Abstract
There is plenty of evidence for improved nutrient acquisition by ectomycorrhizas in trees; however, their role in water uptake is much less clear. In addition to experiments showing improved performance during drought by mycorrhizal plants, there are several studies showing reduced root hydraulic conductivity and reduced water uptake in mycorrhizal roots. The clearest direct mechanism for increased water uptake is the increased extension growth and absorbing surface area, particularly in fungal species with external mycelium of the long-distance exploration type. Some studies have found increased aquaporin function and, consequently, increased root hydraulic conductivity in ectomycorrhizal plants while other studies showed no effect of ectomycorrhizal associations on root water flow properties. The aquaporin function of the fungal hyphae is also likely to be important for the uptake of water by the ectomycorrhizal plant, but more work needs to be done in this area. The best-known indirect mechanism for mycorrhizal effects on water relations is improved nutrient status of the host. Others include altered carbohydrate assimilation via stomatal function, possibly mediated by changes in growth regulator balance; increased sink strength in mycorrhizal roots; antioxidant metabolism; and changes in osmotic adjustment. None of these possibilities has been sufficiently explored. The mycorrhizal structure may also reduce water movement because of different fine root architecture (thickness), cell wall hydrophobicity or the larger number of membranes that water has to cross on the way from the soil to the xylem. In future studies, pot experiments comparing mycorrhizal and nonmycorrhizal plants will still be useful in studying well-defined physiological details. However, the quantitative importance of ectomycorrhizas for tree water uptake and water relations can only be assessed by field studies using innovative approaches. Hydraulic redistribution can support nutrient uptake during prolonged dry periods. In large trees with deep root systems, it may turn out that the most important function of mycorrhizas during drought is to facilitate nutrient acquisition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has become customary to list improved water relations as one of the benefits of ectomycorrhizal symbiosis in general introductions to ectomycorrhizas. This habit is so widespread that, in the outcome of literature searches with key words ‘ectomycorrhiza and water’, a significant part of the papers simply state in the introduction that mycorrhizas are important for water uptake of trees. However, there is actually not much solid information to support this generalisation, particularly in large trees.
It has been shown that the external mycelium of ectomycorrhizal fungi transports water to the host plant (Duddridge et al. 1980), and it has been shown that water taken up by external mycelium can be sufficient to make a difference between the survival and death of a tree seedling (Boyd et al. 1986). It may account for at least some of the frequently observed enhancement of root hydraulic conductance (Muhsin and Zwiazek 2002a). Hence, the external mycelia function as extensions of the root systems in water uptake, as they do in phosphorus and nitrogen uptake (Smith and Read 2008), and the ectomycorrhizal symbiosis can be an advantage for water uptake of tree seedlings. However, an ectomycorrhizal plant is not only a small seedling. Many of these plants grow as large trees with a large canopy and with roots that extend several metres deep into the ground. The contribution of external hyphae to water transport in large trees still remains to be understood and quantified.
There are theoretical and experiment-based reasons to suggest that ectomycorrhizas may not be a major pathway for water uptake by their host plants. However, mycorrhizas may be critically important at times of stress. Read and Boyd (1986) suggested that the benefits of colonisation are likely to arise particularly under stress conditions, in analogue to mycorrhizal benefits in mineral nutrition. They suggested that mycorrhizas could be of benefit through their capacity to provide the minimum requirements for survival of the plant during episodes of drought. Now it has been shown that aquaporin (AQP) expression can be enhanced in ectomycorrhizal compared to nonmycorrhizal seedlings (Marjanović et al. 2005a), and this is suggested to be particularly important in water stress conditions (Marjanović et al. 2005b).
The host plants of ectomycorrhizal associations are perennial woody plants. They occupy very different niches in the world, and many of their habitats are characterised by strong seasonality. In dry regions, soil water is available only for the part of the year when it rains. In cold regions liable to freezing, liquid water is available only for the part of the year when the soil is not frozen. Even then, the rate of water uptake by plants is very low if the soil remains cold (Wan et al. 1999). Parts of the temperate region, where ectomycorrhizal trees are common, are characterised by adequate water for most of the year. However, in much of the temperate forest region, summer droughts are common. Ectomycorrhizal associations also occur in regions with abundant soil moisture such as tropical rainforests.
In this review, our aim is to present what has been achieved in the field of ectomycorrhizal plant water relations, to relate it to present achievements on general water relations studies and to point out priorities for further studies.
Plant water relations concepts
In most of the plant water relations studies, the potential presence of mycorrhizal associations and their effects on the processes of water uptake and transport have been ignored. For plants, the control of water relations is a challenging task even if soil water availability is not a limiting factor to water uptake. The maintenance of the soil–plant–air continuum requires that the rates of water loss and water transport through the plant be balanced by the rates of water uptake from the soil. Therefore, the plant must make a compromise between the photosynthetic CO2 uptake and transpirational H2O loss through the stomata. The effectiveness of this compromise is generally referred to as the water use efficiency (WUE) and it is measured either as the amount of dry matter or as the rate of net photosynthesis per unit of transpirational water loss (Jones 2004).
While osmotic forces contribute to the plant water uptake and slow water movement between the cells and tissues, hydrostatic forces produced by the transpirational water loss are usually the main driving forces for water transport in plants. According to the still widely accepted cohesion–tension theory, they are the only forces that are capable of delivering water up into considerable heights such as those of the tree crowns. The osmotic forces result from local differences in water potentials created largely by the gradients in concentrations of dissolved solutes (osmotic potentials).
The maintenance of the fine balance between water loss and water uptake is aided by the adjustments in tissue hydraulic resistance (inverse of conductance). The radial transport of water from the soil to the root xylem and the transport of water from the leaf xylem to the sites of transpiration have been intensively studied to understand the mechanisms of water flow regulation in these two main sites of hydraulic resistance in plants (Pratt et al. 2008). There is growing evidence that both ecto- and endomycorrhizal associations can drastically affect root hydraulic conductance but, so far, only no effects of ectomycorrhizas on leaf hydraulic conductance have been reported (Calvo-Polanco et al. 2008).
Before reaching the xylem, water passes several layers of root tissues. This radial flow of water can follow (1) the apoplastic pathway outside the protoplasts, through the cell walls and intercellular spaces, or the cell-to-cell pathway. The cell-to-cell water transport can take place through the (2) plasmodesmatal connections (symplastic pathway) or (3) across the plasma membranes (transmembrane or transcellular pathway) (Steudle and Peterson 1998). Aside from the effects of root apoplastic barriers, such as the endodermis, exodermis or suberised dermal tissues, little is understood about the regulation of apoplastic water flow and even less about the regulation of the symplastic pathway. Much of the dynamic regulation of root hydraulic conductance has been attributed to the transmembrane control of hydraulic conductance by the aquaporins.
Aquaporins belong to the ancient and abundant group of membrane intrinsic proteins, which constitute as much as 50% of the total proteins in some cell membranes (Karlsson et al. 2003; Maurel et al. 2008). Aquaporins have been found in bacteria as well as the plant, fungal and animal kingdoms (Zardoya 2005). The main subfamilies of AQPs in plants have been classified largely according to their location and include the plasma membrane intrinsic proteins, tonoplast intrinsic proteins as well as the nodule intrinsic proteins, discovered first in the root nodules of legumes, and small intrinsic proteins found mostly in the endomembranes (reviewed in Wudick et al. 2009). Each subfamily usually consists of several aquaporins that are expressed in the same plant species (Wudick et al. 2009). Aquaporins span the cell membranes producing water channels which can open and close to regulate water flow across the membrane. Although some of the aquaporins show high specificity for water, there are also those that can transport small neutral molecules including N compounds (Holm et al. 2005; Loqué et al. 2005; Kojima et al. 2006), boric acid (Dordas and Brown 2001), glycerol (Biela et al. 1999) and silicic acid (Ma et al. 2006). The posttranslational regulation of the aquaporin-mediated water transport (channel gating) includes protein phosphorylation/dephosphorylation events (Johansson et al. 1998), glycosylation (Vera-Estrella et al. 2004), divalent cations and cytoplasmic pH (Gerbeau et al. 2002), as well as a number of other internal and environmental factors (for a review, see Maurel et al. 2008). The rate of transmembrane water transport, and consequently root hydraulic conductance, can be also regulated by changing the abundance and types of aquaporins in the membranes (protein expression) (Voicu and Zwiazek 2004; Lovisolo et al. 2007).
The hydraulic properties of the whole or parts of the root system are commonly measured using the root pressure probe (Steudle 1993), pressure chamber (Wan and Zwiazek 1999) or high-pressure flow meter (Tyree et al. 1995) techniques and expressed as the root hydraulic conductance (water flux versus driving force) or root hydraulic conductivity (hydraulic conductance expressed on the root surface or volume bases). The cell-to-cell water transport can be directly measured with the cell pressure probe (Huesken et al. 1978). In this technique, a fine tip of a glass microcapillary is inserted into a cell and turgor pressure was measured using a pressure transducer. When the cell sap is pushed into or out of the cells with the pressure probe, rates of water transport out of or into the cells can be measured and converted to cell hydraulic conductivity (Steudle 1993). Cell pressure probe was successfully used to study turgor in fungal hyphae (Money 1990; Amir et al. 1995), but its use for studying water transport in mycorrhizal fungi has been hampered partly by the difficulties with the glass microcapillary insertion into a small-diameter hypha with tough cell walls.
Water uptake by external hyphae
Many, but not all, ectomycorrhizal fungi form an extensive network of hyphae into the surrounding soil. This network is considered to be a functional extension of plant roots (Read 1984), and it also forms functional connections between different plant individuals (Simard et al. 1997). Using tritiated water, Read and Malibari (1979) showed that the mycelial strands of Suillus bovinus took up water and transported it to the roots of Scots pine seedlings. Subsequently, Duddridge et al. (1980) measured the minimum rates of translocation of tritiated water from strands to pine shoots of 27 cm h−1, which is of the same order as the rates measured for transport in the xylem.
It appears that the farther the external mycelia extend from the mycorrhiza the more differentiated they are. In some fungal species, the external mycelium consists of individual hyphae, and the contact with soil or litter is made largely by the mantle (‘contact exploration types’; Agerer 2001). In the other extreme, ‘long-distance exploration type’, the mycelium is highly differentiated into strands (Agerer 2001). The strands may include larger ‘vessel’ hyphae of diameters of 6–20 μm, which generally lack cytoplasmic contents, surrounded by narrower, densely cytoplasmic hyphae often with thick cell walls (Duddridge et al. 1980). The central hyphae may lose their cross walls and thereby form large vessels (Brownlee et al. 1983). However, it is not certain if these large empty central vessels are important for translocation, as there is little evidence for a mechanism that would allow a sufficient solute accumulation, which would generate the necessary hydrostatic pressure for significant translocation (Cairney 2005). Furthermore, it is not certain how widespread these structures are in mycelia in forest soils (Cairney 2005). In any case, the formation of rhizomorphs shifts the zone of uptake farther from the mycorrhiza (Unestam and Sun 1995).
The fungus also has a need to transport carbohydrate along the mycelium towards the growing tips. There are two possibilities on how this may function: either spatial separation within the strands, in the same way as in the vascular structures of plants, or temporal separation, with water flow during the day (due to transpirational pull) and carbohydrate moving the opposite direction during the night (Read and Boyd 1986). Both functions took place simultaneously in the mycelium of Pisolithus tinctorius and S. bovinus mycorrhizas on Pinus spp. and Betula pendula, which supported spatial separation (Boyd 1987). Further evidence and mechanisms for the spatial separation of transport in mycelium have been reviewed by Cairney (1992, 2005).
