Abstract
In this work, sediments were treated with calcium nitrate, aluminum sulfate, ferric sulfate, and Phoslock®, respectively. The impact of treatments on internal phosphorus release, the abundance of nitrogen cycle-related functional genes, and the growth of submerged macrophytes were investigated. All treatments reduced total phosphorus (TP) and soluble reactive phosphorus (SRP) in interstitial water, and aluminum sulfate was most efficient. Aluminum sulfate also decreased TP and SRP in overlying water. Treatments significantly changed P speciations in the sediment. Phoslock® transformed other P species into calcium-bound P. Calcium nitrate, ferric sulfate, and Phoslock® had negative influence on ammonia oxidizers, while four chemicals had positive influence on denitrifies, indicating that chemical treatment could inhibit nitrification but enhance denitrification. Aluminum sulfate had decreased chlorophyll content of the leaves of submerged macrophytes, while ferric sulfate and Phoslock® treatment would inhibit the growth of the root. Based on the results that we obtained, we emphasized that before application of chemical treatment, the effects on submerged macrophyte revegetation should be taken into consideration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) control is critical for mitigating eutrophication, because P is usually the limiting factor of primary productivity in freshwater ecosystems (Carpenter 2008; Schindler et al. 2008). However, after external P loading been blocked, internal P loading can delay the recovery of shallow lakes from cultural eutrophication for decades (Søndergaard et al. 2003; Welch and Cooke 2005). Therefore, the control of internal P release is of great importance for the success of remediation projects.
Internal P loading can be controlled by physical manipulation of the lake environment (e.g., aeration and dredging) and chemical treatment (e.g., oxygenates and active capping agents) (Hickey and Gibbs 2009; Welch and Cooke 2005; Zamparas and Zacharias 2014). Unlike physical measures, chemical treatment can be cost-effective, can cause less disturbance to water ecosystem, and can be easily applied without complex equipments and specialized machineries (Hickey and Gibbs 2009). Previous research has considered a number of chemical restoration agents, including oxygenate (e.g., calcium nitrate, permanganate, and hydrogen peroxide) and active capping agents (e.g., alum salt, iron salt, allophane, modified zeolite, and Phoslock®) (Hickey and Gibbs 2009; Welch and Cooke 2005; Zamparas and Zacharias 2014). Among chemical oxygenate, calcium nitrate has been frequently studied and applied to polluted sediments in many cases (Hemond and Lin 2010; Schauser et al. 2006). The added nitrate can act as an electron acceptor and participate in redox reactions in sediments. Fe(II) (e.g., FeS) is oxidized to FeOOH, and then, phosphate can combine with FeOOH and be removed from water (Yamada et al. 2012). Alum and iron salts are also commonly used in P inactivation (Liu et al. 2009). After adding to the water, the dissolved Al3+ and Fe3+ form Al(OH)3 and Fe hydroxides (e.g., FeOOH), which precipitate with P into the sediment (Guzmán et al. 2016; Li and Davis 2016). Phoslock®, a lanthanum modified bentonite clay, was invented for trapping P in aqueous solutions (Douglas et al. 1999). Phoslock® could reduce dissolved P by the formation of highly insoluble lanthanum–phosphate complex (Reitzel et al. 2013).
Chemical agents will affect the physicochemical environment of the sediment, such as pH (e.g., alum and iron) and redox potentials (e.g., calcium nitate) (Hickey and Gibbs 2009; Welch and Cooke 2005; Zamparas and Zacharias 2014), which could change the activities of microbial communities and thus affects the biogeochemical processes (Barcenas-Moreno et al. 2016; Fierer and Jackson 2006; Jeanbille et al. 2016; Lipson et al. 2015). Moreover, the introduction of metal ions (e.g., Al3+ and La3+) may exert direct toxic effects to microorganisms and thus affect their community structures and function (Amonette et al. 2003; Spears et al. 2013; Yaganza et al. 2004). Among those biogeochemical processes, nitrogen (N) cycling is mainly mediated by microorganisms (Chen et al. 2016). However, little is known about the impact of chemical treatment for P inactivation on N cycling-associated microorganisms.
Chemical treatment methods can be effective in P inactivation in the short term, but revegetation of the submerged macrophytes is crucial for the restoration of the impaired aquatic ecosystem. Submerged macrophytes can provide the essential habitats for fish and invertebrates and thus keep the balance of aquatic ecosystem. Besides, submerged macrophytes can reduce phytoplankton biomass by competing for nutrients and light, secreting allelochemicals, and providing refuge for herbivorous zooplankton, which contribute to the improvement of water quality (Ogdahl and Steinman 2015; Rodrigo et al. 2015; Zhu et al. 2015). However, it is currently unclear whether the chemical treatment can affect the subsequent submerged macrophyte revegetation.
In this work, four chemicals, including calcium nitrate, aluminum sulfate, ferric sulfate, and Phoslock®, were investigated. The aims of this study were to (1) evaluate and compare the abilities of the four chemical agents for P inactivation, (2) evaluate the effects of the four chemicals on the abundance of nitrification- and denitrification-related functional genes, and (3) explore the effects of the four chemicals on the growth of submerged macrophytes using Hydrilla verticillata as an example. Results from this work contribute to a better understanding of the efficiency and influence of chemical treatment on the restoration of aquatic ecosystem.
