Abstract
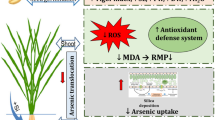
Arsenic (As), a toxic metalloid, is finding its route to human through intake of As-contaminated water and consumption of food grown on contaminated soil. Rice is the most As-affected crop. Present study is aimed to assess the impact of stabilized orthosilicic acid (a proprietary formulation for plant-available silicon (Si) and earlier used as fertilizer for rice to enhance growth and yield) in reducing the accumulation of As in rice grains. Application of arsenic in the form of arsenate (AsV) and arsenite (AsIII) significantly affected plant growth in a dose-dependent manner. Higher doses of AsV and AsIII (50 and 25 mg L−1 respectively) significantly decreased the yield attributes leading to lower yield. A significant accumulation of As in grain was observed in both AsV- and AsIII-exposed plants in a dose-dependent manner. Arsenic exposure also increased the level of Si in rice grains. Application of Si, either in soil or on leaves (foliar), greatly reduced grain As accumulation (up to 67% in AsV and 78% in AsIII) and enhanced the growth and yield of plants under As stress. The level of thiols and activities of antioxidant enzymes were also enhanced under Si application. Foliar Si application was more effective in increasing grain Si level and reducing grain As than soil Si. The level of other trace elements was also significantly enhanced by Si application irrespective of the presence or absence of As in comparison with control. Arsenic exposure constrained some of the trace elements, such as Zn and Co, which were restored by Si application. Results of the present study showed that the application of currently used Si formulation may effectively reduce grain As level even in highly As-contaminated soil and improve grain quality of rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a class one non-threshold carcinogen and toxic to almost all forms of life except a few bacterial species (Stolz and Oremland 1999). Mining activities, use of arsenical pesticides, and irrigation with As-contaminated groundwater are the major sources of As in soil (Zhao et al. 2010). Use of As-contaminated groundwater for irrigation is the primary source of As in agriculture soil. The level of As in the paddy fields varies from 4 to 138 mg Kg−1 in the As-contaminated regions (Khan et al. 2010). From the contaminated soil and irrigation water, As gets accumulated in the crops and cereal grains, particularly in rice grains (Dwivedi et al. 2010a). It has been well documented that Asian rice contains higher proportion of inorganic As than US rice (Meharg et al. 2009; Zhao et al. 2010). Rice constitutes a large proportion of the dietary intake of inorganic As in regions where rice is the staple food (Mondal and Polya 2008; Meharg et al. 2009). Additionally, rice straw widely used as cattle feed also contains high levels of As, thereby serving as an additional route of As entry into the food chain (Panaullah et al. 2009). High level of As has deleterious impacts on human health as well as on plant productivity (Panaullah et al. 2009). In paddy fields, inorganic species, viz, arsenite (AsIII) and arsenate (AsV), are more prevalent than the organic species, viz, methylarsonic acid (MMA), dimethylarsinic acid (DMA), and arsanilic acid (Zhao et al. 2010). A small amount of organic forms are present in soil due to microbial activities and depending on the previous use of soil. In plants, AsV is taken up by phosphate transporters (Tripathi et al. 2007), whereas AsIII and un-dissociated methylated As species are transported through the nodulin 26–like intrinsic proteins (NIPs) along with other neutral solutes like glycerol, ammonia, and silicic acid (Ma et al. 2008; Li et al. 2009).
Silicon is essential for sustainable production of rice (Rao et al. 2017) and can accumulate up to 15% in the stem and leaves of rice (Ma et al. 2006). The silicon transporters, NIP family of transporters, are highly expressed in the rice and are therefore the major route for arsenic, in the form of AsIII, in rice plant and grain (Ma et al. 2008). Moreover, the flooded anaerobic conditions of paddy field are conducive to increased availability of AsIII. These agronomic and physiological conditions make rice an efficient As accumulator (Williams et al. 2007; Xu et al. 2008; Zhao et al. 2010).
Silicon improves plant growth and yield and plays an important role in alleviating various abiotic stresses including heavy metal (Ma et al. 2006; Liu et al. 2009; Adrees et al. 2015). Si supplementation has been reported to reduce the accumulation and toxic effect of arsenic by improving photosynthetic performance and antioxidant system in arsenic-exposed plants (Tripathi et al. 2013; Silva et al. 2015; Sanglard et al. 2016). Grain As level has also been reported to reduce by Si supplementation (Fleck et al. 2013). Thus, in recent years, Si management has been explored as one of the strategies to combat arsenic menace in rice. Although there is an abundance of Si in the earth’s crust, most of it is in insoluble polymeric forms which are not available for plant uptake. Various biogenic and other sources of Si have been used for improving rice plant tolerance and yield enhancement in As- or Cd-contaminated soil (Liu et al. 2009; Seyfferth and Fendorf 2012; Seyfferth et al. 2016). The different sources of Si, however, have different effects on the accumulation of As. For instance, use of fresh rice husk or Si gel increased soil pore water Si and As as well but it decreased the accumulation of As in rice straw and grain particularly inorganic As (Seyfferth and Fendorf 2012; Fleck et al. 2013), while the use of rice husk ash or diatomaceous earth as Si source has either no or little effect on straw and grain As (Seyfferth and Fendorf 2012; Seyfferth et al. 2016). The study concluded that soil pore water Si has more effect on As accumulation than soil pore water As and is negatively correlated with straw and grain As. Norton et al. (2010) established a positive correlation between shoot As and Si concentration. Use of water-soluble silica (as silicic acid or sodium silicate) resulted in different effects on rice seedling under solution culture and pot experiments. In solution culture, a significant decrease in As accumulation and increased plant tolerance were observed (Tripathi et al. 2013) while during soil pot experiment an increase in the level of As in soil solution as well as increased phytotoxicity was observed upon Si fertilization (Lee et al. 2014; Syu et al. 2016). In this regard, foliar application of Si has been suggested as an alternative to avoid Si-induced dissolution of As in soil solution. However, a mixed response to foliar Si application has been reported. While Syu et al. (2016) found no effect of foliar Si application on As accumulation or plant growth, Zhang et al. (2020) reported a significant decrease in As accumulation. Thus, from the above, it is evident that the source of Si and method and time of application are crucial for the availability of Si to plants and thus for Si management strategy to minimize arsenic accumulation and toxicity. Monosilicic acid, also called orthosilicic acid [H4SiO4], is the most plant-available form of Si (Lewin and Reimann 1969). However, orthosilicic acid is highly unstable and readily changes to non available polymeric forms. In the current study, we have used a stabilized orthosilicic acid formulation as a source of Si, which has been developed as a plant growth booster, for the alleviation of As toxicity in rice plant. We have compared both foliar and soil applications of Si in alleviating As toxicity in rice plant, in terms of growth and yield attributing characters, antioxidant enzymes, level of non-protein thiols, accumulation of nutrient elements, and effect on grain As accumulation. Since this Si formulation has been already successfully tested to improve growth and yield of rice without any harmful effect on soil health (Jawahar et al. 2015; Neeru et al. 2016), the present study aimed at assessing if it can be used for mitigation of As in affected areas.
