Abstract
Out of 29 plant species taken into consideration for biodiversity investigations, the present study screened out Cyperus rotundus L., Calotropis procera (Aiton) W.T. Aiton, Croton bonplandianus Baill., Eclipta prostrata (L.) L., and Vernonia cinerea (L.) Less. as the most suitable metal-tolerant plant species (high relative density and frequency) which can grow on metal-laden fly ash (FA) lagoon. Total (aqua-regia), residual (HNO3) and plant available (CaCl2) metal concentrations were assessed for the clean-up of metal-contaminated FA disposal site using naturally colonized plants. The total metal concentration (in mg kg−1) in FA followed an order of Mn (229.8) > Ni (228.4) > Zn (89.4) > Cr (61.2) > Pb (56.6) > Cu (51.5) > Co (41.9) > Cd (9.7). The HNO3- and CaCl2-extracted metals were 0.57–15.68% and 0.03–7.82% of the total metal concentration, respectively. The concentration of Ni and Cr in FA in the present study was highest among the previously studied Indian and average world power plants and Cd, Ni, and Cr were above soil toxicity limit. The variation in total, residual, and plant-available metal (single extraction) concentration indicated the presence of different proportions of metals in FA lagoon which affects the metal uptake potential of the vegetation growing on it. It has been reported that plant-available metal extractant (CaCl2) is the most suitable extractant for assessment of metal transfer from soil to plant. However in the present study, Spearman’s correlation showed best significant correlation between total metal concentration in FA and shoot metal concentration (r = 0.840; p < 0.01) which suggest aqua-regia as the best extractant for understanding the bioavailability and transfer of metal, and in calculation of BCF for moderately contaminated site. It can be stated that plant-available extractant is not always suitable for understanding the availability of metal, but total metal concentration can provide a better insight especially for moderate or low metal-contaminated sites. Principle component analysis revealed that all the plants showed positive correlation with Co and Cd which suggest its subsequent uptake in root and shoot. The biological indices (BCF, BAF, and TF) revealed that E. prostrata (10 mg Cd kg−1) and C. procera (3.5 mg Cd kg−1) can be utilized efficiently for the phytoextraction of Cd and phytostabilization of other potentially toxic metals (Pb, Cr, and Co) from FA lagoon. All the plants were tolerant to Pb pollution (TF > 1, BAF > 1, and BCF > 1); hence, there was a negligible translocation of Pb to the aerial tissues of these plants which shows their suitability in phytostabilization. In addition, V. cinerea accumulated elevated concentration of potentially toxic Cr (50 mg Cr kg−1) and Ni (67 mg Ni kg−1) which could also help in the phytoremediation of FA lagoon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An exceptional intensification in the demand of electricity during the past decades has surpassed all projections and has become a serious menace to the environment. In India, thermal power plants relying on combustion of coal meet this increased demand by contributing 59% of the total electricity production in the country (CEA 2017). The global discharge of fly ash (FA) by thermal power plants has reached to an extent of approximately 600 million tons per year (Ram and Masto 2014). The Indian coal is of low grade, having high ash content of the order of 30–45% (average 35%), generating large quantity of fly ash at coal/lignite-based thermal power stations in the country. For generating 1 MW of electricity using Indian coal, approximately 1800 t of FA is produced (CEA 2014), out of which 20% is wasted due to gravity which is removed as bottom ash and the residual part is FA. This FA is accumulated in ash ponds in the form of thin slurry by using hydraulic transportation techniques. These FA ponds empower global environmental issues by contaminating surrounding aquatic, aerial, and terrestrial ecosystems due to seepage, leaching, and wind erosion.
Phytoremediation is a useful technique in restoring the physical, chemical, and biological properties of metal-laden soils (Prasad 2011; Hattab et al. 2014; Sharma et al. 2015; Antoniadis et al. 2017). The identification of metal-tolerant species from plant communities affected by metal pollution through extensive biodiversity survey is a neglected domain though bioaccumulation/phytoextraction studies with wild plant varieties in FA-affected sites are available in recent literatures (Krgovic et al. 2015; Pandey et al. 2016). However, recent works suggest that screening the metal-tolerant plants following biodiversity sampling methods have better and more logical approach than randomly selected plants for bioaccumulation studies. Hernandez and Pastor (2008) have assessed the effect of metal pollution on the grassland biodiversity of abandoned metal mines in Sierra de Guadarrama, Spain, using biodiversity indices and concluded that Zn has the greatest effect on biodiversity followed by Cd, Cu, and Pb. Chowdhury and Maiti (2016) have compared the mangrove biodiversity of polluted and non-polluted sites to screen out four metal-tolerant halophytes (Avicennia officinalis L., Cryptocoryne ciliata (Roxb.) Fisch. ex Wydler, Heliotropium curassavicum L., and Hemarthria altissima (Poir.) Stapf & C.E.Hubb.) from a mangrove patch exposed to tannery effluent in Indian sundarbans. Similar ecological region exposed to varied level of pollution stress must affect the succession pattern, dominance, and community structure of the plants favoring pollution-tolerant species in stressed areas and normal assemblage in the pollution-free area. Replenishment of vegetation cover can reduce the horizontal or vertical spreading of the contaminants, erosion, and the risk of leaching of potentially toxic contaminants to groundwater. However, it is important to assess the availability of metal for proper establishment of plant community.
It is repeatedly reported in literature that total metal concentration gives an objectionable indicator of metal availability to plants whereas bioavailable/plant-available metal concentration gives better insight for assessment of metal transfer from soil to plant (Hammer and Keller 2002; Szarek-Lukaszewska and Niklinska 2002; Yoon et al. 2006; Remon et al. 2013; Pratas et al. 2013). The bioavailability of these metals can be assessed by evaluating the concentration of extractable metals that are available to plants for uptake. The various in situ methods for metal extraction to study the bioavailable and exchangeable metal concentration have been used by many researchers for studying the phytoavailability of heavy metals (Margui et al. 2006; Gajic et al. 2016). A variety of extractants have been used for accounting exchangeable, carbonate-bound, Fe–Mn oxides bound; organically bound; and residual metals present in FA (Jiao et al. 2016; Dar et al. 2017). CaCl2 is known to be a suitable extractant for assessing the bioavailability of metals (Pueyo et al. 2004). Nitric acid being a strong extractant extracts metals from all fractions excluding the insoluble residual fraction (Lavado and Porcelli 2000).
