Abstract
In the past mines left their waste rock and tailings to weather, filling valleys, lakes and/or rivers. It wasn’t until the end of WWII, that uranium mining wastes became a public concern. Mine waste remediation started gradually with erosion control and prevention of dust storms. Base metal mines started waste remediation gradually later, with the same objectives. Tailings surfaces of base-metal mines were stabilized with lime and grass covers. Some of these abandoned sites were invaded by native plants which were thought to transport radionuclides into the food chain, but no evidence of bioconcentration was found. However, acid and alkaline effluents remained of concern. Government, jointly with the mining industry, funded not only ecological inventories of tailings, but soon also programs addressing acid and alkaline contaminants in waste effluents. Boojum Research was funded within these programs to address these contaminants and provide decommissioning planning. Referring to comprehensive articles for readers interested in details, the chapter explains a main driving force for the improving waste management and effluent containment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Environmental awareness
- Sulfide backfilling
- Radiation safety
- Acid rock drainage
- Reactive Acid Tailings Sulfide Program
- National Uranium Tailings Program
- Mine Environment Neutral Drainage
- Food chain contamination
- Constructed wetlands
- Inorganic contaminants
- Municipal waste
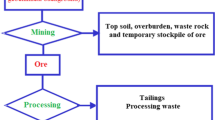
For centuries, mine wastes, were just that – wastes. Miners left waste rock and tailings to natural weathering processes, where atmospheric precipitation carried the weathering products through the wastes, creating acid mine drainage. Calculations based on weathering rates suggest that mine effluent contamination from many mines will continue for hundreds or thousands of years, since weathering occurs both under both aerobic and anaerobic conditions (Kalin & van Everdingen, 1988). Over time, our understanding of the origin and production of mine wastes has led to improvements in mine waste management. Several methods and technologies over the last century have emerged to lessen the impact of these wastes on the environment. Mine management practices have further evolved with the rise of environmental awareness. Table 4.1 summarizes mine waste management in the mining sector, comparing past and present (last 50 years) practices (Kalin, 2004). The comparisons of the past and the present reflect largely an astute awareness that mining wastes are presently confined to the mine waste management area, leaving a smaller footprint. In addition to this progress, the long-term generation of acid mine drainage is recognized through financial assurances since the early nineties, expressed well in an article in the Mining Journal entitled “No Simple Solution” (Knapp & Walsh, 1991). Financial assurances for perpetual treatment are the accepted solution for the decommissioning of a mine waste management area. These changes were initiated mainly in the uranium industry and have been translated to other mining operations. However, they have not necessarily been implemented worldwide. None of the waste management practices listed in Table 4.1 address the role of microbes in generating contaminants. Remediation strategies have been selected based on physical confinement and chemical reactions (neutralization reagents), relying upon retention of contaminants through the reduction or exclusion of oxygen, followed by application of neutralizing agents in water treatment plants.
Several practices have been implemented in the mining industry to reduce the environmental impact. The first practice is segregating the sulfides and backfilling underground workings with a high density paste made from tailings. Initially the practice of backfilling high sulfide wastes was considered an environmentally desirable option, but experience in some Canadian mines demonstrated that the high-sulfide waste used for backfill could catch fire and prevent further mine operation. Thus, for safety reasons, the sulfur content of mine backfill is severely restricted. Generally, though, the volume of broken rock and tailings exceed the volume of the cavities created by mining. Hence, it is not possible for all generated wastes to be accommodated in the mine voids from which they were extracted. The surplus must be stockpiled, unless a use can be found for it as aggregate (sand or gravel), if the sulfur content is negligible. The challenge of isolating or otherwise finding beneficial uses for waste rocks and tailings from open pit operations remains.
The overburden and waste rock from open pit operations must be stockpiled outside the pit during active pit operation and can comprise up to ten times the ore volume. Returning this waste material to the pit when it is mined out is generally prohibitively expensive. Sometimes pit benches are filled with rocks allowing a high hydraulic conductivity. This allows groundwater to flow along the pit walls rather then through the tailings. It is called surround grout construction. Thus, when the pit is mined out, where possible, the void is filled with water by force flooding or allowing the groundwater and rain to gradually fill, creating pit lakes. In Germany, the former coal mining voids (open cast mines) were filled with river water (Jordans, 2018), although not all operations have been successful, as iron-laden water has emerged in the Spree river (IGB [Leibnitz Institut für Gewässerökologie und Binnenfischerei], 2018).
