissues from each species were analyzed using the substrates ASCh, BSCh, and PSCh, utilizing a previously defined range of concentrations (0.005; 0.01; 0.02; 0.04; 0.08; 0.16; 0.32; 0.64; 1.28; 2.56; 5.12; 10.24; and 20.48 mM). All procedures occurred in triplicate at room temperature (25 ± 1 °C), and pH = 7.2. In order to estimate the kinetic parameters–maximum rate of hydrolysis reached (Vmax), and concentration needed to reach one-half of the maximum velocity (Michaelis–Menten constant, Km)–were determined.
The next step was to assess the inhibitory profiles of common and specific inhibitors of ChE, true ChEs, and pseudocholinesterases. At this point, the protocol was performed using the concentrations of preferential substrate, according to the previous procedure, that showed the highest enzyme activity. Therefore, for C. maenas eyes and leg muscle, the substrates were, respectively, PSCh (5.12 mM) and BSCh (5.12 mM); for P. marmoratus eyes and leg muscle, the substrates were, respectively, PSCh (2.56 mM) and BSCh (20.48 mM). In this process, a portion of the homogenized tissue pool was incubated with eserine (6.25, 12.5, 25, 50, 100, and 200 μM), BW284C51 (6.25, 12.5, 25, 50, 100, and 200 μM), and iso-OMPA (0.25, 0.5, 1, 2, 4, and 8 mM), utilizing a previously defined variety of concentrations. Eserine and BW248C51 solutions were formulated in ultra-pure water, and iso-OMPA was dissolved in ethanol (Nunes et al. 2005; Rodrigues et al. 2011; Ramos et al. 2012; Nunes and Resende 2017; Pereira et al. 2019). Following the same protocol of all previous studies described above, 5 μL of each concentration of the inhibitor solution was incubated with 495 μL of homogenate from each tissue, at room temperature (25 ± 1 °C), for 20 min. All these exposures occurred in triplicate, and these reactions happened with the collected homogenized samples in propylene microtubes and were incubated with each concentration of the three inhibitors. In the case of iso-OMPA incubation, an extra control group was introduced to check for potential alterations induced by the solvent, utilizing ethanol. Enzymatic assays and concentrations of proteins were analyzed according to the procedures cited earlier.
Toxicological tests—in vitro assays
Additional incubations similar to those previously mentioned with the ChE inhibitors were conducted to evaluate the in vitro effect of both pesticides on the ChE activity (Nunes et al. 2005; Nunes and Resende 2017; Pereira et al. 2019; Ramos et al. 2012; Rodrigues et al. 2011). The pesticides used were carbofuran and chlorpyrifos in the following concentrations range: 12.5, 25, 50, 100, 200, and 400 μM; this range of concentrations was based on previous work by Pereira et al. (2019) for the same chemical classes of pesticides. In the case of both pesticides’ incubation, a solvent control group was introduced to check for potential alterations induced by the solvent, utilizing acetone. All procedures occurred in triplicate at room temperature (25 ± 1 °C), and pH = 7.2.
Data analysis
The results were statistically evaluated using an ANOVA followed by Dunnett’s test to test for significant differences between the responses observed in treated groups compared to the control treatment. A significance level of 0.05 was always used to infer statistically significant results.
Results
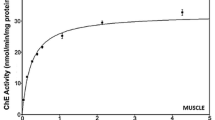
Our results showed that there was not a consistent preferential substrate for any of the tissues, from both species. In the eyes tissue from C. maenas, the preferential substrate—a substrate that leads to the highest ChE activity—was PSChE (Fig. 1). In the leg muscle tissue from the same species, BSChE was more extensively hydrolyzed (Fig. 1). In the eyes tissue of P. marmoratus, there was an almost nearly equal preference for PSCh and BSCh, but this last substrate attained higher hydrolytic rates at higher concentrations (Fig. 2). In the leg muscle from the same species, the substrate BSCh was more rapidly hydrolyzed at higher concentrations of substrate (Fig. 2). Considering these data, the chosen substrates (those that were preferentially hydrolyzed, with which higher hydrolysis rates were attained) were: for, eyes and leg muscle of C. maenas, PSCh (5.12 mM) and BSCh (5.12 mM), respectively; for P. marmoratus eyes and leg muscle, respectively, PSCh (2.56 mM) and BSCh (20.48 mM). An additional important finding was related to the form of the dose-response curves (Figs. 1 and 2). These were not curvilinear (as those described for the typical Michaelis-Menten curves), and a decrease of the measured enzymatic activities was observed for the highest levels of the substrates that were preferentially hydrolyzed. The kinetic parameters support the hypothesis for each enzyme preference for both species (Table 1).