Materials and methods
Materials and chemicals
Water sample and sediment sample were collected from East Lake (30° 32′ 54.91″ N, 114° 21′ 15.61″ E) in Wuhan, China. Water sample was collected with a plexiglass hydrophore, and sediment sample was collected with a stainless steel grab sampler. After transferring back to the laboratory, the sediment sample was homogenized and passed through the 60-mesh sieve to remove coarse particles. Water content, total organic carbon (TOC), total Kjeldahl nitrogen (TKN), and total phosphorus (TP) of the homogenized sediment were 70.01 ± 2.07 %, 3.39 ± 0.12 %, 4.56 ± 0.16 mg N/g, and 1.28 ± 0.02 mg P/g, respectively. The concentrations of NH4 +-N and NO3 −-N of the sediment were 0.11 ± 0.003 and 0.11 ± 0.015 mg N/kg, respectively. Total nitrogen (TN), TP, NH4 +-N, and soluble reactive phosphorus (SRP) of the water sample were 3.22 ± 0.17, 0.14 ± 0.011, 1.09 ± 0.04, and 0.03 ± 0.002 mg/L, respectively.
All chemicals used in the experiment are of analytical-reagent grade. Ferric sulfate (Fe2(SO4)3) is supplied by Tianjin Kermel Chemical Reagent Development Center. Phoslock® (lanthanum-modified bentonite containing 5 % lanthanum) was supplied by the Phoslock® Water Solutions Ltd. Other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd.
Sediment treatment experiment
Water/sediment microcosms were setup in 500-mL glass beakers with a height of 15 cm and a diameter of 7 cm. A total of 30 beakers and five plastic containers were prepared. Beakers were each filled with 6-cm sediment (equal to about 247-g wet sediment) and 8 cm lake water and then were separated into five plastic containers (55 cm × 35 cm × 35 cm) (one for the control and four for treatments). Mixed lake water and tap water (1:1) were added to the plastic container to a height of 20 cm. Chemicals selected for the investigation were first prepared to solutions or slurries and then added to the surface of the sediment without agitation to avoid disturbing the sediment. An aliquot of 1 g of calcium nitrate, aluminum sulfate, Fe2(SO4)3, and Phoslock® was each added to beakers for corresponding treatments. No chemical was added in beakers for the control.
Microcosms were maintained at 24 ± 2 °C with a 12-h light/12-h dark cycle. Three beakers were retreated from each group on day 30 and on day 60 after incubation. Overlying water was siphoned into a sampling bottle. Then, a 50-mL syringe with an upper-end cut was inserted to the sediment to collect sediment samples. The sediment in the syringe was divided into two 3-cm layers, and only the upper layer was collected and measured. The rest of the sediment in the beaker was homogenized and centrifuged to collect interstitial water. The procedures of sampling were performed in the atmosphere of nitrogen gas, and the sampling bottles and bags were filled with nitrogen gas to avoid the oxidation of the samples. Water samples were stored in 4 °C and analyzed within 24 h after sampling.
Measurement and chemical analysis
Water pH and dissolved oxygen (DO) were measured by a Hach HQ40d portable multiparameter meter. For water samples, TN and TP were measured using the alkaline persulfate oxidation and persulfate oxidation methods, respectively, and NH4 +-N was determined by Nessler’s colorimetric method (MEPC 2002). After the water samples were filtered through 0.45-μm cellulose acetate membrane filters, SRP was determined by ammonium molybdate methods (MEPC 2002). For sediment samples, total Kjeldahl nitrogen (TKN) was determined by the automatic Kjeldahl nitrogen analyzer (S5, Behr, Germany), NH4 +-N and NO2 −-N were determined by a spectrophotometric method following potassium chloride extraction, and TP was measured as described by Ruban et al. (1999). The sequential extraction scheme of Psenner et al. (1984) with slightly modifications (Lin et al. 2015) was used for P fractionation. P was separated into loosely sorbed P (NH4Cl-P), reductant-soluble P (BD-P), metal oxide-bound P (NaOH-P), calcium-bound P (HCl-P), and residual P. Residual P was calculated by subtracting the extractable P species from TP.
DNA extraction and real-time quantitative PCR
The upper sediment samples collected on day 60 were used for DNA extraction. DNA was extracted from 0.3-g sediment using the E.Z.N.A. soil DNA kit (Omega Bio-tek, Norcross, GA) according to the manufacturer’s instruction. For the detection of ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB), nitrite reductase-encoding genes (nirK), and nitrous oxide reductase-encoding genes (nosZ), the following primers were used: archaeal amoA: Arch-amoA-F and Arch-amoA-R (Francis et al. 2005); bacterial amoA: amoA1F and amoA2R (Rotthauwe et al. 1997); nirK: nirK876 and nirK1040 (Henry et al. 2004); and nosZ: nosZ2F and nosZ2R (Henry et al. 2006). Quantitative PCR (qPCR) was performed by a real-time PCR instrument (Bio-Rad, USA) in a total volume of 20 μL, which contained 10 μL of iTaq universal SYBR Green Supermix (Bio-Rad, USA), 1 μL of each primer (10 μM), 7 μL of water, and 1 μL of template DNA. The thermal profiles for archaeal amoA genes and bacterial amoA genes consisted of 95 °C for 5 min, followed by 40 cycles of 94 °C for 45 s, 61 °C for 1 min, and 72 °C for 1 min; for nirK genes consisted of 94 °C for 2 min, followed by 40 cycles of 94 °C for 15 s, and 57 °C for 30 s; for nosZ genes consisted of 94 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 64.4 °C for 1 min. Assays used the pMD18-T (Takara, Japan) as the vector, and then, the vectors were transformed into E. coli hosts. Then, the plasmids were extracted by AxyPrep Plasmid Miniprep kits (Axygen, China) and the concentrations of plasmids were measured by a Nanodrop-8000 Spectrophotometer (Thermo, USA).
Standard curves contained the genes ranging from 4.56 × 106 to 1.43 × 1010, 6.96 × 106 to 2.17 × 1010, 5 to 5.00 × 107, and 2.00 × 102 to 2.00 × 107 copies per microliter for AOA, AOB, nirK, and nosZ, respectively. For AOA and AOB, the standard plasmids were five times dilution; for nirK and nosZ, the standard plasmids were ten times dilution. All samples and standard reactions were performed in triplicate, and an average value was calculated. A no-template control was used to exclude any false-positive amplification. Melt curves were generated after each assay to check the specificity of amplification. PCR efficiencies were 93.4 % for AOA, 97.1 % for AOB, 90.7 % for nirK, and 91.2 % for nosZ. Correlation coefficients (r 2) were greater than 0.99 for all runs. The gene abundances were presented as gene copies per gram of the dry sediment.
