Abstract
Bisphenol A (BPA) is a chemical found in environmental xenoestrogen. In the present study, olive oil, curcumin, taurine, BPA, curcumin plus BPA, and taurine plus BPA were exposed to rats for 4 weeks via gavage. Content of malondialdehyde and activities of antioxidant enzymes (GPx, GST, SOD, CAT) and also histopathological and cytopathological changes of heart were studied. No significant changes in all studied parameters were seen between control, olive oil, curcumin, and taurine-treated groups. However, there were significant differences in levels of malondialdehyde and activities of antioxidant enzymes in BPA-exposed rats and some histo/cytopathological changes determined. In curcumin plus BPA-exposed and taurine plus BPA-exposed groups, we measured the preventive effects on some parameters but not exactly. As a result, curcumin and taurine significantly minimized BPA-induced cardiotoxicity in rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Xenoestrogens called as synthetic estrogens are a kind of chemicals in the environmental compounds which mimic the 17β-estradiol (E2) action in estrogen-dependent organs and tissues (Akingbemi et al. 2004). BPA [2,2-(4,4-dihydroxydiphenol) propane] is largely used in polycarbonate plastic construction, which is useful as containers for beverages and foods, also found as a component of dental sealants (Berger et al. 2016; Ortiz-Villanueva et al. 2017; Tarapore et al. 2017). Previous studies have reported that BPA caused several toxicities like testicular toxicity. Akingbemi et al. showed in their study that at low-dose treatment levels, BPA inhibits testicular steroidogenesis (Akingbemi et al. 2004). Berger et al. reported that in utero BPA exposure inhibits germ cell (Berger et al. 2016). BPA is an estrogen-disrupting chemical both in in vivo and in vitro studies (Richter et al. 2007).
We focused in this study to put in order oxidative stress, because it is imbalanced between antioxidants and oxidants in cells. It occurs when a high level of reactive oxygen species (ROS) is present in the cells. Normally, ROS are product of oxygen metabolism in cells. The cells scavenge the ROS by antioxidants like glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT). In addition, exogenous antioxidants have helped these activities such as vitamins and flavonoids (Apaydın et al. 2017). But, if there is an imbalance in this process, oxidative stress can be occurring and oxidative stress-related damages in vital organs may be eventuate in the body.
Curcumin and taurine have also been known as antioxidants. Curcumin is sourced from golden spice turmeric (Curcuma longa) which is a perennial plant from the Zingiberaceae. It has been reported that curcumins have anti-carcinogenic, antioxidant, anti-inflammatory, and antimicrobial activities (Soyalic et al. 2017). Curcumin has a large kind of activities in biological systems. Previous experimental studies have showed its potential as alterative activity against chemical-caused organ toxicity (Kim et al. 2018; Valokola et al. 2018). Taurine is involved in bile formation and it is a natural compound found in animal tissues (Sarkar et al. 2017). It has significant biological roles like membrane stabilization, modulation of calcium signaling, osmoregulation, and antioxidant activity (Sirdah 2015).
In this study. we aim to investigate the relevance of antioxidant properties of curcumin and taurine against BPA-induced cardiotoxicity by assessing some biochemical oxidative parameters and the pathological characteristics namely the transmission electron microscopy and light microscopy.
Materials and methods
Chemicals
Taurine (≥ 99% purity) and curcumin (from Curcuma longa (Turmeric)) were purchased from Sigma. Bisphenol A (≥ 99% purity) was provided by Sigma-Aldrich.
Animals and experimental design
Adult male albino rats (250–300 g) were purchased from GUDAM and were housed in cages, 12 h light/dark cycle, and relative humidity 40%. Water and food (pellet rat chow) were available ad libitum. Experimental studies were confirmed by University of Gazi Animal Ethics Committee (G.U.ET–14.075). After acclimatization (acclimatization period is 10 days), animals were divided into seven groups (n = 6) categorized as follows:
-
Group 1.
(control group): received distilled water (1.0 ml kg−1 bw daily)
-
Group 2.
(olive oil-treated group): received olive oil (1.0 ml kg−1 bw daily)
-
Group 3.
