Abstract
Bisphenol A (BPA) is an estrogenic endocrine disrupting chemical to which humans are frequently exposed during routine daily life. Curcumin and taurine are natural products that have also been used as antioxidants against different environmental toxin–induced hepatotoxicity. Furthermore, they have protective and therapeutic effects against various diseases. The present investigation has been conducted to evaluate the therapeutic potential of curcumin (100 mg kg−1) and taurine (100 mg kg−1) for their hepatoprotective efficacy against BPA (130 mg kg−1)-induced liver injury in rat. BPA significantly elevated the levels of malondialdehyde (MDA), while it reduced the activities of catalase (CAT), total glutathione S-transferase (GST), total glutathione peroxidase (GPx), and total superoxide dismutase (SOD). Besides, these biochemical changes were accompanied by histopathological alterations marked by the destruction of normal liver structure. The histological examinations showed that exposure of BPA caused dilatation of sinusoids, inflammatory cell infiltration, congestion, and necrosis in liver parenchyma. The BPA-induced histopathological alterations in liver were minimized by curcumin and taurine treatment. Furthermore, no necrosis was observed in the liver tissues of curcumin plus BPA and taurine plus BPA-treated rats. Oral administration of curcumin and taurine to BPA-exposed rats significantly reversed the content of lipid peroxidation products, as well as enhanced the activities of GPx and GST, CAT, and SOD enzymes. These findings have indicated that curcumin and taurine might have a protective effect against BPA-induced hepatotoxicity in rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,2-Bis (4-hydroxyphenyl) propane, also defined as bisphenol A (BPA), is a component of epoxy resins and polycarbonate plastics and has one of the highest volume in industrial chemicals manufactured in the World (Khan et al. 2016). The general population is repeatedly exposed to BPA, not only primarily through the diet (packaged food and drinking water) but also through medical procedures/products including cardiopulmonary bypass, hemodialysis, dental sealants (Lakind and Naiman 2011), dermal exposure, and inhalation of household dusts (Ezz et al. 2015), and it has been detected in various human tissues and body fluids (Vandenberg et al. 2010; Nahar et al. 2015; Ye et al. 2015). Therefore, recently, more attention has been focused on its impact on human and animal health.
The vast majority of works on BPA have been conducted on their negative impacts on endocrine system and reproductive system development (Santos-Silva et al. 2018; Zaid et al. 2018; Lv et al. 2019). Several studies have demonstrated that BPA may cause cardiovascular disorders (Ezz et al. 2015) and genotoxic and cytotoxic effects (Dobrzyńska and Radzikowska 2013). In addition, some previous studies reported potential relationships between BPA exposure and chronic diseases, obesity, liver dysfunction, cancer, and diabetes (Jiang et al. 2015; Hassani et al. 2017).
The main organ taken into consideration in toxicological studies is the liver, since this organ has a special role in the metabolism, storage, redistribution, and excretion of endogenous and exogenous substances in the body (Li et al. 2019). In addition, in animals and humans, the liver is the main organ which is responsible for BPA metabolism and transforms BPA to glucurono-conjugated form (Xia et al. 2014). Thus, it could be more vulnerable to BPA-induced toxicity, than other organs (Hassani et al. 2017). Exposure to BPA has been shown to cause defects in oxidative phosphorylation through the inhibition of the first complex of the electron transfer chain in the liver mitochondria (Khan et al. 2016; Mahdavinia et al. 2019). Several studies have reported the negative effects of BPA on liver function and structure in humans and animals. BPA is able to cause hepatic steatosis in human (Martella et al. 2016), increase insulin resistance in HepG2 cells (Geng et al. 2017), affect liver morphology (Nakagawa and Tayama 2000), elevate liver function enzymes [alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and gamma-glutamyltransferase (GGT)] in the serum (Hassan et al. 2012; Mahdavinia et al. 2019).
Many environmental contaminants such as industrial chemicals, fertilizers, ionizing radiation, pesticides, and heavy metals have been reported to disrupt the prooxidant-antioxidant balance of cells, thereby inducing oxidative stress (Ognjanović et al. 2010). Overproduced reactive oxygen species (ROS) like peroxyl radical (ROO), hydrogen peroxide (H2O2), superoxide anion (O2-), and hydroxyl radical (OH) are related to cytotoxicity due to their capacity to induce oxidative stress (Bindhumol et al. 2003). Oxidative stress has been reported to be closely linked with BPA-induced toxicity in the liver. Previous studies showed an impairment of oxidant-antioxidant systems balance and increase of lipid peroxidation in the liver of rodents exposed to BPA (Khan et al. 2016; Hassani et al. 2017). BPA has been reported to generate ROS including hydroxyl radical, hydrogen peroxide, and superoxide anion in the body (Jahan et al. 2016). The study of Chitra et al. (2003) revealed that BPA generate ROS by decreasing the activities of antioxidant enzyme and increasing lipid peroxidation thereby causing oxidative stress (Chitra et al. 2003). Moreover, it has been shown that the BPA can induce the production of ROS in the liver by decreasing the expression of the gene responsible for the prevention of oxidative activity (Hassan et al. 2012; Kazemi et al. 2016). Experimental animal studies have demonstrated BPA-induced toxicity by oxidative stress in different organ systems such as the kidney, pancreas, heart, and testis (Mahmoudi et al. 2015; Moghaddam et al. 2015; Apaydin et al. 2019; Kalender et al. 2019).
Taurine, 2-aminoethanesulfonic acid, the most abundant intracellular amino acid, plays a significant role in several essential biological events such as the regulation of intracellular calcium concentration, conjugation of bile acids, osmoregulation, and biological membrane stabilization (Marcinkiewicz and Kontny 2014). Taurine exhibits antioxidant, antifibrotic, anticancer, and antitumor activities (Rashid et al. 2013; Abdel-Moneim et al. 2015). Additionally, taurine has been shown to protect cells against the cytotoxic effects of inflammation associated with oxidative stress and to provide anti-inflammatory effects (Marcinkiewicz and Kontny 2014). Some researchers have reported its cytoprotective potential in the liver against various toxic agents-induced the hepatotoxicity (Abdel-Moneim et al. 2015; Elwy et al. 2019; Abdel-Daim et al. 2019). The beneficial effects of taurine as an antioxidant have been attributed to its ability to reduce the formation of lipid peroxidation end products, to scavenge ROS such as hydroxyl radicals, and to stabilize biological membranes (Taziki et al. 2018; Abdel-Daim et al. 2019).