Furthermore, the ChE activity was almost completely suppressed following incubation with eserine at all the tested concentrations, and in both tissues from each species (C. maenas eyes: F[6, 14] = 9.062; P < 0.001; C. maenas muscle: F[6, 14] = 22.697; P < 0.001; P. marmoratus eyes: F[6, 14] = 10.489; P < 0.001; P. marmoratus muscle: F[6, 14] = 63.831; P < 0.001), and also with the specific inhibitor BW284C51 at all the tested concentrations in both tissues from each species (C. maenas eyes: F[6, 14] = 11.123; P < 0.001; C. maenas muscle: F[6, 14] = 9.531; P < 0.001; P. marmoratus eyes: F[6, 14] = 9.531; P < 0.001; P. marmoratus muscle: F[6, 14] = 21.163; P < 0.001), in both tissues from each species. The specific inhibitor Iso-OMPA elicited significant inhibition at all the tested concentrations for all tissues except for P. marmoratus muscle (C. maenas eyes: F[7, 16] = 0.883; P < 0.001; C. maenas muscle: F[7, 16] = 1.171; P < 0.001; P. marmoratus eyes: F[7, 16] = 1.172; P < 0.001; P. marmoratus muscle: F[7, 16] = 0.117; P = 0.153) (Fig. 3).
Effects of specific inhibitors (eserine, BW284C51, and ISO-OMPA) on cholinesterase activity of eyes and leg muscle homogenates of C. maenas and P. marmoratus. The substrate for C. maenas eyes was propionylthiocholine (5.12 mM) and butyrylthiocholine (5.12 mM) for leg muscle; the substrate for P. marmoratus eyes was propionylthiocholine (2.56 mM) and butyrylthiocholine (20.48 mM) for leg muscle. Values are the mean of three replicate assays of a pool of tissue from 15 specimens of each species and corresponding standard error bars. *Significant differences, P ≤ 0.05
In vitro exposure to the pesticide chlorpyrifos elicited a significant impairment of ChE activity in both species, at all the tested concentrations for both tissues of C. maenas and eyes of P. marmoratus; in P. marmoratus muscle it elicited a significant impairment of ChE activity in the three highest concentrations (C. maenas eyes: F[6, 14] = 6.139; P < 0.001; C. maenas muscle: F[6, 14] = 10.705; P < 0.001; P. marmoratus eyes: F[6, 14] = 8.584; P < 0.001; P. marmoratus muscle: F[6, 14] = 18.068; P < 0.001). Carbofuran elicited a significant impairment of ChE activity in both species for all tested concentrations (C. maenas eyes: F[6, 14] = 3.646; P < 0.001; C. maenas muscle: F[6, 14] = 10.374; P < 0.001; P. marmoratus eyes: F[6, 14] = 3.712; P < 0.001; P. marmoratus muscle: F[6, 14] = 12.276; P < 0.001) (Fig. 4). A summary of all inhibition results can be seen in Table 2.
In vitro effects of carbofuran and chlorpyrifos on cholinesterase activity of eyes and leg muscle homogenates from C. maenas and P. marmoratus. The substrate for C. maenas eyes was propionylthiocholine (5.12 mM) and butyrylthiocholine (5.12 mM) for leg muscle; the substrate for P. marmoratus eyes was propionylthiocholine (2.56 mM) and butyrylthiocholine (20.48 mM) for leg muscle. Values are the mean of three replicate assays of a pool of tissue from 15 specimens of each species and corresponding standard error bars. *Significant differences, P ≤ 0.05
Discussion
Our results showed that there was not a preferential substrate that was common to any of the tissues and species. In the eyes tissue from C. maenas, the preferential substrate—a substrate that leads to the highest ChE activity—was PSCh (Fig. 1). In the leg muscle tissue from the same species, BSCh was more extensively hydrolyzed (Fig. 1). In the eyes tissue of P. marmoratus, there was an almost nearly equal preference for PSCh and BSCh, but this last substrate attained higher hydrolytic rates at higher concentrations (Fig. 2). In the leg muscle from the same species, the substrate BSCh was more rapidly hydrolyzed at higher concentrations of substrate (Fig. 2). It is known from the literature that different ChE forms may exhibit overlapping hydrolytic capabilities (Rodrigues et al. 2011), justifying that in some cases, ChEs in different tissues from the same species did not show the same preferential substrate. Consequently, and considering this potential variation, the use of ChE inhibition as effect criteria in environmental monitoring requires the full characterization of the enzymatic form present in exposed organisms, especially in terms of its hydrolytic preference, and to know the normal, physiological range of enzymatic activity in non-exposed organisms (Lieberman 2002; Olson and Christensen 1980). This variation in terms of substrate preference may be the consequence of the nature of ChEs, including their genesis and conformation. Several studies showed that ChEs are polymorphic in a great number of species, and distinct forms of ChEs likely show distinct sensitivity to anti-ChE agents (Lieberman 2002; Olson and Christensen 1980). In practice, it is crucial to determine which type of ChE is most abundant in a model organism, because this will distinguish which substrate is the most suitable for monitoring purposes (Ramos et al. 2012).
As stated before, both species of crabs used in this study can be subjected to a large set of anthropogenic pollutants, but without any clear population effect, thereby the species remaining abundant even in contaminated sites (Pedersen et al. 1997). This indicates that compensatory mechanisms that enable these organisms to withstand natural variations in the environment may also grant some resistance to contaminant exposure (Hebel et al. 1997; Brown et al. 2004). Additionally, they have the potential of accumulating various pollutants, including heavy metals, PAHs, and PCBs (Pedersen et al. 1998; Orbea et al. 2002) and, consequently, both species can be an effective bioindicator of environmental pollution from such agents.