(Curcumin-treated group): received curcumin (100 mg kg−1 bw daily in olive oil)
-
Group 4.
(Taurine-treated group): received taurine (100 mg kg−1 bw daily in distilled water)
-
Group 5.
(BPA-treated group): received BPA (130 mg kg−1 bw daily in olive oil)
-
Group 6.
(Curcumin + BPA-treated group): received curcumin and BPA (100 mg kg−1 bw daily +130 mg kg−1 bw daily, respectively)
-
Group 7.
(Taurine + BPA-treated group): received taurine + BPA (100 mg kg−1 bw daily +130 mg kg−1 bw daily, respectively)
Both all solutions were administrated via gavage during 28 days. BPA and other solutions were freshly prepared. After the experimental treatment period, the animals were sacrificed by intracardiac blood discharge under the anesthesia with ketamine hydrochloride (alfamine 10%, 45 mg/kg, i.m.) and xysilazine hydrochloride (alfazyne 2%, 5 mg/kg, i.m.), heart tissues removed quickly.
Measurement of oxidative stress parameters
After dissection of rats, the samples of heart tissues were taken and quickly washed with buffer (sodium phosphate; pH 7.2). Then, they were frozen in liquid nitrogen and kept at − 80 °C. Homogenates of heart tissues were prepared via homogenization buffer at pH 7.4 for antioxidant enzymes or KCl solution for MDA by a homogenizer. We used prepared homogenates in the measurements of antioxidant enzymes and lipid peroxidation levels. We used a spectrophotometer (Shimadzu UV 1700, Kyoto, Japan) for detecting activities of antioxidant enzymes and MDA levels. Malondialdehyde (MDA) is a chemical which is the largest aldehyde that is resulted from lipid peroxidation (LPO) process in membranes of cells. Level of MDA was measured by the thiobarbituric acid (TBA) test which was described by Ohkawa et al.’s study (1979). TBA reacts with MDA and a colored complex forms as a result of this reaction. Absorbance was measured at 532 nm for detecting the MDA level, and the result is assigned as nmol/mg protein. Activity of SOD was determined by the Marklund and Marklund’s procedure which is specified in 1974 by gauging the auto-oxidation and illumination of pyrogallol for 3 min at 440 nm. The SOD activity is given as U/mg of protein. As a control, we used a blank without tissue homogenate for non-enzymatic oxidation of pyrogallol.
Before detecting of the CAT activity, we diluted the homogenates of heart tissues via Triton-X-100. The CAT activity was measured by Aebi’s study (1984) by determining the hydrolysis of hydrogen peroxide (H2O2) at 240 nm. After the necessary calculations, CAT activity is given as mmol/mg of protein. As a control, a blank without homogenate was used for enzymatic hydrolysis of peroxide.
GST activity was evaluated by measuring the formation of 1-chloro 2,4-dinitrobenzene (CDNB) and glutathione conjugate by Habig et al.’s procedure (1974). Absorbance increasing was detected at 340 nm. The activity of GST is assigned as μ/mg of protein. All evaluations were confirmed for non-enzymatic conjugation by CDNB and glutathione in phosphate buffer (pH 7.0).
We measured the activity of GPx enzyme by H2O2 as substrate by the experimental process defined by Paglia and Valentine’s study (1967). The reactions were assayed at 240 nm by measuring the oxidation rate of NADPH. GPx enzyme activity was assigned as nmol/mg of protein. As a control, a blank without homogenate was used for non-enzymatic oxidation of NADPH upon addition of H2O2.
Light microscopic study
For histopathologic study, we fixed the heart samples in formalin (10%). Then, they were dehydrated by passing in decreasing concentrations of ethyl alcohol. Sections were stained with hematoxylin and eosin (H&E) for routine histological investigations. After cleaning with xylene of heart samples, they were embedded in paraffin and sectioned using a microtome (5–7 μm). Ten slides were prepared from each heart tissues. Then, the slides were evaluated by a microscope (light microscope; Olympus BX51, Tokyo, Japan) and photographed (Olympus E-330, Olympus Optical Co., Ltd., Japan). All sections were evaluated for the degree of congestion, infiltration, degeneration, vacuolization, edema, and necrosis. Each hearth slides were examined and assigned for severity of changes using scores on a scale of none (−), mild (+), moderate (++), and severe (+++) damage (Table 1).