Materials and methods
Experimental design and pot experiment
Rice seeds of Saryu-52 were obtained from a commercial seed shop, Lucknow. Seeds were soaked overnight in water and sown in a large tub (6″ × 2.5″) filled with garden soil to prepare seedlings for pot experiments. The pH and EC of soil were measured in 1:2 soil solution (wt/vol) using glass electrode pH meter and conductivity meter, respectively. The soil organic carbon was analyzed by the Walkley-Black chromic acid wet oxidation method (Walkley and Black 1934). Bulk density, particle density, sand, silt, clay, and soil texture were measured by following the protocols given in Okalebo et al. (1993). The porosity of soil was calculated by using the formula: porosity = (1 − bulk density/particle density) × 100. Exchangeable sodium, potassium, and calcium were analyzed by flame photometer after extraction in ammonium acetate at pH 7.0. Available nitrogen, phosphorus, and sulfur were estimated by the method of Subbiah and Asija (1956), Olsen et al. (1954), and Williams and Steinbergs (1962) respectively. The selected physico-chemical properties of soil are given in Table 1.
Twenty-day-old seedlings were transplanted to 12-L earthen pots containing 10 kg of garden soil. Seedlings were transplanted as 3 plants per hill; a total of 9 seedlings were transplanted in each pot. Pots were irrigated with tap water by maintaining a 3-cm flooded condition and kept under natural sunlight. Plants were fertilized by NPK (0.26 g (10:26:26) and 0.46 g urea corresponding to the 160:30:60 kg ha−1 N:P:K, respectively) in three splits, i.e., during tillering, pre-flowering, and post-flowering. With each fertilizer dose, 0.2 g ZnSO4.7H2O per pot (corresponding to the 40 kg Zn ha−1) was also applied, because the level of Zn in soil was below (< 3 mg kg−1, Table 1) the normal range (10–250 mg kg−1). Plants were treated with various combinations of As and Si (Supplementary Table S1). The treatments were applied four times during the course of the experiment, viz, 3 days after transplant and during tillering (45d), pre-flowering (75d), and post-flowering (90d). Si was applied in the form of orthosilicic acid (OSA) either on leaves by using a liquid formulation with 0.4% OSA (foliar Si) or in the soil by using a granular formulation with 5% OSA (soil Si) provided by Privi Life Sciences Pvt. Ltd. as “Silixol.” The doses of Si were selected on the basis of previous studies using Silixol as a rice crop booster (Jawahar et al. 2015; Neeru et al. 2016). Both the formulations were made to 0.2% and 0.4% for foliar Si and 0.4% and 0.8% for soil Si. Arsenic was supplied as sodium arsenate (AsV) and sodium arsenite (AsIII) procured from Sigma-Aldrich. The level of As in contaminated area is significantly high (Khan et al. 2010) and application of Si fertilizer to high As-contaminated soil resulted in increased phytotoxicity to rice seedlings (Lee et al. 2014). Thus, the current study was designed to analyze the long-term effect of Si (i.e., until crop maturity) on grain As level and crop yield in the presence of high As concentration to see the efficacy of Si formulation for high As-contaminated areas. For this purpose, two concentrations of each AsV and AsIII were selected on the basis of the first significant adverse effect on growth and yield parameters (10 mg L−1 AsV and AsIII) and the maximum level at which grain setting was observed, i.e., 25 mg L−1 for AsIII and 50 mg L−1 for AsV. Experiments were repeated twice in the years 2014 and 2015 between July and November, with three replicates for each treatment.
Measurement of plant growth and yield attributing parameters
The growth of plant in terms of plant height and number of tillers was evaluated after 30 and 60 days of transplantation, while the yield attributing characters such as length of panicle, weight of panicle, number of grains per panicle, test weight (wt. of 1000 seeds), and yield in terms of seed dry weight (gram/plant) were evaluated at harvest. The height of plants and length of panicle were measured manually by a metric scale, and the number of tillers was counted.
