Abstract
The objectives of this study were to determine the concentrations of Pb, Cd, As, Cr, Cu, Co, Ni, Zn, Ba, Fe, Al and Ag in Erigeron canadensis L. growing on fly ash landfill of power plant “Kolubara”, Serbia. The content of each element was determined in every part of plant separately (root, stalk and inflorescence) and correlated with the content of elements in each phase of sequential extraction of fly ash. In order to ambiguously select the factors that are able to decidedly characterize the particular part of plant, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed. The bioconcentration factors and translocation factors for each metal were calculated in order to determine the feasibility of the use of plant E. canadensis L. for phytoremediation purpose. There were strong positive correlations between metals in every part of plant samples, and metals from pseudo total form of sequential extraction indicate that the bioavailability of elements in fly ash is similarly correlated with total form. Retained Al, Fe, Cr and Co in the root indicate its suitability for phytostabilization. This plant takes up Cd and Zn from the soil (bioconcentration factors (BCFs) greater than 1), transporting them through the stalk into the inflorescence (translocation factors (TFs) higher than 1). Regarding its dominance in vegetation cover and abundance, E. canadensis L. can be considered adequate for phytoextraction of Cd and Zn from coal ash landfills at Kolubara
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoremediation is an eco-friendly approach for remediation of contaminated soil using plants. It involves processes as removal, transfer, stabilization or degradation of contaminants from soil and sediment water (Hughes et al. 1997). These processes are known as phytoextraction, phytofiltration, phytostabilization and phytovolatilization (Sarma 2011).
Plants used for phytoextraction should be fast growing and deeply rooted, easily propagate and accumulate the target metal (Ghosh and Singh 2005a).
Many of toxic metals enter the environment as a consequence of fossil fuel combustion (Ram and Masto 2010). Lignite is an important source of fossil fuel and is often used for electrical energy production (Ćujić et al. 2014). In Serbia, “Kolubara” power plant, one of the oldest in Serbia, generates annually 6–8 × 109 kg of coal ash. Therefore, important environmental issues concerning ash disposal areas are the need of huge space for deposition as well as the potential pollution of water and air. Combustion waste deposited in landfills displays numerous properties which are unfavorable for plant growth, such as a poor air and water ratio, excessively alkaline reaction and, in some cases, high content of heavy metals (Krzaklewski et al. 2012). From environmental point of view, fly ash not only contains essential macroelements (P, K, Ca, Mg) and microelements (Zn, Fe, Cu, Mn, B, Mo) for plant growth but also non-essential elements and radionuclides. It is important to monitor the processes of metals released to the environment and their bioavailability (Pietrzykowski et al. 2014).
When one of the ash landfill is full, it needs to be remediated to adequate state according to the obligation of power plant directed by national legislation. The chemical or any physical processes are very expensive, and their use is usually reserved for small areas. The introduced vegetation is an important element which initiates the processes of soil formation (Krzaklewski et al. 2012). Over time, the formation of well-developed vegetation cover can significantly reduce further horizontal or vertical spreading of the contaminants, erosion and the risk of leaching of potentially toxic contaminants to groundwater (Vangronsveld et al. 2009).
Plants with extreme levels of metal tolerance are called hyperaccumulators of heavy metals (Barman et al. 2000). This property depends on the plant species, soil conditions (pH, organic matter content, etc.) and heavy metal (Barman et al. 2001). Plants such as Thlaspi caerulescens (Zhao et al. 2002), Arabidopsis halleri L. (Küpper et al. 2000), Rorippa globosa (Sun et al. 2010), Solanum photeinocarpum (Zhang et al. 2011) and Pfaffia glomerata (Gomes et al. 2013) have been identified as Cd hyperaccumulators.
The hyperaccumulators have a high bioconcentration factor (BCF), which is defined as the plant/soil metal concentration; however, the efficiency of phytoextraction depends also of biomass production (Ghosh and Singh 2005b).