Electron microscopic study
For transmission electron microscopic (TEM) evaluations, primary fixation was performed in glutaraldehyde (3%) (Agar Sci. Ltd., Essex, England) in a buffer (sodium phosphate buffer; at pH 7.4) (Merck, Alfred Paluka Co., Turkey) at 4 °C for 3 h. Then, the samples were washed with the same buffer (sodium phosphate buffer; at pH 7.4) and post-fixed in osmium tetroxide (1%) (Agar Sci. Ltd., Essex, England) and sodium phosphate buffer (pH 7.4) at 4 °C for 1 h. Tissue samples were washed with the same buffer at 4 °C for 3 h, then they dehydrated in graded ethyl alcohol mixtures (Agar Sci. Ltd., Essex, England). We embedded the samples in Araldite (Agar Sci. Ltd., Essex, England), and thin sections were taken by an ultramicrotome (Leica EM UC6, Leica Co., Austria). Samples were stained with lead citrate and uranyl acetate (1%). The sections were evaluated and photographed by a TEM (JEOLJEM 1400), and photographs were taken digitally in TEM (Jeol Ltd., Japan) at 80 kV.
Statistical analyses
All the results were signified as mean ± S.E.M. The statistical significance was assessed via Tukey test and one-way analysis of variance (ANOVA) by SPSS version 20.0. If p values were less than 0.05, the results were regarded statistically significant.
Results
Evaluation of MDA level and enzyme activities (CAT, SOD, GST, GPx)
Oxidative stress level and antioxidants were examined to evaluate the action of subacute BPA treatment and to assay whether curcumin and taurine could improve BPA-induced effects.
Both MDA level and also CAT, GST, SOD, and GPx activities, there were no important changes between control group and olive oil-, curcumin-, and taurine-treated groups.
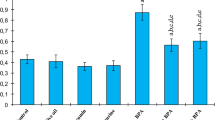
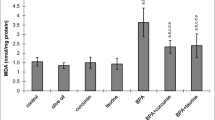
Lipid peroxidation investigation resulted in a statistically major increase of MDA level (an end product of lipid peroxidation) in all BPA-intoxicated groups compared to the control group. CAT, SOD, GST, and GPx activities in all BPA-intoxicated groups were significantly lower than control group. However, a statistically significant decrease in MDA level and significant increment in CAT, SOD, GST, and GPx activities were noted in BPA + curcumin and BPA + taurine groups compared to BPA group (p < 0.05, Figs. 1, 2, 3, 4, and 5).
Effects of curcumin (Curc), taurine (Tau), and bisphenol A (BPA) on GST activities (μmol/mg protein). Each bar represents mean ± SEM. Significance at P < 0.05. aComparison of control and treatment groups. bComparison of olive oil group and treatment groups. cComparison of curcumin group and treatment groups. dComparison of taurine group and treatment groups. eComparison of BPA group and treatment groups
Effects of curcumin (Curc), taurine (Tau), and bisphenol A (BPA) on SOD activities (U/mg protein). Each bar represents mean ± SEM. Significance at P < 0.05. aComparison of control and treatment groups. bComparison of olive oil group and treatment groups. cComparison of curcumin group and treatment groups. dComparison of taurine group and treatment groups. eComparison of BPA group and treatment groups
Effects of curcumin (Curc), taurine (Tau), and bisphenol A (BPA) on GPx activities (nmol/mg protein). Each bar represents mean ± SEM. Significance at P < 0.05. aComparison of control and treatment groups. bComparison of olive oil group and treatment groups. cComparison of curcumin group and treatment groups. dComparison of taurine group and treatment groups. eComparison of BPA group and treatment groups
Effects of curcumin (Curc), taurine (Tau), and bisphenol A (BPA) on CAT activities (mmol/mgprotein). Each bar represents mean ± SEM. Significance at P < 0.05. aComparison of control and treatment groups. bComparison of olive oil group and treatment groups. cComparison of curcumin group and treatment groups. dComparison of taurine group and treatment groups. eComparison of BPA group and treatment groups
Effects of curcumin (Curc), taurine (Tau) and bisphenol A (BPA) on MDA levels (nmol/mg protein). Each bar represents mean ± SEM. Significance at P < 0.05. aComparison of control and treatment groups. bComparison of olive oil group and treatment groups. cComparison of curcumin group and treatment groups. dComparison of taurine group and treatment groups. eComparison of BPA group and treatment groups
Histopathological alterations in heart tissues
The histological examination of the heart tissues of the control, olive oil-, curcumin-, and taurine-treated rats showed normal structure (Fig. 6a). There was congestion, infiltration, degeneration, vacuolization, and edema in myocardial fibers (Figs. 6b–d) in the BPA-treated group. There was infiltration (Fig. 6e) and necrosis shown in curcumin plus BPA-treated group. In taurine plus BPA-treated group, we demonstrated that infiltration (Fig. 6f).
