Abstract
Purpose
The present study was carried out in Roro region, Chaibasa, Jharkhand, India, to assess the impact of chromite–asbestos mine waste (CMW) on a nearby agroecosystem. The role of metal-accumulating grass–legume association in facilitating phytoremediation was investigated.
Materials and methods
Soil and plant samples were collected from (i) chromite–asbestos mine waste (CMW) with Cynodon dactylon, Sorghastrum nutans, and Acacia concinna; (ii) contaminated agricultural soil-1 (CAS1) from a foothill with Cajanus cajan; (iii) contaminated agricultural soil-2 (CAS2) distantly located from the hill, cultivated with Oryza sativa and Zea mays; and (iv) unpolluted control soil (CS). Total metal concentrations were quantified in both soils and plants by digesting the samples using HNO3, HF, HClO4 (5:1:1; v/v/v), and HNO3 and HClO4 (5:1; v/v), respectively, and analyzed under flame atomic absorption spectrophotometry. Metal grouping and site grouping cluster analysis was executed to group the metals and sampling sites. Translocation factor (TF) and bioconcentration factor (BCF) were calculated to determine the phytoremediation efficiency of grasses and legumes.
Results and discussion
Results indicate that total metal concentrations in the CMW were in the order of Cr > Ni > Mn > Cu > Pb > Co > Zn > Cd. High concentrations of Cr (1983 mg kg−1) and Ni (1293 mg kg−1) with a very strong contamination factor were found in the CAS, which exceeds the soil threshold limits. Further, metal and site grouping cluster analysis also revealed that Cr and Ni were closely linked with each other and the CMW was the main source of contamination. Among all the metals, Cr and Ni were mainly accumulated in grasses (C. dactylon and S. nutans) and legumes (A. concinna and C. cajan) as compared to cereals (Z. mays and O. sativa). The TF of Cr was >1 for grasses. Except for Zn, the BCF for all the metals were <1 in roots and shoots of all the plants and cereals.
Conclusions
The present study revealed that abandoned CMW is the source of contamination for agriculture lands. Phytoremediation relies on suitable plants with metal-scavenging properties. Grass–legume cover (C. dactylon, S. nutans, A. concinna, and C. cajan) has the ability to accumulate metals and act as a potential barrier for metal transport, which facilitate the phytoremediation of the CMW. Possibilities for enhancing the barrier function of the grass–legume cover need to be explored with other low-cost agronomic amendments and the role of rhizospheric organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal contamination from active and abandoned mine sites is one of the major environmental concerns throughout the world (Wahsha et al. 2012; Goumih et al. 2013). Improper mine waste disposal causes dispersal of contaminants into air, water, and soil, resulting in heavy metal pollution (Fernandez-Caliani et al. 2009; Barbafieri et al. 2011; Mileusnic et al. 2014). Mine wastes are often prone to water and wind erosion, resulting in the spreading of these toxic wastes into nearby agricultural fields and water bodies (Bech et al. 2012). Because of the scarcity of agricultural lands, small-scale farmers have no option but to cultivate crops on such sites. Many studies have reported a heavy metal contamination problem in the vicinity of chromite and asbestos mines (Singh et al. 2007; Krishna et al. 2013). The abandoned chromite–asbestos mine of Roro region, Chaibasa, India, surrounded by agricultural fields is of considerable interest to understand the fate of heavy metals.
Metal-accumulating crops viz. Helianthus annus and Brassica species are of food value and important for risk-based phytoremediation, being high in productivity. Biomass from such crops is to be used for industrial feed stock and energy production rather than for food (Prasad 2015). Experiments using metal-hyperaccumulating plant suggest their capability for polishing soils with low levels of toxic metals like Pb and Cd. In one such case, multiple crop rotation of Solanum nigram (hyperaccumulator) on Cd-contaminated soil resulted in exclusion of Cd in Chinese cabbage (Niu et al. 2015). Further, naturally growing grasses and legumes are known for phytoremediation of metalliferous soils (Maiti and Maiti 2015). Kumar and Maiti (2014) investigated the translocation and bioaccumulation of Cr, Ni, Pb, and Cd in Oryza sativa (rice) and Zea mays (maize) growing in CAS. In the present study, the phytoremediation facilitating role of the metal-accumulating grass–legume association was investigated.