Growth of submerged macrophytes
H. verticillata was selected in this study, because it has a strong capability for growth in water and is commonly used in the restoration of eutrophicated shallow lakes in China (Yu et al. 2010; Zhang and Liu 2011). H. verticillata were collected in Wuhan Botanical Garden, China, and then were preincubated under laboratory condition before experiment. Thirty days after the modification of chemicals, 45 fine apical shoots with a length of 10 cm were weighed and planted into the beakers through cuttage. Each beaker was planted with 3 shoots.
After 30-day growth, the plants were uprooted from the sediment. After being washed with deionized water and carefully dried with a filter paper, the plants were weighed to measure the fresh weight (including roots, stem, and leaves). The numbers of roots were counted, and the lengths of the roots were measured. The relative growth rate (RGR, g/day) for each treatment was calculated using the following equation: RGR = (ln W t − ln W 0) / t, where W t and W 0 are the final and initial wet weights (g), respectively, and t is the interval (days) between the two measurements.
For RGR, lengths of roots, and numbers of roots, the sum of three plants from one beaker was treated as one sample. For the measurement of chlorophyll, enzyme activity, and malondialdehyde (MDA) content, three plants in one beaker were combined. The chlorophyll content of the leaves was determined by the spectrophotometric method at 663 and 645 nm after 24-h extraction with 80 % ethanol. The contents of protein and MDA of the shoot tip were determined by the bicinchoninic acid and thiobarbituric acid methods, and the activities of superoxide dismutase (SOD) and peroxidase (POD) were determined by the hydroxylamine method and colorimetric method using the assay kits provided by Nanjing Jiancheng Bioengineering Institute, respectively.
Data analysis
All results were presented as the means and standard deviations of three replicates. Differences between treatments and control were tested using one-way ANOVA by a LSD post hoc test using PASW Statistics 18 (SPSS Inc.). Canoco 4.5 (Plant Research International) was used for principal component analysis and plotting. All the other figures were generated using the Origin Pro 9.0 software. A confidence level of 95 % was used.
Results and discussion
Effects of chemical treatment on pH and DO in overlying water
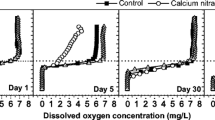
In overlying water, the addition of aluminum sulfate and ferric sulfate caused a minor decrease (p < 0.05) in water pH (Fig. 1). On day 30, the water pHs in control, aluminum sulfate, and ferric sulfate were 7.41, 6.15, and 6.40, respectively. On day 60, water pHs were 7.82, 6.59, and 6.81 in control, aluminum sulfate, and ferric sulfate, respectively. In calcium nitrate treatment, only a slight decrease (p < 0.05) was observed on day 30. Hydrolytic reactions of the added Ca2+, Al3+, and Fe3+ might have caused the increase of hydrogen ion, resulting in the decline of water pH in these treatments. On day 30, the DO concentrations in treatment of calcium nitrate, aluminum salt, and ferric salt were higher than in control (p < 0.05) in the overlying water. On day 60, DO in calcium nitrate and aluminum salt treatment was lower than in control (p < 0.05). The DO concentration in ferric sulfate treatment had no significant difference with control on day 60. The addition of Phoslock® decreased DO concentration (p < 0.05) in the overlying water at both sampling points. The capping of the bentonite clay might have caused the reduction of DO of the sediment/water system. Vopel et al. (2008) used Phoslock® as capping materials in the dose of 200–600 g Phoslock®/m2, and the results showed that O2 saturation was decreased by ∼5 % in the capping layer/water interface. The capping layer of Phoslock® increased the distance for the diffusion of DO from the bottom water to the interstitial water but not interfered with the upward diffusion of reduced solutes, resulting in the upward oxidation zone for reduced compounds. The oxidation of reduced solutes might be oxidized in the overlying water rather than in the sediment, causing the decrease of DO in the bottom water (Vopel et al. 2008).
Effects of chemical treatment on phosphorus
P changes in overlying water and interstitial water
Significant decreases (p < 0.05) of TP and SRP concentrations in the interstitial water were observed in all treatments on day 30 and day 60 (Fig. 2b, d). In the overlying water, the application of aluminum sulfate decreased TP on day 30 and day 60 (Fig. 2c) but decreased SRP only on day 30 (Fig. 2a) (p < 0.05). The calcium nitrate treatment showed no significant influence (p > 0.05) on TP and increased SRP (p < 0.05) in the overlying water. Ferric sulfate and Phoslock® showed no significant impact on TP but caused a significant decrease of SRP (p < 0.05) on day 30 in the overlying water.