In the present study, it was hypothesized that metal pollution must have selection pressure on a similar community, so it would affect the abundance of the species. And, this process could be used to identify/screen pollutant-tolerant plants by comparing a pollution-affected community with a non-polluted site. In addition, it is checked in the study whether really bioavailable extractant is the most suitable for assessment of metal transfer from soil to plant. Various researchers have assessed the total and bioavailable metal concentrations in FA lagoons/deposits of different thermal power plants (Kumari et al. 2016; Pandey and Mishra 2016). Considering the prominence of environmental problems caused by FA generated by thermal power plants, the present study aims at assessment of total, residual, and bioavailable/plant-available metal concentration present in the rhizospheric soil of different plants growing on the FA lagoon, ascertaining spontaneously colonized metal-tolerant plant species growing on FA lagoons by comparing the biodiversity of polluted site with control site. And finally, by using correlation matrix and biological indices, metal-tolerant plants were identified for phytoremediation of metal-contaminated FA lagoon.
Materials and methods
Study site description
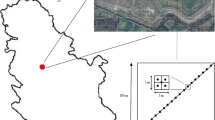
Chandrapura thermal power station (CTPS) is located in the Chandrapura town of Bokaro district in the Indian state of Jharkhand (23°44′23.8″ N, 86°06′46.3″ E; Elevation: 222 m) and operated by Damodar Valley Corporation (Fig. 1). The CTPS has an installed capacity of 920 MW with five units under operation, out of which the first unit was commissioned in October, 1964. It covers an area of 750 ha and is bounded by Damodar river on the south. The climate is humid and sub-tropical characterized by hot and dry summer (maximum temperature reaches upto 46 °C) from March to October and cold winter from November to February.
Biodiversity and dominance assessment
A total of 14 quadrate plots (5 m × 5 m) were randomly selected out of which 7 were located in the control site (CS) and 7 in the FA-polluted site (Fig. 2). In the polluted site, quadrates were selected in the waste land, i.e., in the FA pond, whereas in the control site, they were randomly laid across the vegetation, i.e., the agricultural land in the vicinity of FA pond (about 30 m distance). Work done by Chowdhury and Maiti (2016) highlighted the importance of comparison of plant biodiversity between the community compositions of control and polluted site, prior to the investigation of metal accumulation in selected plant species. In the present study, the same methodology has been followed by comparing the diversity to screen out the plant varieties that have the highest tolerance to a particular stress. A total of 29 different plants from FA and CS were collected and identified, and the five most dominant plant species were selected based on relative density (RD) and frequency (F) for search of a phytostabilizer (in the present study, plants having RD > 10% and F > 85% were considered).
Understory species and ruderals were only considered for biodiversity sampling. The relative density (%) of ruderals and biodiversity indices (Simpson’s Index of Dominance and Diversity, Shannon-Weiner index) were calculated to access the community structure and biodiversity due to heavy metal pollution. Relative density (RD) is an estimate of the numerical strength of a species in relation to all the individuals of all the species, calculated as
Frequency (F) is the distribution or dispersion of an individual species, estimated as percentage of occurrence:
These estimators gives an idea of the status of a particular species in relation to the whole community, which was used in this study to understand the changes in distribution of the plant species between the control and FA-polluted site. Similarity in species composition between the control and polluted areas was calculated by Sorenson’s coefficient of community (CC) (Smith and Smith 2012). This is a widely used index to study the similarity of two communities based on the presence/absence of a particular species, calculated as
where C = common species in both the patches (i.e., polluted and control site), S1 = number of species in polluted site, and S2 = number of species in control site.
The health of the ecosystem was assessed using the Simpson’s index for dominance (D), Simpson’s index of diversity (D′) and Shannon Weiner Index (H′) (Simpson 1949).
Simpson’s index for dominance (D) indicates whether the plant community is evenly distributed or has a biased distribution towards a particular or a group of species (plant association):
Due to the reciprocal relationship between diversity and dominance, the diversity of a particular species increases as the dominance decreases and vice versa. Thus, Simpson’s index of diversity is calculated as
where ni = total number of individual in each species; N = Total number of individual of all species; S = total number of species.
Shannon-Weiner Index (H′) evaluates the health and entropy of the ecosystem which is defined as
where pi = ni/N and ln denotes the natural logarithm.
Collection and chemical characterization of FA and CS
A total of 25 FA samples were collected (five samples from the rhizosphere of each plant species) randomly from the top layer (0–15 cm) of FA lagoon in air tight polyethylene zipper bags and transported to the laboratory. The collected FA samples were air dried at room temperature (30–35 °C) for 1 week, homogenized using a mortar and pestle, and then passed through a 2-mm sieve for further analysis. The pH of FA was measured in FA: water suspension of 1:1 and 1:2.5 (w/v), electrical conductivity (EC) in 1:2.5 (w/v) FA: water suspension (stirring time: 2 h) by using a pH meter (HI 2020, HANNA Instruments, Woonsocket, Rhode island, USA) and conductivity meter (HI 2030, HANNA Instruments, Woonsocket, Rhode island, USA), respectively. Organic carbon was determined as per Walkley and Black (1934) and available nitrogen as per Subbiah and Asija (1956).