Assay of antioxidant enzymes and thiols
The assay of antioxidant enzymes and thiols was performed using leaves collected from 75-day-old plants. Five to six mature leaves were cut from each treatment, washed with Milli-Q water, blotted, frozen in liquid nitrogen, and used for the study of thiols and antioxidant enzymes. The frozen plant material (500 mg) was powdered and homogenized in 100-mM chilled potassium phosphate buffer (pH 7), containing 0.1-mM EDTA and 1% PVP (w/v) at 4 °C. Homogenate was squeezed through four layers of cheese cloth, and the extract was centrifuged at 10000g for 15 min at 4 °C as described earlier (Mishra et al. 2006), except for catalase (CAT) activity which is described below. The supernatant was used to measure the activities of various enzymes which were expressed as units mg−1 protein, i.e., rate substrate conversion min−1 mg−1 protein. The protein content in the supernatant was measured according to Lowry et al. (1951).
The activity of superoxide dismutase (SOD, EC 1.15.1.1) was assayed following the method of Beauchamp and Fridovich (1971) by measuring its ability to inhibit the photochemical reduction of nitrobluetetrazolium (NBT). One unit was the amount of protein required to inhibit 50% initial reduction of NBT under light. Ascorbate peroxidase (APX, EC 1.11.1.11) activity was measured according to the method of Nakano and Asada (1981) by estimating the rate of ascorbate (ASC) oxidation (€ = 2.8 mM−1 cm−1). Enzyme activity was expressed as moles of ASC oxidized min−1 mg−1 protein. Guaiacol peroxidase (GPX, EC 1.11.1.7) activity was assayed according to Hemeda and Klein (1990) with slight modifications as detailed in Mishra et al. (2006). The activity of the enzyme was measured by the increase in absorbance due to the oxidation of guaiacol (€ = 26.6 mM−1 cm−1) at 470 nm. Enzyme activity was expressed as micromoles guaiacol oxidized min−1 mg−1 protein. For the measurement of CAT (EC 1.11.1.6) activity, extraction was done in buffer containing Tris HCl (pH 7), 1 mM EDTA, 1 mM PMSF, and 0.3 g g−1 fw PVP. CAT activity was measured by the method of Aebi (1974). The assay system comprised 50-mM Na2PO4 buffer (pH 7), 20-mM H2O2 (€ = 0.04 cm2 μmole−1), and suitable aliquot of the enzyme in a final volume of 3 mL. Decrease in the absorbance was recorded at 240 nm. Enzyme activity was expressed as μmoles H2O2 degradation min−1 mg−1 protein. Activity of glutathione reductase (GR, EC 1.6.4.2) was assayed by following the method of Smith et al. (1988). The reaction mixture contained 1.0 mL of 0.2-M potassium phosphate buffer (pH 7.5) containing 1-mM EDTA, 0.5 mL 3-mM 5,50-dithiobis (2-nitrobenzoic acid) in 0.01-M phosphate buffer (pH 7.5), 0.25 mL H2O, 0.1 mL 2-mM NADPH, 0.05 mL enzyme extract, and 0.1 mL 20-mM GSSG. The components were added in the order as above directly to a cuvette, and the reaction was started by the addition of GSSG. The increase in absorbance was monitored for 5 min at 412 nm. The rate of enzyme activity was calculated using a standard curve prepared by known amounts of GR (Sigma, USA). The rate of enzyme activity was expressed as μmoles GSSG reduced min−1 mg−1 protein. Dehydroascorbate reductase (DHAR, EC 1.8.5.1) activity was assayed by the formation of ASC at 265 nm (€ = 14 mM−1 cm−1) in a reaction mixture containing 0.1-M Na phosphate buffer (pH 6.2), 2-mM GSH, 1-mM dehydroascorbic acid (DHA), and 100 μg protein with enzyme extract (De Tullio et al. 1998). The rate of non-enzymatic DHA reduction was corrected by subtracting the values obtained in the absence of enzyme extract. The rate of enzyme activity was expressed as μmoles DHA reduced min−1 mg−1 protein. The level of cysteine was estimated by the method of Gaitonde (1967), and estimation of non-protein thiol (NPT) was done by the method of Ellman (1959).
Analysis of arsenic, silica and other trace elements in soil and rice grain
Five hundred milligrams dried soil or 100 mg rice grain samples were finely grounded and acid digested in a microwave digester (HNO3-H2O2, 3:1 v/v) and made to 10 mL by Milli-Q water. Element concentrations (As, Co, Cu, Fe, Mn, Mo, Ni, Se, Zn) were analyzed using inductively coupled plasma mass spectrometer (ICP-MS 7500ex, Agilent Technologies, Japan) following Dwivedi et al. (2010a). Rhodium was added to all samples as an internal standard. Multi-element calibration standards 2A (8500–6940) and 3 (8500–6948; Agilent Technologies, USA) were used for calibration of the instrument. The analytical precision and accuracy of the ICP-MS were maintained as per the requirements of the National Accreditation Board for Testing and Calibration Laboratories (NABL; CSIR-NBRI certification no. T-1381). The calibration and quality assurance for each analytical batch were ensured by repeated analysis (n = 5) of CRMs. Recovery of elements from the samples was found to be 98–105%. The detection limit for each element was 1 μg L−1. Total Si was estimated by the method of Ma et al. (2003) in the digested soil and grain samples.
Statistical analysis
Analysis of variance (ANOVA), Duncan’s multiple range test (DMRT), and correlation analysis were performed to determine the significant differences between treatments by using SPSS 17.0 software.