There are companies that segregate sulfides in tailings and store them under water, pending the day when they can be economically processed. It is also common practice to use and isolate waste rock and tailings as backfill in underground mining operations. Tailings-paste fill operations use the non-sulfide fraction of the tailings backfill after thickening, allowing immediate recycling of water. Sand-fill operations have the option of thickening the slime portion at the concentrate level, producing a thickened product as a valuable, impermeable cover for old tailings and waste rock deposits. Both processes allow the immediate recycling of water, a useful measure.
4.1 Mine Waste Site Ecology: The Beginning and Food Chain Contamination
The generally accepted restoration technique for mining wastes applies lime and fertilizer, followed by crimping of straw (GARD Guide; Verburg et al., 2009). This is usually followed by seeding with a commercial grass seed/legume mixture. The reclamation of uranium tailings in Canada followed the same methodology. In some early mines in the Northwest Territories of Canada, though, the tailings areas were left for indigenous species to repopulate.
The roots of naturally invading trees and shrubs were likely to penetrate deeper than the roots of the grass and legume covers, concentrating toxic metals from the tailings in their tissues. This led governmental regulators and scientists to raise concerns over potential food chain contamination through this indigenous vegetation. Of serious concern were the long-lived radionuclides contained in the uranium mining wastes.
The Institute of Environmental Studies (IES) at the University of Toronto, Ontario, Canada launched investigations of the indigenous flora on alkaline, barren uranium tailings abandoned for 10 to 15 years. A diverse flora of indigenous, terrestrial, and aquatic biota was found (Kalin, 1984). A total of 15 uranium tailings sites were studied, both acidic and alkaline, and re- and un-vegetated, in the province of Ontario, Canada. Later the uranium mine sites in the Northwest Territories and the Province of Saskatchewan (Kalin, 1985) were included. Another part of the funding supported an MSc thesis (Caza, 1983) to study the growth and colonization of Trembling Aspen on uranium tailings (Fig. 4.1a).
Radium-226, Uranium-236, and Lead-210 concentrations were determined in both terrestrial and aquatic vegetation as well as in the tailings around the root areas. As a result of this work, it became evident that indigenous terrestrial plants posed no threat to the food chain, as the radionuclides and most metals remained generally in the roots and surrounding soils. Tree roots form a dense carpet-like structure below the grass cover on seeded tailings (Fig. 4.1b), surrounded by iron precipitate (Figs. 4.1b, c). The vegetated surface covers reduced wind dispersal and erosion of the tailings, while decreasing rainwater infiltration. Figure 4.1c highlights the difference between oxidized and unoxidized areas of the tailings. The oxidized areas (brown) contained elevated levels of radionuclides.
4.2 Boojum and Government/Industry Programs
The uranium industry was one of the first to include environmental issues in their close-out plans, as public awareness raised these issues in the late 1960s. From these efforts, several principles were developed to govern management practices, such as ALARA (As Low as Reasonably Achievable) for radiation safety at uranium operations. It was later followed by BATEA (Best Available Technology Economically Achievable) for all other mining operations (Pouw et al., 2015). Comprehensive historical reviews of risks and environmental policy have been written by Faber & Wagenhals (1988) and Kamieniecki & Kraft (2013). These efforts are commendable and have brought about significant change in the mining industry.
The accepted treatment of contaminated drainages from both tailings and waste rock piles has remained the same for decades. Neutralizing agents, such as lime, are added to acid streams leaving a metal-laden sludge behind which needs further stabilization. The reactivity of the sludge depends on the pH of effluent, the lower the pH the greater the reactivity (McDonald et al., 2006). It is often returned to the tailings piles. The neutralisation leads to an increase in pH with the formation of inorganic particulates, which settle out of the water column, either with time or supported by flocculating agents. With aging of the sludge, and through microbial activity, the metals are released again. A research team at NRCan (Natural Resources Canada) addressed the stability of the resulting neutralizing sludge and concluded: “Current sludge management practices are ad hoc and frequently do not address long-term storage” (Zinck, 2006).