The hydrophobic and hydrophilic properties of the cell walls of mycorrhizal fungi are thought to make a difference for the water transport properties of external mycelia (Unestam 1991; Unestam and Sun 1995). Hydrophilic fungi, such as many Laccaria, Lactarius, Russula and Hebeloma species, are thought to be able to transport water in the apoplast. By contrast, hydrophobic fungi, such as Paxillus involutus and Suillus species, form complex mycelial cords that are thought to transport water in the symplast. Only a small part of their mycelium is hydrophilic, with direct contact to soil water, and this part is responsible for the uptake of water and solutes. Comparisons of the eco-physiological properties of these different types of fungi, combined with actual measurements of the degree of hydrophobicity of the hyphal walls, have not been pursued much since Unestam and Sun published their studies. It would be very relevant to re-define the concepts of hydrophobic and hydrophilic ectomycorrhizal fungus more rigorously and more in relation to other studies on hydrophobicity (Linder et al. 2005). Particularly, comparisons between the water uptake and drought resistance between these different types of fungi would be important. It is quite possible that the hydrophobic and hydrophilic mycorrhizas both differ in survival from drought and their ability to take up water and, concomitantly, nutrients from drying soil. The importance of hydrophobicity has been indicated also in relation to other properties of mycorrhizas, such as heavy metal tolerance (Jentschke and Godbold 2000) and freezing tolerance (Lehto et al. 2008). The mycelia of the ‘contact exploration type’ of Agerer are mostly hydrophilic whilst the ‘long-distance exploration type’ corresponds to the most hydrophobic fungi, with others being intermediate (Agerer 2001).
The increased water uptake by hyphae may not manifest when the soil is near saturation and large pores are filled with water as the root surfaces are also in contact with water. However, as the soil dries and water is retained only in smaller pores where fungal hyphae can grow, but roots cannot, the water uptake function of hyphae becomes more significant for survival (Allen 2007). Boyd et al. (1986) and Read and Boyd (1986) showed in seedlings (B. pendula, Pinus sylvestris) with root systems in dry soil but with the mycorrhizal hyphae extending to moist soil that severing the hyphae rapidly led to the reduction of transpiration and photosynthesis, and the plants died of dehydration. In the same types of experiments, Brownlee et al. (1983) found that Scots pine seedlings remained in an apparently healthy condition for more than 10 weeks with mycelial strands in moist peat as the only source of water. Hence the mycelial water uptake can make a difference between life and death of a small seedling.
Until now, there has been no work done to investigate the importance of aquaporins in water uptake and transport in hyphae of mycorrhizal fungi. The interesting question that also remains unanswered is the contribution of the aquaporin-mediated fungal water transport into the host plant. Aquaporins have been reported from yeast and filamentous fungi, four Ascomycete mould and pathogen species plus Ustilago maydis, a Basidiomycete pathogen (Pettersson et al. 2005), and the AM fungus Glomus intraradices (Aroca et al. 2009).
Mycorrhizal roots and their external mycelium may be less inclined to shrink and thereby form gaps between soil surfaces and absorbing surfaces (Dosskey and Ballard 1980). Similarly, Boyd (1987) suggested that the improved contact between the hyphae and soil particles increased the potential for mycorrhizal water uptake. An evidence for this was found by Bogeat-Triboulot et al. (2004), as Pinus pinaster plants with extensive colonisation by Hebeloma cylindrosporum had a higher root hydraulic conductance compared to other fungi in sand dunes, where H. cylindrosporum also increased the contact of soil and roots more than other fungi; however, this effect was smaller in a drought treatment. It would be worth testing the hypothesis of better contact during droughts in ectomycorrhizal plants also in less extreme environments.
It should also be noted that mycorrhizal mycelium and fine roots do not only grow in soil, but they are part of the soil matrix both alive and after death. Arbuscular mycorrhizal mycelium has been shown to affect soil moisture retention curves and soil particle aggregation (Augé 2004), and there is no doubt that ectomycorrhizal mycelium also influences soil properties strongly.
The pathway of water in mycorrhizal roots
The mycorrhizal structure can affect the pathway of water both within the soil towards the absorbing surface and within the mycorrhizal root. When water is taken up from unsaturated soil, water potential gradients tend to form around absorbing surfaces as water is depleted around them. Mycorrhizal structure generally increases the absorbing surface of the root system (except in experiments in small pots), and therefore it could be assumed that the water uptake rate per unit surface area would be reduced. This would prevent steep gradients of soil water content from occurring around individual absorbing surfaces (Reid 1979). However, if the soil is moist and there are no steep water potential gradients in soil around the mycorrhizal root, the pathway within the mycelium may not be of higher conductance than that of the soil (Oertli 1991). Water may even flow on the surface of mycelium in soil (Allen 2007). Hence, the mycorrhizal benefit depends on soil moisture and moisture gradients, which vary with precipitation, evaporation, soil water conductance and competition for water.
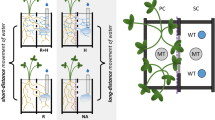
The possible pathways for the transport of water from soil to the stele are the same in mycorrhizal fungi and mycorrhizas as in roots, namely, the apoplastic and cell-to-cell (symplastic or transmembrane) pathways (Figs. 1 and 2). The contributions of cell-to-cell and apoplastic pathways can vary in the root cortex (Steudle and Peterson 1998). The conductance of the apoplastic pathway depends largely on the conductance of the cell walls, and in ectomycorrhizal roots the properties of the root apoplast are modified by the presence of hyphae. There are differences between symbioses in the degree of modification of cell walls of the host and fungus (Smith and Read 1997), but at least in some cases the intercellular space in the Hartig net area does not consist of distinct cell walls of mycelium and host but a communal matrix (Nylund 1987).
Possible pathways for water transport between soil and root xylem in an ectomycorrhizal root of a gymnosperm. FCW, fungal cell wall; PCW, plant cell wall; PM, plant plasma membrane; P, plasmodesma; CB, Casparian band; E, endodermis; C, cortex/Hartig net area; M, mantle; S, strand; H, hypha. Hyphal membrane is not shown separately. Yellow matrix is filled by hyphae and their cell walls. Model pathways for water: (1) Water enters an individual hypha in soil and is transported within the fungal symplast then moves to root symplast through the shared apoplast; (2) same as in (1) except that water enters the symplast of the hypha in the mantle; (3) water enters the apoplast of a mycelial strand and is transported in the fungal and plant apoplast then enters root cell; (4) transmembrane pathway, water passes through several fungal and plant membranes then in the plant symplast; (5) water travels in the fungal and plant apoplast down to the endodermis where the Casparian band forces it into the symplast. Diagram: Pedro Aphalo and Tarja Lehto
The pathway of water in mycelial strands was considered apoplastic by Duddridge et al. (1980), and Muhsin and Zwiazek (2002a) concluded that the increased root hydraulic conductance in ectomycorrhizal Ulmus americanus was due to apoplastic rather than cell-to-cell transport.
The low conductance of roots compared to other parts of the pathway of water within plants is largely due to the presence of apoplastic barriers in the roots. Sands and Theodorou (1978) interpreted their result of lower leaf water potential in mycorrhizal plants as a consequence of different root geometry since the mycorrhizal roots have a larger outer surface area compared to the Casparian band area. The structure of mycorrhizal and nonmycorrhizal seedling root systems is fundamentally different, with the mycorrhizal systems being more branched, having thicker short roots and a variable amount of external mycelium. Therefore, it is not straightforward to compare the hydraulic properties of mycorrhizal and nonmycorrhizal plants. The issue of the differences in root structures between mycorrhizal and nonmycorrhizal roots needs to be further understood as it may affect root water flow properties.
Symplastic transport in hyphae is also possible, and symplastic movement within the hyphae themselves may provide an additional important pathway for water movement in the root cortex of the colonised roots (Reid 1979). Water must move in the fungal symplast in the case of individual external hyphae taking up water from soil. Individual hyphae are not necessarily of higher conductance to water than soil is, in analogue with root hairs (Weatherley 1982). Therefore, the symplastic pathway of water across the cortex could be more arduous in mycorrhizas: in addition to the membranes of the host cells, the water would have to pass through the hyphal membrane as there is no symplastic connection between fungal cells and host root cells. This pathway would be of lower conductance whether it was compared to water moving in the apoplast or symplast of the root cells. Weatherley (1982) calculated that water moving in the transmembrane pathway, or crossing the membrane on entering and leaving each cell, would encounter a drop of water potential of 0.1 to 1.0 MPa per membrane. This might explain the lower shoot water potential found in mycorrhizal plants compared to nonmycorrhizal ones in several studies (Sands and Theodorou 1978; Parke et al. 1983; Dosskey et al. 1991; Lehto 1992b). This concept also agrees with the finding of Duddridge et al. (1980) that radioactivity from tritiated water fed to mycelial strands accumulated in the mantle region possibly because of a decrease in conductivity compared to strands. However, aquaporin function can change the membrane permeability to water.
Mycorrhizas can affect the cell-to-cell pathway at least through effects on plant aquaporin expression, although aquaporins in ectomycorrhizal fungi themselves still remain to be demonstrated. Experiments on hybrid poplar (Populus tremula × Populus tremuloides) seedlings inoculated with Amanita muscaria clearly demonstrated a link between the enhanced expression of root aquaporins and increased root hydraulic conductivity in ectomycorrhizal seedlings (Marjanović et al. 2005a). Also, a several-fold increase in the root hydraulic conductivity of jack pine (Pinus banksiana) seedlings inoculated with ectomycorrhizal Suillus tomentosus was correlated with a similar increase in the hydraulic conductivity of root cortical cells. This increase in the cell-to-cell water transport across root cortex was attributed to the aquaporin-mediated transport (Lee et al. 2010). The maintenance of the aquaporin-mediated root water transport may be especially important for plants growing under stress conditions which affect the rate of water transport such as mild drought, salt stress or low soil temperature (Lee et al. 2008; Marulanda et al. 2010; Sade et al. 2009, 2010).
It appears that the chemical signals originating in plants colonised by the mycorrhizal fungus may, at least in some cases, affect the root hydraulic conductivity more than the fungal penetration of the root cortex. Siemens and Zwiazek (2008) compared the root hydraulic conductivity of balsam poplar (Populus balsamifera) colonised by the ectomycorrhizal Hebeloma crustuliniforme with that of the ectendomycorrhizal Wilcoxina mikolae. As opposed to H. crustuliniforme which showed poor penetration of the root cortex and no presence of Hartig net, W. mikolae formed a well-developed Hartig net deep in the cortex and penetrated root cortical cells. However, root hydraulic conductivity was enhanced by the presence of H. crustuliniforme and not by W. mikolae (Siemens and Zwiazek 2008).
There are still many fundamental questions concerning the pathways of water in ectomycorrhizas which as yet have not received much attention. These include examining the lower conductance to water in ectomycorrhizal plants in some conditions, as well as the location of lowest conductance. As many aspects concerning the pathway of water across the cortex and root conductance to water are still not fully understood, it is conceivable that the study of mycorrhizal and nonmycorrhizal root systems could contribute towards our general knowledge of water uptake and the role of aquaporins in different organisms.
Water relations in ectomycorrhizal vs. nonmycorrhizal seedlings
Most of the published work on mycorrhizal water relations report comparisons between mycorrhizal and nonmycorrhizal tree seedlings grown in pots. Mycorrhizas have improved seedling water relations in many studies. Dixon et al. (1980) reported higher water potentials in both irrigated and drought-stressed Quercus alba with P. tinctorius, although the effect was less clear during drought. In this study, the mycorrhizal plants had larger root systems, and their root extension rates remained higher. Boyd (1987) also found that P. tinctorius increased the transpiration rates and water potentials and thereby the soil–plant conductance to water in B. pendula plants exposed to moderate water stress. As the plants were of comparable size and their shoot P concentrations were not different, a possible explanation was that the increased conductance was caused by the fungal mycelium exploiting the soil volume more efficiently. Mycorrhizas can also induce a higher root/shoot ratio in seedlings, which then leads to sustained water uptake and transpiration during drought even in the absence of nutritional effects (Davies et al. 1996). Larger root systems in inoculated plants were associated with higher CO2 assimilation rates per plant and increased WUE in Pinus pinea (Guehl et al. 1990).