To date, only a few studies have characterized ChEs in marine crustaceans. The characterization of ChEs in the nervous tissues of aquatic vertebrates, namely fish is, in general, marked by a strong preference for ASCh as a substrate (Table 3). The studies by Arufe et al. (2007) with Sparus aurata; Garcia et al. (2000) with Poecilia reticulata; Leticia and Gerardo (2008) with Haemulon plumieri; Monteiro et al. (2005) with Pomatoschistus microps; Nunes et al. (2005) with Gambusia holbrooki; Pereira et al. (2019) with Astyanax altiparanae, Phalloceros harpagos, and Pterygoplichthys pardalis; Rodríguez-Fuentes and Gold-Bouchot (2004) with Oreochromis niloticus, Limanda limanda, and Platichthys flesus; and Sturm et al. (1999) with Serranus cabrilla, showed that the nervous system of most both marine and freshwater fish species have ChE forms that hydrolyze preferentially ASCh. Analogously, the muscle tissue of many fish species also seems to show a similar pattern. Additionally, similar behavior has also been shown for some invertebrates, such as the cockle Cerastoderma glaucum, and the grass shrimp Palaemonetes pugio (Key and Fulton 2002; Ramos et al. 2012). Despite some exceptions, this is a consistent trend. In our case, namely when considering the results for the species C. maenas, a significant finding was the variable preference of the analyzed ChE forms as a function of the level of the substrate. The here obtained results regarding the hydrolytic activity of muscle and eye tissues showed that PSCh was the most hydrolyzed substrate (when in low levels), but higher levels of BSCh attained higher hydrolytic activities. Eyes tissue of P. marmoratus also showed a similar tendency, while muscle tissue of this species exhibited a clear preference for BSCh. This was a distinctive feature of the here tested species concerning the majority of the already tested species for their ChE forms. The biological meaning of these differences is, however, still uncertain.
In general, the substrates attaining the highest hydrolytic rates were also prone to an opposite effect, when present in high levels. From the here obtained results, it was clear that the highest hydrolytic activities were not attained with the highest levels of substrates. It seems that high levels of the substrate may contribute to a competitive inhibitory mechanism, with significant effects on the efficacy of the hydrolytic process, as evidenced by Kato et al. (1972), and Pohanka et al. (2011). In fact, and according to the mechanistic study by Colletier et al. (2006), high levels of the substrate ASCh were able to impair the hydrolytic activity of AChE in the fish Torpedo californica, by preventing the prompt exit of the degradation product (acetate) of this isomer of acetylcholine. This effect resulted in a significant reduction in the hydrolytic rate, similarly to what was observed in our study. Similar results were also found for fish, such as Poecilia reticulata (Garcia et al. 2000); Gambusia holbrooki (Nunes et al. 2005); Phalloceros harpagos and Astyanax altiparanae (Pereira et al. 2019). It thus seems that similar to what has been reported described for other aquatic organisms, ChE forms present in tissues of C. maenas and P. marmoratus are likely to be saturated at high levels of substrate.
Despite the differences in terms of substrate preference, inhibitors tests yielded similar results for all tissues and species, since eserine and BW284C51 obtained almost full inhibition with the lowest tested dose; on the contrary, ISO-OMPA only resulted in partial inhibition, and, in the case of P. marmoratus muscle tissue, did not cause any significant inhibition. In fact, in P. marmoratus muscle tissue, it did not inhibit its ChE activity. Despite this difference, it is consistent with the results obtained for other crustaceans. Varó et al. (2002) obtained similar results for two different Artemia species; Artemia salina ChEs preferred ASCh at low concentrations, however, at high substrate concentrations, it hydrolyzed PSCh at a higher rate. Besides, the ChEs of this anostracan species are also relatively insensitive to ISO-OMPA, similarly to most vertebrate AChE forms. Artemia parthenogenetica also shows miscellaneous characteristics since it prefers PSCh (instead of ASCh), its ChEs are not inhibited by high concentrations of substrate, and it is also susceptible to ISO-OMPA. Nevertheless, A. parthenogenetica ChE is also inhibited by BW284C51. The results obtained by Varó et al. (2002) are in agreement with previous studies carried out with other invertebrates that indicated the complexity of using the ChE vertebrates classification to invertebrate species (Varó et al. 2002). ChEs from invertebrates, displaying intermediate particularities when compared with analogous vertebrate enzymes, could represent the transitional stage of a molecular evolution starting from a hypothetical ancestral enzyme. Kinetic and molecular aspects of this hypothetical enzyme could survive in zoological groups of very ancient phylogenetic origin such as the class of Crustacea (Talesa et al. 1992). Other studies point to this possibility. Forget and Bocquené (1999) found that although results indicate the presence of a single ChE form in the copepod Tigriopus brecornis, it is unclear whether this enzyme is a ChE or a non-specific esterase that also metabolizes acetylcholine. Antó et al. (2009) reported that both AChEs and pseudoChEs were present in the muscle tissues of the crustaceans Aristeus antennatus and Nephrops norvegicus. The classification of invertebrate ChEs is more ambiguous; in insects, for example, a single ChE form metabolizes acetylcholine and BChE (Forget and Bocquené 1999).
Other non-crustacean invertebrates show ChE forms that do not have the greatest affinity for AChE, unlike most vertebrates studied. Nunes and Resende (2017) also obtained unexpected data for Solen marginatus, which indicates the presence of an atypical form of ChE: the hydrolytic profile shows a preference for PSCh, yet the discrimination by the use of specific inhibitors revealed that AChE is probably the predominant form (Nunes and Resende 2017).
