Abstract
Arsenic (As) gets accumulated in plants via phosphorous transporters and water channels and interferes with nutrient and water uptake, adversely affecting growth and productivity. Although, Si and AM have been reported to combat arsenic stress, their comparative and interactive roles in ameliorating As V and As III toxicities have not been reported. Study evaluated effects of Si and Rhizophagus irregularis on growth, As uptake and yield under arsenate and arsenite stress in two pigeonpea genotypes (metal tolerant—Pusa 2002 and metal sensitive—Pusa 991). Higher As accumulation and translocation was observed in As III treated roots of Pusa 991 than those of Pusa 2002 when compared with As V. Roots were more negatively affected than shoots which led to a significant decline in nutrient uptake, leaf chlorophylls, and yield, with As III inducing more negative effects. Pusa 2002 established more effective mycorrhizal symbiosis and had higher biomass than Pusa 991. Si was more effective in inducing shoot biomass while AM inoculation significantly improved root biomass. AM enhanced Si uptake in roots and leaves in a genotype dependent manner. Combined application of Si and AM were highly beneficial in improving leaf water status, chlorophyll pigments, biomass, and productivity. Complete amelioration of negative impacts of both concentrations of As V and lower concentration of As III were recorded under +Si +AM in Pusa 2002. Results highlighted great potential of Si in improving growth and productivity of pigeonpea through R. irregularis under As V and As III stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a ubiquitous toxic metalloid existing in soil and groundwater throughout the world, ranging from 0.5 μg/L to over 1200 mg/L (Mandal and Suzuki 2002; Siddiqui et al. 2015). Due to its adverse effect on human health, its threshold value has been listed as 10 μg/L in drinking water by World Health Organization (WHO) and US Environmental Protection Agency (EPA) (Hettick et al. 2015). In many countries like India, Bangladesh, China, Vietnam, and Thailand, high As contamination has been reported (Dwivedi et al. 2010; McCarty et al. 2011; Talukdar and Talukdar 2014; Sharma et al. 2014), which adversely affect growth and productivity of plants (Sushant and Ghosh 2010). As is generally pervasive in rocks and is released into the environment either by natural processes such as weathering of rocks, hydrothermal ore deposits, volcanic eruptions, geothermal activities, and forest fire (Nriagu et al. 2007) or by anthropogenic activities like mining and fossil fuel combustion, disposal of industrial effluents, sewage sludge, and use of agricultural chemicals (fertilizers, pesticides, herbicides, etc.) (Flora 2015).
As V and As III are the most common forms of As prevalent in aerated and non-aerated (reducing environments) soils, respectively (Danh et al. 2014). Both As V and A III are taken by the plants through different transport pathways and follow the Michaelis–Menten equation (Wang et al. 2002; Li et al. 2011; Wu et al. 2011; Sharma et al. 2014). As V, a chemical analogue of inorganic phosphate (Pi), is transported across the plasma membrane via phosphate transporters (PHT) and competes with P for uptake due to similar size and charge (Ullrich–Eberius et al. 1989; Wang et al. 2002; Garg and Singla 2012). Unlike As V, As III uptake in plants and microbes is facilitated via nodulin 26-like intrinsic membrane proteins (NIP), called aquaporins (Ma et al. 2008; Mosa et al. 2012).
Phytotoxicity of As varies between and within plant species, its oxidation state and edaphic conditions (Sharma et al. 2014). When exposed to excess As, plants exhibit toxicity symptoms such as poor seed germination (Yoon et al. 2016), reduced root and shoot growth (Shaibur and Kawai 2009), decreased plant height (Rahman et al. 2007), wilting and necrosis of leaf margins (Zhang et al. 2009), chlorosis (Singh et al. 2006), nutrient deficiencies (Shaibur et al. 2006; Shaibur and Kawai 2010), biomass inhibition (Zhang et al. 2009), and lower fruit and grain yield (Zhang et al. 2009; Spagnoletti and Lavado 2015]. Both inorganic forms of As, i.e., As V and As III interfere with plant metabolisms by altering various morphological, physiological, and biochemical processes resulting into decline in plant biomass and ultimate yield (Srivastava et al. 2009; Garg and Singla 2012; Farnese et al. 2014). As V competes with Pi for uptake and transport, hence, replacing it in ATP synthesis and oxidative phosphorylation, therefore, disrupting the energy flow in cells (Danh et al. 2014). Once inside the plant cell, As V is rapidly reduced to As III (Garg and Singla 2012; Finnegan and Chen 2012), which is a thiol reactive compound that binds with sulfhydryl groups (-SH) in enzymes and proteins, thus hampering their cellular function and leads to growth retardation (Fu et al. 2014; Farooq et al. 2016).
Contradictory reports have been cited for relative toxicities of As V and As III in crop plants. Abedin and Meharg (2002) reported decrease in percent germination with increasing As (As V and As III) concentration, with As III exhibiting more toxic effects. Relatively more detrimental effects of As III on growth and photosynthesis have been reported in rice (de Andrade et al. 2015) and Withania somnifera (Siddiqui et al. 2015) due to higher reduction of aminolevulinic acid pools and inhibition of enzymes involved in chlorophyll biosynthesis by binding to thiol groups. On the other hand, Abbas and Meharg (2008) reported higher toxicity of As V than As III and DMA in six maize varieties. Therefore, reports on differential toxic responses of As V and As III in crop plants are scanty and need thorough investigations. Moreover, to the best of our knowledge, comparative toxic responses of As V and As III on legume species and their genotypes have not been reported yet.
Arbuscular mycorrhizal (AM) fungi are ecologically important soil microbes playing considerable role in nutrient cycling, plant growth promotion (Krishnamoorthy et al. 2015) and enhanced tolerance to abiotic stresses (Krishnamoorthy et al. 2015; Lenoir et al. 2016). Various studies have indicated the co-occurrence of AMF in As contaminated soils (Meharg and Cairney 1999; Smith et al. 2010, Gonzalez-Chavez et al. 2002, Sun et al. 2016) which suggests that these fungi might have evolved physiological and genetic characteristics beneficial for combating adverse conditions (Sýkorová et al. 2007). AM imparts metal tolerance to plants by reducing bioavailability of metal ions, sequesteration in extraradicular hyphal structures, adsorbtion and formation of complexes of toxic ions with hyphal cell wall components (cellulose, chitin, etc.), and compartmentalizing in vesicles. Furthermore, extracellular hyphae secrete glomalin, a glycoprotein, which binds to metalloid and immobilize them in soil, hence restricting uptake by plants (Bano and Ashfaq 2013; Kapoor et al. 2013). Alleviative role AM for As have been reported in some plant species which acts as a barrier for As uptake, thus leading to increased photosynthesis, growth, and ultimate plant yield (Gonzalez-Chavez et al. 2002; Christophersen et al. 2009; Smith and Read 2008; Garg and Singla 2012; Spagnoletti and Lavado 2015). However, detailed elucidation of the mechanisms adopted by AM fungi on growth and productivity in plants under As V and As III stress are scanty.
Silicon (Si) is the second most prevalent element present on the earth’s crust (Abbas et al. 2015) in the form of silicate (Rizwan et al. 2015). Its concentration in soil solution normally ranges from 0.1 to 0.6 mM. It is absorbed by plant roots in the form of silicic acid ([Si(OH)4] (Garg and Bhandari 2016) and is deposited on cell wall of leaves and stem forming double layer silica-cuticle (Ma et al. 2006). Even though Si has not been considered as an essential element, it is reported to be beneficial for plant growth especially under biotic and abiotic stresses (Raza et al. 2016). Si is accumulated in plants up to 10% of their dry weights; however, its uptake varies among plant species (Epstein 1994; Hodson et al. 2005; Rizwan et al. 2015; Garg and Bhandari 2016). Plants are classified into three categories for Si accumulation, i.e., Si excluders, <0.5% Si of dry weight (Cycas, Pinus, Lycopersicon esculentum) (Epstein 1994; Ma et al. 2007); Si-intermediate, >1–4% Si of dry weight (few legume species such as Cajanus cajan, Glycine max; Cucurbitales and Urticales) (Guerriero et al. 2016), and Si accumulators >4% Si of dry weight (poaceae) (Currie and Perry 2007). Ma et al. (2006), identified Si transporter genes, Lsi1 and Lsi2 in roots and Lsi6 in shoots in rice plants (Mitani and Ma 2005; Ma et al. 2006, 2007; Tubaña and Heckman 2015). Some reports have indicated the role of Si in alleviating As stress in Oryza sativa (Guo et al. 2005; Sanglard et al. 2016; Raza et al. 2016), maize (de Freitas-Silva et al. 2016), and lettuce (Greger et al. 2015). Hammer et al. (2011a) observed Si accumulation in spores and hyphae of Rhizophagus irregularis collected from saline soil, suggesting contribution of AM in silicon uptake. AM have also been reported to increase Si content in plants such as G. max (Yost and Fox 1982), Zea mays (Clark and Zeto 2000), and chickpea (Garg and Bhandari 2016).
