Abstract
Bisphenol A (BPA) received heightened attention in the recent years due to humans continuously being exposed to it. This study explores the effect of taurine or curcumin on subacute BPA treatment-induced nephrotoxicity in rats (Rattus norvegicus). Forty-two adult albino male rats were exposed to BPA (130 mg/kg daily) for 28 days by gastric gavage. BPA led to lipid peroxidation, inhibiting antioxidant enzyme activities like catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GST). BPA exposure also induced histopathological changes like tubular and glomerular degeneration, vascular congestion, and interstitial cell infiltration in kidney tissue. Cotreatment with taurine (100 mg/kg daily) or curcumin (100 mg/kg daily) alleviated the lipid peroxidation level and antioxidant enzyme activities and histological alterations brought about by BPA. In this study, curcumin and taurine application provided protection against renal toxicity caused by BPA but did not prevent toxic effect completely.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is one of the highest capacity chemicals manufactured worldwide. BPA is widely used as a cross-linking chemical in the manufacture of plastic polycarbonates and resin epoxy. It is used in many common consumer products including polycarbonate plastics such as dental sealants, food and drink packaging materials, linings for metal boxes, polyvinyl chloride, toys, baby bottles, thermal paper, water pipes, pharmaceuticals, compact disks, and medical materials (Nam et al. 2010; Huang et al. 2012; Flint et al. 2012). It is known as a kind of endocrine disrupting chemicals with estrogenic activity, and it is present virtually everywhere in our lives (Vandenberg et al. 2009).
BPA is delivered into the environment through waste water–treatment residue, via hydrolysis from plastics and the spontaneously degradation of polycarbonate plastics exposed to acidic or alkaline heat conditions. People can be exposed to BPA through food and beverages. The majority of daily human exposure can result from oral route (Santamaria et al. 2016). However, because BPA is rapidly metabolized, human exposure to BPA might be continuous via various sources, like integumentary and respiratory systems, not only limited for gastrointestinal system (Stahlhut et al. 2009).
Studies have shown the relationships between BPA exposure and carcinogenesis, cardiovascular disease, obesity, hepatotoxicity, functionally impared endocrine system, and female and male reproductive system (Alonso-Magdalena et al. 2006; Zhou et al. 2008; Wang et al. 2012; Hassan et al. 2012; Rochester 2013; Helmestam et al. 2014; Jiang et al. 2016). Besides, BPA causes oxidative stress in liver and kidney (Mourad and Khadrawy 2012). Oxidative stress can have extremely harmful consequences on biological systems (Sorg 2004). Many studies designed to appraise the positive protective effect of various natural and synthetic materials by the antioxidant properties on the BPA-induced toxicity (Aydogan et al. 2010; Li et al. 2014; Popa et al. 2014). Antioxidant therapy may be important to reduce the toxicity caused by BPA (Tamilsevan et al. 2013).
Taurine is an antioxidant, which has been attributed to its ability to inhibit of lipid peroxidation by scavenging reactive oxygen species (ROS) (Agha et al. 2014). Moreover, ıt has substantial physiological functions such as osmoregulation, setting the cytoplasmic and mitochondrial calcium homeostasis, and xenobiotic conjugation (Huxtable 1992). Taurine is (2-aminoethanesulfonic acid) a substantial intracellular amino acid, naturally found in the mammalian tissues (Chesney 1985). Taurine can be found in chicken and turkey meat, beef, processed meat like salami, seafood like tuna fish, shrimp, oystre, mussel, ice cream cow’s milk, and low-fat yogurt. Taurine also can be found in most energy drinks (300, 350 and 400 mg/100 ml). Food supplements contain 750, 800, 900, 1000, and 2000 mg taurine (Granum et al. 2018).
However, it is considered essential for normal development and growth in human infants and therefore is typically added to infant formula (Laidlaw et al. 1990; Wójcik et al. 2010; Rath 2012). Taurine (50, 100,200 mg/kg) in a dose-dependent manner has a preventive effect against acrylamide-caused oxidative stress by increasing antioxidant defense mechanism in rats. However, taurine demonstrated protective effect against the acrylamide-induced histopathological changes in tissues (İnce et al. 2018).
