Abstract
Soil salinity is an increasing problem worldwide, restricting plant growth and production. Research findings show that arbuscular mycorrhizal (AM) fungi have the potential to reduce negative effects of salinity. However, plant growth responses to AM fungi vary as a result of genetic variation in mycorrhizal colonization and plant growth responsiveness. Thus, profitable use of AM requires selection of a suitable combination of host plant and fungal partner. A greenhouse experiment was conducted to compare effectiveness of a native AM fungal inoculum sourced from saline soil and two single exotic isolates, Funneliformis mossseae and Rhizophagus irregularis (single or dual mix), on Cajanus cajan (L.) Millsp. genotypes (Paras and Pusa 2002) under salt stress (0–100 mM NaCl). While salinity reduced plant biomass and disturbed ionic status in both genotypes, Pusa 2002 was more salt tolerant and ensured higher AM fungal colonization, plant biomass and nutrient content with favourable ion status under salinity. Although all AM fungi reduced negative effects of salt stress, R. irregularis (alone or in combination with F. mosseae) displayed highest efficiency under salinity, resulting in highest biomass, yield, nutrient uptake and improved membrane stability with favourable K+/Na+ and Ca2+/Na+ ratios in the host plant. Higher effectiveness of R. irregularis correlated with higher root colonization, indicating that the symbiosis formed by R. irregularis had more stable viability and efficiency under salt stress. These findings enhance understanding of the functional diversity of AM fungi in ameliorating plant salt stress tolerance and suggest the potential use of R. irregularis for increasing Cajanus cajan productivity in saline soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a major abiotic stress which restricts plant growth and productivity in many regions of the world. According to an FAO survey (2008), it is expected that over 800 million ha will be affected by salinity in the near future, making it a major constraint for a steadily increasing population. In India, an area of nearly 9.38 million ha is occupied by salt-affected soils, out of which 5.5 million ha are saline soils (including coastal) (IAB 2000). There are many contaminants in salt-affected soils, the most common being NaCl, which readily dissolves in water to yield toxic sodium (Na+) and chloride (Cl−) ions (Djanaguiraman and Prasad 2013). Excessive soil salinization negatively affects the establishment, growth and development of most plants as well as the rhizosphere microbiota, leading to huge losses in plant productivity and diversity (Evelin et al. 2009).
High salt in soil has a threefold effect on plants: physiological drought due to reduced water potential, ion imbalance and reduced availability of nutrients (Munns and Tester 2008; Ruiz-Lozano et al. 2012; Plaut et al. 2013). Plants take up excessive amounts of Na+ at the cost of K+ in a saline environment, where an important factor in the battle between sodium and potassium is calcium. Ca2+ sustains K+ transport and may directly suppress sodium import mediated by non-selective ion channels. High cytoplasmic Na+/Ca2+ and Na+/K+ ratios may cause an increase in membrane permeability, and this may result in the passive accumulation of Na+ in root and shoot tissues of salt stressed plants (Estrada et al. 2013). Amongst nutrients that are affected, nitrogen is quantitatively the most important for plants. In general, salinity reduces N accumulation and saline environments are associated with nitrogen deficiency (Siddiqui et al. 2010). The influence of salinity on P accumulation in crop plants is variable and depends on the plant and experimental conditions (Dikilitas and Karakas 2010; Duman 2012). In most cases, salinity decreases P concentrations in plant tissue since phosphate ions precipitate with Ca2+, Mg2+ and Zn2+ ions in salt-stressed soils and become unavailable to plants (Grattan and Grieve 1999; Evelin et al. 2009).
Mechanical removal of salt from soils or development of salt-tolerant crops is not economically feasible under the current scenario since most of these processes are long and costly. A viable alternative is the use of microorganisms that mitigate salinity stress to improve plant growth. Amongst these arbuscular mycorrhizal (AM) fungi have received considerable attention due to their extensive benefits to host plant. AM fungi, belonging to the phylum Glomeromycota (Schüβler et al. 2001), are a normal part of the root system in most natural and agro-systems including stressed soils (Ruiz-Lozano et al. 2012). They can be found even under severe saline conditions in nature, both in saline inlands and coasts (Aliasgharzadeh et al. 2001; Yamato et al. 2008) and in salt marshes (Carvalho et al. 2004; Wilde et al. 2009), with Glomus sp. being one of the most commonly observed taxa in saline soils (Wilde et al. 2009; Krishnamoorthy et al. 2014).
Several studies have shown that inoculation with AM fungi can alleviate salt stress (Cekic et al. 2012; Estrada et al. 2013; Garg and Baher 2013; Talaat and Shawky 2014) through improved nutrient uptake (Wilson et al. 2012), water uptake (Auge 2001; Ruiz-Lozano et al. 2012), photosynthetic activity (Aroca et al. 2013) and production of antioxidant molecules in plants (Hajiboland et al. 2010). Mycorrhizal fungi may act as a first barrier for ion selection during nutrient uptake from the soil or during transfer to the plant host, suggesting that they induce a buffering effect on Na+ uptake (Hammer et al. 2011a). Mycorrhizal colonization has also been shown to enhance K+ absorption under saline conditions while preventing Na+ translocation to shoot tissues, resulting in a higher K+/Na+ ratio in mycorrhizal plants (Sannazzaro et al. 2006; Giri et al. 2007; Estrada et al. 2013).
Recent studies indicate the existence of functional diversity in AM as different combinations of host plant and AM fungi have different impacts on morphology, nutritional status, symbiotic efficiency and gene expression patterns in the symbiosis (Jansa et al. 2008; Janouskova et al. 2009; Wagg et al. 2011; Maherali and Klironomos 2012; Tian et al. 2013; Pellegrino and Bedini 2014). This functional diversity has been linked to different life history strategies adopted by different AM fungal species which dictate the nature of competition inside and outside the host for resources (Kiers et al. 2011; Englemoer et al. 2014). It has also been suggested that if a plant is colonized by AM fungal species that are complementary in their functions, they may prove to be more beneficial for the plant as a mixture than any of the species separately (Koide 2000; Jansa et al. 2008; Koch et al. 2012). However, other data do not support the idea of functional complementarity. Some studies indicate that maximum benefits to plants might be achieved with a single, most efficient AM fungal species and that increasing mycorrhizal diversity would not bring further benefits (Janouskova et al. 2009; Tavasolee et al. 2011). Most of the AM fungal inoculation studies have been based on the use of exotic or foreign (non-native) AM fungal isolates selected under non-stressed conditions, and there are only a few reports on the comparative effects of different AM fungal species under salt stress (Porras-Soriano et al. 2009; Peng et al. 2011; Cekic et al. 2012; Estrada et al. 2013; Huang et al. 2013). Peng et al. (2011) found that the symbiosis formed by an isolate of Glomus intraradices (=Rhizophagus irregularis) exhibited a more stable viability and efficiency compared to that formed by an isolate of Glomus mosseae (=Funneliformis mosseae) or Glomus claroideum (=Claroideoglomus claroideum) with increasing salinity. Similar results were also obtained by Alkan et al. (2006). On the other hand, a few studies have pointed to a greater benefit provided by native AM fungi rather than non-native/exotic AM fungal isolates under abiotic stress conditions, as they appear to be physiologically and genetically adapted to the stress conditions (Querejeta et al. 2006; Pellegrino et al. 2011; Estrada et al. 2013; Pellegrino and Bedini 2014). However, a report by Tian et al. (2004) emphasized that a G. mosseae isolate from saline soil did not infer greater tolerance to cotton plants as compared to a non-saline isolate of the same species. Therefore, for a profitable use of AM fungi in agriculture, it is imperative to compare the efficiency of various AM fungal inocula and formulate the most efficient combination under given agricultural constraints.