Metal analysis of FA
The bioavailability of metals in FA was determined by performing single extraction using 0.01 M CaCl2 and 1 N HNO3 with FA/extractant ratio of 1:10 w/v and shaking time of 2 h (Gupta and Sinha 2007a; Maiti 2007) and total metal concentration in FA was determined by digesting 0.5 g of homogenized air-dried FA sample using a mixture of HNO3 (69%; EMPARTA) and HCl (~ 37%; EMPARTA) (3:1) (Maiti and Jaiswal 2008) in a microwave digester system (ETHOS 1, Milestone SrL, Sorisole, BG, Italy). Then, 1% HNO3 solution was added to the digested mass to make up a volume of 50 mL and filtered using Whatman no. 42 filter (pore size 2.5 μm). The samples were refrigerated until analysis for the determination of metal (Cu, Mn, Zn, Pb, Ni, Cr, Co, and Cd) concentration using flame atomic absorption spectrophotometer (FAAS-GBC Avanta PM, Melbourne, Australia). When the concentration of metals was found below detection limit, it has been concentrated to 1:10. The detection limits were 0.001 mg L−1 for Cu, 0.0015 mg L−1 for Mn, 0.008 mg L−1 for Zn, 0.06 mg L−1 for Pb, 0.01 mg L−1 for Ni, 0.003 mg L−1 for Cr, 0.004 mg L−1 for Co, and 0.0004 mg L−1 for Cd.
Collection and metal analysis of naturally colonized plant species
Out of 29 different plant species, 5 most dominant species (identified on the basis of RD and F)—Cyperus rotundus L., Calotropis procera (Aiton) W.T. Aiton, Croton bonplandianus Baill., Eclipta prostrata (L.) L., and Vernonia cinerea (L.) Less.—were collected each in five replicates from the FA lagoon and transported to the laboratory. The collected plant samples were washed with running tap water and then put in an Ultrasonicator (Branson 2200, Bransonic Ultrasonic Corp. Danbury, CT, USA) with deionized water to remove the adhered FA particles. The aboveground and belowground tissues were separated and dried in a hot-air oven (RDHO 80, REMI Ltd., Mumbai, India) overnight at 80 °C to constant weight. The dried tissues were homogenized using a mortar and pestle for the processing of digestion. The dried and powdered portion of 0.5 g of plant tissue was digested with a mixture of HNO3 and HClO4 (5:1, v/v) (Maiti and Nandhini 2006) in a microwave digester system. After that, the volume was made up to 50 mL using 1% HNO3 and filtered using Whatman no. 42 filter (pore size 2.5 μm). The samples were refrigerated until analysis. Metal (Cu, Mn, Zn, Pb, Ni, Cr, Co, and Cd) concentrations in plant tissues were measured using atomic absorption spectrophotometer.
Biological indices
The plant–metal interaction and metal uptake potential of different plant species was assessed using biological indices (Rani et al. 2017; Kumar et al. 2017). Biological concentration factor (BCF) of metals is calculated as the ratio of the metal concentration in root of the plant to the metal concentration in FA. The biological accumulation factor (BAF) is calculated as the ratio of the metal concentration in root and shoot of the plant to the metal concentration in FA. The translocation factor (TF) is defined as the ratio of the metal concentration in shoot to the metal concentration in root.
Quality assurance and quality control
All analytical grade reagents and calibrated glassware were used. Reagent blanks, duplicates, and spiked samples were used. Standard Reference Materials (AccuTrace, Accu Standard Inc., USA; Matrix 2–5% Nitric acid; CRM uncertainty ± 5%) were used for the preparation and calibration of each analytical batch. The recovery percentage of metals in the samples of soil and plant tissues ranged from 81 to 107%. Calibration coefficients were maintained at a high level ≥ 0.99. Limit of detection (LOD) values were calculated as three times the standard deviation of the blank samples for each measured element.
Statistical analyses
Data were checked for normality (Shapiro-Wilk’s test) and homoscedasticity (Levene’s test). Kruskal-Wallis test was used to check the statistical significant difference between the mean values. Spearman's correlation coefficient (r) was performed at p < 0.01 and p < 0.05 level of significance. Principal component analysis (PCA) was performed for the concentrations of total metal accumulated in the roots and shoots of the plants to assess the behavior/relationship of metals with each other. Normalized variables (original variables) were transformed into the rotated components to extract significant principal components (PCs) by suppressing the contribution of variables with minor significance, after Kaiser–Meyer–Olken (KMO) test (> 0.7) and Bartlett’s test (< 0.1) that determines the appropriateness of data reduction through PCA analysis. Furthermore, these PCs were subjected to varimax rotation with loading coefficients (> 0.1) to generate PC factors/groups. There is always a debate between the uses of orthogonal versus oblique rotation methods. Orthogonal rotation classifies the components/factors with the assumption that they are unrelated to each other. But in the case of metal clusters, unrelated components are easy to read, so varimax orthogonal rotation was used in the current study. All statistical calculations were performed using software SPSS, IBM statistics version 16.0 package and graphs were prepared using Origin 8.
Results and discussion
Effect of metal pollution on plant community composition
Understory and ruderal species are the pioneer species to colonize in a stressed and denuded landscape. Sorenson’s coefficient of community (CC) value of 0.8 affirmed that both the FA-polluted site and control site possessed similar plant assemblage. A total of 29 species were recorded in the study site of which 10 species were absent in the polluted site (Table 1). The Dhoob grass (Cynodon dactylon (L.) Pers.) had highest RD of 10 with a 100% frequency. Ageratum conyziodes L., had a relative density of 7.76 and frequency 100%, followed by Lantana camera L. (RD = 6.9, F = 71%). All the three species generally showed invasive habit but in the control site, a low Simpson’s index for dominance value (D = 0.04) indicated that the understory vegetation around the lagoon was not under invasion stress. In contrast in the polluted site, E. prostrata was the most abundant (RD = 18.9, F = 100%) followed by C. bonplandianus (RD = 12.6, F = 86%), C. procera (RD = 10.5, F = 86%), and V. cinerea (RD = 10.5, F = 86%). C. procera is an invasive species reported in Brazil and is highly tolerant to anthropogenic stress (Sobrinho et al. 2013; Frosi et al. 2013). The Simpson’s index for dominance value (D) for the polluted site was 0.1, which was greater than that of the control site (0.04) and so the control site has showed more diversity than the polluted site. Similarly, control site has higher Simpson’s index for diversity (D′ = 0.96) in comparison to polluted site (0.90).