Curcumin is the main curcuminoid of the turmeric rhizome (Curcuma longa L.). Turmeric contains 1.5 and 3% curcumin. It is responsible for the yellow color of turmeric, and it is usually used as a food additive, spice, and colorant (Hanif et al. 1997; Yang et al. 2020). Curcumin possesses antioxidant, anti-inflammatory, anticancer, antiangiogenesis, chemopreventive, and chemotherapeutic properties (Strimpakos and Sharma 2008). Curcumin has been shown to be a potent antioxidant by scavenging of various important ROS, like nitrogen dioxide radical, hydroxyl radical, and superoxide anion (Jijón et al. 2011). Curcumin ameliorated increased malondialdehyde (MDA) level and reduced antioxidant enzyme activities and biochemical and histopathological alterations the caused by tartrazine in liver of rats. (El Desoky et al. 2017). In another study, supplement of curcumin and taurine alone or in combination showed a preservative effect against experimental hepatocarcinogenesis, which may be due to the anticancer activity of curcumin and the antineoplastic effect of taurine (El-Houseini et al. 2016).
The consumption of foods containing antioxidants can help reduce the negative effects of toxic substances. Previous studies have shown that several antioxidants such as cinnamon and alpha tocopherol alleviate BPA-caused oxidative stress and histopathological alterations in liver, testis, kidney, and ovarian tissues (Morgan et al. 2014; Avci et al. 2016). However, in the article screening, no study on the protective actions of curcumin or taurine on BPA-caused possible kidney injury in rats has been found. In our study, the protective potency of taurine or curcumin supplementation against BPA-caused nephrotoxicity was investigated in adult male rats. Blood urea nitrogen, uric acid, and creatinine levels were assayed to evaluate the renal functions in serum of BPA-, BPA + curcumin-, and BPA + taurine-treated rats. However, MDA levels and antioxidant enzyme activities like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S transferase (GST) were measured to determine oxidative stress. However, histopathological alterations were investigated in kidney tissues of BPA-, BPA + curcumin-, and BPA + taurine-treated rats.
Materials and methods
Chemicals, animals, experimental design, and tissue sampling
Taurine (≥ 99% purity), curcumin (from Curcuma longa (Turmeric)), and bisphenol A (≥ 99% purity) were provided from Sigma-Aldrich.
Forty-two adult albino male Wistar rats (Rattus norvegicus) (250–300 g) were acquired from the Gazi University Laboratory Animals Growing and Experimental Research Center. All the experimental animals were kept in an air-conditioned circumference (22 ± 3 °C, 12 h light/12 h dark period) and given the standard rat food and uncontaminated drinking water. Gazi University Committee on the Ethics of Animal Experimentation confirmed experimental procedures (G.U. ET - 14.075).
The doses of BPA, curcumin, and taurine were chosen taking into consideration previous experimental studies (Wu et al. 2013; Yıldız and Barlas 2013; Aly and Khafagy 2014; Lonare et al. 2014; Sangai and Verma 2014).
Seven experimental groups were haphazard formed from the rats, each group containing six animals.
-
Control group: It was treated distilled water (1.0 ml/kg bw daily).
-
Olive oil group: It was treated with olive oil (1.0 ml/kg bw daily).
-
Curcumin group: It was administered curcumin (100 mg/kg bw daily in olive oil).
-
Taurine group: It was treated with taurine (100 mg/kg bw daily in distilled water).
-
BPA group: It received bisphenol A (130 mg/kg bw daily in olive oil).
-
BPA + curcumin group: It was administered both bisphenol A and curcumin (130 mg /kg bw daily + 100 mg/kg bw daily, respectively).
-
BPA + taurine group: It was treated with taurine bisphenol A + taurine (130 mg/kg bw daily + 100 mg/kg bw daily, respectively).
The abovementioned chemicals were treated orally to non-fasted adult rats in the morning (between 09:00 h and 10:00 h) for 28 days. We performed 28 days of application to test the subacute toxicity of BPA.
Animals were sacrificed under anesthesia after the termination of exposure period. The kidney tissues were isolated from other tissues and removed immediately for microscope examinations and oxidative stress assessments and weighed by using automatic balance. The kidney tissues were homogenized using Heidolph Silent Crusher M homogenizer. The supernatants were obtained by centrifuging tissue homogenates. The obtained supernatants were utilized for the evaluation of the lipid peroxidation level and activities of antioxidant enzymes. Furthermore, the tissues were fixed in %10 formaldehyde for light microscopic investigations.
Assessment of MDA level
The malondialdehyde (MDA) level was determined by measuring thiobarbituric acid reactive species (TBARS) as recommended by Ohkawa et al. (1979). TBARS content was determined using Shimadzu UV 1700 spectrophotometer (Kyoto, Japan) at 532 nm. MDA level was presented as nmol/mg protein.