Results
Effect of silicon on growth parameters of rice plant during arsenic stress
Growth parameters under control and during various treatments of Si and As are presented in Tables 2 and 3. In general, application of Si either in soil or foliar slightly enhanced plant growth and number of tillers. Treatment of As inhibited the plant height which was more significant at higher doses, i.e., 25 mg L−1 AsIII and 50 mg L−1 AsV. Arsenite (AsIII) at 25 mg L−1 caused an almost similar reduction in plant height as AsV at 50 mg L−1. Arsenic stress also had a deleterious impact on the number of tillers, and this could be one of the major reasons for yield reduction. Application of Si, either foliar or in soil, to As-stressed plants alleviated the As-induced growth inhibition. In AsV-stressed plants, both higher and lower doses of foliar Si (Silixol Rice 0.2% and 0.4%) showed a positive effect on plant height in comparison with respective AsV alone treatments. The beneficial effect of foliar Si was more significant after 60 days with higher doses of Si (0.4%). While in case of AsIII stress, the plant height significantly increased at both doses of Si. The number of tillers increased with silica application in both AsV- and AsIII-stressed plants. In general, in terms of plant growth, both foliar and soil Si application was more effective against AsV toxicity than AsIII. Among the Si treatments, foliar application was more effective than soil application in improving plant growth under As stress.
Effect of silicon on yield attributing characters of rice plant during arsenic stress
In As-stressed plants, yield attributing characters, such as the number of grain per panicle, weight of panicles, test weight, and the yield, were significantly compromised in comparison with control (Tables 4 and 5). Arsenate caused more reduction in yield than AsIII at 10 mg L−1. Higher doses of AsV (50 mg L−1) and AsIII (25 mg L−1) caused up to 34% and 29% reduction in yield. Both foliar and soil Si significantly enhanced the yield attributing characters and yield of the As-stressed plants. At lower dose of AsV and AsIII, application of Si resulted in 20–30% higher yield in comparison with control plants which was particularly attributed to the increase in the number and weight of seeds. The lower dose (0.4%) of soil Si significantly improved the yield of As-stressed plants with up to 72% and 60% increase in the yield of 50-μM AsV and 25-μM AsIII respectively, which was even higher than that of control plants. Higher dose of soil Si (0.8%) increased the yield of As-stressed plants but the effect was lower than that of 0.4% soil Si. Contrary to this, foliar Si resulted in a continuous increase in yield with an increase in Si concentration with maximum up to 26% and 72% increase in the yield of 25-μM AsIII– and 50-μM AsV–exposed plants, respectively, at 0.4% foliar Si application.
Thus, among the various treatments, 0.4% of both soil and foliar Si showed better growth and yield response and reduced the As-mediated toxicity.
Effect of silicon on the accumulation of arsenic and Si in rice grain during arsenic stress
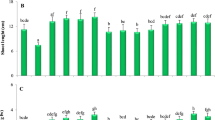
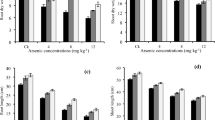
Control plants (not treated with As or Si) exhibited a significant amount of As in the grains, and its level declined in the grains obtained from Si-treated plants. In As-treated plants, the accumulation of As in grains increased significantly in a dose-dependent manner (Fig. 1). At equal concentration of AsV and AsIII (10 mg L−1), accumulation of As in grains was up to 18% higher in AsIII-treated plants than that in AsV-treated plants, while the grain As was almost similar in the plants treated with either 50 mg L−1 AsV (68.25 μg As Kg−1) or 25 mg L−1 AsIII (66.8 μg As Kg−1) indicating higher mobility of AsIII than AsV. Application of soil Si increased grain Si level both in the presence or absence of As in comparison with control (Fig. 2a). Interestingly, the level of grain Si was also increased in As alone treatments (where Si was not supplied) and the level of grain Si was more correlated with application of As (particularly AsV) than Si in soil. In the absence of As, during foliar Si application, the level of grain Si was almost double in comparison with soil Si application. However, in the presence of As, the level of grain Si was comparable to that of soil Si application (Fig. 2b). In contrast, the grain As level was drastically reduced by Si application. Both foliar and soil Si were effectively reducing grain As. During soil Si application, lower dose (0.4%) was more effective in reducing grain arsenic accumulation than the higher dose (0.8%). The 0.4% soil Si caused approximately 66% reduction in grain As in both 25 mg L−1 AsIII– and 50 mg L−1 AsV–exposed plants, while it was only 54% and 61% respectively at 0.8% soil Si application (Fig. 1a). In the case of foliar Si, higher dose (0.4%) was more effective than lower dose (0.2%) in reducing grain As level by 67% and 78% in 50 mg L−1 AsV and 25 mg L−1 AsIII exposure respectively (Fig. 1b).
Effect of soil and foliar Si application on the accumulation of arsenic in the grain of rice cv. Saryu-52 exposed to AsV and AsIII. a Soil Si application and b foliar Si application. Values are mean ± SD from two separate experiments each with three replicates. Different letters indicate significantly different values among treatments (P ≤ 0.05)
Effect of soil and foliar Si application on the accumulation of Si in the grain of rice cv. Saryu-52 exposed to AsV and AsIII. a Soil Si application and b foliar Si application. Values are mean ± SD from two separate experiments each with three replicates. Different letters indicate significantly different values among treatments (P ≤ 0.05)
Effect of silicon on trace element content in rice grain during arsenic stress
Since 0.4% dose of both foliar and soil Si was more effective in reducing grain As accumulation, the trace elements (viz, Co, Cu, Fe, Mn, Mo, Ni, Se, and Zn) and other parameters were analyzed at this Si dose on high As concentrations, i.e., 50 mg L−1 AsV and 25 mg L−1 AsIII. Treatment with either form of As significantly decreased the level of Co, Zn, and Ni in grains, while the level of Se and Mo significantly increased in comparison with that of control (Fig. 3). There was no significant effect on the levels of Fe, Mn, and Cu in the grains of AsV-treated plants; however, in presence of AsIII, the levels of Cu and Mn increased in comparison with those of control. Increasing or decreasing effects on the levels of grain nutrient elements were more prominent with AsIII than AsV. Application of Si, either foliar or in soil, in the presence or absence of As, significantly increased the level of most of the nutrients, viz, Fe, Cu, Mn, Zn, Mo, and Se in grains. Foliar Si resulted in up to 160% and 112% increase in Fe and up to 115% and 62% increase in Zn in comparison with AsV alone– and AsIII alone–treated plants respectively. Levels of Mn in grain increased by 43% and 147% by AsV and AsIII respectively. Application of foliar/soil Si further increased the level of Mn in AsV-treated plant but not in AsIII-treated plant. Levels of Cu, Mo, and Se in grain showed a variable response, while soil Si resulted in increased levels of Cu in grain, foliar Si caused a decrease. Arsenic treatment significantly increased the level of Se in grains which enhanced further upon the application of Si. Levels of Co and Ni decreased with all treatment in comparison with those of control.