The Erigeron canadensis L. plant is distributed widespread across almost the whole world originating from North America (Djurdjevic et al. 2011). In Serbia, it was noted for the first time by Pančić, 1874, on sandy areas and fields. It is a naturalized species (Vrbnicanin 2008) which played a pioneer role inhabiting fields after fire at Deliblato Sands, Serbia. Also, as a pioneer species, it colonized the ash deposit at the “Nikola Tesla” thermoelectric power plant in Obrenovac. E. canadensis L. played the most important role in humus formation as one of the most abundant self-sown plant species which can endure over a long period of time (Djurdjevic et al. 2006). Also, we found that it formed dense cover dominating with aboveground and belowground mass at the coal ash landfills at Kolubara (Serbia).
As a continuation of our previous research on metal content in passive cassettes (landfill) of power plant Kolubara near Lazarevc, Serbia, after five steps of sequential extraction procedure (Krgović et al. 2014) in this study, the concentrations of same elements in E. canadensis L. plant growing on this contaminated site were determined. The goal of this paper was to explore the possibilities to use E. canadensis L. for phytoremediation. In that sense, the content of each element was determined in every part of plant separately (root, stalk and inflorescence) and correlated with the content of elements in landfill ash in each phase of extraction. Moreover, the translocation factors (TFs) and BCFs for each metal were calculated. In order to unambiguously select the factors that are able to decidedly characterize the particular part of plant, principal component analysis (PCA) and partial least square discriminant analysis (PLS-DA) were performed.
Materials and methods
Site characterization
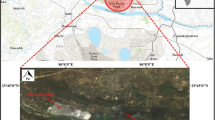
The plant and fly ash samples used in this study were collected from power plant Kolubara landfill (Fig. 1). Thermoelectric power plant Kolubara (Veliki Crljeni, near Lazarevac, Serbia) is the oldest active plant in Serbia, and together with other power plants, it produces electricity for Belgrade area and most of Serbia. Filter ash from power plant Kolubara was mixed with river water and transported to active cassette. The active cassette becomes passive cassette after complete filling which can last up to 10 years or more. Ash deposition is performed into three cassettes, two of which are inactive at any time. The investigation was conducted in the landfill with co-ordinates: 44° 29′ 25″ N and 20° 19′ 6″ E.
Plant and fly ash sampling
According to Braun-Blanquet scale (1964), vegetation cover was 5 with the dominant specie E. canadensis L.. Other plant species were not taken, as their abundance and cover were significantly lower. These plants were up to 60 cm tall.
Plant samples, together with the associated fly ash samples, were collected in October 2012. Sampling was performed by the principle of diagonal grid sampling. A 1-ha area (100 × 100 m) was a sampling site. The scheme of sampling is presented in Fig. 1.
A total of 20 plant samples of E. canadensis L. were collected. After washing, the samples were oven dried at 70 °C until a constant weight was reached (Liu et al. 2007).
Generally, the authors analyze the accumulation of heavy metals in plants by dividing the plant into belowground and aboveground parts. In our study, the plant samples were prepared on two ways: (a) one part of samples was divided into belowground plant part (roots) and aboveground plant parts (leaves, stalk and inflorescences) (data not shown) and (b) the second part of samples was divided into roots (R), stalk without leaves (S) and leaves with inflorescence (I).
After the first statistical analysis, significant difference was observed between aboveground and belowground. In further analysis, the aim was to investigate in details the transport of metals to the end point in the plant.
Also, 20 fly ash samples were taken from 0 to 30 cm, where most of root biomass was found. Each sample was a composite sample consisting of five subsamples (about 5 kg). Samples were air-dried at room temperature for 3 weeks. Then, from the composite samples using the quartering method, 0.5 kg was obtained and only 1 g was analyzed.
Instrumentation
The measurements of fly ash samples were carried out in an inductively coupled atomic emission spectrometer (ICP-OES) (Thermo Scientific, UK), model 6500 Duo, equipped with a CID86 chip detector as the measurements of Al and Fe in the plant samples. This instrument operates sequentially with both radial and axial view. The entire system was controlled with iTEVA software. The measurement of all elements (except Al and Fe) in the plant samples was carried out in an ICP-MS (iCAP Q, Thermo Scientific X series 2). The entire system was controlled with Qtegra Instrument Control Software. 7Li, 45Sc, 115In and 159 Tb were used as internal standards.
Microwave digestion was performed in a microwave oven equipped with a rotor holding 10 PTFE cuvettes (Ethos 1, Advanced Microwave Digestion System; Milestone, Italy).