Pigeonpea (C. cajan (L.) Millsp.) is a grain legume belonging to the Cajaninae sub-tribe of the economically important leguminous tribe Phaseoleae. India is the world’s largest producer of pigeonpea with contribution of over 77% of total production, followed by Myanmar. It is a unique combination of optimal nutritional profile, relatively high tolerance to environmental stresses, high biomass and productivity, and moisture contributions to the soil (Odeny 2007). Pigeonpea can associate very well with AM fungi because of its deep strong and extensive rooting habit (Chikowa et al. 2004) and this symbiotic association might help in Si uptake as well. Despite the agronomic importance of pigeonpea, no information is available on ameliorative role of AM and Si nutrition in reducing As V and As III toxicity. Therefore, the present study would be the first attempt to unravel the relative as well as combined roles of +AM +Si in ameliorating to toxic effects of As species.
The study hypothesizes that the beneficial effects of Si can be achieved to a greater extent in moderate to low Si accumulating plants such as pigeonpea through inoculation with AM. Thus, the present study was undertaken with the following objectives: (i) to study the relative toxic responses As V and As III stress in terms of plant biomass, nutrient uptake, and ultimate yield in tolerant and sensitive pigeonpea genotypes; (ii) to compare the relative roles of Si and AM in combating the negative responses of As species in the two genotypes; and (iii) to investigate the role of AM in Si uptake and the collective response of both in imparting stress tolerance to plants.
Materials and methods
Experimental design
Pot experiments were conducted in Department of Botany, Panjab University, Chandigarh (30.5° N, 76.5° E; 305–366 m a.s.l.), from mid-June to December (minimum temperature 22–29 °C, maximum temperature 30–37 °C, relative humidity 42–92%. Ten pigeonpea (C. cajan (L.) Millsp. genotypes procured from Pulse Laboratory, Indian Agricultural Research Institute, New Delhi, were screened for their relative tolerance for arsenate (As V) and arsenite (III) stress on the basis of metal tolerance index (MTI) and two pigeonpea genotypes, Pusa 2002 (tolerant) and Pusa 991 (sensitive) were selected.
Isolate of R. irregularis (N.C. Schenck & G. S. Sm.) C. Walker & A. Schüβler, (previously called as Glomus intraradices) (NC 100 AZM1/12) was obtained from the Centre for Mycorrhizal Culture Collection, The Energy and Resource Institute, New Delhi, India, in a sterile sandy loam soil. Sinorhizobium fredii strain AR-4, a rhizobial inoculum, was obtained from the Department of Microbiology, Indian Agricultural Research Institute (IARI), New Delhi, India.
Experiments were conducted in earthenware where each pot was filled with 7 kg of autoclaved soil mixture (1:1 sand and loam) obtained from nearby agricultural fields [11.0 mg kg-1 P (Olsen and Sommers 1982), 0.17 mEq/100 g-1 available K, 0.19 meq/100 g-1 Na, 0.82 meq/100 g-1 Ca (Mehlich 1953), 0.42% total N (Nelson and Sommers 1972), pH 7.6 (soil/water 1:1), 0.68% organic carbon (Walkley 1947)]. Pots were arranged in a completely randomized block design with a factorial combination of 3 × 2 × 2 × 2 comprising either of three As V treatments [0, 25 mg/kg, 50 mg/kg] or As III treatments [0 mg/kg, 5 mg/kg, 10 mg/kg], two AM inoculations [with(+) and without(−) AM inoculation), two Si treatments [0 mg/kg (−) and 300 mg/kg K2SiO4 (+)] and two pigeonpea genotypes [Pusa 2002 and Pusa 991]. Seeds were surface sterilized with 10% hydrogen peroxide (v/v) solution for 10 min and then rinsed by soaking in sterile distilled water. Fifty grams of AM inoculums (crude mixture of soil, chopped root segments, and spores) was placed at the depth of 1.5 cm prior to sowing. Seeds were pretreated with rhizobial inoculum and were kept for drying at room temperature. After 2 weeks (Days After Emergence—DAE), seedlings were subjected to arsenate (As V-sodium arsenate Na2HAsO4·7H2O) or arsenite (As III-sodium arsenite NaAsO2) treatments. Deionized water was used to prepare all solutions. Plants were harvested for physiological and biochemical analysis at 80 days after sowing (DAS) and analyzed on six replicate basis. Yield parameters were recorded from the date of first flower initiation till seed setting.
Physiological and biochemical parameters
Plant biomass and productivity
Shoot dry weight (SDW) and root dry weight (RDW) were measured by oven drying at 70 °C for 72 h until they reached constant weight. Root/shoot ratio was calculated. Plant productivity was analyzed by recording various yield attributes such as seed number, seed weight, and above ground biomass per plant. Harvest index (HI) was calculated according to Leport et al. (2006).
Mycorrhizal colonization and mycorrhizal responsiveness
Mycorrhizal Colonization (MC) was estimated by following the grid line intersect method (McGonigle et al. 1990). The roots were cleared with 10% potassium hydroxide and stained with trypan blue (Phillips and Hayman 1970). An index of mycorrhizal responsiveness (MR) was determined by expressing the dry weights of the plants concerned as a percentage of the dry weight of the non-mycorrhizal plants (Hetrick et al. 1992).
Leaf relative water content and chlorophyll pigments
Leaf relative water content (LRWC) was determined according to Weatherley 1950). Chlorophyll (Chl) content and Chl a/b ratio were determined in the first fully expanded leaves by extracting with dimethyl sulphoxide according to the non-maceration method of Hiscox and Israelstam (1979) using the equation of Arnon (1949). Final chl. concentration was expressed as mg g−1F.W.
Determination of mineral elements and Si content
Nitrogen (N) was determined using the colorimetric method of Lindner (1944). Phosphorus (P) was extracted by nitric-perchloric acid digestion and measured using the vanado-molybophosphoric colorimetric method (Jackson 1973). Mg2+ was determined by atomic absorption spectrophotometer (AAS). Ca2+ and K+ contents were measured using a flame photometer (Digital flame photometer MODEL 381E, ESICO, India) and expressed as mg g−1 dry weight (DW) of plant tissue (Allen et al. 1984).
For Si estimation, dried leaves and root tissues (0.1 g) were digested with 2 ml of 50% H2O2 in 100 ml polyethylene tubes (previously rinsed with 0.1 M NaOH and demineralized water). Si in the sample digest was determined by the standard amino molybdate blue colorimetric technique as described by Elliott and Snyder (1991), using UV spectrophotometer (Double Beam UV-190–LUV 100A-Labnics) at 650 nm.
Determination of arsenic and its translocation Factor
Dried plant samples (roots and leaves) were digested with HNO3-H2O2 (1:1 v/v) at 120 °C for 3 h to determine total As content (Tang and Miller 1991). Digested samples were analyzed to determine total As content by wavelength dispersive X-ray fluorescence (WD-XRF-TIGER Bruker Model S8, Sophisticated Analytical Instrumentation Facility, at Panjab University, Chandigarh, India).
Translocation Factor (TF) was calculated as per following equation:
Statistical analyses
Data presented are mean values based on six replicates ± standard error (S.E.) per treatment. A multiple regression model was designed to predict various dependent variables from the independent factors As V, As III, Si, AM, and G. Correlation analysis [Pearson’s correlation (r)] was carried out to investigate the relationship between two dependent variables. Multiple comparisons within the one-way ANOVAs were done with Duncan to evaluate differences among the treatments. All results were subjected to analysis of variance (ANOVA) using the SPSS 16.0 for Windows (SPSS, Inc. Chicago, IL, USA) with genotype (G), As V and As III concentrations, silicon amendment (Si), and mycorrhizal inoculation (AM) as main factors.