a Control rats heart section were observed in normal structure × 400. b–d Heart sections of BPA-treated rats showing congestion (⬆), infiltration (✦), degeneration (⇉), vacuolization (↗), edema in connective tissue (✳) in cardiac muscle cells, × 400. e Heart sections of curcumin plus BPA-treated rats showing infiltration (✦) and necrosis (▲). f Taurine plus BPA-treated rats showing infiltration (✦), × 400
Ultrastructural alterations in myocardial cells
Myocardial cells of control, olive oil-, curcumin-, and taurine-treated group rats were observed in normal ultra-structure. Mitochondria were abundant in myocardial cells, and generally, they were located length widely between myofibrils. Sarcoplasmic reticulum was seen normal in the control group (Fig. 7a). The electron micrographs of myocardial cells of BPA-treated rats showed dilatation of sarcoplasmic reticulum, cytoplasmic edema, mitochondrial vacuolization, and swelling were shown (Figs. 7b, c). The electron micrographs of myocardial cells of curcumin plus BPA-treated rats showed mitochondrial vacuolization and swelling were shown (Fig. 7d). Similarly, the electron micrographs of myocardial cells of taurine plus BPA-treated rats showed mitochondrial vacuolization and swelling were shown (Fig. 7e).
a Electron micrograph of myocardial cell in control rat heart, N: Nucleus, M: Mitochondria, ▲: Myofibrils. b, c Electron micrographs of myocardial cells in BPA-treated rats, ➭: dilatation of sarcoplasmic reticulum, ✳: myeloid figure, M: swelling and vacuolization of mitochondria, ✦: cytoplasmic edema. d Electron micrograph of myocardial cell in curcumin plus BPA-treated groups, M: swelling of mitochondria. e Electron micrograph of myocardial cell in taurine plus BPA-treated groups, M: swelling of mitochondria were shown. × 8000
Discussion
The use of BPA-containing products is harmful to living organisms and also the ecosystem (Poormoosavi et al. 2018). The authors reported that BPA causes oxidative damage in different organs like brain, ovaries, and liver via different pathways (Eid et al. 2015; Berger et al. 2016; El Tabaa et al. 2017). BPA also accumule in the human body such as placenta and human hair (Tzatzarakis et al. 2015). In this study, we examined the effects of BPA exposure on heart tissues of experimental animals. Oral LD50 ratio of BPA in male rats is 3250 mg/kg body weight (Michalowicz 2014). It has been reported that various factors are correlated with cardiovascular diseases such as environmental products (Baş et al. 2014). In the present study, BPA was given at 1/25 of oral LD50 and no rat deaths were observed during the experimental study. Our results show that in heart tissue, BPA exposure decreases GPx, SOD, GST, and CAT activities in BPA, curcumin plus BPA, and taurine plus BPA-treated groups. It may be due to tissue degenerations caused by BPA. SOD and CAT activity reduction may be related to the being free radicals such as hydroxyl radicals, peroxyl radicals and superoxide (Quintana et al. 2018). The enzymes such as GPx, SOD, CAT, and GST play an important mission in preventing cells and tissues from oxidative damages (Baş et al. 2015). In this study, we show that depletion of all antioxidant enzymes may result in oxidative stress. CAT scavenges H2O2 which generates SOD or free radicals (Eraslan et al. 2007). Reduced CAT activity might be explained by reduced proportion of H2O2. GST and GPx are cytosolic enzymes which detoxify various xenobiotics (Djuric et al. 2015).