The aims of the present study are to (i) assess total metal concentrations in the abandoned chromite–asbestos mine waste (CMW), closely and far away located contaminated agricultural soils (CASs), and reference agriculture soil (control soil, CS); (ii) assess metal accumulation in naturally growing grasses, legumes, and cereals; and (iii) analyze soil pollution index and plant factors.
2 Materials and methods
2.1 Study site description
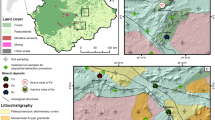
The abandoned CMW is located at Roro hills (N 22° 29′, E 85° 39′), which is about 22 km away from Chaibasa district of Jharkhand, India (Fig. 1a). The chromite ore deposits of this region are spread in three blocks named successively from north to south as Kimsiburu, Kittaburu, and Roroburu-Chitungburu. High-grade chromite ore deposit (30 cm thick) running to an extent of 1.6 km was found in the Roro region.
The Roro mine has undergone extensive open cast and underground mining operations for extraction of magnetite, chromite, and asbestos, was ceased down in 1983, and left approximately 0.7 million tons of toxic mine waste and host rocks. The entire area of the CMW was spread along the slopes of the hill (100 × 400 m) extending to the nearby agriculture fields (500 × 1000 m). The waste is very fine, loose, and homogeneous, lacking essential nutrients. Wind and water erosion of the hill slopes transport a large volume of toxic mine waste, resulting in a deposit of 40 cm of thick chromite–asbestos waste in the agricultural fields.
The climate is dry tropical, characterized by high annual precipitation of approximately 1422 mm (July–October), summer (April–June) and winter (November–March). The CMW is sparsely populated with Cynodon dactylon (Bermuda grass), Sorghastrum nutans (Indian grass), and A. concinna (Shikakai) in an area of about 100 × 400 m. However, the most dominant plant species was C. dactylon. The surrounding agricultural fields are mainly cultivated with Cajanus cajan (pigeon pea) in an area of about 20 × 40 m, and O. sativa (rice), and Z. mays (maize) in 1000 × 500 m.
2.2 Field sampling
To assess the effect of metal pollution, samples were collected in the direction of wind and downward flow of tributaries from the hill. The schematic diagram showing the sampling areas is shown in Fig. 1b. The tributaries from the hill causing gully erosion and the wind are continuously polluting the nearby areas. Soil and plant samples were collected from four sampling locations designated as (i) CMW (n = 9) each with three replicates of C. dactylon (n = 3), S. nutans (n = 3), and A. concinna (n = 3); (ii) contaminated agricultural soil 1 (CAS1) from the foot hill cultivated with the biannual plant C. cajan (n = 5); (iii) contaminated agricultural soil 2 (CAS2) located far away from the hill, cultivated with cereals O. sativa and Z. mays (n = 5); and (iv) CS (n = 3) obtained about 1000 m away from the contaminated hill and assumed to have the background concentration of the analyzed metals. The six plant samples (C. dactylon, S. nutans, A. concinna, C. cajan, O. sativa, and Z. mays) were collected by placing a 10 × 10 m quadrate, and from each quadrate, soil samples were collected for three different profiles (0–15, 15–30, and 30–45 cm). Four sub-samples of 1 kg each were collected (from each corner), mixed, and homogenized together to form a composite sample. All the topsoil samples were collected by removing the top litter layer and pebbles. The samples were sampled between Dec 2012 and May 2013.