Estimation of antioxidant enzyme activities
The renal SOD activity was determined with Marklund and Marklund (1974) method by testing the autooxidation and illumination of pyrogallol for 180 s at 440 nm. One unit SOD activity was calculated as the amount of protein that induced 50% pyrogallol autooxidation inhibition. SOD activity was presented as U/mg protein.
For evaluation of renal CAT activity, tissue samples were diluted with Triton X-100. CAT activity was performed with the procedure defined by Aebi (1984) by testing the hydrolysis of H2O2 and the resulting decline in absorbance at 240 nm for 180 s. The activity of CAT was expressed as mmol/mg protein.
The renal GPx activity was measured using H2O2 as substrate (Paglia and Valentine 1967). The reaction was monitored indirectly as the oxidation rate of NADPH at 240 nm for 180 s. The enzyme activity was presented as nmol/mg protein.
The renal GST activity was estimated by measuring the formation of the glutathione and 1-chloro-2, 4-dinitrobenzene conjugate by the method described by Habig et al. (1974). Renal GST enzyme activity was presented as μmol/mg protein.
The protein levels of the kidney homogenates were analyzed by colorimetric method as proposed by Lowry et al. (1951) using BSA as standard.
Microscopic appraisal
The fixed kidney tissue samples in %10 formaldehyde were embedded in paraffin. A total of 5–7 μm thickness sections were obtained from paraffin blocks. The tissue sections were stained with H&E. All the preparations were examined and photographed with a digital camera (Olympus BX-51) attached to a microscope (Olympus E-330).
Statistical analysis
Statistical evaluation was conducted by SPSS 11.5. Differences among the groups were appraised using one-way ANOVA, followed by Tukey’s procedure for multiple comparisons. The all data were presented as means ± SD. The obtained p < 0.05 was accepted statistically important.
Results
Mortality and behavioral alterations were not observed in rats during the 28-day period.
Determination of kidney weights
At the end of 28 days experimental time, statistically significant differences were not found among the control and olive oil-, curcumin-, taurine-, BPA-, BPA + curcumin-, and BPA + taurine -treated rats in point of left and right absolute and relative kidney weights (p < 0.05) (Table 1).
Evaluation of oxidative stress parameters
The MDA level and antioxidant enzyme activities were measured to investigate the toxic effect of the 28-day BPA exposure and to evaluate whether curcumin and taurine could decline the BPA-caused toxicity.
There were no statistically significant alterations between the control rats and olive oil-, curcumin-, and taurine-treated rats for MDA level and CAT, SOD, GST, and GPx activities.
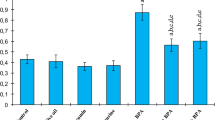
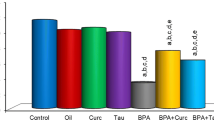
An important enhancement in MDA level (an end product lipid peroxidation) was detected when BPA-treated rats were compared with the control rats. CAT, SOD, GST, and GPx activities in all the BPA-treated rats were importantly lower than the control rats. Also, there was a statistically significant enhancement in SOD, CAT, GST, and GPx activities and a statistically important decline in MDA level when BPA + curcumin and BPA + taurine rats were compared with BPA group. MDA levels of BPA + curcumin and BPA + taurine rats were statistically higher than from all other rats except BPA-treated rats. CAT, SOD, GST, and GPx activities in BPA + curcumin and BPA + taurine rats were significantly lower than from all other rats except BPA-treated (p < 0.05, Figs. 1, 2, 3, 4, and 5).
