Abstract
Mine tailings, generated from the extraction, processing, and utilization of mineral resources, have resulted in serious acid mine drainage (AMD) pollution. Recently, scholars are paying more attention to two alternative strategies for resource recovery and ecological reclamation of mine tailings that help to improve the current tailing management, and meanwhile reduce the negative environmental outcomes. This review suggests that the principles of geochemical evolution may provide new perspective for the future in-depth studies regarding the pollution control and risk management. Recent advances in three recycling approaches of tailing resources, termed metal recovery, agricultural fertilizer, and building materials, are further described. These recycling strategies are significantly conducive to decrease the mine tailing stocks for problematic disposal. In this regard, the future recycling approaches should be industrially applicable and technically feasible to achieve the sustainable mining operation. Finally, the current state of tailing phytoremediation technologies is also discussed, while identification and selection of the ideal plants, which is perceived to be the excellent candidates of tailing reclamation, should be the focus of future studies. Based on the findings and perspectives of this review, the present study can act as an important reference for the academic participants involved in this promising field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The past and present-day mining sector, having been recognized as an economic driver and essential cornerstone, can immensely facilitate economic growth worldwide (Yin et al. 2018a, 2018b). However, with steady societal demands for metal resources at the global scale, the exploitation and utilization of mineral resources tend to be profit maximization-oriented for mining enterprises, and the resulting negative environmental impacts have attracted attention in much research. In addition, owing to the increasing depletion of the high-grade metal deposits, the continuous generation of enormous amounts of profitless solid wastes remains a global challenge in the future.
In the past decades, significant quantities of mine wastes, generally referred to as mine tailings, have been tremendously growing in parallel with the annual extraction, processing and utilization of global mineral resources (Wu et al. 2019). Literature suggests that the amount of solid tailings produced accounts for roughly 97–99 % of total ore processed, whereas only 1–3 % is concentrate (Adiansyah et al. 2015). An estimated 5–7 billion tons of mine tailings is generated each year worldwide (Edraki et al. 2014). The statistical data from National Development and Reform Commission of China shows that the stocks of mine tailings are estimated at 14.6 billion tons by the end of 2013 (NDRCC 2014). In 2013 alone, China had accumulated 1.649 billion tons of tailings (Ye et al. 2017). Tailing discharges, mainly composed of finely crushed materials and commonly enriched with sulfidic polymetallic materials, are historically stacked in open-air tailing impoundments, situated close to mining sites year by year, though only a small part is effectively recycled and utilized (Sibanda et al. 2019). Even more problematic, these tailings not only cover innumerable mining land resources and waste valuable resources but also induce catastrophic environmental accidents (Xiang et al. 2018). It has been reported that the total area of land resource and mined lands damaged by waste tailings is above 2000 ha (Wang et al. 2017). Besides that, coupled with an increasing environmental hazards related to mine tailing repositories, the field researches of effectively ecological remediation and practices in mining areas necessitate the growing awareness within the global scientific community. On the other hand, tailing dam failures worldwide, such as Fundão dam collapse in southeastern Brazil in November 2015, are frequently resulted in enormous property losses, large-scale people mortality, and extensive environmental damage (Queiroz et al. 2018; Quadra et al. 2019). Such catastrophic accidents often occur as a consequence of poor facility performance. In view of their severe social, economic, and environmental impacts, it is of great necessity for mining industries to improve the contemporary tailing management of international scale.
During tailing stockpiling period, acid mine drainage (AMD) has been an unavoidable environmental concern in mining practices among all countries (Park et al. 2019). It is well acknowledged that AMD is an important consequence of the oxidative dissolution of reactive sulfidic minerals, especially pyrite, upon exposure to atmospheric oxygen (O2), water, and microbial activities, which make harmful metallic species become more soluble and mobile (Anju and Banerjee 2010; Elghaliet Elghali et al. 2019; Naidu et al. 2019). Numerous evidences have shown that the migration of various dissolved elements into the neighboring environment receptors, including surface runoffs, soils, sediments, and other local ecosystems, could be traced directly back to the ongoing acid-producing process of AMD (Abraham and Susan 2017; Torres et al. 2018; Park et al. 2019). Thus, it has a long-term deleterious impact on public health and ecological environment (Liao et al. 2016, 2017).
While mining operations subsist, there is an urgent need for local authorities to minimize the pollution risk typically accompanied with the excessive accumulation of hazardous mining discharges generated from processing ores. To address this aim, current mining operations must be conducted in an environmentally sustainable and economically feasible manner, with the aim to significantly contribute to the cleaner production of mineral resources across the globe. In this context, effective recycling and reprocessing of tailing materials have been of great importance to all countries worldwide.
In response to the above concerns, the main purpose of this critical review is therefore to introduce the following three aspects: (i) the potential environmental implications of AMD formation, (ii) the strategies for comprehensive recovery and reutilization of tailing resources, (iii) the current techniques of tailing phytoremediation.
AMD pollution and associated environmental implications
Formation of AMD and secondary minerals
Environmental studies have reported that AMD is primarily formed from the oxidation of pyrite (FeS2), the most abundant sulfide mineral in tailings, as described by the following simplified equations (Park et al. 2019):
The well-understood processes of AMD formation have been reviewed in many scientific literatures (Lowson 1982; Kefeni et al. 2017; Naidu et al. 2019). Pyrite is initially oxidized by O2, resulting in the release of Fe2+, SO42−, and H+ (Eq. (1)). In the presence of atmospheric O2, Fe2+ is subsequently oxidized to Fe3+ (Eq. (2)), which significantly accelerates the oxidative dissolution of more pyrite and AMD acidification and further leads to the solubilization of associated trace metals into pore waters (Eq. (4)). In this reaction, Fe3+ dissolved in acidic solutions becomes the dominant natural oxidant (Singer and Stumm 1970). Fe3+ is precipitated as ferric hydroxides (given as Fe (OH)3), while additional H+ is simultaneously generated (Eq. (3)), which typically depends upon pH values of the systems. Due to the presence of iron oxide precipitates, the oxidation zones of the tailing impoundments are usually recognizable by its yellow-reddish color. It is noteworthy to mention that pyrite oxidation involves spontaneous and microbial-mediated reactions, where the final weathered products are not fully illustrated in formulas (Bao et al. 2018). The overall process is extensively represented by the following reaction (Eq. (5)):
Under AMD environment, the rate of Fe2+ oxidation is quite slow and is identified as the rate-limiting step of the overall reaction (Hao et al. 2017). However, acidophilic chemolithotrophic microorganisms, growing optimally in extremely acidic conditions, can greatly promote the oxidation of Fe2+ to Fe3+ (Gleisner et al. 2006; Diaby et al. 2015). It is reported that, in the existence of acidophilic bacteria like Acidithiobacillus ferrooxidans, the oxidative rate is orders of magnitude faster than that of the previous reaction with pH below 3.5 (Anawar 2015). It has also been noted that microbially enhanced oxidation plays an active role in the precipitation of secondary weathered minerals, characterized especially by jarosite, schwertmannite (Fe16(OH,SO4)12-13O16·10-12H2O), and some iron-bearing secondary minerals, such as hematite (Fe2O3), goethite (α-FeOOH), and lepidocrocite (γ-FeOOH) (Nieva et al. 2019).
