Abstract
The exposure of paddy fields to arsenic (As) through groundwater irrigation is a serious concern that may not only lead to As accumulation to unacceptable levels but also interfere with mineral nutrients in rice grains. In the present field study, profiling of the mineral nutrients (iron (Fe), phosphorous, zinc, and selenium (Se)) was done in various rice genotypes with respect to As accumulation. A significant genotypic variation was observed in elemental retention on root Fe plaque and their accumulation in various plant parts including grains, specific As uptake (29–167 mg kg−1 dw), as well as As transfer factor (4–45%). Grains retained the least level of As (0.7–3%) with inorganic As species being the dominant forms, while organic As species, viz., dimethylarsinic acid and monomethylarsonic acid, were non-detectable. In all tested varieties, the level of Se was low (0.05–0.12 mg kg−1 dw), whereas that of As was high (0.4–1.68 mg kg−1 dw), considering their safe/recommended daily intake limits, which may not warrant their human consumption. Hence, their utilization may increase the risk of arsenicosis, when grown in As-contaminated areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Holocene era aquifers have been extensively utilized through tube wells for drinking water and irrigation of crops that has resulted in severe arsenic (As) contamination in South-East Asia. Epidemiological studies in As-affected regions of West Bengal (India) and Bangladesh found a strong dose–response relationship between As exposure and clinical signs, i.e., melanosis, leucomelanosis, hyperkeratosis, hepatomegaly, neuropathy, edema, and skin, lung, bladder, and urinary tract cancers (Mazumder 2003). The As-exposed villagers had an increase of 8% in melanosis and 4% in keratosis rate as compared to the non-exposed people (Mandal and Biswas 2004). The main cause for As exposure to the human is rice, contributing to more than 60% of dietary As exposure since rice is the major cultivated crop in As-contaminated regions of South-East Asia (Meharg and Rahman 2003). Further, rice is grown in flooded (reduced) conditions where As availability in the form of AsIII remains high (Duxbury et al. 2003) in relation to the soil As contamination (Lu et al. 2009). The total As concentration in rice varies from 0.005 to 0.710 mg kg−1 dw in different varieties, and it also differs from one geographical region to other, e.g., <0.01–2.05 for Bangladesh, 0.31–0.76 for China, 0.03–0.44 for India, and 0.11–0.66 for USA (Zavala and Duxbury 2008). Therefore, the impact of As-contaminated soil on the rice grain quality is especially important, as rice is the major staple food for the population of As-epidemic areas of Bangladesh and India.
Management strategies to reduce As accumulation in rice may include varietal selection on the basis of As accumulation and speciation, iron (Fe) plaque formation, use of aerobic cultivation practices, and suitable fertilization procedures (Tripathi et al. 2007; Tuli et al. 2010). To date, a few studies have been performed on the evaluation of these prospective strategies (Abedin et al. 2002a, b; Meharg and Jardine 2003; Williams et al. 2005; Liu et al. 2004, 2006). Among various strategies to reduce As accumulation, selection of rice cultivars with respect to Fe plaque formation (Chen et al. 1980; Greipsson 1995; Bacha and Hossner 1977; Zhang et al. 1998) is considered to be a feasible approach, as it is suggested that the more the Fe plaque formation on roots, the more will be the As retention in the form of AsV (Liu et al. 2004, 2006). However, Fe plaque formation leads to an enhancement in AsIII uptake and translocation to the shoot (Chen et al. 2005). Further, although AsV is the dominant As species in aerobic soils, AsIII prevails under anaerobic conditions present in rice fields (Tripathi et al. 2007; Smith et al. 2008). Recent studies have unfolded the mystery why rice is a potential accumulator of As and demonstrated that As (in the form of AsIII) follows similar uptake and transport mechanism as that of silica (Si) and As affects trace nutrients in rice (Ma et al. 2008; Williams et al. 2009a; Zhao et al. 2010). It is important to note that rice is one of the best known Si accumulators (Ma et al. 2002). Hence, the suitability of varietal selection on the basis of As sequestration related As contamination in grains and the level of mineral nutrients needs to be tested at the field level.