As plant nutrient status is known to influence photosynthesis and transpiration, many of the mycorrhiza effects are likely to be at least partly mediated by improved mineral nutrition. Parke et al. (1983) found ten times higher CO2 assimilation rate in mycorrhizal than nonmycorrhizal Douglas fir (Pseudotsuga menziesii) seedlings in a drought experiment. In white spruce (Picea glauca) colonised with H. crustuliniforme, root hydraulic conductance was about four times higher compared with nonmycorrhizal seedlings and, although it decreased, it remained higher after severing the external hyphae. These mycorrhizal plants had also higher tissue N and P concentrations (Muhsin and Zwiazek 2002b). Beniwal et al. (2010) showed that the mycorrhizal poplar seedlings kept their photosynthesis up during drought, while it was reduced to nil in nonmycorrhizal seedlings.
In contrast, other studies have found little or no effect of ectomycorrhizas on root water transport and/or plant water relations. Sands et al. (1982) measured the hydraulic conductance of mycorrhizal and nonmycorrhizal Pinus taeda roots and found no effect of mycorrhizal infection. Similarly, Diebolt and Mudge (1987) found no difference in the transpiration and predawn water potential of P. tinctorius mycorrhizal and nonmycorrhizal Scots pine (P. sylvestris), and Coleman et al. (1990) found no positive effect of mycorrhizal colonisation on root hydraulic conductance in Douglas fir (P. menziesii). In experiments on mycorrhizal (P. involutus) and nonmycorrhizal Sitka spruce (Picea sitchensis), mycorrhizas greatly increased the stomatal conductance, photosynthesis, shoot water potential and growth both in well-watered conditions and particularly during drought when mycorrhizal plants were larger and had higher P and K concentrations (Lehto 1992a). However, when mycorrhizal and nonmycorrhizal seedlings were grown to similar size and nutrient concentrations, the mycorrhizal plants did not have any advantage over the nonmycorrhizal plants but a lower shoot water potential (Lehto 1992b). Hence, eliminating the nutrient effect in the same host–fungus combination eliminated any positive effect of the fungus. In these experiments, the extension of external mycelia was prevented by the pot.
The impact of mycorrhizal inoculation on root water flow properties may also be negative. Sands and Theodorou (1978) found no effect of ectomycorrhizal inoculation with Rhizopogon luteolus on the water relations of the radiata pine (Pinus radiata) seedlings when the seedlings were watered, but during drought, the leaf water potentials were lower (more negative) in mycorrhizal plants. Coleman et al. (1987) found a lower root hydraulic conductance in the mycorrhizal seedlings, even though the mycorrhizal seedlings had higher root P concentrations, and P fertilisation as such increased root conductance. Nardini et al. (2000) reported a greater than twofold decrease in the root hydraulic conductance of Quercus ilex inoculated with ectomycorrhizal Tuber melanosporum when compared to noninoculated seedlings. Similarly, H. crustuliniforme significantly decreased the root hydraulic conductance in American elm seedlings, but only if they were growing in well-aerated soil, as opposed to compacted (Calvo-Polanco et al. 2008).
The physical properties of water and the water relations of plants are tied with temperature. At low soil temperatures, water uptake is inhibited by the direct physical effect of temperature on the viscosity of water and reduced plasma membrane permeability. However, aquaporin function can increase membrane permeability particularly at low temperatures. In transgenic Arabidopsis plants overexpressing plasma membrane aquaporins, transmembrane water uptake was not reduced at low substrate temperature to the same extent as the wild type (S.H. Lee and J.J. Zwiazek, unpublished data). As ectomycorrhizal colonisation increased cellular water uptake in jack pine in a comparable manner to the aquaporin overexpression (Lee et al. 2010), it will be a next step in these studies to test the role of temperature on water uptake in mycorrhizal and nonmycorrhizal plants. This study will help determine whether mycorrhizal plants have an ability to keep their water uptake up also at low temperatures and whether this effect is mediated by aquaporins or possibly also through the control of stomata.
The increased hydraulic conductance of ectomycorrhizal roots has been proposed to be a significant factor contributing to the successful growth of trembling aspen trees (P. tremuloides) in cold soils (Landhäusser et al. 2002). In P. tremuloides, inoculation with ectomycorrhizal H. crustuliniforme significantly increased root hydraulic conductance when grown under the 4°C and 8°C soil temperatures. However, in both P. tremuloides and Betula papyrifera, inoculation with H. crustuliniforme and L. bicolor had no effect on root hydraulic conductance of plants grown at higher (20/15°C day/night) temperatures (Yi et al. 2008). The effect of ectomycorrhizas on root water flow properties have been also reported to be influenced by the soil pH (Calvo-Polanco et al. 2009) and soil compaction (Calvo-Polanco et al. 2008). The role of root aquaporins in the responses of plants to ectomycorrhizal colonisation may be further confounded by other biotic factors which may also affect the plants (Dowd et al. 2004). These processes could perhaps provide an important insight that is required to understand why the presence of mycorrhizas does not always translate into improved plant water relations.
In addition to different environmental conditions, different host plant–fungus combinations can show drastically different results from similar experiments. Marjanović (2004) demonstrated that, in contrast to the hybrid poplar (Marjanović et al. 2005a, b), colonisation of Norway spruce (Picea abies) roots with A. muscaria had no effect on the expression of major root aquaporins. Our subsequent measurement of root hydraulic conductivity in these plants showed no differences in root hydraulic conductivity between the mycorrhizal and nonmycorrhizal seedlings (Ž. Marjanović, M.M. Calvo-Polanco, J.J. Zwiazek, N. Uehlein, R. Kaldenhoff and U. Nehls, unpublished manuscript).
In conclusion, there are many reports of positive mycorrhizal effects on seedling water uptake and seedling water relations during drought. However, there are also many publications on those with no effects or negative effects of mycorrhizal colonisation, and it cannot be argued that ectomycorrhizas would always have a beneficial role in water uptake and water relations. Furthermore, publication bias and referencing bias are likely to have affected the publicity of unfavourable mycorrhizal results. Negative effects and no effects are more likely to remain unpublished (either because they are not offered to journals by the scientists or because they are rejected by referees and journals) or published in less-known series or conference abstracts (Kotze et al. 2004). Other authors are likely to quote papers with clearly positive effects (Kotze et al. 2004) or possibly also emphasise the only fungal species out of several that showed a positive effect.
Mechanisms of mycorrhizal effects on plant water uptake and drought resistance
The most-studied and confirmed direct mechanisms for mycorrhizal benefit for seedlings are increased extension of root systems and direct hyphal water uptake during drought by those species that have extensive mycelial systems (Fig. 3). This effect is often inadvertently excluded in experiments in small pots, but as a result of this exclusion other mycorrhizal effects – or lack of other mycorrhizal effects – have been elucidated. The increased amount of absorbing surface directly increases root system conductance unless there is a decrease in conductivity (conductance per unit root or fungus). However, many ectomycorrhizal fungi do not form extensive mycelial structures.
Direct and indirect effects of ectomycorrhizal colonisation on plant water uptake and water relations. In the interest of clarity, some possible effects and interactions are not shown. + indicates a direct positive effect of mycorrhiza, − indicates a direct negative effect, ? indicates that there are no studies published that would show the effect
Enhanced root hydraulic conductance can result in higher stomatal conductance and hence potentially increased carbon assimilation under stress conditions. Larger root systems in mycorrhizas have a higher conductance, which then leads to sustained water uptake and transpiration during drought even in the absence of nutritional effects (Guehl et al. 1990, Davies et al. 1996). However, in another study, root hydraulic conductance was reduced in inoculated as opposed to noninoculated seedlings, even though the inoculated plants had much larger root systems (Nardini et al. 2000).
As discussed above, ectomycorrhizas may increase or decrease the conductivity of an apoplastic route, and lower conductivity may be due to root architecture, cell wall hydrophobicity and the additional need for crossing membranes in mycorrhizal roots. However, for crossing membranes on a cell-to-cell route, the direct benefits of ectomycorrhizal colonisation on root aquaporin function may be important. The aquaporin expression effect on increasing root hydraulic conductivity is established, but much more work is needed to understand why root aquaporins are not always affected by mycorrhiza formation and what the role of the aquaporins of the fungal partner is. If aquaporins are not upregulated, the colonisation may decrease the hydraulic conductance on a symplastic route.
It has been assumed earlier that mycorrhizal structure would affect mainly the soil-to-xylem conductivity; however, Beniwal et al. (2010) showed a smaller number but larger diameter of vessels in stems of ectomycorrhizal compared to noninoculated poplar seedlings after a drought treatment. These results encourage more attention to the whole-plant structure in mycorrhizal vs. nonmycorrhizal plants.
The most obvious indirect way for mycorrhizas to improve water relations is through efficient nutrient uptake. Adequate N nutrition is known to increase the efficiency of the photosynthetic machinery, which will increase WUE if transpiration remains the same. Increased WUE can also follow from improved P nutrition (Guehl and Garbaye 1990). In concert, lowered root hydraulic conductance has been found as a result of N and P deficiency (Radin and Boyer 1982; Radin and Eidenbock 1984; Syvertsen and Graham 1985; Andersen et al. 1989; Coleman et al. 1990). In wheat plants, P and N deprivation reduced root hydraulic conductivity by inhibiting the aquaporin-mediated water transport (Carvajal et al. 1996). However, the nutritional effects on root hydraulic conductivity are not always clear, and there are reports on the inhibitory effects of ammonium (Adler et al. 1996) and nitrate (Glosser et al. 2007) or both (Gao et al. 2010) on root hydraulic conductivity. These inhibitory effects were also thought to be mediated through the aquaporins (Glosser et al. 2007; Gao et al. 2010).
Root and mycelial growth are the strong sinks of photosynthate, and Dosskey et al. (1991) suggested that the sink effect of the vigorous mycelium of Rhizopogon vinicolor was the reason for the lower water potential in inoculated Douglas fir, as opposed to Hebeloma and Laccaria. The sink effect would induce the stomata to remain open for sustained photosynthesis. This might cause a short-term benefit for the fungus at the expense of drying of the whole system in case the drought continues. Nevertheless, maintaining photosynthesis and carbohydrate flow from aboveground parts to roots and mycorrhizas is necessary for continued water and nutrient uptake (Shi et al. 2002) in tree species which are not adapted to summer dormancy.
As with the effects on root water flow, it is still unresolved whether ectomycorrhizas influence the components of water potential or osmotic adjustment in shoots or roots despite many hypotheses involving this effect. Lowered shoot water potential or lowered osmotic pressure as such cannot be considered an advantage as it does not necessarily lead to increased osmotic adjustment (Tyree and Jarvis 1982). Shi et al. (2002) showed that most of the trehalose in beech (Fagus sylvatica) mycorrhizas disappeared in a drought treatment, and mannitol and arabitol concentrations increased. This may be a sign of osmotic adjustment to drought in these mycorrhizas as the splitting of the disaccharide trehalose into two molecules would increase the osmotic potential of solutions. Beniwal et al. (2010) found increased soluble carbohydrate concentrations in root systems of droughted compared to well-watered poplar seedlings; however, the increase was larger in nonmycorrhizal plants which suffered more from water stress. By contrast, no mycorrhizal effect was found in direct determinations of osmotic adjustment (Pallardy et al. 1983; Davies et al. 1996). Trehalose is considered as a stress protectant, also in drought stress, its role being the protection of membranes and proteins (Crowe 2007). However, in mycorrhizal beech roots, trehalose apparently did not have this role (Shi et al. 2002).