Shannon-Weiner Index (H′) indicates the health of the ecosystem where the control site has a better H′ value of 3.04 compared to 2.18 of the polluted site. The control site has a healthier community structure than the polluted site. Many hypothesis are used to understand the changes in forest community structure in response to environmental factors that include geomorphological factors, climatic variability, physio-anatomical variations due to changing abiotic parameters, biotic factors such as herbivory, predation, parasitism, and animal plant interactions (Aston and Macintosh 2002; Smith and Smith 2012). Dominant species generally achieve their majority in the ecosystem at the expense of the non-competitive species (Smith and Smith 2012). Here, five species were seen to have the highest relative density (RD) in the polluted site (C. procera, C. bonplandianus, C. rotundus, E. prostrata, and V. cinerea). So, they must have tolerance towards metal pollutants at the vicinity of the FA lagoon and hence these five species have been selected for bioaccumulation studies. C. rotundus (family: Cyperaceae), a perennial grazable grass and a most invasive weed in tropical and temperate regions. C. procera (family: Apocynaceae) is a flowering plant and possess green globes which are hollow and contains toxic milky sap. C. bonplandianus (family: Euphorbiaceae), a medium-sized shrub which grows wild as a weed. E. prostrata (family: Asteraceae) is a small herbaceous plant finding extensive application in folk medicines and is found in tropical and sub-tropical regions of the world. V. cinerea (family: Asteraceae) is a medicinal plant having antibacterial and anti-inflammatory properties (Iwalewa et al. 2003).
Chemical characterization, total, residual, and bioavailable metals in FA
The pHH2O(1:1) (w/v) and pHH2O(1:2.5) (w/v) of rhizospheric FA was circumneutral (ranged between 5.31–7.04 and 5.54–7.39, respectively) (Table 2). The FA is generally alkaline in nature attributing due to the presence of sulfur in coal, hydroxides, and carbonates of calcium and magnesium. However, the slight alkaline nature of FA could be due to its weathering also. The ECH2O (1:2.5) (w/v) ranged between 24.20 and 42.60 μS/cm. Taking an account of organic carbon and organic matter content in FA is vital as they impact other physical, chemical, and biological properties of the FA. The organic matter content also influences the availability of various metals for the uptake of plants. The organic matter and organic carbon content in the collected FA was below 1%. The low organic matter content can be attributed to the absence of plant litter as there was colonization of limited plants tolerant to anomalous metal concentration. The FA was found to be deficient in the N content. Overall, the control soil was better in nutrient content as compared to FA; however, in spite of low nutrient content, majority of plant species were spontaneously established on FA lagoon.
The rhizospheric FA showed anomalous concentration of metals when extracted using three different extractants. The total metal concentration (in mg kg−1) in FA was in the order of Mn (229.8) > Ni (228.4) > Zn (89.4) > Cr (61.2) > Pb (56.6) > Cu (51.5) > Co (41.9) > Cd (9.7). In the present study, the average total metal concentration at CTPS, Jharkhand (India), along with the usual total metal concentration present in the FA generated around the world is compared in Table 3. A list of total metal concentration (mg kg−1) in FA generated in different regions of India is also tabulated in Supplementary Table. The concentration of Ni and Cr in the present study was highest among the listed power plants. Concentration of Cd, Cr, and Ni were found above critical soil total concentration/permissible level (Alloway 1990, 2013; Kumar et al. 2017; González-Miqueo et al., 2010). Many researchers had reported that estimation of total metal concentration does not always provide the exact information about mobility, bioavailability of metals, and potential toxicity and hence it is important to analyze bioavailable metal concentrations using different extractants such as CaCl2 and nitric acid (Remon et al. 2013; Wojcik et al. 2014). Nevertheless, this justification mainly suits for highly metal-contaminated sites. In case of low or moderately contaminated sites like FA lagoon, it is essential to check the correlation between the metal concentration in substrate and in plant tissues.
To identify the best extractant suitable for assessment of bioavailability of metal in substrate and its subsequent transfer from substrate to plant, three different extractants (aqua-regia, HNO3 and CaCl2) were used. The CaCl2-extracted fraction was very less and accounted for 0.34–0.51% for Mn, 0.86–1.75% for Ni, 0.28–1.29% for Zn, 0.55–0.72% for Cr, 5.28–7.80% for Pb, 0.03–0.06% for Cu, 0.40–2.15% for Co, and 0.19–1.79% for Cd of the total metal concentration and does not demonstrate any correlation with metal concentration in plant tissues. Similarly, HNO3 showed better extracting capability than did CaCl2 and accounted for 6.15–10.38% for Mn, 1.89–2.33% for Ni, 3.15–3.90% for Zn, 0.57–0.83% for Cr, 8.4–15.6% for Pb, 5.39–6.48% for Cu, 2.81–3.85% for Co, and 0.57–2.50% for Cd and showed a low level significant correlation with metal concentration in shoot. The total metal concentration extracted by aqua-regia (total) was much greater than the bioavailable metal fractions obtained by using HNO3 and CaCl2 (Table 4). Spearman’s correlation showed best significant correlation (r = 0.786; p < 0.05) between total metal concentration in FA and shoot metal concentration which suggest aqua-regia as the best extractant for understanding the bioavailability and transfer of metals, and in calculation of BCF for moderately contaminated site (Table 5). Strong significant correlation (r = 0.929; p < 0.01) observed between metal concentration in roots and shoots shows transfer of metals from root to shoot and phytoextraction ability of plant. A low significant correlation was also observed between HNO3-extractable concentration and shoot metal concentration.
Metal accumulation by five dominant plant species
A total of 29 plant species belonging to 17 different families were observed on control site, out of which the same 20 metal-tolerant plant species from 16 different families were naturally colonized on FA lagoon. Five plant species were selected on the basis of its higher relative density and frequency and analyzed for metal accumulation capacity to check its suitability in phytoremediation of FA lagoon. The root and shoot portions of the plants growing on FA lagoon accumulated varying concentration of different metals probably due to plant’s metabolism and mechanisms driving the uptake of metals from the FA (Table 6).