Another important point to consider is that due to the high adsorption capacity of functional groups on Fe hydroxides, Fe plaque may also sequester a number of other metals (zinc (Zn), Ni, Cu, and Pb) and metalloids (selenium (Se)) by adsorption or co-precipitation (Greipsson and Crowder 1992; Ye et al. 1998; Zhang et al. 1999; Batty et al. 2002; Liu et al. 2007). Therefore, selection of a suitable variety of rice with respect to Fe plaque formation and As accumulation should also take into account the accumulation profile of other essential trace metal nutrients. One such important consideration, for example, will be Se level, which is required as a micronutrient in humans and animals and has also been reported to detoxify As in rats, dogs, pigs, rabbits, and humans (Alfthan et al. 1991; Spallholz et al. 2004; Thomson 2004). The dietary requirement of Se (recommended minimum daily intake limit is 55 µg/day) in humans is mainly fulfilled by cereals, in which rice is one of the most commonly consumed cereals in many countries (Liu and Gu 2009). Although Asian cultivars of rice have been, in general, found to be good Se accumulators (Williams et al. 2009a), their grain trace nutrient quality decreased with increasing As content (Williams et al. 2009b). Phosphate uptake is known to be competitively inhibited by AsV (Abedin et al. 2002a), and thus an evaluation of phosphorus (P) levels was also considered worthwhile (Lu et al. 2010). The selection of Fe and Zn was done on the basis of their known importance in plant metabolism including active functioning of a number of enzymes and electron transfer reactions. These points strongly demands for an analysis of nutrient profiling in various rice genotypes differing in Fe plaque formation.
In this backdrop, a field trial was conducted in an As-contaminated area in West Bengal (India) using seven different rice varieties during Boro season. At harvest, plants were analyzed for plaque formation, plaque As sequestration, and specific As uptake (SAU) in plant parts (root, shoot, husk, and grain). Besides, the accumulation of other elements (Fe, P, Zn, and Se) was also analyzed in plaque and plant parts with a view to ascertain whether accumulation pattern of As shares any correlation with the profile of these elements. Various species of As were analyzed in seeds of the selected genotypes.
Materials and methods
Experimental site and growth conditions
A field trial was conducted at As-affected area of Chinsurah (latitude, 22°53′44″N; longitude, 88°24′9″E), Hooghly, West Bengal (India), during Boro season (2008). The seeds of seven rice varieties (IR-68144-127, IR-68144-120, CN1643-3, CN1646-2, IR-36, IR-64, and Gotrabhog (IET-19226)) were selected and cultivated in a randomized block design by following standard agronomic practices. Seedlings (25 days old) of selected cultivars were transplanted in a prepared plot at a spacing of 20 × 15 cm between rows and plants. The N, P, and K were supplied in the form of urea, single super phosphate (P2O5), and muriate of potash (K2O) at a rate of 100, 50, and 50 kg ha−1, respectively. Half of N fertilizer and full dose of P2O5 and K2O were applied as basal dose, whereas remaining half N fertilizer was applied as top dressing in two equal doses: first at the maximum tillering stage and second during panicle initiation stage. The paddy field was irrigated continuously with groundwater, and shallow level of submergence (6 ± 2 cm) was maintained throughout the growth period.
Crop harvest and sample preparation
Harvesting of rice plants was done after maturity. Plants were uprooted carefully from field, and roots were washed thoroughly with running tap water. After blotting, the plants were packed into polythene bags and brought to the laboratory for trace mineral analysis in various plant parts including grains. In lab, plants were divided into root, shoot, husk, and grains. After separation, roots were washed again with double distilled water followed by Milli-Q (thrice). In rice plants, Fe plaque formation significantly affects uptake of nutrients, and this role appears an important consideration for the development of practical approaches to reducing As accumulation. Washed rice roots (1 g) were treated with dithionite citrate bicarbonate (DCB) solution (Taylor and Crowder 1983) to know the level of minerals nutrients adsorbed on the plaque and their relation with As sequestration. DCB mixture (20 ml) contained 0.03 M sodium citrate, 0.125 M sodium bircarbonate, and 0.3 g sodium dithionite. DCB desorbed roots were oven dried at 70°C for 4 days and weighed for further analysis.
Digestion and quantification of trace nutrients and As in rice plants and soil
For the estimation of Fe, Zn, Se, and As in different parts of rice, 0.5 g oven-dried (at 70°C), grinded plant tissues were taken and digested in 3 ml of HNO3. For the estimation of various minerals, viz., Fe, Zn, Se, and As in soil, the analysis was performed after sieving (<2 mm) of powdered paddy soils, which was then oven dried at 70°C. Soil (0.2 g) digestion was done in HNO3: HF (1:1) at 120°C for 2 h and 140°C for 4 h (Lu et al. 2010), then filtered in 10 ml of Milli-Q water and stored at 4°C till the estimation. The metals and metalloids (Fe, Zn, Se, and As) were quantified with the help of inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7500 ce) at SGS India Pvt. Ltd, Gurgaon, Haryana. The P level in rice parts and soil, including DCB solution, was determined by colorimetric method (Jackson 1973). The pH and EC of soil were measured by ion meter (Orion, USA), while water-holding capacity was measured by hydrometery. The available N and total organic C were estimated by following Jackson (1973) and Carter and Gregorich (2007), respectively. SAU indicates the ability of total As uptake while specific Se uptake (SSU) for Se, and it was calculated according to Zhang and Duan (2008) with slight modification as given below.