Results
Mycorrhizal colonization and responsiveness
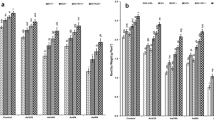
No mycorrhizal colonization was observed in roots of uninoculated plants of both pigeonpea genotypes. Plants inoculated with R. irregularis established the highest degree of colonization with the roots of unstressed plants, which decreased with increase in both As V and As III stresses, with higher reduction in As III treated plants [MC β (As V) = −0.783, β (As III) = −1.040] (Fig. 1, ESM Fig. 1). Although no significant difference in terms of colonization ability was observed between the two genotypes under unstressed conditions, genotypic variability became prominent with increasing stress levels (34% decline over control in Pusa 2002 and 43% in Pusa 991 at 50 mg/kg As V and 46% in Pusa 2002 and 59% in Pusa 991 at 10 mg/kg As III). Pusa 2002 had better ability to colonize with R. irregularis than Pusa 991 under both stressed and unstressed conditions [MC β (G) = 290]. Significant colonization was observed in the two genotypes even at higher As V and As III concentration, indicating metalloid tolerance of R. irregularis. Ability of plants to utilize the benefits provided by mycorrhiza, calculated as mycorrhizal responsiveness (MR), increased with an increase in soil metalloid concentration in both the genotypes, with Pusa 2002 depicting higher responsiveness than Pusa 991 (Fig. 1, ESM Fig. 1). Exogenous application of Si in AM inoculated unstressed plants did not add much to the symbiotic efficiency and the data was almost at par with mycorrhizal plants.
Effect of silicon (Si) and arbuscular mycorrhiza (AM) on a mycorrhizal colonization and b mycorrhizal responsiveness (%) under arsenate (As V—50 mg/kg) and arsenite (As III—10 mg/kg) concentrations in Cajanus cajan genotypes (tolerant—Pusa 2002 and sensitive—Pusa 991). Values are mean of 6 replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by the Duncan multiple range test at p ≤ 0.05. C = Si and AM absent, +Si = Si present, +AM = arbuscular mycorrhiza present, +Si +AM = Si with AM present
Si fertilization increased Si content and decreased As level
With increasing (As V and As III) concentrations in soil, endogenous level of Si decreased significantly in roots and leaves of both the genotypes, decline being more pronounced in Pusa 991 than Pusa 2002 in As III treated plants when compared to As V as revealed through standardized beta coefficient [Si Roots β (As V) = −0.146; β (As III) = −0.589; β (G) = 0.582; Si Leaves β (As V) = −0.135, β (As III) = −0.576; β (G) = 0.456] (Fig. 2, ESM Fig. 2). As content was below detectable limit (BDL) in unstressed plants. Regardless of metalloid treatment, As content was higher in roots than in leaves in both the genotypes. Translocation rate (TR) was lower from leaves to seed when compared to roots and leaves resulting in minimal amount of metal in seeds. However, translocation rate was significantly higher to the aerial parts of Pusa 991 when compared with Pusa 2002 (Table 2, ESM Table 3). Exogenous Si application increased endogenous Si content in both the organs with Pusa 2002 depicting significantly better capacity for uptake when compared with Pusa 991 [Si Roots β (Si) = 0.270; β (G) = 0.582; Si leaves β (Si) = 0.272, β (G) = 0.456] (Fig. 2, ESM Fig. 2). However, Si supplementation decreased As content by 24 and 21% in leaves and roots respectively under As V treatments (50 mg/kg) and 41 and 24% in leaves and roots under As III (10 mg/kg) stress in Pusa 2002. AM was more effective in reducing metalloid uptake [As roots β (Si) = −0.143, β (AM) = −0.255] and further translocation to the leaves [As leaves β (Si) = −0.154, β (AM) = −0.258] as well as seeds when compared to Si. Moreover, Si uptake was enhanced by AM to significant levels in the roots as well as leaves of both pigeonpea genotypes, under As V and As III stress. The combined application of Si and AM further reduced As content and decreased TRs from roots to leaves then to seeds with the result no metal content could be detected in the edible part. Higher percent colonization in Pusa 2002 could be positively correlated with higher Si content in both genotypes with stronger correlation in Pusa 2002 [rp(leaves Si) = 0.995, p = 0.01] than Pusa 991 [rp(leaves Si) = 0.980, p = 0.01], thus resulting into significantly lower metalloid contents.
Effect of silicon (Si) and arbuscular mycorrhiza (AM) on a arsenic content in Pusa 2002 and b arsenic content in Pusa 991 (μg g−1 DW). c Silicon content in leaves. d Silicon content in roots (mg g−1 DW) under arsenate (As V—50 mg/kg) and arsenite (As III—10 mg/kg) concentrations in Cajanus cajan genotypes (tolerant—Pusa 2002 and sensitive—Pusa 991). Values are mean of 6 replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by the Duncan multiple range test at p ≤ 0.05. C = Si and AM absent, +Si = Si present, +AM = arbuscular mycorrhiza present, +Si +AM = Si with AM present
Negative effects of As on growth and yield
Both species of As (As V and As III) significantly reduced root dry weight (RDW) and shoot dry weight (SDW) in both the genotypes (Pusa 2002 and Pusa 991) (Fig. 3, ESM Fig. 3). Regardless of the As treatments, roots growth was more negatively affected than the shoots, with As III exhibiting more detrimental effects in Pusa 991 than Pusa 2002 [RDW β (As V) = −0.167; β (As III) = −0.645 β (G) = 0.449; SDW β (As V) = −0.119, β (As III) = −0.608; β (G) = 0.478]. However, when compared to control, a decline in RDW was variable in the two genotypes with 24 and 46.2% in Pusa 2002 while 36 and 65% in Pusa 991 at 25 and 50 mg/kg As V, respectively. Significantly higher reduction of 34 and 51% in RDW was recorded at 5 mg/kg As III and as much as 64 and 79% at 10 mg/kg As III, in Pusa 2002 and Pusa 991, respectively. Interestingly, exogenous Si application was more effective in improving shoot growth [SDW β (Si) = 0.196; β (AM) = 0.330] while AM was more positive for root biomass [RDW β (Si) = 0.145; β (AM) = 0.337] and improved R/S (Table 1, ESM Table 2) ratio more than Si. However, when the plants were subjected to combined application of both Si and AM, significant positive effects were observed in terms of increased root and shoot biomass.
Effect of silicon (Si) and arbuscular mycorrhiza (AM) on a shoot dry weights and b root dry weights (g plant−1) under arsenate (As V—50 mg/kg) and arsenite (As III—10 mg/kg) concentrations in Cajanus cajan genotypes (tolerant—Pusa 2002 and sensitive—Pusa 991). Values are mean of 6 replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by the Duncan multiple range test at p ≤ 0.05. C = Si and AM absent, +Si = Si present; +AM = arbuscular mycorrhiza present, +Si +AM = Si with AM present
Likewise, crop yield was also reduced by both As V and As III stresses, which was proportionate to the decline in root and shoot dry weights. Comparison of standardized coefficients (β) in regression analysis indicated pronounced negative impacts of As III over As V on seed yield [seed yield β (As V) = −0.176, β (As III) = −0.632, β (G) = 0.472] (Table 2, ESM Table 3). Among the two As stresses, early flowering was observed in As III-treated plants than As V in both the genotypes. Pod setting and seed development were reduced to a greater extent in plants treated with As III with a higher decline observed in Pusa 991. Higher content of metalloid stresses negatively affected the seed development and lowered the seed dry weight, resulting into progressive decline in harvest index [HI β (As V) = −0.151, β (As III) = −0.589] (Table 2, ESM Table 3). However, Si supplementation to the soils improved plant growth and yield significantly with more positive effects under As III stress than As V. Moreover, Pusa 2002 was more responsive to Si than Pusa 991. +Si +AM inoculations could completely ameliorate the negative impacts of both concentrations of As V and lower concentration of As III (5 mg/kg), with significant improvement under higher dose of As III in Pusa 2002. Thus, increase in growth and yield could be directly correlated with reduced As content in plant tissues in +Si +AM plants.