The here-obtained results showed that the in vitro exposure to carbofuran completely inhibited the ChE activity of the two crab species. Carbamates bind to the active site and exert a reversible inhibition of ChEs (Nunes 2011). Nevertheless, this effect is temporary and can be reverted by hydrolysis of the carbamate-enzyme complex, allowing the ChE activity to recover to typical physiological values (Xiao et al. 2017). The inhibition of the in vitro ChE activity has already been reported for other carbamates, for instance, carbaryl for Astyanax altiparanae, Phalloceros harpagos, and Pterygoplichthys pardalis (Pereira et al. 2019); carbofuran in the fish Colossoma macropomum (Assis et al. 2010); bendiocarb, methomyl, propoxur, fenobucarb, and carbosulfan in the species, Astyanax jacuhiensis (Gonçalves et al. 2018), Carassius auratus (Bretaud et al. 2000), Cherax destructor (Pham et al. 2017), Cyprinus carpio (Wang et al. 2015), and Tor tambroides (Ahmad et al. 2016). The here obtained results are in line with the previous findings, showing that both autochthonous marine crab species are also sensitive to such chemicals and may be successfully used in future biomonitoring programs assessing putative contamination of coastal water by this class of pesticides.
Besides carbamates, organophosphates are typical inhibitors of ChE activity. The results of the present study revealed that the in vitro exposure to chlorpyrifos inhibited the ChE activity of the two crab species, following a clear dose-response pattern. Prior in vitro studies with aquatic invertebrates have demonstrated their sensitivity to these pesticides. Individuals of the bivalve Corbicula fluminea exposed to chlorfenvinphos; the snail Potamopyrgus antipodarum (Gagnaire et al. 2008) was sensitive to chlorpyrifos; the marine crustaceans Artemia salina and Artemia parthenogenetica were sensitive to chlorpyrifos and dichlorvos; Gammarus pulex, and Palaemon serratus; the freshwater crustaceans Daphnia magna and Cherax destructor; the midge Chironomus riparius, showed to have their ChE activity significantly inhibited after the exposure to organophosphates, such as, malathion, dichlorvos, parathion, piriminfos-methyl, chlorpyrifos-oxon (Sturm et al. 1999; McLoughlin et al. 2000; Varó et al. 2002; Frasco et al. 2005; Ramos et al. 2012; Pham et al. 2017). These authors concluded that these particular species could be useful for assessing pesticide contamination in saltwater, an assumption that may also be made for the here two tested species.
The recommended preferential substrates for each species, according to our data, are: for Carcinus maenas eyes and leg muscle, respectively, PChE (5.12 mM) and BChE (5.12 mM); for Pachygrapsus marmoratus eyes and leg muscle, respectively, PChE (2.56 mM) and BChE (20.48 mM). Nevertheless, as we stated in our results, the dose-response curves (Figs. 1 and 2) are not curvilinear as the typical Michaelis-Menten curve. Rozengart et al. (2000) stated that out of all diversity of aspects of ChE substrate specificity, the most physiologically important seems to be the inhibition of enzyme activity by high concentrations of substrate. They have sown that the substrate participates in ChE catalysis not only as a passive target of the enzyme action (Rozengart et al. 2000). Both activation and inhibition of enzymatic activity by high substrate concentration have been long known, depending on the nature of ChE forms, which varies from one species to the other. In ChE reactions with reversible and irreversible inhibitors, the substrate performs the so-called protective effect (Rozengart 1996; Rozengart et al. 2000; Rozengart & Basova, 2000; Basova and Kolesov 2000; Basova et al. 2000).
Conclusion
In conclusion, the present research described the prevailing ChEs in two crab species’ eyes and leg muscle tissues, paving the way for their possible use as model organisms in ecotoxicological monitoring and ecotoxicological testing. However, this research made clear the presence of small variations (hydrolytic activity, preference for substrates and inhibition profiles) between the C. maenas ChE types and those found in P. marmoratus. Furthermore, the findings of this research help to demonstrate the complexity in classifying such enzymes solely on the basis of pre-existing classifications used for vertebrates, such as AChE or BChE, because ChEs of invertebrates often exhibit intermediate characteristics between the two forms. Important in vitro effects of both pesticides, chlorpyrifos, and carbofuran, on ChE activity, have been identified for both organisms. This finding is of considerable significance as it highlights both organisms as useful alternatives for aquatic coastal ecotoxicological monitoring and/or testing while evaluating the existence and effects of anti-ChE compounds. The results reported here also show that the two selected species were highly susceptible to the compounds being examined, supporting their use as research organisms in ecotoxicology, to detect the existence and effects of specific pollutants that impair cholinergic neurotransmission.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad AS, Sabullah MK, Shamaan NA, Shukor MYA, Jirangon H, Khalid A, Syed MA (2016) Evaluation of acetylcholinesterase source from fish, Tor tambroides for detection of carbamate. J Environ Biol 37(4):479–484