Our results also indicate that BPA and co-administration with BPA-treated groups increased MDA levels which is a lipid peroxidation end product. In addition, it caused decreased antioxidant enzymes in tissue homogenates. Also, Apaydın et al. (2016) and Kalender et al. (2015) reported enhanced LPO levels caused by many environmental toxicants. Increased MDA level might demonstrate cell membrane damage (Poormoosavi et al. 2018). Our results support this information by cytopathology and histopathologic evidences. Lipid peroxidation has been suggested that mechanisms related to xenobiotic toxicity (Baş et al. 2015).
Light microscopic findings very important to show cardiac cell damage (Baş and Kalender 2011; Valokola et al. 2018). We demonstrate that BPA caused distorted tissue and cell integrity. We showed that in BPA-treated group, serious damages in cells both light microscopic and electron microscopic were investigated. These pathological alterations in the heart could be due to enhanced LPO level and formation of ROS. The light microscopic results support the biochemical assay findings. From ultrastructural evidence of mitochondrial changes, cytoplasmic changes and nucleic changes were clearly identified in BPA-exposed animals.
There is a correlation between all the results in these studies. According to previous studies, natural compounds extracted from herbal products have important therapeutic and protective property against pathological situations (Kim et al. 2018). Electron microscope work is widely used to show the damage of chemical substances to the cells (Degirmenci et al. 2002, 2005). Many xenobiotic caused dysfunction and toxic effects on myocardial cells (Kalender et al. 2004). Cells have many different mechanisms to protect themselves from oxidative stress and to fix up damaged biomolecules in cells. Within the methods, the cells used to do this; non-enzymatic and enzymatic antioxidants scavenge ROS, such as CAT, SOD, or the glutathione peroxidase system, among others. We can say curcumin and taurine are non-enzymatic antioxidants.
Previous studies indicate that BPA cause cell and membrane damage related to oxidative stress (Eid et al. 2015; Apaydın et al. 2018). It also be may related to xenoestrogen properties of BPA. In conclusion, this study shows that preventive effects of taurine and curcumin possibly are due to their antioxidant properties on heart tissues. It has been known that taurine found in the heart is very high level, and it has been used for the treatment of cardiovascular disease because of strengthened cardiac contractility properties (Takatani et al. 2004; Zulli 2011). Curcumin has a wide spectrum of therapeutic properties (Strimpakos and Sharma 2008). The presence of phenolic groups in the structure of curcumin is basic in explaining its ability to scavenge oxygen-derived free radicals responsible for the peroxidation of cell lipids (Sreejayan and Rao 1994). The therapeutic effect of taurine as an antioxidant in biological system has been related to its ability to stabilize biomembranes and also eliminate ROS in animals (Agha et al. 2014). However, in this study, we have not shown completely protection except GPx activity. It may be related to their doses used in this study. However, we show fever histopathological changes in antioxidant supplementation groups than only BPA-treated group.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agha FE, Youness ER, Selim MHH (2014) Nephroprotective potential of selenium and taurine against mercuric chloride induced nephropathy in rats. Ren Fail 36:704–716. https://doi.org/10.3109/0886022X.2014.890012
Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP (2004) Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145(2):592–603. https://doi.org/10.1210/en.2003-1174