Other less-studied mechanisms include growth regulator signals for inducing stomatal closure and changes in the antioxidant system in response to drought-induced increase in reactive oxygen species (ROS). These are fields of study that need more attention in future work on ectomycorrhizal water relations. Signals by zeatin or abscisic acid were not clearly related to differences in root hydraulic conductance between differentially mycorrhizal plants (Coleman et al. 1990). Stomatal conductance is known to decrease also at low soil temperatures, leading to reduced water flow, even if the air temperature is favourable (Wan et al. 1999; Lahti et al. 2002). It is not clear if this is caused only by the reduced ability of roots to take up water or if there is an additional chemical signal from the roots to shoots, possibly abscisic acid (Aphalo et al. 2006), and whether mycorrhizas have a role in such signalling. There is a possible linkage between abscisic acid and ascorbate production in roots (Haberer et al. 2008), and antioxidants as such protect tree roots from drought-induced increase in ROS (Shvaleva et al. 2006). The metabolism of antioxidative enzymes in ectomycorrhizas has been little studied so far, but Alvarez et al. (2009a) showed that the mycorrhizal roots of Nothofagus dombeyi had greater antioxidant enzyme activities than nonmycorrhizal both in non-stress conditions and particularly during water stress, which apparently contributed to the drought resistance of the mycorrhizal plants. The mycorrhizal plants in this study were larger and had higher nutrient concentrations. The expression of the antioxidant systems was greater in mycorrhiza also compared to the mycorrhizal fungi in pure culture (Alvarez et al. 2009a). One of the possible mechanisms for the connection between antioxidants and drought resistance is by aquaporin function, as ROS may inhibit aquaporin function which leads to reduced root hydraulic conductance (Maurel et al. 2009). Further studies into these physiological relationships in mycorrhizal plants are necessary.
The question about mycorrhizal and nonmycorrhizal plants does not have much ecological relevance for species that are regularly 100% ectomycorrhizal in the field, like many conifers. However, it has relevance for tree species that do not always have a high degree of ectomycorrhizal colonisation, such as Eucalyptus species, and it has relevance to the question of whether mycorrhizas are always only beneficial to their host plants. It has practical significance to nursery management because nursery stock is in some cases poorly mycorrhizal, or mycorrhizal with ‘contact exploration types’, which may not be able to form contact with soil quickly.
Survival and recovery from drought episodes
Water stress affects plants in multiple ways, and the time scales for different processes vary. Physiological reactions, such as changes in stomatal conductance, root conductance and aquaporin expression, occur already in short-term experiments, but they can be further acclimated during various drying cycles. Osmotic adjustment may not be expressed in the first drying and rewetting cycle. Changes in nutrient uptake, sustained photosynthesis, growth, growth allocation and short root and mycorrhiza formation are all processes that take more than a few days to occur in the slowly growing ectomycorrhizal species. Many experiments on ectomycorrhizal water relations, particularly on seedlings in pots, have been on a short term and included only one drought cycle and only rarely a recovery period; here we summarise the results of more long-term studies.
Dixon et al. (1980) reported a faster recovery of water potentials and leaf conductances to pre-stress values in mycorrhizal than nonmycorrhizal seedlings. Parke et al. (1983) also found a better performance of R. vinicolor-inoculated Douglas fir (P. menziesii) compared to noninoculated and mycorrhizal with other fungi in a drying and rewatering experiment; however, the mechanism was water-saving by reduced transpiration during drought and quick recovery after rewatering. In another experiment, mycorrhizal plants had lower water potential but they recovered sooner (Parke et al. 1983). Dixon et al. (1980) referred to studies by Shemakhanova, indicating the improved recovery of ectomycorrhizal oak after irrigation in a dry environment. Moreover, in a field experiment with black oak (Quercus velutina) inoculated with P. tinctorius, the mycorrhizal plants had higher water potentials and higher soil-to-plant conductance than nonmycorrhizal ones consistently over the growing season (Dixon et al. 1983), and Ortega et al. (2004) found a better performance of radiata pine inoculated with Rhizopogon roseolus and Scleroderma citrinum particularly in a dry site. In these studies, the root and hyphal extension was a likely mechanism for the improved performance, but in long-term experiments the mycorrhizal effects are probably a sum of different factors.
While the mycorrhizal fungi may survive relatively low water potentials (Mexal and Reid 1973; Coleman et al. 1989), this does not necessarily enhance the survival of the mycorrhiza. If the water potential of the mycorrhizal fungus is lower than that of the root, the direction of the water flow would be out of the root towards the fungus, as Reid (1979) pointed out. This can be advantageous for the survival of the mycelium and for nutrient uptake if water is available through hydraulic redistribution (see below), but not if it leads to the dehydration of the system. The survival of mycorrhizas and roots is a question of both partners of the symbiosis surviving. However, if mycorrhizal fungi survive lower water potentials than fine roots, the mycorrhizal fungus may still remain alive even if the root dies. Mycelium surviving in this way may ensure that there is inoculum for new root growth when the moisture conditions improve again. This is a testable hypothesis.
Nutrient uptake by ectomycorrhizas during water stress conditions has been studied surprisingly little. In a direct tracer study, Reid and Bowen (1979) did not find a mycorrhizal improvement on P uptake in excised roots during drought. However, Wu et al. (1999) showed that mycorrhizal hyphae were able to take up N from an isolated compartment during drought and thereby promoted plant resistance to water stress.
As drought usually reduces plant growth, nutrient concentrations increase if nutrient uptake is reduced less than other processes. This tends to be the result of short-term studies with a quick induction of drought (Lehto 1992a). However, in pot experiments, the importance of the external mycelium in accessing poorly mobile nutrients in soil is usually excluded, and the long-term effects on nutrient acquisition during drought are also difficult to study in pots. In a beech (F. sylvatica) pot experiment (with no mycorrhiza treatment), a 3-week drought reduced P concentrations, presumably because of the poor mobility of P in drying soil (Peuke and Rennenberg 2004). Similarly, a drought treatment reduced foliar P and also K and Mg concentrations in pitch pine seedlings (Schier and McQuattle 2000). In a comparison of Sitka spruce seedlings inoculated with four fungal species over a number of drying and rewetting cycles, Thelephora terrestris was the one that was able to form mycorrhizas also in the driest treatment, and the seedlings inoculated with it also had the largest nutrient concentrations and contents (Lehto 1992c). Alvarez et al. (2009b) showed that ectomycorrhizal N. dombeyi accumulated considerably more N and P during drought than the nonmycorrhizal ones, accompanied with enhanced activities of glutamate synthase (GOGAT), glutamine synthetase, glutamate dehydrogenase, nitrate reductase and acid phosphomonoesterase.
Most of the thoughts about the interactions of water and nutrients arise from studies on mycorrhizal and nonmycorrhizal plants, where mycorrhizal plants may be more drought resistant because of their better nutrient status (see above). However, the survival and sustained function in nutrient uptake could be one of the most important ways for mycorrhizas to promote plant performance during drought and recovery, particularly in mature trees which have access to soil water with the deep, nonmycorrhizal parts of their root systems. In field conditions, drought episodes are sporadic and repeated in most of the regions where ectomycorrhizal plants dominate. The improved nutrient status of the plant may lead to better drought resistance which in turn may lead to increased root and mycorrhiza growth, which in turn will lead to better nutrient uptake. It is a great challenge to devise experiments to assess the relative importance of each process in this cycle in the field.
Water uptake and water relations of large trees
Regarding nutrient uptake, there is no shortage of evidence for increased uptake by mycorrhizas in seedlings, and many of the mechanisms of ectomycorrhizal nutrient uptake have been unravelled on physiological and molecular levels. Nevertheless, in the forest, the main quantitative piece of evidence for the importance of mycorrhizas in nutrient uptake of large trees is the fact that nutrients mostly cycle through the surface organic layers, which is also where the mycorrhizas are located (cf. Lucash et al. 2007). We do not challenge the notion that nutrients are mostly taken up by ectomycorrhizas for large trees, but we rather wish to compare it to water uptake. The case for water uptake is different and probably much more context dependent than nutrient uptake.
Ectomycorrhizas are usually concentrated in the top layer of the soil. In many soils, this is an organic layer, and in any case, aboveground litter falls on the surface of the soil. It is in this layer that ectomycorrhizal fungi spread their mycelial strand networks and induce abundant forking systems of short roots. For example, Jonsson et al. (2000) counted the mycorrhizal tips in a Swedish stand in the organic layer and 5 cm of mineral soil beneath and found that 90% of the mycorrhizas were in the organic layer. Although the distribution is somewhat less steep in different soils (Rosling et al. 2003), the majority of mycorrhizas is in the organic layer and in the mineral layers immediately below this, which also usually contains organic matter. This may suggest that the main function of mycorrhizas and their external mycelium is to scavenge nutrients from decomposing litter, as the topsoil usually contains more N and P than the lower layers. At the same time, the top is the part of the soil profile that is the most liable to drying out in any soil.
Particularly in dry environments, deep-rooted trees are able to meet the minimum requirements during drought by taking up water from deep layers; also in temperate forests, the quantity of coarse roots in a deeper layer was larger in a dry site than a moist site (Bakker et al. 2006). However, most of the absorbing surface area of tree root systems is in the fine roots and mycorrhizas in the top layers of soils. In less dry environments, during periods of adequate moisture in the topsoil and high transpirational pull, the water uptake by fine roots and mycorrhizas is probably very significant. However, it is extremely difficult to separate the role of mycorrhizas, fine roots and deeper coarse roots in the water relations of large trees and therefore such studies are infrequent. In short-term experiments, seedling roots are mostly young, resembling the white fast-growing long-root tips rather than the largest part of the fine root system. Tree fine roots may live for several years and, during this time, they may support mycorrhizal branches that are more short-lived (Smith and Read 2008).
Meta-analysis of publications on drought effects indicated that drought significantly decreased fine root biomass in boreal and temperate ectomycorrhizal trees (Cudlín et al. 2007). Usually, even mild drought reduces fine root growth (Gaul et al. 2008) and the numbers of ectomycorrhizas or the degree of colonisation (Theodorou 1978; Lehto 1992a, b, c; Nilsen et al. 1998; Beniwal et al. 2010). Although resource allocation theories suggest that trees should grow more fine roots during mild drought, the reduced availability of photosynthate for roots and mycorrhizal fungi appears to limit growth when the stomata start closing. On the other hand, the quantity of fine roots (Majdi 2001) and ectomycorrhizas, particularly in relation to foliar biomass (Helmisaari et al. 2009), has been shown to increase towards the north in Sweden and Finland. In this region, nutrient availability decreases and water availability increases towards the north; hence the increased amount of fine roots and mycorrhizas in topsoil is a response to decreasing nutrient availability, a response that is facilitated by the less dry conditions in the north. In these conditions in northern Sweden, irrigation did not have any effect on mycorrhizas in a field study (Fransson et al. 2000), while water was not a limiting factor for Norway spruce productivity (Bergh et al. 1999). By contrast, in an arid climate, a comparison of two soil types with different soil moisture and nutrient retention showed that ectomycorrhizal colonisation was more sensitive to soil moisture variation in the dry soil type (Swaty et al. 1998).
One of the major challenges in finding out the role of the different parts of the root system is to separate the water uptake by fine roots and the mycorrhizas that they support in different field conditions. Intuitively, it is tempting to make an assumption that mycorrhizas are important in taking up water, particularly during drought, because they are located between plant and soil (Garbaye 2000). However, it is also possible to conclude that ectomycorrhizas have little importance for the water uptake of large trees (Kramer and Bullock 1966; Kramer 1988). There is still little evidence to support either suggestion. Quite likely, the answer is that in different conditions and different species combinations the outcome is different. The location of fine roots and mycorrhizas in the topsoil that contains nutrients, but is vulnerable to drying out, could be seen as a similar enigma for plant and fungal life as the impossible task that the stomata have in letting CO2 in without letting water out.