Effect of soil and foliar Si application on the level of mineral nutrient in the grain of rice cv. Saryu-52. Plants were treated with 50 mg L−1 AsV or 25 mg L−1 AsIII during pot experiment. Values are mean ± SD from two separate experiments each with three replicates. Different letters indicate significantly different values among treatments (P ≤ 0.05)
Effect of silicon on the level of thiols and activities of antioxidant enzymes in leaves of arsenic-stressed plants
The level of total non-protein thiols and cysteine and activities of various antioxidant enzymes were investigated in leaves of plants exposed to various combinations of As and Si. The level of cysteine increased by about 70% and 59% in the leaves of AsV- and AsIII-treated plants respectively in comparison with that of control. Application of Si further increased the level of cysteine by about 10% in comparison with respective AsV and AsIII alone treatments (Fig. 4a). In contrast, the level of total non-protein thiols decreased significantly in comparison with that of control in As-treated plants (10% by AsV and 23% by AsIII). However, application of Si resulted in a significant increase in the level of non-protein thiols (Fig. 4b). Foliar application of Si caused more increase in non-protein thiols (34% and 42%) than soil application (23% and 28%) in comparison with AsV and AsIII alone treatments. Among antioxidant enzymes, the activity of SOD, GR, and DHAR increased in comparison with that of control upon As treatment, while the activities of APX, GPX, and CAT decreased with more decrease by AsIII. Application of both foliar and soil Si increased the activity of antioxidant enzymes in comparison with respective As alone treatments or in most cases even higher than control (Fig. 5).
Effect of soil and foliar Si application on the level of thiols in the leaves of rice cv. Saryu-52 exposed to 50 mg L−1 AsV or 25 mg L−1 AsIII. Values are mean ± SD from two separate experiments each with three replicates. Different letters indicate significantly different values among treatments (P ≤ 0.05)
Effect of soil and foliar Si application on the activities of antioxidant enzymes in the leaves of rice cv. Saryu-52 exposed to 50 mg L−1 AsV or 25 mg L−1 AsIII. Values are mean ± SD from two separate experiments each with three replicates. Different letters indicate significantly different values among treatments (P ≤ 0.05)
Discussion
All forms of As are toxic to plants depending on their concentration, availability, uptake, and transport within a plant (Mishra et al. 2017). Inorganic AsIII and AsV are thought to be more toxic than methylated species because of their higher uptake rate. The toxicity of AsV is primarily attributed to its chemical similarity with phosphate (P); thus, it exerts toxicity by replacing P from phosphorylation reaction and interfering with P metabolism. Arsenite, having a high affinity for thiols, interacts with sulfhydryl groups of peptides and proteins, thus hampering their activity. During the present study, a significant reduction in growth, yield attributing parameters, and yield was observed upon treatment with AsV and AsIII. The toxicity mechanism of inorganic As is mainly attributed to hindered pigment biosynthesis (Mishra et al. 2016) resulting in impaired photosynthesis in plants including rice (Mishra et al. 2014; Sanglard et al. 2016). The impaired photosynthesis would eventually result in reduced growth and yield in rice (Sanglard et al. 2016). In the current study, AsIII seems to be more toxic than AsV which was evident by the reduction in various growth and yield parameters at lower AsIII concentration, i.e., 25 mg L−1 in comparison with 50 mg L−1 AsV. This could be attributed to the difference in the uptake and detoxification mechanism of the AsV and AsIII. In a paddy field environment, AsIII has been shown to be more available than AsV due to flooded reducing conditions and is also more efficiently taken up by the rice plant through highly expressed Si transporters (Ma et al. 2008). Furthermore, in rice plants, AsIII has been reported to be the main species for root to shoot transport (Ma et al. 2008). In the present study as well, the ratio of grain to soil As was higher in the plants exposed to AsIII than AsV indicating higher root uptake and translocation of AsIII by rice plant in the flooded pot conditions (Supplementary Table S2). Further treatment with either form of As also significantly altered the mineral nutrients in grains, such as the level of Co, Zn, and Ni decreased significantly. The negative effect of As on the accumulation of mineral nutrients such as Zn, Ni, and Co in rice grain at higher As exposure has been observed in the earlier studies as well (Dwivedi et al. 2010b). The levels of Se, Zn, and Ni have been reported to be constrained in the rice grains grown in As-contaminated fields of Bangladesh (Williams et al. 2009). Constrained mineral nutrient also results in yield reduction and poor grain quality (Williams et al. 2009). Application of foliar and soil Si improved the growth and yield of both AsV- and AsIII-stressed rice plants. The beneficial effect of Si on rice plant has been well understood (Ma et al. 2006; Rao et al. 2017), and the orthosilicic acid formulation used in the present study has already been proved to enhance growth and yield of rice when used as a plant booster (Jawahar et al. 2015; f et al. 2016). Alleviation of As-induced toxicity by Si has been