Curcumin (1,7-bis-[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), a polyphenolic compound, is the main yellow color pigment of turmeric (the isolated form of Curcuma longa rhizome) (Yu et al. 2014). It has a broad spectrum of pharmacological activities, including antioxidant, anti-proliferative, antitumor, immunomodulatory, anti-microbial, and anti-inflammatory agent (Sankar et al. 2015). Most importantly, curcumin has a strong potency in inhibiting the generation of ROS (Wang et al. 2012). In addition, curcumin has been shown to indirectly induce the expression of antioxidant enzyme such as catalase (CAT), glutathione reductase (GR), glutathione S-transferase (GST), glutathione peroxidase (GPx), and superoxide dismutase (SOD) (Trujillo et al. 2013). The hepatoprotective effects of curcumin against toxic chemical-induced liver injury are well known and have been attributed to its intrinsic antioxidant properties (Farombi et al. 2008; Wang et al. 2012; Messarah et al. 2013; Sankar et al. 2015). Geng et al. showed that curcumin diminished insulin resistance, inflammatory response, and oxidative stress induced by BPA in HepG2 cells (Geng et al. 2017). Curcumin has also been shown to have renoprotective (Benzer et al. 2018), neuroprotective (Pluta et al. 2015), and cardio-protective effects (Apaydin et al. 2019) against chemical toxicity. Recently, curcumin and taurine have been reported to ameliorate BPA-induced oxidative stress and histo/cytopathological changes in testis and heart tissues of Wistar rats (Apaydin et al. 2019; Kalender et al. 2019).
It is a known fact that the consumption of antioxidant-rich foods can contribute to reducing the potential toxic effects of environmental contaminants. Various antioxidant agents have been studied for their protective effects against BPA-induced organ toxicity (Avci et al. 2016; Othman et al. 2016; Jahan et al. 2016; Hassani et al. 2017). However, there is not any study about the potential effects of curcumin and taurine on BPA-induced liver damage in rats. Therefore, the current study has been conducted to investigate the possible hepatoprotective effects of taurine and curcumin against subacute BPA intoxication in male rats.
Materials and methods
Chemicals
Bisphenol A (≥ 99% purity), taurine (≥ 99% purity), and curcumin were supplied by Sigma-Aldrich (St. Louis, MO, USA). Other chemicals used in this work were purchased from usual commercial sources [Merck (Germany); Sigma-Aldrich (St. Louis, MO, USA)].
Animals
Forty-two male Wistar rats (250–300 g) were supplied by Laboratory Animals Growing and Experimental Research Center, Gazi University (Ankara, Turkey). All the rats were in quarantine for 10 days before the beginning of the treatment. All the rats were grouped separately and were housed in cages under standard conditions (temperature at 20 ± 2 °C, light-controlled (12 h on–12 h off), a relative humidity 40–45%). Rats were provided with standard rodent pellets diet and were given drinking water ad libitum during the experimental period. The Local Ethics Committee on Animal Experiments of the University (Approval number: G.U.ET-14.075) approved the experimental protocol.
Animals were exposed to all tested compounds by oral gavage during 28 days. At the first day of the experiment (day 0), they were separated into two main groups: control (n = 12) and experimental groups (n = 30). Based on the solvents, two control groups were selected, each consisting of six rats: distilled water and olive oil groups. The experimental group was separated into five subgroups, each consisting of six rats: curcumin, taurine, BPA, curcumin plus BPA, and taurine plus BPA. The experimental design was shown in Table 1.
The dose of BPA in this study was determined from previous experimental studies of Yıldız and Barlas (2013) and Wu et al. (2013). The doses of curcumin and taurine were selected on the basis of previous studies (Lonare et al. 2014; Aly and Khafagy 2014) that reported protection against the toxicity of environmental contaminant.
All the rats were anesthetized via intramuscular enjection with a combination of ketamin-xylazine at the end of a 4-week treatment period. The liver of the rats was isolated to assess the activities of antioxidant enzyme, the levels of MDA, and light microscope examinations.
Evaluation of body and liver weights
The body weight of the rats was recorded on day 0 and thereafter on day 28. On day 28, the rats were dissected under anesthesia. The liver tissues were isolated, weighed, and recorded. The relative weight of liver was calculated according to the following formula:
Relative liver weight = (liver weight/body weight of the animal on sacrifice day) × 100
Determination of liver malondialdehyde and antioxidant enzyme activities
After the isolation of dissected rat liver tissues, they were rinsed with sodium phosphate buffer (pH 7.2), and kept at − 80 °C for subsequent analysis. Liver homogenates were prepared using Heidolph Silent Crusher M, then were centrifuged. In order to determine the malondialdehyde (MDA) levels and antioxidant markers and the absorbance of the samples, they were measured with a Shimadzu UV 1700 spectrophotometer (Kyoto, Japan). Protein concentrations in homogenates were estimated by colorimetric method described by Lowry et al. (1951), using bovine serum albumin as standard.
The MDA level has been frequently used as an indicator of membrane lipid peroxidation. The MDA level in the liver was determined, based on the thiobarbituric acid (TBA) test at 532 nm, according to the methods of Ohkawa et al. (1979). MDA levels were defined as nanomoles of MDA per milligram of protein−1.
The activity of hepatic total SOD was detected as described by Marklund and Marklund’s study (1974) by assaying the illumination and autooxidation of pyrogallol at 440 nm for 3 min. One unit of enzyme activity was calculated as the amount of protein that caused 50% pyrogallol autooxidation inhibition. A blank without homogenate was used as a control for non-enzymatic oxidation of pyrogallol in Tris–EDTA buffer (50 mM Tris, 10 mM EDTA, pH 8.2). The specific activity of SOD was defined as U mg protein−1.
For CAT activity determination, samples were diluted with Triton X-100.The activity of hepatic CAT was assayed using H2O2 as substrate according to the procedure described by Aebi (1984). The enzyme activity was determined by monitoring the decline in absorbance at 240 nm as H2O2 was consumed. The specific activity of CAT was defined as mmol mg protein−1.
