Abstract
In this study, the in situ restoration of urban landscape water through the combined application of sponge iron (SI) and calcium nitrate (CN) was conducted in the Xi’an Moat of China. The combination effect of SI and CN on the phosphorus (P) control was explored through laboratory and field experiments. Results showed that the optimum mass ratio of SI and CN was 4:1, and the optimum dosage of combined SI and CN was 1.4 g/L for controlling eutrophication in the water body at Xi’an Moat. The field experiment demonstrated that SI and CN efficiently controlled P concentration in overlying and interstitial water and obtained a maximum efficiency of 88.6 and 65.2% in soluble reactive P locking, respectively. The total P, organic P, and Ca-bound P contents in sediment simultaneously increased by 7.7, 15.2, and 2.4%, respectively, after 56 days. Therefore, the combined application of SI and CN achieved the goal of transferring the P from overlying and interstitial water to the sediment. Considering the environmental effect and economic investment, the combined application of SI and CN at a mass ratio of 4:1 and dosage of 1.4 g/L is an excellent choice for the in situ rehabilitation of eutrophic water with a high internal P load.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The severity of eutrophication is measured in both nutritional factors and biological factors, the latter being affected by the former. Among nutritional factors, nitrogen (N) and phosphorus (P) are the most critical factors that cause eutrophication (Smith and Schindler 2009). Increasing concentrations of N and P in the water body leads to a higher algal and chlorophyll a (chla) concentrations correspondingly, which eventually decrease the water transparency and dissolved oxygen (DO) concentration. The Organization for Economic Co-operation and Development (OECD) found that among 200 investigated lakes in the world, 80% are P-controlled type eutrophic lakes. Unsurprisingly, most of eutrophic lakes in China are controlled by their P concentration. Thus, P plays the most important role in water eutrophication (Kopácek et al. 2005). Although sufficiently reducing the P loading is important for controlling eutrophication, the remains of P loading in sediments may maintain waters in a eutrophic state for decades, thereby hampering the recovery of eutrophic environments (Søndergaard et al. 2007). Thus, even if no additional external P sources are introduced, the self-purification process and recovery from heavily eutrophic status in an urban landscape water system would remain a big challenge for an environmental scientist. Therefore, how to degrade the remains of P concentration in eutrophic waters has gotten the attention of experts worldwide.
In recent decades, several approaches have been developed to control eutrophication and recover the water body. Sediment dredging, an ectopic treatment, is one of the physical methods for removal of internal nutrients, particularly P, from the water body. The application of the sediment dredging in a system, however, is limited by its high labor and mechanical costs. Moreover, the sediment dredging process exposes the surrounding environment to the risk of new hazards (Jing et al. 2013; Liu et al. 2015a) if its follow-up treatment and disposal process are conducted improperly. In addition, the transportation of dredged sediment can also cause traffic problems when the landscape water body is in urban districts. Therefore, the in situ treatment of sediment has become highly popular in recent years. This treatment method is characterized by its low cost and high efficiency of P removal or at least P locking in severely eutrophic water (Hansen et al. 2003; Meis et al. 2013) and is particularly effective in the treatment of urban landscape waters when the accumulated sediment is not large enough for dredging, but the P load has already caused damage to the water environment.
In situ treatment with specific chemical reagents, such as aluminum and iron salts, is effective for controlling internal nutrient load (Cooke et al. 1993; Hansen et al. 2003; Hong et al. 2008; Liu et al. 2009; Meis et al. 2013). However, aluminum salt can be toxic to aquatic organisms (Pessot et al. 2014), and iron salt may be sensitive to the redox and pH conditions of waters (Burley et al. 2001; Immers et al. 2013). Recent research has shown that the addition of nitrate eliminates the malodors of black and anoxic water, suppresses the internal P loading of contaminated sediment, and improves the redox condition of waters (Hemond and Lin 2010; Ripl 1976; Wang et al. 2017; Xu et al. 2014; Yamada et al. 2012). At the same time, sponge iron (SI), an inorganic material used for P adsorption, can significantly, stably, and persistently lock soluble P in waters (Cheng et al. 2013; Wang et al. 2017). The effect of treatment with only the use of SI or calcium nitrate (CN), individually, on P levels of eutrophic water has been confirmed by our previous studies (Wang et al. 2017). However, the combination effect of SI and CN on eutrophic water is unclear. In this study, the pollution or eutrophication control was implemented in Xi’an Moat via in situ restoration through the combined application of SI and CN. Xi’an Moat was selected as a typical urban landscape water body where external P-sources are mostly controlled.
As a historical scenic spot located in the center of Xi’an, China, Xi’an Moat is presently used to control urban flooding and store rainwater. Xi’an Moat was historically used for 600 years to guard the city against military offense. Severe pollution and eutrophication have become the most important problems for the management of Xi’an Moat. The severe eutrophication of Xi’an Moat during summer causes the water body to become strongly oxygen-deficient, which results in blackish water, strong odors, massive death of fish, and disappearance of other aquatic organisms. The Xi’an Municipal Bureau had conducted several dredging works before 1998. Maintaining the water quality of Xi’an Moat via dredging has become more arduous in recent years, however, given rapidly increasing urban populations and growing traffic congestions in Xi’an. Thus, the discovery of another suitable method for the water rehabilitation in Xi’an Moat is urgent. The in situ rehabilitation is a potential water rehabilitation method to be applied in Xi’an Moat.
This study aims to explore the combination effect of SI and CN on the P control in Xi’an Moat. A laboratory experiment was first conducted to study the effect of the mass ratio of SI and CN (MSI:MCN) on the P concentration in the water body. In this experiment, P was released from Xi’an Moat sediments through a turbulent motion process. Then, to explore the optimal application dosage of SI and CN for controlling eutrophication, a field experiment was conducted in a severely eutrophic section of Xi’an Moat. Furthermore, the cost-benefit ratio of this combined treatment was compared with that of the popular lanthanum-modified bentonite Phoslock® treatment (Miquel and Frank 2013). This study is particularly important for the rehabilitation of similar urban landscape waters, like Xi’an Moat, whose treatment is limited by the geological locations.
Materials and methods
Sampling point and study site
The sediment used in the laboratory experiment were collected from a section of Xi’an Moat (34° 16′ 37.68″ N, 108° 55′ 13.81″ E) wherein the sediment was easy sampled and fertile. In this section, the mean water depth was approximately 1.0 m with a total area of approximately 58.8 ha. A stainless-steel grab sampler was used to collect the surface sediment at a depth of 10 cm. The collected sediment samples were immediately sealed in polyethylene bags, while squeezing air out to preclude sediment oxidation. Then, the samples were taken back to the laboratory and sieved with a filter of 15 mesh coarse to remove large particles such as stones and sand immediately. The sediment was used for the P-locking test after homogenization.