2.3 Soil analyses
The collected samples were air dried for 3 days, oven dried at 40 °C, allowed to pass through a nylon sieve <2 mm, and kept in polypropylene airtight zip bags for subsequent analyses. The physico-chemical characteristics of the CMW, the CAS, and the CS were determined using standard methods. Particle size distribution was determined by sieving method (Gee and Bauder 1986). Water holding capacity was determined using Keen’s box and bulk density by metal core sampler method (Mukhopadhyay et al. 2013). Pore space (porosity) was determined from bulk density measurements with an assumed particle density of 2.65 mg m−3 (Sobek et al. 1978). The pH and electrical conductivity (EC) were measured in a soil–water suspension (1:1; w/v) using a pH meter (Cyberscan 510) and a conductivity meter (EI 601), respectively (Kumar and Maiti 2015). organic carbon (OC, rapid dichromate oxidation technique, Walkley and Black 1934). available phosphorus (Avl. P, Olsen method, Olsen and Sommers 1982) using a UV–visible spectrophotometer (UV 265, Shimadzu), and available nitrogen (Avl. N, alkaline permanganate method, Subbiah and Asija 1956) were determined. Cation exchange capacity (CEC) was determined using 1 N ammonium acetate extraction method (Jackson 1973) using a flame photometer (Systronics 128). For total metal analysis, 1 g of soil sample was taken in a Teflon beaker and digested using HNO3 conc. (69–71 %), HF (40 %), and HClO4 (71–73 %) (5:1:1; v/v/v) (Ho et al. 2013). The digested sample was warmed with 1 % HNO3, filtered, and transferred into a standard volumetric flask, and metal concentrations were analyzed using a flame atomic absorption spectrophotometer (FAAS-GBC Avanta, Australia).
2.4 Plant analyses
Two grass species (C. dactylon and S. nutans), two leguminous plants (A. concinna and C. cajan), and two cereals (Z. mays and O. sativa) were collected, washed properly, and finally rinsed with deionized Millipore water (pH 6.9; Milli-Q system) to remove the unwanted adhered soil particles completely. The washed plant samples were divided into roots, stems, and leaves, cut into 1–2 cm in length, and oven dried at 70 °C for constant weight. The samples were ground using a mortar and pestle and sieved through a 1000-μm stainless steel sieve. Accurately weighed 0.5 g of powdered plant sample was digested using HNO3 (69–71 %) and HClO4 (71–73 %) (5:1; v/v) (Zou et al. 2012). Metal concentrations were analyzed in the filtered supernatant using FAAS.
2.5 Soil pollution index and plant factors
To study the impact of metal pollution on soil, contamination factor (CF) has been widely and frequently used by researchers for predicting the level of contamination in multi-metal CASs (Gemici and Tarcan 2007; Machender et al. 2013). It is the assessment of metal contamination in respect to the control soil and is calculated as CF = [C]heavy metal/[C]background, where [C]heavy metal is the concentration of a particular metal in contaminated soil and [C]background is the concentration of a particular metal in non-contaminated or unpolluted control soil. The level of contamination can be assessed as 0 = none, 1 = none to medium, 2 = moderate, 3 = moderate to strong, 4 = strongly polluted, 5 = strong to very strong, and 6 = very strong (Tomlinson et al. 1980).
Translocation factor (TF) is the ability of the plant to transfer metal from roots to shoots and calculated as TF = [C]shoot/[C]root, where [C]shoot is the concentration of metal in shoots and [C]root is the concentration of metal in roots (Maiti and Jaiswal 2008). Bioconcentration factor (BCF) is defined as the transfer of metal from soil to different parts of the plant and calculated as bioconcentration factor of roots (BCF)root = [C]root/[C]soil, and bioconcentration factor of shoot (BCF)shoot = [C]shoot/[C]soil, where [C]root or [C]shoot indicates the metal concentration accumulated in the plant tissue (root or shoot), and C (soil/substrate) indicates metal concentration in the substrate (soil/mine waste) (Bech et al. 2012).
2.6 Quality assurance and quality control
All glassware used were soaked in nitric acid and cleaned properly. Reagent blanks, duplicates, and spiked samples were used. Samples were prepared using deionized Millipore water (Milli-Q system, Millipore). Standard reference materials (SRM, AccuTrace, AccuStandard Inc., USA; matrix 2–5 % nitric acid; CRM uncertainty ±5 %; verified against NIST SRM# 3108 for Cd, 3112a for Cr, 3136 for Ni, 3128 for Pb, 3132 for Mn, 3168a for Zn, 3113 for Co, and 3114 for Cu) were used for the preparation and calibration of each analytical batch. Calibration coefficients were maintained at a high level ≥0.99. The recovery percentage of metals in the SRM samples for soil and plants ranged between 81–102 and 84–121 %, respectively. The accuracy was checked by running standard solutions after every 15 samples.