In the case of potentially toxic but essential metals (Cu, Mn, Zn, and Ni), among all five plants, C. rotundus had accumulated maximum concentration of 28.50 mg Cu kg−1; however, in the case of shoot, it was maximum for C. procera (20.28 mg Cu kg−1). The Cu concentration in the root or shoot portions was within the safe limits as per Alloway (1990) and Kabata and Pendias (2001). The critical Mn concentration in plants range between 300 and 500 mg kg−1 above which toxic effects are likely to occur (Alloway 1990); however, all the plants were below toxicity levels and observed maximum in the shoot portion of V. cinerea (106.16 mg kg−1). Availability of Zn to plants depends upon pH, organic matter, moisture regime, microbial activity, and adsorption sites and the normal concentration of Zn in plants ranges between 10 and 300 mg kg−1 (Alloway 1990). The present study revealed the highest Zn concentration in the roots of E. prostrata (152.62 mg kg−1), and its concentration in all the plants were within safe limits. Due to deficiency of organic matter and slightly acidic pH, the Zn was not much accumulated by plants and TFZn < 1 for C. rotundus, C. bonplandianus, and E. alba further suggests its hindered mobility in plant shoot. TF > 1 for Mn in all the studied plants and in most of the plants in case of Zn suggests its transfer from root to shoot which is essential for plant growth. The mobility of Ni in the soil increases with a decrease in the pH and cation exchange capacity. Tripathi et al. (2004) demonstrated Ni accumulation in Cassia siamea Lamk growing on FA between 60 and 120 mg Ni kg−1, and the normal and critical concentration range of Ni in plants reported by Alloway (1990) were 10–50 and 10–100 mg kg−1, respectively. In the present study, the range of Ni concentration in plants was between 25 and 72 mg Ni kg−1 which was within the safe limits. Overall, all the potentially toxic but essential metals (Cu, Mn, Zn, and Ni) were accumulated in significant quantity but within the safe limits which further helps in plant growth and development.
In case of other potentially toxic metals (Pb, Co, Cr, and Cd), maximum concentration was for Pb followed by Co > Cr > Pb. The normal range of Pb in plants, i.e., 0.2–20 mg kg−1 (Alloway 1990), and the critical Pb concentration (30–300 mg kg−1) above which toxicity effects are likely to occur has not been surpassed by any of the plant species (Alloway 1990). However, significant concentration of metal was accumulated in plants with maximum concentration of Pb in the roots of C. procera (38.56 mg kg−1). Cr was found to have its maximum concentration in the roots of V. cinerea (34.28 mg kg−1). Along with this, shoots of V. cinerea (16.32 mg kg−1) and roots of E. prostrata (14.60 mg kg−1) exhibited metal toxicity due to Cr as per limits defined by Shanker et al. (2005). Cr concentration above 5 mg kg−1 sometimes results in the necrosis, chlorosis, and detrimental effects on the plants (Shanker et al. 2005; Kumar et al. 2017). Maximum concentration of Co was in the roots of E. prostrata (35.61 mg kg−1) which indicates all the plant species are within safe limits. Cadmium is an effective inhibitor of photosynthesis (Krupa et al. 1993) so it becomes toxic if its concentration is high. The maximum Cd concentration was in shoot of E. prostrata (6.70 mg kg−1) while the lowest Cd concentration in roots of C. procera (1.44 mg kg−1).
Principal component analysis (PCA) was performed only for more potentially toxic metals (Pb, Cr, Co, and Cd) which could be possibly harmful for human health if exposed in higher concentration. The PCA revealed three principal components with eigenvalues > 1 were extracted, together explaining about 74.13% of total variability in the data (Fig. 3). PC1 has high positive correlation with Cd and Co and negative correlation with Cr and Pb. Except for V. cinerea and E. prostrata, all the shoots and roots of other three studied plant species showed high positive correlation with Cd and Co. However, all the plants showed positive correlation with Co and Cd which suggest its subsequent uptake in root and shoot. PC2 shows high positive correlation of Cr with Cd and Co. Among shoot part of all five plants, maximum accumulation of Cd was found in E. prostrata with high correlation of Cd and its subsequent uptake in shoots of E. prostrata.