Toxic effects of As on photosynthetic pigments, nutrient, and water status
Relative leaf water content (RLWC) indicates the hydration state of the leaf, which may impact photosynthetic chlorophyll pigments (Table 1, ESM Table 2). The decline in plant biomass had a direct bearing on the water status of the leaves as well as the chlorophyll pigments under As V and As III stress in a concentration and genotype dependent manner. At higher concentrations of both As V and As III, RLWC decreased to 35 and 57% in Pusa 2002 and 51 and 71% in Pusa 991; TChls degenerated by 42.61 and 59.9% in Pusa 2002 and 56.37 and 73.22% in Pusa 991, which disturbed the Chl a/b ratio. Si supplementation or AM inoculation improved the leaf water status in both genotypes with lesser positive effects of Si amendment when compared with AM. Comparison of standardized beta coefficients for RLWC indicated that AM symbiosis could maintain higher water status when compared with Si nutrition in both the genotypes, with more positive effects observed in Pusa 2002 [RLWC β (Si) = 0.187; β (AM) = 0.322; β (G) = 0.438]. Higher benefits of AM and Si application was observed in plants treated with As III compared to As V in terms of RLWC, TChl, and Chl a/b ratio in Pusa 2002. Higher improvement in leaf water status observed in Pusa 2002 was commensurated with its higher colonizing ability Pusa 2002 [rp(RLWC) = 0.989, p = 0.01] and Pusa 991 [rp (RLWC) = 0.987, p = 0.01]. Combined application of Si and AM (+Si +AM) were highly beneficial in improving leaf water status and arresting the degeneration of the chlorophyll pigments. Interestingly, the percent benefit of +Si and +AM was significantly higher under As III stress when compared with As V. Improvement in leaf water status and TChl content could be related to restricted metalloid concentration in plant tissues under +Si +AM fertilization.
Mineral elements, such as N, P, K, Mg, and Ca, play an important role in maintaining plant growth and productivity. As V and As III concentrations in soil arrested nutrient uptake and status in both roots and their translocation to shoots, overall higher negativity was observed in As III treated plants (Fig. 4, Table 1, ESM Fig. 4, ESM Fig. 5, ESM Fig. 6, ESM Table 1, ESM Table 4). With the exception of N, which was more negatively affected under As V treatments as indicated by standardized beta coefficient in [N roots β (As V) = −0.435; β (As III) = −0.432; leaves β (As V) = −0.473; β (As III) = −0.432). As III had significantly more negative effects on other nutrients as compared to As V [P root β (As V) = −0.158; β (As III) = −0.594; β (G) = 0.477; leaves β (As V) = −0.162; β (As III) = −0.572; β (G) = 0.471; K root β (As V) = −0.128; β (As III) = −0.565; β (G) = 0.567; leaves β (As V) = −0.131; β (As III) = −0.586; β (G) = 0.465; Ca root β (As V) = −0.180; β (As III) = −0.678; β (G) = 0.402; leaves β (As V) = −0.137; β (As III) = −0.632; β (G) = 0.419; Mg root β (As V) = −0.176; β (As III) = −0.558; β (G) = 0.668; leaves β (As V) = −0.156; β (As III) = −0.603; β (G) = 0.554], with a decline more pronounced in Pusa 991. Si application and mycorrhizal inoculation individually enhanced the nutrient status of both roots and shoots with higher improvement under AM inoculation when compared with Si supplementation as revealed through as revealed through regression analysis [N roots β (Si) = 0.136; β (AM) = 0.258; leaves β (Si) = 0.172; β (AM) = 0.316; P roots β (Si) = 0.165; β (AM) = 0.335; leaves β (Si) = 0.174; β (AM) = 0.339; K roots, β (Si) = 0.149; β (AM) = 0.280; leaves β (Si) = 0.158; β (AM) = 0.290; Ca roots β (Si) = 0.139; β (AM) = 0.268; leaves β (Si) = 0.137; β (AM) = 0.250; Mg 2+ roots β (Si) = 0.131; β (AM) = 0.237; leaves β (Si) = 0.137; β (AM) = 0.253]. Plants were benefited to the most when Si treatment was given along with AM, leading to restoration of nutrient imbalance under both As stresses. Pusa 2002 displayed the potential to accumulate more nutrients, which could be correlated with an ability of this genotype to establish a more efficient mycorrhizal symbiosis as determined by Pearson’s correlation value (r) in Pusa 2002 [root rP(P) = 0.996, p = 0.01; rP(N) = 0.989, p = 0.01; rP(K) = 0.992, p = 0.01; rP(Ca) = 0.996, p = 0.01; rP(Mg) = 0.998, p = 0.01 than Pusa991 [root rP(P) = 0.994, p = 0.01; rP(N) = 0.971, p = 0.01; rP(K) = 0.989, p = 0.01; rP(Ca) = 0.988, p = 0.01; rP(Mg) = 0.991, p = 0.01]. Elevated levels of Mg contents enhanced Chl biosynthesis and a strong correlation was observed between Mg and TChl content in Pusa 2002 [rp(SMg) = 0.999, p = 0.01] and improved Chl a/b ratio, thereby, leading to increase in ultimate yield (HI).
Effect of silicon (Si) and arbuscular mycorrhiza (AM) on a phosphorous, b potassium, c nitrogen, and d magnesium contents (mg g−1 DW) in roots under arsenate (As V—50 mg/kg) and arsenite (As III—10 mg/kg) concentrations in Cajanus cajan genotypes (tolerant—Pusa 2002 and sensitive—Pusa 991). Values are mean of 6 replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by the Duncan multiple range test at p ≤ 0.05. C = Si and AM absent, +Si = Si present, +AM = arbuscular mycorrhiza present, +Si +AM = Si with AM present
Discussion
The present study compared the negative effects of As V and As III stress on plant biomass and yield in two differentially metal-tolerant pigeonpea genotypes. A reduction in root, shoot dry weights, root to shoot ratio (RSR), and nutrient uptake was observed under both As species, with more drastic effects of As III over As V. The higher negative effects of As III could be directly correlated to higher total As accumulation under this treatment in both roots and leaves of the Pusa 991 when compared with Pusa 2002. Reduced root to shoot ratio in stressed plants could be attributed to reduced plant growth due to alteration of morphological and physiological characteristics of roots (Khudsar et al. 2000), decreasing their capacity to explore the rhizosphere, consequently resulting into reduced water and mineral uptake capacity. Variable responses of the two genotypes observed in the present study could be related to their differential ability for uptake, translocation, and accumulation of As in the plant organs as well as their inherited physiological and biochemical adaptation strategies (Chaturvedi 2005), often as a result of single or multiple gene difference (Baker 1987; Macnair et al. 1992; Mahdieh et al. 2013). In the present study, even though As V concentration applied to the plants was five times higher than that of As III, yet phytotoxic effects of As III were significantly higher than As V. This could be due to the fact that As III is more water soluble and is readily taken up by the plant roots and translocated to shoots more efficiently than As V due to different transport mechanisms adopted by both As V and As III in plants (Chaturvedi 2005; Brackhage et al. 2014). Metal(loid)s generally cause physiological drought either by restricting water transport activities (Maurel and Chrispeels 2001) or by blocking its transport in the plant tissues (Barcelo and Poschenrieder 1990) which might have led to reduced leaf water status in our study. Reduction in photosynthetic pigments may possibly be due to inhibition of enzymes involves in Chl biosynthesis, alteration of thylakoid membrane, reduction of aminolevulinic acid pool, and reduction in chloroplast density resulting into a decrease in photosynthetic efficiency and plant growth (de Andrade et al. 2015).
In the present study, nutrients uptake was significantly reduced under both As V and As III stress, with As III causing a higher reduction in P, K, Mg, and Ca contents while As V was more toxic for N uptake. A decline in P and K contents in As III-treated plants could be due to downregulation of genes involved in absorption and utilization of these nutrients while in case of As V, N-related genes are downregulated (Wang et al. 2010). Since Pi is replaced by As, plants are unable to carry out energy transfer process, thus disrupting various physiological activities, ultimately resulting into reduced growth and yield (Farnese et al. 2014). A reduction in the Ca concentration could be correlated with reduced P content as roots absorb Ca together with negatively charged phosphate (Caldwell and Haug 1982; Shaibur and Kawai 2010). Furthermore, a higher decrease in N content might be due to uncoupling of oxidative phosphorylation due to disruption of ETC by As V and inhibition enzymes involved in photosynthesis and respiration by As III. In the current study, onset of flowering was early in the plants treated with As III; this trait of early flowering could be an adaptive mechanism to escape stress and minimize the time of exposure to the stress (Araújo et al. 2015; Duc et al. 2015; Farooq et al. 2017). Formation of pods, development of seeds, and seed weight were significantly reduced under both As stresses, with higher detrimental effects of As III, ultimately resulting into reduced HI. Pod abortion is induced due to restrained carbohydrate flux from leaves to pods as well as a decrease in water potential and an increase in ABA content in flowers and pods at early reproductive development stages, thus compromising seed yield under abiotic stresses (Liu et al. 2004; Araújo et al. 2015). Reduced harvest index as observed in the present study was the direct result of reduced absorption and utilization of nutrients, leading to reduced photosynthetic efficiency and stunted growth under both As species.