Theoretically, all four chemicals (i.e., calcium nitrate, aluminum sulfate, ferric sulfate, and Phoslock®) are able to remove P from water by the formation of FeOOH, alum flocs, ferric flocs, and LaPO4, respectively (Yamada et al. 2012; Guzmán et al. 2016; Li and Davis 2016; Reitzel et al. 2013). In this study, high P removal efficiency in the interstitial water was observed in four treatments, showing the great abilities of lowering the risk of P release from the interstitial water. This was of great significance for controlling the release of endogenous P. Among the four, aluminum sulfate was the most effective agent for P removal and immobilization in the overlying water and the interstitial water, followed by ferric sulfate. However, the abilities of other chemicals in controlling P concentration in overlying water were weak. What is more, the addition of calcium nitrate caused an increase in SRP in the overlying water, whereas Phoslock® caused the increase in TP in the overlying water on day 30. The following reasons might be involved: First, the added nitrate could penetrate into sediment and promote denitrification, resulting in the production of nitrogen gases in the sediment (Xu et al. 2014). This was verified by the observation of gaps in the sediment and on the sediment surface. When rising from the sediment, the gas can loosen the sediment and release P in the interstitial water, resulting in an increase of P flux from sediment to overlying water. Second, the dosage for Phoslock® in this experiment (about 260 g/m2) might be not enough to retain P in the sediment. Meis et al. (2013) indicated that an addition of 510 g/m2 Phoslock® was required to control the P release from sediment. Previously, when a dosage of 510 g/m2 Phoslock® was used, the P concentration in the overlying water was maintained at a lower level throughout the experimental period (Lin et al. 2015). The capping of Phoslock® could lead to a decrease in DO concentration (even zero) below the capping layer, leading to an anaerobic condition at the capping layer/sediment interface (Vopel et al. 2008). If the dosage of Phoslock® was not enough to retain P, the flux of P into the overlaying water might occur, leading to an increase of TP in the overlying water.
Changes of P species in the sediment
NH4Cl-P is the most bioavailable form of P and has a high likelihood of release (Jing et al. 2015; Meis et al. 2012). In Fig. 3a, 60 days after treatments, aluminum sulfate, ferric sulfate, and Phoslock® significantly decreased NH4Cl-P content (p < 0.05), indicating a favorable tendency to control the release of P from sediments.
In Fig. 3b, the application of calcium nitrate and ferric sulfate caused an increase in BD-P (p < 0.05), while aluminum sulfate and Phoslock® treatment decreased BD-P (p < 0.05). BD-P represents the redox-sensitive P bound to Fe-hydroxides and Mn compounds (Kaiserli et al. 2002; Lukkari et al. 2007). The added nitrate in the calcium nitrate treatment acted as an electron acceptor, resulting in the oxidation of sulfides (e.g., iron sulfide) and the release of Fe2+. Since the addition of calcium nitrate had increased DO concentration in the overlying water (Fig. 2), the released Fe2+ can be oxidized to Fe-hydroxides (Yamada et al. 2012) under aerobic conditions or with the existence of adequate nitrate. The addition of ferric sulfate increased DO concentration on day 30, favoring the formation of FePO4 and Fe-hydroxides, resulting in an increase in BD-P content, whereas, on day 30, the addition of Phoslock® decreased DO content in the overlying water, which might result in the reduction of Fe-hydroxides and thus caused a decrease in BD-P in this treatment.
Figure 3c shows the changes of NaOH-P. NaOH-P includes P bound to aluminum oxides, non-reducible Fe, and some organic P with complex components that are easy to mineralize (Kaiserli et al. 2002; Lukkari et al. 2007; Meis et al. 2012). P can be combined to different kinds of aluminum-containing minerals, such as wavellite (Al3(OH)3(PO4)2·5H2O) and variscite (AlPO4·2H2O) (Lukkari et al. 2007). The added aluminum salt had significantly increased the NaOH-P content (p < 0.05) by the formation of aluminum oxides. Besides, a decrease in water pH favors the retention of NaOH-P because NaOH-P is sensitive to high pH. A slight decrease in water pH was observed in the calcium nitrate treatment, and no significant change was observed in the Phoslock® treatment, whereas NaOH-P in both calcium nitrate and Phoslock® treatments decreased. The released P in NaOH-P might be retained in NH4Cl-P and BD-P forms in the calcium nitrate treatment and in HCl-P form in the Phoslock® treatment.
HCl-P and residual P were the more stable P fractions. Both of them can be considered as nonbioavailable forms (Jing et al. 2015). In this study (Fig. 3e), the four chemicals had no significant influence on residual P content owing to its stability. However, the addition of aluminum salts and ferric salts caused the decrease of HCl-P content on day 60 (p < 0.05). HCl-P contains P bound to carbonates and apatite P (Meis et al. 2012), and this P form is very sensitive to lower pH. Addition of aluminum and ferric salts decreased pH and therefore decreased HCl-P. Phoslock® application led to an increase in HCl-P on both day 30 and day 60 (p < 0.05). On day 60, the differences of HCl-P content between the Phoslock® treatment and the control was 0.11 mg P/g, while the sum of decreased P content, including NH4Cl-P, BD-P, and NaOH-P, in the Phoslock® treatment on day 60 was 0.09 mg P/g. A coincident decrease of P was observed in the interstitial water. Thus, decreased P in the interstitial water, NH4Cl-P, BD-P, and NaOH-P contents in the Phoslock® treatment was likely be retained in the form of HCl-P.
Effects of chemical treatment on nitrogen, ammonia-oxidizing microorganisms, and denitrifying microorganisms
NH4 +-N concentration in the overlying water treated by aluminum salt was higher (p < 0.05) than that of the control at both sampling points. Ferric sulfate also caused an increase in NH4 +-N concentration (p < 0.05) in the overlying water on day 30, but not on day 60. NH4 +-N concentration in the overlying water treated by ferric salt decreased significantly (p < 0.05), while TN concentration increased (p < 0.05) on day 60. NH4 +-N concentration in the overlying water treated by Phoslock® was higher than that of the control (p < 0.05) at both sampling points. In the interstitial water and sediment treated with calcium nitrate, NH4 +-N concentration increased (p < 0.05) at both sampling points. On day 30, NH4 +-N concentration in the interstitial water treated with aluminum salt and ferric salt was higher (p < 0.05) than that of the control. The four chemicals had minor effects on TKN in the sediment (Fig. 4).