The presence of neutralizing carbonates minerals like calcite and dolomite is able to neutralize the strong acidity generated by the oxidative weathering of pyrite in tailings. Gypsum is another typical secondary weathering product and also key cemented mineral. The mineralogical transformation of Ca-bearing carbonates to gypsum by consuming H+ occurs by the following reaction (Lindsay et al. 2015; Liu et al. 2018a):
Geochemical properties of AMD
The geochemical properties of AMD vary with tailing types. Table 1 summarizes the recent case studies regarding the geochemical characteristics of AMD in metal mines worldwide. As clearly presented in Table 1, low pH values, elevated concentrations of sulfate ions (SO42−), dissolved iron (Fe), manganese (Mn), aluminum (Al), and various trace metals such as cadmium (Cd), copper (Cu), lead (Pb), zinc (Zn), and arsenic (As) are typically characteristic for AMD.
Environmental implications of AMD
In China, most metal mines are sulfide ore deposits (Chen et al. 2018). As a consequence, AMD pollution in mining areas resulting from the continuous weathering and oxidation of exposed sulfide tailings is of considerable concern. It can be expected that the resulting oxidative dissolutions of various sulfide minerals, such as pyrite (FeS2), chalcopyrite (CuFeS2), sphalerite (ZnS), and galena (PbS), are potential sources of released trace elements (Lindsay et al. 2015). Reich et al. (2013) documented that pyrite commonly hosts significant concentrations of toxic accessory elements, including Ni, Co, Cu, Pb, Zn, As, Sb, Se, Te, Hg, Tl, Bi, Au, and Ag. Furthermore, untreated AMD in tailing deposits significantly enhances the leaching, dissolution, and mobility of trace elements born in tailings and in turn has long-lasting detrimental effects on the nearby environmental media. For instance, Zhang et al. (2018) concluded that contamination of Anshan mine tailings and associated transportation are the principal sources of Cr, Cd, Cu, Zn, and Pb released to the soil environment. From this perspective, an increasing concern from mine tailing deposits is the migration and mobilization of large amounts of toxic heavy metals towards their surrounding areas, which are ecologically unacceptable to mine operators and environmental protection authorities.
As sulfide oxidation progresses, the cemented layers will widely develop on the surface of both fresh and aged sulfide tailings, which are hereafter referred to as crusts or even hardpans (Stumbea et al. 2019). These hardpan layers are typically comprised of primary and secondary mineral phases, as well as adsorbed heavy metals. In addition, various secondary minerals may precipitate within the oxidized tailings, with the most common being Fe(III) oxyhydroxides, Fe(III) hydroxysulfates, and gypsum (Kohfahl et al. 2010). On the one hand, the appearance of this oxidized layer may limit pore-water migration and oxygen ingress, and their barrier effects further inhibit the exposure of metal sulfides to oxygen within un-weathered tailings (Liu et al. 2018a). On the other hand, the newly formed hardpans may act as temporary sinks for polymetallic pollutants released through AMD (Blowes et al. 1991; Bao et al. 2018). The latter can slowly reduce the mobility of contaminative elements through natural attenuation mechanisms, such as re-adsorption, co-precipitation, and substitution (Liu et al. 2018b). This implies that in AMD systems, the secondary weathered minerals can exert a profound impact on the distribution pattern and potential mobility of hazardous trace metals released from tailings and largely control their potential environmental risks (Chen et al. 2018; Ouyang et al. 2019). Nevertheless, the AMD precipitates are generally unstable because of poor crystallinity and being highly soluble in acid waters (Chen et al. 2018; Liu et al. 2018b). As a result, once physico-chemical changes happen to AMD, toxic elements adsorbed in hardpans are most likely to be released into the surrounding water bodies. In light of these facts, a conceptual illustration of identified geochemical weathering mechanisms within mine tailings, commonly related to the mineralogical, geochemical, and sedimentological status, is summarized in Fig. 1. It is expected that the favorable mechanisms might be found from geochemical evolution, which would guide the local authorities to take remedial actions.
Recycling strategies of mine tailing resources
To date, the main treatment methods for huge amounts of mine tailings are their reuse as cemented paste backfill (CPB) in open pits or underground mines and storage in tailing impoundments, aimed at improving the current tailing management at mine sites (Qi et al. 2018; Yao et al. 2019). For example, Lu et al. (2018) investigated that a new backfill procedure was applied to an engineering instance, Shirengou Iron Mine, Hebei province, China, where the recovery efficiency of waste tailings were 100%. In another related study, Sun et al. (2018) presented an approach where mining solid wastes such as tailings and rocks were utilized to prepare a paste for backfilling the subsidence areas and preventing secondary disasters. Ercikdi et al. (2015) and Lu et al. (2018) have also reported recycling waste tailings as CPB as an ideal option of tailing disposal, which can significantly facilitate cleaner and safer production in the mining industry worldwide. At present, several acceptable approaches of resources recycled from mine tailings, reported in most previous studies, are as the following: recovery of useful minerals and metals, production of economical building materials, and preparation of soil modifier and agricultural fertilizer (Li et al. 2010; Yin et al. 2018a, 2018b). In practical terms, these recycling strategies have positive effects on reducing the burden of tailings discharged, with the additional benefits of protecting precious resources, saving energy consumption, and minimizing security risks.