Both the genotypes of pigeonpea had an ability to develop efficient mycorrhizal colonization under unstressed conditions which declined under both As V and As III stress, with higher colonization observed in Pusa 2002. A decline in mycorrhizal colonization (MC) could be due to toxic effects of As on pre-symbiotic stage of mycorrhizal colonization resulting into reduced percent spore germination and hyphal length, thereby lowering colonization (Hajiboland et al. 2010; Spagnoletti and Lavado 2015). However, in our study, significant and differential colonization was observed even under higher As concentrations with Pusa 2002 displaying much higher responsiveness when compared with Pusa 991. Variability in percent colonization that occurs among plant genotypes to same fungus has been reported in plants such as peanut (Kesava Rao et al. 1990), cowpea (Mercy et al. 1990), tomato (Gao et al. 2001), pigeonpea (Garg and Kaur 2013; Garg and Pandey 2015), Helianthus annuus (Turrini et al. 2016), soya bean (Salloum et al. 2016), chickpea (Garg and Bhandari 2016), and Phaseolus mungo (Sharma et al. 2016) which is believed to be due to strong genetic influence affecting differences in mycorrhizal colonization and host plant responsiveness (Linderman and Davis 2004). Pusa 2002 had the ability to accumulate higher biomass which might have increased C allocation to the roots and thus made more carbon available to the fungus resulting in higher root colonization (Garg and Pandey 2015) which in turn increased its efficiency for N and P transport (Hammer et al. 2011b; Fellbaum et al. 2012). Although Si content was low even in the Si supplemented pigeonpea genotypes, its uptake further declined when the plants were subjected to As stress. Low Si content in Si supplemented unstressed plants could be due to the reason that legumes are relatively low Si accumulators (Broadley et al. 2011). Significantly higher Si content was observed in +Si +AM-treated plants indicating a positive correlation between mycorrhization and Si uptake. Pusa 2002 which displayed a higher percent AM colonization also recorded significantly higher Si content in both roots and shoots. An increase in Si content in mycorrhizal plants could be due to the ability of AM for upregulation of aquaporin which mediates Si uptake. Ma et al. 2001 reported that Si was mainly absorbed by secondary roots and AM is reported to modify root system architecture by enhancing lateral root formation (Gutjahr and Paszkowski 2013). Thus, in our study, AM mediated improved root rhizosphere and soil exploration might have resulted in enhanced Si uptake.
In the present study, Si was relatively more effective in reducing As content in As III-treated plants when compared to As V-treated plants while AM reduced As content more efficiently under As V stress. A higher reduction in As content in As III-treated plants might be due to the reason that both Si and As III share the same influx transporter (low silicon rice 1, Lsi1) as reported in rice (Ma et al. 2008; Guo et al. 2009). A reduction in metalloid uptake could also be due to the fact that Si reduces the apoplasmic transport of metalloid by decreasing its concentration in the apoplasm (Adrees et al. 2015). Moreover, in the present study, a decline in As content in +Si +As V-treated plants might be due to Si-mediated increase in soil pH (Owino-Gerroh and Gascho 2005) which limits the solubility of As by its precipitation as Ca or Fe arsenates and thus retain As V in soil (Xie and Naidu 2006; Zhang and Selim 2008; Moreno-Jiménez et al. 2012). More positive impact of AM in reducing As content as observed could be due to the fact that AM colonization suppresses high affinity arsenate/phosphate transporters in the epidermis and root hairs (Meharg and Macnair 1992; Gonzalez-Chavez et al. 2002), and P uptake is carried out via mycorrhizal pathway which selectively prefer P over As, resulting into reduced As accumulation in the plants (Yun-sheng et al. 2007). The presence of common transporters for Si-As III uptake and AM mediated P- As V uptake could be the reason for differential but positive effects of both Si and AM on As content observed in the present study in roots, shoots, and seeds. This reduction in As content might also be due to the fact that Si and AM stimulate production of root exudates which can chelate metals and reduce their uptake by roots (Kidd et al. 2001; Gaur and Adholeya 2004; Adrees et al. 2015). Moreover, Si acts as a physical barrier for metal translocation by the deposition in the endodermis which reduces the cell wall porosity in root tissues, (Shi et al. 2005; da Cunha and do Nascimento 2009; Keller et al. 2015) while AM adsorb metalloid on its surface as well as sequester in vesicles, thus reducing translocation to shoots (Kapoor et al. 2013; Ambrosini et al. 2015).
In our study, beneficial effects of Si were more pronounced in shoots while AM inoculation significantly improved root biomass more vigorously. Zhang et al. (2015) reported that Si increased the cell wall extensibility, structurally alter xylem diameter, mesophyll and epidermis thickness, and arrangement of chollenchyma resulting into decreased As translocation to the shoots (Sahebi et al. 2015). AM inoculated plants had highly developed and dense root system which allowed the plants roots to penetrated deeper in the soil and thus improve water uptake, nutrient status, higher flower initiation, and superior seed setting, leading to higher yield in these plants. Functional complementarity was observed between AM and Si in terms of significant improvement in both root and shoot biomass, nutrient uptake, and harvest index through their individual affirmative roles in reducing metal uptake and translocation. Possible mechanism for improved nutrient and water uptake in pigeonpea plants supplemented with Si could be due to Si mediated activation of H+- ATPase in plasma membrane, which promotes uptake and utilization of water and minerals by modulating physiological activities (Gul Bakhat 2012). Moreover, AM can improve water and nutrient uptake from the soil directly by activation of specific transporters for P (PHT1 family) (Harrison et al. 2002; Maeda et al. 2006), N (AMT1) (Lopez-Pedrosa et al. 2006), and K+ (ZmAKT2) (Ouziad et al. 2006) and aquaporins (Aroca et al. 2007; Giovannetti et al. 2012) making them available to the plants (Krajinski et al. 2000; Porcel et al. 2006; Hu et al. 2009). One of the reason for improved water status and nutrient uptake in mycorrhizal plants could also be due to increased absorbing surface by soil-growing hyphae combined with the fungal ability to take up water and nutrients in much higher proportions (Augé 2001; Ruiz-Lozano 2003; Lehto and Zwiazek 2011; Bárzana et al. 2012). In our results, both Si and AM significantly reverted As-mediated impairment of photosynthetic pigments which could be due to alteration in morphological, anatomical, and ultrastructural photosynthetic apparatus (Adrees et al. 2015). Since Si gets deposited on the leaf epidermis, it might have directly reduce metal translocation to the leaves, thereby improving the leaf area for more efficient photosynthesis. On the other hand, AM might have improved photosynthesis by increasing its ability to use light energy and by improving the electron transport, thereby improving the efficiency of PSII photochemistry (de Andrade et al. 2015). Differential ability of the two genotypes to tolerate As toxicity could be related to their relative colonization ability and the resultant differential Si uptake. Enhanced metal tolerance in +Si and/or +AM pigeonpea plants might be due to reduced As content in plants explained by dilution effects due to growth enhancement by Si and AM fungal colonization (Al-Karaki 2006; Garg and Pandey 2015; Rizwan et al. 2015).
Conclusion
The results affirmed negative correlations between As stress and growth potential of both the genotypes. Although, individual treatments of Si and AM reduced As uptake and translocation to a certain extent, their combined application proved to be highly beneficial in arresting the toxic effects of both species of As (As V and As III) to the degree that no As could be detected in the seeds of metal-tolerant genotype. The study highlighted the importance of selecting a genotype with higher ability for AM colonization in order to achieve maximum Si effectiveness in pigeonpea plants.