The activity of hepatic GPx was determined using H2O2 as substrate according to the methods of Paglia and Valentine (1967). The reaction was monitored indirectly as the oxidation rate of NADPH at 240 nm for 3 min. A blank without homogenate was used as a control for non-enzymatic oxidation of NADPH upon addition of hydrogen peroxide in 0.1 M Tris buffer, pH 8.0. GPx activity was defined as nmol mg protein−1.
The activity of hepatic total GST was analysed by measuring the generation of the glutathione and 1-chloro-2, 4-dinitrobenzene conjugate by the procodure specified by Habig et al. (1974). GST activity is defined as μmol mg protein−1.
Light microscopic examination
Regarding the histopathologic analysis, small pieces of the liver tissues from each rat were cut and placed in 10% formalin solution for fixation. The liver tissues were dehydrated with different concentrations of ethanol series and embedded in paraffin. Sections of 4–6 μm thickness were cut from liver blocks and then stained with H&E (hematoxylin-eosin) and examined by Olympus BX-51 light microscopy. The histological photographs of the liver tissues were acquired using an Olympus E-330 digital photograph machine. All liver slides were evaluated for the degree of histopathological alterations. Each slide was examined and assigned for severity of changes using scores on a scale of none (0), slight (1), moderate (2), and severe (3) damage.
Statistics
Statistical analysis of the present study was conducted using SPSS 17.0 software. All the data were given as mean ± S.D. (standard deviation). The significance of the differences among groups was determined by Tukey’s procedure for multiple comparisons using one-way analysis of variance (ANOVA). The statistical significance was determined at P value of < 0.05.
Results
There was no treatment-related mortality recorded in all seven groups. No significant differences were shown between control groups, curcumin-treated groups, and taurine-treated groups on examining parameters (Table 2) (Figs. 1, 2, and 3). Furthermore, the distilled water–treated group was considered the main control group for the comparison with the experimental groups.
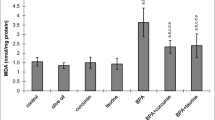
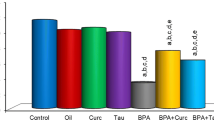
Effects of subacute treatment of BPA, curcumin and taurine on MDA level in the liver tissue of rats. Each bar represents mean ± S.D. of six animals in each group. Significance at p < 0.05. a Comparison of control and other groups. b Comparison of olive oil-treated group and other groups.
c Comparison of curcumin-treated group and other groups. d Comparison of taurine-treated group group and other groups. e Comparison of BPA-treated group and other groups
Effects of subacute treatment of BPA, curcumin and taurine on antioxidant enzyme activities in the liver tissue of rats. a SOD activity. b CAT activity. c. GPx activity. d. GST activity. Each bar represents mean ± S.D. of six animals in each group. Significance at p < 0.05. a Comparison of control and other groups. b Comparison of olive oil-treated group and other groups. c Comparison of curcumin-treated group and other groups. d Comparison of taurine–treated group group and other groups. e Comparison of BPA-treated group and other groups
Representative photomicrograph of rat livers stained with H&E (× 200). a The liver of a control rats showed normal architecture of the hepatocytes, hepatic cords radiating from the central vein (CV) and separated by the hepatic sinusoids ( ), Histologic structures of olive oil-, curcumin-, and taurine-treated rats were similar with those of the control group. b–e Liver sections of BPA-treated rats: b Dilation of sinusoids (⇈), c inflammatory cell infiltration (♦), d congestion (↗) and necrosis (✱), e necrosis (✱). f, g Liver sections of curcumin plus BPA-treated rats: f Dilation of sinusoids (⇈), g inflammatory cell infiltration (♦) and congestion (↗). h, ı Liver sections of taurine plus BPA-treated rats: h Dilation of sinusoids (⇈), and ı inflammatory cell infiltration (♦) and congestion (↗)
), Histologic structures of olive oil-, curcumin-, and taurine-treated rats were similar with those of the control group. b–e Liver sections of BPA-treated rats: b Dilation of sinusoids (⇈), c inflammatory cell infiltration (♦), d congestion (↗) and necrosis (✱), e necrosis (✱). f, g Liver sections of curcumin plus BPA-treated rats: f Dilation of sinusoids (⇈), g inflammatory cell infiltration (♦) and congestion (↗). h, ı Liver sections of taurine plus BPA-treated rats: h Dilation of sinusoids (⇈), and ı inflammatory cell infiltration (♦) and congestion (↗)
Changes of body and organ weights
Body, liver, and relative liver weights did not show any significant changes, when BPA, curcumin plus BPA, and taurine plus BPA groups were compared with control group (Table 2).
MDA levels
MDA levels in the liver tissues were significantly increased in BPA, curcumin plus BPA, and taurine plus BPA groups compared to the control group, while they decreased in the curcumin plus BPA and taurine plus BPA groups compared to the only BPA-treated group (Fig. 1) (p < 0.05).
Antioxidant enzyme activities
The antioxidant markers (SOD, CAT, GPx, and GST) in the livers of the rats treated with BPA were shown in Fig. 2a–d.
Antioxidant enzyme activities significantly decreased when BPA, curcumin plus BPA, and taurine plus BPA groups were compared with the control group (Fig. 2) (p < 0.05). However, antioxidant enzyme activities were significantly increased in the curcumin plus BPA– and taurine plus BPA–treated groups compared to the BPA-treated group (Fig. 2a–d).
Histopathological findings
Light micrographs of histological examinations were shown in Fig. 3. The liver of control-, olive oil-, curcumin-, and taurine-administered rats showed normal hepatic architecture characterized by polygonal shape hepatocytes, sinusoidal spaces, and a central vein (Fig. 3a). The liver of BPA-exposed rats showed loss of cellular architecture with dilatation of sinusoids, inflammatory cell infiltration, congestion, and necrosis (Fig. 3b–e). In the liver tissues of curcumin plus BPA (Fig. 3f, g) and taurine plus BPA (Fig. 3 h, ı)-treated rats, dilation of sinusoids, congestion, and inflammatory cell infiltration were observed. Histopathologically, the antioxidant supplementation caused a significant decrease in the inflammatory cell infiltration in liver (Table 3) (p < 0.05). Furthermore, no necrosis was observed in the liver tissues of curcumin plus BPA– and taurine plus BPA–treated rats (Table 3). The histopathological changes were graded and summarized in Table 3.