There has been an effort throughout the world to come up with proper strategies for decreasing the volume of mine tailings and increasing the associated economic benefits (Ahmari and Zhang 2012). It is reported that the utilization of tailings in China has been increasing from 13.3 % in 2013 to 28.9 % in 2015 (Lv et al. 2019). However, despite these efforts, the rates remain far lower than the average rates in developed countries (Shettima et al. 2016).
Recovery of precious metal resources
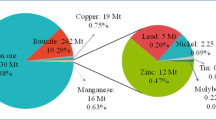
Tailings was defined as valuable metal stocks in the technosphere by Johansson et al. (2013), indicating that reprocessing might also be categorized into an innovative reclamation technology. There are various types of mine tailings discharged, including iron, gold, copper, manganese, lead-zinc, vanadium, rare earth, and platinum tailings (Abraham and Susan 2017; Galvão et al. 2018; Gandarillas et al. 2019; Yang et al. 2003). Thus, there have been significant interests in studying and developing technically feasible and environmentally acceptable technologies for metal recovery from different types of mine tailings. These technologies include, but are not limited to, the following representative technologies: acid leaching, bioleaching, and magnetic separation. Recently, studies have demonstrated that effectively recovering rare, precious, and strategic metals from mine tailings is feasible (Lan et al. 2019; Zhang et al. 2019). A considerable number of studies have been focused on valuable metal resources recycled from various types of mine tailings, as summarized in Table 2. As suggested by incomplete statistics, the typical amount of Au in gold tailings ranges between 0.2 and 0.6 g/t, the Fe grade of iron tailings ranges from 8 to 12 %, Cu concentration in copper tailings varies from 0.02 to 0.1 %, and the amount of Pb and Zn accounts for 0.2–0.5 % of lead and zinc tailings (Zhang 2012). In the near future, it in particular is of concern to evaluate the metal recovery potential from mine tailings through the investigated amounts and grades of the valuable metals in combination with metallurgical test work campaigns (Yin et al. 2018a, 2018b).
Production of building materials
As shown in Table 2, mine tailings are rich in various major elements such as Si, Ca, Mn, Fe, and Al and their main phase composition is carbonate, silicate, and quartz. In comparison with common building materials, tailings have similar physico-chemical, compositional, and mechanical characteristics in industrial application. In recent decade, more industrial researches are required to develop technical and economic routes that tailing materials are used to produce building materials (Onuaguluchi and Eren 2016). As summarized in the recent advances (Table 3), different types of mine tailings have been utilized as alternative raw materials to produce environmentally friendly building materials, such as bricks, concrete, ceramics, glass fibers, and paint.
Preparation of agricultural fertilizer
Mine tailings contain abundant various trace elements such as B, V, Mn, Cu, Zn, Fe, Mo, and P, which are essential micronutrients for plant growth and soil supplements (Zhang et al. 2009). As a result, there has been an increasing expectation that mine tailing can be reprocessed as various microelement fertilizers (Guo et al. 2009). Hu et al. (2017) have reported that low-release silicon fertilizers were prepared from iron tailings using solid-phase sintering, whose available SiO2 was far greater than current Chinese agricultural standard for silicon fertilizers, and where trace elements would improve the growth of pakchoi. It is noteworthy that, unlike organic fertilizer, agricultural fertilizers prepared from tailings cannot be easily decomposed and meanwhile have insufficient fertility. To our knowledge, no other similar reports are found in Web of Science. It can be seen that recycling and reusing tailings as raw fertilizer materials are of great difficulty using new technical approaches, because the operational costs and technical difficulty will be significantly increased, and the successful industrial application is also greatly limited.
Ecological reclamation of tailing impoundments
Appropriate and cost-effective ecological rehabilitation at metal mines is an important measure for building green mines and also an important ecological practice to follow the green development concept. In comparison with traditional physical-chemical methods, phytoremediation, which might be a promising bioremediation technique, proves to be an eco-friendly and potentially cheap remediation strategy. This biological method has sparked renewed interests, because it is an adequate option for the in situ rehabilitation of highly polluted sites (Wei et al. 2019). Phytoremediation removes the pollutants without affecting soil aggregations, thus improving soil fertility and increasing organic matter and nutrient content for later uses (Salt et al. 1995; Álvarez-Mateos et al. 2019). However, compared with other common treatment procedures, phytoremediation has some drawbacks, such as slow growth rate of plant species and low bioavailability of heavy metals (Ashraf et al. 2019; Li et al. 2019a, 2019b). During the past decades, phytoremediation has often been carried out for rehabilitating tailing landscapes, in combination with multidisciplinary studies (Jia et al. 2017; Acosta et al. 2018; Hammond et al. 2018). As an example, Gil-Loaiza et al. (2018) found that the phytoremediation field trial at the Iron King Mine and Humboldt Smelter Superfund site could significantly decrease dust emissions and metal transport from mine tailings. Furthermore, mine spoil dumpsites and acid-generating tailings are widely regarded as an extreme and challenging case for rehabilitation, primarily as a result of nutritional deficiency, poor physical structure, and high levels of heavy metals, which inhibit natural plant growth (Wang et al. 2017). At the same time, the scarcity of natural top soils to reconstruct functional root system for vegetation establishment severely limits the rehabilitation progress of mine tailings (Wu et al. 2019). For this reason, proper measures should be proposed to improve the physical, chemical, and biological properties of mine tailings to enhance the colonization of plants and their metal accumulation capacity.
The concept of phytoremediation
Phytoremediation is mainly subdivided into phytovolatilization, phytostabilization, and phytoextraction, depending on different plant properties (Wang et al. 2017). The advantages and disadvantages of different phytoremediation types are given in Table 4. Phytovolatilization is the uptake of metal pollutants by plants, followed by their translocation into the aerial parts and then their release from plant foliage (Leguizamo et al. 2017). Phytostabilization is to immobilize toxic metals via sorption, precipitation, or complexation of plant’s root systems and further reduce their bioavailability in the environment (Shim et al. 2013). Among these, phytoextraction is a widely applicable option of tailing reclamation, since this mechanism transports and concentrates metal pollutants into the aerial harvestable parts (Tang et al. 2019; Mahar et al. 2016). However, after harvest, the safe disposal of metal-enriched biomass of plants is quite challenging.