Transfer factor (TF) for As was calculated as per the following formula:
Arsenic speciation
The oven-dried (80°C) grain powder was used for the analysis of different As species. The procedure of analysis was performed by following the protocol of Abedin et al. (2002b). The speciation was done on ICP-MS coupled with high-performance liquid chromatography (Agilent 7500 ce), and standard solutions were prepared fresh from stocks for calibration.
Quality control and quality assurance
The standard reference materials of metals (E-Merck, Germany) were used for the calibration and quality assurance for each analytical batch. Analytical data quality of metals was ensured with repeated analysis (n = 3) of quality control samples, and the results were found within (±2.82) the certified values. Recovery of Fe, Zn, and Se from the plant tissue was found to be more than 96.5%, as determined by spiking samples with a known amount of metal, while for As, rice flour NIST 1568a was used as a reference material with known spiked samples, and recovery of total As were 85.3% (±2.8; n = 5) and 89.5% (±3.1; n = 5), respectively. The detection limit for Fe and Zn were 1 and 0.2 mg l−1, respectively, while for As and Se, it was 1 µg l−1.
Statistical analysis
The field experiment was conducted following a randomized block design. Two-way analysis of variance and Duncan's multiple range test were performed with all the data. Correlation analysis was performed which has been given within text at relevant places (***p < 0.001, **p < 0.01, *p < 0.1; NS non-significant; Gomez and Gomez 1984).
Results
Physicochemical properties of paddy soil
The pH of selected paddy field soil was around neutral (pH 7.6). The levels of available nitrogen (87.77%) and water-holding capacity (0.54%) were high, while EC, porosity, and total organic carbon were 74.16%, 77.69%, and 0.69%, respectively. The paddy soils were high in Fe (48,326 mg kg−1 dw) and As (12.43 mg kg−1 dw) content. Se, Zn, and total P were 7.24, 93.52, and 448.4 mg kg−1 dw, respectively.
Iron plaque formation and sequestration of metals and metalloids
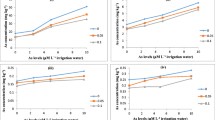
During the field trial, all selected cultivars showed reddish brown coating on the root surface, but the amount of DCB-Fe differed significantly among the genotypes (Fig. 1a). The Fe (mg kg−1 fw) adsorbed on the root surface was the maximum for IR-36 (25361) while the least for CN1643-3 (7847). Thus, the DCB-Fe order was IR-36 > IR68144-127 > IR68144-120 > CN1664-2 > Gotrabhog > IR-64 > CN1643-3. During the present study, a significant amount of P, Zn, Se, and As remained sequestered in the plaque, and their levels (mg kg−1 fw) differed significantly from one genotype to the other. DCB-P ranged from 59 to 121 in the selected cultivars (Fig. 1b), with the maximum level in CN1643-3 and the least in IR-36. It thus showed a negative correlation to DCB-Fe (*R = −0.661). DCB-Zn on the root surface was significantly correlated with the amount of Fe plaque (**R = 0.906; Fig. 1c), with the maximum being in IR68144-120 (610). The sequestration of both As (***R = 0.973) and Se (**R = 0.904) was also positively correlated with DCB-Fe. The highest Se was found in the plaque of IR68144-127 (5; Fig. 1d), whereas the maximum sequestration of As was found in IR68144-120 (72; Fig. 1e).
a–f Sequestration of Fe (a), P (b), Zn (c), Se (d), and As (e) on root surface during plaque formation and specific As uptake (SAU; e) of seven rice cultivars. All the values are mean of five replicates ± SD. Analysis of variance significant at p ≤ 0.01. Different letters indicate significant difference among rice genotypes (Duncan's multiple range test, p ≤ 0.05)
As accumulation and its relation with mineral elements in root and shoot
Roots were processed and analyzed for determination of nutrients (Fe, P, Zn, and Se; mg kg−1 dw) and As (mg kg−1 dw; Fig. 2a–e). IR68144-120 showed the maximum Fe accumulation (49622; Fig. 2a) but the least P accumulation (62; Fig. 2b). IR-64 showed the maximum accumulation of both P (1313) and Zn (1013; Fig. 2c) but the minimum accumulation of Fe (12858), Se (0.25; Fig. 2d), and As (23; Fig. 2e). The highest level of Se was recorded in roots of CN1646-2 (3) while the lowest in IR-64 (0.3). The concentration of As in roots differed among the genotypes and was two- to 23-fold higher than that observed in shoot. The maximum accumulation of As (Fig. 2e) in roots was found in CN1643-3 (155) and the least in IR-64 (22).