The abundance of archaeal amoA (AOA), bacterial amoA (AOB), nirK, and nosZ genes was measured in the sediment sampled from day 60 (Fig. 5). AOA and AOB gene copies ranged from 3.72 × 1010 to 5.69 × 1010 and 2.68 × 109 to 5.56 × 109 g−1 dry weight soil, respectively. Significant decreases (p < 0.05) of AOA and AOB genes were observed in sediment treated by ferric sulfate and calcium nitrate. A significant decrease (p < 0.05) in the abundance of AOA genes was also found in sediment treated with Phoslock®. No significant difference was found between the aluminum sulfate treatment and the control. The denitrifying bacteria genes showed different tendencies compared to the AOA and bacteria. The abundance of nirK genes increased (p < 0.05) by the application of the four chemicals. The abundance of nosZ genes increased dramatically in the calcium nitrate treatment, while the other three treatments had no significant influence on the abundance of nosZ genes. The nosZ gene number treated with calcium nitrate was 3.42 × 108 g−1 dry weight soil, and it was about ten times than the other groups, which ranged from 1.99 × 107 to 3.54 × 107 g−1 dry weight soil.
Reduced pH can affect the balance between ammonia (NH3) and ammonium (NH4 +), leading to the increase of NH4 +-N concentration (Beman et al. 2011; Watanabe 2015). AOA and AOB tend to use ammonia as the substrate rather than ammonium; thus, reduced pH can slow ammonia oxidation rates and inhibit AOB and AOA growth (Beman et al. 2011; Ward 1987; Wang and Gu 2014). In our study, a decrease in pH was found in the overlying water treated by calcium nitrate, aluminum salt, and ferric salt on day 60, which might explain the decrease in AOA and AOB gene copies in calcium nitrate and ferric salt treatments. However, a decrease in AOA and AOB gene copies was not found in aluminum salt treatment, which had the lowest water pH on day 60. Moreover, water pH in the Phoslock® treatment showed no significant difference with control on day 60, but AOA decreased in the Phoslock® treatment. Lots of evidences had shown that some ammonia-oxidizing microorganisms could survive in acidic soil (De Boer and Kowalchuk 2001; Allison and Prosser 1993; Nicol et al. 2008). Nicol et al. (2008) even found that the abundance of AOA genes decreased significantly as soil pH increased, while AOB genes increased with the increase of soil pH. The composition and diversity of bacterial communities can be influenced by a wide range of biotic and abiotic factors. So we guessed that in addition to pH, there were other factors being involved, such as DO, ammonia availability, and organic carbon. However, it is difficult to determine which of these factors had led to the phenomena of AOA and AOB in aluminum salt treatment based on the available results. But it was likely that a decrease in the AOA abundance in the sediment treated with Phoslock® might be caused by a decrease in DO as AOA can oxidize ammonia into nitrite in the presence of oxygen (Könneke et al. 2005). Meanwhile, a decrease in DO in the Phoslock® treatment might have led to other changes. When the reduction of DO in the Phoslock® treatment occurred, some other nitrogen forms might be reduced to ammonium, leading to an increase in NH4 +-N in the overlying water and the interstitial water (Fig. 4). However, an increase in NH4 +-N concentration might also be caused by the NH4 +-N contamination in Phoslock®. We had checked the NH4 +-N contamination in the four chemicals, and ∼1 g chemicals were added to ∼300 mL of pure water, respectively. Results showed that Phoslock® could result in an increase in NH4 +-N in water. Reitzel et al. (2013) also stated that ammonium might be released from Phoslock® when Phoslock® was dispersed in water.
Denitrification can be affected by many factors, such as DO, nitrate content, organic matter, redox potential, temperature, pH, and soil type, but the determining factors of denitrification were nitrate concentration, DO, and pH (Ligi et al. 2014; Shao et al. 2011). A few studies found that denitrification could be enhanced by nitrate addition and oxygen supply could inhibit denitrification (Firestone et al. 1979; Thomas et al. 1994; Shao et al. 2011). Similar results were observed in this study that the application of calcium nitrate increased the number of nirK gene copies and promoted denitrification. In ferric sulfate treatment on day 60, NH4 +-N decreased while TN increased in the overlying water. It was possible that part of NH4 +-N was oxidized to NO3 −-N. Therefore, the addition of ferric sulfate caused an increase in nirK genes and enhanced denitrification. Besides, the application of aluminum sulfate and Phoslock® also significantly increased the abundance of nirK genes. We assumed that the decrease in DO on day 60 caused by aluminum salt and Phoslock® addition had promoted the denitrification activity. Our results indicated that all four chemicals could promote denitrification process. However, the direct addition of nitrate had the largest impact. The addition of calcium nitrate dramatically increased the nosZ gene copies. The nitrous oxide reductase was encoded by nosZ gene, and this enzyme catalyzes the reduction of N2O to N2 (Thomas and Moselio 2012). An increase in the abundance of nosZ genes may benefit the controlling of greenhouse emission from sediment (Cui et al. 2016; Iribar et al. 2015).
Principal component analysis of different treatments
Principal component analysis (PCA) of 17 environmental factors in the overlaying water, interstitial water, and surface sediments was proceeded for 30 samples in this work. The first two PCs account for 32.35 and 22.34 % of the total variance explained, respectively, with a cumulative variance of 55.69 %. The PC1 axis was significantly positively correlated to (p < 0.01) NH4 +-N in interstitial water (r = 0.7406) and NaOH-P in sediment (r = 0.8879), while significantly negatively correlated (p < 0.01) to pH in the overlaying water (r = −0.9303), TP (r = −0.738), and SRP (r = −0.647) in the interstitial water and BD-P (r = −0.6545) and HCl-P (r = −0.6068) in the sediments. The PC2 axis was significantly positively correlated (p < 0.01) with SRP (r = 0.8344), DO (r = 0.647), and TN (r = 0.7659) in the overlaying water and NH4Cl-P (r = 0.785) in the sediments. Overall, the PC1 and PC2 represented the gradient of P in the sediments and environmental factors in the overlaying water, respectively.