Principal component (PC) analysis ordination diagram grouping five plant species with respect to their metal concentration (Pb, Cr, Co, and Cd) in shoot and root. Numbers in the parenthesis show percent of explained variance. CRr C. rotundus root. CRs C. rotundus shoot. CPr C. procera root. CPs C. procera shoot. CBr C. bonplandianus root. CBs C. bonplandianus. EPr E. prostrata root. EPs E. prostrata shoot. VCr V. cinerea root. VCs V. cinerea shoot
Biological indices for assessment of potentiality of studied plants in phytoremediation
To understand the potentiality of plants in phytoremediation of metal-polluted sites, three different factors are generally evaluated, bioconcentration factor (BCF) of root, biological accumulation factor (BAF) of shoot, and translocation factor (TF) (Figs. 4a, b and 5). The mobility of toxic and non-essential metals from belowground tissues to aboveground tissues is restricted as they are not much required by the plants and gets sequestered in the vacuoles of the roots which favor the plant’s behavior against heavy metal toxicity. However, various other properties of the rhizospheric FA drive the uptake of metals by plant roots such as pH, electrical conductivity, plant-associated bacteria, mycorrhizal fungi, etc. (Redon et al. 2009; Alloway 2013). The BCF of metals in root portion of a plant indicates the phytoextraction potential of plants from the FA. The BCF values were in the range of 0.1–0.5 for Cu, 0.1–0.2 for Mn, 0.7–1.6 for Zn, 0.5–0.7 for Pb, 0.2–0.4 for Ni, 0.1–0.6 for Cr, 0.2–0.9 for Co, and 0.2–0.4 for Cd. In the case of BAF, the values ranged between 0.3 and 0.8 for Cu, 0.2 and 0.7 for Mn, 0.9 and 2.3 for Zn, 0.4 and 1.1 for Pb, 0.4 and 0.6 for Ni, 0.1 and 0.8 for Cr, 0.4 and 1.1 for Co, and 0.5 and 1.0 for Cd. The TF of different metals in plants under investigation ranged between 0.30 (Cr in E. prostrata) to 4.12 (Mn in C. rotundus). The highest BCFroot (0.55) of Cu was in C. rotundus, and the highest BAF (0.85) of Cu was observed in E. prostrata. The accumulation of Cu in shoots of C. procera have been confirmed by several researchers (Gupta and Sinha 2007b, 2008; Ahmad et al. 2011; Kumar et al. 2013) which was confirmed in the present study by considerable BAF (0.63) of Cu in C. procera in spite of low BCFroot (0.23). Except for Zn, such low BCF and BAF values clearly indicate the excluding strategy in all plant species naturally inhabiting the waste deposits studied. In most of the plant species, low BCF of Mn and Zn was found due to its translocation to the shoot tissue (TF > 1). In fact, BCF and BAF is a function of both the metal transfer from the soil to the plant root and root to shoot translocation efficiency, and thus, it depends not only on the metal concentration in the growth medium but also on its mobility (Wojcik et al. 2014). BCFroot of Cr in C. rotundus, C. procera, E. prostrata, and V. cinerea was found high in comparison to BAF due to its low translocation to shoot tissue of the plants. Similar findings in studies conducted on C. rotundus (Rai 2009), C. procera (Kumar et al. 2013; Kumar et al. 2017), E. prostrata (Dwivedi et al. 2008; Ahmad et al. 2011), and V. cinerea (Jaison and Muthukumar 2017) have also supported this behavior of these plants towards Cr. Because of low mobility of Pb, its TF was at minimum as compared to other metals for all the studied plants. Zn and Cd are highly mobile and get easily transported from soil to root to shoot. Zn is a competing ion with Cd (Mengel and Kirkby 1978) as the transportation of both the metals is facilitated by a common carrier at the root plasma membrane which has comparatively higher affinity for Cd than Zn (Hart et al. 2005). Both the metals showed higher BAF values for most of the plant species. Except for Pb, C. procera was able to translocate (TF > 1) all the studied metals from root to shoot. In addition, it also showed higher BAF and BCF values for Pb as compared to other plants which suggest its applicability in phytoextraction. The TF of Co was observed in the order of C. procera (1.86) > V. cinerea (1.17) > C. bonplandianus (1.02) > C. rotundus (0.71) > E. prostrata (0.32). The high TF in three of the plant species can be attributed to the vital role that Co plays in biological N fixation (Alloway 1990). The TF of Cd in E. prostrata was high (1.93) despite its toxicity. However, E. prostrata has also shown high TF for Cd (4.93) in a study conducted by Ahmad et al. (2011).
Phytoextraction is definitely not an option for decontamination of mine tailings or smelter wastes as was comprehensively explained by Ernst (2005). However, it may be applied to soils with low and moderate concentrations of metals. FA upholds moderate concentration of metals and could be toxic when leached into the environment. E. prostrata was found to have BAF and TF > 1 which suggests its phytoextraction ability for Cd which is also evidenced from PCA and can act as a suitable plant for phytoremediation of FA lagoon. In addition, it can also act in phytostabilization of other highly toxic metals such as Pb, Co, and Cr. V. cinerea accumulates high concentration of Cr in root (above toxicity limit without showing any detrimental effect) with BCF, BAC, and TF < 1which suggests its applicability in phytostabilization of FA lagoon. C. procera accumulated significant concentration of all the metals and could be used in phytoremediation of moderately contaminated sites.
Conclusions
The present study strongly encourages the assessment of biodiversity between the polluted and control site in bioaccumulation studies while identifying the plant species which are tolerant to metal contamination and could help in restoration of fly ash lagoon. Moderate concentration of metals was found in rhizospheric fly ash. However, the concentration of Ni and Cr in the present study was highest among the previously studied Indian and world power plants. After the comparison of biodiversity between polluted and control site, the polluted site (i.e., flyash lagoon) was found to have lesser biodiversity and higher dominance indicating a stressed ecosystem facilitating the proliferation of pollution-tolerant plant species. Out of 29 plant species taken into consideration for biodiversity investigations, the present study screened out C. rotundus, C. procera, C. bonplandianus, E. prostrata, and V. cinerea as the most suitable metal-tolerant plant species which can grow on metal laden fly ash lagoon. The variation in total, residual, and plant-available metal concentration indicated the presence of different proportions of metals in fly ash lagoon which affects the metal uptake potential of the vegetation growing on it. It is being reported in literature that bioavailable/plant-available metal concentration is the most suitable for assessment of metal transfer from soil to plant. However, in the present study, positive correlation was observed between total metal concentration and shoot metal concentration which suggests importance of correlation study for selection of extractant for calculation of biological indices. It can be stated that total metal concentration can provide a better insight for moderately or low metal-contaminated sites. The biological indices (BCF, BAF, and TF) revealed that E. prostrata and C. procera can be utilized efficiently for the phytoextraction of toxic metals from FA lagoon. Multivariate analysis (PCA) indicated that Cd was found to have an independent route of transport to shoots whereas all the plants were tolerant to Pb pollution hence there was a negligible translocation of Pb to the aerial tissues of the plants. V. cinerea accumulated elevated concentration of potentially toxic Cr and Ni which could also help in the phytoremediation of FA lagoon. The study concluded that five species (C. rotundus, C. procera, C. bonplandianus, E. prostrata, and V. cinerea) can be used for phytoremediation of FA lagoons. and these species are commonly found in India.
Future perspectives and recommendations
Use of different extractants and correlation study are needed to identify the best suitable extractant for calculation of biological indices such as bioconcentration and bioaccumulation factor to know exactly the strategy (phytoextraction, phytostabilization, hyperaccumulation, etc.) of the plant species for moderate or low metal-polluted sites. Sequential extraction can be done for better insight for assessment of metal mobility and immobilization of metals in soil.