INAP (International Network for Acid Prevention) has created a guideline, the GARD Guide (Global Acid Rock Drainage: GARD), which is an internationally recognized guide to the prediction, prevention, and management of drainage produced from sulfide mineral oxidation (Verburg et al., 2009; Kleinmann & Chatwin, 2011). In accordance with the guide, most current mine operation practices emphasize containment of the wastes, thereby reducing the volume of effluent, not its quality. These containment practices require significant financial commitment from the operating mining company. But, while the management practices outlined in the GARD Guide certainly reduce the immediate environmental impacts, they may, in many ways, delay the onset of longer-term mine drainage issues. These entrenched practices are a hindrance to novel approaches to mine waste management and the acid challenge.
Remediation efforts and drainage treatment are viewed by some in industry and government as ‘the price to be paid’ and therefore accepted as part of mining and metal extraction. To some degree, physical and chemical aspects of natural weathering processes are abated by present mining practices, but the fundamental contribution of microbial populations is ignored. Herein lies the long-term challenge. Only when the microbial oxidation is controlled will long-term weathering processes subside. Hence, current mine environmental management practices are, in a true sense, not sustainable.
While discussions on the food chain were ongoing, some regulators were starting to think about the idea of declaring mine wastes as hazardous materials. Mine wastes are rocks, broken or ground, and certainly not hazardous. They need appropriate handling, as rocks are part of nature, supplying essential elements supporting living systems in the aquatic, terrestrial and even in the atmospheric areas of the planet. The challenge arises due to the very large surface area of mineralized rock that is exposed. The weathering of this rock releases an excess of some elements which, in many cases are toxic to the surrounding ecosystems, altering the pH and the electrical conductivity- two key drivers of ecosystem change.
As food chain contamination was no longer a pressing issue, concerns turned to contaminated fresh- and groundwater. The uranium industry in Canada anticipated the development of more stringent environmental regulations, and was seeking sustainable, ecological approaches to address drainages from their wastes and for decommissioning of mine waste management areas. The Canadian government then funded a 5-year program, the National Uranium Tailings Program (NUTP), in 1981, to address the long-term environmental impact of uranium tailings. The long-term goal for these was to seek a sustainable approach to the decommissioning of mine waste management areas. With the encouragement of the government and the uranium industry, Boojum Research Ltd. (Boojum) was founded in 1982 as an R&D company. Its objective was to find long-term, sustainable, economic solutions to mine closures.
Boojum Research’s first assignment under NUTP was to remove contaminants from alkaline uranium mine waste holding ponds. Several abandoned, pH-neutral, tailings ponds were investigated for their indigenous aquatic floras. In base metal and gold tailings, extensive meadows of Chara vulgaris were found growing (Figs. 4.2a, b). These algae appeared to be ideal biological polishers, as they were also fast growing, and did not transport contaminants from the sediment back into the water when the biomass decayed, but relegated the biomass and contaminants into the sediment.
Since phosphate is often limiting to aquatic plant growth, supplementing phosphate to Chara was investigated to alleviate one of the forcing functions restricting their growth and productivity. The effects of phosphate on the growth of Chara supported by NRC IIPAP funds, the Masters thesis of M. P. Smith (1988). Forcing functions are defined as one or more resources that halt or slow progression of further development (see Chapter 5 for details). This research was supported by Boojum and an IRAP grant (Industrial Research Assistance Program of the National Research Council of Canada) and lead to introduction of Chara as bio-polishers to ponds where they did not previously exist. After several failures, the algae were finally established in several tailings ponds, supporting metal and radionuclide removal (Kalin & Smith, 1986).
The NUTP research program was followed in 1983 by the Reactive Acid Tailings Sulfide Program (RATS). This program focused on modeling, prediction, and methodologies to reduce or remediate the long-term environmental effects of acid-generating materials (John & Joe, 1987). Boojum’s first project under the RATS program took place on a tailings site covered with a hard-oxidized crust of pyrrhotite (FeS). When unoxidized pyrrhotite is exposed to moisture, it starts to burn. Rains produced acid run-off. Further, the mining company could not risk using heavy re-vegetation equipment to establish a cover, as the crust could not carry the weight of the heavy equipment. The crust would break, exposing the un-oxidized FeS, and rain or moisture would ignite it.