The translocation of metals from root to shoot differed among various genotypes and was correlated with the amount of As in the shoot (Fig. 3a–e). IR-36 (1548) represented the least Fe accumulation while the maximum was found in Gotrabhog (5911). The P content in shoot also differed significantly among selected genotypes (Fig. 3b). IR68146-120 (599) accumulated the maximum amount of Zn, while IR68144-127 (156) accumulated the least amount (Fig. 3c). The Se concentration in shoot of selected rice genotypes ranged between 0.2 and 1.2 (Fig. 3d). Due to sequestration of most of the As (15.5–72) in Fe plaque, only about 4–27% was translocated from root to shoot (Fig. 3e), and a significant variation was observed in selected rice cultivars. Gotrabhog (7) was found to accumulate the maximum amount of As (Fig. 3e), while CN1646-2 (2.5) accumulated the least amount.
a–f Accumulation of Fe (a), P (b), Zn (c), Se (d), and As (e) in shoot during plaque formation and As transfer factor (TF; f). All the values are mean of five replicates ± SD. Analysis of variance significant at p ≤ 0.01. Different letters indicate significant difference among rice genotypes (Duncan's multiple range test, p ≤ 0.05)
As accumulation and its relation with nutrients in husk and grain
Mineral nutrient level (mg kg−1 dw) in husk and grain also varied significantly among selected cultivars. IR-36 (2695) was found to accumulate the maximum amount of Fe (Fig. 4a), while the minimum content was found in CN1646-2 (974). Significant variation was observed in P content of these varieties that ranged from 261 to 364 (Fig. 4b). However, a lower range of variation in accumulation of Zn was recorded (118–175; Fig. 4c). Cultivar CN1643-3 accumulated the maximum amount of Se (0.8; Fig. 4d) in husk, while the lowest accumulation was observed in IR-64 (0.1). There was a significant variation in As content of the different varieties, and it was observed that about 2–7% (Fig. 4e) of the total As was translocated to husk. The maximum level of As was found in IR68144-120 (6; 7% translocation) followed by CN1643-3 (4.5; 3% translocation).
a–f Accumulation of Fe (a), P (b), Zn (c), Se (d), and As (e) in husk during plaque formation and specific Se uptake (SSU; f) of seven rice cultivars. All the values are mean of five replicates ± SD. Analysis of variance significant at p ≤ 0.01. Different letters indicate significant difference among rice genotypes (Duncan's multiple range test, p ≤ 0.05)
The Fe content in grain varied significantly among the different rice germplasms. Gotrabhog (174) accumulated the maximum amount, while IR68144-120 (62) accumulated the least amount (Fig. 5a). Both P and Zn content also varied significantly in different cultivars (Fig. 5b, c). Se accumulation ranged from 0.04 to 0.11 in different cultivars, with the minimum being in grains of Gotrabhog (0.04) and the maximum in CN1643-3 (0.11; Fig. 5d). The total As (Table 1) accumulation ranged from 0.4 to 1.7 with the highest accumulation in IR68144-127 (1.7) and the least in CN1646-2 (0.4). In general, the maximum amount of As was retained in roots (64.5–93%) followed by shoot (4–29%), husk (2–7%), and grains (0.65–3%) of total SAU. The maximum As retention was in the roots of CN1664-3 (93%), while the minimum was in Gotrabhog (64.5%), with only about 0.65% and 1.26% translocation to grains, respectively. The SSU of selected genotypes differ significantly, and it was maximum for CN1646-2 (5; Fig. 4f), while TF (Fig. 5f) showed different trend, and instead of CN1646-2 (35), CN1643-3 (90) showed maximum transfer of Se from root to shoot. The recovery of inorganic As species varies and ranges between 18.38% and 35.51% in all the genotypes.
a–f Accumulation of Fe (a), P (b), Zn (c), Se (d), and As (e) in grain during plaque formation and Se transfer factor (TF; f). All the values are mean of five replicates ± SD. Analysis of variance significant at p ≤ 0.01. Different letters indicate significant difference among rice genotypes (Duncan's multiple range test, p ≤ 0.05)
As speciation
The inorganic (AsIII and AsV) and organic (dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA)) As species (mg kg−1 dw) were analyzed in the grains of rice genotypes (Table 1). Results showed a very typical feature that DMA and MMA were absent in seeds, and only inorganic As was detected in all seven cultivars. The concentration of inorganic As was the least in IR-36 (0.125), while the maximum total inorganic As was found in the grains of IR68144-127 (0.413).
Discussion
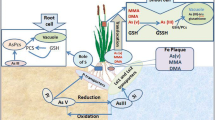
The formation of Fe plaque is considered to be a consequence of oxidation of Fe from ferrous (II) to ferric (III) and precipitation of Fe oxide on the root surface (Taylor et al. 1984; Liu et al. 2004). During the present study, rice cultivars showed Fe plaque formation in the form of reddish brown coating on the root surface, and the DCB-Fe significantly varied among the genotypes, which demonstrated that rice varieties differ significantly with respect to Fe plaque formation. This is concurrence to the previous study of Liu et al. (2006). Fe plaque is commonly formed on the rice roots due to release of oxygen and oxidants into the rhizosphere (Liu et al. 2006), and thus differential ability of rice genotypes in terms of oxygen evolution from roots leads to variable Fe plaque-forming ability and, subsequently, variable tendency to accumulate metals and metalloids.