Pigeon pea (Cajanus cajan), a perennial member of the family Fabaceae, is an important grain legume of the semiarid tropics and sub-tropics and is a hardy, widely adapted and drought-tolerant crop, which allows its cultivation in a wide range of environments and cropping systems. India is the primary pigeonpea-growing country in the world, accounting for 3.53 mha area and 2.51 million tons of production (Varshney et al. 2010). Its seeds have 20–22 % protein and are consumed as green peas, whole grain or split peas. It has the ability to produce more N per unit area from plant biomass than many other legumes making it an ideal crop of sustainable agriculture in the tropical and sub-tropical regions of India (Saxena and Nadarajan 2010). However, it does not grow well in saline soil, which poses a major constraint to its production (Srivastava et al. 2006; Varshney et al. 2010). The use of AM fungi in pigeonpea cultivation could help overcome these constraints and increase productivity under saline conditions. In order to optimize the benefits of AM, it is imperative to select the best AM fungal-host combination under the given stress conditions.
Starting from the evidence that pigeonpea responds positively to exotic AM fungal inocula (Garg and Manchanda 2009; Garg and Chandel 2011), the present study was aimed to evaluate the effectiveness of native and exotic AM fungal species on two genotypes of pigeonpea, varying in their salinity tolerance. The study was undertaken to investigate whether (1) the two genotypes of pigeonpea showed differential mycorrhizal responsiveness under salt stress, (2) a native AM fungal community, sourced from saline soils, promoted greater growth under salt stress than non-native or exotic AM fungal isolates used as single or dual species inocula, and (3) the functional diversity of these AM fungi is reflected in terms of difference in root colonization and effects on plant growth, nutrient uptake and ion accumulation capacity.
Materials and methods
Biological material
Soil samples from a saline area (Hissar, 29° 10′ N–75° 46′ E, Haryana, India,; ECe 10.2 dSm−1; N 118 mg/kg; olsen P 7.3 mg/kg; Ca 23.0 mmol/l; Na 44.5 mmol/l) were collected and indigenous AM fungal spores isolated by wet sieving and sucrose density gradient centrifugation (Gerdemann and Nicolson 1963). Spores were identified under a compound microscope (http://invam.caf.wvu.edu). The soil contained a consortium of Glomus sp., Acaulospora sp. and Gigaspora sp. (each constituting approximately 60, 30 and 10 %, respectively, in the consortium). This inoculum was labelled as saline mix inoculum (SMix). Single isolates of Funneliformis mosseae, (T. H. Nicolson & Ged.) C. Walker & A. Schüβler (formerly Glomus mosseae) isolate UTMU 128 WM1/1 and Rhizophagus irregularis (N.C. Schenck & G.S. Sm.) C. Walker & A. Schüβler, (formerly Glomus intraradices) isolate NC 100 AZM1/12 (Schüβler and Walker 2010) were obtained from the Centre for Mycorrhizal Culture Collection, The Energy and Resources Institute (TERI), New Delhi, India. The mycorrhizal fungi were bulked in open-pot cultures of Zea mays L., Sorghum bicolor L. and Coriandrum sativum L. The crude mixture of soil, root fragments and spores obtained from the pots was used as AM fungal inoculum. Inoculum of salt-tolerant Sinorhizobium fredii strain AR-4 was obtained from the Department of Microbiology, Indian Agricultural Research Institute (IARI), New Delhi, India. The experimental plant material consisted of two genotypes of pigeonpea (Cajanus cajan (L.) Millsp., Paras (salt sensitive) and Pusa 2002 (salt tolerant), procured from CCS Haryana Agricultural University, Hissar, India, and Indian Agricultural Research Institute, New Delhi, India, respectively. These genotypes were selected in previous studies, after preliminary screening of ten genotypes on the basis of their differential salt tolerance index (STI). [STI = (dry weight of plant at stressed condition − dry weight of plant at controlled condition) / dry weight of plant at controlled condition × 100].
Experimental setup
Greenhouse experiments were conducted in the Department of Botany, Panjab University, Chandigarh (30.5° N, 76.5° E; elevation = 305–366 m) with minimum temperature 22–29 °C, maximum temperature 30–39 °C, morning relative humidity 35–78 % and afternoon relative humidity 30–52 %. Seeds of pigeonpea were surface sterilized with 10 % hydrogen peroxide (v/v) solution for 8 min and then rinsed by soaking in sterile distilled water to remove any traces of chemical that could interfere with seed germination. The seeds were pre-treated with rhizobial inoculum of Sinorhizobium fredii AR-4 and sown in circular pots (30 cm × 25 cm × 25 cm), disinfected with 70 % ethanol before filling them with soil (Liu et al. 2011); a thick wad of glass wool was placed on the central drainage hole, which was covered by a clean watch glass. The pots were lined with polythene bags (to avoid leaching of the salt during irrigation) and were filled with 7 kg of soil mixture. The experimental soil (a mixture of sand and loam in a ratio of 1:1 by volume) was obtained from the nearby agricultural fields [11.0 mg kg−1 P (Olsen and Sommers 1982), 0.17 meq/100 g available K, 0.19 meq/100 g Na, 0.82 meq/100 g Ca (Mehlich 1953), 0.42 % total N (Nelson and Sommers 1972), pH 7.6 (soil/water 1:1), ECe 0.88 dSm−1, 0.68 % organic carbon (Walkley 1947)]. It was autoclaved (121 °C, 1 h twice at 48-h interval) to eliminate existing AM fungal propagules. For AM inoculation, 50-g soil-based inoculum containing an average of 40 spores per gramme soil and 75 % of AM fungal colonized root fragments were placed in a layer beneath the seeds in the pots to facilitate fungal colonization of plant roots. The fungal inoculants used were saline mix (SMix), F. mosseae (F1) and R. irregularis (F2) and the dual species mix (FMix) obtained by mixing equal quantities of the two single species (F1 + F2; 1:1). Non-AM treatments received the same weight of autoclaved inoculums (equal quantities of all AM fungi mixed together). Finally, to ensure a common microflora, all pots received 10-ml aliquot of inoculum filtrate (inoculum containing a mixture of all AM fungi), obtained by filtering through Whatman No. 1 filter paper.
The experimental design consisted of five different mycorrhizal treatments: NM (non-mycorrhizal), SMix, F1, F2 and FMix. Pigeonpea plants were established for 2 weeks and then subjected to four NaCl treatments: 0, 60, 80 and 100 mM. The soil was salinized step-wise for three consecutive days to avoid osmotic shock. Addition of 60, 80 and 100 mM NaCl increased initial soil electrical conductivity (ECe) from 0.18 (0 NaCl) to 6.2, 8.3 and 10.1 dSm−1, respectively. The pots were watered as necessary with tap water and once a week with the different salt solutions to maintain the desired levels of salinity. If leaching occurred, the leachate was collected and added to the soil to maintain salinity treatments near the target levels. Pots were arranged in a completely randomized block design with a factorial combination of 5 × 4 × 2 (AM fungi, salinity, genotype) in six replicates. Plants were harvested for physiological and biochemical analyses at 80 days after sowing (DAS).
Mycorrhizal colonization and mycorrhizal responsiveness
The extent of AM fungal colonization was determined by staining the cleared roots with trypan blue. Root samples were cleared with 10 % KOH solution and stained with 0.05 % trypan blue (Phillips and Hayman 1970) and microscopically examined for root colonization. The mycorrhizal colonization was evaluated using the method of Giovannetti and Mosse (1980).