Effects of 28 days BPA treatment and curcumin or taurine on MDA levels in the kidney tissues of rats. Values are means ± S.D. for six rats in each group. Significance at p < 0.05. (a) Comparison of control group and other groups, (b) comparison of olive oil group and other groups, (c) comparison of curcumin group and other groups, (d) comparison of taurine group and other groups, (e) comparison of BPA group and other groups
Effects of 28 days BPA treatment and curcumin or taurine on CAT activities in the kidney tissues of rats. Values are means ± S.D. for six rats in each group. Significance at p < 0.05. (a) Comparison of control group and other groups, (b) cmparison of olive oil group and other groups, (c) comparison of curcumin group and other groups, (d) comparison of taurine group and other groups, (e) comparison of BPA group and other groups
Effects of 28 days BPA treatment and curcumin or taurine on SOD activities in the kidney tissues of rats. Values are means ± S.D. for six rats in each group. Significance at p < 0.05. (a) Comparison of control group and other groups, (b) comparison of olive oil group and other groups, (c) comparison of curcumin group and other groups, (d) comparison of taurine group and other groups, (e) comparison of BPA group and other groups
Effects of 28 days BPA treatment and curcumin or taurine on GST activities in the kidney tissues of rats. Values are means ± S.D. for six rats in each group. Significance at p < 0.05. (a) Comparison of control group and other groups, (b) comparison of olive oil group and other groups, (c) comparison of curcumin group and other groups, (d) comparison of taurine group and other groups, (e) comparison of BPA group and other groups
Effects of 28 days BPA treatment and curcumin or taurine on GPx activities in the kidney tissues of rats. Values are means ± S.D. for six rats in each group. Significance at p < 0.05. (a) Comparison of control group and other groups, (b) comparison of olive oil group and other groups, (c) comparison of curcumin group and other groups, (d) comparison of taurine group and other groups, (e) comparison of BPA group and other groups
Histological findings
The microscopic examinations of the kidney tissues of the control, olive oil-, curcumin-, and taurine-treated rats indicated normal arrangement of cells, with no histological alterations in the kidney tissues of the four groups (Fig. 6a).
a Kidney section of control rats: proximal tubules (P), distal tubules (D), glomerulus (G), b–e Kidney section of BPA rats: tubular degeneration (→), glomerular degeneration (*), vascular congestion (⇇), interstitial cell infiltration (◀). f Kidney section of BPA + curcumin rats. g Kidney section of BPA + taurine rats, × 200, H&E
The microscopic examinations of the kidney tissues of the BPA-exposed animals showed that BPA induced tubular and glomerular degeneration, vascular congestion, and interstitial cell infiltration (Fig. 6b–e).
In the BPA + curcumin and BPA + taurine groups, tubular degeneration was occurred, and also, in the BPA + taurine group, vascular congestion was observed (Fig. 6f, g).
Discussion
BPA is an endocrine disruptor, and many studies have reported that BPA has adverse effects on the reproductive system (Othman et al. 2016; Avci et al. 2016). Also, BPA causes disrupt effects in several organs except reproductive system (Hassan et al. 2012; Popa et al. 2014). Besides, exposure to BPA can induce oxidative damage in the tissues by enhancing free radical production owing to disrupting the redox status of BPA (Kabuto et al. 2003; Obata and Kubota 2000). It was reported that BPA generated many of ROS, which induce oxidative tissue damage (Avci et al. 2016). Enhanced MDA level is commonly known as a marker of lipid peroxidation (Sangai and Verma 2014; Pandır 2015; Pandır 2016). In our study, the enhancement in renal oxidative stress was indicated by a marked elevation in the MDA levels and a decline in the activities of the enzymatic antioxidants CAT, SOD, GST, and GPx in BPA-treated rats. BPA induced enhanced MDA level in different tissues, like brain, testes, ovarian, and liver tissues (Korkmaz et al. 2010; Jain et al. 2011; Othman et al. 2016; Avci et al. 2016).The increased level of MDA might be a concequence of elevated formation of ROS induced by BPA. Besides, the rising of renal MDA level could be as a consequence of the significant inhibition in enzymatic antioxidant activities. There is a dynamic equilibrium between the amount of free-radicals produced in the body and endogenous antioxidant defense system such as CAT, SOD, GST, and GPx under normal cellular conditions. Cells and tissues are protected from free-radical damage with endogenous antioxidant defense system (Selvakumar et al. 2011; İlce et al. 2019). Earlier investigations have shown that BPA treatment led to depletion in CAT, SOD, GST, and GPx enzyme activities in various tissues (Hassan et al. 2012; El-Beshbishy et al. 2012; Tamilsevan et al. 2013; Sangai and Verma 2014). Our findings corroborate with their findings. SOD protects tissues and cells from oxidative damage by catalyzing the superoxide radicals to turn into hydrogen peroxide (Fridovich 1997). CAT and GPx convert hydrogen peroxide to water (El-Demerdash 2011). Major function of GST catalyzes the conjugation of glutathione with some toxic substances. A decrement activity of SOD leads to enhancing the level of superoxide radicals, in this way contributing to increased oxidative stress. Moreover, a reduction in GPx and CAT activities results in the increased H2O2 concentration; thus, it contributes to the enhancement of oxidative stress. A decline in GST activity leads to an increase in the formation of ROS (El-Beshbishy et al. 2012). In our study, the inhibition of the activities of CAT, SOD, GST, and GPx enzymes might be owing to excessive increase ROS formation due to BPA exposure.