References
Abbas MHH, Meharg AA (2008) Arsenate, arsenite and dimethyl arsinic acid (DMA) uptake and tolerance in maize (Zea mays L.) Plant Soil 304:277–289
Abbas T, Balal RM, Shahid MA, Pervez MA, Ayyub CM, Aqueel MA, Javaid MM (2015) Silicon-induced alleviation of NaCl toxicity in okra (Abelmoschus esculentus) is associated with enhanced photosynthesis, osmoprotectants and antioxidant metabolism. Acta Physiol Planta 37(2):1–15
Abedin MJ, Meharg AA (2002) Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.) Plant Soil 243:57–66
Adrees M, Ali S, Rizwan M, Zia-ur-Rehman M, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Al-Karaki GN (2006) Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hortic 109:1–7
Allen SF, Grimshaw HF, Rowl AB (1984) Chemical analysis. In: Moor PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 185–344
Ambrosini VG, Voges JG, Canton L, da Rosa Couto R, Ferreira PAA, Comin JJ, de Melo GWB, Brunetto G, Soares CRFS (2015) Effect of arbuscular mycorrhizal fungi on young vines in copper-contaminated soil. Braz J Microbiol 46(4):1045–1052
Araújo SS, Beebe S, Crespi M, Delbreil B, González EM, Gruber V, Lejeune-Henaut I, Link W, Monteros MJ, Prats E, Rao I, Vadez V, Vaz Patto MC (2015) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci 34(1–3):237–280
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenyloxidase in Beta vulgaris. Plant Physiol 24:1–15
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173:808–816
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Bano SA, Ashfaq D (2013) Role of mycorrhiza to reduce heavy metal stress. Nat Sci 5:16–20
Barcelo J, Poschenrieder C (1990) Plant water relations as affected by heavy metal stress: a review. Water Air Soil Pollut 47:287–319
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM (2012) Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Ann Bot 109(5):1009–1017
Brackhage C, Huang J-H, Schaller J, Elzinga EJ, Dudel EG (2014) Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.) Sci Rep 4:4944
Broadley M, Brown P, Cakmak I, Ma J, Rengel Z, Zhao F (2011) Beneficial elements. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants: third edition. Elsevier, London, pp 249–269
Caldwell CR, Haug A (1982) Divalent cation inhibition of barley root plasma membrane-bound Ca2+-ATPase activity and its reversal by monovalent cations. Physiol Planta 54:112–118
Chaturvedi I (2005) Effects of arsenic concentrations and forms on growth and arsenic uptake and accumulation by Indian mustard (Brassica juncea L.) genotypes. J Cent Eur Agri 7:31–40
Chikowa R, Mapfumo P, Nyamugafata P, Giller KE (2004) Woody legume fallow productivity, biological N2-fixation and residual benefits to two successive maize crops in Zimbabwe. Plant Soil 262:303–315
Christophersen HM, Smith FA, Smith SE (2009) Arbuscular mycorrhizal colonisation reduces arsenate uptake in barley via down-regulation of transporters in the direct epidermal phosphate uptake pathway. New Phytol 184:962–974
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23:867–902
Currie HA, Perry CC (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 100:1383–1389
de Andrade SAL, Domingues AP Jr, Mazzafera P (2015) Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 134:141–149
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Poll 197:323–330
de Freitas-Silva L, de Araújo TO, da Silva LC, de Oliveira JA, de Araujo JM (2016) Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol Environ Saf 124:1–9
Danh LT, Truong P, Mammucari R, Foster N (2014) A critical review of the arsenic uptake mechanisms and phytoremediation potential of Pteris vittata. Int J Phytoremediat 16:429–453
Duc G, Agrama H, Bao S, Berger J, Bourion V, De Ron AM, Gowda CLL, Mikic A, Millot D, Singh KB, Tullu A, Vandenberg A, Vaz Patto MC, Warkentin TD, Zong X (2015) Breeding annual grain legumes for sustainable agriculture: new methods to approach complex traits and target new cultivar ideotypes. Crit Rev Plant Sci 34:381–411
Dwivedi S, Tripathi RD, Srivastava S, Singh R, Kumar A, Tripathi P, Dave R, Rai UN, Chakrabarty D, Trivedi PK, Tuli R, Adhikari B, Bag MK (2010) Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown on arsenic-contaminated soils of West Bengal. Protoplasma 245(1–4):113–124
Elliott CL, Snyder GH (1991) Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J Agric Food Chem 39:1118–1119
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A 91:11–17
Farnese FS, Oliveira JA, Farnese MS, Gusman GS, Silveira NM, Siman LI (2014) Uptake arsenic by plants: effects on mineral nutrition, growth and antioxidant capacity. IDESIA 32:99–106
Farooq MA, Islama F, Ali B, Najeeb U, Mao B, Gill RA, Yan G, KHM S, Zhou W (2016) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot 32:42–52
Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS and Siddique, KHM (2017) Drought stress in grain legumes during reproduction and grain filling. J Agron Crop Sci. 203:81–102
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bucking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 109(7):2666–2671
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182
Flora SJS (2015) Arsenic: chemistry, occurrence, and exposure. In: Flora SJS (ed) Handbook of arsenic toxicology. Academic Press, Oxford S.J.S, pp 1–49
Fu SF, Chen PY, Nguyen QTT, Huang LY, Zeng GR, Huang TL, Zeng G-R, Huang T-L, Lin C-Y, Huang H-J (2014) Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol 14:94
Gao L-L, Delp G, Smith SE (2001) Colonization patterns in a mycorrhiza-defective mutant tomato vary with different arbuscular-mycorrhizal fungi. New Phytol 151:477–491
Garg N, Bhandari P (2016) Silicon nutrition and mycorrhizal inoculations improve growth, nutrient status, K+/Na+ ratio and yield of Cicer arietinum L. genotypes under salinity stress. Plant Growth Regul 78:371–387
Garg N, Kaur H (2013) Impact of cadmium-zinc interactions on metal uptake, translocation and yield in pigeonpea genotypes colonized by arbuscular mycorrhizal fungi. J Plant Nutr 36:67–90
Garg N, Pandey R (2015) Effectiveness of native and exotic arbuscular mycorrhizal fungi on nutrient uptake and ion homeostasis in salt-stressed Cajanus cajan L. (Millsp.) genotypes. Mycorrhiza 25(3):165–180
Garg N, Singla P (2012) The role of Glomus mosseae on key physiological and biochemical parameters of pea plants grown in arsenic contaminated soil. Sci Hortic 143:92–101
Gaur A, Adholeya A (2004) Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr Sci 86:528–534
Giovannetti M, Balestrini R, Volpe V, Guether M, Straub D, Costa A, Ludewig U, Bonfante P (2012) Two putative-aquaporin genes are differentially expressed during arbuscular mycorrhizal symbiosis in Lotus japonicus. BMC Plant Biol 12:186
Gonzalez-Chavez MC, Harris PJ, Dodd J, Meharg AA (2002) Arbuscular mycorrhizal fungi enhanced arsenate resistance on Holcus lanatus. New Phytol 155:163–171
Greger M, Bergqvist C, Sandhi A, Landberg T (2015) Influence of silicon on arsenic uptake and toxicity in lettuce. J App Bot Food Qual 88:234–240
Guerriero G, Hausman J-F, Legay S (2016) Silicon and the plant extracellular matrix. Front Plant Sci 7:463
Gul Bakhat, HFS (2012) Role of silicon in plasmalemma H –ATPase hydrolytic and pumping activity in maize (Zea mays L.). Phd. Thesis
Guo HW, Zhou Wong YS, Tam NF (2005) Isolation of PAH-degrading bacteria from mangrove sediments and their biodegradation potential. Mar Pollut Bull:1054–1061
Guo W, Zhang J, Teng M, Wang LH (2009) Arsenic uptake is suppressed in a rice mutant defective in silicon uptake. J Plant Nutr Soil Sci 172:867–874
Gutjahr C, Paszkowski U (2013) Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front Plant Sci 4:20
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Hammer EC, Nasr H, Pallon J, Olsson PA, Wallander H (2011a) Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 21(2):117–129
Hammer EC, Pallon J, Wallander H, Olsson PA (2011b) Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol 76:236–244
Harrison MJ, Dewbre GR, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 14:2413–2429
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hettick BE, Cañas-Carrell JE, French AD, Klein DM (2015) Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J Agric Food Chem 63(32):7097–7107