The field experiment (mesocosm) was carried out upstream Moat of Xi’an to avoid flood strikes on the experiment in summer. Three similar cylindrical stainless enclosures were installed in the same region of Xi’an Moat, as shown in Fig. 1. The main indicators of water and sediment samples in Xi’an Moat include the total P (TP), soluble reactive P (SRP), organic P (OP), Fe/Al-bound P (Fe/Al-P), and Ca-bound P (Ca-P) content. The corresponding values for the sediment and water samples in mesocosm are shown in Table 1.
P-locking materials
The SI is a rough, porous, and spongy inorganic product made from the carbon-reduced iron mineral under a melting temperature. The SI used in this experiment had the iron content of 96.6%, density of 2.62 g/cm3, and size of less than 500 μm. The CN used in this study was a colorless and transparent crystal with the chemical purity of more than 99%. The CN was procured from Tianli Chemical Reagent Co., Ltd. of Tianjin, China.
Laboratory experimental design
The laboratory experiment (microcosm) was performed to study the combination effect of SI and CN on the water quality and sediments of Xi’an Moat. It was conducted to determine the optimal mass ratio of SI and CN (MSI:MCN).
Four columnar glass vessels with an effective volume of 10 L each were used in parallel. The vessels were 23 cm in diameter, 38 cm in height, and had rubber lids that were 10 cm in diameter. Three glass tubes of 6 mm in diameter were installed on the lids. The tube for water sampling was 26 cm long, that for N2 agitation was 31 cm, and that for the exhausts was 5 cm. All vessels were covered with package foil to prevent the light exposure.
At the beginning of the experiment, the bottom of each vessel was filled with 2.5 L of fresh sediments (~ 500 g dry sediment). Subsequently, 100 g of SI and CN were added to the three vessels at MSI:MCN = 9:1, 4:1, and 1:1, respectively. Then, the sediments were gently mixed with SI and CN. The fourth vessel, which did not contain SI and CN, was used as the control group. Finally, the total volume of each vessel was brought up to 10 L by adding distilled water via the siphon method. To simulate the DO of the moat water, the DO concentration of each vessel was adjusted to less than 1 mg/L by pumping N2 into the vessel at the beginning of the experiment. The nitrogen was also used for mixing during the experiment. All vessels were maintained at room temperature in a static and dark state. The overlying water sample of each vessel was collected near the water–sediment interface on the 1st, 5th, 10th, 15th, 20th, 30th, and 40th day after SI and CN were introduced.
Field experimental design
The field experiment (mesocosm) was performed to study the combination effect of SI and CN on water quality and sediments in Xi’an Moat. It was conducted to identify the optimal dosage of SI and CN during the summer season from July to September of 2016, when the seasonal temperature ranges from 21 to 27 °C.
Three days before the experiment, three similar cylindrical stainless enclosures were installed upstream of Xi’an Moat, as shown in Fig. 1. No fish were present inside and outside of the enclosures during the experiment. The enclosures were hollow at both ends with a diameter of 1.2 m and a height of 1.6 m. The effective water depth and wet sediment depth in the enclosures were 1.05 and 0.2 m, respectively, which were equivalent to 1.2 m3 of water and 0.23 m3 of wet sediment. Both the inner and outer walls of the enclosures were coated with the epoxy resin to prevent corrosion.
A dosage (2.8 g/L) of combined SI and CN used in the mesocosm experiment was based on the dosage applied in the microcosm experiment. The P stock in the enclosures was estimated from 1.2 m3 of water and 0.23 m3 of wet sediment as mentioned above.
One cylindrical enclosure without SI and CN addition was used as the control group. The combined SI and CN were added at dosages of 1.4 and 2.8 g/L at MSI:MCN = 1:4 to the other two cylindrical enclosures. The water and sediments in the three enclosures were mixed completely by mechanical stirring at the beginning of the experiment. Water and sediment samples were taken at 50 cm below the water surface and on the surface of the water and sediment.
The sediment and water were sampled from the three enclosures on the 1st, 7th, 14th, 21st, 35th, and 56th days in the 56 days of operation. The samples were collected and transported to the laboratory for the further investigations.
Analytical methods
The wet sediment was centrifuged at 7000 rpm for 4 min to obtain the interstitial water and then freeze-dried. The sediment was sieved with a 100-mesh screen after freeze-drying process. All analyses for the overlying water, interstitial water, and sediment from both the microcosm and mesocosm experiments were performed in the Key Laboratory of Ministry of Education, China.
Water samples
The water samples were analyzed for TP, pH, turbidity, chla, and algal cell density. The TP concentration was determined via ascorbic acid-molybdate method. The pH was determined via glass electrode method. The turbidity was determined via spectrophotometric method. The chla concentration was determined using the phytoplankton delayed fluorescence spectrometer (TDF-3DC). The Algal cell density was counted under a microscope (ECLIPSE90i, Nikon, Japan) from 1 ml of water sample that was collected and immediately preserved in Lugol’s iodine solution for 24 h.
To determine the dissolved nutrient concentrations, water samples were filtered through 0.45-μm cellulose acetate membranes. The SRP concentration was calculated via ascorbic acid–molybdate method. The nitrate concentration was measured via UV spectrophotometry method. The nitrite concentration was obtained via N-(1-naphthalene)-diaminoethane method. The sulfate concentration was tested via anion chromatography method. All analytical results were obtained in accordance with standard methods (EPAC 2002).
Sediment samples
The TP content in sediment was tested via sodium hydroxide melt-Mo-Sb colorimetry. The P fraction, IP, OP, Fe/Al-P, and Ca-P in sediments were measured by continuous extraction in reference to the standard measurement and test (SMT) procedure of P forms in freshwater sediments (Hupfer et al. 1995, Ruban et al. 2001, Ruttenberg 1992). The sediment oxidation-reduction potential (ORP) was measured in situ using a pressed ORP meter. The total organic carbon (TOC) content in the sediment was tested via potassium dichromate oxidation–spectrophotometric method. The elemental C and S composition in the sediment was measured with an isotope ratio mass spectrometer (Isoprime 100).