Antioxidant supplementation is known to mitigate ROS-caused detriments. There were many studies on the use of antioxidants against oxidative damage caused by BPA (Anjum et al. 2011; Othman et al. 2016). Curcumin and taurine have ability to scavenge ROS and diminish lipid peroxidation. Thus, taurine and curcumin preserve membrane wholeness (Kandemir et al. 2011; Agha et al. 2014). Previous investigations showed that curcumin and taurine provide protection against the harmful effects of several chemical compounds (Gürer et al. 2001; Ahmad et al. 2013; Zhang et al. 2014; Abdel-Moneim et al. 2015; Desai et al. 2015). In our study, the concurrent supplementation of curcumin or taurine to the BPA-trated rats lead to a decrease in the MDA levels and an increase antioxidant enzyme activities of the kidney tissues and histopathologic alterations. The therapeutic effect could be clarified restricting lipid peroxidation by scavenging ROS by the role of curcumin and taurine and thus preserving the degenerated cell membrane wholeness due to lipid peroxidation. Besides, the enhancement in SOD, CAT, GPx, and GST activities caused by taurine and curcumin might be due to increased activation of Nrf2 signaling pathway, which is associated with the expression of these enzymes, by curcumin and taurine. However, this study found that BPA + curcumin- and BPA + taurine-treated groups had high MDA levels and low antioxidant enzyme activities compared with non-BPA-treated groups. This result means that concomitant administration of curcumin and taurine with BPA reduces lipid peroxidation and increases antioxidant enzyme levels but cannot completely prevent oxidative damage. It was correlated with histopathological findings.
The evaluation of organ weights in toxicologic studies may be one of the indicators of general health condition and changes in the organ weights (Uzunhisarcikli and Aslanturk 2019). In the present study, there were no alterations absolute and relative kidney weights. Our results are compatible with the results of earlier studies (Yıldız and Barlas 2013). These results may result from dose and/or administration period.
BPA caused histopathological changes in several tissues (Yıldız and Barlas 2013; Popa et al. 2014; Kalb et al. 2016). In our study, the administration of BPA provoked histopathological alterations like glomerular and tubular degenerations, vascular congestion, and cell infiltration in the kidney tissues. The histologic alterations might be because of the generation of free radicals formed by BPA. These radicals disrupt the wholeness and permeability of cell and organelle membranes (Stajn et al. 1997; Lin et al. 2013). It may be suggested that BPA may function as a membrane labilizer (Abdel-Wahab 2014). Histopathological alterations caused by BPA were slighter in the cotreated curcumin or taurine with BPA groups.
The result of this study showed that BPA caused kidney damage due to oxidative stress. Increased MDA level and decreased endogenous SOD, CAT, GST, and GPX enzyme activities show that BPA leads to the oxidant/antioxidant imbalance and induces ROS formation and caused oxidative stress. Supplementation of curcumin or taurine significantly can reversed the increased lipid peroxidation and decreased antioxidant enzyme activities and histopathological abnormalities caused by 28 days oral administration of BPA in the kidney tissue of male rats but not precisely eliminate. The protective role of curcumin and taurine may be associated with their antioxidant roles and their capability to behave as scavengers for ROS. Consequently, the curcumin or taurine could be proposed as potential nephro-preventive agents.
All experimental procedures were confirmed by University of Gazi Animal Ethics Committee (G.U.ET–14.075).