Hiscox TD, Israelstam GF (1979) A method for extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hodson MJ, White PJ, Mead A (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot 96:1027–1046
Hu J, Lin X, Wang J, Dai J, Cui X, Chen R, Zhang J (2009) Arbuscular mycorrhizal fungus enhances crop yield and P-uptake of maize (Zea maysL.): a field case study on a sandy loam soil as affected by long-term P-deficiency fertilization. Soil Biol Biochem 41:2460–2465
Jackson ML (1973) Soil chemical analysis. Published by Printice Hall, New Delhi, p 485
Kapoor R, Evelin H, Mathur P, Giri B (2013) Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In: Tuteja N, Gill SS (eds) Plant acclimation to environmental stress. Springer Science+Business Media, New York, pp 359–401
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidumL.) grown in hydroponics and exposed to 0 to 30lM cu. Planta 241:847–860
Kesava Rao PS, Tilak KVB, Arunachalam V (1990) Genetic variation for VA mycorrhiza-dependent phosphate mobilization in groundnut (Arachis hypogaea L.) Plant Soil 122:137–142
Khudsar T, Mahmooduzzafar Soh WY, Iqbal M (2000) Morphological and anatomical variations of Cajanus cajan (Linn.) Huth raised in cadmium-rich soil. J Plant Biol 43(3):149–157
Kidd P, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.) J Exp Bot 52:1339–1352
Krajinski F, Biela A, Schubert D, Gianinazzi-Pearson V, Kaldenhoff R, Franken P (2000) Arbuscular mycorrhiza development regulates the mRNA abundance of Mtaqp1 encoding a mercury-insensitive aquaporin of Medicago truncatula. Planta 211(1):85–90
Krishnamoorthy R, Kim C-G, Subramanian P, Kim K-Y, Selvakumar G, Sa T-M (2015) Arbuscular mycorrhizal fungi community structure, abundance and species richness changes in soil by different levels of heavy metal and metalloid concentration. PLoS One 10(6):e0128784
Lehto T, Zwiazek JJ (2011) Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21:71–90
Lenoir I, Fontaine J, Sahraoui AL-H (2016) Arbuscular mycorrhizal fungal responses to abiotic stresses: a review. Phytochemistry 123:4–15
Leport L, Turner NC, Dauies SL, Siddique KHM (2006) Variation in pod production and abortion among chickpea cultivars under terminal drought. Eur J Agron 24(3):236–246
Li H, Wu C, Ye ZH, Wu SC, Wu FY, Wong MH (2011) Uptake kinetics of different arsenic species in lowland and upland rice colonized with Glomus intraradices. J Hazard Mater 194:414–421
Linderman RG, Davis EA (2004) Varied response of marigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci Hortic 99:67–78
Lindner RC (1944) Rapid analytical method for some of the more inorganic constituents of plants tissue. Plant Physiol 19:76–89
Liu F, Jensen CR, Andersen MN (2004) Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: its implication in altering pod set. Field Crop Res 86:1–13
Lopez-Pedrosa A, González-Guerrero M, Valderas A, Azcón-Aguilar C, Ferrol N (2006) GintAMT1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genet Biol 43:102–110
Ma JF, Goto S, Tamai K, Ichii M (2001) Role of root hairs and lateral roots in silicon uptake by rice. Plant Physiol 127(4):1773–1780
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Ma JF, Yamaji N, Tamai K, Mitani N (2007) Genotypic difference in silicon uptake and expression of silicon transporter genes in rice. Plant Physiol 145:919–924
Ma JF, Yamaji N, Mitani N, Xu X-Y, Yu-Hong Su Y-H, McGrath SP, Zhao F-J (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105(29):9931–9935
Macnair MR, Cumbes QJ, Meharg AA (1992) The genetics of arsenate tolerance in Yorkshire fog, Holcus lanatus L. Heredity 69:325–335
Maeda D, Ashida K, Iguchi K, Chechetka SA, Hijikata A, Okusako Y, Deguchi Y, Izui K, Hata S (2006) Knockdown of an arbuscular mycorrhiza-inducible phosphate transporter gene of Lotus japonicus suppresses mutualistic symbiosis. Plant Cell Physiol 47:807–881
Mahdieh S, Ghaderian SM, Karimi N (2013) Effect of arsenic on germination, photosynthesis and growth parameters of two winter wheat varieties in Iran. J Plant Nutr 36:651–664
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Maurel C, Chrispeels MJ (2001) Aquaporins. A molecular entry into plant water relations. Plant Physiol 125:135–138
McCarty KM, Hanh HT, Kim K-W (2011) Arsenic geochemistry and human health in South East Asia. Rev Environ Health 26(1):71–78
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115(3):495–501
Meharg AA, Cairney JWG (1999) Co-evolution of mycorrhizal symbionts and their hosts to metal contaminated environments. Adv Ecol Res 30:70–112
Meharg AA, Macnair MR (1992) Suppression of the high-affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Mehlich A (1953) Determination of P, Ca, Mg, K, Na and NH4. North Carolina Soil Test Division (Mimeo), Raleigh
Mercy MA, Shivashankar G, Bagyaraj DJ (1990) Mycorrhizal colonization in cowpea is host dependent and heritable. Plant Soil 121:292–294
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261
Moreno-Jiménez E, Esteban E, Peñalosa JM (2012) The fate of arsenic in soil-plant systems. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology. Springer Science+Business Media, New York, pp 1–37
Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, White JC, Dhankher OP (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21(6):1265–1277
Nelson DW, Sommers LE (1972) A simple digestion procedure for estimation of total nitrogen in soil and sediments. J Environ Qual 1:423–425
Nriagu JO, Bhattacharya P, Mukherjee AB, Bundschuh J, Zevenhoven R, Loeppert RH (2007) Arsenic in soil and groundwater: an overview. In: Bhattacharya P, Mukherjee AB, Bundschuh J, Zevenhoven R, Loeppert RH (eds) Trace metals and other contaminants in the environment, 9. Elsevier, Amsterdam, pp 3–60
Odeny DA (2007) The potential of pigeonpea (Cajanus cajan (L.) Millsp.) in Africa. Nat Res Forum 31:297–305
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil analysis, Agron. No. 9, part 2—chemical and microbiological properties, 2nd edn. Am. Soc. Agron, Madison, pp 403–430
Ouziad F, Wilde P, Schmelzer E, Hildebrandt U, Bothe H (2006) Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ Exp Bot 57:177–186
Owino-Gerroh C, Gascho GJ (2005) Effect of silicon on low pH soil phosphorus sorption and on uptake and growth of maize. Commun Soil Sci Plant Anal 35:2369–2378
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. T Brit Mycol Soc 55(1):158–161
Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 60:389–404
Rahman MA, Hasegawa H, Rahman MM, Islam MN, Miah MAM, Tasmin A (2007) Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere 67:1072–1079
Raza MM, Ullah S, Ahmad Z, Saqib S, Ahmad S, Bilal HM, Wali F (2016) Silicon mediated arsenic reduction in rice by limiting its uptake. Agric Sci 7:1–10
Rizwan M, Ali S, Ibrahim M, Farid M, Adrees M, Bharwana SA, Zia-ur-Rehman M, Qayyum MF, Abbas F (2015) Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: a review. Environ Sci Poll Res 22:15416–15431
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Sahebi M, Hanafi MM, Akmar ASN, Rafii MY, Azizi P, Tengoua FF, Azwa JNM, Shabanimofrad M (2015) Importance of silicon and mechanisms of biosilica formation in plants. Biomed Res Int 2015:396010
Salloum MS, Guzzo MC, Velazquez MS, Sagadin MB, Luna CM (2016) Variability in colonization of arbuscular mycorrhizal fungi and its effect on mycorrhizal dependency of improved and unimproved soybean cultivars. Can J Microbiol. doi:10.1139/cjm-2016-0383
Sanglard LMVP, Detmann KC, Martins SCV, Teixeira RA, Pereira LF, Sanglard ML, Fernie AR, Araújo WL, DaMatta FM (2016) The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ Exp Bot 123:22–36
Shaibur MR, Kawai S (2009) Effect of arsenic on visible symptom and arsenic concentration in hydroponic Japanese mustard spinach. Environ Exp Bot 67:65–70
Shaibur MR, Kawai S (2010) Effect of arsenic on nutritional composition of Japanese mustard spinach: an ill effect of arsenic on nutritional quality of a green leafy vegetable. Nat Sci 8:186–194
Shaibur MR, Kitajima N, Sugawara R, Kondo T, Imamul Huq SM, Kawai S (2006) Physiological and mineralogical properties of arsenic–induced chlorosis in rice seedlings grown hydroponically. Soil Sci Plant Nutr 52:691–700