2.7 Statistical analyses
The soil and plant data were analyzed by one-way analysis of variance (ANOVA) and a test of homogeneity of variance was carried out. Mean separation was performed by Tukey’s test at 5 % level of significance after the normality and homogeneity of variances were verified. Non-normal data were analyzed by the Kruskal–Wallis non-parametric test to compare the soil sites and plant species. Hierarchical cluster analysis (CA) was performed using Ward’s method with Euclidean distances as measure of similarity (Varol et al. 2013). All the data were executed using SPSS 20.0 (SPSS Inc. Chicago, USA) and XLSTAT 2007 package.
3 Results
3.1 Physico-chemical characteristics and heavy metal concentrations in mine waste and soils
The physical and chemical characteristics of the CMW, CAS1, CAS2, and CS for all the three profiles are presented in Table S1 (Electronic Supplementary Material). Significant differences were found between different sites of the same profile. Particle size distribution in mine-influenced areas was sandy in texture with higher percentage of sand in the top profile of 0–15 cm as compared to lower profiles. Bulk density (BD) was higher in CMW, CAS1, and CAS2 soil as compared to CS. The pH value and EC for all the soil samples ranged between 6.45–7.5 and 0.02–0.23 dS m−1, respectively, in the top profile. The OC content, Avl. N, and Avl. P were found low for CMW and CS in all the profiles; however, they were high in CAS1. Maximum CEC (13.55 cmol kg−1) was found in CAS1 and minimum in CAS2 (3.47) for the top profile. Mine waste-influenced agriculture soils namely CAS2 were deficient in nutrient content (available nitrogen, 70 mg kg−1; available phosphorous, 0.21 mg kg−1; and exchangeable K, 0.16 cmol + kg−1); however, because of the use of manures or P-fertilizers by local farmers, the CAS1 contained larger amounts of NPK and other nutrients (Supplementary Table; Kumar and Maiti 2015).
The total metal concentrations of the eight analyzed heavy metals in the CMW, CAS1, CAS2, and CS for three different depths are statistically (p < 0.05) presented in Table 1. Cr had the greatest concentrations across soils followed by Ni > Mn > Cu > Pb > Co > Zn > Cd. The average total metal concentrations across soil depth of 0–45 cm of the CMW, CAS1, and CAS2 ranged between 412 and 2418 mg Cr kg−1, 182 and 1344 mg Ni kg−1, 40 and 74 mg Zn kg−1, 331 and 544 mg Mn kg−1, 27 and 73 mg Co kg−1, 40 and 129 mg Cu kg−1, 41 and 90 mg Pb kg−1, and 0.6 and 1.3 mg Cd kg−1. The CF of the metals were very strong for Ni and Cr and found in the order of Ni > Cr > Cu > Co > Pb > Mn > Cd > Zn (Fig. 2). The critical ranges of metal concentration (mg kg−1) in soil are 7–100 for Cr, 100 for Ni, 1500–3000 for Mn, 70–400 for Zn, 25–50 for Co, 60–125 for Cu, 100–400 for Pb, and 3–8 for Cd (Alloway 1990, 2013). Detailed reference may be made to Kumar and Maiti (2015) for guidelines of safe limits of metals in agricultural soil.
Contamination factor (CF) of different analyzed metals for the chromite–asbestos mine waste (CMW), contaminated agricultural soil 1 (CAS1), and contaminated agricultural soil 2 (CAS2). Different letters for the same metal represent significant difference at p < 0.05. CF is the ratio of heavy metal concentration in contaminated soil to the heavy metal concentration in background soil
3.2 Metal and spatial site grouping cluster analysis
The hierarchical cluster analysis (CA) produced three clusters: the first cluster contains Co, Pb, Cu, Zn, and Cd, the second cluster contains Mn linked with Zn, and the third cluster contains Cr and Ni. The results obtained from CA revealed that Cr, Ni, and Mn were closely linked to each other (Fig. 3a).