Field surveys showed that cattails (Typha sp.), moss, horsetails (Equisitum sp.), and blue-green algae (cyanophytes) grew along the banks of a nearby alkaline mine slime stream. The alkaline stream resulted from washing explosives from the underground walls or using shotcrete to cover acid-generating walls (Jones & Wong, 1994). Similar organisms were found growing on the tailings, mostly associated with sticks or rocks, despite the reactivity of the pyrrhotite. On the edge of the barren tailings crust, vegetation was noted, similar to that along the alkaline creek. The distribution patterns were comparable to those observed on uranium tailings several years earlier (Kalin, 1984). Detailed measurements in the colonized areas generally produced hints that physical topography (rocks providing shade or decaying wood) and/or chemical conditions were growth-supporting factors.
The FeS tailings needed to be covered to reduce the acid run-off. The first level of ‘vegetation’ included a moss cover as it could colonize bare surfaces. Early attempts to grow moss in greenhouses and onsite were unsuccessful, but with the proper fertilizer and shade, a green haze developed in some of the boxes in the greenhouse trials (Fig. 4.3a). The treatment that produced the greenest boxes was translated to the field, where trials were run to determine the best season for starting (Fig. 4.3b). One example of successful plant growth was an island overgrown with horsetails (Equisitum sp.; Fig. 4.3c). This overgrowth had established without our help and served as an example for our trials. At the end of the RATS program, we recommended to the mine operators that they create permeable dikes (composed of coarse, un-compacted, larger waste rocks) and divert mine slime streams into these dikes. The mine manager implemented the recommendations and after several growing seasons, native horsetails and moss covered the site. A photo taken 10 years later shows the success of the treatment (Fig. 4.3d).
Ecological engineering measures for stabilization and cover for pyrrhotite tailings. (a) Tailings covered with alkaline mine slimes fosters colonization by indigenous plants. (b) Experiments to fertilize the pyrrhotite tailings surface at various times during the season. Fall fertilization was successful. (c) Horsetails colonized the alkaline mine slimes without any fertilizer. In background are the permeable waste rock dikes to accumulate mine slimes. (d) Tailings several years after decommissioning recommendations implemented. (Photographs by Boojum Research)
In addition to the alkaline stream on the pyrrhotite tailings, a slow-moving, acidic (pH 2.5) creek was chosen on the site to address the forcing functions for aquatic acid systems (Fig. 4.4a). The creek water contained high concentrations of sulfate (4–6 g.L−1 SO4) and dissolved iron (1–2 g.L−1). Loose straw (not bales) was used as an organic carbon amendment for microbial growth. The straw was added to several sections of the creek (Fig. 4.4b). In the winter, while drilling holes in the ice cover, hydrogen sulfide was released. In the spring, clear water was found in the straw-filled section. Within the straw the pH was up to about 3.5, a remarkable increase from the low of 2.5 (Fig. 4.4c).
The improvements in the creek water had been clearly induced by microbial activity. The ice cover on the creek slowed the flow, while reducing wind-driven mixing and oxygen diffusion. Heterotrophic microbes growing on the straw consumed oxygen, lowering the redox potential of the water. The combination of low oxygen and organic carbon fostered the growth of anaerobic, iron- and sulfate-reducing microbes. Stumm & Morgan (1996; p. 477) provided a clear explanation for geomicrobiology processes. In general, a group of heterotrophic microbes alters the surrounding growth conditions by making them a little less oxidative. This, in turn, provides the proper conditions for the next microbe group to lower the redox state even further. When the local conditions are reducing, microbes such as iron and sulfate-reducing microbes will precipitate iron onto the straw while increasing the pH. The emerging smell of H2S, the rotten egg smell noted in the winter suggested that not enough oxidized iron had remained in the water to form iron sulfide. If the pH had been high enough and the Eh low enough some pyrite could possibly have formed (Fernández-Remolar et al., 2003; Reitner & Thiel, 2011).