Reagents and chemicals
All chemicals were of analytical grade and were supplied by Merck (Darmstadt, Germany). Ultrapure water was prepared by passing doubly de-ionized water from a Milli-Q system (˃18 MΩ). All glassware was soaked in 10 % HNO3 for minimum 12 h and rinsed well with distilled water. The multi-element stock solution containing 1000 mg/L of 22 elements was used to prepare intermediate multi-element standard solutions for ICP-OES measurements, and the multi-element stock solution containing 10 mg/L of each element was used to prepare intermediate multi-element standard solutions for ICP-MS measurements. Internal standards (10 μg/L for Bi, Ga, In, Tb and Y and 50 μg/L for Li and Sc) were prepared from internal standard mix solution (VHG standards; Manchester, UK). In order to check the accuracy and precision of instrument condition, the standard reference material was applied by ERM-CD281 (rye grass; JRC-IRMM, Belgium) (Supplementary data Table A).
Procedure for plant sample preparation
Plant samples (0.5 g) were transferred into PTFE cuvettes, and 7 mL of 65 % HNO3 and 1 mL of 30 % H2O2 were added. Digestion was performed under the following programme: warm-up for 10 min at 200 °C and held for 15 min at that temperature. After cool off period, the samples were quantitatively transferred into a volumetric flask (25 mL) and diluted with distilled water.
Sequential extraction procedure for fly ash samples
A five-step sequential extraction procedure for fly ash samples was applied (Popovic et al. 2011). The extractants used in each extraction step and extraction fractions are given in Table 1. Detailed description of sequential extraction procedure was given in our previous paper (Krgović et al. 2014).
Calculation of bioconcentration and translocation factors
The BCF estimates the efficiency of a plant in taking up metals from fly ash and is calculated as follows:
where c root is the total concentration (μg/g) of the target metal in the plant root and c ash is the concentration (μg/g) of the same metal in the first three phases in fly ash. The c ash was calculated as the sum of the first three phases instead of pseudo total concentration in fly ash regarding the fractions that are bioavailable for plants. The TF is calculated separately for stalk (s) and inflorescence with leaves (i) as follows:
where c s, c i and c root are the total concentrations (μg/g) of target metal in the plant stalk, inflorescence and root, respectively.
Statistical analysis
PCA and PLS-DA were carried out by the means of PLS ToolBox, v.6.2.1, for MATLAB 7.12.0 (R2011a). All data were autoscaled prior to any multivariate analysis. PCA was carried out as an exploratory data analysis using a singular value decomposition (SVD) algorithm and a 0.95 confidence level for Q and T 2 Hotelling limits for outliers. PCA was performed in order to reduce the dimensionality of data space, visualize the structure of data, identify important variables and confirm the presence of outliers. The PLS-DA was performed using the SIMPLS algorithm without forcing orthogonal conditions to the model in order to condense Y-block variance into first latent variables. PLS-DA is a classical PLS regression where the response variable is categorical, i.e. indicating the classes or categories of the samples. The aim of this supervised technique is to build a mathematical model that could be used in further classification of unknown samples. The models were validated using a venetian blind-validation procedure.
The quality of the models was monitored with the following parameters: (1) R 2 cal, the cumulative sum of squares of the Ys explained by all extracted components, and R 2 CV, the cumulative fraction of the total variation of the Ys that can be predicted by all extracted components, and these two values should be as high as possible, and (2) root mean square errors of calibration (RMSEC) and root mean square errors of cross-validation (RMSECV); these two values should be as low as possible and with the lowest difference between the two.
Results and discussion
Fly ash properties at studied landfill: reference data
The produced ash is aluminosilicate (approximately 80 %) with a significant amount of Fe, Ca, Mg, K and Ti oxides (approximately 17 %), and it is of alkaline reaction (pH 8). Leaching of elements from fly ash obtained in the Kolubara power plant during the storage was investigated in our previous paper (Krgović et al. 2014). We discovered that the contents of Cr, Cu, Pb, Zn and Co are usually higher in the samples from filter ash compared to the samples from landfill, indicating their leaching during the storage. Then, the significant fraction of Zn, Cr and Cu in filter ash was associated with oxide of iron and manganese (third phase of sequential extraction). Contrary, these metals were found in the first two easily exchangeable phases in the samples from landfill. It means that under reduction conditions and lower pH, Cr, Cu and Zn migrated to the adsorbed and ion exchangeable form which are bioavailable for plants. Cadmium was present in the samples in the smallest amount compared to all other elements, but the highest content was observed for second extraction phase (Krgović et al. 2014).