Due to the high adsorption capacity of functional groups on Fe hydroxides, Fe plaque sequesters a number of metals and metalloids by adsorption or co-precipitation (Liu et al. 2007). In the present study, a number of elements were sequestered in the plaque in an order of Fe > Zn > P > As > Se. Other workers have also demonstrated that Fe plaque could adsorb P (Zhang et al. 1999; Batty et al. 2002), Zn, Pb, Ni, Cu (Greipsson and Crowder 1992; Ye et al. 1998), and Cd (Liu et al. 2007). Otte et al. (1989) reported that amount of Fe and Zn in the Fe plaque was positively correlated in Aster tripolium. The present study also demonstrated the positive correlation between Fe in the plaque and Zn sequestration in rice. Similarly, a positive correlation between amount of Fe plaque and Se adsorption was observed (Zhou and Shi 2007). The sequestration of As by Fe plaque on the root of rice (Liu et al. 2004; Chen et al. 2005), macrophytes (Taggart et al. 2009), and cattail (Blute et al. 2004) has been demonstrated. The adsorption of As by the Fe plaque may be an efficient strategy to reduce As contamination of rice grains. Since formation of Fe plaque varies among genotypes, a variety having significant Fe plaque formation and As adsorption on the root surface may thus be a suitable candidate for cultivation in As-contaminated regions. In the present study, the paddy field had around 12.5 mg kg−1 dw As. Recently, Norton et al. (2009) estimated the As level of two (Nonaghata (latitude, 23°42′N; longitude, 88°44′E) and De Ganga (latitude, 22°87′N; longitude, 88°76′E)) Indian field sites in As-affected area of West Bengal and found As levels of 6.3 and 14.9 mg kg−1 dw, respectively. During our trial, rice cultivars were grown in the same field, but As in DCB-Fe was significantly varied and high (up to 72 mg kg−1 fw), which might be due to the variation in Fe plaque thickness on the rice roots (Zhang et al. 1998). On the other hand, P showed a negative correlation (*R = −0.644) with DCB-Fe, probably due to a competition between As and P for binding to Fe plaque, and As presumably had a higher affinity than P that resulted in low P binding to the plaque (Wang et al. 2002).
The As (mg kg−1 dw) concentration in roots showed significant difference among the rice genotypes, which ranged from 23 to 155 and showed the following order: CN1646-3 (155) > IR68144-120 (74) > CN1646-2 (46.5) > IR68144-127 (46) > IR-36 (42) > Gotrabhog (27) > IR-64 (23). These findings are in contrast to the earlier observations of Liu et al. (2004) who found no significant difference in root As among cultivars. However, Zhang and Duan (2008) found significant genotypic difference in As uptake and translocation between hydroponically grown rice genotypes. High concentration of As and low concentration of P in rice roots indicate that As can competitively inhibit P uptake by roots (Zhang and Duan 2008) owing to the fact that AsV is a phosphate analogue and thus both compete for the same transporters (Meharg and Macnair 1992). However, Zhang et al. (1999) suggested that shoot P concentration of rice plants with Fe plaque was higher than those without plaque, but during the present field trial, the shoot P concentration of various tested genotypes decreased due to the increased concentration of As (Zhang and Duan 2008) barring two cultivars such as CN1643-3 and IR68144-120. Further, IR8144-120, IR68144-127, and IR-36 showed higher amount of DCB-Fe, thus it was possible that the thick coating of Fe plaque might become a barrier preventing P on root interface (Zhang et al. 1999) in these cultivars.
The Zn uptake by plants depends on the uptake capacity of root and Zn concentration in the medium (Howeler 1973). Fe plaque sequestered higher amount (346–610 mg kg−1 fw) of Zn on the root surface than that of the paddy soil (93.5 mg kg−1 dw). Zn uptake by plants with Fe plaque might be enhanced if plants could take up that Zn (Zhang et al. 1998). In the present study, the concentration of accumulated Zn was higher (625–1,013) than the Zn present in DCB-Fe, thus it has been suggested that Zn adsorption in Fe plaque represents a weaker binding mechanism than chemical binding, and plant roots can take up that Zn (Otte et al. 1989). During the present study, 0.3–5 mg kg−1 fw of Se (DCB-Se) was sequestered into the Fe plaque, and 37–74% was accumulated by roots, 13.5–33% by shoot, 9–26% by husk, and 2–11% by grains. Earlier, Zhou and Shi (2007) demonstrated that high Fe plaque formation resulted in more Se sequestration in the plaque and hence decreased Se concentration in above ground parts.