Combined techniques used for phytoremediation purposes
It should be noted that one reclamation method is on its own insufficient for rehabilitation, due to some limitations and weakness (Wang et al. 2017). Hence, phytoremediation is often combined with one or more of other traditional approaches to make it more effective, considering the extreme physical and environmental characteristics of tailing impoundments. In most recent cases, phytoremediation is generally assisted with common remediation techniques, namely soil amelioration, microbiological inoculation, as well as biogenetic engineering, which are conducive to provide an appropriate substrate for reducing heavy metal bioavailability, as well as increasing metal-accumulated plant biomass (Shim et al. 2013; Babu et al. 2014; Li et al. 2019a, 2019b). Some case studies of tailing phytoremediation, in conjunction with some assisted approaches, are presented in Table 5. Regarding future remediation challenges of mine tailings, plant-based remediation technologies may be the most potentially promising and effective method in metal mining areas, which have increasingly become an international research hotspot.
Role of amelioration in phytoremediation
Phytoremediation efficiency can be accelerated with the assistance of inorganic and organic ameliorations. These ameliorations can reduce the mobility of heavy metals, increase the biomass yield of plants, and also ameliorate the condition stress of polluted sites. For example, Yu et al. (2019) reported that Mn remediation efficacy by Polygonum pubescens was enhanced in the unexplored soil, mining soil, and tailing soil, with the chemical chelate (EDTA) treatments. This was because the application of EDTA greatly increased the water-extractable Mn content in all three soils. Another study carried out by Beauchemin et al. (2018) indicated that the application of oxygen-consuming organic covers for 4 to 5 years could greatly enhance tailing rehabilitation, because they reduced the water-soluble metals and increased nutrient and organic carbon contents in the oxidized Cu-Ni pyrrhotite tailings, while improving the microbial activity and diversity. In addition, Gandarillas et al. (2019) demonstrated that either pig slurries or their solid organic fractions that were incorporated into copper tailings significantly increase organic matter and nutrient contents in tailings, as well as the productivity and Zn accumulation of ryegrass.
Role of biogenetic engineering in phytoremediation
Recent results have shown that transgenic plants have gradually become an attractive candidate for increasing phytoremediation efficiency, due to their excellent performance regarding significant metal accumulation (Rizwan and Ali 2018; Rostami and Azhdarpoor 2019). For instance, Shim et al. (2013) suggested that after being transformed with heavy metal resistance gene (ScYCF1), poplar trees planted in mine tailing soil under greenhouse increased the accumulated amounts of Cd, Zn, and Pb in the root due to their enhanced root systems, in comparison with the non-transgenic plants. In addition, an early study performed by Bennett et al. (2003) also reported that all three types of transgenics significantly reduced the metal concentrations of tailing soil, in amounts ranging between 6% for Zn and 25% for Cd of the total soil metal content, and confirmed the importance of metal-binding peptides for the enhanced metal tolerance of plants.
Role of microorganisms in phytoremediation
It was confirmed that some species of plants can form mutualistic associations with selected bacterial or fungi strains for phytoremediation enhancement (Deng and Cao 2017; Li et al. 2019a, 2019b). It was reported that the symbiotic association among Setosphaeria rostrata, arbuscular mycorrhiza fungi (AMF), and rhizobia greatly increased S. rostrata plant uptake of uranium in uranium contaminated soils and its biomass (Ren et al. 2019). AMF could improve plants resistance to heavy metals, followed by sequestrating them between the mycorrhizosphere and the mycorrhizal roots (Chen et al. 2004). In addition, AMF hyphae could also alter the soil microbial community to increase the tolerant capacity of plants to environmental stress (Chen et al. 2019; Li et al. 2019a, 2019b). According to Yu et al. (2017), inoculating P. pinnata with rhizobia strain (PZHK1), isolated from the V-Ti magnetite tailing soils, greatly promoted the translocation of Fe, Ni, and Cu to shoots. It has been argued that P. pinnata formed an effectively nitrogen fixing nodules with rhizobia, and this symbiotic association increased the biomass production of plants and its stress tolerance to metals (Arpiwi et al. 2013; Yu et al. 2017).
Plant species for phytoremediation
It is being increasingly recognized that identification and selection of suitable native wild plant species from metal-contaminated areas for planting on mine tailings is an effective route to meet the objectives of phytoremediation (Haque et al. 2008; García-Carmona et al. 2019). Qian et al. (2018) investigated 259 wild plants from the Wanshan District, eastern Guizhou Province, China, and proposed Erica ciliaris and Acromyrmex hispidus as potential candidates for phytoremediation of Hg mining-polluted soils. Likewise, Midhat et al. (2019) conducted a botanical survey in three abandoned mining sites in Morocco, and eight plants are found to be the suitable candidates for phytostabilization of mining sites due to their much higher ability to accumulate metals. Plant species for bioremediation should be extremely tolerant to a wide range of adverse growth conditions of the metal-impacted regions, such as high concentrations of toxic heavy metals, as well as variations in humidity, salinity, acidity, and temperature at site-specific systems. Besides that, the plants should be abundant in the specific areas and able to grow rapidly, develop an extensive root system, and produce large biomass (Shi et al. 2017). As described by Baker et al. (1981), metallophytes, termed hyperaccumulators, are currently recognized as the most ideal and attractive plants, due to their tolerance mechanisms that enable them to accumulate extremely high levels of heavy metals in their shoots rather than roots. However, there is a crucial consideration that the slow growth and low biomass yields of most hyperaccumulators are limiting factors of the remediation efficiency. Furthermore, plants obtained from harsh conditions are often performed better than those introduced from non-polluted areas, in terms of survival, growth, and reproduction (Yoon et al. 2006). As a consequence, studies on investigating native pioneer plant species in highly metal polluted areas, understanding their metal accumulation patterns, and evaluating their potential use have been performed by many scientists (Yoon et al. 2006; Qian et al. 2018).