Similarly, spatial CA was applied to group the different sampling sites with similar characteristics. Hierarchical CA was performed along the sites with three different profiles of 0–15, 15–30, and 30–45 cm (Fig. 3b). Cluster 1 consists of non-metal-influenced control soil and low CASs of deeper profile: CS (0–15 cm), CS (15–30 cm), CS (30–45 cm), CAS2 (15–30 cm), and CAS2 (30–45 cm). Cluster 2 contains (CMW1 0–15 cm, CMW 15–30 cm, and CMW 30–45 cm) very strongly Cr and Ni contaminated sites. The third cluster contains the moderate to highly contaminated agricultural soils (CAS1 0–15, 15–30, 30–45 cm and CAS2 0–15 cm) due to the CMW.
3.3 Heavy metal accumulation in grasses, legumes, and cereals
Heavy metal concentrations in different parts of the plants are statistically presented in Fig. 4. Cr concentration was significantly high (p < 0.05) in grasses (C. dactylon and S. nutans) and legumes (A. concinna and C. cajan) growing on the CMW and CAS1 with significant (p < 0.05) variability in their standard deviation whereas low Cr was accumulated in the cereals (Z. mays and O. sativa) growing on the CAS2. Maximum concentration of Cr in roots (72 mg kg−1) and shoots (58 mg kg−1) was found in A. concinna and S. nutans, respectively, whereas minimum concentrations were observed in roots (11 mg kg−1) and shoots (8 mg kg−1) of Z. mays. The TF for Cr were >1 for grasses and <1 for legumes and cereals whereas BCFroot and BCFshoot were very low. The Ni content in roots (18–35 mg kg−1) and shoots (28–46 mg kg−1) of grass–legumes was significantly higher (p < 0.05) as compared to that of cereals. However, none of the plant had exceeded critical plant concentration of 10–100 mg kg−1 (Kabata-Pendias 2011). Except for C. dactylon, Ni mobility was high for grass–legumes (TF > 1). However, BCFroot and BCFshoot were very low for all the six plants. Accumulation of Zn and Mn was quite higher in grass–legumes as compared to cereals. Zn content was maximum in the shoot of C. cajan (110 mg kg−1) and minimum in Z. mays (24 mg kg−1) whereas for Mn it was maximum for A. concinna. All the plants were capable of transporting Zn and Mn in the shoot part (TF >1). BCFshoot > 1 were found for grasses and cereals and BCFroot < 1 for cereals (Fig. 5).
a–g Concentration of metals (mg kg−1) in the different plant parts of Cynodon dactylon, Sorghastrum nutans, Acacia concinna, Cajanus cajan, Zea mays, and Oryza sativa. Different small letters for the roots, capital letters for the leaves, and numbers for the stems represent significant difference at p < 0.05
a Translocation factor (TF), b bioconcentration factor of roots (BCFroot), and c bioconcentration factor of shoots (BCFshoot) of grasses, legumes, and cereals growing on the CMW, CAS1, and CAS2. Different letters among the six plants for the same metal represent significant difference at p < 0.05. TF is the ratio of the metal concentration in shoots to that of roots, BCFroot is the ratio of the metal concentration in roots to that of the substrate, and BCFshoot is the ratio of the metal concentration in shoots to that of the substrate
Concentrations of Co in the plants were in the order of A. concinna > C. dactylon > C. cajan > S. nutans > Z. mays > O. sativa. Except for C. dactylon, all the plants were with TF >1 whereas the BCF values of roots and shoots for grasses, legumes, and cereals were very low, with the maximum BCFroot and BCFshoot values in C. dactylon (0.076) and A. concinna (0.096), respectively. Cu concentrations in grass–legume and cereals ranged from 8 to 43 and 2 to 5 mg kg−1, respectively, in roots and 6–26 and 2–6 mg kg−1 in shoots, respectively. Pb content in the roots and shoots of grasses and legumes was many folds higher than cereals and in order of A. concinna > S. nutans > C. cajan > C. dactylon > Z. mays > O. sativa. The TF >1 and BCFs <1 were found for all the plants. Cd was very low or below detection limit (<0.03) in all the plants growing on all the sites (data not shown).
