Abstract
A hydroponic experiment was conducted to establish the response of exogenous silicon [Si] in alleviating arsenate [As (V)] prompted alterations on antioxidant enzyme activities and thiol metabolism in wheat (Triticum aestivum L. cv PBW 343) seedlings. Objective of the work was to validate the hypothesis whether silicate may alleviate arsenate-provoked oxidative stress in wheat through diverse metabolic pathways with an endeavor to improve food safety and health. Arsenate treatment significantly enhanced oxidative stress and was associated with modifications in non-enzymatic and enzymatic antioxidants. The activities of arsenate reductase [AR] and the enzymes related to thiol metabolism revealed dose-dependent enhancements with increase in arsenate along with enhanced production of phytochelatins [PCs] in the cultivar. Simultaneous supplementations of silicate with arsenate in the nutrient formulation reduced arsenate uptake along with arsenate reductase activity and consequently lowered arsenite [As (III)] accumulation. The antioxidative defense was upregulated and phytochelatin production was lowered causing an appreciable revival from the arsenate-imposed consequences that eventually augmented growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The non-essential metalloid arsenic (As) is found in soil at concentrations ranging from 0.1 to 40 mg kg−1. Escalating arsenic concentrations in groundwater has become one of the major environmental adversities and has accentuated perturbations in natural ecosystems. The carcinogenic metalloid becomes a part of the environment through natural and anthropogenic causes (Hettick et al. 2015). The Indo-Gangetic basin is regarded as the most intensely arsenic-contaminated environmental zone in the world where arsenic levels in water have been documented up to 3200 μg L−1 over the permissible limit of 10 μg L−1 specified by World Health Organization (WHO) (McCarty et al. 2011). Consumption of cereals and vegetables grown in such metalloid-polluted soils along with use of contaminated water forms a route for arsenic exposure and has led to the incidence of arsenic-related disorders in more than 3 lakhs of natives. Arsenic build-up in soil from groundwater is alarming due to its undesirable consequences on food safety (Mishra et al. 2014). Arsenic pollution not only affects humans but also has severe effects on metabolic processes of plants and influences agricultural productivity.
Arsenic uptake occurs primarily as arsenate [As (V)], though arsenite [As (III)] is absorbed as well. Inside the plant cell arsenate reductase (AR), mediated reduction of As (V) to As (III) occurs. The strong propensity of As (III) to bind sulfhydryl (–SH) groups interferes with protein functioning, inactivates essential enzymes, and inhibits cellular functions (Sharma 2012). Arsenic toxicity induces production of reactive oxygen species (ROS) during transformation from As (V) to As (III) that results in unrestricted oxidative destruction to the cellular machinery and damages nucleic acids, cellular proteins causing per oxidation of membrane lipids leading to electrolyte leakage. This eventually provokes oxidative stress (Hasanuzzaman and Fujita 2013). To combat such undesirable inconveniences, plant cells are equipped with admirable antioxidant defense mechanisms that detoxify ROS and defend cellular machinery. These are manifested in a broad array of phenomena that include modifications in non-enzymatic and enzymatic antioxidants as well as production of thiols that have high affinity for toxic metals (Jozefczak et al. 2012). Synthesis and production of low molecular weight thiols and phytochelatins (PCs) help to bind As (III) and sequester it to vacuole (Dixit et al. 2015). Another low molecular weight tripeptide (γ-glutamylcysteine—Gly), glutathione (GSH), is also a non-protein thiol, which exists abundantly in plant cells and is the substrate for PC synthesis required for heavy metal detoxification (Zagorchev et al. 2013). It participates in ROS scavenging through ascorbate-glutathione (ASA-GSH) cycle and maintains redox homeostasis in the cells. Formation of As (V) to As (III) is one of the key steps for detoxification since As (III) can only bind to PCs; consequently AR activity becomes crucial (Li et al. 2015).
The underappreciated quasi-essential metalloid silicon (Si), in group IVA of the periodic table, is ubiquitous with multiple beneficial functions and has been documented to sustain plants in combinatorial complexities of environmental adversities. The tetravalent metalloid mitigates several stresses by external as well as internal mechanisms (Liu et al. 2017; Pontigo et al. 2017; Sil et al. 2018, 2019a, b; Das et al. 2019; Dwivedi et al. 2020). Results obtained from our previous research affords evidences that supplemental Si has the potentiality to efficiently repair As (V)-incited damage by elevating the levels of plastidial pigments along with considerable enhancements in photosynthetic parameters. Silicon-mediated enhancement in polyamine production appreciably restored ionic homeostasis that improved photosynthesis and eventually reinstated growth under As (V) excess (Sil et al. 2019b). Silicate co-treatment with As (V) also lowered the oxidative stress markers and improved the activities of the respiratory cycle enzymes as well as gamma amino butyric acid (GABA) synthesis by enhancing the availability of phosphorus that helped to counteract the effects of As (V) toxicity (Sil et al. 2018). Silicate amendments further improved nitrogen metabolism by enhancing nitrate uptake and elevated the activities of nitrogen-metabolizing enzymes under As (V) stress. This, in turn, upregulated the activities of ammonia assimilatory enzymes and enhanced amino acid and protein contents (Sil et al. 2019a). Supplemental silicate thus appreciably revived arsenate-imposed reduction in growth indicating ameliorative interaction of silicate in wheat (Sil et al. 2018, 2019a, b). Utilization of Si nanoparticles (Si-NPs) in farming systems also has the potential to afford solutions to numerous agricultural setbacks including pathogenicity, drought, and productivity and consequently improve crop yield (Rastogi et al. 2019).
Wheat is extensively cultivated in tropical and subtropical regions under both rain-fed and irrigated agriculture. Approximately 85% of the global population depends on this cereal for basic calories and protein (Caverzan et al. 2016). Heavy metal pollution in arid and semi-arid lands induces oxidative stress in this cereal crop with severe consequences on diverse physiological processes that include seed germination, plant growth and adversely affects crop production causing substantial yield loss ultimately affecting human health (Rahaie et al. 2013).
The study aimed to investigate responses of different concentrations (25 μM, 50 μM, and 100 μM) of As (V) with or without silicate (5 mM) in connection with some non-enzymatic and enzymatic antioxidants [ascorbate, α-tocopherol, superoxide dismutase (SOD), catalase (CAT) and catechol peroxidase (CPX), ascorbate peroxidase (APX), ascorbate oxidase (AAO)] in wheat seedlings. Accumulations of As and As (III) along with the activity of enzyme AR responsible for conversion of As (V) to As (III) and the enzymes involved in thiol metabolism were also determined together with PC synthesis. We endeavored to explore whether Si supplementation was competent enough to lower As (V)-induced phytotoxicity through reduction of As (V) uptake, amelioration of oxidative stress, regulation of ASA-GSH cycle thereby facilitating defense against As (V) stress in the test seedlings.
Materials and methods
Growth conditions and stress treatments
Wheat (Triticum aestivum L. cv. PBW-343) seeds were disinfected using sodium hypochlorite (5% v/v) for 15 min and rinsed extensively in distilled water. Around 100 seeds for individual treatment were placed in petri dishes (ɸ 20 cm) containing moist pre-sterilized blotting sheets for germination in dark for 48 h at 30 ± 2 °C. Germinated seedlings were spiked with modified Hoagland’s solution (Zhu et al. 2006) and sodium arsenate (Na2HAsO4·7H2O; Loba-Chemie, India) in absence or presence of sodium silicate (Na2SiO3·9H2O Loba-Chemie, India) at 16 h photoperiod (260 μmol m−2 s−1 PFD) for 19 days. Nutrient solution supplemented individually with either Na2HAsO4·7H2O (25 μM, 50 μM, and 100 μM) and Na2SiO3·9H2O (5 mM) or with Na2SiO3·9H2O (5 mM) and Na2HAsO4·7H2O (25 μM, 50 μM, and 100 μM) were the treatment sets while the control comprised only modified Hoagland’s solution. Solutions were replaced every alternate day. The mentioned concentrations of As (V) are environmentally relatable and correspond to soil conditions (Choudhury et al. 2010). Again from screening experiments with different concentrations of sodium silicate, 5 mM silicate exhibited maximum growth promotion when applied singly or in combination with 25 μM, 50 μM, and 100 μM As (V) and was therefore selected. Treatments with 25 μM, 50 μM, and 100 μM As (V) have been represented as 25 As (V), 50 As (V), and 100 As (V), respectively, while the corresponding silicate treatments were 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si. The plantlets were collected after 21 days, weighed in equivalent amounts, and kept at − 40 °C until analyses.