During the field trial, it was observed that As (mg kg−1 dw) translocation from root to shoot, husk, and grains decreased sequentially, and most of the As was accumulated in husk (1–6), and only about 0.5–1.7 was accumulated in grains; thus, a two- to 3.5-fold difference was observed in husk to grain As level, which was in accordance with previous reports. Rahman et al. (2008) reported that husk of BRRI hybrid dhan I contains 3.8-fold higher As than grains, while it was 3.4-fold higher for BRII dhan 28. Rice seeds used for human consumption are the main source of As exposure (Abedin et al. 2002b) causing serious health problems (Zavala and Duxbury 2008). Meharg and Rahman (2003) reported that grain As concentration reached above 1.7 mg kg−1 dw in some cultivars; however, the global normal range of As is 0.08–0.20 mg kg−1 dw. However, as per the maximum tolerable daily intake of As (2 μg kg−1 body weight per day), even the As level as low as 0.1 mg kg−1 dw may contribute to significant exposure to a person having rice-based subsistence diet (Williams et al. 2005). Although, Fe plaque restricted the entry of high amount of As to the plants, still As levels in the grains were considerably high in all the varieties in the present analysis. Further, As speciation plays an important role in contributing to toxicity caused by its accumulation. Speciation analysis of grains indicated that only inorganic species (AsV and AsIII) were present in the grains, while organic species (MMA and DMA) were absent, suggesting that rice plants presumably lacked the ability to methylate As. This is in contrast to previous reports showing the presence of organic As species, particularly DMA in rice grains (Williams et al. 2005). Norton et al. (2008) recently demonstrated an upregulation of potential gene involved in AsV methylation in rice. It has recently been suggested that as As level rise, US rice contains more methylated As, the less toxic form, whereas rice grown in Asia and Europe contains more toxic inorganic As (Zavala and Duxbury 2008; Williams et al. 2005). The concentration of total inorganic As (mg kg−1 dw) in grains varied significantly. IR68144-127 accumulated high amounts of both AsV (0.295) and AsIII (0.118), whereas IR-36 accumulated only AsIII (0.125), indicating genotypic characteristics of a particular rice cultivar. The absence of methylated species and presence of only inorganic As content in Bengal rice poses threat to the regional human population in Bengal delta, not because it is non-threshold class I carcinogen but also because rice is a staple diet in this region. Our results on grain As speciation revealed 18.38–35.51% recovery of inorganic As. Similarly, Abedin et al. (2002b) found less recovery of different As species using methanol extraction method.
Grain Se (mg kg−1 dw) content varied remarkably (0.04–0.11) revealing that different rice genotypes exhibited difference in Se accumulation and its translocation from root to shoot. Similarly, Zhang et al. (2006a, b) reported that Se content in brown rice grains was positively correlated with that in shoot. Further, Zhang et al. (2006a, b) reported the significant difference in Se accumulation in grains of two japonica rice cultivars. The Se levels detected in the present analysis were significantly low than that may be required to fulfill the recommended daily intake of Se of 55 μg/day from the rice-based diet. Even the highest grain Se-accumulating cultivar CN1643-3 (0.11 mg kg−1 dw) would not fulfill the Se requirement of a person consuming up to 450 g of rice as subsistence diet. Though, Williams et al. (2009a, b) reported higher level of Se in rice grain from India but low accumulation of Se in different plant parts, and its lower fractions in grains might be due to the decreased Se availability in soil in West Bengal. Kirk (2004) reported that under reduced conditions, Se is in insoluble form because of the thermodynamic stability of selenite (SeO 2−3 ) and selenide (Se2−). Thus, flooded condition (paddy habitat) appears to be an important factor for decreasing soil Se availability, which is the source to rice grains (Yadav et al. 2008). In general, As constrained the levels of Zn, P, and Se in different plant parts; however, a positive correlation was observed for As and Fe. Similarly, Williams et al. (2009a, b) reported that As affects the trace mineral (Se, Zn, and Ni) nutrition in rice grains.
In conclusion, results provide information regarding the different levels of SAU and SSU among rice cultivars and As transfer in rice plant parts. Results showed genotypic differences with respect to Fe plaque formation, As sequestration, and accumulation. Se level in all the rice genotypes were low, while As content was high. It is well demonstrated that Se is antagonist to As toxicity and carcinogenicity in mammalian models as evident through multiple mechanism (Zhu et al. 2009). Thus, low dietary intake of Se for those persons having rice-based diet may increase the risk of arsenicosis. Although, these cultivars are popularly grown in various Indian states, consumption of these rice cultivars might prove toxic when grown on As-contaminated soil and hence unsuitable for human consumption.