An index of mycorrhizal responsiveness was determined by expressing the dry weights of the plants concerned as a percentage of the dry weight of the non-mycorrhizal plants (Hetrick et al. 1992).
Plant biomass and yield
For dry weight measurements, the samples (roots and leaves) were oven-dried at 70 °C for 72 h until they reached a constant weight. Six plants per treatment were analysed, and data were calculated on per plant basis by taking the means. Root/shoot ratio was calculated. Yield parameters (seed dry weight per plant, above ground biomass) were recorded from flowering till seed development and maturity stage. Harvest index (HI) was calculated according to Leport et al. (2006) [the proportion of above-ground biomass production invested into harvestable organs].
Determination of nitrogen (N) and phosphorus (P) contents
Dried ground shoots and roots (1 g) were digested in a mixture of boiling sulphuric acid and hydrogen peroxide. When the fumes were white and the solution was completely clear, it was cooled to room temperature and filled up to 10 ml with deionized water. Reagent blanks were prepared by carrying out the whole extraction procedure but in the absence of sample. Nitrogen content was determined using the colorimetric method of Lindner (1944). Phosphorus (P) was extracted by nitric-perchloric acid digestion and measured using the vanado-molybophosphoric colorimetric method (Jackson 1973).
Determination of sodium (Na), potassium (K) and calcium (Ca) ion contents
A dilute acid extraction was done according to Munns et al. (2010). Dried plant tissue 50 mg was weighed out in 10-ml plastic tubes with lids. After capping, all tubes were placed on a shaker for 2 days at room temperature. The extract was then centrifuged, and sodium, potassium and calcium contents were measured using flame photometer (Allen et al. 1984) and expressed as milligram per gram dry weight of plant tissue.
Relative membrane permeability (MP)
Relative membrane permeability was measured by the electrolyte leakage (EL) method following Dionisio-Sese and Tobita (1998). Fresh leaf samples (third leaf) were cut into small pieces (5-mm length) and placed in 10-ml distilled deionized water contained in a test tube. The tubes were heated to 32 °C in a water bath. After 2 h of incubation, the initial electrical conductivity of the medium (EC1) was measured using a digital conductivity metre. Thereafter, all samples were autoclaved at 121 °C for 20 min to kill the tissues so as to release all the electrolytes. The samples were then cooled to 25 °C to record final electrical conductivity (EC2). EL was calculated using the following formula: EL = (EC1 / EC2) × 100
Statistical analyses
Data presented are mean values based on six replicates ± standard error (S.E.) per treatment. All results were subjected to analysis of variance (ANOVA) using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA) with genotype (G), salinity level (S) and mycorrhizal inoculation (AM) as main factors. Multiple comparisons within the one-way ANOVAs were done with orthogonal contrasts to compare the effect within different AM fungal assemblages. A multiple regression model was designed to predict various dependent variables from the independent factors S, AM and G. Pearson’s correlation (r) was used to determine the relationship between two dependent variables.
Results
Mycorrhizal colonization (MC) and mycorrhizal responsiveness (MR)
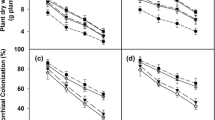
Data of percent AM fungal colonization in the roots of Cajanus cajan (pigeonpea) genotypes with the different AM fungal inocula under salt stress are presented in Fig. 1. No root colonization was observed in uninoculated plants. The salt-tolerant genotype Pusa 2002 showed significantly higher AM fungal colonization than Paras. Results of ANOVA show S, AM and G as well as S × AM, S × G, G × AM interactions to be significant (Table 4). As is evident from Table 1, increasing salt concentration reduced root colonization level, irrespective of pigeonpea genotype. The decrease was much greater in Paras than Pusa 2002. Reduction in root colonization by SMix and F1 was greater than those colonized by F2 and FMix under NaCl application. Colonization by F2 and FMix was not much affected by salinity and was maintained at 52 and 54 %, respectively, in Paras and 62 and 66 %, respectively, in Pusa 2002, even at 100 mM NaCl (Table 1). Orthogonal contrasts showed that mycorrhizal colonization was significant in all AM fungal inoculations. At all saline levels, significant differences were observed between SMix and single isolates (F1 and F2). There was also a significant difference between the two single isolates, F1 and F2. However, FMix did not differ significantly from F2 (Table 2). This implies an overall higher colonization by F2 and FMix at all salinity levels in both pigeonpea genotypes.
Seed yield (g/plant) and harvest index (HI) in pigeonpea genotypes (Paras, Pusa 2002) inoculated with different AM fungi (SMix native inoculum, F1 Funneliformis mosseae, F2 Rhizophagus irregularis, FMix mix of F1 and F2) under salt stress (0–100 mM NaCl). Values are means based on six replicates ± standard error (S.E.)
Mycorrhizal responsiveness (MR, Fig. 2) was higher in Pusa 2002 than in Paras, indicating that Pusa 2002 was much more responsive to AM fungal colonization, and MR increased as salinity intensified from control to higher salinity levels. It was also observed that as AM fungal colonization decreased with increasing NaCl stress, MR increased indicating a negative correlation between the two (r(Paras) = −0.288, p = 0.05; r(Pusa 2002) = −0.233, p = 0.05).
Mycorrhizal responsiveness of pigeonpea genotypes (Paras, Pusa 2002) inoculated with different AM fungi (SMix native inoculum, F1 Funneliformis mosseae, F2 Rhizophagus irregularis, FMix mix of F1 and F2) under salt stress (0–100 mM NaCl). Values are means based on six replicates ± standard error (S.E.)
Plant biomass and productivity
Salinity reduced dry matter production, and detrimental effects on growth were observed in both genotypes of pigeonpea (supplementary material ESM 1). ANOVA revealed significant effects of S, AM and G on root dry weight (RDW) and shoot dry weight (SDW). There was a significant interaction between S × AM, S × G, G × AM (Table 4). Paras was more severely affected by salinity than Pusa 2002; such decrease was more evident at the highest salt level applied (100 mM NaCl). Comparison of standardized coefficients (β) in regression analysis revealed high negative effects of salinity on root and shoot biomass while AM fungi and plant genotype had a positive impact (RDW, β(S) = −0.792, β(AM) = 0.394, β(G) = 0.375; SDW, β(S) = −0.719, β(AM) = 0.494, β(G) = 0.392, p < 0.01). The impact of salinity was more pronounced in roots than shoots resulting in decreased R/S ratios (Table 1). Although pigeonpea genotypes did not differ in terms of R/S ratios in controlled conditions, the decrease was more pronounced in Paras as compared to Pusa 2002 under increasing salinity. However, inoculation with AM fungi significantly improved root and shoot biomass in all salt treatments, as indicated by a significant SxAM interaction (p < 0.05) (Table 4). Higher RDW than SDW improved the R/S ratio in mycorrhizal plants although the increase was not statistically significant (Table 2). The dry weight of plants (roots as well as shoots) inoculated with F2 or FMix was significantly higher than that with SMix and F1 at all saline concentrations indicating greater effectiveness of F2 (alone or in combination) in alleviating salt stress. No significant variation was observed in R/S ratio within the different AM fungal inoculations.