These experiments provided key observations for mine waste and water management, such as:
-
No microbes were needed to seed the acidic water; they invade or awake when food is available.
-
Iron precipitate covered the straw, reducing access to the organic carbon, not desirable.
-
Ice cover reduced oxygen access, giving anaerobic microbes a chance to flourish.
Iron reduction by microbes raises pH, and this, in turn, leads to the in-situ metal precipitation. To reproduce these conditions in any mine effluent, two things needed to be developed. First, a living, floating vegetation cover would replace the ice cover. This would provide a continuous supply of organics, through decomposing litter and root exudates, and it would also decrease wind mixing of the water. Second, an iron-precipitation pond is needed upstream of the living cover to prevent intense iron encrustation of the root systems. In the creek, the straw became encrusted with iron, forming secondary mineral spheres (Fig. 4.5). The straw provided organic carbon supporting the establishment of oxygen-consuming microbes. The microbial-based treatment system thus developed was named Acid Reduction Using Microbiology or ARUM.
The RATS program was followed by the Mine Environment Neutral Drainage (MEND) program in 1989. All programs were under the auspices CANMET (Canada Centre for Mineral and Energy Technology) of the NRC (Natural Resources Canada). MEND funding supported projects developing constructed wetlands for the treatment of AMD. Officials of these programs expected the same successes found while treating organic waste waters. They anticipated that same processes would take place with inorganic substances (Kadlec & Knight, 1996; Mitsch & Gosselink, 2000).
The stated objective for MEND, as Boojum understood it, was to “...develop technologies to prevent and control acidic drainage, or -- how to stop the lime trucks.” These technologies were to address decommissioning of mine waste and water management areas, working within the wastewater management areas, before discharge to the receiving environment. The technologies to be developed were to provide acceptable conditions for a sustainable, walk-away so that the treatment plant could eventually shut down.
Although Boojum Research obtained funding under all government/industry programs it continued under MEND, but eventually differences in objectives lead to a divergence. Boojum Research focused on containing the weathering products within the waste and water management area (In Chapters 8 and 9 detail), whereas MEND reviewers were seeking solutions in wetlands (Kadlec & Knight, 1996).
The construction of microbially-active sediments and the addition of targeted nutrients to reduce or deactivate the predominantly oxidative environment within wastes is the focus of our ecological engineering solutions. The goal was the development of ecological tools which would improve the drainage leaving the site. Hence, Boojum’s challenge was to determine the forcing functions within the mine wastes, alleviate them, and have natural bio-geochemical processes transfer contaminants to sediments and transform them back into ore bodies of the future (Debus, 1990).
References
Caza, C. (1983). “Biology of P. tremuloides on abandoned Uranium mill tailings sites near Bancroft, Ontario”. M.Sc. Thesis, University of Toronto, Department of Botany.
Debus, K. (1990). Mining with microbes. Technology Review, 93(6), 50–57.
Faber, M., & Wagenhals, G. (1988). Towards a long-term balance between economics and environmental protection. In W. Salmons & U. Förstner (Eds.), Environmental management of solid waste (pp. 227–242). Springer.
Fernández-Remolar, D. C., Rodriguez, N., Gómez, F., & Amils, R. (2003). Geological record of an acidic environment driven by iron hydrochemistry: The Tinto River system. Journal of Geophysical Research: Planets, 108(E7).
IGB. (2018). Sulfate in River Spree and Lake Müggelsee. https://www.igb-berlin.de/en/project/sulfate-river-spree-and-lake-Muggelsee
John, R., & Joe, E. (1987). CANMET’s Tailings Research Programs—An update. In Proceedings of the 11th annual British Columbia mine reclamation symposium in Campbell River, BC, The Technical and Research Committee on Reclamation (pp. 105–115). Available online.
Jones, C. E., & Wong, J. Y. (1994). Shotcrete as a cementitious cover for acid generating waste rock piles. In Proceedings of the international land reclamation and mine drainage conference and 3rd international conference on the Abatement of Acidic Drainage (Vol. 24, pp. 104–112).