Estimation of total arsenic, arsenite contents, and determination of arsenate reductase activity
Oven-dried plant samples (2 g each) were digested in 20 ml freshly prepared aqua regia containing 65% HNO3 and 37% HCl (in the ratio of 1:3) followed by boiling in a water bath (at 95 °C) until the sample had completely dissolved (Ang and Lee 2005). Each extract was then cooled and filtered and final volume was adjusted to 25 ml with milli Q water. Total As and As (III) contents were estimated under different conditions using flow injection-hydride generation-atomic absorption spectrophotometry (FI-HG-AAS) following Abdel-Lateef et al. (2013) and Chooto et al. (2015). The atomic absorption spectrophotometer (Spectra AA50 Varian) with hydride generator (Agilent VGA-77) was equipped with an electrically heated quartz tube furnace having an arsenic electrodeless discharge lamp (As-EDL) as radiation source. From an aliquot (1 ml) of each digested sample, only As (III) content was determined. The extract was transferred into the polypropylene auto sampler tube and then diluted to 10 ml with 10% v/v HCl for analysis. From another 1 ml of each digested extract, total arsenic [sum of As (V) and As (III)] content was determined. The extract was transferred to the polypropylene auto sampler tube and pre reduced from As (V) to As (III) with 1 ml concentrated HCl and 1 ml of solution containing 3% (w/v) potassium iodide (KI) and ascorbic acid. The treated sample was kept at room temperature for 45 min and diluted to 10 ml with 10% v/v HCl before analysis. The sample loop on the flow injection valve with the acidified sample was switched to the inject position where 500 μl of the sample mixed with the stream of reductant [sodium borohydride (NaBH4) (0.3% (w/v) in 0.05 M NaOH)] at a flow rate of 6 ml/min. The concentration of HCl was maintained at 5% (v/v) at a flow rate of 10 ml/min. Reaction of NaBH4 with acidified sample generated volatile hydride, arsine (AsH3) which was carried by argon gas into quartz cell at a flow rate of 40 ml/min where arsine decomposed to gaseous arsenic (As) atom and was detected. Absorbance was recorded at 193.7 nm. Standards and blank were run similarly and compared periodically for response consistency. Peak areas were used for quantification.
Arsenate reductase (AR) activity was determined in terms of GR-specific NADPH oxidation in an enzymatic reaction according to Duan et al. (2005).
Assay of non-enzymatic and enzymatic antioxidants
Ascorbate content and α-tocopherol contents were estimated following the methodologies of Mukherjee and Chaudhuri (1983) and Backer et al. (1980), respectively. The amount of ascorbate and α-tocopherol present in the plant material was computed using a standard curve with known concentrations of ascorbic acid and DL-α tocopherol, respectively.
Procedures for enzyme extraction were performed at 4–8 °C. One (1) gram of plant material was crushed in 5 ml of pre-chilled 0.1 M sodium phosphate buffer (pH 7.0) followed by its centrifugation at 12,000g for 20 min. The supernatant was used for determining the activities of enzymes.
Superoxide dismutase [SOD; EC 1.15.1.1] activity was estimated following Giannopolitis and Ries (1977). Catalase [CAT; EC 1.11.1.6] and catechol peroxidase [CPX; EC 1.11.1.7] activities were determined according to Gasper and Laccoppe (1968) and Chance and Maehly (1955), respectively, while activities of ascorbate peroxidase [APX; E.C. 1.11.1.11] and ascorbic acid oxidase [AAO; EC 1.10.3.3] were calculated as stated by Nakano and Asada (1981) and Olliver (1967), respectively.
Extraction and estimation of thiol compounds and assays of glutathione-metabolizing enzymes
Cysteine content and total glutathione (GSH) content were estimated following Gaitonde (1967) and Sedlak and Lindsay (1968), respectively.
Glutathione reductase [GR; EC1.6.4.2] activity was estimated as stated by Smith et al. (1988). The activities of glutathione peroxidase [GPx; EC1.11.1.9] and glutathione-S-transferase [GST; EC2.5.1.18] were measured in accordance with Elia et al. (2003) and Ando et al. (1988), respectively.
Extraction and analysis of phytochelatins
For extraction of phytochelatins, root and shoot samples were washed carefully with deionized water and frozen in liquid nitrogen and preserved at − 80 °C until sample preparation. Phytochelatin analysis was performed following Sneller et al. (2000). Water RF-551 fluorescence detector was used to monitor fluorescence at emission and excitation wavelengths 470 nm and 380 nm, respectively, for a period of 60 min. The phytochelatin contents were determined from the peak heights of respective chromatograms of derivatized samples with respect to the standard.
Protein estimation
Protein content was determined following Lowry et al. (1951), by employing bovine serum albumin (BSA, Sigma) as standard.
Statistical analyses
The experiments were executed in a completely randomized design (CRD) thrice having two replications in each treatment; each set composed of a single petri plate with 100 seeds on an average. Data were computed using one-way analysis of variance (ANOVA) followed by balanced ANOVA to compare the significance among concentrations of arsenic in presence and/or absence of silicon independently. Significant differences between the means for every assay were compared by Fisher’s least significant difference (LSD) method at p ≤ 0.05. Regression analysis was performed with Minitab 18 software to examine the effects of arsenic and silicon. Multivariate scatter plots to establish correlations between different parameters were determined using SPSS software (version 22).
Results
Influence on total arsenic, arsenite content, and arsenate reductase activity
Significant (p ≤ 0.05) dose-dependant enhancements in total As and As (III) contents were observed with As (V) imposition under 25 As (V), 50 As (V), and 100 As (V) treatments in both root and shoot (Fig. 1a and b). Such variations were also significant (p ≤ 0.05) among the doses of As (V) administered. Total As and As (III) content in root was more than shoot. Silicon supplementation in As (V) containing media reduced the stated contents in both root as well as shoot. Results of balanced ANOVA from both the parameters also demonstrated significant (p ≤ 0.05) interaction between As (V) and silicate. Total As contents under doses of As (V) and silicate can be explicated by regression equations Yr = 40.4 + 1.094 As − 3.60 Si (R2 = 68.09%) and Ys = 2.802 + 0.1148 As − 0.725 Si (R2 = 80.31%), respectively. Similarly, the equations Yr = 19.72 + 0.4131 As − 3.046 Si (R2 = 69.60%) and Ys = 1.445 + 0.05535 As − 0.4207 Si, (R2 = 84.58%) explained the variations in As (III) content in root and shoot under different doses of As (V) and silicate, respectively. Thus, total As and As (III) contents were positively correlated with As (V) treatments and enhanced with As (V) treatments. Silicate supplementation on the contrary was negatively correlated with As (V) and decreased total As content.