also reported in several recent studies (Sanglard et al. 2016; Gang et al. 2018). This is primarily attributed to the reduction in the As uptake and translocation, and at physiological and biochemical levels by improving photosynthetic performance, stomatal conductance, mineral nutrients, and cellular redox state of As-stressed plants (Fleck et al. 2013; Tripathi et al. 2013; Sanglard et al. 2014). In the present study, application of Si significantly reduced the level of grain As which was more in AsIII-treated plants than that in AsV-treated plants. Higher reduction in grain As accumulation upon Si application in AsIII-exposed plants might be due to antagonism during uptake and probably also during translocation through shared transporters. In rice plants, up to 84% of accumulated AsV has been reported to be reduced to AsIII in roots (Mishra et al. 2017); thus, Si application may as well suppress the As transport and grain accumulation in AsV-exposed plants after reduction to AsIII. Inorganic As, particularly AsIII, and dimethylarsinic acid (DMA) are the main As species in rice grain (Zhao et al. 2010; Fleck et al. 2013). It has been reported that while inorganic As is readily taken up by rice roots, its translocation to shoot is relatively low, whereas organic As though poorly absorbed by the roots is very efficiently exported to the shoots and also to the grains (Zhao et al. 2010; Mishra et al. 2017). Since Asian rice is dominated by inorganic As, inhibiting As uptake by Si application may prove to be an efficient method in reducing total grain As concentration because the study of Fleck et al. (2013) showed that the reduction in total grain As concentration in rice was solely attributed to the lowered accumulation of AsIII. In a short-term experiment, Si priming of rice seeds has been shown to increase As tolerance in rice by lowering the expression of As(III) transporters OsLsi1, OsLsi2, and OsLsi6 (Khan and Gupta 2018). In the present study, since the pots were flooded up to 3 cm during the course of the experiment to simulate paddy field conditions, partial reduction of AsV to AsIII already in soil solution cannot be excluded leading to lowered uptake and subsequent grain accumulation due to Si application. In short-term experiments up to the seedling stage, high Si application resulted in increased As accumulation and phytotoxicity (Lee et al. 2014; Syu et al. 2016); in contrast, the long-term experiment reported a significant decrease in grain As only at high Si fertilizer application (Wang et al. 2016). However, in the current study, no toxic effect of Si application on growth of plant under As stress was observed at any time point, i.e., 30 days, 60 days, and after maturity. Furthermore, the Si formulation (stabilized orthosilicic acid) was more effective in reducing grain As as well with up to 66% by soil Si and 78% by foliar Si application. The higher effectiveness of foliar Si than soil Si application may be attributed to the different mechanisms involved in the two application methods. Soil application of Si has been shown to increase the level of Fe, As, and P along with Si concentration in soil solution (Fleck et al. 2013). An increase in As concentration in soil solution has been also observed in other studies as a result of competitive sorption between Si and As for the adsorption sites of soil minerals (Li et al. 2009b; Syu et al. 2016). The increased availability of As in soil solution may facilitate As uptake. However, the observed lower grain As upon 0.4% soil Si application in the current study and in other studies indicates that the inhibition of As uptake by Si outweighed the effect of an increased availability of As (Li et al. 2009b; Fleck et al. 2013; Syu et al. 2016). The comparatively lower efficiency of higher soil Si (0.8%) application in reducing grain As may be related to the higher desorption of As. Nevertheless, it still reduced the grain As by more than half of that of As alone treatment. In a similar way, the increase in the level of Si in the grain of As alone–treated plants may also be explained by the increased dissolution of Si by As. In contrast to soil application, foliar application of Si excludes the possibility of As release in soil solution. In a recent study, foliar Si application markedly reduced husk and grain As concentration in rice (Zhang et al. 2020). Foliar application of Si during the tillering stage resulted in an increased concentration of Si in root and shoot which downregulated the Si transporters (Lsi1 and Lsi2 in roots and Lsi6 in leaves), leading to lower As uptake and transport. Furthermore, Carey et al. (2010) showed that in rice grains inorganic As is mostly (90%) fed through phloem transport while in husk primarily via the xylem. Thus, apart from less root transport of As, the downregulation of Lsi6, which transports Si in leaves and panicles (Yamaji and Ma 2009), might lower the As concentration in leaves leading to further lowering of As in grain through phloem transport without compromising other nutrients and Si content. In the current study, the level of grain Si was almost double during foliar application than that in soil application showing efficient Si absorption by leaves and its transport to the grain. Further Si application, either foliar or in soil, significantly increased the level of most of the nutrients, viz, Fe, Cu, Mn, Zn, Mo, and Se in grains leading to improved yield and grain quality.