The present study revealed that although salinity induced early flowering and yield parameters like seed yield (g/plant), the harvest index (HI) decreased with increased salinity (Fig. 1a, b). A genotypic variation was observed in the yield parameters of pigeonpea with increasing salinity stress. ANOVA results show a significant effect of S, AM and G on seed yield and HI. Significant S × AM, S × G, G × AM interactions were observed in seed yield, whereas for HI, only the S × G interaction was significant. Comparison of standardized coefficients (β) in regression analysis indicated pronounced negative impacts of salinity on seed yield and HI (seed yield β(S) = −0.809, β(AM) = 0.290, β(G) = 0.452; HI β(S) = −0.819, β(AM) = 0.073, β(G) = 0.493) while AM had a positive impact on both. A higher decline in seed yield and HI in Paras compared to Pusa 2002 indicated higher sensitivity of the former to salt stress. Inoculation with AM fungi significantly reduced the toxicity generated by NaCl and improved these parameters in salt stressed plants (Table 3). Although the benefit in terms of seed yield and HI was not significant under control conditions, on exposure to salinity there was a significant improvement in both parameters. Host benefit in terms of seed yield (seed weight per plant) for SMix, F1, F2 and FMix treatments at 80 mM NaCl was 34.6, 39.7, 62.2 and 67.3 % in Paras and 42.3, 48.3, 64.5 and 69.9 % in Pusa 2002, respectively. When results were compared amongst the AM inocula, a significantly higher seed yield was recorded by plants inoculated with F2 and FMix over F1 and SMix-inoculated plants. Higher HI was recorded in mycorrhizal plants although no significant variation was observed within different AM inoculations.
Nutrient uptake (N, P)
With increasing levels of salinity, N and P were reduced in shoots of both plant genotypes, the decline being more pronounced in Paras than Pusa 2002 (Fig. 3a, b). ANOVA showed a significant effect of S, AM, G as well as S × AM, S × G, G × AM interactions on N and P concentrations (Table 4). All mycorrhizal plants had significantly higher tissue concentrations of N and P compared to non-mycorrhizal plants under salt stress, as revealed by orthogonal contrasts (Table 3). The functional diversity of AM fungi was evident in N and P acquisition. The symbiosis formed by F2 and FMix resulted in significantly higher N and P concentrations as compared to F1 and SMix at all saline levels in both plant genotypes, as revealed by orthogonal contrasts (Table 3). A strong positive correlation between N, P levels and shoot dry weight was observed in both genotypes (N r(Paras) = 0.991, p = 0.01, r(Pusa 2002) = 0.985, p = 0.01; P r(Paras) = 0.991, p = 0.01, r(Pusa 2002) = 0.987, p = 0.01).
Concentration of a nitrogen (N) and b phosphorus (P) in shoots of pigeonpea genotypes (Paras, Pusa 2002) inoculated with different AM fungi (NM non-mycorrhizal, SMix native inoculum, F1 Funneliformis mosseae, F2 Rhizophagus irregularis FMix mix of F1 and F2) under salt stress (0–100 mM NaCl). Values are means based on six replicates ± standard error (S.E.)
Ion homeostasis
Salinity caused an increase in Na+ levels with a concomitant decrease in K+ and Ca2+ in roots as well as shoots. ANOVA showed a significant effect of S, AM, G as well as S × AM, S × G, AM × G interactions on ion (Na, K and Ca) concentrations. A significant S × G (p < 0.05) interaction suggested a genotypic variation in ion uptake with Paras showing significantly higher Na+ and lower K+ and Ca2+ uptake than Pusa 2002 (Table 4). Higher Na+ content in roots as compared to shoots (data not shown) indicated less translocation to shoots resulting in higher K+/Na+ ratios in shoots. The K+/Na+ and Ca2+/Na+ ratios were negatively affected by salinity in both pigeonpea genotypes (Fig. 4a, b). Higher K+/Na+ and Ca2+/Na+ ratios were observed in non-mycorrhizal Pusa 2002 plants as compared to Paras under saline treatments, indicating inherent salt tolerance of the former genotype. AM plants exhibited reduced Na+ content and enhanced K+ and Ca2+ uptake over non-mycorrhizal pigeonpea plants resulting in significantly higher K+ /Na+ and Ca2+/Na+ ratios (Fig. 4a, b). Plants inoculated with F2 and FMix exhibited a greater recovery of ionic homeostasis with highest K+ and Ca2+ and lowest Na+ under all saline treatments (Table 3). K+ and Ca2+ in shoots showed a positive correlation (K r(Paras) = 0.989, p = 0.01, r(Pusa 2002) = 0.980, p = 0.01; Ca r(Paras) = 0.975, p = 0.01, r(Pusa 2002) = 0.987, p = 0.01) while Na+ showed a strong negative correlation (Na r(Paras) = −0.847, p = 0.01, r(Pusa 2002) = −0.882, p = 0.01) with SDW in both genotypes, indicating the role of ionic balance in the overall plant growth .
a K+/Na+ ratio and b Ca2+/Na+ ratio in shoots of pigeonpea genotypes (Paras, Pusa 2002) inoculated with different AM fungi (NM non-mycorrhizal, SMix native inoculum, F1 Funneliformis mosseae, F2 Rhizophagus irregularis, FMix mix of F1 and F2) under salt stress (0–100 mM NaCl). Values are means based on six replicates ± standard error (S.E.)
Relative membrane permeability (MP)
Shoot and root MP increased with increase in salinity, with highest permeability observed at the highest salt concentration (100 mM NaCl). Higher membrane permeability in Paras was recorded at all saline levels as compared to Pusa 2002. ANOVA results revealed significant effects of S, AM and G on MP. There was a significant interaction between S × AM, S × G, AM × G (Table 4). A significant AM × G (p < 0.05) interaction indicated a genotypic variation in plant response to AM fungal inoculation with greater improvement in Pusa 2002 compared to Paras. Inoculation with AM fungi imparted greater membrane stability in both genotypes as expressed by lower membrane permeability or electrolyte leakage (Fig. 5). Lowest electrolyte leakage was observed in the roots and shoots of F2 and FMix-inoculated plants at all salinity levels, suggesting that R. irregularis (alone or in combination) imparted maximum stability to the plasma membranes compared to the other inoculated AM fungi. Orthogonal contrasts show a significant difference between SMix and single isolates as well as between single isolates F1 and F2. MP showed a negative correlation with P [r(Paras) = −0.879, p = 0.01, r(Pusa 2002) = −0.909, p = 0.01] and a positive correlation with Na+ [r(Paras) = 0.985, p = 0.01, r(Pusa 2002) = 0.973, p = 0.01] for shoots of both genotypes.
Membrane permeability (MP) in shoots of pigeonpea genotypes (Paras, Pusa 2002) inoculated with different AM fungi (NM non-mycorrhizal, SMix native inoculum, F1 Funneliformis mosseae, F2 Rhizophagus irregularis, FMix mix of F1 and F2) under salt stress (0–100 mM NaCl). Values are means based on six replicates ± standard error (S.E.)