Influence of arsenate and/or silicate on a total arsenic content, b arsenite content, c arsenate reductase activity, and d scatter plot representing the relationship between total arsenic content, arsenite content, and arsenate reductase activity of 21-day-old wheat (cv PBW 343) seedlings. Each data point depicts the mean ± SE obtained from three independent experiments with two replications for an individual treatment (n = 6). Asterisk indicates significant difference between the treatments according to Fisher’s LSD method at p ≤ 0.05 in comparison with control
The activity of AR increased by about 248%, 303%, and 323% in root and by about 42%, 143%, and 163% in shoot in response to 25 As (V), 50 As (V), and 100 As (V) treatments over control, respectively, and was significant according to Fisher’s LSD method at p ≤ 0.05 over control (Fig. 1c). The increase in AR activity was narrowed down with supplemental silicate in comparison with sole As (V) treatments depicting significant (p ≤ 0.05) increments by about 156%, 178%, and 212% in root under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, respectively, over control. The enzyme activity in shoot enhanced insignificantly by about 9% under 25 As (V) + Si treatment and significantly (p ≤ 0.05) by about 37% and 124% under 50 As (V) + Si and 100 As (V) + Si treatments over control. Results from balanced ANOVA illustrated significant (p ≤ 0.05) variations among different doses of As (V) and significant (p ≤ 0.05) arsenate-silicate interaction. Regression equations Yr = 361.3 + 3.886 As − 27.26 Si (R2 = 67.07%) and Ys = 196.0 + 2.389 As − 14.31 Si (R2 = 84.14%) explained the variations in activity of AR in root and shoot under doses of As (V) and silicate, respectively. Positive relationship with As (V) treatment and enzyme activity depicted arsenate-induced increase in enzymatic activity in the test seedlings but negative relationship with silicate demonstrated reduction in enzyme activity with silicate supplementation (Fig. 1d).
Influence on non-enzymatic and enzymatic antioxidants
Increasing concentrations of As (V) treatment registered a decline in ascorbate contents that exhibited significant (p ≤ 0.05) variations among doses of As (V) administered (Table 1). Such decline was significant by about 40%, 45%, and 50% in root under the mentioned doses of As (V) treatments compliant with Fisher’s LSD method at p ≤ 0.05 over control. In shoot, however, an insignificant decline of about 8% and 16% was evident under 25 As (V) and 50 As (V) treatments while the decline of about 36% under 100 As (V) was considered significant (p ≤ 0.05) with respect to control. Co-application of silicate along with As (V) narrowed the decline in ascorbate contents by about 20%, 25%, and 35% in root under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, respectively, over control showing significant (p ≤ 0.05) change only under 100 As (V) + Si treatment. Comparable doses in shoot narrowed down the said decline by 4%, 12%, and 20% as well. Ascorbate contents under concentrations of As (V) and silicate in root and shoot can be put forward by the regression equations Yr = 5.319 − 0.02289 As + 0.161 Si (R2 = 38.90%) and Ys = 7.868 − 0.02211 As + 0.0870 Si (R2 = 36.49%), respectively. The negative correlation of ascorbate with As (V) led to a decline in ascorbate content under different doses of As (V) while positive correlation with silicate enhanced ascorbate content in the seedlings.
α-Tocopherol contents enhanced with increase in As (V) concentrations by about 11%, 18%, and 28% in root and by about 5%, 7%, and 22% in shoot under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control (Table 1). Further enhancements in α-tocopherol contents by about 19%, 33%, and 41% occurred in root under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, respectively, depicting significant (p ≤ 0.05) variations under 50 As (V) + Si and 100 As (V) + Si treatments. In shoot, likewise, an enhancement of about 13%, 21%, and 36% was recorded but difference only under 100 As (V) + Si treatment was statistically significant (p ≤ 0.05) according to Fisher’s LSD method. Regression equations Yr = 16.126 + 0.0484 As + 0.336 Si (R2 = 38.81%) and Ys = 17.065 + 0.0488 As + 0.330 Si (R2 = 44.08%) represented the correlation between α-tocopherol contents of root and shoot with doses of As (V) and silicate, respectively. Enhancements in α-tocopherol contents were positively correlated with both As (V) and silicate treatments but arsenate-silicate treatments enhanced the content to a greater extent in comparison with the corresponding As (V) treatments.
Superoxide dismutase (SOD) activity registered an increasing trend in both root as well as shoot and indicated significant (p ≤ 0.05) variations under different As (V) regimes (Fig. 2a). The enzymatic activity enhanced by about 10%, 14%, and 16% in root in response to 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control. Correspondingly, the increment was by about 7%, 16%, and 24% in shoot under similar treatments and changes at 50 As (V) and 100 As (V) treatments were statistically significant (p ≤ 0.05) over control. Inclusion of silicate in As (V) containing media further increased SOD activity by about 15%, 21%, 23% and 11%, 22%, 25% in root and shoot, respectively, where changes under 50 As (V) + Si and 100 As (V) + Si treatments were regarded significant (p ≤ 0.05) with respect to control (Fig. 2a). Variations in activity of SOD under different doses of As (V) and silicate can be explained by the equations Yr = 0.07214 + 0.000138 As + 0.000515 Si (R2 = 31.27%) and Ys = 0.05513 + 0.000146 As + 0.000150 Si (R2 = 59.80%) for root and shoot, respectively. Both As (V) as well as silicate were positively correlated with SOD activity and enhanced enzyme activity but silicate induced an enhancement to a greater extent in comparison with As (V) treatment in the test cultivar.
Influence of arsenate and/or silicate on the activities of a superoxide dismutase, b catalase, c catechol peroxidase, d ascorbate peroxidase, and e ascorbate oxidase in root and shoot of 21-day-old wheat (cv PBW 343) seedlings. Each data point depicts the mean ± SE obtained from three independent experiments with two replications for an individual treatment (n = 6). Asterisk indicates significant difference between the treatments according to Fisher’s LSD method at p ≤ 0.05 in comparison with control
A gradual decline in catalase (CAT) activity was noted with significant (p ≤ 0.05) variations among As (V) treatments (Fig. 2b). CAT activity declined by about 23%, 36%, 75% in root under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, where only 100 As (V) concentration was statistically significant (p ≤ 0.05) over control. Likewise in shoot, CAT activity declined insignificantly by about 15% under 25 As (V) treatment and significantly (p ≤ 0.05) by about 30% and 48% under 50 As (V) and 100 As (V) treatments, respectively, with respect to control. Incorporation of silicate with equivalent concentrations of As (V) narrowed down the decline in enzyme activity by 3% and 17% on an average, in root and shoot, respectively, with respect to control and was greater than As (V) treatment alone (Fig. 2b). The said decline in enzyme activity was only significant (p ≤ 0.05) at 100 As (V) + Si treatment over control. Activity of CAT in root and shoot under As (V) and silicate treatments can be put forward by regression equations Yr = 39.61 − 0.2928 As + 1.722 Si (R2 = 69.75%) and Ys = 51.12 − 0.2729 As + 1.253 Si (R2 = 65.34%), respectively. The negative relationship of CAT activity with As (V) treatment paralleled with the decrease in enzyme activity with As (V) treatment whereas silicate was positively correlated with the enzyme activity and reversed the trend.
Catechol peroxidase (CPX) activity in the test cultivar declined significantly (p ≤ 0.05) by about 30%, 38%, and 45% in root and by about 21%, 38%, and 40% in shoot under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control and demonstrated significant (p ≤ 0.05) variations among levels of As (V) (Fig. 2c). Incorporation of silicate under different As (V) regimes, in contrast, narrowed down the decrease in enzyme activity significantly (p ≤ 0.05) in both root as well as shoot. The enzyme activity declined by about 23%, 33%, and 40% in root and by about 19%, 31%, and 36% in shoot under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, respectively, over control. However, with exogenous silicate, the enzyme activity was enhanced in comparison with individual As (V) treatments (Fig. 2c). CPX activity in root and shoot under As (V) and silicate treatments can be explained by equations Yr = 0.5508 − 0.002760 As + 0.01050 Si (R2 = 64.04%) and Ys = 0.3920 − 0.001760 As + 0.00600 Si (R2 = 61.94%), respectively. Negative correlation of CPX activity with As (V) decreased the activity of the enzyme under As (V) exposure whereas positive correlation with silicate application enhanced enzyme activity in the seedlings.