Results
Laboratory experiment
The variation in the P and N concentrations of the overlying water that was treated with different MSI:MCN at the same total dosage was presented in Fig. 2. As seen from Fig. 2a, compared with the control, the addition of SI and CN at different ratios obviously reduced SRP concentration in the overlying water. Moreover, SRP concentration was maintained below 0.09 mg/L from the beginning to the end of the experiment. On the 40th day and with MSI:MCN of 9:1, 4:1, and 1:1, the SRP concentrations of the overlying water were 0.09, 0.01, and 0.02 mg/L, which were 88.3, 98.7, and 97.4% lower than those of the control group, respectively. These results indicated that adding SI and CN into the system had locked the majority of the SRP in the overlying water. However, the different ratios of MSI:MCN had exhibited different SRP locking efficiencies in overlying water as follows: MSI:MCN = 4 > MSI:MCN = 1 > MSI:MCN = 9, although the difference was not so significant statistically.
As seen in Fig. 2b, the addition of SI and CN at different ratios evidently increased the nitrate concentration of the overlying water at the beginning of the experiment when compared with the control group. The nitrate concentration of the overlying water increased with the increasing ratio of CN. On the first day, nitrate concentration was 68.08, 152.05, and 449.35 mg/L at the corresponding MSI:MCN of 9:1, 4:1, and 1:1, respectively. Then, the nitrate concentration gradually decreased over time after treatment with combined SI and CN. By the end of the experiment, the nitrate concentration in overlying water decreased to 0.27, 23.24, and 111.8 mg/L at the corresponding MSI:MCN of 9:1, 4:1, and 1:1, respectively. These results indicated that although the combined application of SI and CN clearly increased nitrate concentration in overlying water at the beginning of the experiment, the nitrate concentration in the overlying water would gradually decrease eventually.
The nitrite concentrations in the overlying water would also inevitably increase with the combined application of SI and CN, as shown in Fig. 2c. However, the time course of nitrite concentration in the overlying water varied with different ratios of the mass of SI and CN, although the nitrite concentration was first increased to maximum values and then gradually decreased. The nitrite concentration decreased to 0.01, 0.69, and 10.12 mg/L from the corresponding maximum values of 3.54, 10.61, and 29.74 mg/L at the ratios of mass of SI and CN of 9:1, 4:1, and 1:1, respectively.
Considering the toxic effect of nitrite on aquatic organisms (Liu et al. 2015b) and the efficiency of the SRP locking in overlying water, the optimum mass ratio of 4:1 for SI and CN was chosen to control P concentration in a water body in this study.
Field experiment
Effect of different dosages of combined SI and CN on overlying water
Change in pH and turbidity of overlying water
The variation in pH and turbidity of the overlying water that was treated with different dosages of combined SI and CN at MSI:MCN = 4 is presented in Fig. 3. As seen from Fig. 3a, the pH values of the overlying water that was treated with the combined SI and CN method at the doses of 1.4 and 2.8 g/L were higher than those of the control group during the whole experimental period. Concurrently, the pH value of the water treated with 2.8 g/L combined SI and CN was always higher than that of the water treated with 1.4 g/L combined SI and CN. Therefore, the treatment with the combination of SI and CN increased the pH value of the overlying water, and the higher doses lead to a higher pH value. As seen from Fig. 3b, the turbidity of the overlying water treated with the combined SI and CN method at the doses of 1.4 and 2.8 g/L respectively, distinctly lowered during the first 21 days of the operation compared with that of the control group. The turning point occurred at the 21st day when the turbidity of the treated overlying water was higher than that of the control group. From then on, the turbidity in the water treated with the combined SI and CN method at the dose of 1.4 g/L was similar to that of the control group. However, in the water that was treated with 2.8 g/L combined SI and CN, the turbidity sharply increased on the 35th day when the turbidity reached a maximum value of 100.27 NTU, which was almost 1.6 times higher than that of the control. Turbidity then decreased to 65.22 NTU and finally reached the same level as of the control group. Therefore, treatment with 2.8 g/L combined SI and CN brought a higher pH value and destabilized the turbidity of the water compared with treatment with 1.4 g/L combined SI and CN.
Change in TP and SRP concentrations of overlying water
The variation in TP and SRP concentration in overlying water that was treated with different dosages of combined SI and CN at MSI:MCN = 4:1 is presented in Fig. 4. As seen from Fig. 4, both the TP and SRP concentrations in the overlying water decreased under treatment with 1.4 and 2.8 g/L combined SI and CN. During the entire experiment, the TP concentration decreased by 54.2–69.2% in overlying water when treated with 1.4 g/L combined SI and CN method and by 32.4–74.4% in overlying water when treated with 2.8 g/L combined SI and CN. The TP concentration reached the minimum value on the 14th day for both the samples treated with 1.4 and 2.8 g/L combined SI and CN. At the end of the experiment, the TP concentrations in the overlying water decreased to 0.11 and 0.09 mg/L, respectively, for the different treatment with 1.4 and 2.8 g/L combined SI and CN, whereas TP concentration in the control group remained stable at 0.24 mg/L. Similarly, SRP concentration decreased by 62–88.6% in the overlying water when treated with1.4 g/L combined SI and CN and by 72.9–90.5% in the overlying water when treated with 2.8 g/L combined SI and CN doses. The SRP concentration in both samples reached the minimum value on the 14th day when treated with 1.4 and 2.8 g/L combined SI and CN. On the 56th day of the experiment, the SRP concentrations were 0.05 mg/L and 0.03 mg/L in the overlying water treated with 1.4 and 2.8 g/L combined SI and CN, respectively, whereas the corresponding concentration for the control group remained constant at 0.13 mg/L. Therefore, the treatment with the combined SI and CN method at MSI:MCN = 4 and dosage of 1.4 and 2.8 g/L obviously controlled both the concentration of TP and SRP in the overlying water. Given that the TP and SRP concentrations in the overlying water were similar at the end of experiment for both samples treated with 1.4 and 2.8 g/L combined SI and CN doses, respectively, the treatment dosage of 1.4 g/L may be more effective for P control in terms of choosing the reagent dose.