Sharma P, Jha AB, Dubey RS (2014) Arsenic toxicity and tolerance mechanisms in crop plants. In: Pessarakli M (ed) Handbook of plant and crop physiology. CRC Press, Taylor & Francis Group, LLC, New York, pp 733–782
Sharma N, Yadav K, Aggarwal A (2016) Growth response of two Phaseolus mungo L. cultivars induced by arbuscular mycorrhizal fungi andTrichoderma viride. Inter J Agron 2016:1524304
Shi X, Zhang C, Wang H, Zhang F (2005) Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 272:53–60
Siddiqui F, Tandon PK, Srivastava S (2015) Analysis of arsenic induced physiological and biochemical responses in a medicinal plant, Withania somnifera. Physiol Mol Biol Plant 21(1):61–69
Singh N, Ma LQ, Srivastava MM, Rathinasabapathi B (2006) Metabolic adaptations to arsenic induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Smith S, Read D (2008) Mycorrhizal symbiosis. Academic Press, London
Smith SE, Christophersen HM, Pope S, Smith FA (2010) Arsenic uptake and toxicity in plants: integrating mycorrhizal influences. Plant Soil 327:1–21
Spagnoletti F, Lavado RS (2015) The arbuscular mycorrhiza Rhizophagus intraradices reduces the negative effects of arsenic on soybean plants. Agronomy 5(2):188–199
Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60:3419–3431
Sun Y, Zhang X, Wu Z, Hu Y, Wu S, Chen B (2016) The molecular diversity of arbuscular mycorrhizal fungi in the arsenic mining impacted sites in Hunan Province of China. J Environ Sci 39:110–118
Sushant KS, Ghosh AK (2010) Effect of arsenic on photosynthesis, growth and its accumulation in the tissues of Allium cepa (onion). Intern J Environ Eng Manag 1:39–50
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14
Talukdar T, Talukdar D (2014) Response of antioxidative enzymes to arsenic-induced phytotoxicity in leaves of a medicinal daisy, Wedelia chinensis Merrill. J Nat Sci Biol Med 4(2):383–388
Tang T, Miller DM (1991) Growth and tissue composition of rice grown in soil treated with inorganic copper, nickel, and arsenic. Commun Sci Plant Anal 22:19–20
Tubaña BS, Heckman JR (2015) Silicon in soils and plants. In: Rodrigues FA, Datnoff LE (eds) Silicon and plant diseases. Springer International Publishing, Switzerland, pp 7–51
Turrini A, Giordani T, Avio L, Natali L, Giovannetti M, Cavallini A (2016) Large variation in mycorrhizal colonization among wild accessions, cultivars, and inbreds of sunflower (Helianthus annuus L.) Euphytica 207:331
Ullrich–Eberius C, Sanz A, Novacky A (1989) Evaluation of arsenic and vanadate–associated changes of electrical membrane potential and phosphate transport in Lemna gibba G L. J Exp Bot 40:119–128
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils: effect of variations in digestion conditions and of organic soil constituents. Soil Sci 63:251–263
Wang J, Zhao FJ, Meharg AA, Raab A, Feldman J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata: uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Wang HB, He HB, Yang GD, Ye CD, Niu BH, Lin WX (2010) Effects of two species of inorganic arsenic on the nutrient physiology of rice seedlings. Acta Physiol Plant 32:245–251
Weatherley PE (1950) Studies in the water relations of cotton plant. I. The field measurement of water deficits in leaves. New Phytol 49:81–97
Wu Z, Ren H, McGrath SP, Wu P, Zhao F-J (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157(1):498–508
Xie ZM, Naidu R (2006) Factors influencing bioavailability of arsenic to crops. In: Naidu R, Smith E, Owens G, Bhattacharya P (eds) Managing arsenic in the environment. From soils to human health. CSIRO Pub, Collingwood, pp 223–234
Yoon Y, Lee W-M, An Y-J (2016) Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res Int 22(14):11047–11056
Yost RS, Fox RL (1982) Influence of mycorrhizae on the mineral contents of cowpea and soybean grown in an oxisol. Agron J 74:475–481
Yun-sheng X, Bao-dong C, Peter C, Andrew SF, You-shan W, Xiao-lin L (2007) Arsenic uptake by arbuscular mycorrhizal maize (Zea mays L.) grown in an arsenic-contaminated soil with added phosphorus. J Environ Sci 19:1245–1251
Zhang H, Selim HM (2008) Reaction and transport of arsenic in soils: equilibrium and kinetic modeling. Adv Agron 98:45–115
Zhang WD, Liu DS, Tian JC, He FL (2009) Toxicity and accumulation of arsenic in wheat (Triticum aestivum L.) varieties of China. Inter J Exp Bot 78:147–154
Zhang Q, Liua J, Lu H, Zha S, Wang W, Du J, Yan C (2015) Effects of silicon on growth, root anatomy, radial oxygen loss (ROL) and Fe/Mn plaque of Aegiceras corniculatum (L.) Blanco seedlings exposed to cadmium. Environ Nanotechnol Monitor Manag 4:1–6
Acknowledgments
We gratefully acknowledge the University Grants Commission (UGC-No.f.25-1/2013-14(BSR)/7-151/2007(BSR) and Department of Biotechnology (DBT- BT/PR9466/AGR/21/231/2007), Government of India, for providing financial support in undertaking the research work. We are also thankful to TERI, New Delhi, and Pulse laboratory, IARI, New Delhi, for providing the biological research material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
ESM Fig. 1
Effect of Silicon (Si) and Arbuscular Myorrhiza (AM) on (a) Mycorrhizal colonization (b) Mycorrhizal responsiveness (%) under Arsenate (As V-25 mg/kg) and Arsenite (As III-5 mg/kg concentrations in Cajanus cajan genotypes (Tolerant-Pusa-2002 and Sensitive- Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si + AM = Si with AM present (GIF 159 kb)
ESM Fig. 2
Effect of Silicon (Si) and Arbuscular Myorrhiza (AM) on (a) Arsenic content in Pusa 2002 (b) Arsenic content in Pusa 991 (μg g−1 DW) (c) Silicon content in leaves (d) Silicon content in roots (mg g−1 DW) under Arsenate (As V-25 mg/kg) and Arsenite (As III-5 mg/kg) concentrations in Cajanus cajan genotypes (Tolerant-Pusa-2002 and Sensitive- Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si + AM = Si with AM present (GIF 349 kb)
ESM Fig. 3
Effect of Silicon (Si) and Arbuscular Myorrhiza (AM) on (a) Shoot Dry weights (b) Root Dry weights (g plant−1) under Arsenate (As V-25 mg/kg) and Arsenite (As III-5 mg/kg) concentrations in Cajanus cajan genotypes (Tolerant-Pusa-2002 and Sensitive- Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si + AM = Si with AM present (GIF 226 kb)
ESM Fig. 4
Effect of Silicon (Si) and Arbuscular Mycorrhiza (AM) on (a) Phosphorous (b) Potassium (c) Nitrogen (d) Magnesium contents (mg g−1 DW) in leaves under Arsenate (As V-25 mg/kg) and Arsenite (As III-5 mg/kg) concentrations in Cajanus cajan genotypes (Tolerant- Pusa-2002 and Sensitive - Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si +AM = Si with AM present (GIF 375 kb)
ESM Fig. 5
Effect of Silicon(Si) and Arbuscular Myorrhiza(AM) on (a) Phosphorous (b) Potassium (c) Nitrogen (d) Magnesium contents (mg g−1 DW) in roots under Arsenate (As V-25 mg/kg) and Arsenite (As III-5 mg/kg) concentrations in Cajanus cajan genotypes (Tolerant-Pusa 2002 and Sensitive- Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si + AM = Si with AM present (GIF 452 kb)
ESM Fig. 6
Effect of Silicon(Si) and Arbuscular Myorrhiza(AM) on (a) Phosphorous (b) Potassium (c) Nitrogen (d) Magnesium contents (mg g−1 DW) in leaves under Arsenate (As V-50 mg/kg) and Arsenite (As III-10 mg/kg) concentrations in Cajanus cajan genotypes (Tolerant-Pusa-2002 and Sensitive- Pusa 991). Values are mean of Six replicates ± standard error (SE). Different letters above the bar indicate significant differences among the treatments assessed by Duncan multiple range test at p ≤ 0.05. C = Si and AM absent; +Si = Si present; +AM = Arbuscular mycorrhiza present; +Si + AM = Si with AM present (GIF 446 kb)
ESM Table 1
(DOCX 36 kb)
ESM Table 2
(DOCX 65 kb)
ESM Table 3
(DOCX 51 kb)
ESM Table 4
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Garg, N., Kashyap, L. Silicon and Rhizophagus irregularis: potential candidates for ameliorating negative impacts of arsenate and arsenite stress on growth, nutrient acquisition and productivity in Cajanus cajan (L.) Millsp. genotypes. Environ Sci Pollut Res 24, 18520–18535 (2017). https://doi.org/10.1007/s11356-017-9463-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9463-x