Discussion
BPA is a synthetic xenoestrogenic compound that is commonly used in the manufacturing of polycarbonate plastic including water and infant bottles and fungicides, flame retardants, and epoxy resins (e.g., lining food cans) (Kazemi et al. 2016; Khan et al. 2016). Humans are exposed to BPA due to its increased use in food containers and polycarbonate plastics (Carwile et al. 2009). In the present study, we investigated the protective effects of taurine and curcumin on BPA-induced hepatotoxicity in albino rats.
The analysis of body and organ weights in toxicology studies may be one of the indicators of animals’ general health condition and alterations in the organ weights are one of the important criteria for the evaluation of organ toxicity (Crissman et al. 2004; Ping et al. 2013; Abdel-Wahab 2014). In the present study, no significant difference was found in the body and liver weight in BPA-treated rats. Similarly, previous studies showed that subacute BPA exposure in male rats did not significantly influence body weight (Youn et al. 2002; Hassani et al. 2017) and body weight gain (Wu et al. 2013; Li et al. 2016). Yamasaki et al. reported significant change in body weight in the animals treated with 600 mg/kg/day BPA (Yamasaki et al. 2002). No change in body weight caused by BPA treatment may be due to unchanged metabolic activity of animals (Chitra et al. 2003). In accordance with our study, Kourouma et al. demonstrated that absolute and relative liver weight did not change significantly in the BPA-treated rats (Kourouma et al. 2015). These results might be due to the fact that the toxic effect of BPA depends on dose and/or treatment period.
Oxidative stress is induced by an excess accumulation of ROS and can change the basic cellular processes and viability (El-Missiry et al. 2014). Several studies have demonstrated the toxic effect of BPA on various organs by increasing oxidative stress (Ezz et al. 2015; Avci et al. 2016; Elswefy et al. 2016). Othman et al. reported that BPA disturbs the balance between ROS and antioxidant defence system and causes oxidative stress in the testis of rats (Othman et al. 2016).
MDA is one of the last products of polyunsaturated fatty acids peroxidation in the cell membrane, and its enhanced level is a major marker of lipid peroxidation. However, MDA level indirectly reflects the degree of tissue and cell damage (Su et al. 2008). In this study, an increase in the MDA level in the liver tissue was observed in the BPA-treated rats. Other studies showed that the administration of BPA caused an increment in MDA level in a variety of tissues such as kidney (Mahmoudi et al. 2015), liver (Eid et al. 2015), testis (Kalender et al. 2019), ovarium (Avci et al. 2016), heart (Apaydin et al. 2019), and brain (El-Missiry et al. 2014) of rats. The increase in MDA level can be considered as a marker of tissue injury induced by BPA. It was correlated with histopathological findings.
Antioxidant markers such as CAT, SOD, GST, and GPx play a major role in the cellular protection against oxidative stress induced by ROS (Uzunhisarcikli et al. 2016). SOD, the first line of the defence system against free radicals, destroys the free radical superoxide by converting it to less noxious hydrogen peroxide (Stinghen et al. 2014). CAT is a hemeprotein which catalyzes the reduction of hydrogen peroxide to molecular oxygen and water and protects the cell from oxidative damage of hydroxyl radical and hydrogen peroxide (Safhi et al. 2016). GPx is a selenoenzyme that catalyzes the reduction of hydrogen peroxide to water utilizing glutathione (GSH), thereby can prevent oxidative damage of mammalian cell (Bhattacharjee et al. 2014). GST is one of the major phase II detoxifying enzymes and plays an essential role in the detoxification and excretion of toxins in the liver by conjugating them with GSH (Abdel-Daim et al. 2015). In this study, the activities of CAT, SOD, GST, and GPx were significantly reduced in the liver of BPA-treated rats. These results are consistent with previous studies (Othman et al. 2016; Sangai et al. 2014; Suthar and Verma 2014). The reduction in the antioxidant enzyme activities may have resulted from increment-free radical generation depending on the increase in lipid peroxidation. In addition, Hassan et al. demonstrated a decrease in the gene expression levels of antioxidant enzyme such as CAT, SOD, GST, and GPx in liver tissues of the BPA-treated rats (Hassan et al. 2012).
Foods rich in antioxidants or antioxidant supplements may be used to help the human body in reducing oxidative damage by active oxygen and free radicals (Rajbanshi and Pandanaboina 2014). Curcumin and taurine are natural products that have also been used as antioxidants, against different environmental toxins-induced toxicity (Nagai et al. 2016; Messarah et al. 2013). Recent studies reported that taurine and curcumin showed ameliorative effects on BPA-induced cardiotoxicity and testicular toxicity in rat (Apaydin et al. 2019; Kalender et al. 2019).