References
Abdel-Moneim AM, El-Toweissy MY, Ali AM, Awad Allah AAM, Darwish HS, Sadek IA (2015) Curcumin ameliorates lead (Pb2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168:206–220. https://doi.org/10.1371/journal.pone.0144509
Abdel-Wahab WM (2014) Thymoquinone attenuates toxicity and oxidative stres induced by bisphenol A in liver of male rats. PJBS 17:1152–1160. https://doi.org/10.3923/pjbs.2014.1152.1160
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agha FE, Youness ER, Selim MHH, Ahmed HH (2014) Nephroprotective potential of selenium and taurine against mercuric chloride induced nephropathy in rats. Ren Fail 36:704–716. https://doi.org/10.3109/0886022X.2014.890012
Ahmad MK, Khan AA, Mahmood R (2013) Taurine ameliorates potassium bromate-induced kidney damage in rats. Amino Acids 45:1109–1121. https://doi.org/10.1007/s00726-013-1563-4
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A (2006) The estrogenic effect of bisphenol A disrupts pancreatic β cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112. https://doi.org/10.1289/ehp.8451
Aly HAA, Khafagy RM (2014) Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem Toxicol 64:1–9. https://doi.org/10.1016/j.fct.2013.11.007
Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H, Ansari RAA, Raisuddin S (2011) Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol 49:2849–2854. https://doi.org/10.1016/j.fct.2011.07.062
Avci B, Bahadir A, Tuncel OK, Bilgici B (2016) Influence of α-tocopherol and α-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicol Ind Health 32:1381–1390. https://doi.org/10.1177/0748233714563433
Aydogan M, Korkmaz A, Barlas N, Kolankaya D (2010) Pro-oxidant effect of vitamin C coadministration with bisphenol A, nonylphenol, and octylphenol on the reproductive tract of male rats. Drug Chem Toxicol 33:193–203. https://doi.org/10.3109/01480540903286468
Chesney RW (1985) Taurine: its biological role and clinical implications. Adv Pediatr Infect Dis 32:1–42
Desai KR, Rajput DK, Patel PB, Highland HN (2015) Ameliorative effects of curcumin on artesunate-induced subchronic toxicity in testis of swiss albino male mice. Dose-Response: Int J: April–June:1–9. https://doi.org/10.1177/1559325815592393
El Desoky GE, Ghaffari AA, Al-Othman AZ, Habıla MA, Al-Sheikh YA, Ghneim HK, Giesy JP, Abdoul Soud MAM (2017) Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur Rev Med Pharmacol Sci 21:35–645
El-Beshbishy HA, Aly HAA, El-Shafey M (2012) Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health 29:875–887. https://doi.org/10.1177/0748233712446728
El-Demerdash FM (2011) Oxidative stress and hepatotoxicity induced by synthetic pyrethroids organophosphate insecticides mixture in rat. J Environ Sci Health A 29:145–158. https://doi.org/10.1080/10590501.2011.577679
El-Houseini ME, El-Agoza IA, Sakr MM, El-Malky GM (2016) Novel protective role of curcumin and taurine combination against experimental hepatocarcinogenesis. Exp Ther Med 2:29–36. https://doi.org/10.3892/etm.2016.3952
Flint S, Markle T, Thomson S, Wallace E (2012) Bisphenol A exposure effects and policy; a wild life perspective. J Environ Manag 104:19–34. https://doi.org/10.1016/j.jenvman.2012.03.021
Fridovich I (1997) Superoxide anion radical (O・−), superoxide dismutases and related matters. J BiolChem 272:18515–18517
Granum B, Bruzell EM, Hetland BR, Rohloff THJ, Steffensen IL (2018) Risk assessment of "other substances" –taurine. Eur J Nutr Food Safe 8(4):170–173. https://doi.org/10.9734/EJNFS/2018/42541
Gürer H, Özgünes H, Saygin E, Ercal N (2001) Antioxidant effect of taurine against lead-induced oxidative stress. Arch Environ Contam Toxicol 41:397–402. https://doi.org/10.1007/s002440010265
Habig WH, Pabst MJ, Jokoby WB (1974) Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hanif R, Qiao L, Shiff SJ, Rigas B (1997) Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med 130:576–584. https://doi.org/10.1016/S0022-2143(97)90107-4
Hassan ZK, Elobeid MA, Virk P, Omer SA, El Amin M, Daghestani MH, Al Olayan EM (2012) Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxidative Med Cell Longev 2012:1–6. https://doi.org/10.1155/2012/194829
Helmestam M, Davey E, Stavreus-Evers A, Olovsson M (2014) Bisphenol A affects human endometrial endothelial cell angiogenicactivity in vitro. Reprod Toxicol 46:69–76. https://doi.org/10.1016/j.reprotox.2014.03.002