Summary and future perspectives
Large amounts of abandoned tailings generated from different types of mines have resulted in a series of society, economy, resource, and environment-related concerns. This critical review underscores (i) AMD pollution and associated environmental implications, (ii) recycling strategies of tailing resources, (iii) ecological reclamation of tailing. AMD remains quite challenging due to its typically geochemical characteristics. AMD formation has crucial implications on the natural reduction of potential pollution risks. In addition, the current recycling strategies of tailing resources for different industries are described. Nevertheless, little information can be found regarding tailings utilized as microelement fertilizers. Finally, this review indicates that the combination of phytoremediation and other traditional techniques such as amelioration, genetic modifications, and biostimulation-assisted phytoremediation, can increase reclamation efficiency. Overall, this review shows that tailing management with related restoration efforts is of great importance to all countries worldwide.
More knowledge is needed on possible management strategies designed to prevent AMD generation for the mitigation of potential health threats. Moreover, the secondary exploration targets for mining industries will focus on the research and development of innovative and modern technologies, which are universal, suitable, and cost-effective for different tailing types to improve the utilization, while ensuring economic financial returns. In addition, future work on health risk assessment of population exposure to various types of hazardous tailings must be considered for remediation potential of tailing impoundments. Subsequently, how to combine multidisciplinary approaches and various restoration technologies to enhance the phytoremediation efficiency of tailings is an important direction. There is a crucial consideration that the tolerant capacity of ideal plants to metals is influenced by the combined effects of geochemical and environmental characteristics at tailings impacted sites. It is therefore important to identify the quantitative relationships between multiple factors and metal-accumulated amounts of plants using mathematical statistics.
References
Abraham MR, Susan TB (2017) Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites in Western Uganda; implications for domestic water quality. Chemosphere 169:281–287
Acosta JA, Abbaspour A, Martínez GR, Martínez-Martínez S, Zornoza R, Gabarrón M, Faz A (2018) Phytoremediation of mine tailings with Atriplex halimus and organic/inorganic amendments: a five-year field case study. Chemosphere 204:71–78
Adiansyah JS, Rosano M, Vink S, Keir G (2015) A framework for a sustainable approach to mine tailings management: disposal strategies. J Clean Prod 108:1050–1062
Ahmari S, Zhang LY (2012) Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr Build Mater 29:323–331
Álvarez-Mateos P, Alés-Álvarez FJ, García-Martín JF (2019) Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J Environ Manag 231:886–895
Anawar HM (2015) Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J Environ Manag 158:111–121
Anju M, Banerjee DK (2010) Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 78:1393–1402
Arpiwi NL, Yan GL, Barbour EL, Plummer JA, Watki E (2013) Phenotypic and genotypic characterisation of root nodule bacteria nodulating Millettia pinnata (L.) Panigrahi, a biodiesel tree. Plant Soil l367:363–377
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox Environ Safe 174:714–727
Babu AG, Shim J, Shea PJ, Oh BT (2014) Penicillium aculeatum PDR-4 and Trichoderma sp. PDR-16 promote phytoremediation of mine tailing soil and bioenergy production with sorghum-sudangrass. Ecol Eng 69:186–191
Baker AJM (1981) Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutrition 3(1-4):643–654
Bao YP, Guo CL, Lu GN, Yi XY, Wang H, Dang Z (2018) Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine. Sci Total Environ 616-617:647–657
Beauchemin S, Clemente JS, Thibault Y, Langley S, Gregorich EG, Tisch B (2018) Geochemical stability of acid-generating pyrrhotite tailings 4 to 5 years after addition of oxygen-consuming organic covers. Sci Total Environ 645:1643–1655
Bennett LE, Burkhead JL, Hale KL, Terry N, Pilon M, Pilon-Smits EA (2003) Analysis of transgenic Indian mustard plants for phytoremediation of metal-contaminated mine tailings. J Environ Qual 32:432–440
Blowes DW, Reardon EJ, Jambor JL, Cherry JA (1991) The formation and potential importance of cemented layers in inactive sulfide mine tailings. Geochim Cosmochim Ac 55:965–978
Chen BD, Liu Y, Shen H, Li XL, Christie P (2004) Uptake of cadmium from an experimentally contaminated calcareous soil by arbuscular mycorrhizal maize (Zea mays L.). Mycorrhiza 14:347–354
Chen MQ, Lu GN, Wu JX, Yang CF, Niu XC, Tao XQ, Shi ZQ, Yi XY, Dang Z (2018) Migration and fate of metallic elements in a waste mud impoundment and affected river downstream: a case study in Dabaoshan Mine, South China. Ecotox Environ Safe 164:474–483
Chen XW, Wu L, Luo N, Hui C, Wong MH, Li H (2019) Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 337:749–757
Choi HJ, Lee SM (2015) Heavy metal removal from acid mine drainage by calcined eggshell and microalgae hybrid system. Environ Sci Pollut R 22:13404–13411
Clyde EJ, Champagne P, Jamieson HE, Gorman C, Sourial J (2016) The use of a passive treatment system for the mitigation of acid mine drainage at the Williams Brothers Mine (California): pilot-scale study. J Clean Prod 130:116–125
Deng ZJ, Cao LX (2017) Fungal endophytes and their interactions with plants in phytoremediation: a review. Chemosphere 168:1100–1106
Diaby N, Dold B, Rohrbach E, Holliger C, Rossi P (2015) Temporal evolution of bacterial communities associated with the in situ wetland-based remediation of a marine shore porphyry copper tailings deposit. Sci Total Environ 533:110–121