Antó M, Arnau S, Buti E, Cortijo V, Gutiérrez E, Solé M (2009) Characterisation of integrated stress biomarkers in two deep-sea crustaceans, Aristeus antennatus and Nephrops norvegicus, from the NW fishing grounds of the Mediterranean Sea. Ecotoxicol Environ Saf 72(5):1455–1462. https://doi.org/10.1016/j.ecoenv.2009.02.007
Arufe MI, Arellano JM, García L, Albendín G, Sarasquete C (2007) Cholinesterase activity in gilthead seabream (Sparus aurata) larvae: characterization and sensitivity to the organophosphate azinphosmethyl. Aquat Toxicol 84(3):28–336. https://doi.org/10.1016/j.aquatox.2007.06.009
Assis CR, Castro PF, Amaral IP, Carvalho EV, Carvalho LB Jr, Bezerra RS (2010) Characterization of acetylcholinesterase from the brain of the Amazonian tambaqui (Colossoma macropomum) and in vitro effect of organophosphorus and carbamate pesticides. Environ Toxicol Chem 29(10):2243–2248. https://doi.org/10.1002/etc.272
Basova TV, Kolesov BA (2000) Raman spectra of copper phthalocyanin: experiment and calculation. J Struct Chem 41(5):770–777. https://doi.org/10.1023/a:1004802000669
Basova NE, Rozengart EV, Khovanskikh AE (2000) Kinetic analysis of the “substrate protective effect” in cholinesterases of different origin. J Evol Biochem Physiol 36(2):130–137. https://doi.org/10.1007/bf02754325
Bonansea RI, Amé MV, Wunderlin DA (2013) Determination of priority pesticides in water samples combining SPE and SPME coupled to GC–MS. A case study: Suquía River basin (Argentina). Chemosphere 90(6):1860–1869. https://doi.org/10.1016/j.chemosphere.2012.10.007
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bretaud S, Toutant J-P, Saglio P (2000) Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus). Ecotoxicol Environ Saf 47(2):117–124. https://doi.org/10.1006/eesa.2000.1954
Brown R, Galloway T, Lowe D, Browne M, Dissanayake A, Jones M, Depledge M (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66(3):267–278. https://doi.org/10.1016/j.aquatox.2003.10.001
Colletier J-P, Fournier D, Greenblatt HM, Stojan J, Sussman JL, Zaccai G, Silman I, Weik M (2006) Structural insights into substrate traffic and inhibition in acetylcholinesterase. EMBO J 25(12):2746–2756. https://doi.org/10.1038/sj.emboj.7601175
Cooper NL, Bidwell JR (2006) Cholinesterase inhibition and impacts on behavior of the Asian clam, Corbicula fluminea, after exposure to an organophosphate insecticide. Aquat Toxicol 76(3–4):258–267. https://doi.org/10.1016/j.aquatox.2005.09.012
Cunha I, García LM, Guilhermino L (2005) Sea-urchin (Paracentrotus lividus) glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination. J Environ Monit 7(4):288–294. https://doi.org/10.1039/b414773a
Deli T, Fratini S, Ragionieri L, Said K, Chatti N, Schubart CD (2016) Phylogeography of the marbled crab Pachygrapsus marmoratus (Decapoda, Grapsidae) along part of the African Mediterranean coast reveals genetic homogeneity across the Siculo-Tunisian Strait versus heterogeneity across the Gibraltar Strait. Mar Biol Res 12(5):471–487. https://doi.org/10.1080/17451000.2016.1154972
Diamantino TC, Almeida E, Soares AM, Guilhermino L (2003) Characterization of cholinesterases from Daphnia magna Straus and their inhibition by zinc. Bull Environ Contam Toxicol 71(2):219–225. https://doi.org/10.1007/s00128-003-0153-7
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Eto M (1974) Organophosphorus pesticides. Organic and biological chemistry. CRC Press, Cleveland
Forget J, Bocquené G (1999) Partial purification and enzymatic characterization of acetylcholinesterase from the intertidal marine copepod Tigriopus brevicornis. Comp Biochem Physiol C Toxicol Pharmacol 123(4):345–350. https://doi.org/10.1016/S0305-0491(99)00073-5
Forget J, Livet S, Leboulenger F (2002) Partial purification and characterization of acetylcholinesterase (AChE) from the estuarine copepod Eurytemora affinis (Poppe). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 132(1):85–92. https://doi.org/10.1016/s1532-0456(02)00050-9
Franzellitti S, Capuzzo A, Viarengo A, Fabbri E (2011) Interactive effects of nickel and chlorpyrifos on Mediterranean mussel cAMP-mediated cell signaling and MXR-related gene expressions. Comp Biochem Physiol C Toxicol Pharmacol 154(4):377–382. https://doi.org/10.1016/j.cbpc.2011.07.006
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2005) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10(5):360–375. https://doi.org/10.1080/13547500500264660