Apaydın FG, Bas H, Kalender S, Kalender Y (2016) Subacute effects of low dose lead nitrate and mercury chloride exposure on kidney of rats. Environ Toxicol Pharmacol 41:219–224. https://doi.org/10.1016/j.etap.2015.12.003
Apaydın FG, Bas H, Kalender S, Kalender Y (2017) Bendiocarb induced histopathological and biochemical alterations in rat liver and preventive role of vitamins C and E. Environ Toxicol Pharmacol 49:148–155. https://doi.org/10.1016/j.etap.2016.11.018
Apaydın FG, Uzunhisarcıklı M, Aslantürk A, Kalender S (2018) Bisphenol A-induced histopathological alterations on small intestine tissues of rats: the protective role of taurine and curcumin. Iğdır Univ J Inst Sci Tech 8:43–47. https://doi.org/10.21597/jist.427870
Baş H, Kalender Y (2011) Chlorpyrifos induced cardiotoxicity in rats and the protective role of quercetin and catechin. GU J Sci 24(3):387–395
Baş H, Kalender S, Apaydın FG (2014) Adverse effects of lead treatment: relationship of histopathological changes and protective role of sodium selenite on non-diabetic and diabetic rat hearts. GU J Sci 27(2):855–859
Baş H, Kalender S, Karaboduk H, Apaydın FG (2015) The effects on antioxidant enzyme systems in rat brain tissues of lead nitrate and mercury chloride. GU J Sci 28(2):169–174
Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA (2016) The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol 60:39–52. https://doi.org/10.1016/j.reprotox.2015.12.004
Degirmenci I, Kalender S, Ustuner MC, Kalender Y, Gunes HV, Unal H, Basaran A (2002) The effects of acarbose and Rumex patientia on liver ultrastructure in streptozotocin-induced diabetic (type II) rats. Drug Exp Clin Res 28(6):229–234
Degirmenci I, Ustuner MC, Kalender Y, Kalender S, Gunes HV (2005) The effects of acarbose and Rumex patientia L. on ultrastructural and biochemical changes of pancreatic B cells in streptozotocin-induced diabetic rats. J Ethnopharmacol 97:555–559. https://doi.org/10.1016/j.jep.2005.01.002
Djuric A, Begic A, Gobeljic B, Stanojevic I, Ninkovic M, Vojvodic D, Pantelic A, Zebic G, Prokic V, Dejanovic B, Stojanovic I, Pavlica M, Djukic D, Saso L, Djurdjevic D, Pavlovii M, Topic A, Vujanovic D, Stevnovic I, Djukic M (2015) Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem Toxicol 86:25–33. https://doi.org/10.1016/j.fct.2015.09.004
Eid JI, Eissa SM, El-Ghor AA (2015) Bispheol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspring. JOBAZ 71:10–19. https://doi.org/10.1016/j.jobaz.2015.01.006
El Tabaa MM, Sokkar SS, Ramadan ES, Abd El Salam IZ, Zaid A (2017) Neuroprotective role of Ginkgo biloba against cognitive deficits associated with bisphenol a exposure: an animal model study. Neurochem Int 108:199–212. https://doi.org/10.1016/j.neuint.2017.03.019
Eraslan G, Saygi S, Essiz D, Aksoy A, Gul H, Macit E (2007) Evaluation of aspect of some oxidative stress parameters using vitamin E, proanthocyanidin and nacetylcysteine against exposure to cyfluthrin in mice. Pestic Biochem Physiol 88:43–49. https://doi.org/10.1016/j.pestbp.2006.08.010
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Kalender S, Kalender Y, Ogutcu A, Uzunhisarcıklı M, Durak D, Acikgoz F (2004) Endosulfan-induced cardiotoxicity and free radical metabolism in rats: the protective effect of vitamin E. Toxicology 202:227–235. https://doi.org/10.1016/j.tox.2004.05.010
Kalender S, Apaydin FG, Baş H, Kalender Y (2015) Protective effects of sodium selenite on lead nitrate-induced hepatotoxicity in diabetic and non-diabetic rats. Environ Toxicol Pharmacol 40:568–574. https://doi.org/10.1016/j.etap.2015.08.011
Kim KS, Lim H, Lim JS, Son JY, Lee J, Lee BM, Chang SC, Kim HS (2018) Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem Toxicol 114:34–40. https://doi.org/10.1016/j.fct.2018.02.007
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Michalowicz J (2014) Bisphenol A—sources, toxicity and biotransformation. Environ Toxicol Pharmacol 27:738–758. https://doi.org/10.1016/j.etap.2014.02.003