Abbreviations
- As:
-
Arsenic
- DCB:
-
Dithionite citrate bicarbonate
- DMA:
-
Dimethylarsinic acid
- GW:
-
Groundwater
- Fe:
-
Iron
- MMA:
-
Monomethylarsonic acid
- ND:
-
Not detectable
- P:
-
Phosphorus
- Se:
-
Selenium
- SAU:
-
Specific arsenic uptake
- SSU:
-
Specific selenium uptake
- TF:
-
Transfer factor
- Zn:
-
Zinc
References
Abedin MJ, Feldmann J, Meharg AA (2002a) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Abedin MJ, Cresser M, Meharg AA, Feldmann J, Cotter-Howells J (2002b) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Tech 36:962–968
Alfthan GAA, Arvilommi H, Huttunen JK (1991) Selenium metabolism and platelet glutathione peroxidase activity in healthy finnish men: effects of selenium yeast, selenite, and selenate. Am J Clin Nutr 53:120–125
Bacha RE, Hossner LR (1977) Characteristics of coatings formed on rice roots as affected by iron and manganese addition. Soil Sci Soc Am J 41:931–935
Batty LC, Baker AJM, Wheeler BD (2002) Aluminium and phosphate uptake by Phragmites australis: the role of Fe, Mn and Al root plaques. Ann Bot 89:443–449
Blute NK, Brabander DJ, Hemond HF, Sutton SR, Newville MG, Rivers ML (2004) Arsenic sequestration by ferric iron plaque on cattail roots. Environ Sci Technol 38:6074–6077
Carter MR, Gregorich EG (2007) Soil sampling and methods of analysis, 2nd edn. CRC Press, Taylor and Francis Group, Mortimer House, 37–41 Mortimer Street, London, W1T 3JH, p 264
Chen CC, Dickso JB, Turner FT (1980) Iron coating on rice roots: morphology and models of development. Soil Sci Soc Amer J 44:1113–1119
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Duxbury JM, Mayer AB, Lauren JG, Hassan N (2003) Food chain aspects of arsenic contamination in Bangladesh: effect on quality and productivity of rice. Environ Sci Health Part A 38:61–69
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Greipsson S (1995) Effect of iron plaque on roots of rice on growth of plants in excess zinc and accumulation of phosphorus in plants in excess copper or nickel. J Plant Nutr 18:1659–1665
Greipsson S, Crowder AA (1992) Amelioration of copper and nickel toxicity by iron plaque on roots of rice (Oryza sativa). Can J Bot 70:824–830
Howeler RH (1973) Iron-induced oranging disease of rice in relation to physic chemical change in a flooded oxisol. Soil Sci Soc Am Proc 37:898–903
Jackson ML (1973) Soil chemical analysis. Printice Hall of India Private Ltd., New Delhi
Kirk G (2004) The biochemistry of submerged soils. Wiley, Hoboken
Liu K, Gu Z (2009) Selenium accumulation in different brown rice cultivars and its distribution in fraction. J Agric Food Chem 57:675–700
Liu WJ, Zhu YG, Smith FA, Smith SE (2004) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55:1707–1713
Liu WJ, Zhu YG, Hu Y, Williams PN, Gualt AG, Meharg AA, Charnock JM, Smith FA (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol 40:5730–5736
Liu HJ, Zhang JL, Zhang FS (2007) Role of iron plaque in Cd uptake by and translocation within rice (Oryza sativa L.) seedlings grown in solution culture. Environ Experim Bot 59:314–320
Lu Y, Adomako EE, Solaiman ARM, Islam MR, Deacon C, Williams PN, Rahman GKMM, Meharg AA (2009) Baseline soil variation is a major factor in arsenic accumulation in Bengal delta paddy rice. Environ Sci Technol 43:1724–1729
Lu Y, Dong F, Deacon C, Chen H-J, Raab A, Meharg AA (2010) Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South China. Environ Pollut 158:1536–1541
Ma JF, Tamai K, Ichii M, Wu GF (2002) A rice mutant defective in Si uptake. Plant Physiol 130:2111–2117
Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao F-J (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105:9931–9935
Mandal NK, Biswas R (2004) A study on arsenical dermatosis in rural community of West Bengal. India J Public Health 48:30–33
Mazumder D (2003) Chronic arsenic toxicity: clinical features, epidemiology and treatment: experience in West Bengal. J Env Sci Health AA 38:141–163
Meharg AA, Jardine L (2003) Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol 157:39–44
Meharg AA, Macnair MR (1992) Suppression of the high-affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Mehrag AA, Rahman M (2003) Arsenic contamination of Bangladesh paddy soils: Implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice–arsenate interactions in hydroponics: whole genome transcriptional analysis. J Experem Bot 59:2267–2276
Norton GJ, Islam MR, Deacon CM, Zhao FJ, Stroud JL, Mcgrath SP, Islam S, Jahiruddin M, Feldmann J, Price AH, Meharg AA (2009) Identification of low inorganic and total grain arsenic rice cultivars from Bangladesh. Environ Sci Technol 43:6070–6075