Jordans, F. (2018). Germany turns former coal mines into vast lakeside resorts. The Durango Herald, 6, 22–2018. https://durangoherald.com/articles/229327
Kadlec, R. H., & Knight, R. L. (1996). Treatment wetlands. CRC Lewis Publisher sop 881, ISBN 0-87371-930-1.
Kalin, M. (1984). Port Radium, Northwest Territories: An evaluation of environmental effects of the uranium and silver tailings. University of Toronto, Institute for Environmental Studies. Retrieved from https://zone.biblio.laurentian.ca/handle/10219/3016
Kalin, M. (1989). Ecological engineering and biological polishing: methods to economize waste management in hard rock mining. In W.J. Mitch & S.E. Jorgensen (Eds.), Ecological engineering (pp. 443–461). wiley & Sons. ISBN 0-471-62559-0
Kalin, M. (1998). The Role of Applied Biotechnology in Decommissioning Mining Operations. Proceedings of the 30th Annual Meeting of the Canadian Mineral Processors, Ottawa, January 20–22 (pp. 154–167).
Kalin, M. (2004). Slow progress in controlling acid mine drainage (AMD): A perspective and a new approach. Peckiana, Staatliches Museum für Naturkunde Görlitz, 3, 101–112.
Kalin, M., & Smith, M. P. (1986). Biological polishing agents for mill wastewater. An example: Chara. In R. W. Lawrence, R. M. R. Branion, & H. G. Ebner (Eds.), Fundamental and applied biohydrometallurgy (p. 491). Elsevier.
Kalin, M., & van Everdingen, R.O. (1988). Ecological Engineering: Biological and geochemical aspects. Phase I experiments, In W. Salomons & U. Foerstner (Eds.), Environmental management of solid waste (pp. 114–128). Springer-Verlag. ISBN 3-540-18232-2.
Kamieniecki, S., & Kraft, M. (2013). The Oxford handbook of US environmental policy. Oxford University Press. Retrieved from https://global.oup.com/academic/product/the-oxford-handbook-of-us-environmental-policy-9780199744671?cc=ch&lang=en&#
Kleinmann, R. L., & Chatwin, T. (2011). The GARD Guide and its general applicability to mine water issues. In Proceedings, American Society of mining and reclamation, Bismarck, North Dakota Reclamation: Sciences Leading to Success June 11–16 (pp. 317–325).
Knapp, R., & Walsh, D. (1991). No simple solution, CIM Bulletin, June 1991, 63–66.
McDonald, D. M., Webb, J. A., & Taylor, J. (2006). Chemical stability of acid rock drainage treatment sludge and implications for sludge management. Environmental Science and Technology, 40(6), 1984–1990. https://doi.org/10.1021/es0515194
Mitsch, W. J., & Gosselink, J. G. (2000). Wetlands, 3rd Edition (p. 920).
Reitner, J., & Thiel, V. (Eds.). (2011). Encyclopedia of Geobiology (Encyclopedia of earth science series). Springer.
Pouw, K., Campbell, K., & Babel, L. (2015). Best Available Technologies Economically Achievable to manage effluent from mines in Canada. In 10th International Conference on Acid Rock Drainage and IMWA Annual Conference (pp. 1–10).
Smith, M. P. (1988). Phosphorus Nutrition of Chara vulgaris L. MSc Thesis University of Toronto, 69 pp.
Stumm, W., & Morgan, J. (1996). Aquatic chemistry: Chemical equilibria and rates in natural waters (3rd ed.). Wiley.
Verburg, R., Bezuidenhout, N., Chatwin, T., & Ferguson, K. (2009). The global acid rock drainage guide (GARD Guide). Mine Water and the Environment, 28(4), 305.
Zinck, J. (2006). Disposal, reprocessing and reuse options for acidic drainage treatment sludge. In 7th International Conference on Acid Rock Drainage (ICARD) (pp. 2604–2617).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kalin-Seidenfaden, M. (2022). Waste Management: A Brief History and the Present State. In: Kalin-Seidenfaden, M., Wheeler, W.N. (eds) Mine Wastes and Water, Ecological Engineering and Metals Extraction. Springer, Cham. https://doi.org/10.1007/978-3-030-84651-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-84651-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84650-3
Online ISBN: 978-3-030-84651-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)