In root, the activity of ascorbate peroxidase (APX) increased significantly (p ≤ 0.05) by about 60%, 64%, and 70% in response to 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control (Fig. 2d). In shoot, however, the activity increased by about 16%, 17%, and 31% in response to identical treatments. Increments in enzyme activity also divulged significant (p ≤ 0.05) variations among As (V) treatments. Under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, the enzyme activity in root further increased significantly (p ≤ 0.05) by about 80%, 103%, and 138%, respectively, over control. Again, in shoot, the activity increased insignificantly by about 21% and 25% under 25 As (V) + Si and 50 As (V) + Si treatments while increment of about 37% under 100 As (V) + Si treatment was considered significantly different at 5% probability level from control. The relationship between APX activity in root and shoot in response to concentrations of As (V) and silicate can be described by the equations Yr = 0.04592 + 0.000310 As + 0.003225 Si (R2 = 73.56%) and Ys = 0.01965 + 0.000055 As + 0.000210 Si (R2 = 35.28%), respectively. Positive correlation of APX activity with both As (V) and silicate indicated enhancement in enzyme activity with both As (V) and silicate treatment. This enhancement was, however, to a greater extent with silicate application than As (V).
Ascorbate oxidase (AAO) activity of the seedlings depicted dose-dependant increments with As (V) treatment and indicated significant (p ≤ 0.05) variations among the treatments (Fig. 2e). The enzyme activity in root enhanced significantly (p ≤ 0.05) by about 18%, 27%, 45% under 25 As (V), 50 As (V), and 100 As (V) treatments, but in shoot, the enhancement was by about 6%, 22%, and 61%, respectively, under analogous doses where increments under 50 As (V) and 100 As (V) treatments were regarded statistically significant (p ≤ 0.05) in comparison with control. The enzyme activity further escalated significantly (p ≤ 0.05) by about 32%, 73%, 123% in root and by about 17%, 39%, 72% in shoot, respectively, under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments, respectively, over control (Fig. 2e). Differences in AAO activity in root and shoot under concentrations of As (V) and silicate can be elucidated by equations Yr = 0.1930 + 0.001760 As + 0.01600 Si (R2 = 83.46%) and Ys = 0.16100 + 0.001349 As + 0.00200 Si (R2 = 89.21%), respectively. Both As (V) as well as silicate treatments were positively correlated with the enzyme activity and enhanced the same but silicate induced enhancement in the said activity to a greater extent compared with As (V) treatment.
Influence on thiol compounds and activities of glutathione-metabolizing enzymes
Cysteine content in root increased by about 10%, 22%, and 31% under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, where changes under 50 As (V) and 100 As (V) treatments were considered significantly different according to Fisher’s LSD method at 5% probability level from control. In shoot, however, significant (p ≤ 0.05) increments of about 33%, 42%, and 50% were registered under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control (Table 2). The stated increments in both root as well as shoot, however, revealed significant (p ≤ 0.05) differences among As (V) levels. Cysteine content further increased by about 15%, 27%, and 32% in root, under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si treatments where increments under 50 As (V) + Si and 100 As (V) + Si treatments were statistically significant (p ≤ 0.05) with respect to control. Likewise, in shoot increments of about 48%, 54% and 58% were statistically significant (p ≤ 0.05) under identical doses of As (V) and silicate over control. Changes in cysteine content of root and shoot under As (V) and silicate treatments can be clarified by regression equations Yr = 134.32 + 0.3697 As + 1.150 Si (R2 = 64.00%) and Ys = 103.30 + 0.3417 As + 2.60 Si (R2 = 55.40%), respectively.
Glutathione content in the test seedlings increased significantly (p ≤ 0.05) by about 27%, 77%, and 86% in root under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively. In shoot, increments of about 18%, 42%, and 85% were registered under comparable concentrations, respectively, and increments at 50 As (V) and 100 As (V) arsenate treatments were significant (p ≤ 0.05) (Table 2). Glutathione content further increased significantly (p ≤ 0.05) by about 47%, 96%, and 109% in root at 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si concentrations with respect to control. Conversely, in shoot, the contents increased by about 31%, 66%, and 102% under the same treatments where variations among As (V) levels were statistically significant (p ≤ 0.05). The variations in glutathione content of root and shoot under As (V) and silicate treatments can be put to the regression equations Yr = 76.43 + 0.6953 As + 2.35 Si (R2 = 77.84%) and Ys = 45.90 + 0.4446 As + 1.400 Si (R2 = 77.90%). Cysteine and glutathione were both positively correlated with As (V) as well as silicate and enhanced these contents but As (V)-induced increment was lesser compared with that of silicate supplementation.
Glutathione reductase (GR) activity was upregulated under As (V) treatments and exhibited significant (p ≤ 0.05) variations among the As (V) treatments (Fig. 3a). The enzyme activity enhanced by about 4%, 15%, and 24% in root at 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, over control. In shoot, the enzyme activity enhanced by 3%, 12%, and 45% under the similar concentrations and activity under 100 As (V) treatment was statistically significant (p ≤ 0.05). Exogenous silicate combined with identical As (V) treatments further increased the enzyme activity in comparison with the solely As (V)-treated sets by about 23%, 43%, 72% in root and 33%, 62%, 65% in shoot, respectively, where activities under 50 As (V) + Si and 100 As (V) + Si concentrations were considered significantly different at 5% probability level from control. Changes in GR activity under different doses of As (V) and silicate in root and shoot can be explained by the regression equations Yr = 46.90 + 0.2543 As + 2.587 Si (R2 = 59.47%) and Ys = 47.53 + 0.2798 As + 2.675 Si (R2 = 62.24%), respectively. Positive correlation among As (V) as well as silicate treatment with GR activity enhanced the enzyme activity. However, this increment was greater with silicate treatment than As (V) in the test seedlings.
Influence of arsenate and/or silicate on a glutathione reductase, b glutathione peroxidase, c glutathione-S-transferase activities in root and shoot of 21-day-old wheat (cv PBW 343) seedlings. Each data point depicts the mean ± SE obtained from three independent experiments with two replications for an individual treatment (n = 6). Asterisk indicates significant difference between the treatments according to Fisher’s LSD method at p ≤ 0.05 in comparison to control
A gradual decline in glutathione peroxidase (GPx) activity was noted under different As (V) treatments that was significant (p ≤ 0.05) among the As (V) concentrations (Fig. 3b). GPx activity declined by about 24%, 32%, 40% in root and 16%, 25%, 46% in shoot at 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, where activities under 50 As (V) and 100 As (V) treatments were statistically significant (p ≤ 0.05) with respect to control. On the contrary, the decrease in enzyme activity was narrowed in comparison with the As (V)-treated sets and was about 16%, 27%, 32% in root and 12%, 14%, 20% in shoot at 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si concentrations, respectively, over control. Decrease in enzyme activity in root was also significant (p ≤ 0.05) under 50 As (V) + Si and 100 As (V) + Si treatments compared with control. Variations in GPx activity in response to As (V) and silicate treatments in root and shoot can be denoted by regression equations Yr = 51.24 − 0.1617 As + 0.198 Si (R2 = 37.49%) and Ys = 70.28 − 0.2161 As + 1.237 Si (R2 = 46.27%), respectively. Negative correlation of GPx activity with As (V) treatment led to a decline in the said activity with As (V) treatment. On the contrary, silicate treatment lowered enzyme activity and was positively correlated with silicate.
Activity of glutathione-S-transferase (GST) increased by about 21%, 58%, and 68% in root under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, and changes under 50 As (V) and 100 As (V) treatments were significant (p ≤ 0.05) over control. The enzyme activity equally increased in shoot by about 46%, 86%, and 156% over control and was statistically significant (p ≤ 0.05) under similar treatments (Fig. 3c). ANOVA analysis also disclosed significant (p ≤ 0.05) differences among various levels of As (V). Co-treatments of As (V) and silicate, however, narrowed down the increase in GST activity in comparison with the individual As (V) treatments and was about 14%, 46%, and 65% in root showing significant (p ≤ 0.05) changes under 50 As (V) + Si and 100 As (V) + Si concentrations. In shoot, however, increment of about 58%, 74%, and 98% was registered under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si concentrations, respectively, and revealed significant (p ≤ 0.05) change under 50 As (V) + Si and 100 As (V) + Si treatments. Alterations in GST activity of root and shoot under As (V) and silicate can be presented by the regression equations Yr = 64.40 + 0.3978 As − 0.555 Si (R2 = 72.62%) and Ys = 47.67 + 0.6001 As − 1.056 Si (R2 = 87.49%), respectively. The activity of GST was positively correlated with As (V) treatment and enhanced enzyme activity; contrarily, negative correlation with silicate supplementation decreased GST activity in the test cultivar.