References
Ahmad A, Ghufran R, Zularisam AW (2011) Phytosequestration of metals in selected plants growing on a contaminated Okhla industrial areas, Okhla, New Delhi, India. Water Air Soil Pollut 217(1–4):255–266
Alloway BJ (1990) Heavy metals in soils. Blackie Academic & Professional, Glasgow, p 339
Alloway BJ (2013) Heavy metals in soils. Trace metals and metalloids in soil and their bioavailability. Springer, New York
Antoniadis V, Levizou E, Shaheen SM, Ok YS, Sebastian A, Baum C, Prasad MN, Wenzel WW, Rinklebe J (2017) Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—a review. Earth Sci Rev 171:621–645
Aston EC, Macintosh DJ (2002) Preliminary assessment of the plant diversity and community ecology of Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manage 166(1):111–129
Baba A (2000) Leaching characteristics of wastes from Kemerkoy (Mugla-Turkey) power plant. Global Nest Int J 2(1):51–57
Baba A, Gurdal G, Sengunalp F, Ozay O (2008) Effects of leachant temperature and pH on leachability of metals from fly ash. A case study: Can thermal power plant, province of Canakkale, Turkey. Environ Monit Assess 139:287–298
Central Electronic Authority (CEA) (2014) Report on fly ash generation at coal/lignite based thermal power stations and its utilization in the country for the year 2013–14, New Delhi. http://cea.nic.in/reports/others/thermal/tcd/flyash_yearly_201314.pdf
Central Electronic Authority (CEA) (2017) All India installed power capacity (In MW), New Delhi http://www.cea.nic.in/reports/monthly/installedcapacity/2017/installed_capacity-03.pdf
Chowdhury A, Maiti SK (2016) Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: a case study from Indian Sunderban. Environ Earth Sci 75(9):1–21
Dar MI, Green ID, Naikoo MI, Khan FA, Ansari AA, Lone MI (2017) Assessment of biotransfer and bioaccumulation of cadmium, lead and zinc from fly ash amended soil in mustard–aphid–beetle food chain. Sci Total Environ 584:1221–1229
Dwivedi S, Srivastava S, Mishra S, Dixit B, Kumar A, Tripathi RD (2008) Screening of native plants and algae growing on fly-ash affected areas near National Thermal Power Corporation, Tanda, Uttar Pradesh, India for accumulation of toxic heavy metals. J Hazard Mater 158(2):359–365
Ernst WHO (2005) Phytoextraction of mine wastes—options and impossibilities. Chem Erde 65:29–42
Frosi G, Oliveira MT, Almeida-Cortez J, Santos MG (2013) Ecophysiological performance of Calotropis procera: an exotic and evergreen species in Caatinga, Brazilian semi-arid. Acta Physiol Plant 35(2):335–344
Gajic G, Djurdjević L, Kostic O, Jaric S, Mitrovic M, Stevanovic B, Pavlovic P (2016) Assessment of the phytoremediation potential and an adaptive response of Festuca rubra L. sown on fly ash deposits: native grass has a pivotal role in ecorestoration management. Ecol Eng 93:250–261
González-Miqueo L, Elustondo D, Lasheras E, Santamaría JM (2010) Use of native mosses as biomonitors of heavy metals and nitrogen deposition in the surroundings of two steel works. Chemosphere 78(8):965–971
Gupta AK, Sinha S (2007a) Assessment of single extraction methods for the prediction of bioavailability of metals to Brassica juncea L. Czern. (var. Vaibhav) grown on tannery waste contaminated soil. J Hazard Mater 149(1):144–150
Gupta AK, Sinha S (2007b) Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour Technol 98(9):1788–1794
Gupta AK, Sinha S (2008) Decontamination and/or revegetation of fly ash dykes through naturally growing plants. J Hazard Mater 153(3):1078–1087
Hammer D, Keller C (2002) Changes in the rhizosphere of metal-accumulating plants evidenced by chemical extractants. J Environ Qual 31(5):1561–1569
Hart JJ, Welch RM, Norvell WA, Clarke JM, Kochian LV (2005) Zinc effects on cadmium accumulation and partitioning in near-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol 167(2):391–401
Hattab N, Motelica-Heino M, Bourrat X, Mench M (2014) Mobility and phytoavailability of cu, Cr, Zn, and As in a contaminated soil at a wood preservation site after 4 years of aided phytostabilization. Environ Sci Pollut Res 21(17):10307–10319
Hernandez AJ, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30(2):127–133
Iwalewa EO, Iwalewa OJ, Adeboye JO (2003) Analgesic, antipyretic, anti-inflammatory effects of methanol, chloroform and ether extracts of Vernonia cinerea less leaf. J Ethnopharmacol 86(2):229–234
Jaison S, Muthukumar T (2017) Chromium accumulation in medicinal plants growing naturally on tannery contaminated and non-contaminated soils. Biol Trace Elem Res 175:223–235
Jiao F, Zhang L, Dong Z, Namioka T, Yamada N, Ninomiya Y (2016) Study on the species of heavy metals in MSW incineration fly ash and their leaching behavior. Fuel Process Technol 152:108–115
Kabata A, Pendias H (2001) Trace elements in soils and plants. CRC press, Washington
Kapicka A, Petrovsky E, Ustjak S, Machackova K (1999) Proxy mapping of fly-ash pollution of soils around a coal-burning power plant: a case study in the Czech Republic. J Geochem Explor 66(1):291–297
Krgovic R, Trifkovic J, Milojkovic-Opsenica D, Manojlovic D, Markovic M, Mutic J (2015) Phytoextraction of metals by Erigeron canadensis L. from fly ash landfill of power plant “Kolubara”. Environ Sci Pollut Res 22(14):10506–10515
Krupa Z, Oquist G, Huner N (1993) The effects of cadmium on photosynthesis of Phaseolus vulgaris—a fluorescence analysis. Physiol Plant 88(4):626–630
Kumar N, Bauddh K, Kumar S, Dwivedi N, Singh DP, Barman SC (2013) Accumulation of metals in weed species grown on the soil contaminated with industrial waste and their phytoremediation potential. Ecol Eng 61:491–495