Discussion
In the present work, the performance of one native AM fungal inoculum (isolated from saline area) and two exotic (non-native) isolates was assessed and their performance compared in imparting salt tolerance to two genotypes of pigeonpea with differential salt sensitivity. Percentage of root length colonized primarily determines the extent of AM associations between plants and fungi. Both pigeonpea genotypes exhibited a high degree of mycotrophy, since all fungal treatments developed an AM symbiotic association under unstressed and stressed conditions, although levels declined with salt stress. The decline in mycorrhizal colonization (MC) could be a consequence of direct effects of NaCl on spore germination, hyphal spread and/or mycorrhiza formation (Juniper and Abbott 2006; Tian et al. 2004; Hajiboland et al. 2010). Sannazzaro et al. (2006) reported that G. intraradices (=R. irregularis) established a more efficient symbiosis with a salt-tolerant genotype of Lotus glaber where root colonization little affected by saline conditions, suggesting that the tolerant genotype offered the fungal partner higher protection and better chances of growth within host tissues than a salt-sensitive genotype. The higher mycorrhizal colonization observed in the tolerant pigeonpea genotype Pusa 2002 under saline stress could account for its higher mycorrhizal responsiveness. A recent theory of preferential host-carbon (C) allocation (Kiers et al. 2011) may explain the differential responsiveness amongst the pigeonpea genotypes. The tolerant genotype may increase C allocation to the roots and thus make more carbon available to the fungus resulting in higher root colonization. This in turn could trigger N and P transport in the AM symbiosis (Hammer et al. 2011b; Fellbaum et al. 2012), resulting in increased plant growth and mycorrhizal responsiveness. Increasing MR with increase in salinity (in both pigeonpea genotypes) indicates that the AM association was strengthened in the saline environment, providing evidence for the ecological importance of AM for plant survival and growth in saline soils.
Plant biomass production is an integrative measurement of plant performance under many types of abiotic stress conditions, and symbiotic efficiency of AM fungi is generally measured in terms of plant growth improvement (Evelin et al. 2009; Ruiz-Lozano et al. 2012; Estrada et al. 2013). Several researchers have shown that shoot growth is more sensitive to salinity than root growth (Lauchli and Grattan 2007; Munns and Tester 2008). Contrary to this, in the present study, root growth was more severely affected by salinity resulting in reduced R/S ratios. This might be due to higher allocation of assimilates from the root to shoot under stress. In most cases, environmental signals, such as excess salt concentration, trigger plant response to growing conditions through changes in phytohormone concentrations, controlling the assimilate partitioning between different sink tissues (Hartig and Beck 2006). The reduced plant growth under high salinity is mainly attributed to the negative effect of the high osmotic potential of the saline soil solution which tends to reduce nutrient and water uptake, thus restricting plant growth (Munns and Tester 2008). A higher root than shoot biomass in mycorrhizal pigeonpea plants (increased R/S ratio) indicates a more highly developed and dense root system in response to mycorrhization which could allow the plant greater exchange between the roots and the aerial parts and more efficient water uptake. The main mechanism for enhanced salinity tolerance in the mycorrhiza pigeonpea plants seems to be improvement in nutrient uptake and translocation in the stressed environment, as revealed by positive correlations between N, P concentration and root, shoot dry weight. AM fungi have been reported to directly take up both organic and inorganic N from the soil solution and transfer it to their host plants (Ferrol and Pérez-Tienda 2009). Pigeonpea, being a legume, also shows a direct positive effect of AM fungal inoculation on rhizobial symbiosis, nitrogen fixation and N acquisition (data not shown), which would also contribute to growth enhancement (Barea et al. 2005; Garg and Chandel 2011; Abd-Alla et al. 2014). Increased P due to AM development is consistently associated with an increase in N accumulation due to increased nitrogen fixation (Saia et al. 2014). AM fungi provide a very effective pathway by which P is scavenged from large volumes of soil and rapidly delivered to cortical cells within the root, bypassing direct uptake and reducing the impact of Pi depletion in the rhizosphere (Smith et al. 2011).
The decline in yield parameters of the two pigeonpea genotypes, concomitant with decline in root and shoot biomass and nutrient status, under saline stress could be due to a reduction in photosynthesis as a result of salt stress which, at this point, might have directly affected seed weight leading to reduced harvest index. The better plant growth and nutrition stimulated by AM in inoculated pigeonpea plants could account for the higher flower initiation and seed setting, leading to higher yield in these plants. AM fungal inoculation has been reported to increase productivity by as much as 75 % in chickpea (Pellegrino and Bedini 2014) and 77 % in Trifolium alexandrinum (Pellegrino et al. 2011),.
In saline conditions, plants increasingly accumulate Na+ ions which compete with cellular K+ (Ruiz-Lozano et al. 2012). Excess Na+ in plant cells directly damages membrane systems and organelles, disturbs essential cellular metabolisms such as protein synthesis and enzyme activity resulting in plant growth reduction and abnormal development prior to plant death (Davenport et al. 2005; Quintero et al. 2007). A lower K+/Na+ ratio generated due to salinity disrupts the ionic balance in the cytoplasm, consequently decreasing cell turgor maintenance and disrupting various metabolic pathways, which hampers growth (Giri et al. 2007; Evelin et al. 2009). K+/Na+ ratio can thus be used as a physiological indicator of salt tolerance (Munns et al. 2000; Flowers 2004; Chen et al. 2007). Higher K+/Na+ ratio in the pigeonpea genotype Pusa 2002, compared to Paras, indicated its greater tolerance to salinity which may have been brought about due to the intrinsic differences in rates of toxic ion uptake, transport, and distribution within the plant (Garg and Manchanda 2009; Fatehi et al. 2012). Chen et al. (2007) found that an efficient Na+ extrusion (through a plasma membrane Na+/H+ exchanger) as well as a better retention of K+ (primarily through higher H+-ATPase activity) makes a crucial contribution to tolerance in salt-tolerant varieties of barley. Also, the higher Ca2+/Na+ ratio in Pusa 2002 under saline conditions might have helped to maintain membrane integrity and selectivity (Lauchli and Grattan 2007) and so protect Pusa 2002 plants against salt damage. The ability of a cell membrane to control the rate of ion movement in and out of cells (electrolyte leakage), taken as a measure of membrane permeability (MP), has been reported as an effective selection criterion for salt tolerance in various crops (Flowers and Flowers 2005; Mansour 2013). The higher MP observed in the Paras genotype as compared to Pusa 2002 underlines the sensitivity of this genotype to salt stress. Salinity induced oxidative stress (i.e., oxidative damage of membrane proteins and lipid peroxidation) contributes to increased membrane permeability in salt-sensitive plant species, whereas the improved membrane integrity and thus maintained permeability in salt resistant species is a result of reduced oxidative damage (Mansour 2013). Shoot Na+ concentration in the Paras genotype of pigeonpea was positively correlated to high MP in the present study, suggesting that membrane damage resulted in excess Na+ uptake. Maintenance of a high cytosolic K+/Na+ ratio in AM plants is an important mechanism for the enhancement of salt tolerance (Garg and Manchanda 2009; Estrada et al. 2013). Reduced Na+ uptake and enhanced K+ in Pusa 2002 correlated with higher AM fungal colonization in this genotype compared to Paras.
It has recently been suggested that the AM fungal mycelium may act as a first barrier for ion selection by selectively uptaking K+ and Ca2+ and alleviate salt stress in plants by pre-selecting nutrients and preventing toxic ions like Na+ from entering the plant (Hammer et al. 2011a). If the mycorrhizal uptake pathway is the dominant pathway for K+ and Na+ (and other ions) uptake in mycorrrhizal plants, as shown for P, it can be hypothesized that the peri-arbuscular membrane would serve as another barrier for selective delivery of various ions to the host plant (Hajiboland 2013). In this case, dual ion selectivity (by mycelial membrane and peri-arbuscular membrane) might account for reduced uptake of Na+ and increase in K+ ions in the mycorrhizal pigeonpea plants. Reduced Na+ content in mycorrhizal plants might also be explained by dilution effects due to growth enhancement by AM fungal colonization (Al-Karaki 2006). Recently Estrada et al. (2013) correlated higher K+/Na+ ratios with regulation of ZmAKT2, ZmSOS1 and ZmSKOR gene expression in mycorrhizal maize roots, contributing to K+ and Na+ homeostasis in plants. In the pigeonpea genotypes, the mycorrhizal symbiosis strengthened membrane stability contributing to greater ion homeostasis and better salt stress tolerance in plants. This increased membrane stability in the mycorrhizal plants may be attributed to mycorrhiza-mediated enhanced P uptake (Feng et al. 2002) as shown by correlation studies in the present investigation. Membrane permeability to water and ions is also involved in the control of cellular water potential and turgor (Bray 2001) through aquaporins and ion transporters. Higher K+ retention due to increased AKT2 and SKOR channels in membranes (Estrada et al. 2013) increased membrane stability. The strengthening of membrane stability in mycorrhizal plants possibly leads to maintenance of turgor in cells, causing a reversal of growth inhibition due to water deficit.