Influence on phytochelatin contents
To characterize the thiols induced by As (V) imposition in the test cultivar, PC standards were utilized for HPLC analysis (Supplementary Fig. 3). The HPLC profiles of thiols in the control and As (V)-treated samples revealed low intensity thiol and mono bromobimane (mBBr) peaks in control (root and shoot) along with monothiol cysteine (Cys) and glutathione (GSH). However, in As (V)-treated samples, thiol peaks for PC2 and PC4 were noted besides Cys and GSH with retention time (RT) ranging between 16 and 33 min, respectively. The peak with retention time (RT) around 15 min was identified as PC2 and the second peak which eluted after PC2 with RT around 32 min was identified as PC4 with respect to the standard. The PC3 peak at 19 min was not observed in the test cultivar (Supplementary Fig. 4). The contents of PC2 and PC4 were determined from the peak heights of respective chromatograms of derivatized samples with respect to the standard. Arsenate-treated roots showed PC2 and PC4 in abundance compared with that of shoots. PC2 contents significantly (p ≤ 0.05) increased by about 249%, 275%, and 333% in root and by about 217%, 272%, and 327% in shoot under 25 As (V), 50 As (V), and 100 As (V) treatments, respectively, compared with control. PC4 contents also increased by about 87%, 98%, and 108% in root and by about 22%, 58%, and 94% in shoot under identical treatments over control (Fig. 4). In silicate-supplemented seedlings, the contents of both PC2 and PC4 decreased than the corresponding As (V)-treated sets. The PC2 contents significantly (p ≤ 0.05) increased by about 32%, 141%, and 317% in root and by about 56%, 176%, and 297% in shoot under 25 As (V) + Si, 50 As (V) + Si, and 100 As (V) + Si concentrations. Likewise, PC4 contents also increased by about 12%, 58%, and 104% in root, where changes under 50 As (V) + Si and 100 As (V) + Si treatments were statistically significant at 5% probability level over control. In shoot, equally, PC4 contents enhanced by about 10%, 12%, and 14% under similar concentrations. Balanced ANOVA indicated significant (p ≤ 0.05) differences among As (V) doses as well as significant arsenate-silicate interaction in both PC2 and PC4 contents of root and shoot. Regression equations Yr = 0.3618 + 0.005878 As − 0.03325 Si (R2 = 81.49%) and Ys = 0.3401 + 0.005962 As − 0.02850 Si (R2 = 86.12%) represented the relation between As (V) and silicate treatment with PC2 contents in both root and shoot, respectively, while the equations Yr = 1.1233 + 0.00871 As − 0.0515 Si (R2 = 77.28%) and Ys = 0.9964 + 0.005050 As − 0.0652 Si (R2 = 76.76%) depicted the relationship between As (V) and silicate treatment with PC4 contents in both root and shoot, respectively. Positive correlation of As (V) with both PC2 and PC4 implied an elevation of PC2 and PC4 content with As (V) treatment in the test cultivar while the negative correlation with silicate reversed the trend.
Influence of arsenate and/or silicate on PC2 and PC4 contents in root and shoot of 21-day-old wheat (cv PBW 343) seedlings. Each data point depicts the mean ± SE obtained from three independent experiments with two replications for an individual treatment (n = 6). Asterisk indicates significant difference between the treatments according to Fisher’s LSD method at p ≤ 0.05 in comparison to control
Discussion
Arsenic toxicity has emerged a severe crisis affecting crop productivity worldwide. The present study was designed to substantiate the hypothesis that silicate amendments at pertinent concentrations might mitigate As (V)-induced perturbations in the test seedlings.
Influence on total arsenic, arsenite, and arsenate reductase activity
Arsenic accumulation, translocation, and compartmentalization represent decisive parameters in As tolerance. The total As and As (III) contents in root were more compared with shoot and were dependant on the concentration of As (V) in the growth media (Fig. 1a and b). Although As (V) is rapidly reduced to As (III) following its uptake, the present study registered greater content of arsenate [total As–As (III)] in comparison with that of As (III) and is in agreement to the study conducted by Abedin et al. (2002). A probable reason for this decrease might be the efflux of As (III) via aquaporin channels (Shi et al. 2015). The activity of AR also coordinated with As (III) contents (Fig. 1c). Total As content was positively correlated with As (III) (R2 = 0.945) and AR activity (R2 = 0.811) (Fig. 1d). Retention of As in root is considered to occur as a result of reduction of As (V) to As (III) by AR and its consequent immobilization in vacuole and forms an imperative strategy for As detoxification. Since As (III) can only bind to PCs to form As (III)-PC complexes that are sequestered to vacuoles, consequently the activity of AR becomes crucial for As tolerance in plants (Li et al. 2015). Si-induced decrease in total As and As (III) contents was noted in both roots and shoots of As+Si-treated seedlings along with increments in Si contents (Supplementary Table 1). Such Si-stimulated reduction in total As and As (III) has been attributed to the decreased uptake of As in lettuce as Si modified the functional groups of the cell wall and promoted tighter binding of As. These structural alterations in the cell wall blocked apoplasmic transport of As (Greger et al. 2015). Reduction in transport from root to shoot is thus one of the plausible mechanisms by which Si ameliorates As (V)-imposed toxicity. Silicate interacts with As (V) at the high-affinity phosphate transporters and the presence of Si has been documented to stimulate uptake and mobilization of phosphate which restricts As entry (Moreno-Jiménez et al. 2012; Kostic et al. 2017). Decrease in As contents due to Si supplementation further downregulated AR activity and decreased As (III) contents in comparison with sole As (V)-treated sets.
Influence on non-enzymatic and enzymatic antioxidants
Arsenate potentiated oxidative injuries disrupted the antioxidative defense system by overproducing ROS and triggered multifarious biochemical responses that altered non-enzymatic as well as enzymatic antioxidants in the test cultivar. These perturbations exacerbated cellular damage and inhibited growth (Supplementary Fig. 1). The lipid-soluble antioxidant, tocopherol, reduces lipid peroxy radical generated during oxidative stress to tocopheroxyl radical and stabilizes cellular membranes (Szarka et al. 2012). Enhancement in α-tocopherol contents under As (V) stress could be due to its scavenging ability to counteract the toxic effects of potential ROS and prevent membrane damage. Recent report suggests activation of gene expression responsible for tocopherol synthesis in plants during oxidative stress (Singh et al. 2016). Ascorbate, the water-soluble antioxidant, is also a potent ROS scavenger that protects membranes by scavenging superoxide (O2−), hydroxyl radical (OH−), and regenerates α-tocopherol via the ascorbate-glutathione cycle. Decrease in ascorbate contents in our study might be due to an enhancement in APX activity which consumed ascorbate subsequently lowering its content. Reduced level of ascorbate under As (V) stress has been previously documented (Kumar et al. 2013; Singh et al. 2016). Dual application of silicate and arsenate contrarily enhanced the ascorbate pool and stimulated defense by quenching the tocopherol radical along with ROS and lowered oxidative load in the test cultivar. This further enhanced α-tocopherol contents that possibly counteracted lipid peroxidation (MDA contents) by quenching reactive oxidative anions and helped to stabilize membrane integrity in the Si-supplemented seedlings (Table 1; Supplementary Table 2).