Kumar A, Maiti SK, Tripti PMNV, Singh RS (2017) Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite–asbestos mine. J Soils Sediments 17(5):1358–1368
Kumari A, Lal B, Rai UN (2016) Assessment of native plant species for phytoremediation of heavy metals growing in the vicinity of NTPC sites, Kahalgaon, India. Int J Phytoremediation 18(6):592–597
Lavado RS, Porcelli CA (2000) Contents and main fractions of trace elements in Typic Argiudolls of the Argentinean pampas. Chem Speciat Bioavailab 12(2):67–70
Maiti SK (2007) Bioreclamation of coalmine overburden dumps—with special emphasis on micronutrients and heavy metals accumulation in tree species. Environ Monit Assess 125(1–3):111–122
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess 136(1–3):355–370
Maiti SK, Nandhini S (2006) Bioavailability of metals in fly ash and their bioaccumulation in naturally occurring vegetation: a pilot scale study. Environ Monit Assess 116(1–3):263–273
Margui E, Queralt I, Carvalho ML, Hidalgo M (2006) Assessment of metal availability to vegetation (Betula pendula) in Pb–Zn ore concentrate residues with different features. Environ Pollut 145(1):179–184
Mengel K, Kirkby EA (1978) Principles of plant nutrition. International Potash Institute, Worblaufen-Bern
Pandey VC, Mishra T (2016) Assessment of Ziziphus mauritiana grown on fly ash dumps: prospects for phytoremediation but concerns with the use of edible fruit. Int J Phytorem Article in press doi:10.1080/15226514.2016.1267703
Pandey SK, Bhattacharya T, Chakraborty S (2016) Metal phytoremediation potential of naturally growing plants on fly ash dumpsite of Patratu thermal power station, Jharkhand, India. Int J Phytoremediation 18(1):87–93
Pavlovic P, Mitrovic M, Djurdjevic L (2004) An ecophysiological study of plants growing on the fly ash deposits from the “Nikola Tesla–A” thermal power station in Serbia. Environ Manag 33(5):654–663
Prasad MNV (2011) A state-of-the-art report on bioremediation, its applications to contaminated sites in India. Ministry of Environment and Forests, New Delhi
Pratas J, Favas PJC, D’Souza R, Varun M, Paul MS (2013) Phytoremedial assessment of flora tolerant to heavy metals in the contaminated soils of an abandoned Pb mine in Central Portugal. Chemosphere 90:2216–2225
Pueyo M, Lopez-Sanchez JF, Rauret G (2004) Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal Chim Acta 504(2):217–226
Rai PK (2009) Heavy metals in water, sediments and wetland plants in an aquatic ecosystem of tropical industrial region, India. Environ Monit Assess 158(1–4):433–457
Ram LC, Masto RE (2014) Fly ash for soil amelioration: a review on the influence of ash blending with inorganic and organic amendments. Earth Sci Rev 128:52–74
Rani P, Kumar A, Arya RC (2017) Stabilization of tannery sludge amended soil using Ricinus communis, Brassica juncea and Nerium oleander. J Soils Sediments 17:1449–1458
Redon PO, Beguiristain T, Leyval C (2009) Differential effects of AM fungal isolates on Medicago truncatula growth and metal uptake in a multimetallic (Cd, Zn, Pb) contaminated agricultural soil. Mycorrhiza 19(3):187–195
Remon E, Bouchardon JL, Le Guédard M, Bessoule JJ, Conord C, Faure O (2013) Are plants useful as accumulation indicators of metal bioavailability? Environ Pollut 175:1–7
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31(5):739–753
Sharma S, Singh B, Manchanda VK (2015) Phytoremediation: role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environ Sci Pollut Res 22(2):946–962
Simpson EH (1949) Measurement of diversity. Nature 163(4148):688. doi:10.1038/163688a0
Smith TM, Smith RL (2012) Elements of ecology, 3rd edn. Pearson, Glenview
Sobrinho MS, Tabatinga GM, Machado IC, Lopes AV (2013) Reproductive phenological pattern of Calotropis procera (Apocynaceae), an invasive species in Brazil: annual in native areas; continuous in invaded areas of caatinga. Acta Bot Bras 27(2):456–459
Subbiah BV, Asija GL (1956) A rapid procedure for the determination of available nitrogen in soils. Curr Sci 25:259–260
Szarek-Lukaszewska G, Niklinska M (2002) Concentration of alkaline and heavy metals in Biscutella laevigata L. and Plantago lanceolata L. growing on calamine spoils (S. Poland). Acta Biol Cracov Ser Bot 44:29–38
Tripathi RD, Vajpayee P, Singh N, Rai UN, Kumar A, Ali MB (2004) Efficacy of various amendments for amelioration of fly ash toxicity: growth performance and metal composition of Cassia siamea Lamk. Chemosphere 54:1581–1588
Tripodi RA, Cheremissinof PN (1980) Coal ash disposal solid waste impact. Technomica, Westport, pp 11–26
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wojcik M, Sugier P, Siebielec G (2014) Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci Total Environ 487:313–322
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2):456–464
Acknowledgements
This research was financially supported by the Ministry of Human Resources and Development of the Government of India. The authors are grateful to Indian Institute of Technology (Indian School of Mines), Dhanbad, Jharkhand (India), for providing laboratory and e-research facilities for carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
ESM 1
(DOCX 27 kb).
Rights and permissions
About this article
Cite this article
Mukhopadhyay, S., Rana, V., Kumar, A. et al. Biodiversity variability and metal accumulation strategies in plants spontaneously inhibiting fly ash lagoon, India. Environ Sci Pollut Res 24, 22990–23005 (2017). https://doi.org/10.1007/s11356-017-9930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9930-4