Change in the nitrate and nitrite concentrations of overlying water
The variation in the nitrate and nitrite concentrations of the overlying water that was treated with different dosages of combined SI and CN at MSI:MCN = 4:1 is presented in Fig. 5. As seen from Fig. 5a, compared with the control group, the treatment with combined SI and CN substantially increased nitrate concentration in the overlying water at the beginning of the experiment. When the experiment continued, the nitrate concentration in the overlying water considerably increased with increasing treatment dosages of combined SI and CN. The nitrate concentrations reached 54.06 and 106.54 mg/L on the first day of the experiment with 1.4 and 2.8 g/L combined SI and CN doses, respectively. The nitrate concentration gradually decreased until the 35th day of the experiment when the nitrate concentration in the overlying water treated with both 1.4 and 2.8 g/L combined SI and CN doses were close to the control group and with a value below 1.0 mg/L. By contrast, the nitrite concentrations in the overlying water gradually increased at the beginning of treatment and then decreased at the end of the experiment, as shown in Fig. 5b. The maximum nitrite concentration in the overlying water occurred on day 14. The maximum nitrite concentration was 0.98 mg NO2−/L in the overlying water when treated with 1.4 g/L combined SI and CN and 1.11 mg NO2−/L in the overlying water when treated with 2.8 g/L combined SI and CN.
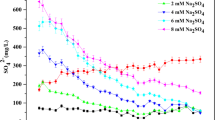
Change in sulfate concentration of overlying water
The variation in sulfate concentration of the overlying water that was treated with different dosages of combined SI and CN at MSI:MCN = 4:1 is presented in Fig. 6.
As seen from Fig. 6, the sulfate concentration of the overlying water obviously increased when treated with the combined SI and CN method, while the control group remained at a stable level of sulfate concentration during the entire experiment. The sulfate concentration of the overlying water reached the maximum value on the 14th day of the experiment. The maximum sulfate concentration was 121.5 and 139.2 mg/L when treated with 1.4 and 2.8 g/L combined SI and CN, respectively. The sulfate concentration was 1.75 and 2 times higher than that of the control group on the 14th day during the experiment. Then, the sulfate concentration gradually decreased and stabilized after 35 days of the experiment. By the end of the experiment, the sulfate concentrations in the overlying water were 92.6, 84.2, and 66.6 mg/L for the 1.4 and 2.8 g/L combined SI and CN and control group (no treatment), respectively. The treatment with combined SI and CN enhanced the sulfate concentration of the overlying water. Moreover, a higher treatment dosage increased sulfate concentration in the overlying water of Xi’an Moat.
Change in algal cell density and chla concentration of overlying water
The variation in the algal cell density and chla concentration in overlying water that was treated with different dosages of combined SI and CN at MSI:MCN = 4:1 is presented in Fig. 7. As seen from Fig. 7, both the algal cell density and chla concentration in the overlying water that was treated with combined SI and CN decreased before the 14th day of the experiment compared with the control. From the 21st day onwards, both the algal cell density and chla concentration in the overlying water in the samples that were treated with the combined SI and CN were higher than in the control group. However, the treatment with different dosages of combined SI and CN resulted in different variation trends in both the algal cell density and chla concentration in the overlying water. The algal cell density and chla concentration exponentially increased under the treatment with 2.8 g/L combined SI and CN dose, whereas the treatment with 1.4 g/L combined SI and CN dose caused fluctuations in both the algal cell density and chla concentration of the overlying water. At the end of the experiment, the algal cell density of overlying water was 256 × 103 and 373 × 103 cells/mL under treatment with 1.4 and 2.8 g/L combined SI and CN, respectively, which were 1.23 and 1.79 times higher than that in the control group. Meanwhile, the chla concentration in the overlying water in the experiment was 53.68 and 109.61 μg/L under treatment with 1.4 and 2.8 g/L combined SI and CN doses, respectively, and were 2 and 4.16 times higher than that in the control group, respectively. Consequently, the treatment with combined SI and CN inhibited algal growth within the first 14 days but promoted algal growth afterwards during the experiment. Moreover, although a high treatment dosage evidently inhibited algal growth within the first 14 days, the high treatment dosage also promoted algal growth in overlying water afterwards.
Effect of different dosages of combined SI and CN on sediment
Change in TP and SRP concentrations in interstitial water from sediment
The variation in TP and SRP concentrations in the interstitial water from sediments treated with different dosages of combined SI and CN at MSI:MCN = 4:1 was presented in Fig. 8. As seen from the figure, both the TP and SRP concentrations in the interstitial water decreased except for on the 7th day of the experiment when the combined SI and CN was added into the samples. The TP locking efficiency in the interstitial water of the combined SI and CN reached maximum values on the 14th day of the experiment. The TP locking efficiency was 54.6 and 70.5% under treatment with 1.4 and 2.8 g/L combined SI and CN methods, respectively. At the end of the experiment, TP concentrations in the interstitial water were 1.35 and 1.01 mg/L under treatment with1.4 and 2.8 g/L combined SI and CN methods, respectively, with locking efficiencies of 41 and 55.9% correspondingly. Similarly, on the 14th day of the experiment, the maximum efficiencies for locking SRP also reached their highest point, which were 65.2 and 80.4% under the treatment with 1.4 and 2.8 g/L combined SI and CN methods, respectively. By contrast, on the 56th day of the experiment, the SRP concentrations in the interstitial water were similar, which were 0.40 and 0.38 mg/L under treatment with 1.4 and 2.8 g/L combined SI and CN methods, respectively.
Change in C and S contents and ORP of sediment
The mass percentages of carbon (C) and sulfur (S) in the sediment also changed after treatment with combined SI and CN at MSI:MCN = 4:1, as shown in Table 2 and Fig. 9a.
As seen from Table 2, the C and S content of sediment obviously decreased upon treatment with the combined SI and CN method. However, the C and S contents in the sediment decreased distinctly more under treatment with 2.8 g/L combined SI and CN than under treatment with 1.4 g/L combined SI and CN. After 56 days, compared with the control, the C and S contents in the sediment decreased by 30.9 and 60.4%, respectively, under treatment with 2.8 g/L combined SI and CN, and by 28.3 and 55.8%, respectively, under treatment with 1.4 g/L combined SI and CN. The change in the TOC contents of the sediments also indicated that the C content decreased, as shown in Fig. 9a. The treatment with 1.4 and 2.8 g/L combined SI and CN method decreased the TOC contents in the sediment during the entire experiment. By the end of the experiment, the TOC content in the sediment was 63.77 and 61.54 mg/g under treatment with 1.4 and 2.8 g/L combined SI and CN method, respectively, indicating that the TOC content decreased by 24.9 and 27.6%, respectively. These values were consistent with the aforementioned 28.3 and 30.9% reduction in the C content in the sediment.