Antioxidative enzymes play imperative role in oxidative stress management. Superoxide dismutase (SOD), catalase (CAT), catechol peroxidase (CPX), ascorbate peroxidase (APX), and ascorbic acid oxidase (AAO) are considered to be the major enzymes involved in antioxidative defense. Arsenate imposition in the present study altered the activities of antioxidant enzymes in a differential manner (Fig. 2a–e). Substantial increments in SOD, APX, and AAO activities were accompanied by decline in CAT and CPX activities. SOD, regarded as the major O2·− scavenger, constitutes a frontline in defense against ROS produced during environmental stress. Arsenate-incited enhancement in SOD activity in the present study coincides with the study conducted in Oryza sativa under As (V) stress (Choudhury et al. 2011). Excess H2O2 generated due to SOD activity is reduced to water and molecular oxygen by CAT and CPX at different locations in the cell. A linear decline in the activities of these enzymes depicted inferior ability in protecting the cell against H2O2 detoxification. Additionally, escalation in SOD activity might have resulted in rapid dismutation of highly reactive O2− radical to H2O2 causing its accumulation (Fig. 2a–c; Supplementary Table 2). Alteration in activities of CAT and CPX under As (V) stress in the test cultivar could be due to high H2O2 levels that might have possibly inactivated the enzymes. Furthermore, enhanced AAO and APX activities in the test cultivar were incapable to quench ROS due to low availability of its co-factor, reduced ascorbate. Our study is in conformity with the study conducted in Oryza sativa under As stress (Rahman et al. 2015). Contrastingly, silicate supplementation along with As (V) efficiently activated the plant defense by augmenting the activities of ROS scavenging antioxidant enzymes viz., SOD, CAT, CPX, APX, and AAO in the test cultivar and protected the cells from oxidative injuries (Fig. 2a–e). This was apparent from the positive correlations between silicon application and the activities of the antioxidant enzymes in both roots and shoots that led to a noticeable decline in H2O2 contents and improved growth (Supplementary Table 2). Such enhancements in the activities of antioxidant enzymes by silicon application have also been documented in rice (Dwivedi et al. 2020).
Influence on components of ascorbate-glutathione cycle
Cysteine acts as a source of reduced sulfur and is the precursor of S-containing compounds, such as amino acids, vitamins, Fe-S cluster, reduced glutathione (GSH), and thiol-containing proteins that play vital role in detoxification of As (V) toxicity (Khan and Gupta 2018). A sufficient amount of cysteine helps to maintain the optimum level of GSH (Bashir et al. 2015). Induction of cysteine in the test cultivar also corresponds to enhancements in GSH and PCs that help in the detoxification process. Comparable results have been attained in Oryza sativa under As (V) stress where increase in cysteine content also enhanced GSH synthesis (Tripathi et al. 2013; Singh et al. 2016). Heavy metals have been documented to enhance the sulfur reduction pathway including the activity of its enzymes viz., ATP sulfurylase, APS reductase, serine acetyl transferase, and cysteine synthase which increases accumulation of cysteine (Rausch and Wachter 2005).
The tripeptide glutathione in reduced form acts as an antioxidant that directly reduces ROS generated during stress and is employed as a stress marker. Arsenate-accrued enhancement in GSH contents in our study could be ascribed to the increased requirement of sulfur for biosynthesis of antioxidants under stressful conditions (Gill et al. 2013). Enhanced GSH content in the arsenate As (V)-exposed plants is due to the upregulation in transcription of γ-glutamyl cysteine synthetase and glutathione synthetase genes that increases GSH biosynthesis (Xiang and Oliver 1998). This is of pivotal importance, since GSH is required for PC synthesis which facilitates the detoxification of xenobiotics and heavy metals by forming PC-metal complexes (Sharma 2012). Furthermore, GSH acts as reductant for AR-catalyzed reduction of As (V) to As (III), which is also a prerequisite for the complex formation (Rosen 2002; Dhankher 2005). Simultaneous supplementations of silicate along with respective doses of As (V)-induced cysteine to a greater extent in both root and shoot and were well coordinated with elevated GSH contents in the test cultivar (Table 2). Similar studies on silicon induced intensification of non-protein thiols and GSH also coincided with the study conducted in two rice cultivars: Triguna and IET-4786 under As exposure (Tripathi et al. 2013).
Glutathione reductase (GR), a flavo-protein oxidoreductase localized predominantly in chloroplasts, catalyzes the conversion of oxidized glutathione (GSSG) to GSH and is necessary for functioning of ascorbate-glutathione (ASA-GSH) cycle as well as for PC synthesis (Cobbett and Goldsbrough 2002). It sustains the reduced status of GSH by maintaining the ratio of GSH/GSSG and consequently functions in defense against ROS (Rao and Reddy 2008). Upregulation of GR activity in the test cultivar under As (V) imposition is concurrent to the study conducted in Phaseolus aureus (Singh et al. 2007). Elevated GR activity also increased GSH contents in the test seedlings (Fig. 3a; Table 2). As-inflicted oxidative stress has been documented to induce increments in GR activity which is vital for detoxification of ROS and PC synthesis (Sharma 2012). Silicon supplementation on the other hand, further upregulated GR activity, enhanced GSH contents thereby antioxidant capacity of the As (V)-stressed seedlings that possibly improved defense against oxidative stress. Silicon induced increase in GR activity during As stress in rice cultivars; Triguna and IET-4786 has been ascribed to the induction of GR isozymes (Tripathi et al. 2013).
Glutathione peroxidases (GPxs) are a large family of diverse isozymes that scavenge H2O2 by utilizing GSH to form GSSG and protect cells from oxidative stress. Decline in GPx activity in the present study points to H2O2 accumulation indicating ineffective detoxification and enhanced vulnerability to ROS-induced damages. On the other hand, Si-induced upregulation in GPx activity lowered H2O2 and mitigated the As-provoked effects in the test cultivar (Fig. 3b; Supplementary Table 2).
Glutathione-S-transferases (GST) catalyze the conjugation of electrophilic xenobiotic substrates with GSH and facilitate their sequestration to the vacuoles by removing genotoxic compounds that can react or damage DNA, RNA and proteins thus defend the cell against oxidative damage (Jozefczak et al. 2012). Arsenate-induced enhancement in GST activity in the test seedlings is parallel to that reported in rice (Singh et al. 2016). Such augmentation of GST activity might stimulate free metal binding and renders detoxification of As (III)-PC complexes to the vacuoles. Silicon fertilization, on the contrary, down-regulated GST activity with respect to the only As (V)-treated seedlings probably because it reduced the entry of As, diminished the buildup of cytotoxic compounds, and thereby lowered generation of ROS as evidenced by decline in H2O2 contents in the present scenario that decreased PC production in the test cultivar (Figs. 3c and 4; Supplementary Table 2).
Influence on phytochelatins
Phytochelatins synthesized in the cytosol are metal-binding peptides with cysteine thiol groups that chelate heavy metals to form phytochelatin-metal complexes and sequester these to vacuoles. The imperative role of PCs in detoxification of As has been documented (Solanki and Dhankhar 2011). Present study revealed substantial enhancements in the levels of both PC2 and PC4 under As (V) imposition and is well coordinated with enhanced GSH production (Fig. 4). On the contrary, PC3 was not obtained in the test cultivar. As documented earlier, dominance of dithiol complexes with As(III)-(PC2) due to the formation of ring structures causes differential stability for the synthesis of As-PC complexes with longer chains (Schulz et al. 2008). The presence of PC4, however, indicates its synthesis from its substrate, PC3, and chances might be that PC3 had been utilized during PC4 synthesis and could not be detected in the chromatograms. Comparable observation in Cuscuta reflexa initiated the synthesis of PC3 and PC4 under high and low concentrations of cadmium, respectively, while its substrate PC2 could not be detected (Srivastava et al. 2004). Our results are in contrast with Shi et al. (2017) that documented only the presence PC2 in wheat subjected to arsenate stress with no traces of PC3 or PC4. Such differential distribution of PCs may therefore be concentration dependant or species specific. Positive correlations of As content with GSH (R2 = 0.536) and PCs [PC2, R2 = 0.852, PC4, R2 = 0.798] enhanced GSH and PC production in the test cultivar that ultimately decreased growth (R2 = 0.802) (Fig. 5). Similar increase in GSH and PC has also been reported in rice under As stress (Kumar et al. 2014). Arsenate exposure induced production of thiols to a greater extent in root compared with that of shoot under identical treatments indicating superior detoxification potential in roots that further reduced mobility of As to shoot. Formation of As (III)-PC complexes in root possibly restricted root to shoot translocation of As thereby lowering risk of food-chain contamination. Our results are consistent to that obtained in rice cultivars where enhanced complexation of As in root induced PC synthesis in response to free metal ions in the cytoplasm (Batista et al. 2014). However, Si-induced drop in PCs possibly occurred due to decreased As (V) uptake that lowered As availability and reduced oxidative stress suggesting the alleviation of Si-mediated As toxicity due to inhibition of As uptake rather than PC accumulation (Fig. 5). Such silicon-elicited decline in PC synthesis probably lowered utilization of GSH pool for PC production and elevated production of cysteine as well as GSH. However, in spite of decreased As (V) uptake, translocation and improved compartmentation along with enhanced antioxidant capacity, silicon supplementation still failed to restore the PC level comparable with the control probably due to the severity of stress involved.