Hydraulic redistribution and ectomycorrhizas
Hydraulic redistribution of soil water is a term used for the vertical or lateral transport of water through roots or hyphae, where water transport occurs passively along a soil water potential gradient. This transport is much more rapid than the movement of soil water (Richards and Caldwell 1987), and in coarse-textured soils it is practically the only means of upward or lateral water movement. Hydraulic redistribution, or the particular case of hydraulic lift, was first shown in ecosystems with severe summer droughts, when the surface soil dries, but there is still water available in deeper layers (Richards and Caldwell 1987 and references therein). During the day, plants transpire. At night, when the stomata close, water flows into roots in the deep soil, is transported upwards within the root, and if the soil water potential is lower than that of the upper roots, water flows into the surface soil. More recently, it has been shown that hydraulic redistribution is a significant factor also in tropical and temperate forests, where it allows continued transpiration during dry seasons (Lee et al. 2005; Warren et al. 2007). Although leakage of water from roots to dry surface soil may lead to increased evaporational water loss from soil, there are significant advantages for continued nutrient uptake and soil microbial activity; furthermore, water acquisition of shallow-rooted plants and seedlings is improved, and the competitive balance between species can be affected (Richards and Caldwell 1987; Allen 2009).
In ectomycorrhizal roots in dry surface soil, hydraulically lifted water can move from root to the fungal partner, which can directly enhance the survival and activity of the mycorrhiza (Querejeta et al. 2003). Quercus agrifolia trees in a California savanna were efficient enough in keeping the roots in the topsoil hydrated; there was not much decline in ectomycorrhizal mycelium in soil during severe soil drying, suggesting continued hyphal growth (Querejeta et al. 2007). The authors pointed out that the soil hyphal length was reduced much more during summer in a temperate Douglas fir stand (Hunt and Fogel 1983).
An intriguing suggestion on the role of ectomycorrhizas in water uptake in the field comes from a chaparral environment, where plants are known to depend on water stored in cracks in the bedrock. As ectomycorrhizal mycelium was found also in the weathered bedrock itself, far from cracks, it appears possible that mycorrhizal mycelium was taking up water – and nutrients – from the weathering bedrock (Egerton-Warburton et al. 2003). Growth of mycorrhizal hyphae into weathering bedrock and their nutrient uptake is known also in boreal and temperate forests (van Scholl et al. 2008). On shallow soils and cliffs, these ‘rock-eating fungi’ might have a role in providing water during dry periods also in humid climates, but this has so far not been studied. Better characterisation of water recharge and depletion in bedrock may have profound influence on models of climate prediction and global vegetation (Schwinning 2010).
Water flow into the mycorrhizal fungus may reduce water leakage from roots to the soil (Allen 2009), but water may be also leaked from hyphal tips into soil, and the leaked water may be subsequently reabsorbed (Sun et al. 1999). As the addition of water into dry soil will increase nutrient availability, the leaking and reabsorbing is important for the continued nutrient uptake function by mycorrhizas (Querejeta et al. 2003, 2007). Water taken from deeper layers may also promote sporocarp formation and thereby sexual reproduction of the fungi. Considerably larger amounts of water are needed for forming sporocarps than maintaining soil mycelium, as epigeous sporocarps are exposed to the atmosphere, but they do not have similar controls for water loss as plants have in their stomata. Water loss from epigeous sporocarps was directly related to vapour pressure deficit and the morphology (surface to volume ratio) in different ectomycorrhizal species (Lilleskov et al. 2009). Nevertheless, some species are able to form epigeous sporocarps in dry soil, as they are able to transport enough water from deep water sources either via root hydraulic lift or directly with deep hyphae (Lilleskov et al. 2009).
Egerton-Warburton et al. (2007) and Plamboeck et al. (2007) showed in chamber experiments, using deuterium and dyes as tracers, that water transport from plant to plant via both arbuscular and ectomycorrhizal mycelium is possible between the same plant species and different plant species. However, in a Douglas fir stand, there was no evidence for hydraulic redistribution by mycelium as seedlings isolated from the mycelia in surrounding soil received as much deuterium-labelled water from mature (‘donor’) Douglas fir trees as did seedlings with a potential for mycelial connections (Schoonmaker et al. 2007). In this region, there is usually a distinct dry period during summer, although the study year was relatively moist. By contrast, Warren et al. (2008) found the transport of water to Pinus seedlings from a recently cut stump through a hyphal connection, although the water provided was a small proportion of the water used. The authors suggested that the hydraulic redistribution via the fungus could be of benefit to the seedling under a very dry spell and, importantly, contributes to the survival of the mycelial network.
Teste et al. (2009) showed the transfer of labelled carbon and nitrogen from large Douglas fir trees to seedlings through mycelium; however, in stands with large trees, the mother tree is also a powerful competitor for resources. In areas of natural regeneration or gaps, water can be the most important limiting resource for seedlings, while their nutrient requirements are small. Lehto (1956) tested the effect of trenching on the survival of Scots pine seedlings in a natural regeneration stand and showed that cutting the connection to the mother tree strongly increased seedling survival; this occurred in the humid boreal zone, which still has dry periods critical for seedling establishment during the short summer. Fitter et al. (2000) introduced the term ‘mycocentric’ to emphasise that processes in the mycorrhizal mycelium largely occur to fulfil the needs of the mycorrhizal fungus and the benefits for the plant can be side effects of the transport of substances within the mycelia.
For the host tree, transporting water to mycorrhizas might be of key importance for nutrient uptake during droughts. Further studies are needed for establishing the occurrence of hydraulic redistribution in different vegetation zones and ecosystems and the role of mycorrhizas in these processes. Stable isotopes of oxygen and hydrogen have proven as a very efficient tool for this, both utilising natural differences in oxygen isotopes in different sources of water and as labelled tracers alongside with dye tracers.
Fungal species and genotype differences in reactions to drought
There are differences between mycorrhizal fungal species in the way that they affect plant water relations as has been reviewed above. Early studies attempted to find drought-tolerant mycorrhizal fungal species and strains in pure culture experiments, but pure culture studies did not always correlate with the performance of seedlings inoculated with the same strain (Mexal and Reid 1973; Theodorou 1978; Parke et al. 1983; Coleman et al. 1989). However, Boyle and Hellenbrand (1991) and Dunabeitia et al. (2004) found that the fungi that performed best in pure culture generally improved the performance of conifer seedlings during water stress. The discrepancies in pure culture studies may be due to the continued carbohydrate availability in pure culture, while root exudation is reduced by drought (Theodorou 1978). The differences between species may be caused either by the drought tolerance of the fungus or the ability of the fungus to tolerate reduced carbohydrate availability from the host. Furthermore, it is not known how well related the occurrence of different fungi is to their performance in providing their host plants with water.
The proportion of Cenococcum geophilum (C. graniforme) mycorrhizas has been found to be considerably increased during drought as other species decline (Worley and Hacskaylo 1959; Pigott 1982; Querejeta et al. 2009). Some caution is in place because the mycelium of this species may be less easily decomposable than that of some other fungi (Meyer 1974), and the mycelium may simply remain in the soil without being active. C. geophilum is considered to be hydrophilic (Unestam 1991) and belonging to a short-distance exploration type (Agerer 2001). It is not known if the superior survival of this species confers advantage to the host in water or nutrient uptake during drought, and the potential importance of C. geophilum in tree and forest water relations remains to be clarified (Jany et al. 2003; di Pietro et al. 2007; Smith and Read 2008), Moreover, the species is probably not as uniform as has been earlier assumed (Douhan and Rizzo 2005). The proportion of C. geophilum mycorrhizas has been brought up as a potential indicator of environmental change; however, this species is resistant also to other stress factors than drought. This nonspecifity, together with the other issues (above), has hindered the usage of C. geophilum in environmental assessments (Cudlín et al. 2007).
The ability of P. tinctorius to improve seedling performance as opposed to forest tree nursery fungi and native soil fungi in some conditions is well established (Marx et al. 1984). Pisolithus has shown a great improvement of water relations, which has been associated with improved nutrient uptake particularly in difficult sites in the southeast of the USA (Walker et al. 1989). Afterwards, there have been conflicting reports about this species, which are at least partly due to problems in identifying related species in different parts of the world (Martin et al. 2002).
Other fungi that are suggested to be more drought tolerant than some others include species with strong external mycelia, such as Rhizopogon species (Parke et al. 1983; Dunabeitia et al. 2004; Boyle and Hellenbrand 1991). In addition to between-species variation, within-species variation may also make a difference in different conditions. For example, the colonisation by P. involutus was decreased in a severe, cyclic drought treatment on Sitka spruce while T. terrestris was not (Lehto 1992c); however, P. involutus was superior compared to H. crustuliniforme and Laccaria laccata in an oak field experiment (Garbaye and Churin 1997). Lamhamedi et al. (1992a, b) showed that different genotypes of Pisolithus sp. had a positive correlation between the performance during drought conditions and the formation of external hyphal systems. Comparison of different mycorrhizal fungi and their traits in relation to plant water relations is an issue of great potential both for understanding ecosystem function and for practical applications in forest tree nurseries. However, this is a topic that would require more extensive and long-term experimentation than has been possible in most cases up to now.
It is often suggested that mycorrhizal fungi with extensive external mycelia are better at taking up water for the host plant (Garbaye 2000). Hence, it would be favourable for plants if these types are common in dry soils or if they become more common during dry times. As was expected, Bakker et al. (2006) showed a larger proportion of contact exploration types in a humid site and more short-distance and long-distance exploration types (Agerer 2001) in a dry site. As also the nutrient availability was higher in the humid stand than the dry stand, it was not possible to separate the effects of water and nutrient relations. This is a common situation in the comparison of forest stands (e.g. Cajander 1949; Swaty et al. 1998; Giesler et al. 1998; Querejeta et al. 2009) as soil formation is a result of many interacting factors. Furthermore, the host species are different in mesic and dry sites. It is not understood if there is co-adaptation of the fungal species and host tree species to water availability of the sites that they occupy together. It seems likely that one of the traits of drought-tolerant fungi is limited evaporation from their sporocarps, and therefore one possible approach is to study water loss from sporocarps of mycorrhizal fungal species (Lilleskov et al. 2009) occurring in habitats with different water availability and with different hosts.
Ectomycorrhizas and other types of mycorrhizas
The role of arbuscular mycorrhizas (AM) in plant water relations was reviewed by Augé (2001, 2004) and the role of aquaporins in AM plants by Uehlein et al. (2007). There is ample evidence that AM are often beneficial for providing their hosts with water. As in ectomycorrhizas, the best-known mechanisms for this are improved nutrition and water uptake by external hyphae, and the role of aquaporin expression is emerging. However, the effects of AM on water acquisition are “not as dramatic and consistent as those on P acquisition and host growth and one would not expect them to be” (Augé 2001).
Ectomycorrhizal and arbuscular mycorrhizal root systems provide a natural comparison between two possibly different pathways of water transport. These types of comparisons may complement or replace comparisons to nonmycorrhizal roots (cf. Jones et al. 1998). This approach could yield insights into the water uptake process. Calvo-Polanco et al. (2008) found twice as high hydraulic conductance in American elm seedlings that probably had a spontaneous AM infection compared to the ones inoculated with ectomycorrhizal fungi. This may be due to the nutritional effects of the different symbionts, but it may also be due to a different pathway of water in the two types of mycorrhizas or a difference in the modification of aquaporins by the two types of infection. In the case of salinity stress, it was recently shown (Aroca et al. 2009) that a signalling communication between salt-treated mycelium and untreated mycelium took place to regulate the gene expression of both the fungal (G. intraradices) and host plant (Phaseolus vulgaris) root aquaporins. This communication was thought to be involved in the facilitation of water transport between mycelium and root tissues. A similar mechanism could operate in ectomycorrhizal associations in response to environmental stresses including drought.
In ectendomycorrhizas, the fungus penetrates cortex cells in addition to forming the Hartig net (Mikola 1965). Therefore, the pathway of water could be different from that in ectomycorrhizas, and the conductance might be larger as the water would be transported directly into host cells. However, in the only study comparing ectendomycorrhizas formed by W. mikolae to ectomycorrhizas formed by H. crustuliniforme, the root hydraulic conductance was lower in the ectendomycorrhizas despite their otherwise beneficial effects on their hosts as shown by increased growth (Siemens and Zwiazek 2008).