Several studies have implied greater physiological and genetic adaptability of native AM fungi to stress conditions of the target environment than non-native isolates (Querejeta et al. 2006; Pellegrino and Bedini 2014). Estrada et al. (2013) observed that a native isolate of R. irregularis (=G. intraradices) from saline soils showed an increased colonization capacity and plant growth improvement under saline conditions as compared to a non-native isolate of the same species. Contrary to this, the lower percent colonization and effectiveness displayed by saline mix (SMix) inoculum compared to the single, exotic (non-native) AM fungal isolates (F. mosseae and R. irregularis) observed under salinity in the present study suggest that it may be the inherent genetic composition of AM fungi rather than adaptability which influence colonization under saline stress. This is in agreement with some earlier observations which indicate that single exotic inocula were more efficient than mixed native ones (Cantrell and Linderman 2001; Tian et al. 2004; Baslam et al. 2014). Higher colonization by R. irregularis (F2), even at 100 mM NaCl, indicated higher tolerance of R. irregularis to external saline stress compared to the native inoculum mix (SMix) or F. mosseae (F1). A more efficient symbiosis by G. intraradices (=R. irregularis) under increasing salinity levels compared to that formed by G. mosseae (=F. mosseae) has also been reported by Alkan et al. (2006) and Peng et al. (2011). Growth improvement of pigeonpea plants by R. irregularis was higher than F. mosseae, and it became more obvious under 100 mM NaCl, which demonstrated the symbiotic capacity of this AM fungus particularly under stress conditions. Although similar results of higher growth enhancement by R. irregularis over F. mosseae in salt-stressed plants have previously been reported (Estau’n et al. 2003; Peng et al. 2011), many studies have shown F. mosseae to be more beneficial under abiotic stress (Porras-Soriano et al. 2009; Zaefarian et al. 2011; Huang et al. 2013). Differences in benefits conferred to hosts are generally associated with different life history strategies employed by AM fungal species (Maherali and Klironomos 2012). For example, AM fungal species differ in the amount of carbon they extract from their host (Li et al. 2008; Olsson et al. 2010), their ability to acquire P (Smith et al. 2000; Burleigh et al. 2002) and their nutrient storage strategies (Kiers et al. 2011; Englemoer et al. 2014). Functional diversity between AM fungal species is not only apparent at the level of mycorrhiza formation and plant nutrient uptake but also in expression of plant genes involved in P uptake (Burleigh et al. 2002; Tian et al. 2013). Recently, Estrada et al. (2013) found evidence that functional diversity can also be detected in the expression of host genes involved in ion homeostasis. Higher K+/Na+ ratio correlated with regulation of ZmAKT2, ZmSOS1 and ZmSKOR (genes involved in Na+ exclusion and K+ acquisition) expression in the roots of maize subjected to salt stress, contributing to K+ and Na+ homeostasis in plants colonized by the most efficient AM fungi.
It has also been suggested that establishment of mixed communities by different AM fungal species may be more beneficial to the growth of plants than any of individual species (Koide 2000; Mwangi et al. 2011; Wagg et al. 2011; Koch et al. 2012). Recently, Koch et al. (2011) also found intra-specific synergism between two isolates of G. intraradices suggesting that functional complementarity may not only occur amongst but also within AM fungal species. However, no significant difference in effectiveness (in terms of plant parameters studied) of the mixture of R. irregularis and F. mosseae compared to the respective single-species inoculations under salt stress indicated that simply increasing AM fungal richness may not necessarily enhance plant performance synergistically. It has been suggested that it is rather the dominant AM fungal isolate that influences responses in the isolate mixtures (Jansa et al. 2008; Janouskova et al. 2009; Peng et al. 2011; Lewandowski et al. 2013). Similar levels of colonization in FMix and F2 (R. irregularis) suggested a predominance of R. irregularis in the symbiosis formed by FMix. Dominance of R. irregularis in Astralagus sinicus roots inoculated with a mixture of R. irregularis, F. mosseae and Claroideoglomus claroideum (=G. intraradices, G. mosseae, and G. claroideum) under salt stress has been confirmed by nested PCR analysis, suggesting its major influence on the symbiotic performance of A. sinicus plants colonized by the mixed inoculum (Peng et al. 2011). High abundance of R. irregularis following mixed inoculation of a single root system may result from direct competitive interactions between fungal partners (Jansa et al. 2008) or due to preferential host-carbon enrichment to the more co-operative fungus (Kiers et al. 2011; Verbruggen et al. 2012; Englemoer et al. 2014).
The existence of AM functional diversity suggests that introduction of exotic AM fungal species may be an effective strategy for sustainable agriculture under saline conditions. However, before the inoculation of non-native AM fungi can be promoted for low-input agriculture, effects on soil microbial biodiversity and the functioning of natural ecosystems need to be carefully studied. In one study, inoculation of an agricultural soil with a commercial isolate of G. intraradices at the rate of application recommended by the supplier promoted plant growth without affecting the resident AM fungal community in maize roots (Antunes et al. 2009). In contrast, however, a greenhouse study in pots (Koch et al. 2011) and a field inoculation trial (Douds et al. 2011) with some strains of the AM fungal species, G. intraradices, found that, at least in the short term, inoculants disturbed natural AM fungal communities. Also, Pellegrino et al. (2012) using molecular genetic tracing tools recently found that two foreign isolates of F. mosseae successfully competed with members of the natural AM fungal community as root colonizers and enhanced yield. Such disturbance of native AM fungi or their exclusion from colonizing roots raises concerns about the bio-invasion potential of non-native inoculants that will need further study in the light of possible biodiversity losses resulting from anthropogenic movement of biota between biogeographic regions.
In conclusion, the significant interactions between pigeonpea genotypes and AM fungal species underline the importance of selecting the appropriate host-AM fungi combination for improving performance of pigeonpea worldwide under saline conditions. In the present investigation, the pigeonpea genotype Pusa 2002 displayed highest mycorrhiza development under salt tolerance which correlated with improved plant biomass, nutrient uptake and a more balanced ionic status. Exotic or non-native single isolates conferred higher salt tolerance to pigeonpea than native inoculum isolated from saline soil: R. irregularis (alone or in combination) proved to be more effective than F. mosseae under salt stress, which was reflected in terms of highest growth and yield. However, the interaction of non-native isolates with the local microflora needs to be explored, and future studies need to be directed towards evaluating the performance of non-native AM fungi in field conditions where a local AM fungal community may already be present.