There were clear separations of most of the treatments in different sampling dates in PCA ordination plot, except the Phoslock® treatment and the control (Fig. 6), implying that the Phoslock® treatment in this study might have less impact environmental gradients in PC1 and PC2. The separations of different treatments in the plot represented the different effects of these treatments. For example, the samples in the calcium nitrate treatment were separated from the other samples along the direction of the PC2 axis, indicating that compared to the other treatments and the control, the calcium nitrate treatment could increase SRP, DO, and TN in the overlaying water and NH4Cl-P in the sediments, while the samples in aluminum sulfate treatment and ferric sulfate treatment were separated from the other samples along the PC1 axis, denoting that these two treatments could increase NH4 +-N in the interstitial water and NaOH-P in the sediment and decrease the pH in the overlaying water, TP and SRP in the interstitial water, and BD-P and HCl-P in the sediments. Moreover, the distances between treatment sites and control sites became smaller in day 60 compared with those in day 30, which that indicated the effects of the treatments would fade through time.
PCA of 17 environmental factors in overlaying water (O), interstitial water (I), and sediment (S), showing horizontally the PC1 axis and vertically the PC2 axis. Sampling sites were represented by symbols and environmental factors were represented by solid arrows. The length of the arrow was a measure of the importance of the variable, and the arrowhead pointed in the direction of increasing influence
Effects on submerged macrophyte revegetation
The relative growth rate (RGR), the root number, the root length, the chlorophyll, the activities of SOD and POD, and the MDA content are shown in Fig. 7. The application of the four chemicals showed no significant influence (p > 0.05) on RGR. Ferric sulfate and Phoslock® treatment significantly decreased the number of the roots, and the root length was shorter in Phoslock® treatment than that of the control (p < 0.05). Phoslock® capping might reduce the diffusion of oxygen to the sediment, thus depressed the root growth of the plants. Chlorophyll content in plant leaves was lower in aluminum treatment than in the control (p < 0.05) but did not differ significantly between the control and the other three treatments (p > 0.05). NH4 +-N stress can damage the photosynthetic system and inhibit photosynthesis in aquatic plants (Zhu et al. 2015). Wang et al. (2008) found that chlorophyll decreased by about 12 % in leaves treated with 0.4 mM NH4Cl (equal to 5.6 mg/L NH4 +-N) for 4 days. In our study, the NH4 +-N concentration treated by aluminum salt was 5.23 mg/L on day 60. Thus, we assumed that the decrease in chlorophyll treated by aluminum salt was caused by NH4 + stress. SOD activity (p < 0.05) decreased in plant leaves treated by calcium nitrate but no such trends for the other three treatments. Furthermore, no significant difference was observed in POD activities in the four treatments. Environment stress can cause the imbalance of cell activities and induce the excess production of reactive oxygen species (ROS) (Apel and Hirt 2004; Mittler et al. 2004). Excess ROS results in the response of plants to oxidative stress, including enhancement or suppression of activities of antioxidative enzymes (Wang et al. 2008). Among the antioxidative enzymes, SOD can defend against ROS by catalyzing superoxide to H2O2, and POD can decompose H2O2 to water and oxygen (Wang et al. 2008). MDA is an end product of polyunsaturated fatty acid oxidation and has been used to detect the degree of lipid peroxidation, and the MDA concentration is an important index of physiological stress (Wang et al. 2008; Zhu et al. 2015). In this study, MDA concentration in plant leaves treated by aluminum salt, ferric salt, and Phoslock® decreased significantly (p < 0.05) compared with control. However, no increased SOD activities, POD activities, or MDA concentration was found in this study, indicating that there might be no oxidative stress on plants by the application of the four chemicals, or after growing for 30 days, the oxidative stress caused by the chemicals may not be observed due to the self-healing.
Effects of chemical treatment on a relative growth rate (RGR); b root numbers; c root length; d chlorophyll content, activities of e superoxide dismutase (SOD) and f peroxidase (POD), and g malondialdehyde (MDA) content of Hydrilla verticillata. Asterisks indicate where a significant difference was observed between treatments and controls (p < 0.05)
Conclusion
Overall, our results showed that all of the four tested chemicals had the abilities of reducing P release from interstitial water. Calcium nitrate, ferric sulfate, and Phoslock® inhibited nitrification, and all treatment promoted denitrification process. Aluminum sulfate was the most efficient in reducing P in the overlying water and the interstitial water and reducing the risk of releasing P from sediment. However, negative effect on chlorophyll of H. verticillata was observed which should be paid attention in subsequent submerged macrophyte revegetation. Ferric sulfate was also an efficient chemical for P inactivation, and it had the efficiency of reducing P inferior to aluminum sulfate. Calcium nitrate is not recommended when P content in lake water is much lower than in the sediment, since the application of calcium nitrate might lead to P release from the sediment to the overlying water, which might trigger the outbreak of algal blooms. Phoslock® can be effective in reducing P in overlying water only when added at enough dosage. Phoslock® was much more expensive than the other three chemicals; therefore, the application of Phoslock® may not be cost-effective. Another consequence of Phoslock® application is that capping of Phoslock® might cause the lack of oxygen in sediment and thus harmful to the growth of plant roots, which is unfavorable for the submerged macrophyte revegetation.