4 Discussion
The physico-chemical characteristics of the CMW and CAS were poor and deficient in nutrient content (NPK); however, agricultural soils (CAS1) supplemented with manures or P-fertilizer by the local farmers resulted in enhanced NPK content (Alloway 2013; Barbafieri et al. 2011). Because of the high sandy texture and BD, water holding capacity and pore space for the CMW and CASs were found low. Along the depth, the NPK content decreased because of poor availability of nutrients in the lower profiles. Spreading and thin layer deposition of the CMW from the hill into the surrounding area during monsoon and summer seasons resulted in lower availability of nutrients in the top profile (0–15 cm) of the CAS2; however, it was better in other profiles (15–30 and 30–45 cm). Das et al. (2013) had also reported low availability of nutrients mainly N, P, and OC in the chromite overburden dump. Exchangeable cations (exc. K, Ca, and Na) were found very low in the CMW and CAS maybe because of the runoff CMW deposited on the surface of the soil (Kumar and Maiti 2015).
Significant differences in metal concentration were found for Cr-contaminated soil and CS for the same profile. However, for most of the metals, no significant differences were found in the CMW, CAS1, and CAS2, suggesting the possibility of the CMW as a source of metal contamination for the agricultural soils.
Metal grouping cluster analysis showed Cr was most distantly linked with the first and second groups of clusters and closely linked with Ni, revealing that the Cr has a single source of origin. Site grouping cluster analysis for different depths showed a thin layer of the CMW was deposited on the top profile because of the distance between the CAS2 and the CMW, resulting in high contamination, whereas it was moderate to low in lower profiles. C. dactylon was the most dominant plant species followed by S. nutans, A. concinna, and C. cajan growing on the CMW and CAS1 sites. Therefore, it may be opined that grass–legumes are thriving towards adaptation of metal contamination and acting as a potential barrier for water and wind erosion.
Cr concentration in grasses and legumes was high and above the critical plant total metal concentration of 5–30 mg kg−1 (Kabata-Pendias 2011). Irrespective of high concentration of Cr in the soil, its accumulation was found low (<10 mg kg−1) in the cereals growing on the CASs maybe because of its highly immobile nature (>80 % of Cr present in immobile residual fraction) and grass–legumes were acting as a potential barrier for metal uptake by cereals (Alloway 2013). Further, addition of farmyard manure reduces the Cr mobility in soil, resulting in lower Cr accumulation in crops (Singh et al. 2007). Mertz (1969) and Petrunina (1974) reported that the native plants of chromite deposits can accumulate only a small fraction of 0.3 to 3.4 % of Cr. The toxicity limit of Cr for the crops growing on the serpentine soils of California ranged between 1 and 10 mg kg−1 (Alexander et al. 2007). The TF of C. dactylon, S. nutans, and C. cajan were found >1 which implies higher accumulation of Cr in the leaves as compared to roots. BCF for all the plants were found very low (0.005–0.030) may be due to lower availability. Similar observations were found by Brunetti et al. (2009). with TF >1 and BCF <1 for Cr in the wild plants growing on a multi-metal-contaminated site of Apulia region, Southern Italy. It is known that Ni has a high mobility in serpentine soil (Kumar and Maiti 2015). Grass–legumes were found to translocate metals to leaves TF >1; however, the BCF values were found to be very low because of the high concentration of Ni in the soil. Brunetti et al. (2009) reported TF >1 and BCF <1 for Ni in the wild plants growing on a multi-metal-contaminated site of Apulia region of Southern Italy. The Zn concentrations were found within the normal range of 15–150 mg kg−1 (Chaney 1989). High TF (TF >1) and low BCFs (BCF <1) for Zn indicate its accumulation in the aerial parts and low translocation from soil, respectively.
None of the grass–legumes and cereals has exceeded the critical plant total Mn concentration of 300–500 mg kg−1 and faced the Mn deficiency of 15–25 mg kg−1. Overall, it was found that Mn mainly accumulated in the plant shoot than root which may be due to its higher mobility (Kabata-Pendias 2011), highlighting higher translocation ability (TF >1) of the plants. The BCFroot and BCFshoot of grass–legumes and cereals were <1, with maximum BCFshoot values in cereal species because of the presence of significant concentration of Mn in contaminated soil. The maximum BCFshoot was found for A. concinna followed by C. cajan > S. nutans > Z. mays > O. sativa > C. dactylon. For Co, Cu, and Pb, all the grasses, legumes, and cereals were found within the toxicity limit for Co, Cu, and Pb. Except for S. nutans, TF >1 was reported in all the grasses, legumes, and cereals, showing its high mobility of Pb (Brunetti et al. 2009).