We have not found reports on the water uptake by ericoid mycorrhizas or their possible influence on plant water relations. Unlike AM and ECM, ericoid mycorrhizas do not have a system of external hyphae extending far from roots, but their hyphae are short and the fine roots of ericoid plants are mostly responsible for exploring the soil space (Read 1991). Nevertheless, ericoid mycorrhizas should benefit their host water relations at least through improved nutrition, particularly N. Other possibilities include modification of aquaporins as in AM and EM.
Read (1984, 1991; Smith and Read 1997, 2008) has presented and developed a global view on the distribution of different types of mycorrhizas in the major vegetation zones of the world. Briefly, the dominance of different mycorrhiza types is thought to be a result of climatic factors but not so much directly as through the influence of climate on soil formation and, consequently, nutrient availability. According to this, the most growth-limiting nutrient is one of the key determinants for the global distribution of plant species through their mycorrhizal types. AM are very efficient in P uptake but not in N uptake: AM species dominate in warm regions where P is the most limiting nutrient. In the temperate and boreal regions, EM species dominate while they are more efficient in taking up N. The role of N as the limiting nutrient tends to increase towards colder regions. In coldest regions, ericoid mycorrhizas dominate through their better ability to use organic N compounds.
However, it is difficult to distinguish the indirect effects of climate factors through soil formation from their direct effects on organisms at the present time. Humidity (balance between precipitation and evaporation) tends to be higher where temperatures are lower; this is where recalcitrant N compounds accumulate in the soil. By contrast, the humidity of the growing season is low, where P is the most growth-limiting nutrient and AM species dominate. Although the ectomycorrhiza-forming families Pinus and Quercus include species that grow on seasonally very dry sites, their survival of driest periods is thought to be due to coarse root systems that reach several metres deep (e.g. Nadezhdina et al. 2008), hydraulic lift and summer dormancy. In regions with still more frequent and severe summer droughts, forests are replaced by grasslands (Lambers et al. 2008). Allen et al. (1995) suggested that AM tolerate dry conditions better than EM, and this is likely to be one of the reasons for the dominance of AM in dry environments. In many grassland species, the strategy for survival is death of the aboveground parts during the driest periods, and then the activity of AM declines because of lack of photosynthate (Querejeta et al. 2007). The competition between an ECM tree, pinyon pine (Pinus edulis) and an AM shrub was shown to have negative impacts for the EM colonisation and growth of the pine during drought (McHugh and Gehring 2006).
In species that can form both EM and AM, the dominance of EM has been shown in more humid conditions and dominance of AM in drier conditions both in riparian cottonwood (Populus angustifolia) (Gehring et al. 2006) and in California coast live oak (Q. agrifolia) (Querejeta et al. 2009). In the oak trees, AM were not affected by drought, while EM declined especially during severe droughts in hillside soil. However, the EM mycorrhizas and external mycelium recovered when water was available again. It remains to be shown if this pattern is favourable for the host plant for N uptake. Gehring et al. (2006) suggested that the lower carbon cost of forming AM is a factor contributing to their prevalence in drier conditions. Dual mycorrhizal systems occur in a number of woody plants and further studies into the dominance of different types and their function during environmental stress will yield important insights into the role of mycorrhizas in different ecosystems.
In the other extreme, in regions where soil water freezes during the winter, trees may have problems in acquiring water in the spring as the soil remains frozen when light levels and air temperatures are already sufficient for photosynthesis. It would be intriguing to find out whether ectomycorrhizas have a particularly significant role in water uptake in situations where the soil has been frozen and the surface thaws first compared to growing season conditions.
Conclusions
Ectomycorrhizas have been shown to be important in plant water uptake, but these studies are mostly limited to small seedlings. The direct mechanisms for improved water relations include at least water uptake by external mycelium and enhanced aquaporin function which may lead to increased root hydraulic conductance. However, in some conditions, ectomycorrhizas can have no effect or a negative effect on the hydraulic conductance of the root system, and the reasons for this are not yet fully understood. Some mycorrhizal benefits in water relations, particularly aquaporin expression in ectomycorrhizas, may not be seen in optimal water availability, but they will manifest during droughts and other environmental stresses, such as low soil temperature. Improved nutrient acquisition is the major indirect mechanism of mycorrhizal benefits. Adequate mineral nutrition, together with increased water acquisition during drought, leads to sustained photosynthesis and improved whole-plant performance. However, the role of mycorrhizas is poorly known especially during long-term and repeated droughts.
Pot experiments have been an important tool for mycorrhizal water relations based on the concept that a comparison between mycorrhizal and nonmycorrhizal plants is a useful model for studying the mycorrhizal effects. By excluding the most obvious mycorrhizal effects, namely, the extension by mycelium and the nutritional effects, it has been possible to reveal the other functions of mycorrhizas – and often the lack of other functions. In future studies, pot experiments comparing mycorrhizal and nonmycorrhizal plants will still be useful in studying well-defined physiological details. However, the quantitative importance of ectomycorrhizas for tree water uptake and water relations can only be assessed by field studies using innovative approaches.
In the field, ectomycorrhizas and their external mycelia are mostly located in the topsoil which is likely to dry out before the deeper layers. Ectomycorrhizal trees often have a well-developed root system which can provide for water from deeper layers particularly in drought conditions. In dry regions, ectomycorrhizal hydraulic lift has been shown to function, and hydraulic redistribution to ectomycorrhizas appears to have a role also in temperate forests with less water stress. The hydraulic lift to mycelium is most probably important also for the host tree, and it remains to be shown quantitatively how important it is in different environments. Nutrient uptake during dry spells, survival and recovery of nutrient uptake after dry spells and after soil thawing may turn out to be among the most important features of ectomycorrhizas in regard to water.
References
Adler PR, Wilcox GE, Markhart AH III (1996) Ammonium decreases muskmelon root system hydraulic conductivity. J Plant Nutr 19:1395–1403
Agerer R (2001) Exploration types of ectomycorrhizae—a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114
Allen MF (2007) Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone J 6:291–297
Allen MF (2009) Bidirectional water flows through the soil–fungal–plant mycorrhizal continuum. Commentary. New Phytol 182:290–293
Allen EB, Allen MF, Helm FJ, Trappe JM, Molina R, Rincon E (1995) Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170:47–62
Alvarez M, Huygens D, Fernandez C, Gacitua Y, Olivares E, Saavedra I, Alberdi M, Valenzuela E (2009a) Effect of ectomycorrhizal colonization and drought on reactive oxygen species metabolism of Nothofagus dombeyi roots. Tree Physiol 29:1047–1057
Alvarez M, Huygens D, Olivares E, Saavedra I, Alberdi M, Valenzuela E (2009b) Ectomycorrhizal fungi enhance nitrogen and phosphorus nutrition of Nothofagus dombeyi under drought conditions by regulating assimilative enzyme activities. Physiol Plant 136:426–436
Amir R, Steudle E, Levanon D, Hadar Y, Chet I (1995) Turgor changes in Morchella esculenta during translocation and sclerotial formation. Exp Mycol 19:129–136
Andersen CP, Sucoff EI, Dixon RK, Markhart AH III (1989) Effects of phosphorus deficiency on root hydraulic conductivity in Fraxinus pennsylvanica. Can J Bot 67:472–476
Aphalo PJ, Lahti M, Lehto T, Repo T, Rummukainen A, Mannerkoski H, Finér L (2006) Responses of silver birch saplings to low soil temperature. Silva Fenn 40:429–442
Aroca R, Bago A, Sutka M, Paz JA, Cano C, Amodeo G, Ruiz-Lozano JM (2009) Expression analysis of the first arbuscular mycorrhizal fungi expression between salt-stressed and nonstressed mycelium. MPMI 22:1169–1178
Augé R (2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé R (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Bakker MR, Augusto L, Achat DL (2006) Fine root distribution of trees and understory in mature stands of maritime pine (Pinus pinaster) on dry and humid sites. Plant Soil 286:37–51
Beniwal RS, Langenfeld-Heyser R, Polle A (2010) Ectomycorrhiza and hydrogel protect hybrid poplar from water deficit and unravel plastic responses of xylem anatomy. Environ Exp Bot 69:189–197
Bergh J, Linder S, Lundmark T, Elfving B (1999) The effect of water and nutrient availability on the productivity of Norway spruce in northern and southern Sweden. Forest Ecol Manage 119:51–62
Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotiana tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18:565–570
Bogeat-Triboulot M-B, Bartoli F, Garbaye J, Marmeisse R, Tagu D (2004) Fungal ectomycorrhizal community affect root hydraulic properties and soil adherence to roots of Pinus pinaster seedlings. Plant Soil 267:213–223
Boyd R (1987) The role of ectomycorrhiza in the water relations of plants. Ph.D. thesis, University of Sheffield, UK, p 136
Boyd R, Furbank RT, Read DJ (1986) Ectomycorrhiza and the water relations of trees. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetic aspects of mycorrhizae. INRA, Paris, pp 689–694
Boyle CD, Hellenbrand KE (1991) Assessment of the effect of mycorrhizal fungi on drought tolerance of conifer seedlings. Can J Bot 69:1764–1771
Brownlee C, Duddridge JA, Malibari A, Read DJ (1983) The structure and function of mycelia systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport. Plant Soil 71:433–443
Cairney JWG (1992) Translocation of solutes in ectomycorrhizal and saprotrophic rhizomorphs. Mycol Res 96:135–141
Cairney JWG (2005) Basidiomycete mycelia in forest soils: dimensions, dynamics and roles in nutrient distribution. Mycol Res 109:7–20
Cajander AK (1949) Forest site types and their significance. Acta For Fenn 56:1–71
Calvo-Polanco M, Zwiazek JJ, Voicu MC (2008) Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant Soil 308:189–200
Calvo-Polanco M, Jones MD, Zwiazek JJ (2009) Effects of pH on NaCl resistance of American elm (Ulmus americana) seedlings inoculated with Hebeloma crustuliniforme and Laccaria bicolor. Acta Physiol Plant 31:515–522
Carvajal M, Cooke DT, Clarkson DT (1996) Responses of wheat plants to nutrient deprivation may involve regulation of water-channel function. Planta 199:372–381
Coleman MD, Bledsoe CS, Smit-Spinks B (1987) Ectomycorrhizae decrease Douglas-fir root hydraulic conductivity. In: Sylvia DM, Hung LL, Graham JH (eds) Mycorrhizae in the next decade. Practical applications and research priorities. Institute of Food and Agricultural Sciences, University of Florida, Gainesville, p 243