In the present study, curcumin and taurine treatments significantly reversed the adverse effects of BPA on antioxidant enzyme activities and MDA levels in liver tissue. Curcumin has a strong potency in inhibiting the generation of ROS (Wang et al. 2012). Curcumin was notified to inhibit hydroxyl radical and superoxide anion formation by preventing oxidation of Fe2+ to Fe3+ via Fenton reaction (Messarah et al. 2013). Recent studies show that curcumin could reduce the hepatotoxicity induced by heavy metals, chemicals, and drugs; prevent lipid peroxidation, glutathione depletion, and histological injury; protects against mitochondrial dysfunction; and maintains the liver antioxidant enzyme status (Wang et al. 2012; Messarah et al. 2013; Yu et al. 2014; Garcia-Nino and Pedraza-Chaverri 2014). Moreover, curcumin has hepatoprotective effects due to its anti-inflammatory, antioxidant, and anticancer activities (Wang et al. 2012; Messarah et al. 2013). These experimental data and our present findings support that curcumin treatment significantly ameliorated BPA-induced oxidative stress mainly through the preservation of hepatic endogenous antioxidants. In addition, there is also evidence that curcumin protects against oxidative stress by increasing nuclear factor erythroid 2-related factor 2 (Nrf2) activation and decreasing ROS generation. Nrf2 is responsible for regulation of expression of genes encoding the majority of phase II detoxification and antioxidant enzymes (Xie et al. 2017). It has been reported that curcumin activates the transcription factor Nrf2 in the liver of mice (Gao et al. 2013). Furthermore, it was also demonstrated that taurine increased the expression of Nrf2 (Yang et al. 2017). Taurine is a non-protein sulfur-containing essential amino acid and plays an important role in several essential biological processes such as osmoregulation, bile formation, calcium binding and transporter regulation, antioxidant, detoxification, and modulation of neurotransmission (Yang et al. 2015; Abdel-Moneim et al. 2015; Abdel-Daim et al. 2019). It is known that taurine has the ability to reduce lipid peroxidation, improve cellular antioxidant enzyme activities, and stabilize biological membranes, thus preventing apoptosis and necrotic cell death (Hagar 2004). Moreover, taurine plays a role as a scavenger of hydroxyl radical, singlet oxygen molecule, and superoxide (Ozden et al. 2009). Consistent with our results, some studies showed that taurine supplementation decreases the oxidative stress induced by various hepatotoxins (Sinha et al. 2009; Ozden et al. 2009; El-Sayed et al. 2011; Nagai et al. 2016). In the present study, the protective effects of taurine and curcumin against BPA-induced oxidative stress might be attributed to their ability of reducing and scavenging the ROS and inducing the activities of antioxidant enzyme. Moreover, the increment in antioxidant enzyme activities induced by taurine and curcumin might be due to enhanced activation of Nrf2 signaling pathway, which is associated with the expression of antioxidant enzymes, by taurine and curcumine. The results of previous studies also support this opinion (Gao et al. 2013; Xie et al. 2017; Yang et al. 2017).
Microscopic evaluation of histopathological changes is accepted as a major vehicle to assess the impacts of environmental toxicants in cells, tissues, and organs. Some studies on the toxicological effect of BPA have shown that it causes pathological changes in the various tissues (Othman et al. 2016; Mahmoudi et al. 2015; Gear et al. 2017). In this study, BPA treatment caused severe pathological alterations such as dilatation of sinusoids, inflammatory cell infiltration, congestion, and necrosis in the liver tissue. Our results are supported by the findings of Hussein and Eid (2013) and Hassani et al. (2017). In the present study, the pathological findings were supported by the data obtained from biochemical analysis. These histopathological alterations may reflect the significantly increased hepatic MDA levels and reduced antioxidative enzyme activities in the liver of rats in BPA group. Nakagawa and Tayama reported that BPA causes cytotoxicity, cell damage, and lysis in isolated rat hepatocytes (Nakagawa and Tayama 2000). Elswefy et al. showed that BPA administration caused hepatic injury and fibrosis related to oxidative stress, inflammation, and apoptosis (Elswefy et al. 2016). They reported BPA mediated this deleterious effect through increasing the proinflammatory cytokine (IL-1b) and decreasing the anti-inflammatory/antifibrotic correlations between hepatic GSH, CAT, BCL2, and fibrotic markers (Elswefy et al. 2016). We showed that the histopathological alterations in the taurine and curcumin-treated rats were more mild. The antioxidants caused a marked decrease in the inflammatory cell infiltration and no necrosis was visible. These therapeutic beneficial effects of curcumin and taurine as an antioxidant in organ pathology might be attributed to their ability of stabilizing biological membrane, scavenging ROS, and inducing the activities of antioxidant enzyme (Gao et al. 2013; Aly and Khafagy 2014). The protection against necrosis might be due to intracellular calcium-mobilizing and membrane stabilizing properties of taurine (Marcinkiewicz and Kontny 2014). Furthermore, several studies have demonstrated that taurine attenuated necrosis through reduction of oxidative stress (Redmond et al. 1996; Erdem et al. 2000). It has been demonstrated that taurine and curcumin reduced the inflammatory responses by inhibiting proinflammatory cytokines (Su et al. 2014; Garcia-Nino and Pedraza-Chaverri 2014). Similary, Kalender et al. reported that taurine and curcumin administration prevented necrosis induced by BPA in testis tissues (Kalender et al. 2019).
Considering the results obtained from this study, it can be said that BPA causes hepatic injury by way of oxidative stress. Increased levels of lipid peroxidation and changed activities of antioxidant enzyme reveal that BPA disrupts the prooxidant/antioxidant balance and increases the generation of ROS, thereby causing oxidative stress. Oral administration of curcumin and taurine to BPA-exposed rats significantly reversed the content of lipid peroxidation, as well as enhanced the level of GPx and GST, CAT, and SOD activities compared to only BPA-treated rats. Moreover, they reduced inflammatory cell infiltration and prevented necrosis in the liver tissue of rats. The findings of this research suggest that curcumin and taurine applied at the tested doses could partially protect the BPA-induced hepatotoxicity in rats.