Distribution of metals in different plant parts
The summarized parameters of descriptive statistics obtained from the metal content analysis in three separate parts of plant (root, stalk and inflorescence) E. canadensis L., collected from the power plant landfill ash, are presented in Table 2.
Plant samples are characterized with a higher amount of Al, Cr, Fe, Pb and Co in the root, and As, Cd, Cu, Zn and Ag in the inflorescence, while the content of all analyzed metals is smallest in the stalk except Cd and Zn, although with gradually increased concentrations from the root to the inflorescence. Although descriptive statistics provided information regarding general metal composition and some specific metals that can differentiate the samples from roots and shoots, still it is not possible to ambiguously select the factors that are able to decidedly characterize the particular part of plant. In order to further analyze the obtained results and evaluate a distribution of metals in plant, sophisticated chemometric techniques, such as PCA and PLS-DA, were applied. The PCA analysis provides the insight in the very structure of data. It is usually carried out at the exploratory (introductory) level; therefore, it is not used as a classification model, but rather as a hint what could be expected from the current data and to check if there is some logical patterns in the data that might be explained. PLS-DA was served as a classification method. Metal content was served as an input data matrix.
A PCA resulted in a two-component model which explains 75.88 % of total variance. The first principal component, PC1, accounted for 51.45 % of the overall data variance and the second one, PC2, for 24.43 %. Mutual projections of factor scores and their loadings for the first two principal components (PCs) are presented in Fig. 2. Taking into account PC1 and PC2 score values (Fig. 2a) three distinctive groups of the samples belonging to three different parts of the plant are obtained.
There is some overlapping of Hotelling T 2 ellipses among the stalk and inflorescence samples. In spite of that, the root samples are firmly clustered and distinguish in relation to shoot. The loading plot (Fig. 2b) reveals that the most influential parameters discriminating between inflorescence and samples from two other parts of plant are Zn, Cu and Cd. On the other hand, the contents of Al, Cr, Fe and Co are the major factors which lead to the separation of root samples from the stalk and flower. The content of Ag, Ni and Ba differentiate root and inflorescence samples from stalk. The results of PCA indicated the different metal accumulation characteristics among the tissues of E. canadensis L.
Samples of different location origins were also modelled simultaneously using PLS-DA. The number of the latent variables (LVs) was selected on the basis of the minimum value of RMSECV, which was achieved with two LVs. Classification and validation results were expressed as R 2 cal, R 2 CV, RMSEC and RMSECV values. Statistical results of PLS-DA models for the E. canadensis L. samples are presented in Supplementary data Table F. All three models were statistically significant, with relatively high values of R 2 cal and R 2 CV and low difference between RMSEC and RMSECV values. Score plot of data for the three different parts of the plant, also, confirmed good predictive ability of PLS-DA modelling (Fig. 3a).
The assessment of variables that have the greatest influence on differentiation was done based on variable importance in the projection (VIP) scores. The variables with a VIP score higher than 1 was considered as the most relevant for explaining a certain class of samples, while the others are of extremely low or almost no contribution. In addition, the standardized regression coefficient reveals the significance of an individual variable in the regression models. The most important factors that discriminate root samples from shoot were Zn, Cu, Ag and Al, in descending order (Fig. 3b). The highest negative impacts on a model have Zn and Cu, suggesting that these two elements are accumulating in the root but do not retain in there; instead, it is transported further in the shoot (Fig. 3c). The highest positive influence on discrimination of root samples has Al, indicating its retention in it (Fig. 3c). Apoplastic route is limited by endodermis, and in order to reach the vascular system of the plant, a metal ion needs to be in a non-cationic metal chelate form and transported proteins (Raskin et al. 1997). Good example is aluminium ions which move through the apoplast, but are unable to enter the cytosol of endodermal cells.