In addition to lower As accumulation and improved mineral nutrients, the redox balance (in terms of thiols and antioxidant enzymes) during Si application may also be a reason for improved growth and yield of rice plant under As stress. Thiols play a pivotal role under abiotic stress conditions by maintaining cellular redox balance and by deactivating the reactivity of metal, metalloid, or other xenobiotics, thus constituting the first line of defense under metal/metalloid stress. Antioxidant enzymes constitute a secondary detoxification pathway by minimizing the oxidative stress induced by As. Glutathione (GSH) and phytochelatins (PCs) are the major constituents of non-protein thiols and cysteine is the precursor to the GSH and PCs. Glutathione is particularly important playing a multifarious role during As stress in plants (Mishra et al. 2019). It is involved in the reduction of AsV to AsIII, and both GSH and PCs are involved in the complexation of AsIII, thus detoxifying its toxicity. Several AsIII-thiol complexes including PCs, GSH, and cysteine has been identified in the As-exposed rice plant (Mishra et al. 2017). Increased level of cysteine in the present study indicates As-induced stimulation of thiol biosynthesis pathway to meet with the increased demand of thiols under As stress. The decrease in total non-protein thiol in the leaves under As stress could be due to their rapid transport to roots to meet the high demand in roots as discussed by Mishra et al. (2017). Furthermore, deactivation of enzymes involved in thiol biosynthesis, e.g., GSH synthetase or PC synthase, by As may also be a reason for reduced thiols. Si application however facilitated the synthesis of non-protein thiols in leaves, probably by augmenting the nutrients, such as S and N, as observed for trace elements as well as by reducing As concentration in leaves. An increase in the level of cysteine upon Si application in rice leaves has been observed earlier as well (Tripathi et al. 2013). In As-exposed plants, the activity of SOD, GR, and DHAR increased significantly. SOD is the most important enzyme of the antioxidant system constituting the first line of defense against ROS in a cell (Alscher et al. 2002). SOD converts superoxide radical to hydrogen peroxide (H2O2), which is then reduced to water and oxygen either by APX in an ascorbate-glutathione cycle or by GPX and CAT in the cytoplasm and other cellular compartments. GR and DHAR are another important enzymic constituent of the ascorbate-glutathione cycle that helps reduce oxidized glutathione (GSSG) and dehydroascorbate to GSH and ascorbate, respectively, thus maintaining cellular redox balance, crucial for protection against oxidative damage. Since thiol also directly scavenge ROS, at high concentration, probably until a certain threshold, the consumption of thiols drastically increases than their synthesis, leading to cellular redox imbalance (Mishra et al. 2019). Si-mediated reduction in As accumulation lower than this threshold might be the reason for an increase in the level of thiol, which will improve the redox status of the cell during Si supplementation leading to enhanced growth. The observed decrease in the activities of APX, GPX, and CAT in As-exposed plants in comparison with that in control might also be due to the inactivation of these enzymes by As. However, application of both foliar and soil Si increased the activity of antioxidant enzymes in comparison with respective As alone treatments or in most cases even higher than control.
Thus, in the present study, the reduction in rice grain As was found to be significantly higher by the used stabilized Si formulation, both through foliar and soil Si application, than those reported in earlier studies. The level of thiols and antioxidant enzyme activities were also enhanced upon Si application probably due to increased mineral nutrients and decrease in As concentration. There was no toxic effect of Si application on the growth of plant under As stress. Foliar application of Si proved to be more beneficial in reducing grain As than soil application, also the level of Si in grain was higher during foliar application than soil application. The absorption of Si was probably more efficient through leaves increasing internal Si concentration. This may lead to lower root uptake of Si as well as As. Among the various treatments of Si, 0.4% of both soil and foliar Si showed better growth and yield response and reduced the As-mediated toxicity. The result showed that the doses used in the current study for both foliar and soil Si will be effective even for highly As-contaminated soil without causing phytotoxicity or yield reduction.
Conclusion
Application of Si in the form of OSA (either foliar or in soil) significantly enhanced the growth and yield of rice plants in uncontaminated (background arsenic contamination) as well as arsenic-contaminated soil. Additionally, it improved grain quality by reducing As accumulation in grain and enhancing nutrients elements. Foliar application of Si was found more effective than soil application and 0.4% of soil Si showed better growth and yield response than 0.8%. Thus, 0.2% and 0.4% foliar Si and 0.4% soil Si, in the form of stabilized OSA which has already been proved beneficial for the rice crop, could also significantly mitigate arsenic stress though the optimum dose of Si application in different soil types needs to be optimized.
References
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Safe 119:186–197
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie/Academic Press Inc., Weinheim/NewYork, p 673–680. https://doi.org/10.1016/b978-0-12-091302-2.50032-3
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53:1331–1341
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice. Plant Physiol 152(1):309–319
De Tullio MC, De Gara L, Paciolla C, Arrigoni O (1998) Dehydroascorbate-reducing proteins in maize are induced by the ascorbate biosynthesis inhibitor lycorine. Plant Physiol Biochem 36:433–440
Dwivedi S, Tripathi RD, Srivastava S, Singh R, Kumar A, Tripathi P, Dave R, Rai UN, Chakrabarty D, Trivedi PK, Tuli R, Adhikari B, Bag MK (2010a) Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown under arsenic contaminated soils of West Bengal. Protoplasma 245:113–124
Dwivedi S, Tripathi RD, Tripathi P, Kumar A, Dave R, Mishra S, Singh R, Sharma D, Rai UN, Chakrabarty D, Trivedi PK, Adhikari B, Bag MK, Dhankher OP, Tuli R (2010b) Arsenate exposure affects amino acids, mineral nutrient status and antioxidants in rice (Oryza sativa L.) genotypes. Environ Sci Technol 44:9542–9549
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Fleck AT, Mattusch J, Schenk MK (2013) Silicon decreases the arsenic level in rice grain by limiting arsenite transport. J Plant Nutr Soil Sci 176(5):785–794