References
Abdel-Daim MM, Abd Eldaim MA, Hassan AGA (2015) Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem Cell Biol 93:192–198. https://doi.org/10.1139/bcb-2014-0122
Abdel-Daim MM, Dessouki AA, Abdel-Rahman HG, Eltaysh R, Alkahtani S (2019) Hepatorenal protective effects of taurine and N-acetylcysteine against fipronil-induced injuries: the antioxidant status and apoptotic markers expression in rats. Sci Total Environ 650:2063–2073. https://doi.org/10.1016/j.scitotenv.2018.09.313
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 10:e0144509. https://doi.org/10.1371/journal.pone.0144509
Abdel-Wahab WM (2014) Thymoquinone attenuates toxicity and oxidative stress induced by bisphenol A in liver of male rats. Pak J Biol Sci 17:1152–1160. https://doi.org/10.3923/pjbs.2014.1152.1160
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Aly HAA, Khafagy RM (2014) Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem Toxicol 64:1–9. https://doi.org/10.1016/j.fct.2013.11.007
Apaydin FG, Aslanturk A, Uzunhisarcikli M, Bas H, Kalender S, Kalender Y (2019) Histopathological and biochemical studies on the effect of curcumin and taurine against bisphenol A toxicity in male rats. Environ Sci Pollut Res 26:12302–12310. https://doi.org/10.1007/s11356-019-04578-4
Avci B, Bahadir A, Tuncel OK, Bilgici B (2016) Influence of α-tocopherol and α-lipoic acid on bisphenol-A induced oxidative damage in liver and ovarian tissue of rats. Toxicol Ind Health 32:1381–1390. https://doi.org/10.1177/0748233714563433
Benzer F, Kandemir FM, Kucukler S, Comaklı S, Caglayan C (2018) Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: by modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch Physiol Biochem 124:448–457. https://doi.org/10.1080/13813455.2017.1422766
Bhattacharjee A, Basu A, Ghosh P, Biswas J, Bhattacharya S (2014) Protective effect of selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J Biomater Appl 29:303–317. https://doi.org/10.1177/0885328214523323
Bindhumol V, Chitra KC, Mathur V (2003) Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188:117–124. https://doi.org/10.1016/S0300-483X(03)00056-8
Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB (2009) Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect 117:1368–1372. https://doi.org/10.1289/ehp.0900604
Chitra KC, Latchoumycandane C, Mathur PP (2003) Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 185:119/127. https://doi.org/10.1016/S0300-483X(02)00597-8
Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, Riley JH, Seaman WJ, Thake DC (2004) Best practice guideline: toxicologic histopathology. Toxicol Pathol 32:126–131. https://doi.org/10.1080/01926230490268756
Dobrzyńska MM, Radzikowska J (2013) Genotoxicity and reproductive toxicity of bisphenol A and X-ray/bisphenol A combination in male mice. Drug Chem Toxicol 36:19–26. https://doi.org/10.3109/01480545.2011.644561
Eid JI, Eissa SM, El-Ghor AA (2015) Bisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. J Basic Appl Zool 71:10–19. https://doi.org/10.1016/j.jobaz.2015.01.006
El-Missiry MA, Othman AI, Al-Abdan MA, El-Sayed AA (2014) Melatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against bisphenol-A-induced apoptosis. J Neurol Sci 347:251–256. https://doi.org/10.1016/j.jns.2014.10.009
El-Sayed WM, Al-Kahtania MA, Abdel-Moneima AM (2011) Prophylactic and therapeutic effects of taurine against aluminum-induced acute hepatotoxicity in mice. J Hazard Mater 192:880–886. https://doi.org/10.1016/j.jhazmat.2011.05.100
Elswefy SES, Abdallah FR, Atteia HH, Wahba AS, Hasan RA (2016) Inflammation, oxidative stress and apoptosis cascade implications in bisphenol A-induced liver fibrosis in male rats. Int J Exp Pathol 97:369–379. https://doi.org/10.1111/iep.12207
Elwy AEM, El-Agousa IMA, Azzazy AE (2019) Taurine as a drug for protection of liver and kidney against toxicity of dinitrotoluene on male rats (applicable study). Int J Pharm Res Allied Sci 8:102–114
Erdem A, Gündoğan NÜ, Usubütün A, Kılınç K, Erdem ŞR, Kara A, Bozkurt A (2000) The protective effects of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant 15:1175–1182. https://doi.org/10.1093/ndt/15.8.1175
Ezz HSA, Khadrawy YA, Mourad IM (2015) The effect of bisphenol A on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology 67:145–155. https://doi.org/10.1007/s10616-013-9672-1
Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ (2008) Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol 46:1279–1287. https://doi.org/10.1016/j.fct.2007.09.095
Gao S, Duan X, Wang X, Dong D, Liu D, Li X, Sun G, Li B (2013) Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol 59:739–747. https://doi.org/10.1016/j.fct.2013.07.032
Garcia-Nino WR, Pedraza-Chaverri J (2014) Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxicol 69:182–201. https://doi.org/10.1016/j.fct.2014.04.016
Gear R, Kendziorski JA, Belcher SM (2017) Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: a clarity-BPA study. Toxicol Lett 275:123–135. https://doi.org/10.1016/j.toxlet.2017.05.011
Geng S, Wang S, Zhu W, Xie C, Li X, Wu J, Zhu J, Jiang Y, Yang X, Li Y, Chen Y, Wang X, Meng Y, Zhu M, Wu R, Huang C, Zhong C (2017) Curcumin attenuates BPA-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways. Toxicol Lett 15:75–83. https://doi.org/10.1016/j.toxlet.2017.03.011
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hagar HH (2004) The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett 151:335–343. https://doi.org/10.1016/j.toxlet.2004.03.002
Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, Alolayan EM (2012) Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med Cell Longev:1–6. https://doi.org/10.1155/2012/194829
Hassani FV, Mehri S, Abnous K, Birner-Gruenberger R, Hosseinzadeh H (2017) Protective effect of crocin on BPA-induced liver toxicity in rats through inhibition of oxidative stress and downregulation of MAPK and MAPKAP signaling pathway and miRNA-122 expression. Food Chem Toxicol 107:395–405. https://doi.org/10.1016/j.fct.2017.07.007
Hussein RM, Eid JI (2013) Pathological mechanisms of liver injury caused by oral administration of bisphenol A. Life Sci J 10:663–673