Coleman MD, Bledsoe CS, Lopushinsky W (1989) Pure culture response of ectomycorrhizal fungi to imposed water stress. Can J Bot 67:29–39
Coleman MD, Bledsoe CS, Smit B (1990) Root hydraulic conductivity and xylem sap levels of zeatin riboside and abscisic acid in ectomycorrhizal Douglas fir seedlings. New Phytol 115:275–284
Crowe JH (2007) Trehalose as a “chemical chaperone”: facts and fantasy. Adv Exp Med Biol 594:143–158
Cudlín P, Kieliszewska-Rokicka B, Rudawska M, Grebenc T, Alberton O, Lehto T, Bakker M, Børja I, Konopka B, Leski T, Kraigher H, Kuyper T (2007) Fine roots and ectomycorrhizas as indicators of environmental change. Plant Biosystems 141:406–425
Davies FT, Svenson SE, Cole JC, Phavaphutanon L, Duray SA, Olalde-Portugal V, Meier CE, Bo SH (1996) Non-nutritional stress acclimation of mycorrhizal woody plants exposed to drought. Tree Physiol 16:985–993
Diebolt K, Mudge KW (1987) Do ectomycorrhizae influence host plant response to drought. In: Sylvia DM, Hung LL, Graham JH (eds) Mycorrhizae in the next decade. Practical applications and research priorities. Institute of Food and Agricultural Sciences, University of Florida, Gainesville, p 246
di Pietro M, Churin J-L, Garbaye J (2007) Differential ability of ectomycorrhizas to survive drying. Mycorrhiza 17:547–550
Dixon RK, Wright GM, Behrns GT, Tesky RO, Hinckley TM (1980) Water deficits and root growth of ectomycorrhizal white oak seedlings. Can J For Res 10:545–548
Dixon RK, Pallardy SK, Garrett HE, Cox GS, Sander IL (1983) Comparative water relations of container-grown and bare-root ectomycorrhizal and nonmyocorrhizal Quercus velutina seedlings. Can J Bot 61:1559–1565
Dordas C, Brown PH (2001) Evidence of channel mediated transport of boric acid in squash (Cucurbita pepo). Plant Soil 235:95–103
Dosskey MG, Ballard TM (1980) Resistance to water uptake by Douglas-fir seedlings in soils of different texture. Can J For Res 10:530–534
Dosskey MG, Boersma L, Linderman RG (1991) Role for the photosynthate demand of ectomycorrhizas in the response of Douglas fir seedlings to drying soil. New Phytol 117:327–334
Douhan GW, Rizzo DM (2005) Phylogenetic divergence in a local population of the ectomycorrhizal fungus Cenococcum geophilum. New Phytol 166:263–271
Dowd C, Wilson LW, McFadden H (2004) Gene expression profile changes in cotton root and hypocotyls tissues in response to infection with Fusarium oxysporum f. sp vasinfectum. Mol Plant-Microb Interact 17:654–667
Duddridge JA, Malibari A, Read DJ (1980) Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287:834–836
Dunabeitia MK, Hormilla S, Garcia-Plazaola JI, Txarterina K, Arteche U, Becerril JM (2004) Differential response of three fungal species to environmental factors and their role in the mycorrhization of Pinus radiata D. Don Mycorrhiza 14:11–18
Egerton-Warburton LM, Graham RC, Hubbert KR (2003) Spatial variability in mycorrhizal hypae and nutrient and water availability in a soil–weathered bedrock profile. Plant Soil 249:331–342
Egerton-Warburton LM, Querejeta JI, Allen MF (2007) Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J Exp Bot 58:1473–1483
Fitter AH, Heinemeyer A, Staddon PL (2000) The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol 147:179–187
Fransson PMA, Taylor AFS, Finlay RD (2000) Effects of continuous optimal fertilization on belowground ectomycorrhizal community structure in a Norway spruce forest. Tree Physiol 20:599–606
Gao YX, Li Y, Yang XX, Li HL, Shen QR, Guo SW (2010) Ammonium nutrition increases water absorption in rice seedlings (Oryza sativa L.) under water stress. Plant Soil 331:183–201
Gaul D, Hertel D, Borken W, Matzner E, Leuschner C (2008) Effects of experimental drought on the fine root system of mature Norway spruce. Forest Ecol Manage 256:1151–1159
Garbaye J (2000) The role of ectomycorrhizal symbiosis in the resistance of forests to water stress. Outlook Agric 29:63–69
Garbaye J, Churin JL (1997) Growth stimulation of young oak plantations inoculated with the ectomycorrhizal fungus Paxillus involutus with special reference to summer drought. Forest Ecol Manage 98:221–228
Gehring CA, Mueller RC, Whitham TG (2006) Environmental and genetic effects on the formation of ectomycorrhizal and arbuscular mycorrhizal associations in cottonwoods. Oecologia 149:158–164
Gerbeau P, Amodeo G, Henzler T, Santoni V, Ripoche P, Maurel C (2002) The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J 30:71–81
Giesler R, Högberg M, Högberg P (1998) Soil chemistry and plants in Fennoscandian boreal forest as exemplified by a local gradient. Ecology 79:119–137
Glosser V, Zwieniecki MA, Orians CM, Holbrook NM (2007) Dynamic changes in root hydraulic properties in response to nitrate availability. J Exp Bot 58:2409–2415
Guehl JM, Garbaye J (1990) The effects of ectomycorrhizal status on carbon-dioxide assimilation capacity, water-use efficiency and response to transplanting in seedlings of Pseudotsuga menziesii (Mirb.) Franco. Ann Sci For 47:551–563
Guehl JM, Mousain D, Falconnet G, Gruez J (1990) Growth, carbon dioxide assimilation capacity and water-use efficiency of Pinus pinea L. seedlings inoculated with different ectomycorrhizal fungi. Ann Sci For 47:91–100
Haberer K, Herbinger K, Alexou M, Rennenberg H, Tausz M (2008) Effects of drought and canopy ozone exposure on antioxidants in fine roots of mature European beech (Fagus sylvatica). Tree Physiol 28:713–719
Helmisaari H-S, Ostonen I, Lõhmus K, Derome J, Lindroos A-J, Merilä P, Nöjd P (2009) Ectomycorrhizal root tips in relation to site and stand characteristics in Norway spruce and Scots pine stands in boreal forests. Tree Physiol 29:445–456
Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D et al (2005) NH3 and NH +4 permeability in aquaporin-expressing Xenopus oocytes. Pflügers Arch 450:415–428
Huesken D, Steudle E, Zimmermann U (1978) Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol 61:158–163
Hunt GA, Fogel R (1983) Fungal hyphal dynamics in a Western Oregon Douglas-fir stand. Soil Biol Biochem 15:641–649
Jany JL, Martin F, Garbaye J (2003) Respiration activity of ectomycorrhizas from Cenococcum geophilum and Lactarius sp. in relation to soil water potential in five beech forests. Plant Soil 255:487–494
Jentschke G, Godbold DL (2000) Metal toxicity and ectomycorrhizas. Physiol Plant 109:107–116
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10:451–460
Jones H (2004) What is water use efficiency? In: Bacon MA (ed) Water use efficiency in plant biology. Blackwell, Oxford, pp 27–41. ISBN 1-4051-1434-7
Jones MD, Durall DM, Tinker PB (1998) Comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: growth response, phosphorus uptake efficiency and external hyphal production. New Phytol 140:125–134
Jonsson L, Dahlberg A, Brandrud T-E (2000) Spatiotemporal distribution of an ectomycorrhizal community in an oligotrophic Swedish Picea abies forest subjected to experimental nitrogen addition: above- and below-ground views. Forest Ecol Manage 132:143–156
Karlsson M, Fotiadis D, Sjövall S, Johansson I, Hedfalk K, Engel E, Kjellbomm P (2003) Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett 537:69–72
Kojima S, Bohner A, von Wirén N (2006) Molecular mechanisms of urea transport in plants. J Membr Biol 212:83–91
Kotze DJ, Johnson CA, O’Hara RB, Vepsäläinen K, Fowler MS (2004) Editorial. JNR 1:1–15
Kramer PJ (1988) Changing concepts regarding plant water relations. Plant, Cell Environ 11:565–568
Kramer PJ, Bullock HC (1966) Seasonal variations in the proportions of suberized and unsuberized roots of trees in relation to the absorption of water. Amer J Bot 53:200–204
Lahti M, Aphalo PJ, Finér L, Lehto T, Leinonen I, Mannerkoski H, Ryyppö A (2002) Soil temperature, gas exchange and nitrogen status of 5-year-old Norway spruce seedlings. Tree Physiol 22:1311–1316
Lambers H, Chapin FSIII, Pons TL (2008) Plant physiological ecology, 2nd edn. Springer, New York
Lamhamedi MS, Bernier PY, Fortin JA (1992a) Growth, nutrition and response to water stress of Pinus pinaster inoculated with ten dikaryotic strains of Pisolithus sp. Tree Physiol 10:153–167
Lamhamedi MS, Bernier PY, Fortin JA (1992b) Hydraulic conductance and soil water potential at the soil–root interface of Pinus pinaster seedlings inoculated with different dikaryons of Pisolithus sp. Tree Physiol 10:231–244
Landhäusser SM, Muhsin TM, Zwiazek JJ (2002) The effect of ectomycorrhizae on water relations in aspen (Populus tremuloides) and white spruce (Picea glauca) at low soil temperatures. Can J Bot 80:684–689
Lee J-E, Oliveira RS, Dawson TE, Fung I (2005) Root functioning modifies seasonal climate. Proc Natl Acad Sci USA 102:17576–17581
Lee SH, Zwiazek JJ, Chung GC (2008) Light-induced transpiration alters cell water relations in figleaf gourd (Cucurbita ficifolia) seedlings exposed to low root temperatures. Physiol Plant 133:354–362
Lee SH, Calvo Polanco M, Chung GC, Zwiazek JJ (2010) Cell water flow properties in root cortex of ectomycorrhizal (Pinus banksiana) seedlings. Plant Cell Environ 33:769–780
Lehto J (1956) Studies on the natural reproduction of Scots pine on the upland soils of Southern Finland. Acta For Fenn 66:1–106
Lehto T (1992a) Mycorrhizas and drought resistance of Picea sitchensis. I. In nutrient deficient conditions. New Phytol 122:661–668
Lehto T (1992b) Mycorrhizas and drought resistance of Picea sitchensis. II. In conditions of adequate nutrition. New Phytol 122:669–673
Lehto T (1992c) Effect of drought on Picea sitchensis seedlings inoculated with mycorrhizal fungi. Scand J For Res 7:177–182
Lehto T, Brosinsky A, Heinonen-Tanski H, Repo T (2008) Freezing tolerance of ectomycorrhizal fungi in pure culture. Mycorrhiza 18:385–392
Lilleskov EA, Bruns TD, Dawson TE, Camacho FJ (2009) Water sources and controls of water-loss rates of epigeous ectomycorrhizal fungal sporocarps during summer drought. New Phytol 182:483–494
Linder MB, Szilvay GR, Nakari-Setälä T, Penttilä ME (2005) Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol Rev 29:877–896
Loqué D, Ludewig U, Yuan L, vonWirén N (2005) Tonoplast intrinsic proteins AtTIP2;1 and AtTIP2;3 facilitate NH3 transport into the vacuole. Plant Physiol 137:671–680
Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A (2007) Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiol Plant 130:543–552
Lucash MS, Eissenstat DM, Joslin JD, McFarlane KJ, Yanai RD (2007) Estimating nutrient uptake by mature tree roots under field conditions: challenges and opportunities. Trees 21:593–603
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S et al (2006) A silicon transporter in rice. Nature 440:688–691
Majdi H (2001) Changes in fine root production and longevity in relation to water and nutrient availability in a Norway spruce stand in northern Sweden. Tree Physiol 21:1057–1061
Marjanović Ž (2004) Impact of mycorrhiza formation and drought stress on the expression and function of aquaporins in Norway spruce (Picea abies (L.) Karst.) and hybrid aspen (Populus tremula L. × Populus tremuloides Mich.) Ph.D. thesis, Karl-Eberhard Universität Tübingen, Germany
Marjanović Ž, Uehlein N, Kaldenhoff R, Zwiazek JJ, Weiß M, Hampp R, Nehls U (2005a) Aquaporins in poplar: what a difference a symbiont makes! Planta 222:258–268
Marjanović Z, Nehls U, Hampp R (2005b) Mycorrhiza formation enhances adaptive response of hybrid poplar to drought. Ann NY Acad Sci 1048:496–499
Martin F, Díez J, Dell B, Delaruelle C (2002) Phylogeography of the ectomycorrhizal Pisolithus species as inferred from nuclear ribosomal DNA ITS sequences. New Phytol 153:345–357
Marulanda A, Azcon R, Chaumont F, Ruiz-Lozano JM, Aroca R (2010) Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 232:533–543
Marx DH, Cordell CE, Kenney DS, Mexal JG, Artman JD, Riffle JW, Molina RJ (1984) Commercial vegetative inoculums of Pisolithus tinctorius and inoculation techniques for development of ectomycorrhizae on bare-root tree seedlings. Forest Sci Monograph 25 (Suppl to vol 30)