References
Allison SM, Prosser JI (1993) Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil Biol Biochem 25:935–941. doi:10.1016/0038-0717(93)90096-t
Amonette JE, Russell CK, Carosino KA, Robinson NL, Ho JT (2003) Toxicity of Al to Desulfovibrio desulfuricans. Appl Environ Microbiol 69:4057–4066. doi:10.1128/aem.69.7.4057-4066.2003
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
Barcenas-Moreno G, Baath E, Rousk J (2016) Functional implications of the pH-trait distribution of the microbial community in a re-inoculation experiment across a pH gradient. Soil Biol Biochem 93:69–78. doi:10.1016/j.soilbio.2015.10.024
Beman JM et al (2011) Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc Natl Acad Sci U S A 108:208–213. doi:10.1073/pnas.1011053108
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci U S A 105:11039–11040. doi:10.1073/pnas.0806112105
Chen J, Zhou HC, Pan Y, Shyla FS, Tam NFY (2016) Effects of polybrominated diphenyl ethers and plant species on nitrification, denitrification and anammox in mangrove soils. Sci Total Environ 553:60–70. doi:10.1016/j.scitotenv.2016.02.052
Cui PY et al (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93:131–141. doi:10.1016/j.soilbio.2015.11.005
De Boer W, Kowalchuk GA (2001) Nitrification in acid soils: micro-organisms and mechanisms. Soil Biol Biochem 33:853–866. doi:10.1016/s0038-0717(00)00247-9
Douglas GB, Adeney JA, Robb MS (1999) A novel technique for reducing bioavailable phosphorus in water and sediments. International Association Water Quality Conference on Diffuse Pollution: 517–523. http://www.phoslock.eu/media/7476/Novel_Technique_-_CSIRO_Paper_Douglas_etal_1999.pdf
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi:10.1073/pnas.0507535103
Firestone MK, Smith MS, Firestone RB, Tiedje JM (1979) Influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of dentrification in soil. Soil Sci Soc Am J 43:1140–1144. doi:10.2136/sssaj1979.03615995004300060016x
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688. doi:10.1073/pnas.0506625102
Guzmán A, Nava JL, Coreño O, Rodríguez I, Gutiérrez S (2016) Arsenic and fluoride removal from groundwater by electrocoagulation using a continuous filter-press reactor. Chemosphere 144:2113–2120. doi:10.1016/j.chemosphere.2015.10.108
Hemond HF, Lin K (2010) Nitrate suppresses internal phosphorus loading in an eutrophic lake. Water Res 44:3645–3650. doi:10.1016/j.watres.2010.04.018
Henry S, Baudoin E, Lopez-Gutierrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 59:327–335. doi:10.1016/j.mimet.2004.07.002
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189. doi:10.1128/aem.00231-06
Hickey CW, Gibbs MM (2009) Lake sediment phosphorus release management-decision support and risk assessment framework. N Z J Mar Freshwat Res 43:819–854. doi:10.1080/00288330909510043
Iribar A, Hallin S, Perez JMS, Enwall K, Poulet N, Garabetian F (2015) Potential denitrification rates are spatially linked to colonization patterns of nosZ genotypes in an alluvial wetland. Ecol Eng 80:191–197. doi:10.1016/j.ecoleng.2015.02.002
Jeanbille M, Buee M, Bach C, Cebron A, Frey-Klett P, Turpault MP, Uroz S (2016) Soil parameters drive the structure, diversity and metabolic potentials of the bacterial communities across temperate beech Forest soil sequences. Microb Ecol 71:482–493. doi:10.1007/s00248-015-0669-5
Jing LD, Liu XL, Bai S, Wu CX, Ao HY, Liu JT (2015) Effects of sediment dredging on internal phosphorus: A comparative field study focused on iron and phosphorus forms in sediments. Ecol Eng 82:267–271. doi:10.1016/j.ecoleng.2015.04.099
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments—lakes Volvi and Koronia. N Greece Chemosphere 46:1147–1155. doi:10.1016/s0045-6535(01)00242-9
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546. doi:10.1038/nature03911
Li JK, Davis AP (2016) A unified look at phosphorus treatment using bioretention. Water Res 90:141–155. doi:10.1016/j.watres.2015.12.015
Ligi T, Truu M, Truu J, Nolvak H, Kaasik A, Mitsch WJ, Mander U (2014) Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex. Ecol Eng 72:47–55. doi:10.1016/j.ecoleng.2013.07.015
Lin J, Qiu P, Yan X, Xiong X, Jing L, Wu C (2015) Effectiveness and mode of action of calcium nitrate and Phoslock® in phosphorus control in contaminated sediment, a microcosm study. Water Air Soil Pollut 226:1–12. doi:10.1007/s11270-015-2590-4
Lipson DA, Raab TK, Parker M, Kelley ST, Brislawn CJ, Jansson J (2015) Changes in microbial communities along redox gradients in polygonized Arctic wet tundra soils. Environ Microbiol Rep 7:649–657. doi:10.1111/1758-2229.12301
Liu GR, Ye CS, He JH, Qian Q, Jiang H (2009) Lake sediment treatment with aluminum, iron, calcium and nitrate additives to reduce phosphorus release. J Zhejiang Univ Sci A 10:1367–1373. doi:10.1631/jzus.A0920028
Lukkari K, Hartikainen H, Leivuori M (2007) Fractionation of sediment phosphorus revisited. I: fractionation steps and their biogeochemical basis. Limnol Oceanogr Methods 5:433–444
Meis S, Spears BM, Maberly SC, O'Malley MB, Perkins RG (2012) Sediment amendment with Phoslock (R) in Clatto reservoir (Dundee, UK): investigating changes in sediment elemental composition and phosphorus fractionation. J Environ Manag 93:185–193. doi:10.1016/j.jenvman.2011.09.015
Meis S, Spears BM, Maberly SC, Perkins RG (2013) Assessing the mode of action of Phoslock® in the control of phosphorus release from the bed sediments in a shallow lake (loch Flemington, UK). Water Res 47:4460–4473. doi:10.1016/j.watres.2013.05.017
MEPC (2002) Standard methods for examination of water and wastewater, fourth edn. Chinese Environmental Sciences Press, Beijing
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498. doi:10.1016/j.tplants.2004.08.009