Jahan S, Ain QU, Ullah H (2016) Therapeutic effects of quercetin against bisphenol A induced testicular damage in male Sprague Dawley rats. Syst Biol Reprod Med 62:114–124. https://doi.org/10.3109/19396368.2015.1115139
Jiang Y, Xia W, Yang J, Zhu Y, Chang H, Liu J, Huo W, Xu B, Chen X, Li Y, Xu S (2015) BPA-induced DNA hypermethylation of the master mitochondrial gene PGC-1a contributes to cardiomyopathy in male rats. Toxicology 329:21–31. https://doi.org/10.1016/j.tox.2015.01.001
Kalender S, Apaydin FG, Kalender Y (2019) Testicular toxicity of orally administrated bisphenol A in rats and protective role of taurine and curcumin. Pak J Pharm Sci 32:1043–1047. https://doi.org/10.1007/s11356-019-04578-4
Kazemi S, Mousavi SN, Aghapour F, Rezaee B, Sadeghi F, Moghadamnia AA (2016) Induction effect of bisphenol A on gene expression involving hepatic oxidative stress in rat. Oxidative Med Cell Longev:1–5. https://doi.org/10.1155/2016/6298515
Khan S, Beigh S, Chaudhari BP, Sharma S, Abdi SAH, Ahmad S, Ahmad F, Parvez S, Raisuddin S (2016) Mitochondrial dysfunction induced by bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol 31:1922–1934. https://doi.org/10.1002/tox.22193
Kourouma A, Quan C, Duan P, Qi S, Yu T, Wang Y, Yang K (2015) Bisphenol A induces apoptosis in liver cells through induction of ROS. Adv Toxicol:1–10. https://doi.org/10.1155/2015/901983
Lakind JS, Naiman DQ (2011) Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National health and nutrition examination survey. J Expo Sci Environ Epidemiol 21:272–279. https://doi.org/10.1038/jes.2010.9
Li J, Mao R, Zhou Q, Ding L, Tao J, Ran MM, Gao ES, Yuan W, Wang JT, Hou LF (2016) Exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of ERK signal pathway. Toxicol Mech Methods 26:180–188. https://doi.org/10.3109/15376516.2016.1139024
Li S, Zhou J, Xu S, Li J, Liu J, Lu Y, Shia J, Zhou S, Wu Q (2019) Induction of Nrf2 pathway by Dendrobium nobile Lindl. alkaloids protects against carbon tetrachloride induced acute liver injury. Biomed Pharmacother 117:109073. https://doi.org/10.1016/j.biopha.2019.109073
Lonare M, Kumar M, Raut S, More A, Doltade S, Badgujar P, Telang A (2014) Evaluation of imidacloprid-induced neurotoxicity in male rats: a protective effect of curcumin. Neurochem Int 78:122–129. https://doi.org/10.1016/j.neuint.2014.09.004
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 19:265–275
Lv Y, Li L, Fanga Y, Chen P, Wu S, Chen X, Ni C, Zhu Q, Huanga T, Lian Q, Ge RS (2019) In utero exposure to bisphenol A disrupts fetal testis development in rats. Environ Pollut 246:217–224. https://doi.org/10.1016/j.envpol.2018.12.006
Mahdavinia M, Alizadeh S, Vanani AR, Dehghani MA, Shirani M, Alipour M, Shahmohammadi HA, Asl SR (2019) Effects of quercetin on bisphenol A-induced mitochondrial toxicity in rat liver. Iran J Basic Med Sci 22:499–505. https://doi.org/10.22038/ijbms.2019.32486.7952
Mahmoudi A, Ghorbel H, Bouallegui Z, Marrekchi R, Isoda H, Sayadi S (2015) Oleuropein and hydroxytyrosol protect from bisphenol A effectsin livers and kidneys of lactating mother rats and their pups’. Exp Toxicol Pathol 67:413–425. https://doi.org/10.1016/j.etp.2015.04.007
Marcinkiewicz J, Kontny E (2014) Taurine and inflammatory diseases. Amino Acids 46:7–20. https://doi.org/10.1007/s00726-012-1361-4
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Martella A, Silvestri C, Maradonna F, Gioacchini G, Allara M, Radaelli G, Overby DR, Marzo VD, Carnevali O (2016) Bisphenol A induces fatty liver by an endocannabinoid-mediated positive feedback loop. Endocrinology 157:1751–1763. https://doi.org/10.1210/en.2015-1384
Messarah M, Amamra W, Boumendjel A, Barkat L, Bouasla I, Abdennour C, Boulakoud MS, Feki AE (2013) Ameliorating effects of curcumin and vitamin E on diazinon-induced oxidative damage in rat liver and erythrocytes. Toxicol Ind Health 29:77–88. https://doi.org/10.1177/0748233712446726
Moghaddam HS, Samarghandian S, Farkhondeh T (2015) Effect of bisphenol A on blood glucose, lipid profile and oxidative stress indices in adult male mice. Toxicol Mech Methods 25:507–513. https://doi.org/10.3109/15376516.2015.1056395
Nagai K, Fukuno S, Oda A, Konishi H (2016) Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-Cancer Drug 27:17–23. https://doi.org/10.1097/CAD.0000000000000299
Nahar MS, Liao C, Kannan K, Harris C, Dolinoy DC (2015) In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 124:54–60. https://doi.org/10.1016/j.chemosphere.2014.10.071
Nakagawa Y, Tayama S (2000) Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol 74:99–105. https://doi.org/10.1007/s002040050659
Ognjanović BI, Marković SD, Ethordević NZ, Trbojević IS, Stajn AS, Saicić ZS (2010) Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: Protective role of coenzyme Q 10 and Vitamin E. Reprod Toxicol 29:191–197. https://doi.org/10.1016/j.reprotox.2009.11.009
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Othman AI, Edrees GM, El-Missiry MA, Ali DA, Aboel-Nour M, Dabdoub BR (2016) Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health 32:1537–1549. https://doi.org/10.1177/0748233714561286
Ozden S, Catalgol B, Gezginci-Oktayoglu S, Arda-Pirincci P, Bolkent S, Alpertunga B (2009) Methiocarb-induced oxidative damage following subacute exposure and the protective effects of vitamin E and taurine in rats. Food Chem Toxicol 47:1676–1684. https://doi.org/10.1016/j.fct.2009.04.018
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70:158–165
Ping KY, Darah I, Chen Y, Sreeramanan S, Sasidharan S (2013) Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomed Res Int:182064. https://doi.org/10.1155/2013/182064
Pluta R, Bogucka-Kocka A, Ulamek-Koziol M, Furmaga-Jablonska W, Januszewski S, Brzozowska J, Jablonski M, Kocki J (2015) Neurogenesis and neuroprotection in postischemic brain neurodegeneration with Alzheimer phenotype: is there a role for curcumin? Folia Neuropathol 53:89–99. https://doi.org/10.5114/fn.2015.52405
Rajbanshi SLA, Pandanaboina CS (2014) Alcohol stress on cardiac tissue - ameliorative effects of Thespesia populnea leaf extract. J Cardiol 63:449–459. https://doi.org/10.1016/j.jjcc.2013.10.015