References
Abd-Alla MH, El-Enany AWE, Nafady NA, Khalaf DM, Morsy FM (2014) Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol Res 169:49–58
Aliasgharzadeh N, Saleh Rastin N, Towfighi H, Alizadeh A (2001) Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 11:119–122
Alkan N, Gadkar V, Yarden O, Kapulnik Y (2006) Analysis of quantitative interactions between two species of arbuscular mycorrhizal fungi, Glomus mosseae and G. intraradices, by real-time PCR. Appl Environ Microbiol 72:4192–4199
Al-Karaki GN (2006) Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci Hortic 109:1–7
Allen SF, Grimshaw HF, Rowl AB (1984) Chemical analysis. In: Moor PD, Chapman SB (eds) Methods in Plant Ecolgy. Blackwell, Oxford, pp 185–344
Antunes PM, Koch AM, Dunfield KE, Hart MM, Downing A, Rillig MC, Klironomos JN (2009) Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 317:257–266
Aroca R, Ruiz-Lozano JM, Zamarreno AM, Antonio Paz J, Garcia-Mina JM, Pozo MJ, Lopez-Raez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Auge RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56(417):1761–1778
Baslam M, Qaddoury A, Goicoechea N (2014) Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28:161–172
Bray, EA (2001) Plant response to water-deficit stress. Encyclopedia of Life Sciences, Nature Publishing Group, pp 1–5
Burleigh SH, Cavagnaro T, Jakobsen I (2002) Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J Exp Bot 53:1593–1601
Cantrell IC, Linderman RG (2001) Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 233:269–281
Carvalho LM, Correia PM, Martins-Loucão MA (2004) Arbuscular mycorrhizal fungal propagules in a salt marsh. Mycorrhiza 14:165–170
Cekic FA, Unyayar S, Ortas I (2012) Effects of arbuscular mycorrhizal inoculation on biochemical parameters in Capsicum annuum grown under long term salt stress. Turk J Bot 36:63–72
Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabal S (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145(4):1714–1725
Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiol 137:807–818
Dikilitas M, Karakas S (2010) Salts as potential environmental pollutants, their types, effects on plants and approaches for their phytoremediation. In: Ashraf M, Ozturk M, Ahmad MSA (eds) Plant adaptation and phytoremediation. Springer, London, pp 357–381
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Djanaguiraman M, Prasad PVV (2013) Effects of salinity on ion transport, water relations and oxidative damage. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 89–113
Douds DD, Nagahashi G, Wilson DO, Moyer J (2011) Monitoring the decline in AM fungus populations and efficacy during a long term bare fallow. Plant Soil 342:319–326
Duman F (2012) Uptake of mineral elements during abiotic stresses In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, pp 267–281
Englemoer DJP, Behm JE, Kiers ET (2014) Intense competition between arbuscular mycorrhizal mutualists in an in vitro root microbiome negatively affects total fungal abundance. Mol Ecol 23:1584–1593
Estau’n V, Cambrubı´ A, Calvet C, Pinochet J (2003) Nursery and field response of olive trees inoculated with two arbuscular mycorrhizal fungi, Glomus intraradices and Glomus mosseae. Am Soc Hort Sci 128:767–75
Estrada B, Aroca R, Maathuis FJM, Barea JM, Ruiz-Lozano JM (2013) Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ 36(10):1771–1782
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
FAO (2008) FAO Land and Plant Nutrition Management Service. http://www.fao.org/ag/agl/agll/spush/
Fatehi F, Hosseinzadeh A, Alizadeh H, Brimavandi T, Struik PC (2012) The proteome response of salt-resistant and salt-sensitive barley genotypes to long-term salinity stress. Mol Biol Rep 39:6387–6397
Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, Kiers ET, Bucking H (2012) Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 109(7):2666–71
Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12:185–90
Ferrol N, Pérez-Tienda J (2009) Coordinated nutrient exchange in arbuscular mycorrhiza interface. In: Azcon-Aguilar C, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (eds) Mycorrhizas: functional processes and ecological impact. Springer, Berlin, Heidelberg, Germany, pp 73–87
Flowers TJ, Flower SA (2005) Why does salinity pose such a difficult problem for plant breeders? Agric Water Manag 78:15–24
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Garg N, Baher N (2013) Role of arbuscular mycorrhizl symbiosis in proline biosynthesis and metabolism of Cicer arietinum L. (chickpea) genotypes under salt stress. J Plant Growth Regul 32:767–778
Garg N, Chandel S (2011) The effects of salinity on nitrogen fi xation and trehalose metabolism in mycorrhizal Cajanus cajan (L.) Millsp. plants. J Plant Growth Regul 30:490–503
Garg N, Manchanda G (2009) Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp. pigeonpea. J Agron Crop Sci 195:110–123
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Giovannetti M, Mosse B (1980) Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microbiol Ecol 54:753–760
Grattan SR, Grieve CM (1999) Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 203–226
Hajiboland R (2013) Role of arbuscular mycorrhiza in amelioration of salinity. In: Ahmad P, Azooz MM, Prasad MNV (eds) Salt stress in plants: signalling, omics and adaptations. Springer, New York, pp 301–354
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Hammer EC, Nasr H, Pallon J, Olsson PA, Wallander H (2011a) Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 21:117–129
Hammer EC, Pallon J, Wallander H, Olsson PA (2011b) Tit for Tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol 76:236–44
Hartig K, Beck E (2006) Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol 8:389–396
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Huang JC, Lai WA, Singh S, Hameed A, Young CC (2013) Response of mycorrhizal hybrid tomato cultivars under saline stress. J Soil Sci Plant Nutri 13(2):469–484
IAB (2000) Indian agriculture in brief. (27th edition). Agriculture Statistics Division, Ministry of Agriculture, Govt. of India, New Delhi
Jackson ML (1973) Soil chemical analysis. Published by Printice Hall, New Delhi, p 485
Janouskova M, Seddas P, Mrnka L, van Tuinen D, Dvorackova A, Tollot M, Gianinazzi-Pearson V, Vosatka M, Gollotte A (2009) Development and activity of Glomus intraradices as affected by co-existence with Glomus claroideum in one root system. Mycorrhiza 19(6):393–402
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16:371–379
Kiers ET, Duhamel M, Beesetty Y et al (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Koch AM, Antunes PM, Barto EK, Cipollini D, Mummey DL, Klironomos JN (2011) The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol Invasions 13:1627–1639
Koch AM, Antunes PM, Klironomos JN (2012) Diversity effects on productivity are stronger within than between trophic groups in the arbuscular mycorrhizal symbiosis. PLoS ONE 7(5):e36950. doi:10.1371/journal.pone.0036950
Koide RT (2000) Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol 147:233–235
Krishnamoorthy R, Kim K, Kim C, Sa T (2014) Changes of arbuscular mycorrhizal traits and community structure with respect to soil salinity in a coastal reclamation land. Soil Biol Biochem 72:1–14
Lauchli A, Grattan SR (2007) Plant growth and development under salinity stress. In: M.A. Jenks et al. (eds.), Advances in molecular breeding toward drought and salt tolerant crops, Springer, pp:1–32
Leport L, Turner NC, Dauies SL, Siddique KHM (2006) Variation in pod production and abortion among chickpea cultivars under terminal drought. Eur J Agron 24(3):236–246