The most important variables that discriminate stalk samples were Co, Ni, Fe, Al, Cr, Ag and Ba, in descending order (Fig. 3b). Regression coefficients of all these variables in a model are negative, indicating their lower concentration in stalk compared to root and flower (Fig. 3c). The most relevant variables for explaining inflorescence with leaf samples were Zn, Cu, Cd and Ag (Fig. 3b). These four elements have the highest positive impact on discrimination of inflorescence samples, suggesting their accumulation in this part of plant (Fig. 3c).
Metal content in the associated soil samples, after five extraction steps, is given in Table 3. The content of each element of ash by phases is given in Fig. 4.
In order to process the data in terms of the possible correlation between metals in root, stalk and inflorescence, a correlation analysis was applied (Supplementary data Tables B, C and D). According to the values of Pearson correlation coefficients, i.e. t test for correlation, among the root samples, a strong positive correlation exists between Fe, Al, Ba, Cr and Co concentrations (0.9250 < r < 0.9921, 10.327 < t < 33.533, t cr(0.05;18) = 2.101), indicating their similar accumulation behaviour. Also, positive correlations between Ni, Ag and Co (r = 0.9792, t = 9.455; r = 0.9124, t = 20.477, respectively) show their connection (Supplementary data Table B). Similar correlations were observed between metal concentrations in stalk and inflorescence samples (Supplementary data Tables C and D). It should be emphasized that the inflorescence samples were characterized with high correlation between Cd and Zn (r = 0.8166, t = 6.002), which can indicate a similar deposition and atmospheric transport behaviour (Supplementary data Table D). Water, nutrients as well as metal ions move across the plant tissue by transmembrane transport, apoplastic or symplastic pathway. Nevertheless, due to their insolubility, most metals are retained as carbonate, sulphate or phosphate precipitated in extracellular or intracellular chambers (Khan et al. 2011).
Mean values of concentration of each element in root, stalk and inflorescence samples were correlated with mean values of elements determined for each phase of extraction of soil samples (Supplementary data Table E). Strong positive correlations were observed between metals in root samples and metals determined after phase I (r = 0.9579, t = 10.015, t cr(0.05;9) = 2.262), phase III (r = 0.9404, t = 8.295) and phase V (r = 0.9788, t = 14.335) of sequential extraction. Identical trend was obtained for stalk and inflorescence samples.
Also, there were strong positive correlations between metals in root, stalk and inflorescence with pseudo total metal concentration in soil (r = 0.8365, t = 4.579, t cr(0.05;9) = 2.262; r = 0.8404, t = 4.651, t cr(0.05;9) = 2.262; r = 0.8652, t = 5.176, t cr(0.05;9) = 2.262, respectively). In addition, the pseudo total content of heavy metals in every part of plant is similarly correlated as that in phase I. It means that the bioavailability of elements in fly ash is similarly correlated with total form.
BCFs and TFs
The bioconcentration factor expresses the ability of a plant to accumulate metal from soils. The optimum conditions for phytoremediation include high concentrations of heavy metals in soil and plants and high BCFs (Xue et al. 2014). Calculated BCFs are presented in Table 4. BCF values lower than 1 showed metals Cu, Pb, Ni, Fe, Cr, Ba and As, in descending order. The lowest BCF value has As (0.07). Plants were classified as potential hyperaccumulators if the BCF shoot values are higher than 10 (Ma et al. 2001). In our case, BCF values suggest that E. canadensis L. is not hyperaccumulator. On the other hand, a high BCF value is not an enough parameter to qualify for high phytoextraction efficiency (Ghosh and Singh 2005b). Indicator plants in comparison to hyperaccumulators have a lower metal bioaccumulation but have at least 10 times the biomass production, so that the actual amount of extraction is higher (Alloway 1995).
As presented in Table 4, BCF values higher than 1 were obtained only for Cd and Zn (1.33 and 1.34, respectively). A strong adsorption of Cd on root apoplast might act as a driving force to extract the metal from the soil, compete with the symplast absorption and contribute to the amount of metals taken up by the accumulator, at least in its roots (Redjala et al. 2009). The major storage site for Zn and Cd in plants is cell wall of roots, vacuoles of epidermis and bundle sheath of leaves (Hu et al. 2009). It is not unusual that Zn-accumulating species have been also identified as Cd accumulators (Yang et al. 2004).