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Maurel C, Santoni V, Luu D-T, Wudick MM, Verdoucq L (2009) The cellular dynamics of plant aquaporin expressions and functions. Curr Opin Plant Biol 12:690–698
Meyer FH (1974) Physiology of mycorrhiza. Ann Rev Plant Physiol 25:567–586
McHugh TA, Gehring CA (2006) Below-ground interactions with arbvuscular mycorrhizal shrubs decrease the performance of pinyon pine and the abundance of its ectomycorrhizas. New Phytol 171:171–178
Mexal J, Reid CPP (1973) The growth of selected mycorrhizal fungi in response to induced water stress. Can J Bot 51:1579–1588
Mikola P (1965) Studies on the ectendotrophic mycorrhiza on pine. Acta For Fenn 79(2):1–56
Money NP (1990) Measurement of hyphal turgor. Exp Mycol 14:416–425
Muhsin TM, Zwiazek JJ (2002a) Ectomycorrhizas increase apoplastic water transport and hydraulic conductivity in Ulmus americana seedlings. New Phytol 153:153–158
Muhsin TM, Zwiazek JJ (2002b) Ectomycorrhizae increase water conductance and protect white spruce (Picea glauca) seedlings against salt stress. Plant Soil 238:217–225
Nadezhdina N, Ferreira MI, Silva R, Pacheco CA (2008) Seasonal variation of water uptake of a Quercus suber tree in Central Portugal. Plant Soil 305:105–119
Nardini A, Salleo S, Tyree MT, Vertovec M (2000) Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann For Sci 57:305–312
Nilsen P, Børja I, Knutsen H, Brean R (1998) Nitrogen and drought effects on ectomycorrhizae of Norway spruce [Picea abies L. (Karst.)]. Plant Soil 198:179–184
Nylund J-E (1987) The ectomycorrhizal infection zone and its relation to acid polysaccharides of cortical cell walls. New Phytol 106:505–516
Oertli JJ (1991) Transport of water in the rhizosphere and in roots. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York
Ortega U, Duñabeitia A, Menendez S, Gonzalez-Murua C, Majada J (2004) Effectiveness of mycorrhizal inoculation in the nursery on growth and water relations of Pinus radiata in different water regimes. Tree Physiol 24:65–73
Pallardy SG, Parker WC, Dixon RK, Garrett HE (1983) Tissue water relations of roots and shoots of droughted ectomycorrhizal shortleaf pine seedlings. In: Thielges BA (ed) Proceedings of the 7th North American Forest Biology Workshop “Physiology and Genetics of intensive culture”. University of Kentucky, Lexington, KY, pp 386–373
Parke JL, Linderman RG, Black CH (1983) The role of ectomycorrhizas in drought tolerance of Douglas-fir seedlings. New Phytol 95:83–95
Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S (2005) Aquaporins in yeasts and filamentous fungi. Biol Cell 97:487–500
Peuke AD, Rennenberg H (2004) Carbon, nitrogen, phosphorus, and sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): phosphorus most affected by drought. Trees 18:639–648
Pigott CD (1982) Survival of mycorrhiza formed by Cenococcum geophilum Fr. in dry soils. New Phytol 92:513–517
Plamboeck AH, Dawson TE, Egerton-Warburton LE, North M, Bruns TD, Querejeta JI (2007) Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza 17:439–447
Pratt RB, Jacobsen AL, North GB, Sack L, Schenk HJ (2008) Plant hydraulics: new discoveries in the pipeline. New Phytol 179:590–593
Querejeta JI, Egerton-Warburton LM, Allen MF (2003) Direct nocturnal transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134:55–64
Querejeta JI, Egerton-Warburton LM, Allen MF (2007) Hydraulic lift may buffer rhizosphere hyphae against the negative effects of severe soil drying in a California Oak savanna. Soil Biol Biochem 39:409–417
Querejeta JI, Egerton-Warburton LM, Allen MF (2009) Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 90:649–662
Radin JW, Boyer JS (1982) Control of leaf expansion by nitrogen nutrition in sunflower plants. Role of hydraulic conductivity and turgor. Plant Physiol 69:771–775
Radin JW, Eidenbock MP (1984) Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton plants. Plant Physiol 75:372–377
Read DJ (1984) The structure and function of the vegetative mycelium of mycorrhizal roots. In: Jennings DH, Rayner ADM (eds) The ecology and physiology of the fungal mycelium. Cambridge University Press, Cambridge, pp 215–240
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Read DJ, Malibari A (1979) Water transport through mycelial strands to ectomycorrhizal roots of pine. In: Riedacker A, Cagnair-Michard J (eds) Root physiology and symbiosis. CNFR, Nancy, France, pp 410–423
Read DJ, Boyd R (1986) Water relations of mycorrhizal fungi and their host plants. In: Ayres PG, Boddy L (eds) Water, fungi and plants. Cambridge University Press, Cambridge, pp 215–240
Reid CPP (1979) Mycorrhizae and water stress. In: Riedacker A, Gagnaire-Michard J (eds) Root physiology and symbiosis. Proceedings of the IUFRO symposium. Nancy, France, pp 392–409
Reid CPP, Bowen GD (1979) Effect of water stress on phosphorus uptake by mycorrhizas of Pinus radiata. New Phytol 83:103–107
Richards JH, Caldwell MM (1987) Hydraulic lift: substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73:486–489
Rosling A, Landeweert R, Lindahl BD (2003) Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytol 159:775–783
Sade N, Vincour BJ, Diber A, Shatil A, Ronen G, Nissan H, Wallach R, Karchi H, Moshelion M (2009) Improving plant stress tolerance and yield production: is the tonoplast aquaporin SITIP2;2 a key to isohydric to anisohydric conversion? New Phytol 181:651–661
Sade N, Gebretsadik K, Seligmann R, Scwartz A, Wallach R, Moshelion M (2010) The role of tobacco aquaporin 1 in improving water use efficiency, hydraulic conductivity and yield production under salt stress. Plant Physiol 152:245–254
Sands R, Theodorou C (1978) Water uptake by mycorrhizal roots of radiata pine seedlings. Aust J Plant Physiol 5:301–309
Sands R, Fiscus EL, Reid CPP (1982) Hydraulic properties of pine and bean roots with varying degrees of suberization, vascular differentiation and mycorrhizal infection. Aust J Plant Physiol 9:559–569
Schier GA, McQuattle CJ (2000) Effect of water stress on aluminium toxicity in pitch pine seedlings. J Plant Nutr 23:637–647
Schoonmaker AL, Teste FP, Simard SW, Guy RD (2007) Tree proximity, soil pathways and common mycorrhizal networks: their influence on the utilization of redistributed water by understory seedlings. Oecologia 154:455–466
Schwinning S (2010) The ecohydrology of roots in rocks. Ecohydrol 3:238–245
Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12:303–311
Siemens AJ, Zwiazek JJ (2008) Root hydraulic properties and growth of balsam poplar (Populus balsamifera) mycorrhizal with Hebeloma crustuliniforme and Wilcoxina mikolae var. mikolae. Mycorrhiza 18:393–401
Simard SW, Perry DA, Jones MD, Myrolds DD, Durall DM, Molina R (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388:579–582
Shvaleva AL, Silva FCE, Breia E, Jouve L, Hausman JF, Almeidea MH, Maroco JP, Rodrigues ML, Pereira JS, Chaves MM (2006) Metabolic responses to water deficits in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiol 26:239–248
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd ed. Academic Press, p 603
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3 rd ed. Academic Press, p 787
Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and to studies of water and solute relations at cell, tissue and organ. In: Smith JAC, Griffiths H (eds) Water deficits: plant responses from cell to community. Bios Scientific Publishers Ltd., Oxford, pp 5–36
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49:775–788
Sun Y-P, Unestam T, Lucas SD, Johanson KJ, Kenne L, Finlay RD (1999) Exudation-reabsorption in a mycorrhizal fungus, the dynamic interface for interaction with soil and soil microorganisms. Mycorrhiza 9:137–144
Syvertsen JP, Graham JH (1985) Hydraulic conductivity of roots, mineral nutrition, and leaf gas exchange of Citrus rootstocks. J Amer Soc Hort Sci 110:865–869
Swaty RL, Gehring CA, van Ert M, Theimer TC, Keim P, Whitham TG (1998) Temporal variation in temperature and rainfall differentially affects ectomycorrhizal colonization at two contrasting sites. New Phytol 139:733–739
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL (2009) Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822
Theodorou C (1978) Soil moisture and the mycorrhizal association of Pinus radiata D.Don. Soil Biol Biochem 10:33–37
Tyree MT, Jarvis PG (1982) Tissue water relations. In: Lange O, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, new ser vol 12B. Springer, pp 34–78
Tyree MT, Patino S, Bennink J, Alexander J (1995) Dynamic measurements of root hydraulic conductance using a high-pressure flowmeter in the laboratory and field. J Exp Bot 46:83–94
Uehlein N, Fileschi K, Eckert M, Bienert GP, Bertl A, Kaldenhoff R (2007) Arbuscular mycorrhizal symbiosis and plant aquaporin expression. Phytochemistry 68:122–129
Unestam T (1991) Water repellency, mat formation and leaf-stimulated growth of some ectomycorrhizal fungi. Mycorrhiza 1:13–20
Unestam T, Sun YP (1995) Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 5:301–311
Van Scholl L, Kuyper TW, Smits MM, Landeweert R, Hoffland E, van Breemen N (2008) Rock-eating mycorrhizas: their role in plant nutrition and biogeochemical cycles. Plant Soil 303:35–47
Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (2004) Novel regulation of aquaporins during osmotic stress. Plant Physiol 135:2318–2329
Voicu MC, Zwiazek JJ (2004) Cycloxeximide inhibits root water flow and stomatal conductance in aspen (Populus tremuloides) seedlings. Plant Cell Environ 27:199–208
Walker RF, West DC, McLaughlin SB, Amundsen CC (1989) Growth, xylem pressure potential and nutrient absorption of loblolly pine on a reclaimed surface mine as affected by an induced Pisolithus tinctorius infection. Forest Sci 35:569–581
Wan X, Zwiazek JJ (1999) Mercuric chloride effects on root water transport in aspen (Populus tremuloides) seedlings. Plant Physiol 121:939–946
Wan X, Landhäusser SM, Zwiazek JJ, Lieffers VJ (1999) Root water flow and growth of aspen (Populus tremuloides) at low root temperatures. Tree Physiol 19:879–884
Warren JM, Meinzer FC, Brooks JR, Domec J-C, Coulombe R (2007) Hydraulic redistribution of soil water in two old-growth coniferous forests: quantifying patterns and controls. New Phytol 173:753–765
Warren JM, Brooks JR, Meinzer FC, Eberhart JL (2008) Hydraulic redistribution of water from Pinus ponderosa trees to seedlings: evidence for an ectomycorrhizal pathway. New Phytol 178:382–394
Weatherley PE (1982) Water uptake and flow in roots. In: Lange O, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, new ser vol 12B. Springer, New York, pp 79–108
Worley JF, Hacskaylo E (1959) The effect of available soil moisture on the mycorrhizal association of Virginia pine. Forest Sci 5:267–268
Wu B, Watanabe I, Hayatsu M, Nioh I (1999) Effect of ectomycorrhizae on the growth and uptake and transport of N-15-labeled compounds by Pinus tabulaeformis seedlings under water-stressed conditions. Biol Fert Soils 28:136–138
Wudick MM, Luu DT, Maurel C (2009) A look inside: localization patterns and functions of intracellular plant aquaporins. New Phytol 184:289–302
Yi H, Calvo-Polanco M, MacKinnon MD, Zwiazek JJ (2008) Responses of ectomycorrhizal Populus tremuloides and Betula papyrifera seedlings to salinity. Environ Exp Bot 62:357–363
Zardoya R (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97:397–414
Acknowledgements
We thank Pedro J. Aphalo for the constructive comments on the manuscript. The Academy of Finland (decision no.: 123637) provided funding for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehto, T., Zwiazek, J.J. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21, 71–90 (2011). https://doi.org/10.1007/s00572-010-0348-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-010-0348-9