Gagnaire B, Geffard O, Xuereb B, Margoum C, Garric J (2008) Cholinesterase activities as potential biomarkers: characterization in two freshwater snails, Potamopyrgus antipodarum (Mollusca, Hydrobiidae, Smith 1889) and Valvata piscinalis (Mollusca, Valvatidae, Müller 1774). Chemosphere 71(3):553–560. https://doi.org/10.1016/j.chemosphere.2007.09.048
Garcia LM, Castro B, Ribeiro R, Guilhermino L (2000) Characterization of cholinesterases from guppy (Poecilia reticulata) muscle and its in vitro inhibition by environmental contaminants. Biomarkers 5(4):274–284. https://doi.org/10.1080/135475000413827
Gonçalves CR, Marins AT, do Amaral AMB, Leitemperger J, Severo ES, Moraes BS, Zanella R, Loro VL (2018) Biochemical responses in freshwater fish exposed to insecticide propoxur. Bull Environ Contam Toxicol 100(4):524–528. https://doi.org/10.1007/s00128-018-2285-9
Hebel D, Jones M, Depledge M (1997) Responses of crustaceans to contaminant exposure: a holistic approach. Estuar Coast Shelf Sci 44(2):177–184. https://doi.org/10.1006/ecss.1996.0209
Jbilo O, Bartels CF, Chatonnet A, Toutant J, Lockridge O (1994) Tissue distribution of human acetylcholinesterase and butyrylcholinesterase messenger RNA. Toxicon 32(11):1445–1457. https://doi.org/10.1016/0041-0101(94)90416-2
Kato G, Tan E, Yung J (1972) Acetylcholinesterase. Kinetic studies on the mechanism of atropine Ikhibition. J Biol Chem 247(10):3186–3189
Key PB, Fulton MH (2002) Characterization of cholinesterase activity in tissues of the grass shrimp (Palaemonetes pugio). Pestic Biochem Physiol 72(3):186–192. https://doi.org/10.1016/S0048-3575(02)00006-8
Leticia A-G, Gerardo G-B (2008) Determination of esterase activity and characterization of cholinesterases in the reef fish Haemulon plumieri. Ecotoxicol Environ Saf 71(3):787–797. https://doi.org/10.1016/j.ecoenv.2008.01.024
Lieberman JA (2002) Managing anticholinergic side effects. Prim Care Companion J Clin Psychiatry 4(6):240–241
Lionetto M, Caricato R, Giordano M, Pascariello M, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46(3):324–330. https://doi.org/10.1016/s0025-326x(02)00403-4
Lionetto MG, Caricato R, Calisi A, Giordano ME, Schettino T (2013) Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. Biomed Res Int 2013:1–8. https://doi.org/10.1155/2013/321213
Madeira D, Narciso L, Diniz MS, Vinagre C (2014) Synergy of environmental variables alters the thermal window and heat shock response: an experimental test with the crab Pachygrapsus marmoratus. Mar Environ Res 98:21–28. https://doi.org/10.1016/j.marenvres.2014.03.011
McLoughlin N, Yin D, Maltby L, Wood RM, Yu H (2000) Evaluation of sensitivity and specificity of two crustaceans biochemical biomarkers. Environ Toxicol Chem 19(8):2085–2092. https://doi.org/10.1002/etc.5620190818
Monserrat J, Bianchini A (1998) Some kinetic and toxicological characteristics of thoracic ganglia cholinesterase of Chasmagnathus granulata (Decapoda, Grapsidae). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120(2):193–199. https://doi.org/10.1016/s0742-8413(98)00040-1
Monteiro M, Quintaneiro C, Morgado F, Soares AMVM, Guilhermino F (2005) Characterization of the cholinesterases present in head tissues of the estuarine fish Pomatoschistus microps: application to biomonitoring. Ecotoxicol Environ Saf 62(3):341–347. https://doi.org/10.1016/j.ecoenv.2004.12.007
Mora P, Fournier D, Narbonne J (1999) Cholinesterases from the marine mussels Mytilus galloprovincialis Lmk. and M. edulis L. and from the freshwater bivalve Corbicula fluminea Müller. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 122(3):353–361. https://doi.org/10.1016/s0742-8413(98)10130-5
Mugni H, Demetrio P, Paracampo A, Pardi M, Bulus G, Bonetto C (2012) Toxicity persistence in runoff water and soil in experimental soybean plots following chlorpyrifos application. Bull Environ Contam Toxicol 89(1):208–212. https://doi.org/10.1007/s00128-012-0643-6
Narra MR, Begum G, Rajender K, Rao JV (2012) Toxic impact of two organophosphate insecticides on biochemical parameters of a food fish and assessment of recovery response. Toxicol Ind Health 28(4):343–352. https://doi.org/10.1177/0748233711412423
Nunes B (2011) The use of cholinesterases in ecotoxicology. In: Reviews of environmental contamination and toxicology, vol 212. Springer, New York, pp 29–59. https://doi.org/10.1007/978-1-4419-8453-1_2
Nunes B, Resende ST (2017) Cholinesterase characterization of two autochthonous species of Ria de Aveiro (Diopatra neapolitana and Solen marginatus) and comparison of sensitivities towards a series of common contaminants. Environ Sci Pollut Res 24(13):12155–12167. https://doi.org/10.1007/s11356-017-8761-7
Nunes B, Carvalho F, Guilhermino L (2005) Characterization and use of the total head soluble cholinesterases from mosquitofish (Gambusia holbrooki) for screening of anticholinesterase activity. J Enzyme Inhib Med Chem 20(4):369–376. https://doi.org/10.1080/14756360500094094