Edraki M, Baumgartl T, Manlapig E, Bradshaw D, Franks DM, Moran CJ (2014) Designing mine tailings for better environmental, social and economic outcomes: a review of alternative approaches. J Clean Prod 84:411–420
Elghali A, Benzaazoua M, Bussière B, Kennedy C, Parwani R, Graham S (2019) The role of hardpan formation on the reactivity of sulfidic mine tailings: a case study at Joutel mine (Québec). Sci Total Environ 654:118–128
Ercikdi B, Külekci G, Yılmaz T (2015) Utilization of granulated marble wastes and waste bricks as mineral admixture in cemented paste backfill of sulphide-rich tailings. Constr Build Mater 93:573–583
Galvão JLB, Andrade HD, Brigolini GJ, Peixoto RAF, Mendes JC (2018) Reuse of iron ore tailings from tailings dams as pigment for sustainable paints. J Clean Prod 200:412–422
Gandarillas M, España H, Gardeweg R, Bas F, Arellano EC, Brown S, Ginocchio R (2019) Integrated management of pig residues and copper mine tailings for aided phytostabilization. J Environ Qual 48:430–438
García-Carmona M, García-Robles H, Torrano CT, Ondoño EF, Moreno JL, Aragón MS, Peinado FJM (2019) Residual pollution and vegetation distribution in amended soils 20 years after a pyrite mine tailings spill (Aznalcóllar, Spain). Sci Total Environ 650:933–940
Gil-Loaiza J, Field JP, White SA, Csavina J, Felix O, Betterton EA, Sáez AE, Maier RM (2018) Phytoremediation reduces dust emissions from metal (Ioid)-contaminated mine tailings. Environ Sci Technol 52:5851–5858
Gleisner M, Herbert RB, Kockum PCF (2006) Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen. Chem Geol 225:16–29
Grawunder A, Merten D, Büchel G (2014) Origin of middle rare earth element enrichment in acid mine drainage-impacted areas. Environ Sci Pollut R 21:6812–6823
Guo JW, Wang JH, Yang GH (2009). Current situation and comprehensive utilization of iron ore tailings resources in our country. Modern Min 25(10):23–25 (in Chinese)
Hammond CM, Root RA, Maier RM, Chorover J (2018) Mechanisms of arsenic sequestration by Prosopis juliflora during the phytostabilization of metalliferous mine tailings. Environ Sci Technol 52:1156–1164
Han BS, Altansukh B, Haga K, Stevanović Z, Jonović R, Avramović L, Urosević D, Takasaki Y, Masuda N, Ishiyama D, Shibayama A (2018) Development of copper recovery process from flotation tailings by a combined method of high-pressure leaching solvent extraction. J Hazard Mater 352:192–203
Hao CB, Wei PF, Pei LX, Du ZR, Zhang Y, Lu YC, Dong HL (2017) Significant seasonal variations of microbial community in an acid mine drainage lake in Anhui Province, China. Environ Pollut 223:507–516
Haque N, Peralta-Videa JR, Jones GL, Gill TE, Gardea-Torresdey JL (2008) Screening the phytoremediation potential of desert broom (Baccharis sarothroides Gray) growing on mine tailings in Arizona, USA. Environ Pollut 153:362–368
Hu P, Zhang YH, Zhou YR, Ma X, Wang XK, Tong WS, Luan XL, Chu PK (2017) Preparation and effectiveness of slow-release silicon fertilizer by sintering with iron ore tailings. Environ Prog Sustain Energy 37:1011–1019
Jia T, Cao MW, Jing JH, Liu JX, Chai BF (2017) Endophytic fungi and soil microbial community characteristics over different years of phytoremediation in a copper tailings dam of Shanxi, China. Sci Total Environ 574:881–888
Johansson N, Krook J, Eklund M, Berglund B (2013) An integrated review of concepts and initiatives for mining the technosphere: towards a new taxonomy. J Clean Prod 55:35–44
Kefeni KK, Msagati TAM, Mamba BB (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Clean Prod 151:475–493
Kim Y, Kim M, Sohn J, Park H (2018) Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J Clean Prod 203:957–965
Kim Y, Lee Y, Kim M, Park H (2019) Preparation of high porosity bricks by utilizing red mud and mine tailing. J Clean Prod 207:490–497
Kohfahl C, Graupner T, Fetzer C, Pekdeger A (2010) The impact of cemented layers and hardpans on oxygen diffusivity in mining waste heaps: a field study of the Halsbrucke lead-zinc mine tailings (Germany). J Hazard Mater 408:5932–5939
Krawczyk-Bärsch E, Lünsdorf H, Arnold T, Brendler V, Eisbein E, Jenk U, Zimmermann U (2011) The influence of biofilms on the migration of uranium in acid mine drainage (AMD) waters. Sci Total Environ 409:3059–3065
Lam EJ, Cánovas M, Gálvez ME, Montofré ÍL, Keith BF, Faz Á (2017) Evaluation of the phytoremediation potential of native plants growing on a copper mine tailing in northern Chile. J Geochem Explor 182:210–217
Lan X, Gao JT, Li Y, Guo ZC (2019) A green method of respectively recovering rare earths (Ce, La, Pr, Nd) from rare-earth tailings under super-gravity. J Hazard Mater 367:473–481
Leguizamo MAO, Gómez WDF, Sarmiento MCG (2017) Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands - a review. Chemosphere 168:1230–1247
Lei C, Yan B, Chen T, Wang XL, Xiao XM (2018) Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J Clean Prod 181:408–415
Li C, Sun HH, Bai J, Li LT (2010) Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J Hazard Mater 174:71–77
Li WS, Lei GY, Xu Y, Huang QF (2018) The properties and formation mechanisms of eco-friendly brick building materials fabricated from low-silicon iron ore tailings. J Clean Prod 204:685–692
Li X, Chen AY, Yu LY, Chen XX, Xiang L, Zhao HM, Mo CH, Li YW, Cai QY, Wong MH, Li H (2019a) Effects of β-cyclodextrin on phytoremediation of soil co-contaminated with Cd and BDE-209 by arbuscular mycorrhizal amaranth. Chemosphere 220:910–920
Li XX, Wang XL, Chen YD, Yang XY, Cui ZJ (2019b) Optimization of combined phytoremediation for heavy metal contaminated mine tailings by a field-scale orthogonal experiment. Ecotox Environ Safe 168:1–8
Liao JB, Wen ZW, Ru X, Chen JD, Wu HZ, Wei CH (2016) Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: public health implications in Guangdong Province, China. Ecotox Environ Safe 124:460–469