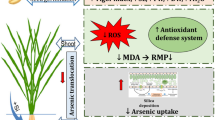
Taken together, As (V) treatment upregulated the activity of AR and led to As (III) production that persuaded generation of ROS in the test cultivar. ROS-incited oxidative damages activated the stress-induced responses and altered the non-enzymatic and enzymatic (SOD, CAT, CPX, APX, AAO) anti-oxidative defense mechanisms. This coordinately affected the ASA-GSH cycle and activities of GPx and GST along with thiol metabolism that enhanced PC production (Fig. 6). Silicon co-treatments, however, reduced endogenous As contents and directly lowered the stress-related parameters (MDA, proline, and H2O2). Supplemental Si further facilitated alleviation of As (V) toxicity by activating the non-enzymatic and enzymatic defense cascade along with ASA–GSH cycle that efficiently lowered H2O2 and helped to regenerate antioxidant metabolites α-tocopherol, ASA, GSH in the test seedlings. Silicate amendments also lowered PC accumulation by lowering As (III) availability in the test seedlings. Supplementation of silicon, thus, undeniably remitted the detrimental effects of arsenate incited stress to an appreciable extent and was actively involved in counteracting ROS leading to improved growth.
Schematic representation of silicon-mediated modulation of antioxidative defense and thiol metabolism [abbreviations used: SOD: superoxide dismutase, CAT; catalase, CYS: cysteine, APX: ascorbate peroxidase, MDHAR: mono dehydro ascorbate reductase, DHAR: dehydroascorbate reductase, GR: glutathione reductase, ASA: ascorbate, GSH: reduced glutathione, GSSG: oxidized glutathione, AR: arsenate reductase, PC: phytochelatin, GST: glutathione-S-transferase, As (V): arsenate, As(III): arsenite]
Conclusion
Silicon supplementation was therefore competent enough in lowering As contents and induced an upsurge in the antioxidative defense as well as ASA–GSH cycle that alleviated the negative effects of reactive oxygen species and helped to combat the arsenic-imposed consequences reinstating growth. Results from our study offer insight to the plausible efficacy of silicate nutrition in mitigation of arsenic stress in wheat seedlings that could form a strategy for maintaining the crop productivity and minimize arsenic health risk among the consumers. However, our work was conducted under controlled laboratory conditions, but in the field scenario, stresses are collective and complex depending on several factors that are interdependent and deserve further studies before recommendation to cultivators. Field trials are, therefore, of utmost importance to validate the precise extent of Si fertilization that could be employed to expunge arsenate toxicity to some extent that might have encouraging implications in agro biological systems.
References
Abdel-Lateef AM, Mohamed RA, Mahmoud HH (2013) Determination of arsenic (III) and (V) species in some environmental samples by atomic absorption spectrometry. Adv Chem Sci 2(4):110–113 ACS02524110113
Abedin MJ, Cresser M, Meharg A, Feldmann J, Howells JC (2002) Arsenic accumulation and metabolism in rice (Oryza sativa L). Environ Sci Technol 36:962–968
Ando K, Honma M, Chiba S, Tahara S, Mizutani JK (1988) Glutathione transferase from Mucor javanicus. Agric Biol Chem 52:135–139
Ang HH, Lee KL (2005) Analysis of mercury in Malaysian herbal preparations. J Microbiol Biotechnol Res 4:31–36
Backer H, Frank O, De Angells B, Feingold S (1980) Plasma tocopherol in man at various times after ingesting free or ocetylaned tocopherol. Nutr Rep Int 21:531–536
Bashir H, Ibrahim MM, Bagheri R, Ahmad J, Arif IA, Baig MA, Qureshi MI (2015) Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 7:plv001. https://doi.org/10.1093/aobpla/plv001
Batista BL, Nigar M, Mestrot A, Rocha BA, Barbosa F Jr, Price AH, Raab A, Feldmann J (2014) Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot 65:1467–1479. https://doi.org/10.1093/jxb/eru018
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39(1):1–6. https://doi.org/10.1590/1678-4685-GMB-2015-0109
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–817
Chooto P, Wararattananurak P, Kangkamano T, Innuphata C, Sirinawin W (2015) Determination of inorganic arsenic species by hydride generation atomic absorption spectrophotometry and cathodic stripping voltammetry. Sci Asia 41:187–197. https://doi.org/10.2306/scienceasia1513-1874.2015.41.187
Choudhury B, Mitra S, Biswas AK (2010) Regulation of sugar metabolism in rice (Oryza sativa L.) seedlings under arsenate toxicity and its improvement by phosphate. Physiol Mol Biol Pla 16:59–68. https://doi.org/10.1007/s12298-010-0008-8
Choudhury B, Chowdhury S, Biswas AK (2011) Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J Plant Interact 6(1):15–24. https://doi.org/10.1080/17429140903487552
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Das P, Manna I, Sil P, Bandyopadhyay M, Biswas AK (2019) Exogenous silicon alters organic acid production and enzymatic activity of TCA cycle in two NaCl stressed indica rice cultivars. Plant Physiol Biochem 136:76–91. https://doi.org/10.1016/j.plaphy.2018.12.026
Dhankher OP (2005) Arsenic metabolism in plants: an inside story. New Phytol 168:503–505
Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwivedi S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251. https://doi.org/10.1016/j.jhazmat.2015.06.008
Duan GL, Zhu YG, Tong YP, Cai C, Kneer R (2005) Characterization of arsenate reductase in the extract of roots and fronds of chinese brake fern, an arsenic hyperaccumulator. Plant Physiol 138:461–469. https://doi.org/10.1104/pp.104.057422
Dwivedi S, Kumar A, Mishra S, Sharma P, Sinam G, Bahadur L, Goyal V, Jain N, Tripathi RD (2020) Orthosilicic acid (OSA) reduced grain arsenic accumulation and enhanced yield by modulating the level of trace element, antioxidants, and thiols in rice. Environ Sci Pollut Res 27:24025–24038. https://doi.org/10.1007/s11356-020-08663-x
Elia AC, Galarini R, Taticchi MI, Dörr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55(2):162–167
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gasper T, Laccoppe J (1968) The effect of CCC and AMO-1618 on growth, catalase, peroxidase, IAA oxidase activity of young barley seedlings. Physiol Plant 21:1104–1109
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212. https://doi.org/10.1016/j.plaphy.2013.05.032
Greger M, Bergqvist C, Sandhi A, Landberg T (2015) Influence of silicon on arsenic uptake and toxicity in lettuce. J Appl Bot Food Qual 88:234–240. https://doi.org/10.5073/JABFQ.2015.088.034
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596. https://doi.org/10.1007/s10646-013-1050-4
Hettick BE, Cañas-Carrell JE, French AD, Klein DM (2015) Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J Agric Food Chem 63:7097–7107. https://doi.org/10.1021/acs.jafc.5b02487
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175. https://doi.org/10.3390/ijms13033145
Khan E, Gupta M (2018) Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep 8:10301. https://doi.org/10.1038/s41598-018-28712-3
Kostic L, Nikolic N, Bosnic D, Samardic J, Nikolic M (2017) Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 419:447–455 10..1007/s11104-017-3364-0
Kumar N, Mallick S, Yadava RN, Singh AP, Sinha S (2013) Co-application of selenite and phosphate reduces arsenite uptake in hydroponically grown rice seedlings: toxicity and defence mechanism. Ecotoxicol Environ Saf 91:171–179. https://doi.org/10.1016/j.ecoenv.2013.01.027