Oliva M, De Marchi L, Cuccaro A, Casu V, Tardelli F, Monni G, Pretti C (2019) Effects of copper on larvae of the marbled crab Pachygrapsus marmoratus (Decapoda, Grapsidae): toxicity test and biochemical marker responses. Comp Biochem Physiol C Toxicol Pharmacol 223:71–77. https://doi.org/10.1016/j.cbpc.2019.05.007
Oliveira M, Maria VL, Ahmad I, Serafim A, Bebianno MJ, Pacheco M, Santos MA (2009) Contamination assessment of a coastal lagoon (Ria de Aveiro, Portugal) using defence and damage biochemical indicators in gill of Liza aurata–an integrated biomarker approach. Environ Pollut 157(3):959–967. https://doi.org/10.1016/j.envpol.2008.10.019
Olson DL, Christensen GM (1980) Effects of water pollutants and other chemicals on fish acetylcholinesterase (in vitro). Environ Res 21(2):327–335. https://doi.org/10.1016/0013-9351(80)90034-1
Orbea A, Ortiz-Zarragoitia M, Solé M, Porte C, Cajaraville MP (2002) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat Toxicol 58(1–2):75–98. https://doi.org/10.1016/s0166-445x(01)00226-0
Pacheco M, Santos MA, Teles M, Oliveira M, Rebelo JE, Pombo L (2005) Biotransformation and genotoxic biomarkers in mullet species (Liza SP.) from a contaminated coastal lagoon (Ria De Aveiro, Portugal). Environ Monit Assess 107(1–3):133–153. https://doi.org/10.1007/s10661-005-5308-z
Pedersen S, Lundebye A, Depledge M (1997) Field application of metallothionein and stress protein biomarkers in the shore crab (Carcinus maenas) exposed to trace metals. Aquat Toxicol 37(2–3):183–200. https://doi.org/10.1016/s0166-445x(96)00816-8
Pedersen SN, Pedersen KL, Højrup P, Knudsen J, Depledge MH (1998) Induction and identification of cadmium-, zinc- and copper-metallothioneins in the shore crab Carcinus maenas (L.). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120(2):251–259. https://doi.org/10.1016/s0742-8413(98)10003-8
Peña-Llopis S, Ferrando M, Peña JB (2003) Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol 65(4):337–360. https://doi.org/10.1016/s0166-445x(03)00148-6
Pereira BVR, Silva-Zacarin ECM, Costa MJ, Dos Santos ACA, do Carmo JB, Nunes B (2019) Cholinesterases characterization of three tropical fish species, and their sensitivity towards specific contaminants. Ecotoxicol Environ Saf 173(October 2018):482–493. https://doi.org/10.1016/j.ecoenv.2019.01.105
Pérez J, Monteiro MS, Quintaneiro C, Soares AM, Loureiro S (2013) Characterization of cholinesterases in Chironomus riparius and the effects of three herbicides on chlorpyrifos toxicity. Aquat Toxicol 144-145:296–302. https://doi.org/10.1016/j.aquatox.2013.10.014
Pham B, Miranda A, Allinson G, Nugegoda D (2017) Evaluating the non-lethal effects of organophosphorous and carbamate insecticides on the yabby (Cherax destructor) using cholinesterase (AChE, BChE), glutathione S-transferase and ATPase as biomarkers. Ecotoxicol Environ Saf 143:283–288. https://doi.org/10.1016/j.ecoenv.2017.05.035
Pohanka M, Hrabinova M, Kuca K, Simonato J-P (2011) Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman’s method. Int J Mol Sci 2011(12):2631–2640. https://doi.org/10.3390/ijms12042631
Quintaneiro C, Monteiro M, Soares AM, Ranville J, Nogueira AJ (2014) Cholinesterase activity on Echinogammarus meridionalis (Pinkster) and Atyaephyra desmarestii (Millet): characterisation and in vivo effects of copper and zinc. Ecotoxicology 23(3):449–458. https://doi.org/10.1007/s10646-014-1204-z
Ramos AS, Gonçalves F, Antunes SC, Nunes B (2012) Cholinesterase characterization in Corbicula fluminea and effects of relevant environmental contaminants: a pesticide (chlorfenvinphos) and a detergent (SDS). J Environ Sci Health B 47(6):512–519. https://doi.org/10.1080/03601234.2012.665661
Rodrigues ET, Pardal MÂ (2014) The crab Carcinus maenas as a suitable experimental model in ecotoxicology. Environ Int 70:158–182. https://doi.org/10.1016/j.envint.2014.05.018
Rodrigues SR, Caldeira C, Castro BB, Gonçalves F, Nunes B, Antunes SC (2011) Cholinesterase (ChE) inhibition in pumpkinseed (Lepomis gibbosus) as environmental biomarker: ChE characterization and potential neurotoxic effects of xenobiotics. Pestic Biochem Physiol 99(2):181–188. https://doi.org/10.1016/j.pestbp.2010.12.002
Rodrigues AP, Oliveira PC, Guilhermino L, Guimarães L (2012) Effects of salinity stress on neurotransmission, energy metabolism, and anti-oxidant biomarkers of Carcinus maenas from two estuaries of the NW Iberian Peninsula. Mar Biol 159(9):2061–2074. https://doi.org/10.1007/s00227-012-1992-8
Rodríguez-Fuentes G, Gold-Bouchot G (2004) Characterization of cholinesterase activity from different tissues of Nile tilapia (Oreochromis niloticus). Mar Environ Res 58(2–5):505–509. https://doi.org/10.1016/j.marenvres.2004.03.037
Rozengart EV (1996) The cholinesterase reactivity of the Komandor squid Berryteuthis magister. Substrates and organophosphorus inhibitors. Zh Evol Biokhim Fiziol 32(5):576–583