Lewandowski TJ, Dunfield KE, Antunes PM (2013) Isolate identity determines plant tolerance to pathogen attack in assembled mycorrhizal communities. PLoS ONE 8(4):e61329. doi:10.1371/journal.pone.0061329
Li H, Smith FA, Dickson S, Holloway RE, Smith SE (2008) Plant growth depressions in arbuscular mycorrhizal symbioses: not just caused by carbon drain? New Phytol 178:540–544
Lindner RC (1944) Rapid analytical method for some of the more inorganic constituents of plants tissue. Plant Physio 19:76–89
Liu LZ, Gong ZQ, Zhang YL, Li PJ (2011) Growth, cadmium accumulation and physiology of marigold (Tagetes erecta L.) as affected by arbuscular mycorrhizal fungi. Pedosphere 21(3):319–327
Maherali H, Klironomos JN (2012) Phylogenetic and trait-based assembly of arbuscular mycorrhizal fungal communities. PLoS ONE 7:e36695
Mansour MMF (2013) Plasma membrane permeability as an indicator of salt tolerance in plants. Boil Plant 57(1):1–10
Mehlich A (1953) Determination of P, Ca, Mg, K, Na and NH4. North Carolina Soil Test Division (Mimeo 1953)
Munns R, Hare RA, James RA, Rebetzke GJ (2000) Genetic variation for improving the salt tolerance of durum wheat. Aust J Agric Res 51:69–74
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Physiol Plant Mol Biol 59:651–681
Munns R, Wallace PA, Teakle NL, Colmer TD (2010) Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In: Sunkar R (ed) Plant Stress Tolerance, Methods in Molecular Biology. Springer, Berlin, pp 371–382
Mwangi MW, Monda EO, Okoth SA, Jefwa JM (2011) Inoculation of tomato seedlings with Trichoderma harzianum and arbuscular mycorrhizal fungi and their effect on growth and control of wilt in tomato seedlings. Braz J Microbiol 42:508–513
Nelson DW, Sommers LE (1972) A simple digestion procedure for estimation of total nitrogen in soil and sediments. J Environ Qual 1:423–425
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil analysis, Agron. No. 9, part 2—chemical and microbiological properties, 2nd edition, Am. Soc. Agron., Madison, WI, USA, pp 403–430
Olsson PA, Rahm J, Aliasgharzad N (2010) Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol Ecol 72:125–131
Pellegrino E, Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem 68:429–439
Pellegrino E, Bedini S, Avio L, Bonari E, Giovannetti M (2011) Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol Biochem 43:367–376
Pellegrino E, Turrini A, Gamper HA, Cafa G, Bonari E, Young JPW, Giovannetti M (2012) Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol 194:810–822
Peng J, Li Y, Shi P, Chen X, Lin H, Zhao B (2011) The differential behavior of arbuscular mycorrhizal fungi in interaction with Astragalus sinicus L. under salt stress. Mycorrhiza 21:27–33
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Plaut Z, Edelstein M, Ben-Hur M (2013) Overcoming salinity barriers to crop production using traditional methods. Crit Rev Plant Sci 32(4):250–291
Porras-Soriano A, Soriano-Martín ML, Porras-Piedra A, Azcón R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359
Querejeta JI, Allen MF, Caravaca F, Rolda’n A (2006) Differential modulation of host plant δ13C and δ18O by native and non native arbuscular mycorrhizal fungi in a semiarid environment. New Phytol 169(3):79–387
Quintero JM, Fournier JM, Benlloch M (2007) Na+ accumulation in shoot is related to water transport in K+-starved sunflower plants but not in plants with a normal K+ status. J Plant Physiol 164:60–67
Ruiz-Lozano JM, Porcel R, Azcón C, Aroca R (2012) Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J Exp Bot 63(11):4033–4044
Saia S, Amato G, Frenda AS, Giambalvo D, Ruisi P (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS ONE 9:e90738
Sannazzaro AI, Ruíz OA, Alberto EO, Menendez AB (2006) Alleviation of salt stress in Lotus glaber by Glomus intraradices. Plant Soil 285:279–287
Saxena KB, Nadarajan N (2010) Prospects of pigeonpea hybrids in Indian agriculture. Elect J Plant Breed 1(4):1107–1117
Schüβler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Schüβler A, Walker C (2010) The Glomeromycota: a species list with new families and genera. Edinburgh & Kew, UK, The Royal Botanic Garden; Munich, Germany: Botanische Staatssammlung Munich and Oregon, USA: Oregon State University. Available from http://schuessler.userweb.mwn.de/amphylo/Schuessler&Walker2010_Glomeromycota.pdf
Siddiqui MH, Mohammad F, Khan MN, Al-Whaibi MH, Bahkali AHA (2010) Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in brassica genotypes grown under salt stress. Agric Sci China 9:671–680
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular fungi in symbiosis with mycorrhizal Medicago truncatula. New Phytol 147(2):357–366
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating phosphorus acquisition. Plant Physiol 156:1050–1057
Srivastava N, Vadez V, Upadhyaya HD, Saxena KB (2006) Screening for intra and inter specific variability for salinity tolerance in pigeonpea (Cajanus cajan) and its related wild species. e-Journal of SAT Agric Res 2(1):1–12
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Tavasolee A, Aliasgharzad N, Salehi-Jouzani G, Mardi M, Asgharzadeh A (2011) Interactive effects of arbuscular mycorrhizal fungi and rhizobial strains on chickpea growth and nutrient content in plant. Afr J Biotechnol 10(39):7585–7591
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl Soil Ecol 26:143–148
Tian H, Drijber RA, Xiaolin L, Miller DN, Wienhold BJ (2013) Arbuscular mycorrhizal fungi differ in their ability to regulate the expression of phosphate transporters in maize (Zea mays L.). Mycorrhiza 23:507–514
Varshney RK, Penmetsa RV, Dutta S et al (2010) Pigeonpea genomics initiative (PGI): an international effort to improve crop productivity of pigeonpea (Cajanus cajan L.). Mol Breed 26:393–408
Verbruggen E, Van Der Heijden MGA, Weedon JT, Kowalchuk GA, Roling WFM (2012) Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol Ecol 21:2341–2353
Wagg C, Jansa J, Stadler M, Schmid B, Van der Heijden MGA (2011) Mycorrhizal fungal identity and diversity relaxes plant – plant competition. Ecology 92:1303–1313
Walkley A (1947) A critical examination of a rapid method for determining organic carbon in soils: Effect of variations in digestion conditions and of organic soil constituents. Soil Sci 63:251–263
Wilde P, Manal A, Stodden M, Sieverding E, Hildebrandt U, Bothe H (2009) Biodiversity of arbuscular mycorrhizal fungi in roots and soils of two salt marshes. Environ Microbiol 11:1548–1561
Wilson BAL, Ash GJ, Harper JDI (2012) Arbuscular mycorrhizal fungi improve the growth and nodulation of the annual legume Messina (Melilotus siculus) under saline and non-saline conditions. Crop Pasture Sci 63:164–178
Yamato M, Ikeda S, Iwase K (2008) Community of arbuscular mycorrhizal fungi in a coastal vegetation on Okinawa island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 18:241–249
Zaefarian F, Rezvani M, Rejali F, Ardakani MR, Noormohammadi G (2011) Effect of heavy metal and arbuscular mycorrhizal fungal on growth and nutrients (N, P, K, Zn, Cu and Fe) accumulation of alfalfa (Medicago sativa L.). Am Eurasian J Agric Environ Sci 11(3):346–352
Acknowledgments
We gratefully acknowledge Department of Biotechnology (DBT), Government of India for providing financial support in undertaking the present research work. We are also thankful to TERI, New Delhi and Pulse laboratory, IARI, New Delhi for providing the biological material for the research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Garg, N., Pandey, R. Effectiveness of native and exotic arbuscular mycorrhizal fungi on nutrient uptake and ion homeostasis in salt-stressed Cajanus cajan L. (Millsp.) genotypes. Mycorrhiza 25, 165–180 (2015). https://doi.org/10.1007/s00572-014-0600-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-014-0600-9