Otte ML, Rozema J, Koster L, Haarsma MS, Broekman RA (1989) Iron plaque on roots of Aster tripolium L. intraction with zinc uptake. New Phytol 111:309–317
Rahman MA, Hasegawa H, Rahman MM, Miah MMA, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Smith E, Juhasz AL, Weber J, Naidu R (2008) Arsenic uptake and speciation in rice plants grow under green house conditions with arsenic contaminated irrigation water. Sci Total Environ 392:277–283
Spallholz JE, Mallory BL, Rahman MM (2004) Environmental hypothesis: is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bengladesh and West Bengal. India Sci Total Environ 323:21–32
Taggart MA, Mateo R, Charnock JM, Bahrami F, Green AJ, Meharg AA (2009) Arsenic rich iron plaque on macrophytes roots an ecotoxicology risk? Environ Pollut 157:945–954
Taylor GJ, Crowder AA (1983) Use of DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70:1254–1257
Taylor GJ, Crowder AA, Rodden R (1984) Formation and morphology of an iron plaque on the roots of Thyha latifolia L. grown in solution culture. Am J Bot 71:666–675
Thomson CD (2004) Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr 58:391–402
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Mathuis FJM (2007) Arsenic hazards; strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Tuli R, Chakrabarty D, Trivedi PK, Tripathi RD (2010) Recent advances in arsenic accumulation and metabolism in rice. Mol Breed. doi:10.1007/s11032-010-9412-6
Wang J, Zhao F-J, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulator in Chinese brake (Pteris vittata). Environ Pollut 124:223–230
Williams PN, Prince AH, Raab A, Hossain SA, Feldmann J, Meharg AA (2005) Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol 39:5531–5540
Williams PN, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman ARM, Rahman GKMM LuY, Deacon C, Zhu Y-G, Meharg AA (2009a) Arsenic limits trace mineral nutrition (selenium, zinc and nickel) in Bangladesh rice grain. Environ Sci Technol 43:8430–8436
Williams PN, Lombi E, Sun G-X, Scheckel K, Zhu YG, Feng X, Zhu J, Carey AM, Adomako E, Lawgali Y, Deacon C, Meharg AA (2009b) Selenium characterization in the global rice supply chain. Environ Sci Technol 43:6024–6030
Yadav SK, Singh I, Sharma A, Singh D (2008) Selenium status grains of northern districts of India. J Environ Manag 88:770–774
Ye ZH, Baker AJM, Wong MH, Willis AJ (1998) Zinc, lead and cadmium accumulation and tolerance in Typha latifolia as affected by iron plaque on the root surface. Aquat Bot 61:55–67
Zavala YJ, Duxbury JM (2008) Arsenic in rice: estimating normal levels of total arsenic in rice grains. Environ Sci Technol 42:3856–3860
Zhang J, Duan GL (2008) Genotypic difference in arsenic and cadmium accumulation by rice seedlings grown in hydroponics. J Plant Nut 31:2168–2182
Zhang XK, Zhang FS, Mao DR (1998) Effect of iron plaque outside roots on nutrients uptake by rice (Oryza sativa L.): zinc uptake by Fe-deficient rice. Plant Soil 202:33–39
Zhang XK, Zhang FS, Mao DR (1999) Effect of iron plaque outside roots on nutrients uptake by rice (Oryza sativa L.): phosphorus uptake. Plant Soil 209:187–192
Zhang LH, Shi WM, Wang XC (2006a) Difference in selenite absorption between high-and low-selenium rice cultivars and its mechanism. Plant Soil 282:183–193
Zhang LH, Shi WM, Wang XC (2006b) Difference in selenium accumulation in shoots of two rice cultivars. Pedosphare 16:646–653
Zhao F-J, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:7.1–7.25
Zhou XB, Shi WM (2007) Effect of root surface iron plaque on Se translocation and uptake by Fe-deficient rice. Pedosphere 17:580–587
Zhu YG, Elizabeth AH, Smits P, Zhao FJ, Williams PN, Meharg AA (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14:436–442
Acknowledgements
This work was supported by Network Project (NWP-19) of Council of Scientific and Industrial Research, Government of India. SD is grateful to SERC Division, Department of Science and Technology, New Delhi, India, for the award of Young Scientist. The authors are also thankful to the Director, Department of Agriculture, Government of West Bengal for providing the lab and field facility to conduct the field trial.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Dwivedi, S., Tripathi, R.D., Srivastava, S. et al. Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown on arsenic-contaminated soils of West Bengal. Protoplasma 245, 113–124 (2010). https://doi.org/10.1007/s00709-010-0151-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0151-7