The treatment with combined SI and CN also changed the ORP in the sediment, as shown in the Fig. 9b. The addition of combined SI and CN increased the ORP in the sediment. Moreover, the ORP increased with increasing treatment dosage. In addition, the treatment with combined SI and CN caused visibly fluctuations in the ORP of the sediment during the whole experiment.
Change in P content in sediment
The variation in different P forms in sediment that was treated with different dosages of combined SI and CN at MSI:MCN = 4:1 is presented in Fig. 10.
As shown in Fig. 10a, compared with the control group, the treatment with combined SI and CN method increased the TP content. Moreover, the TP content increased with increasing treatment dosage. On the 7th day of the experiment, the TP content was 2.30 and 2.37 mg/g in the sediment treated with 1.4 and 2.8 g/L combined SI and CN doses, respectively, and were 1.22 and 1.26 times higher than that of the control group, respectively. Both values then remained relatively stable value until the end of the experiment. At the end of the experiment, the TP content was 2.23 and 2.30 mg/g in the sediment that was treated with 1.4 and 2.8 g/L combined SI and CN, respectively, whereas in the control group the TP content was 2.07 mg/g.
As shown in Fig. 10b, at the initial stage of the experiment, the OP content remained relatively stable in the treated sediment compared with that of the control sediment. From the 14th day onward, the OP content was higher in the sediments which were treated with the combined SI and CN than in the control sediment. The maximum OP values were observed on the 21st day and were 0.56 and 0.52 mg/g in the sediments treated with 1.4 and 2.8 g/L combined SI and CN, respectively. These values were 1.27 and 1.18 times higher than that of the control sediment. On the 56th day, the OP content was 0.53 and 0.52 mg/g in the sediment that was treated with 1.4 and 2.8 g/L combined SI and CN, respectively, which were 1.15 and 1.13 times higher than that of the control sediment. Therefore, the treatment with the combined SI and CN method increased the OP content in the sediment. Moreover, the OP content in the sediments increased with increasing treatment dose.
Fe/Al-P, a potential bioavailable form of P in sediment, has a high potential for releasing phosphate into water (Wang et al. 2009). As seen from Fig. 10c, treatment with the combined SI and CN increased Fe/Al-P contents in the sediment to a maximum point on the 7th day. Fe/Al-P content then decreased gradually until the end of the experiment. Fe/Al-P content increased with increasing treatment dosages. Meanwhile, Fe/Al-P content in the treated sediment was always higher than that of the control sediment except on the 56th day of the experiment. This deviation may be attributed to the increased Fe/Al-P content in the control sediment and the decreased Fe/Al-P content in the treated sediment. Given that Ca-P is refractory (Kaiserli et al. 2002), with a lower potential for releasing P, Ca-P is a relatively stable form of P in sediment. As seen from Fig. 10d, during the entire experiment, Ca-P content was higher in the treated sediment than in the control sediments. A higher treatment dosage led to a higher Ca-P content in sediments. By the end of the experiment, the Ca-P content was 0.85 and 0.89 mg/g in the sediment that was treated with 1.4 and 2.8 g/L combined SI and CN, respectively. These values were 102.4% and 107.2% higher than the Ca-P content in the control sediment.
Discussion
Comprehensive effect of combined SI and CN on the water body
The treatment with combined SI and CN doses increased the pH value of eutrophic water (Fig. 3). The increased pH is beneficial for the formation of FePO4 when Fe3+ and PO43− coexist (Immers et al. 2013), thus promoting the fixation of P in water (Fig. 4). Meanwhile, the iron ions from SI were dissolved in the water, thus promoting the flocculation of the suspended solids and colloids (Wang et al. 2008). Therefore, the application of the combined SI and CN method reduced the turbidity and increased the transparency of the water body during the first 14 days of the experiment (Fig. 3b). However, the aquatic algae could use CN as a N source, which might cause the persistent growth of the algae even though the ammonia N is deficient (Li et al. 2016). Therefore, the turbidity increased and destabilized during the first 14 days when treated with the combined SI and CN. Moreover, the turbidity later increased after the first 14 days, when a high treatment dosage of combined SI and CN was used.
A relatively high nitrate concentration (> 12 mg N L−1) was observed in the overlying water at the beginning of the field experiment (Fig. 5a). The higher nitrate concentration is an unavoidable consequence of adding CN to control P in water. A comprehensive review of nitrate toxicity data revealed that the observed aqueous nitrate concentration exceeded the recommended safety level of 10 mg N L−1 (Camargo et al. 2005) for freshwater species. This result implied a potential risk of incurring intolerable nitrate toxicity to sensitive aquatic organisms when CN is introduced into the water body. Fortunately, although a slightly higher nitrate concentration occurred in the initial stage of the experiment, the nitrate concentration remained at a safe level for the majority of the experiment (Fig. 5a).
Nitrite is a common intermediate product of denitrification and nitrate reduction to ammonium in an anoxic environment. On the 14th day of the study, the nitrite concentrations in the overlying water increased and reached the maximum values of 0.30 and 0.34 mg N/L at the treatment dosages of 1.4 and 2.8 g/L, respectively (Fig. 5b). This result, in terms of the aqueous nitrite concentration, coincides with that observed in a previous study on the toxicity of CN addition (Liu et al. 2015b; Yamada et al. 2012) and provided evidences that this level of nitrite concentration may be toxic to sensitive freshwater invertebrates and fishes (Camargo and Alonso 2006). Based on the water quality criteria for protecting freshwater aquatic life from short-term toxicity, the recommended concentration of nitrite concentration is 0.1 mg N/L (Yamada et al. 2012). Therefore, for a short period, e.g., 14 days, in this experiment, the nitrite concentration in the overlying water for both dosages was higher than the established guideline (Fig. 5b).
Certain concentrations of nitrate and nitrite are toxic to living aquatic species (Camargo and Alonso 2006, Liu et al. 2015b). However, these toxic effects could become weaker and weaker or even disappear completely with time (Yamada et al. 2012). Given that CN can damage the aquatic ecosystem, the severity of pollution should be evaluated before applying CN to control eutrophication. In this experiment, the tested sites in Xi’an Moat were severely eutrophic and contained limited aquatic life, and were dominated by phytoplankton. In this case, the application of combined SI and CN to decrease eutrophication was appropriate.
Adding the combined SI and CN into the water increased sulfate concentration in the overlying water (Fig. 6) and at the same time decreased total carbon and sulfide in the sediment (Table 2). This result indicated that the sulfides and organic carbon in the sediment could be oxidized to sulfate and CO2, whereas additional NO3− was reduced by the indigenous microorganisms into N or N oxides via denitrification. Therefore, the addition of the combined SI and CN could improve the current blackish color and foul odor of water in Xi’an Moat when no additional external sources of pollution were introduced into this system.