Gaitonde MK (1967) Spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gang LI, Zheng M, Jianfeng TA, Hojae SH, Chao CA (2018) Effect of silicon on arsenic concentration and speciation in different rice tissues. Pedosphere 28:511–520
Hemeda HM, Klein BP (1990) Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci 55:184–185
Jawahar S, Jain N, Suseendran K, Kalaiyarasan C, Kanagarajan R (2015) Effect of silixol granules on silicon uptake, stem borer and leaf folder incidence in rice. Int J Curr Res Acad Rev 3:168–174
Khan E, Gupta M (2018) Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep 8(1):10301
Khan MA, Stroud JL, Zhu YG, McGrath SP, Zhao FJ (2010) Arsenic bioavailability to rice is elevated in Bangladeshi paddy soils. Environ Sci Technol 44:8515–8521
Lee CH, Huang HH, Syu CH, Lin TH, Lee DY (2014) Increase of As release and phytotoxicity to rice seedlings in As-contaminated paddy soils by Si fertilizer application. J Hazard Mater 276:253–261
Lewin J, Reimann BE (1969) Silicon and plant growth. Annu Rev Plant Physiol 20:289–304
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009b) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783
Liu C, Li F, Luo C, Liu X, Wang S, Liu T, Li X (2009) Foliar application of two silica sols reduced cadmium accumulation in rice grains. J Hazard Mater 161:1466–1472
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Ma JF, Higashitani A, Sato K, Takeda K (2003) Genotypic variation in silicon concentration of barley grain. Plant Soil 249:383–387
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. PNAS 105:9931–9935
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, Raab A, Zhao FJ, Islam R, Hossain S, Yanai J (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Mishra S, Stärk HJ, Küpper H (2014) A different sequence of events than previously reported leads to arsenic-induced damage in Ceratophyllum demersum L. Metallomics 6:444
Mishra S, Alfeld M, Sobotka R, Andresen E, Falkenberg G, Küpper H (2016) Analysis of sub-lethal arsenic toxicity to Ceratophyllum demersum: subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J Exp Bot 67:4639–4646
Mishra S, Mattusch J, Wennrich R (2017) Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci Rep 7:40522. https://doi.org/10.1038/srep40522
Mishra S, Dwivedi S, Mallick S, Tripathi RD (2019) Redox homeostasis in plants under arsenic stress. In: Redox homeostasis in plants. Springer, Cham, pp 179–198
Mondal D, Polya DA (2008) Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl Geochem 23:2987–2998
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Neeru J, Shaliesh C, Vaishali T, Purav S, Manoherlal R (2016) Role of orthosilicic acid (OSA) based formulation in improving plant growth and development. Silicon 1-5
Norton GJ, Islam MR, Duan G, Lei M, Zhu YG, Decon CM, Moran AC, Islam S, Zhao FJ, Stroud JL, McGrath SP, Feldmann J, Price AH, Meharg AA (2010) Arsenic shoot-grain relationships in field grown rice cultivars. Environ Sci Technol 44:1471–1477
Okalebo, J.R., Gathua, K.W. and Woomer, P.L (1993) Laboratory methods of soil and plant analysis: a working manual. Soil Science Society of East Africa Technical Publication No. 1 UNESCO/ Rosta. Nairobi, Kenya
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with NaHCO3. USDA Cir. 939. U.S. Washington
Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Ahmed ZU, Duxbury JM (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 317:31–39
Rao GB, Pi PY, Syriac EK (2017) Silicon nutrition in rice: a review. J Pharma Phytochem 6:390–392
Sanglard LMVP, Martins SCV, Detmann KC, Silva PEM, Lavinsky AO, Silva MM, Detmann E, Araújo WL, DaMatta FM (2014) Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: an analysis of the key limitations of photosynthesis. Physiol Plant 152:355–366
Sanglard LMVP, Detmann KC, Martins SCV, Teixeira RA, Pereira LF, Sanglard ML, Fernie AR, Araújo WL, DaMatta FM (2016) The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ Exp Bot 123:22–36
Seyfferth AL, Fendorf S (2012) Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ Sci Technol 46:13176–13183
Seyfferth AL, Morris AH, Gill R, Kearns KA, Mann JN, Paukett M, Leskanic C (2016) Soil-incorporation of silica-rich rice husk decreases inorganic As in rice grain. J Agric Food Chem 64:3760–3766
Silva AJ, Nascimento CW, Neto G, da Silva A, Silva Junior EA (2015) Effects of silicon on alleviating arsenic toxicity in maize plants. R Bras Ci Solo 39:289–296
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175:408–413
Stolz JF, Oremland RS (1999) Bacterial respiration of arsenic and selenium. FEMS Microbiol Rev 23:615–627
Subbiah B, Asija G (1956) A rapid procedure for the estimation of available nitrogen in soils. Curr Sci India 25:259–260
Syu CH, Huang CC, Jiang PY, Chien PH, Wang HY, Su JY, Lee DY (2016) Effects of foliar and soil application of sodium silicate on arsenic toxicity and accumulation in rice (Oryza sativa L.) seedlings grown in As-contaminated paddy soils. Soil Sci Plant Nutr 62:357–366
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Goutam D, Shri M, Trivedi PK, Chakrabarty D (2013) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wang HY, Wen SL, Chen P, Zhang L, Cen K, Sun GX (2016) Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ Sci Pollut Res 23(4):3781–3788
Williams CH, Steinbergs A (1962) The evaluation of plant-available sulphur in soils. Plant Soil 17:279–294
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Williams PN, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman AR, Rahman GK, Lu Y, Deacon C, Zhu YG, Meharg AA (2009) Arsenic limits trace mineral nutrition (selenium, zinc, and nickel) in Bangladesh rice grain. Environ Sci Technol 43:8430–8436
Xu XY, McGrath SP, Meharg AA, Zhao F-J (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Yamaji N, Ma JF (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21(9):2878–2883
Zhang S, Geng L, Fan L, Zhang M, Zhao Q, Xue P, Liu W (2020) Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil. Sci Total Environ 704:135239
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Acknowledgments
Authors are thankful to Director, CSIR-National Botanical Research Institute, Lucknow, for providing the infrastructure and laboratory facilities. SERB-DST and CSIR, New Delhi, are acknowledged for the award of Young Scientist Fellowship (SB/YS/LS-381/2013) and SRA (letter No. IA-27594, dated 02-11-2017) respectively to SM.
Funding
Privi Life Science Pvt. Ltd., Mumbai, is also acknowledged for financial support for this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 133 kb)
Rights and permissions
About this article
Cite this article
Dwivedi, S., Kumar, A., Mishra, S. et al. Orthosilicic acid (OSA) reduced grain arsenic accumulation and enhanced yield by modulating the level of trace element, antioxidants, and thiols in rice. Environ Sci Pollut Res 27, 24025–24038 (2020). https://doi.org/10.1007/s11356-020-08663-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08663-x