One of the strategies of phytoremediation of metal-contaminated soil is phytoextraction, through uptake and accumulation of metals into plant shoots, which can then be harvested and removed from the site. Translocation factor indicates the efficiency of a plant in translocating heavy metals from roots to shoots. The ratio of metals between roots and plant parts is an important criterion for the selection of model plant species for phytoextraction, and the ratio higher than 1 means higher accumulation of metals in shoots than in roots (Barman et al. 2000). Calculated TFs were presented in Table 4. E. canadensis L. contained several heavy metals with valuable degree of absorption and transportation from the root to the shoot area (Table 4). This plant showed efficient translocation of metal ions Zn, Cd, As, Cu, Ba, Ni, Pb, Fe and Cr, in descending order. Retained Al, Fe, Cr and Co in the root indicate its suitability for phytostabilization. Higher values of Zn, Cu and Cd content could be expected in the shoot of the E. canadensis L. The bioconcentration and translocation factors of Cd and Zn are higher than 1 which indicated that E. canadensis L. is favourable for phytoextraction of Cd and Zn from the landfill at Kolubara basin. The possibility to use Cd for remediation of contaminated soil was also described by Wei et al. (2009). E. canadensis L. can be considered as a satisfactory specie for phytoextraction of Cd and Zn from contaminated soils.
However, some technical solution and field application of this specie in phytoextraction require more detailed investigation of biomass, cover abundance as well as ecological and phytosociological studies of vegetation on fly ash disposal.
Conclusions
The metal content in various parts of E. canadensis L. from fly ash deposit of power plant Kolubara (Serbia) was determined in order to estimate its metal accumulation potential. The most influential parameters which distinguished shoot from root samples were Zn, Cu and Cd. Content of Al, Cr, Fe and Co was the major indicator of root samples in comparison to the stalk and inflorescence. The strong correlations among Al, Ba, Cr and Fe could result from their similar accumulation behaviour. Retained Al, Fe, Cr and Co in the root indicate its suitability for phytostabilization. Higher values of Zn, Cu and Cd content could be expected in the shoot of the E. canadensis L. This plant takes up Cd and Zn from the soil (BCFs greater than 1), transporting them through the stalk into the inflorescence (TFs higher than 1). Strong positive correlations between metals in every part of plant samples and metals from phase I and the pseudo total form of sequential extraction indicate that the bioavailability of elements in fly ash is similarly correlated with total form as an easy soluble form. Regarding its dominance in vegetation cover and abundance, the E. canadensis L. can be considered adequate for phytoextraction of Cd and Zn from coal ash landfills at Kolubara.
References
Alloway BJ (1995) Cadmium. In: Alloway BJ (ed) Heavy metals on soils. Blackie Academic and Professional, London, pp 123–151
Barman SC, Sahu RK, Bhargava SK, Chatterjee C (2000) Distribution of heavy metals in wheat, mustard and weed grown in fields irrigated with industrial effluents. Bull Environ Contam Toxicol 64:489–496
Barman SC, Kisku GC, Salve PR, Misra D, Sahu RK, Ramteke PW, Bhargava SK (2001) Assessment of industrial effluent and its impact on soil and plants. J Environ Biol 22:251–256
Braun-Blanquet J (1964) Pflanzensoziologie. Grundzüge der Vegetationskunde 3. Springer, Wien
Ćujić M, Dragović S, Sabovljević M, Slavković-Beškoski L, Kilibarda M, Savović J, Onjia A (2014) Use of mosses as biomonitors of major, minor and trace element deposition around the largest thermal power plant in Serbia. Clean—Soil Air Water 42:5–11
Djurdjevic L, Mitrovic M, Pavlovic P, Gajic G, Kostic O (2006) Phenolic acids as bioindicators of fly ash deposit revegetation. Arch Environ Contam Toxicol 50:488–495
Djurdjevic L, Mitrovic M, Gajic G, Jaric S, Kostic O, Lj O, Pavlovic P (2011) An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L. Flora 206:921–927