Rozengart EV, Basova NE (2000) Species-specific differences in the substrate-inhibitory specificity of cholinesterases from optical ganglia of squids of the Gonatidae family. J Evol Biochem Physiol 36(3):249–253. https://doi.org/10.1007/bf02737039
Rozengart EV, Basova NE, Moralev SN, Khovanskikh AE (2000) Effect of the substrate structure on the backward inhibition of cholinesterases of various origin. Zh Evol Biokhim Fiziol 36(4):298–303
Sanchez-Hernandez JC, Walker CH (2000) In vitro and in vivo cholinesterase inhibition in lacertides by phosphonate- and phosphorothioate-type organophosphates. Pestic Biochem Physiol 67(1):1–12. https://doi.org/10.1006/pest.1999.2471
Sturm A, Silva de Assis HC, Hansen P-D (1999) Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar Environ Res 47(4):389–398. https://doi.org/10.1016/S0141-1136(98)00127-5
Talesa V, Contenti S, Mangiabene C, Pascolini R, Rosi G, Principato G (1990) Propionylcholinesterase from Murex brandaris: comparison with other invertebrate cholinesterases. Comp Biochem Physiol C Toxicol Pharmacol 96(1):39–43. https://doi.org/10.1016/0742-8413(90)90041-7
Talesa V, Contenti S, Principato GB, Pascolini R, Giovannini E, Rosi G (1992) Cholinesterases from Maia verrucosa and Palinurus vulgaris: a comparative study. Comp Biochem Physiol C Toxicol Pharmacol 101(3):499–503. https://doi.org/10.1016/0742-8413(92)90077-K
Varó I, Navarro JC, Amat F, Guilhermino L (2002) Characterisation of cholinesterases and evaluation of the inhibitory potential of chlorpyrifos and dichlorvos to Artemia salina and Artemia parthenogenetica. Chemosphere 48(6):563–569. https://doi.org/10.1016/S0045-6535(02)00075-9
Vryzas Z, Alexoudis C, Vassiliou G, Galanis K, Papadopoulou-Mourkidou E (2011) Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicol Environ Saf 74(2):174–181. https://doi.org/10.1016/j.ecoenv.2010.04.011
Walday P, Fonnum F (1989) A comparative pharmacological characterization of cholinesterases in salmon (Salmo Salar) brain and sealice (Lepeophtheirus Salmonis). Comp Biochem Physiol C Toxicol Pharmacol 92(2):197–199. https://doi.org/10.1016/0742-8413(89)90040-6
Wang Y, Chen C, Zhao X, Wang Q, Qian Y (2015) Assessing joint toxicity of four organophosphate and carbamate insecticides in common carp (Cyprinus carpio) using acetylcholinesterase activity as an endpoint. Pestic Biochem Physiol 122:81–85. https://doi.org/10.1016/j.pestbp.2014.12.017
Xiao Q, Zhou H, Wei H, Du H, Tan W, Zhan Y, Pistolozzi M (2017) A new method to characterize the kinetics of cholinesterases inhibited by carbamates. J Pharm Biomed Anal 144:175–182. https://doi.org/10.1016/j.jpba.2017.04.007
Xuereb B, Noury P, Felten V, Garric J, Geffard O (2007) Cholinesterase activity in Gammarus pulex (Crustacea Amphipoda): characterization and effects of chlorpyrifos. Toxicology 236(3):178–189. https://doi.org/10.1016/j.tox.2007.04.010
Acknowledgments
Bruno Nunes is hired by ECO-R-pharmplast-Ecotoxicity of realistic combinations of pharmaceutical drugs and microplastics in marine ecosystems, Fundação para a Ciência e a Tecnologia, FCT (reference POCI-01-0145-FEDER-029203). Thanks, are also due for the financial support to CESAM (UIDB/50017/2020+UIDP/50017/2020), to FCT/MEC through national funds, and the co-funding by the ERDF, within the PT2020 Partnership Agreement and Compete 2020.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia, FCT (project ECO-R-pharmplast-ecotoxicity of realistic combinations of pharmaceutical drugs and microplastics in marine ecosystems, reference POCI-01-0145-FEDER-029203). FCT also funded the research center CESAM, in which the research was conducted (UIDB/50017/2020+UIDP/50017/2020); CESAM was also co-funded by ERDF, within the PT2020 Partnership Agreement and Compete 2020. None of these sources of funding had any role in the design of the study and collection, analysis, and interpretation of data and in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Ana Filipa Ferreira was involved in formal analysis; investigation; methodology; and writing of the original draft.
Bruno Nunes was involved in conceptualization; data curation; formal analysis; funding acquisition; project administration; resources; supervision; validation; and in writing, namely, reviewing and editing the manuscript.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable. Animals used in this assay are from an invertebrate species, which do not require previous ethics approval.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nogueira, A.F., Nunes, B. Cholinesterase characterization and effects of the environmental contaminants chlorpyrifos and carbofuran on two species of marine crabs, Carcinus maenas and Pachygrapsus marmoratus. Environ Sci Pollut Res 28, 14681–14693 (2021). https://doi.org/10.1007/s11356-020-11492-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11492-7