Rashid K, Das J, Sil PC (2013) Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol 51:317–329. https://doi.org/10.1016/j.fct.2012.10.007
Redmond HP, Wang JH, Bouchier-Hayes D (1996) Taurine attenuates nitric oxide-and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg 131:1280–1288. https://doi.org/10.1001/archsurg.1996.01430240034004
Safhi MM, Khuwaja G, Alam MF, Hussain S, Siddiqui MHA, Islam F, Islam F (2016) Cadmium-induced nephrotoxicity via oxidative stress in male Wistar rats and capsaicin protects its toxicity. Bull Environ Pharmacol Life Sci 5:05–11
Sangai NP, Verma RJ, Trivedi MH (2014) Testing the efficacy of quercetin in mitigating bisphenol A toxicity in liver and kidney of mice. Toxicol Ind Health 30:581–597. https://doi.org/10.1177/0748233712457438
Sankar P, Telang AG, Kalaivanan R, Karunakaran V, Manikam K, Sarkar SN (2015) Effects of nanoparticle-encapsulated curcumin on arsenic-induced liver toxicity in rats. Environ Toxicol 30:628–637. https://doi.org/10.1002/tox.21940
Santos-Silva AP, de Moura EG, Pinheiro CR, Oliveira E, Lisboa PC (2018) Short-term and long-term effects of bisphenol a (BPA) exposure during breastfeeding on the biochemical and endocrine profiles in rats. Horm Metab Res 50:491–503. https://doi.org/10.1055/a-0628-6708
Sinha M, Manna P, Sil PC (2009) Induction of necrosis in cadmium- induced hepatic oxidative stress and its prevention by the prophylactic properties of taurine. J Trace Elem Med Biol 23:300–313. https://doi.org/10.1016/j.jtemb.2009.03.010
Stinghen AEM, Chillon JM, Massy ZA, Boullier A (2014) Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd.3). Toxins 6:1742–1760. https://doi.org/10.3390/toxins6061742
Su L, Wabg M, Yin ST, Wang HL, Chen L, Sun LG, Ruan DY (2008) The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol Environ Saf 70:483–489. https://doi.org/10.1016/j.ecoenv.2007.05.018
Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 266:56–65. https://doi.org/10.1016/j.neuroscience.2014.02.006
Suthar H, Verma RJ (2014) Bisphenol A induces hepatotoxicity through oxidative stress in mice. Int J Adv Lif Sci 7:11–18
Taziki S, Khori V, Jahanshahi M, Seifi A, Babakordi FB, Nikmahzar EN (2018) Protective role of taurine against hepatotoxicity induced by pyrazinamide in rats. Natl J Physiol Pharm Pharmacol 8:824–828. https://doi.org/10.5455/njppp.2018.8.010180522018
Trujillo J, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J (2013) Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol 17:448–456. https://doi.org/10.1016/j.redox.2013.09.003
Uzunhisarcikli M, Aslanturk A, Kalender S, Apaydin FG, Bas H (2016) Mercuric chloride induced hepatotoxic and hematologic changes in rats: the protective effects of sodium selenite and vitamin E. Toxicol Ind Health 32:1651–1662. https://doi.org/10.1177/0748233715572561
Vandenberg LN, Chahoud I, Heinde JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G (2010) Urinary, circulating, and tissue biomonitoring studies ındicate widespread exposure to bisphenol A. Environ Health Perspect 1181:1055–1070. https://doi.org/10.1289/ehp.0901716
Wang ME, Chen YC, Chen IS, Hsieh SC, Chen SS, Chiu CH (2012) Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. J Nutr Biochem 23:1352–1366. https://doi.org/10.1016/j.jnutbio.2011.08.004
Wu HJ, Liu C, Duan WX, Xu SC, He MD, Chen CH, Wang Y, Zhou Z, Yu ZP, Zhang L, Chen Y (2013) Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res 752:57–67. https://doi.org/10.1016/j.mrgentox.2013.01.005
Xia W, Jiang Y, Li Y, Wan Y, Liu J, Ma Y, Mao Z, Chang H, Li G, Xu B, Chen X, Xu S (2014) Early-life exposure to bisphenol A induces liver injury in rats involvement of mitochondria-mediated apoptosis. PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0090443
Xie YL, Chu JG, Jian XM, Dong JZ, Wang LP, Li GX, Yang NB (2017) Curcumin attenuates lipopolysaccharide/D-galactosamine-induced acute liver injury by activating Nrf2 nuclear translocation and inhibiting NF-kB activation. Biomed Pharmacother 91:70–77. https://doi.org/10.1016/j.biopha.2017.04.070
Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M (2002) Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the ‘Enhanced OECD Test Guideline no. 407. Arch Toxicol 76:65–74. https://doi.org/10.1007/s00204-001-0319-1
Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J (2015) Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 47:1549–1558. https://doi.org/10.1007/s00726-015-1995-0
Yang W, Huang J, Xiao B, Liu Y, Zhu Y, Wang F, Sun S (2017) Taurine protects mouse spermatocytes from ionizing radiation-induced damage through activation of Nrf2/HO-1 signaling. Cell Physiol Biochem 44:1629–1639. https://doi.org/10.1159/000485762
Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM (2015) Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000-2014. Environ Sci Technol 49:11834–11839. https://doi.org/10.1021/acs.est.5b02135
Yıldız N, Barlas N (2013) Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum Exp Toxicol 32:675–686. https://doi.org/10.1177/0960327112464796
Youn JY, Park HY, Lee JW, Jung IO, Choi KH, Kim K, Cho KH (2002) Evaluation of the immune response following exposure of mice to bisphenol a: Induction of Thl cytokine and prolactin by BPA exposure in the mouse spleen cells. Arch Pharm Res 25:946–953
Yu C, Mei XT, Zheng YP, Xu DH (2014) Zn (II)–curcumin protects against hemorheological alterations, oxidative stress and liver injury in a rat model of acute alcoholism. Environ Toxicol Pharmacol 37:729–737. https://doi.org/10.1016/j.etap.2014.02.011
Zaid SSM, Othman S, Kassim NM (2018) Protective role of Ficus deltoidea against BPA-induced impairments of the follicular development, estrous cycle, gonadotropin and sex steroid hormones level of prepubertal rats. J Ovarian Res 11:1–9. https://doi.org/10.1186/s13048-018-0466-0
Acknowledgments
The authors are thankful to Prof. Dr. Yusuf KALENDER and Assoc. Prof. Dr. Fatma Gokce APAYDIN for their interest and support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uzunhisarcikli, M., Aslanturk, A. Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ Sci Pollut Res 26, 37242–37253 (2019). https://doi.org/10.1007/s11356-019-06615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06615-8