Liao JB, Ru X, Xie BB, Zhang WH, Wu HZ, Wu CF, Wei CH (2017) Multi-phase distribution and comprehensive ecological risk assessment of heavy metal pollutants in a river affected by acid mine drainage. Ecotox Environ Safe 141:75–84
Lindsay MBJ, Moncur MC, Bain JG, Jambor JL, Ptacek CJ, Blowes DW (2015) Geochemical and mineralogical aspects of sulfide mine tailings. Appl Geochem 57:157–177
Liu TY, Tang Y, Han L, Song J, Luo ZW, Lu AX (2017) Recycling of harmful waste lead-zinc mine tailings and fly ash for preparation of inorganic porous ceramics. Ceram Int 43:4910–4918
Liu QY, Chen BH, Haderlein S, Gopalakrishnan G, Zhou YZ (2018a) Characteristics and environmental response of secondary minerals in AMD from Dabaoshan Mine, South China. Ecotox Environ Safe 155:50–58
Liu YJ, Wu SL, Nguyen TAH, Southam G, Chan TS, Lu YR, Huang LB (2018b) Microstructural characteristics of naturally formed hardpan capping sulfidic copper-lead-zinc tailings. Environ Pollut 242:1500–1509
Lowson RT (1982) Aqueous oxidation of pyrite by molecular oxygen. Chem Rev 82:461–497
Lu HJ, Qi CC, Chen QS, Gan DQ, Xue ZL, Hu YJ (2018) A new procedure for recycling waste tailings as cemented paste backfill to underground stopes and open pits. J Clean Prod 188:601–612
Lv XD, Shen WG, Wang L, Dong Y, Zhang JF, Xie ZQ (2019) A comparative study on the practical utilization of iron tailings as a complete replacement of normal aggregates in dam concrete with different gradation. J Clean Prod 211:704–715
Ma BG, Cai LX, Li XG, Jian SW (2016) Utilization of iron tailings as substitute in autoclaved aerated concrete: physico-mechanical and microstructure of hydration products. J Clean Prod 127:162–171
Macías F, Pérez-López R, Caraballo MA, Cánovas CR, Miguel Nieto JM (2017) Management strategies and valorization for waste sludge from active treatment of extremely metal-polluted acid mine drainage: a contribution for sustainable mining. J Clean Prod 141:1057–1066
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li RH, Zhang ZQ (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotox Environ Safe 126:111–121
Midhat L, Ouazzani N, Hejjaj A, Ouhammou A, Mandi L (2019) Accumulation of heavy metals in metallophytes from three mining sites (Southern Centre Morocco) and evaluation of their phytoremediation potential. Ecotox Environ Safe 169:150–160
Migaszewski ZM, Gałuszka A, Dołęgowska S (2016) Rare earth and trace element signatures for assessing an impact of rock mining and processing on the environment: Wiśniówka case study, south-central Poland. Environ Sci Pollut R 23:24943–24959
Mohamed S, van der Merwe EM, Altermann W, Doucet FJ (2016) Process development for elemental recovery from PGM tailings by thermochemical treatment: preliminary major element extraction studies using ammonium sulphate as extracting agent. Waste Manag 50:334–345
Naidu G, Ryu S, Thiruvenkatachari R, Choi Y, Jeong S, Vigneswaran S (2019) A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ Pollut 247:1110–1124
National Development and Reform Commission of China (2014) Annual report on China’s comprehensive utilization of resources. National Development and Reform Commission Press, Beijing, China
Nieva NE, Bia G, Garcia MG, Borgnino L (2019) Synchrotron XAS study on the As transformations during the weathering of sulfide-rich mine wastes. Sci Total Environ 669:798–811
Odoh CK, Zabbey N, Sam K, Eze CN (2019) Status, progress and challenges of phytoremediation - an African scenario. J Environ Manag 237:365–378
Onuaguluchi O, Eren O (2016) Reusing copper tailings in concrete: corrosion performance and socioeconomic implications for the Lefke-Xeros area of Cyprus. J Clean Prod 112:420–429
Ouyang BJ, Lu XC, Li J, Liu H (2019) Microbial reductive transformation of iron-rich tailings in a column reactor and its environmental implications to arsenic reactive transport in mining tailings. Sci Total Environ 670:1008–1018
Park I, Tabelin CB, Jeon S, Li XL, Seno K, Ito M, Hiroyoshi N (2019) A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 219:588–606
Qi CC, Fourie A, Chen QS, Tang XL, Zhang QL, Gao RG (2018) Data-driven modelling of the flocculation process on mineral processing tailings treatment. J Clean Prod 196:505–516
Qian XL, Wu YG, Zhou HY, Xu XH, Xu ZD, Shang LH, Qiu GG (2018) Total mercury and methylmercury accumulation in wild plants grown at wastelands composed of mine tailings: insights into potential candidates for phytoremediation. Environ Pollut 239:757–767
Quadra GR, Roland F, Barros N, Malm O, Lino AS, Azevedo GM, Thomaz JR, Andrade-Vieira LF, Praca-Fontes MM, Almeida RM, Mendonça RF, Cardoso SJ, Guida YS, Campos JMS (2019) Far-reaching cytogenotoxic effects of mine waste from the Fundao dam disaster in Brazil. Chemosphere 215:753–757
Queiroz HM, Nóbrega GN, Ferreira TO, Almeida LS, Romero TB, Santaella ST, Bernardino AF, Otero XL (2018) The Samarco mine tailing disaster: a possible time-bomb for heavy metals contamination? Sci Total Environ 637-638:498–506
Reich M, Deditius A, Chryssoulis S, Li JW, Ma CQ, Parada MA, Barra F, Mittermayr F (2013) Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: a SIMS/EMPA trace element study. Geochim Cosmochim Ac 104:42–62
Ren CG, Kong CC, Wang SX, Xie ZH (2019) Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere 217:773–779
Rizwan M, Ali S, Rehman MZU, Rinklebe J, Tsang DCW, Bashir A, Maqbool A, Tack FMG, Ok YS (2018) Cadmium phytoremediation potential of Brassica crop species: a review. Sci Total Environ 631-632:1175–1191
Rostami S, Azhdarpoor A (2019) The application of plant growth regulators to improve phytoremediation of contaminated soils: a review. Chemosphere 220:818–827
Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I (1995) Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 13:468–474