Adding both doses (1.4 and 2.8 g/L combined SI and CN) into eutrophic water was very effective in terms of controlling the algal growth within the first 14 days period (Fig. 7). This result likely occurred because nitrite concentration in overlying water exceeded the threshold within the first 14 days (Fig. 5b), thus decreasing algal biomass. Nitrite toxicity gradually decreased below the threshold after 21 days. Moreover, nitrate is a N source for algal growth (Bradley et al. 2010; Cochlan et al. 2008), thus increasing algal cell density and chla concentration in overlying water after 21 days. In addition, the algal growth was evidently promoted when a high dosage of combined SI and CN was applied. Therefore, both the background of the aquatic system and the duration of treatment should be considered when using the combined SI and CN method to control water quality. In this study, 1.4 g/L combined SI and CN is recommended for controlling algal growth in Xi’an Moat, and the treatment should be limited to within 14 days in the summer when the water temperature was about 25 °C (23~27 °C). The combination of SI and CN under the dosage and mass ratio in our paper was proposed as an effective approach for controlling the water quality in the summer in Xi’an Moat. However, this is not a method that can be completed once for all, but as an alternative method for emergency eutrophication eruption in the summer. In terms of the other seasons, the application dosage of this treatment depends on the algae density in the water.
Effect of combined SI and CN on P concentration in eutrophic water and P forms in sediment
The maximum efficiency of locking SRP reached 88.6 and 90.5% in the overlying water (Fig.4b), and 65.2 and 80.4% in the interstitial water (Fig. 8b) when treated with 1.4 and 2.8 g/L combined SI and CN, respectively. Thus, the addition of combined SI and CN obviously decreased P concentration in eutrophic water.
SI, a P-locking material, was used to fix P from water into sediments in this study. SI can stably and persistently lock P in eutrophic water (Wang et al. 2017). In addition, given that Fe2+ oxidation is mediated by microorganisms, and flocculation and precipitation are chemically dominated by Fe3+, a strong synergy and mutual promoting effect on P fixation may exist between SI and microorganisms (Wang et al. 2015).
CN has been reported numerous times as a P-locking compound in previous literature (Lin et al. 2015; Wang et al. 2017; Wauer et al. 2005; Yamada et al. 2012). The main mechanism of P-locking is as follows: nitrate acts as an electron acceptor, resulting in the oxidation of organic matter and other reduced substrates, such as sulfides and iron compounds. In turn, these oxidized compounds may oxidize Fe2+ into Fe3+ and further enhance iron oxide to absorb more P into the sediment. By contrast, calcium phosphate precipitates when the Ca2+ and PO43− combine together in water, eventually increasing Ca–P content in the sediment (Fig. 10d), and inhibiting P release from the sediments.
The addition of combined SI and CN increased TP, OP, and Ca-P contents in the sediment to a certain extent. Under the treatment, however, the Fe/Al-P content in the sediment was higher than that in the control group before the 35th day of the experiment and was lower than that in the control at the end of the experiment (Fig. 10). P-locking in the overlying and interstitial water increased the different P forms in the sediment. Meanwhile, the higher concentrations of N available for usage stimulated the growth of microorganisms in the sediment and transformed parts of the bioavailable Fe/Al-P into OP (Hemond and Lin 2010; Wang et al. 2017; Xu et al. 2014; Yamada et al. 2012). However, a previous study (Lin et al. 2015) showed that at 66 days after the addition of CN to control P in the sediment, Fe/Al-P increased and OP only slightly increased in sediment. By contrast, Ca-P content did not markedly change. The difference between this result and that of the current study could be related to the background environment of the sediment, as well as to the dosage and types of P-locking materials. Overall, the addition of combined SI and CN changed the chemical environment of the aquatic system, evidently decreased the P levels in the overlying and interstitial water, and increased TP, OP, and Ca-P contents in the sediment.
Comparison of SRP locking efficiency among CN, SI, and the combination of SI and CN
In our field experiment, when treated with 1.4 g/L combined SI and CN, the efficiency of SRP locking was 62.2% in the overlying water and 62.3% in the interstitial water after day 56. The results of our previous experiment in the laboratory (Wang et al. 2017) showed that the dosage of 15 g/L CN had an obvious effect on lowering the phosphorus concentration in the overlying water and interstitial water. The phosphorus-locking efficiency for SRP in the overlying water and interstitial water was 99.1 and 95.5%, respectively, after the treatment of 68 days. Under the same conditions, the phosphorus-locking efficiency of SRP in the overlying water and interstitial water with the SI treatment at 15 g/L was 78.5 and 77.9%, respectively.
Although there were differences for SRP locking efficiency among CN, SI, and SI+CN as described above, the difference actually depended on not only the reagent type but the application dosage. It is obvious that high P-locking efficiency was based on the large dosage applied. However, harmful effects may occur when excessive amounts of calcium nitrate and sponge iron are applied to control the phosphorus concentration in eutrophic water. Too much calcium nitrate may be toxic to sensitive aquatic organisms, whereas too much sponge iron would increase the total mass of the sediment. Thus, from considerations based on the environmental effect and the economic investment required, the dosage of 1.4 g/L combination of SI and CN is a better choice for the in situ restoration of a water body with high internal P loading.
Optimal dosage of combination agent and its cost-effect analysis
The efficiency of SRP locking was only slightly higher at the dosage of 2.8 g/L than that at 1.4 g/L. Moreover, both dosages significantly, stably, and persistently locked P in eutrophic water. However, the application dosage of 2.8 g/L substantially promoted algal growth, further aggravating the eutrophication of the water body. In addition, the application dosage of 2.8 g/L is double the cost of using 1.4 g/L instead. Considering the environmental effect and economic investment, the combination dosage of 1.4 g/L and MSI:MCN = 4:1 are recommended for the in-situ restoration of eutrophic water with high internal P loads.