Ghosh M, Singh SP (2005a) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Env Res 3:1–18
Ghosh M, Singh SP (2005b) A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut 133:365–371
Gomes MP, Sá e Melo Marques TCLL, Soares AM (2013) Cadmium effects on mineral nutrition of the Cd-hyperaccumulator Pfaffia glomerata. Biologia 68:223–230
Hu PJ, Qiu RL, Senthilkumar P, Jiang D, Chen ZW, Tang JT, Liu FJ (2009) Tolerance accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ Exp Bot 66:317–325
Hughes JB, Shanks J, Vanderford M, Lauritzen J, Bhadra R (1997) Transformation of TNT by aquatic plants and plant tissue cultures. Environ Sci Technol 31:266–271
Khan MS, Zaidi A, Goel R, Musarrat J (2011) Biomanagement of metal-contaminated soils, environmental pollution. Springer, Dordrecht
Krgović R, Trifković J, Milojković-Opsenica D, Manojlović D, Mutić J (2014) Leaching of major and minor elements during the transport and storage of coal ash obtained in power plant. Sci World J 2014:212506
Krzaklewski W, Pietrzykowski M, Woš B (2012) Survival and growth of alders (Alnus glutinosa (L.) Gaertn. and Alnus incana (L.) Moench) on fly ash technosols at different substrate improvement. Ecol Eng 49:35–40
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212:75–84
Liu J, Donga Y, Xua H, Wang D, Xu J (2007) Accumulation of Cd, Pb and Zn by 19 wetland plant species in constructed wetland. J Hazard Mater 147:947–953
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579–579
Pietrzykowski M, Socha J, van Doorn N (2014) Linking heavy metal bioavailability (Cd, Cu, Zn and Pb) in Scots pine needles to soil properties in reclaimed mine areas. Sci Total Environ 470–471:501–510
Popovic A, Đorđevic D, Relic D, Mihajlidi-Zelic A (2011) Speciation of trace and major elements from coal combustion products of Serbian power plants (II)-Obilic power plant. Energy Sources Part A 33:2309–2318
Ram LC, Masto RE (2010) An appraisal of the potential use of fly ash for reclaiming coal mine spoil. J Environ Manage 91:603–617
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Redjala T, Sterckeman T, Morel JL (2009) Cadmium uptake by roots: contribution of apoplast and of high- and low-affinity membrane transport systems. Environ Exp Bot 67:235–242
Sarma H (2011) Metal hyperaccumulation in plants: a review, focusing on phytoremediation technology. J Environ Sci Technol 4:118–138
Sun R, Jin C, Zhou Q (2010) Characteristics of cadmium accumulation and tolerance in Rorippa globosa (Turcz.) Thell., a species with some characteristics of cadmium hyperaccumulation. Plant Growth Regul 61:67–74
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794
Vrbnicanin S (2008) The distribution of some economically damaging, invasive and quarantine weed species in Serbia. Part 2: spatial distribution and presence of nine weed species in Serbia. Plant Doct 36:408–418
Wei S, Zhou Q, Saha UK, Xiao H, Hu YY, Ren L, Ping G (2009) Identification of a Cd accumulator Conyza Canadensis. J Hazard Mater 163:32–35
Xue L, Liu J, Shi S, Wei Y, Chang E, Gao M, Chen L, Jiang Z (2014) Uptake of heavy metals by native herbaceous plants in an antimony mine (Hunan, China). Clean—Soil Air Water 42:81–87
Yang XE, Ye HB, Long XX, He B, He ZL, Stoffella PJ, Calvert DV (2004) Uptake and accumulation of cadmium and zinc by Sedum alfredii Hance at different Cd/Zn supply levels. J Plant Nutr 27:1963–1977
Zhang X, Xia H, Li Z, Zhuang P, Gao B (2011) Identification of a new potential Cd-hyperaccumulator Solanum photeinocarpum by soil seed bank-metal concentration gradient method. J Hazard Mater 189:414–419
Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP (2002) Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot 53:535–543
Acknowledgments
This research was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, project Nos. 172030 and 172017.
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Krgović, R., Trifković, J., Milojković-Opsenica, D. et al. Phytoextraction of metals by Erigeron canadensis L. from fly ash landfill of power plant “Kolubara”. Environ Sci Pollut Res 22, 10506–10515 (2015). https://doi.org/10.1007/s11356-015-4192-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4192-5