5 Conclusions
In the present study, metal and spatial site grouping cluster revealed the abandoned CMW is the source of contamination for the agriculture lands. The success of phytoremediation depends upon the selection of plants with metal-scavenging properties. Grass–legume cover (C. dactylon, S. nutans, A. concinna, and C. cajan) investigated in this study had the ability to accumulate metals and act as a potential barrier for transport which facilitate phytoremediation of chromite–asbestos mine waste (Hoflich et al. 1994; Zong et al. 2015). Possibilities for enhancing the barrier function of the grass–legume cover need to be explored with other low-cost agronomic amendments and the role of rhizospheric organisms. Use of high-biomass grasses (Cymbopogon sp., Chrysopogon sp.) and leguminous plants (Stylosanthes sp., Sesbania sp.) with biochar, farmyard manure, and chicken manure along with Pseudomonas sp. and Bacillus sp. which has plant growth-promoting activities could help in the remediation of Cr-contaminated soil.
References
Alexander EB, Coleman RG, Keeler-Wolf T, Harrison S (2007) Serpentine geoecology of western North America. Oxford University Press, New York
Alloway BJ (1990) Heavy metals in soils. Wiley, New York
Alloway BJ (2013) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Environ Pollut 22. Springer, Dordrecht
Barbafieri M, Dadea C, Tassi E, Bretzel F, Fanfani L (2011) Uptake of heavy metals by native species growing in a mining area in Sardinia, Italy: discovering native flora for phytoremediation. Int J Phytorem 13(10):985–997
Bech J, Duran P, Roca N, Poma W, Sanchez I, Roca-Perez L, Boluda R, Barcelo J, Poschenrieder C (2012) Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. J Geochem Explor 123:109–113
Brunetti G, Soler-Rovira P, Farrag K, Senesi N (2009) Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region, Southern Italy. Plant Soil 318:285–298
Chaney RL (1989) Toxic accumulation in soils and crops: protecting soil fertility and agricultural food-chains. In: Bar-Yosef B, Barrow NJ, Goldschmid J (eds) Inorganic contaminants in the vadose zone. Springer, Berlin, pp 140–158
Das S, Ram SS, Sahu HK, Rao DS, Chakraborty A, Sudarshan M, Thatoi HN (2013) A study on soil physico-chemical, microbial and metal content in Sukinda chromite mine of Odisha, India. Environ Earth Sci 69:2487–2497
Fernandez-Caliani JC, Barba-Brioso C, Gonzalez I, Galan E (2009) Heavy metal pollution in soils around the abandoned mine sites of the Iberian pyrite belt (Southwest Spain). Water Air Soil Pollut 200:211–226
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis, part 1, 2nd edn. ASA and SSSA, Madison, WI, pp 383–411
Gemici U, Tarcan G (2007) Assessment of the pollutants in farming soils and waters around untreated abandoned Turkonu mercury mine (Turkey). Bull Environ Contam Toxicol 79:20–24
Goumih A, El-Adnani M, Hakkou R, Benzaazoua M (2013) Geochemical behavior of mine tailings and waste rock at the abandoned Cu–Mo–W Azegour Mine (Occidental High Atlas, Morocco). Mine Water Environ 32:121–132
Ho CP, Hseu ZY, Chen NC, Tsai CC (2013) Evaluating heavy metal concentration of plants on a serpentine site for phytoremediation applications. Environ Earth Sci 70:191–199
Hoflich G, Wiehe W, Kuhn G (1994) Plant growth stimulation with symbiotic and associative rhizosphere microorganisms. Experientia 50:897–905
Jackson ML (1973) Soil chemical analysis. Prentice Hall Pvt. Ltd., New Delhi
Kabata-Pendias A (2011) Trace elements in soils and plant, 4th edn. Taylor and Francis Group, LLC, Boca Raton
Krishna AK, Mohan KR, Murthy NN, Periasamy V, Bipinkumar G, Manohar K, Rao SS (2013) Assessment of heavy metal contamination in soils around chromite mining areas, Nuggihalli, Karnataka, India. Environ Earth Sci 70:99–708
Kumar A, Maiti SK (2014) Translocation and bioaccumulation of metals in Oryza sativa and Zea mays growing in chromite-asbestos contaminated agricultural fields, Jharkhand, India. Bull Environ Contam Toxicol 93:434–441