In this study, the costs of our combination agent and the P-locking material were calculated and compared based on the present market price of SI, CN, and Phoslock®. Lürling and Oosterhout (2013) used Phoslock® (Phoslock®:Preleasable = 100:1) to control releasable P in overlying water and in sediment from the 5-cm layer of a eutrophic lake in Rauwbraken, Netherlands. In their study, Phoslock® cost approximately ¥18,300 per ton, indicating that the cost per unit mass of P locked by Phoslock® was approximately ¥1.83/g P. To compare the economic benefits of Phoslock® and combined SI and CN (MSI:MCN = 4:1) and to calculate releasable P, the overlying water and 5-cm layer sediment were similarly selected as research objects in the present study. In our experiment, the total releasable P in the overlying water and 5-cm layer sediment was approximately 6.7 g, and the total dosage of combined SI and CN used was approximately 2 kg based on a 1.4 g/L dosage, thus providing a combined agent to releasable P mass ratio of 300:1. Given the mass ratio of the combination agent (MSI:MCN = 4:1) dosage and the releasable P was 300:1, the cost of industrial CN was approximately ¥1800 per ton and that of SI was approximately ¥4500 per ton. Thus, the cost per unit mass of P that was locked by the combined SI and CN in our experiment was approximately ¥1.19/g P.
The cost to lock the same mass of P by the combined SI and CN was approximately 35% cheaper than that by Phoslock®. Thus, the economic benefit of the combined SI and CN in P locking in a eutrophic water system was competitive compared with Phoslock®.
Conclusions
The laboratory and field experiments were successively performed to study the combination effect of SI and CN on water and sediment quality of Xi’an Moat. The following conclusions were drawn:
In the laboratory experiment, different combinations of SI and CN at different ratios of mass obviously decreased P concentration in the overlying water. However, considering the toxicity of nitrate and nitrite to aquatic organisms, the optimum MSI:MCN = 4:1 was proposed for P control in water bodies.
In the field experiment, the addition of 1.4 and 2.8 g/L combined SI and CN to eutrophic water increased the pH value of the water body for the entire duration of the experiment and decreased the turbidity of the water body within the 21 days. In addition, the treatment with 2.8 g/L combined SI and CN increased pH but destabilized turbidity in the water compared with the treatment with 1.4 g/L dose. The addition of combined SI and CN temporarily increased nitrate and nitrite concentrations in the overlying water. Certain levels of nitrate and nitrite concentrations may exert toxic effects on the sensitive freshwater species. In this study, nitrate and nitrite concentrations quickly lowered to safe levels over time. Meanwhile, the addition of 1.4 and 2.8 g/L combined SI and CN to eutrophic water inhibited algal growth within the first 14 days of the experiment. The treatment with 2.8 g/L combined SI and CN, however, evidently promoted algal growth after 14 days. Therefore, treatment with 1.4 g/L combined SI and CN is recommended for controlling algal growth in Xi’an Moat. Moreover, the effective duration of application should be limited to within 14 days during the summer.
Considering the environmental effect and economic investment required, the dose of 1.4 g/L combined SI and CN is a better choice for the in situ restoration of a water body with high internal P loads. At the dose of 1.4 g/L combined SI and CN, the efficiency of SRP locking was 62.2% in the overlying water and 62.3% in the interstitial water after 56 days of the experiment. Under this treatment, TP, OP, and Ca-P contents in sediment increased by 7.7, 15.2, and 2.4%, respectively. Meanwhile, sulfides and organic carbon in sediment were oxidized into sulfate and CO2 as a result of improved redox conditions in the sediment. The economic benefit of the combination agent was competitive when compared with that of Phoslock® in terms of P-locking in a eutrophic water system.
Despite the temporary negative biological impacts observed in both laboratory and field experiments, the results of this study indicated that the combined application of SI and CN at MSI:MCN = 4:1 and dosage of 1.4 g/L is an excellent approach to transfer P from eutrophic water to the sediments, a more stable form. Therefore, the combined application of SI and CN is a promising technology for the remediation of significantly polluted water bodies such as Xi’an Moat.
Despite the temporary negative biological impacts observed in both laboratory and field experiments, the results of this study indicated that the combined application of SI and CN at MSI:MCN = 4:1 and dosage of 1.4 g/L is an excellent approach to transfer P from eutrophic water to the sediments, a more stable form. Therefore, the combined application of SI and CN is a promising technology for the remediation of severely polluted water bodies such as Xi’an Moat.
References
Bradley PB, Sanderson MP, Frischer ME, Brofft J, Booth MG, Kerkhof LJ, Bronk DA (2010) Inorganic and organic nitrogen uptake by phytoplankton and heterotrophic bacteria in the stratified Mid-Atlantic Bight. Estuarine Coastal Shelf Sci 88(4):429–441. https://doi.org/10.1016/j.ecss.2010.02.001
Burley KL, Prepas EE, Chambers PA (2001) Phosphorus release from sediments in hardwater eutrophic lakes: the effects of redox-sensitive and -insensitive chemical treatments. Freshw Biol 46(8):1061–1074. https://doi.org/10.1046/j.1365-2427.2001.00789.x
Camargo JA, Alonso A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int 32(6):831–849. https://doi.org/10.1016/j.envint.2006.05.002
Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58(9):1255–1267. https://doi.org/10.1016/j.chemosphere.2004.10.044
Cheng J, Jia L, He Y, Zhang B, Kirumba G, Xie J (2013) Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. J Colloid Interf Sci 402:246–252
Cochlan WP, Herndon J, Kudela RM (2008) Inorganic and organic nitrogen uptake by the toxigenic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 8(1):111–118. https://doi.org/10.1016/j.hal.2008.08.008
Cooke GD, Welch EB, Martin AB, Fulmer DG, Hyde JB, Schrieve GD (1993) Effectiveness of Al, Ca, and Fe salts for control of internal phosphorus loading in shallow and deep lakes. Springer, Netherlands, pp 323–335
EPAC (Environmental Protection Agency of China) (2002) Standard Methods for the Examination of Water and Wastewater, 4th ed. Chinese Environmental Science Press, Beijing (in Chinese)