Santos ODH, Carvalho CD, da Silva GA, dos Santos CG (2015) Manganese ore tailing: optimization of acid leaching conditions and recovery of soluble manganese. J Environ Manag 147:314–320
Shettima AU, Hussin MW, Ahmad Y, Mirza J (2016) Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr Build Mater 120:72–79
Shi X, Wang SF, Sun HJ, Chen YT, Wang DX, Pan HW, Zou YZ, Liu JF, Zheng LY, Zhao XL, Jiang ZP (2017) Comparative of Quercus spp. and Salix spp. for phytoremediation of Pb/Zn mine tailings. Environ Sci Pollut R 24:3400–3411
Shim D, Kim S, Choi YI, Song WY, Park J, Youk ES, Jeong SC, Martinoia E, Noh EW, Lee Y (2013) Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere 90:1478–1486
Sibanda T, Selvarajan R, Msagati T, Venkatachalam S, Meddows-Taylor S (2019) Defunct gold mine tailings are natural reservoir for unique bacterial communities revealed by high-throughput sequencing analysis. Sci Total Environ 650:2199–2209
Singer PC, Stumm W (1970) Acid mine drainage-rate determining step. Sci 167:1121–1123
Stumbea D, Chicoș MM, Nica V (2019) Effects of waste deposit geometry on the mineralogical and geochemical composition of mine tailings. J Hazard Mater 368:496–505
Sun W, Wang HJ, Hou KP (2018) Control of waste rock-tailings paste backfill for active mining subsidence areas. J Clean Prod 171:567–579
Tang L, Hamid Y, Zehra A, Sahito ZA, He ZL, Hussain B, Gurajala HK, Yang XE (2019) Characterization of fava bean (Vicia faba L.) genotypes for phytoremediation of cadmium and lead co-contaminated soils coupled with agro-production. Ecotox Environ Safe 171:190–198
Torres E, Lozano A, Macías F, Gomez-Arias A, Castillo J, Ayora C (2018) Passive elimination of sulfate and metals from acid mine drainage using combined limestone and barium carbonate systems. J Clean Prod 182:114–123
Vargas F, Lopez M (2018) Development of a new supplementary cementitious material from the activation of copper tailings: mechanical performance and analysis of factors. J Clean Prod 182:427–436
Viers J, Grande JA, Zouiten C, Freydier R, Masbou J, Valente T, de la Torre ML, Destrigneville C, Pokrovsky OS (2018) Are Cu isotopes a useful tool to trace metal sources and processes in acid mine drainage (AMD) context? Chemosphere 193:1071–1079
Wang L, Ji B, Hu YH, Liu RQ, Sun W (2017) A review on in situ phytoremediation of mine tailings. Chemosphere 184:594–600
Wei ZW, Hao ZK, Li XH, Guan ZB, Cai YJ, Liao XR (2019) The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci Total Environ 670:950–960
Wu SL, Liu YJ, Southam G, Robertson L, Chiu TH, Cross AT, Dixon KW, Stevens JC, Zhong HT, Chan TS, Lu YJ, Huang LB (2019) Geochemical and mineralogical constraints in iron ore tailings limit soil formation for direct phytostabilization. Sci Total Environ 651:192–202
Xi CP, Zheng F, Xu JH, Yang WG, Peng YQ, Li Y, Li P, Zhen Q, Bashir S, Liu JL (2018) Preparation of glass-ceramic foams using extracted titanium tailing and glass waste as raw materials. Constr Build Mater 190:896–909
Xiang JY, Huang QY, Lv W, Pei GS, Lv XW, Bai CG (2018) Recovery of tailings from the vanadium extraction process by carbothermic reduction method: thermodynamic, experimental and hazardous potential assessment. J Hazard Mater 357:128–137
Yang B, Shu WS, Ye ZH, Lan CY, Wong MH (2003) Growth and metal accumulation in vetiver and two Sesbania species on lead/zinc mine tailings. Chemosphere 52:1593–1600
Yao G, Liu Q, Wang JX, Wu P, Lyu XJ (2019) Effect of mechanical grinding on pozzolanic activity and hydration properties of siliceous gold ore tailings. J Clean Prod 217:12–21
Ye MY, Yan PF, Sun SY, Han DJ, Xiao X, Zheng L, Huang SS, Chen Y, Zhuang SW (2017) Bioleaching combined brine leaching of heavy metals from lead-zinc mine tailings: transformations during the leaching process. Chemosphere 168:1115–1125
Yin SH, Wang LM, Wu AX, Kabwe E, Chen X, Yan RF (2018a) Copper recycle from sulfide tailings using combined leaching of ammonia solution and alkaline bacteria. J Clean Prod 189:746–753
Yin ZG, Sun W, Hu YH, Zhang CH, Guan QJ, Wu KP (2018b) Evaluation of the possibility of copper recovery from tailings by flotation through bench-scale, commissioning, and industrial tests. J Clean Prod 171:1039–1048
Yoon J, Cao XD, Zhou QX, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Yu XM, Li YX, Li YM, Xu CH, Cui YL, Xiang QJ, Gu YF, Zhao K, Zhang XP, Penttinen P, Chen Q (2017) Pongamia pinnata inoculated with Bradyrhizobium liaoningense PZHK1 shows potential for phytoremediation of mine tailings. Appl Microbiol Biotechnol 101:1739–1751
Yu FM, Li Y, Li FR, Li CM, Liu KH (2019) The effects of EDTA on plant growth and manganese (Mn) accumulation in Polygonum pubescens Blume cultured in unexplored soil, mining soil and tailing soil from the Pingle Mn mine, China. Ecotox Environ Safe 173:235–242
Zhang L (2012) Recycling and utilization of mine tailings as construction material through geopolymerization. US EPA Hardrock Mining Conference 3:19–25
Zhang YL, Li HM, Yu XJ (2012) Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation. J Hazard Mater 213-214:167–174
Zhang X, Yang HH, Cui ZJ (2018) Evaluation and analysis of soil migration and distribution characteristics of heavy metals in iron tailings. J Clean Prod 172:475–480
Zhang Y, Zhang TA, Dreisinger D, Lv CX, Lv GZ, Zhang WG (2019) Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J Hazard Mater 369:632–641
Acknowledgments
The authors are very grateful to the anonymous reviewers for their revising suggestions.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 41603117).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, DM., Zhan, CL., Liu, HX. et al. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ Sci Pollut Res 26, 35657–35669 (2019). https://doi.org/10.1007/s11356-019-06555-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06555-3