Kumar A, Maiti SK (2015) Assessment of potentially toxic heavy metal contamination in agricultural fields, sediment, and water from an abandoned chromite asbestos mine waste of Roro hill, Chaibasa, India. Environ Earth Sci 74(3):2617–2633
Machender G, Dhakate R, Rao GT, Loukya G, Reddy MN (2013) Assessment of trace element contamination in soils around Chinnaeru River Basin, Nalgonda District, India. Environ Earth Sci 70:1021–1037
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess 136:355–370
Maiti SK, Maiti D (2015) Ecological restoration of waste dumps by topsoil blanketing, coir-matting and seeding with grass–legume mixture. Ecol Engg 77:74–84
Mertz W (1969) Chromium occurrence and function in biological systems. Physiol Rev 49:163–239
Mileusnic M, Mapani BS, Kamona AF, Ruzicic S, Mapaure I, Chimwamurombe PM (2014) Assessment of agricultural soil contamination by potentially toxic metals dispersed from improperly disposed tailings, Kombat mine, Namibia. J Geochem Explor 144:409–420
Mukhopadhyay S, Maiti SK, Masto RE (2013) Use of Reclaimed Mine Soil Index (RMSI) for screening of tree species for reclamation of coal mine degraded land. Ecol Engg 57:133–142
Niu M, Wei S, Bai J, Wang S, Ji D (2015) Remediation and safe production of Cd contaminated soil via multiple cropping hyperaccumulator Solanum nigrum L. and low accumulation Chinese cabbage. Int J Phytorem 17(7):657–661
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL et al (eds) Methods of soil analysis, part 2, 2nd edn. ASA and SSSA, Madison, WI, pp 403–430
Petrunina NS (1974) Geochemical ecology of plants from the provinces of high trace element contents. In: Problems of Geochemical Ecology of Organisms, Izd. Nauka, Moscow, Russia
Prasad MNV (2015) Bioremediation and bioeconomy. Elsevier, USA, p 730. ISBN 978-012-802872-8
Singh G, Brar MS, Malhi SS (2007) Decontamination of chromium by farm yard manure application in spinach grown in two texturally different Cr-contaminated soils. J Plant Nutr 30:289–308
Sobek AA, Schuller WA, Freeman JR, Smith RM (1978) Field and laboratory methods applicable to overburden and minesoils. USEPA Rep. 600/2-78-054, U.S. Gov. Print. Office, Washington, DC
Subbiah BV, Asija GL (1956) A rapid procedure for the determination of available nitrogen in soil. Curr Sci 25:259–260
Tomlinson DJ, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgoland Mar Res 33(1–4):566–575
Varol M, Gokot B, Bekleyen A (2013) Dissolved heavy metals in the Tigris River (Turkey): spatial and temporal variations. Environ Sci Pollut Res Int 20(9):6096–6108
Wahsha M, Bini C, Argese E, Minello F, Fontana S, Wahsheh H (2012) Heavy metals accumulation in willows growing on Spolic Technosols from the abandoned Imperina Valley mine in Italy. J Geochem Explor 123:19–24
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Zong K, Huang J, Nara K, Chen Y, Shen Z, Lian C (2015) Inoculation of ectomycorrhizal fungi contributes to the survival of tree seedlings in a copper mine tailing. J Forest Res 20:493–500
Zou T, Li T, Zhang X, Yu H, Huang H (2012) Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ Earth Sci 65:621–630
Acknowledgments
The main authors would like to thank the Indian School of Mines, Dhanbad, India, for providing the research facilities to conduct the experiments. The authors would also like to appreciate Sinku N for providing assistance during the field sampling. Thanks are due to Urfu for e-research facility supported by the Act 211 Government of the Russian Federation, agreement № 02.A03.21.0006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: María Luisa Andrade
Rights and permissions
About this article
Cite this article
Kumar, A., Maiti, S.K., Tripti et al. Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite–asbestos mine. J Soils Sediments 17, 1358–1368 (2017). https://doi.org/10.1007/s11368-015-1323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1323-z