Huang Y, Wong C, Zheng J, Bouwman H, Barra R, Wahlstrom B, Neretin L, Hong M (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels and potential human impacts. Environ Int 42:91–99. https://doi.org/10.1016/j.envint.2011.04.010
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
İlce F, Pandır D, Gök G (2019) Acute effects of LPS on kidney of rats and preventive role of vitamin E and sodium selenite. Hum Exp Toxicol 38(5):47–560. https://doi.org/10.1177/0960327118817106
İnce S, Acaröz U, Acaröz DA, Varol N, Gürler Z, Küçükkurt İ, Demirel HH, Eryavuz A (2018) Protective effect of taurine against acrylamide-induced oxidative stress in rats. Kocatepe Vet J 11(4):479–490
Jain S, Kumar CHM, Suranagi UD, Mediratta PK (2011) Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem Toxicol 49:1404–1409. https://doi.org/10.1016/j.fct.2011.03.032
Jiang X, Chen HQ, Cui ZH, Yin L, Zhang WL, Liu WB, Han F, Ao L, Cao J, Liu JY (2016) Low-dose and combined effects of oral exposure to bisphenol A and diethylstilbestrol on the male reproductive system in adult Sprague-Dawley rats. ETAP 43:94–102. https://doi.org/10.1016/j.etap.2016.02.014
Jijón EM, Tapia E, Zazueta C, El Hafidi M, Zatarain-Barrón ZL, Hernández-Pando R, Medina-Campos ON, Zarco-Márquez G, Torres I, Pedraza-Chaverri J (2011) Curcumin prevents Cr (VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radical Bio Med 51:1543–1557. https://doi.org/10.1016/j.freeradbiomed.2011.07.018
Kabuto H, Hasuike S, Minagawa N, Shishibori T (2003) Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res 93:31–35. https://doi.org/10.1016/S0013-9351(03)00062-8
Kalb AC, Kalb AL, Cardoso TF, Fernandes CG, Corcini CD, Junior ASV, Martinez PE (2016) Maternal transfer of bisphenol a during nursing causes sperm impairment in male offspring. Arch Environ Contam Toxicol 70:793–801. https://doi.org/10.1007/s00244-015-0199-7
Kandemir FM, Benzer F, Yildirim NC, Ozdemir N (2011) Compensatory effects of curcumin on cisplatin-induced toxicity in rabbit testis. J Med Plant Res 5:456–461
Korkmaz A, Aydogan Ahbab M, Kolankaya D, Barlas N (2010) Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem Toxicol 48:2865–2871. https://doi.org/10.1016/j.fct.2010.07.019
Laidlaw SA, Grosvenor M, Kopple JD (1990) The taurine content of common foodstuffs. J Parenter Enter Nutr 14(2):183–188
Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R, Wu J, Geng S, Zhong C (2014) Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res 28:1553–1560. https://doi.org/10.1002/ptr.5167
Lin Y, Sun X, Qiu L, Wei J, Huang Q, Fang C, Ye T, Kang M, Shen H, Dong S (2013) Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis 206:1–10. https://doi.org/10.1038/cddis.2012.206
Lonare M, Kumar M, Raut S, More A, Doltade S, Badgujar P, Telang A (2014) Evaluation of imidacloprid-induced neurotoxicity in male rats: a protective effect of curcumin. Neurochem Int 78:122–129. https://doi.org/10.1016/j.neuint.2014.09.004
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 19:265
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. EJB 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Morgan AM, El-Ballal SS, El-Bialyc BE, EL-Borai NB (2014) Studies on the potential protective effect of cinnamon against bisphenol A- and octylphenol-induced oxidative stress in male albino rats. Toxicol Rep 1:92–101. https://doi.org/10.1016/j.toxrep.2014.04.003
Mourad IM, Khadrawy YA (2012) The sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of bisphenol A. Int J Life Sci Pharma Res 2:19–28
Nam SH, Seo YM, Kim MG (2010) Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 79:949–952. https://doi.org/10.1016/j.chemosphere.2010.02.049
Obata T, Kubota S (2000) Formation of hydroxyl radicals by environmental estrogen-like chemicals in rat striatum. Neurosci Lett 296:41–44. https://doi.org/10.1016/s0304-3940(00)01619-0
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Othman AI, Edrees GM, El-Missiry MA, Ali DAA, Aboel-Nour M, Dabdoub BR (2016) Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health 32:1537–1549. https://doi.org/10.1177/0748233714561286
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J Lab Clin Med 70:158–165