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ortiz-Villanueva E, Navarro-Martín L, Jaumot J, Benavente F, Sanz-Nebot V, Pina B, Tauler R (2017) Metabolic disruption of zebrafish (Danio rerio) embryos by bisphenol A. An integrated metabolomics and transcriptomic approach. Environ Pollut 231:22–36. https://doi.org/10.1016/j.envpol.2017.07.095
Paglia DE, Valentine WN (1967) Studies on the quantative and qualitative characterization of erytrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Poormoosavi SM, Najafzadehvarzi H, Behmanesh MA, Amirgholami R (2018) Protective effects of Asparagus officinalis extract against bisphenol A induced toxicity in Wistar rats. Toxicol Rep 5:427–433. https://doi.org/10.1016/j.toxrep.2018.02.010
Quintana MM, Osimani VR, Magnarelli G, Rovedatti MG, Guiñazú N (2018) The insecticides chlorpyrifos and acetamiprid induce redox imbalance in umbilical cord blood erythrocytes in vitro. Pestic Biochem Physiol 148:87–92. https://doi.org/10.1016/j.pestbp.2018.04.001
Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenberg JG, Walser-Kuntz DR, vom Saal FS (2007) In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224. https://doi.org/10.1016/j.reprotox.2007.06.004
Sarkar P, Basak P, Ghosh S, Kundu M, Sil PC (2017) Prophylactic role of taurine and its derivatives against diabetes mellitus and its related complications. Food Chem Toxicol 110:109–121. https://doi.org/10.1016/j.fct.2017.10.022
Sirdah MM (2015) Protective and therapeutic effectiveness of taurine in diabetes mellitus: a rationale for antioxidant supplementation. Diabetol Metab Syndr 9:55–64. https://doi.org/10.1016/j.dsx.2014.05.001
Soyalic H, Gevrek F, Karaman S (2017) Curcumin protects against acoustic trauma in the rat cochlea. Int J Pediatr Otorhinolaryngol 99:100–106. https://doi.org/10.1016/j.ijporl.2017.05.029
Sreejayan N, Rao MNA (1994) Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 46:1013–1016. https://doi.org/10.1111/j.2042-7158.1994.tb03258.x
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545. https://doi.org/10.1089/ars.2007.1769
Takatani T, Takahashi K, Uozumi Y, Shikata E, Yamamoto Y, Ito T, Matsuda T, Schaffer SW, Fujio Y, Azuma J (2004) Taurine inhibits apoptosis by preventing formation of the Apaf-1/caspase-9 apoptosome. Am J Physiol Renal Physiol 287:C949–C953. https://doi.org/10.1152/ajpcell.00042.2004
Tarapore P, Hennesy M, Son D, Ying J, Outang B, Govindarajah V, Leung Y, Ho S (2017) High butter-fat diet and bisphenol A additively impair male rats spermatogenesis. Reprod Toxicol 68:191–199. https://doi.org/10.1016/j.reprotox.2016.09.008
Tzatzarakis MN, Vakonaki E, Kavvalakis MP, Barmpas M, Kokkinakis EN, Xenos K, Tsatsakis AM (2015) Biomonitoring of bisphenol A in hair of Greek population. Chemosphere 118:336–341. https://doi.org/10.1016/j.chemosphere.2014.10.044
Valokola MG, Karimi G, Razavi BM, Kianfar M, Jafarian AH, Jaafari MR, Imenshahidi M (2018) The protective activity of nanomicelle curcumin in bisphenol A-induced cardiotoxicity following subacute exposure in rats. Environ Toxicol 34:1–9. https://doi.org/10.1002/tox.22687
Zulli A (2011) Taurine in cardiovascular disease. Curr Opin Clin Nutr Metab Care 14:57–60. https://doi.org/10.1097/MCO.0b013e328340d863
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Experimental studies were confirmed by University of Gazi Animal Ethics Committee (G.U.ET–14.075).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Apaydin, F.G., Aslanturk, A., Uzunhisarcikli, M. et al. Histopathological and biochemical studies on the effect of curcumin and taurine against bisphenol A toxicity in male rats. Environ Sci Pollut Res 26, 12302–12310 (2019). https://doi.org/10.1007/s11356-019-04578-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04578-4