Hansen J, Reitzel K, Jensen HS, Andersen FØ (2003) Effects of aluminum, iron, oxygen and nitrate additions on phosphorus release from the sediment of a Danish softwater lake. Hydrobiologia 492(1-3):139–149. https://doi.org/10.1023/A:1024826131327
Hemond HF, Lin K (2010) Nitrate suppresses internal phosphorus loading in an eutrophic lake. Water Res 44(12):3645–3650. https://doi.org/10.1016/j.watres.2010.04.018
Hong XU, Zhang J, Gao Y (2008) Experiment study on the removal of phosphorus in eutrophic water bodies by the utilization of mineral calcite. Earth Sci Front 15:138–141
Hupfer M, Gächter R, Giovanoli R (1995) Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci 57(4):305–324. https://doi.org/10.1007/BF00878395
Immers AK, Sande MTVD, Zande RMVD, Geurts JJM, Donk EV, Bakker ES (2013) Iron addition as a shallow lake restoration measure: impacts on charophyte growth. Hydrobiologia 710(1):241–251. https://doi.org/10.1007/s10750-011-0995-7
Jing LD, Wu CX, Liu JT, Wang HG, Ao HY (2013) The effects of dredging on nitrogen balance in sediment-water microcosms and implications to dredging projects. Ecol Eng 52:167–174. https://doi.org/10.1016/j.ecoleng.2012.12.109
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments—lakes Volvi and Koronia. N Greece Chemosphere 46(8):1147–1155. https://doi.org/10.1016/S0045-6535(01)00242-9
Kopácek J, Borovec J, Hejzlar J, Ulrich KU, Norton SA, Amirbahman A (2005) Aluminum control of phosphorus sorption by lake sediments. Environ Sci Technol 39(22):8784–8789. https://doi.org/10.1021/es050916b
Li J, Zhang J, Wei H, Kong F, Yue L, Min X, Zheng Z (2016) Comparative bioavailability of ammonium, nitrate, nitrite and urea to typically harmful cyanobacterium Microcystis aeruginosa. Mar Pollut Bull 110(1):93–98. https://doi.org/10.1016/j.marpolbul.2016.06.077
Lin J, Qiu P, Yan X, Xiong X, Jing L, Wu C (2015) Effectiveness and mode of action of calcium nitrate and Phoslock® in phosphorus control in contaminated sediment, a microcosm study. Water Air Soil Pollut 226:1–12
Liu GR, Chun-Song YE, Jing-Hao HE, Qian Q, Jiang H (2009) Lake sediment treatment with aluminum, iron, calcium and nitrate additives to reduce phosphorus release. J Zhejiang Univ-Sci A 10(9):1367–1373. https://doi.org/10.1631/jzus.A0920028
Liu C, Shen Q, Zhou Q, Fan C, Shao S (2015a) Precontrol of algae-induced black blooms through sediment dredging at appropriate depth in a typical eutrophic shallow lake. Ecol Eng 77:139–145. https://doi.org/10.1016/j.ecoleng.2015.01.030
Liu T, Yuan J, Dong W, Wu H, Wang H (2015b) Effects on inorganic nitrogen compounds release of contaminated sediment treatment with in situ calcium nitrate injection. Environ Sci Pollut Res 22(2):1250–1260. https://doi.org/10.1007/s11356-014-3421-7
Lürling M, Oosterhout FV (2013) Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res 47(17):6527–6537. https://doi.org/10.1016/j.watres.2013.08.019
Meis S, Spears BM, Maberly SC, Perkins RG (2013) Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (Loch Flemington, UK). Water Res 47(13):4460–4473. https://doi.org/10.1016/j.watres.2013.05.017
Miquel L, Frank VO (2013) Controlling eutrophication by combined bloom precipitation and sediment phosphorus inactivation. Water Res 47:6527–6537
Pessot CA, Åtland Å, Liltved H, Lobos MG, Kristensen T (2014) Water treatment with crushed marble or sodium silicate mitigates combined copper and aluminium toxicity for the early life stages of Atlantic salmon ( Salmo salar L.) Aquac Eng 60:77–83. https://doi.org/10.1016/j.aquaeng.2014.04.001
Ripl W (1976) Biochemical oxidation of polluted lake sediment with nitrate: a new lake restoration method. Ambio 5:132–135
Ruban V, López-Sánchez JF, Pardo P, Rauret G, Muntau H, Quevauviller P (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments--a synthesis of recent works. Anal Bioanal Chem 370(2-3):224–228. https://doi.org/10.1007/s002160100753
Ruttenberg KC (1992) Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol Oceanogr 37(7):1460–1482. https://doi.org/10.4319/lo.1992.37.7.1460
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24(4):201–207. https://doi.org/10.1016/j.tree.2008.11.009
Søndergaard M, Jeppesen E, Lauridsen TL, Skov C, Van Nes EH, Roijackers R, Lammens E, Portielje R (2007) Lake restoration: successes, failures and long-term effects. J Appl Ecol 44(6):1095–1105. https://doi.org/10.1111/j.1365-2664.2007.01363.x
Wang S, Jin X, Zhao H, Wu F (2009) Phosphorus release characteristics of different trophic lake sediments under simulative disturbing conditions. J Hazard Mater 161:1551–1559
Wang Y, Gao B, Yue Q, Wei J, Li Q (2008) The characterization and flocculation efficiency of composite flocculant iron salts–polydimethyldiallylammonium chloride. Chem Eng J 142(2):175–181. https://doi.org/10.1016/j.cej.2007.11.022
Wang Y, Li J, Zhai S, Wei Z, Feng J (2015) Enhanced phosphorus removal by microbial-collaborating sponge iron. Water Sci Technol A J Int Assoc Water Pollut Res 72(8):1257–1265. https://doi.org/10.2166/wst.2015.323
Wang G, Wang Y, Guo Y, Peng D (2017) Effects of four different phosphorus-locking materials on sediment and water quality in Xi'an moat. Environ Sci Pollut Res 24(1):264–274. https://doi.org/10.1007/s11356-016-7796-5
Wauer G, Gonsiorczyk T, Casper P, Koschel R (2005) P-immobilisation and phosphatase activities in lake sediment following treatment with nitrate and iron. Limnologica - Ecol Manag Inland Waters 35(1-2):102–108. https://doi.org/10.1016/j.limno.2004.08.001
Xu M, Zhang Q, Xia C, Zhong Y, Sun G, Guo J, Yuan T, Zhou J, He Z (2014) Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J 9:1932–1944
Yamada TM, Sueitt APE, Beraldo DAS, Botta CMR, Fadini PS, Nascimento MRL, Faria BM, Mozeto AA (2012) Calcium nitrate addition to control the internal load of phosphorus from sediments of a tropical eutrophic reservoir: microcosm experiments. Water Res 46:6463–6475
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant number: 21677115) and the Technology Bureau of Xi’an (Project No. SF1430). The authors wish to express great gratitude to the City Wall Management Committee of Xi’an for their supporting to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wang, Gb., Wang, Y. & Zhang, Y. Combination effect of sponge iron and calcium nitrate on severely eutrophic urban landscape water: an integrated study from laboratory to fields. Environ Sci Pollut Res 25, 8350–8363 (2018). https://doi.org/10.1007/s11356-017-1161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-1161-1