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978. doi:10.1111/j.1462-2920.2008.01701.x
Ogdahl ME, Steinman AD (2015) Factors influencing macrophyte growth and recovery following shoreline restoration activity. Aquat Bot 120:363–370. doi:10.1016/j.aquabot.2014.10.006
Psenner R, Pucsko R, Sager M (1984) Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten–Versuch einer Definition ökologisch wichtiger Fraktionen. Arch Hydrobiol Suppl 70:111–155
Reitzel K, Lotter S, Dubke M, Egemose S, Jensen HS, Andersen FØ (2013) Effects of Phoslock® treatment and chironomids on the exchange of nutrients between sediment and water. Hydrobiologia 703:189–202. doi:10.1007/s10750-012-1358-8
Rodrigo MA, Rojo C, Segura M, Alonso-Guillen JL, Martin M, Vera P (2015) The role of charophytes in a Mediterranean pond created for restoration purposes. Aquat Bot 120:101–111. doi:10.1016/j.aquabot.2014.05.004
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Ruban V, López-Sánchez J, Pardo P, Rauret G, Muntau H, Quevauviller P (1999) Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediment. J Environ Monit 1:51–56. doi:10.1039/a807778i
Schauser I, Chorus I, Lewandowski J (2006) Effects of nitrate on phosphorus release: comparison of two Berlin lakes. Acta Hydrochim Hydrobiol 34:325–332. doi:10.1002/aheh.200500632
Schindler DW et al (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci U S A 105:11254–11258. doi:10.1073/pnas.0805108105
Shao MF, Zhang T, Fang HHP, Li XD (2011) The effect of nitrate concentration on sulfide-driven autotrophic denitrification in marine sediment. Chemosphere 83:1–6. doi:10.1016/j.chemosphere.2011.01.042
Søndergaard M, Jensen JP, Jeppesen E (2003) Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506:135–145. doi:10.1023/B:HYDR.0000008611.12704.dd
Spears BM et al (2013) Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: an analysis of water column lanthanum data from 16 case study lakes. Water Res 47:5930–5942. doi:10.1016/j.watres.2013.07.016
Thomas MS, Moselio S (2012) Topics in ecological and environmental microbiology. Science Press, Beijing
Thomas KL, Lloyd D, Boddy L (1994) Effects of oxygen, pH and nitrate concentration on denitrification by Pseudomaonas species. FEMS Microbiol Lett 118:181–186. doi:10.1016/0378-1097(94)90616-5
Vopel K, Gibbs M, Hickey CW, Quinn J (2008) Modification of sediment-water solute exchange by sediment-capping materials: effects on O2 and pH. Mar Freshw Res 59:1101–1110. doi:10.1071/MF08130
Wang YF, Gu JD (2014) Effects of allylthiourea, salinity, and pH on ammonia/ammonium-oxidizing prokaryotes in mangrove sediment incubated in laboratory microcosms. Appl Microbiol Biotechnol 98:3257–3274. doi:10.1007/s00253-013-5399-3
Wang C, Zhang SH, Wang PF, Hou J, Li W, Zhang WJ (2008) Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquat Toxicol 87:88–98. doi:10.1016/j.aquatox.2008.01.009
Ward BB (1987) Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus. Arch Microbiol 147:126–133. doi:10.1007/bf00415273
Watanabe Y et al (2015) Response of the ammonia oxidation activity of microorganisms in surface sediment to a controlled sub-seabed release of CO2. Int J Greenhouse Gas Control 38:162–170. doi:10.1016/j.ijggc.2014.11.013
Welch EB, Cooke GD (2005) Internal phosphorus loading in shallow lakes: importance and control. Lake Reserv Manage 21:209–217. doi:10.1080/07438140509354430
Xu MY et al (2014) Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J 8:1932–1944. doi:10.1038/ismej.2014.42
Yaganza ES, Rioux D, Simard M, Arul J, Tweddell RJ (2004) Ultrastructural alterations of Erwinia carotovora subsp atroseptica caused by treatment with aluminum chloride and sodium metabisulfite. Appl Environ Microbiol 70:6800–6808. doi:10.1128/aem.70.11.6800-6808.2004
Yamada T et al (2012) Calcium nitrate addition to control the internal load of phosphorus from sediments of a tropical eutrophic reservoir: microcosm experiments. Water Res 46:6463–6475. doi:10.1016/j.watres.2012.09.018
Yu HC, Ye C, Song XF, Liu J (2010) Comparative analysis of growth and physio-biochemical responses of Hydrilla verticillata to different sediments in freshwater microcosms. Ecol Eng 36:1285–1289. doi:10.1016/j.ecoleng.2010.06.004
Zamparas M, Zacharias I (2014) Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci Total Environ 496:551–562. doi:10.1016/j.scitotenv.2014.07.076
Zhang XF, Liu ZW (2011) Interspecific competition effects on phosphorus accumulation by Hydrilla verticillata and Vallisneria natans. J Environ Sci 23:1274–1278. doi:10.1016/s1001-0742(10)60548-7
Zhu Z, Yuan H, Wei Y, Li P, Zhang P, Xie D (2015) Effects of ammonia nitrogen and sediment nutrient on growth of the submerged plant Vallisneria natans. Clean-Soil Air Water 43:1653–1659. doi:10.1002/clen.201300878
Acknowledgments
The authors would like to thank the support from the Major Scientific and Technological Innovation Projects of the Hangzhou City (20131813A04), the Science and Technology Project of the Ministry of Housing and Urban-Rural Development of China (2014-K7-014), and the National Major Science and Technology Projects for Pollution Control and Management (2012ZX07104-002-005, 2012ZX07101-007-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hein
Rights and permissions
About this article
Cite this article
Lin, J., Zhong, Y., Fan, H. et al. Chemical treatment of contaminated sediment for phosphorus control and subsequent effects on ammonia-oxidizing and ammonia-denitrifying microorganisms and on submerged macrophyte revegetation. Environ Sci Pollut Res 24, 1007–1018 (2017). https://doi.org/10.1007/s11356-016-7828-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7828-1