Kumar A, Singh RP, Singh PK, Awasthi S, Chakrabarty D, Trivedi PK, Tripathi RD (2014) Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicol 23:1153–1116. https://doi.org/10.1007/s10646-014-1257-z
Li N, Wang J, Song WY (2015) Arsenic uptake and translocation in plants. Plant Cell Physiol 0(0):1–10. https://doi.org/10.1093/pcp/pcv143
Liu C, Lu W, Ma Q, Ma C (2017) Effect of silicon on the alleviation of boron toxicity in wheat growth, boron accumulation, photosynthesis activities, and oxidative responses. J Plant Nutr 40:2458–2467. https://doi.org/10.1080/01904167.2017.1380817
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
McCarty KM, Hanh HT, Kim KW (2011) Arsenic geochemistry and human health in. South East Asia Rev Environ Health 26(1):71–78 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128386
Mishra BK, Dubey CS, Shukla DP, Bhattacharya P, Usham AL (2014) Concentration of arsenic by selected vegetables cultivated in the Yamuna flood plains (YFP) of Delhi, India. Environ Earth Sci 72(9):3281–3291
Moreno-Jiménez E, Esteba E, Peñalosa JM (2012) The fate of arsenic in soil-plant systems. Rev Environ Contam Toxicol 215:1–37
Mukherjee SP, Chaudhuri MA (1983) Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Olliver M (1967) Ascorbic acid estimation. In: Sebrell WH, Harris RS (eds) The vitamins VI. Academic Press, New York, p 338
Pontigo S, Godoy K, Jiménez H, Gutiérrez-Moraga A, Mora ML, Cartes P (2017) Silicon-mediated alleviation of aluminum toxicity by modulation of Al/Si uptake and antioxidant performance in ryegrass plants. Front Plant Sci 8:642. https://doi.org/10.3389/fpls.2017.00642
Rahaie M, Xue GP, Schenk PM (2013) The role of transcription factors in wheat under different abiotic stresses. In: Vahdati K, Leslie C (eds) Abiotic stress - plant responses and applications in agriculture. InTech, Rijeka, pp 367–385
Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int 2015:1–12. https://doi.org/10.1155/2015/340812
Rao ASVC, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stresses in plants. Springer, Berlin: Verlag, pp 111–147. https://doi.org/10.1007/978-3-540-76326-0_6
Rastogi A, Tripathi DKT, Yadav S, Chauhan DK, Živčak M, Ghorbanpour M, El-Sheery NI, Brestic M (2019) Application of silicon nanoparticles in agriculture. 3Biotech 9:90. https://doi.org/10.1007/s13205-019-1626-7
Rausch T, Wachter A (2005) Sulphur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10(10):503–509
Rosen BP (2002) Biochemistry of arsenic detoxicfication. FEBS Lett 529:86–92
Schulz H, Härtling S, Tanneberg H (2008) The identification and quantification of arsenic-induced phytochelatins—comparison between plants with varying As sensitivities. Plant Soil 303:275–287. https://doi.org/10.1007/s11104-007-9507-y
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bond and non-protein sulfhydryl groups in tissue with Ell-man’s reagent. Anal Biochem 25:192–205
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67(3):447–453. https://doi.org/10.2478/s11756-012-0024-y
Shi GL, Zhu S, Meng JR, Qian M, Yang N, Lou LQ, Cai QS (2015) Variation in arsenic accumulation and translocation among wheat cultivars: the relationship between arsenic accumulation, efflux by wheat roots and arsenate tolerance of wheat seedlings. J Hazard Meter 289:190–196. https://doi.org/10.1016/j.jhazmat.2015.02.045
Shi GL, Lou LQ, Li DJ, Hu ZB, Cai QS (2017) Phytochelatins play key roles for the difference in root arsenic accumulation of different Triticum aestivum cultivars in comparison with arsenate uptake kinetics and reduction. Chemosphere 175:192–199. https://doi.org/10.1016/j.chemosphere.2017.02.017
Sil P, Das P, Biswas AK (2018) Silicon induced mitigation of TCA cycle and GABA synthesis in arsenic stressed wheat (Triticum aestivum L.) seedlings. S Afr J Bot 119:340–352
Sil P, Das P, Biswas AK (2019a) Impact of exogenous silicate amendments on nitrogen metabolism in wheat seedlings subjected to arsenate stress. Silicon 12:535–545. https://doi.org/10.1007/s12633-019-00158-w
Sil P, Das P, Biswas S, Mazumdar A, Biswas AK (2019b) Modulation of photosynthetic parameters, sugar metabolism, polyamine and ion contents by silicon amendments in wheat (Triticum aestivum L.) seedlings exposed to arsenic. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-04896-7
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth ReguL 53:65–73. https://doi.org/10.1007/s10725-007-9205-z
Singh AP, Dixit G, Kumar A, Mishra S, Singh PK, Dwivedi S, Trivedi PK, Chakrabarty D, Mallick S, Pandey V, Dhankher OP, Tripathi RD (2016) Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci 6:1272. https://doi.org/10.3389/fpls.2015.01272
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5-dithiobis-(2-nitrobenzoic acid). Anal Biochem 175:408–413
Sneller FEC, Van Heerwaarden LM, Koevoets PLM, Vooijs R, Schat H, Verkleij JAC (2000) Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman’s reagent and monobromobimane. J Agric Food Chem 48:4014–4019. https://doi.org/10.1021/jf9903105
Solanki R, Dhankhar R (2011) Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia. 66(2):195–204
Srivastava S, Tripathi RD, Dwivedi UN (2004) Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa – an angiospermic parasite. J plant Physiol 161(6):665–674. https://doi.org/10.1078/0176-1617-01274
Szarka A, Tomasskovics B, Bánhegyi G (2012) The Ascorbate glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci 13:4458–4483. https://doi.org/10.3390/ijms13044458
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Goutam D, Shri M, Trivedi PK, Chakrabarty D (2013) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103. https://doi.org/10.1016/j.ecoleng.2012.12.057
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550. https://doi.org/10.1105/tpc.10.9.1539
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432. https://doi.org/10.3390/ijms14047405
Zhu YG, Geng CN, Tong YP, Smith SE, Smith FA (2006) Phosphate (Pi) and arsenate uptake by two wheat (Triticum aestivum) cultivars and their doubled haploid lines. Ann Bot 98:631–636
Acknowledgments
The authors acknowledge the infrastructural assistance provided by the Centre of Advanced Study, Department of Botany, University of Calcutta (UGC-CAS Phase VII), DST-FIST and DBT-IPLS facility for completion of the work. The authors are thankful to the Central Instrument Facility, Bose Institute, Kolkata, India for providing the HPLC facilities. The authors also acknowledge the assistance of Prof. Uttam Bandopadhyay, Department of Statistics, University of Calcutta, for statistical analyses.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, financial assistance to A.K.B. was from the University Grants Commission, New Delhi, for completion of the work.
Author information
Authors and Affiliations
Contributions
Both the authors contributed significantly to the research article. P.S. conducted the experiments, compiled and analyzed data, and prepared draft of the manuscript. A.K.B. conceived the idea, designed experiments, and finalized the manuscript. Both authors equally approve the publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sil, P., Biswas, A.K. Silicon nutrition modulates arsenic-inflicted oxidative overload and thiol metabolism in wheat (Triticum aestivum L.) seedlings. Environ Sci Pollut Res 27, 45209–45224 (2020). https://doi.org/10.1007/s11356-020-10369-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10369-z