Pandır D (2015) Assesment of the DNA damage in human sperm and lymphocytes exposed to the carcinogen food contaminant furan with comet assay. Brazilian Arch Biol Tech 58(5):773–780. https://doi.org/10.1590/S1516-9132015050269
Pandır D (2016) DNA damage in human germ cell exposed to the some food additives in vitro. Cytotechnology 68(4):725–733. https://doi.org/10.1007/s10616-014-9824-y
Popa DS, Bolfa P, Kiss B, Vlase L, Paltinean R, Pop A, Cato C, Crisan G, Loghin F (2014) Influence of Genista tinctoria L or methylparaben on subchronic toxicity of bisphenol A in rats. Biomed Environ Sci27:85–96.https://doi.org/10.3967/bes2014.021
Rath M (2012) Energy drinks: what is all the hype? The dangers of energydrink consumption. J Am Acad Nurse Pract 24(2):70–76. https://doi.org/10.1111/j.1745-7599.2011.00689.x.Epub
Rochester JR (2013) Bisphenol A and human hesalth: a review of the literature. Reprod Toxicol 42:132–155. https://doi.org/10.1016/j.reprotox.2013.08.008
Sangai NP, Verma RJ (2014) Testing the efficacy of quercetin in mitigating bisphenol A toxicity in liver and kidney of mice. Toxicol Ind Health 30:581–597. https://doi.org/10.1177/0748233712457438
Santamaria C, Durando M, Muñoz de Toro M, Luque EH, Rodriguez HA (2016) Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. J Steroid Biochem 158:220–230. https://doi.org/10.1016/j.jsbmb.2015.11.016
Selvakumar S, Karrunakaran CM, Rao MRK, Balasubramanian MP (2011) Inhibitory effects of Indıgofera aspalathoides on 20-methylcholanthrene-induced chemical carcinogenesis in rats. J Carcinog 10:1. https://doi.org/10.4103/1477-3163.75458
Sorg O (2004) Oxidative stress: a theoretical model or a biological reality? C R Biol 327:649–662. https://doi.org/10.1016/j.crvi.2004.05.007
Stahlhut RW, Welshons WV, Swan SH (2009) Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure or both. Environ Health Perspect 117:784–789. https://doi.org/10.1289/ehp.0800376
Stajn A, Ziki RV, Ognjanovic B, Pavlovic SZ, Kostic MM, Petrovic VM (1997) Effect of cadmium and selenium on the antioxidant defence system in rat kidneys. Comp Biochem Physiol 2:167–172. https://doi.org/10.1016/S0742-8413(97)00063-7
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545. https://doi.org/10.1089/ars.2007.1769
Tamilsevan P, Bharathiraja K, Vijayaprakash S, Balasubramanian MP (2013) Protective role of lycopene on bisphenol A induced changes in sperm characteristics, testicular damage and oxidative stres in rats. Int J Pharm Bio Sci 4:131–143
Uzunisarcikli M, Aslanturk A (2019) Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ Sci Pollut Res 26:37242–37253. https://doi.org/10.1007/s11356-019-06615-8
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95. https://doi.org/10.1210/er.2008-0021
Wang L, Hao J, Hu J, Pu J, Lu Z, Zhao L, Wang Q, Yu Q, Wang Y, Li G (2012) Protective effects of ginsenosides against bisphenol A-induced cytotoxicity in 5P-1 sertoli cells via extracellular signal-regulated kinase 1/2 signalling and antioxidant mechanisms. BCPT 111:42–49. https://doi.org/10.1111/j.1742-7843.2012.00857.x
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y (2010) The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208:19–25. https://doi.org/10.1016/j.atherosclerosis.2009.06.002
Wu HJ, Liu C, Duan WX, Xu SC, He MD, Cen CH, Wang Y, Zhou Z, Yu ZP, Zhang L, Chen Y (2013) Melatonin amaliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat Res 752:57–67. https://doi.org/10.1016/j.mrgentox.2013.01.005
Yang QQ, Farha AK, Kima G, Gula K, Ganb RY, Corke H (2020) Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci Technol 97:341–354. https://doi.org/10.1016/j.tifs.2020.01.023
Yıldız N, Barlas N (2013) Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum Exp Toxicol 32:675–686. https://doi.org/10.1177/0960327112464796
Zhang Z, Liu D, Yi B, Liao Z, Tang L, Yin D, He M (2014) Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol Med Rep 10:2255–2262. https://doi.org/10.3892/mmr.2014.2544
Zhou W, Liu J, Liao L, Han S, Liu J (2008) Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol 283:12–18. https://doi.org/10.1016/j.mce.2007.10.010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslanturk, A., Uzunhisarcikli, M. Protective potential of curcumin or taurine on nephrotoxicity caused by bisphenol A. Environ Sci Pollut Res 27, 23994–24003 (2020). https://doi.org/10.1007/s11356-020-08716-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08716-1